Abstract
Background: The authors describe the developmental process of intravenous anti-COVID-19 hyperimmune immunoglobulin from anti-SARS-CoV-2 neutralizing antibody-containing plasma. Furthermore, the authors investigated its safety and protective activity in animal models. Materials & methods: The manufacturing process included standard ethanol fractionation, chromatographic purification steps and virus removal or inactivation. Results: The authors produced pure and safe immunoglobulin for intravenous administration, with 98.1 ± 6.5 mg/ml protein content, of which 97.6 ± 0.7% was IgG. The concentration factor of SARS-CoV-2 neutralizing antibodies was 9.4 ± 1.4-times. Safety studies in animals showed no signs of acute/chronic toxicity or allergenic or thrombogenic properties. Intravenous anti-COVID-19 hyperimmune immunoglobulin protected immunosuppressed hamsters against SARS-Cov-2. Conclusion: The obtained results can allow the start of clinical trials to study the safety and efficacy in healthy adults.
Keywords: : COVID-globulin, hyperimmune immunoglobulin, IgG, immunoglobulin against COVID-19, preclinical evaluation, protective efficacy, SARS-CoV-2
Plain language summary
An intravenous immunoglobulin with a high concentration of SARS-CoV-2-neutralizing antibodies was prepared from COVID-19 convalescent plasma, which could be utilized as a passive immunization tool in regard to COVID-19 treatment. The manufacturing process employed conforms to commonly held business standards within the intravenous immunoglobulin industry and includes plasma ethanol fractionation following chromatographic purification and special virus removal or inactivation steps. The results of the preclinical in vitro and in vivo experiments demonstrate that the immunoglobulin produced in this study is pure and safe enough to be considered for intravenous applications. The SARS-CoV-2 neutralizing antibody concentration was found to have increased 9.4 ± 1.4-times compared with human plasma. The anti-COVID-19 hyperimmune immunoglobulin showed no signs of toxicity and did not cause any blood clot formations when administered to rabbits. Furthermore, the anti-COVID-19 hyperimmune immunoglobulin was demonstrated to protect immunosuppressed hamsters against SARS-CoV-2.
Tweetable abstract
Pure and safe intravenous immunoglobulin with a high concentration of SARS-CoV-2 neutralizing antibodies was produced from #COVID19 convalescent plasma and demonstrated protective effects against SARS-CoV-2 in immunosuppressed hamsters.
COVID-19 is an acute viral disease, caused by SARS-CoV-2, that predominantly causes lesions in the upper respiratory tract; it escalated into a pandemic in 2020. As of 19 April 2022, there had been more than 505 million laboratory-confirmed cases of SARS-CoV-2 infection and more than 6.2 million deaths worldwide [1]. Several therapeutic options are now available for the treatment of COVID-19, such as antiviral medications, IL-6 inhibitors, kinase inhibitors and corticosteroids, but morbidity and mortality persist and effective and specific medications are still needed.
One of the first healthcare system decisions worldwide was the use of COVID-19 convalescent plasma (CCP) for disease treatment. This method has been historically used in many outbreaks since the Spanish flu [2,3]. Published results of CCP treatment's effectiveness are contradictory [4–7]. The results of several studies support the beneficial effect of CCP in the treatment of COVID-19 patients at various disease stages and indicate decreasing mortality risk [8,9]. Other studies demonstrate no improvement in hospitalized patients with COVID-19 [10,11]. There are several disadvantages associated with this treatment, such as the uncontrolled consistency of cytokines, blood clotting factors and other molecules that can worsen the condition of recipient patients [12]. Adverse effects can include allergy/anaphylaxis, transfusion-related acute lung injury, transfusion-associated circulatory overload, unintended infection and antibody-dependent enhancement [5]. According to the latest WHO recommendations, CCP is not effective for the treatment of patients with nonsevere, severe and extremely severe COVID-19 [7].
Another method of passive antibody therapy is the use of monoclonal antibodies. Due to the urgency and rapid onset of the pandemic, several preparations of monoclonal anti-SARS-CoV-2 neutralizing antibodies (nAbs) have received emergency use authorization [13]. However, the fact that virus mutations within the epitope region affect antibody binding could lead to partial or even complete loss of effectiveness with the spread of new variants of the virus. As a possible alternative, polyclonal immunoglobulins manufactured from CCP can be used to treat COVID-19. Hyperimmune immunoglobulins have long been proven effective against bacterial and viral infections [14].
Compared with direct CCP transfusion, hyperimmune immunoglobulin use has several advantages, such as a lower infusion volume, an easier administration route and easier preservation, a higher nAb titer, a standard nAb amount, higher pathogen safety ensured by the presence of special virus inactivation and removal steps in the production process, a lessened allergic and anaphylactic reaction, no requirement for ABO and RH compatibility and the absence of complement proteins and coagulation factors [15,16]. Commonly used techniques of plasma fractionation with purification and virus removal/inactivation steps ensure the safety of hyperimmune immunoglobulins through the elimination of IgA, IgM, albumin, fibrinogen, prekallikrein and high-molecular-weight aggregates of IgG in comparison with that of plasma transfusions [17]. In addition, the broad specificity of immunoglobulins against different isolates of infectious agents provides neutralizing benefits. Therefore, a hyperimmune anti-SARS-CoV-2 immunoglobulin G formulation produced from a large CCP pool could be effective against different isolates of SARS-CoV-2 in COVID-19 patients without the CCP-associated transfusion disadvantages [12,18]. Despite vaccines being the primary strategy in managing the ongoing COVID-19 pandemic, their effectiveness is limited for individuals with lymphopenia and primary/secondary antibody deficiencies. In such cases, immunoglobulin preparations are a strong alternative therapeutic option [19].
In April 2020, a group of plasma manufacturers (e.g., Takeda, Biotest, CSL Behring, Octapharma) formed the CoVIg19 Plasma Alliance [20] to jointly develop unbranded anti-SARS-CoV-2 hyperimmune IgG products. Other manufacturers, such as Grifols [21], Emergent BioSolutions, Kedrion Biopharma S.p.A. and Kamada Ltd also reported the findings of their efforts in this area [22,23].
The Plasma Alliance conducted a randomized, double-blind, placebo-controlled, phase III trial (ITAC) to evaluate the safety and clinical efficacy of anti-SARS-CoV-2 hyperimmune intravenous immunoglobulin (hIVIG) in addition to the standard of care, including the antiviral remdesivir in 593 patients hospitalized with COVID-19 without end organ failure. The authors concluded that hIVIG confers no clinical benefit for hospitalized patients with COVID-19. It was found that the number of adverse events after hIVIG infusion varied according to nAb status, with significantly more adverse events in patients with nAbs at baseline compared with placebo than the nAb-negative group when compared with placebo. An insufficient effectiveness of hIVIG could be associated with the progressive disease stages when augmenting the humoral immune response might not be useful. Besides, antibody therapy might not benefit patients who have already mounted an immune response; therefore, hIVIG could be effective at earlier disease stages or in immunosuppressed patients or groups with persistent failure to mount humoral immune responses to infection [24]. A randomized, controlled, single-blind trial of anti-SARS-CoV-2 hIVIG in severe and critical COVID-19 patients in Pakistan (phase I/II) showed that a single dose of anti-COVID-19 hIVIG in combination with the standard of care was found to be safe and efficacious while increasing the chances of survival and reducing the risk of disease progression. That study found that survival distribution was significantly better in the group with 5% anti-COVID-19 hIVIG dose 0.15 g/kg with the standard of care compared with the control group. However, it was only a phase I/II study of 50 patients, and further studies of the safety and effectiveness of this treatment are needed [25].
The creation of a drug based on hyperimmune immunoglobulin that has minimal side effects and is pure and safe would add tools to the healthcare system to treat COVID-19. When creating a production technology of hyperimmune immunoglobulin, it is advisable to use the experience accumulated in IgG production. IgG preparations have traditionally been manufactured from human plasma by the cold ethanol fractionation method developed in the 1940s and its modifications. Historically, first IgG preparations could only be administered intramuscularly or subcutaneously because of the adverse effects associated with their intravenous infusion. Next, efforts were concentrated on further IgG purification and aggregate removal, improving intravenous tolerability and increasing its viral safety [26].
Currently, there are many ways for the production of human IVIG, using various plasma fractionation methods, such as ethanol fractionation, polyethylene glycol or caprylate precipitation, chromatography-based fractionation. [27]. Generally, IVIG preparations are manufactured from plasma pool of thousands of liters fractionated in highly sophisticated facilities using complex and highly regulated, large-scale technologies. However, sometimes small-scale, easy-to-use technology is needed, especially during infectious outbreaks. Caprylic acid precipitation is one possible way to do so. In one study, it resulted in the production of nearly 90% pure immunoglobulin fraction [28]. A caprylic acid precipitation method was utilized to produce the mixed immunoglobulin IgG–IgA–IgM [29]. Combining caprylic acid precipitation with anion exchange chromatography, IgG with purity greater than 95% could be obtained [26,30].
Industrial fractionation processes should also be designed to keep antibody molecules as intact as much as possible during the process, to maintain the integrity of its Fc region and prevent the formation of aggregates. It is known that the fractionation process can influence IgG subclass distribution. Since most anti-SARS-CoV-2 nAb responses in the IgG class have been shown to be associated with IgG1 and IgG3 subclasses, it is necessary to maintain the subclass ratio in total IgG during the purification process to obtain effective aanti-SARS-CoV-2 IVIG [16].
This study presents the developmental process of CCP-derived intravenous anti-COVID-19 hyperimmune globulin (COVID-globulin) containing G-class-only immunoglobulins and its in vivo virus neutralizing activity and safety assessment results. The key features of the COVID-globulin development process are the CCP selection approach, the degree of IgG purification and approach to the study of the anti-SARS-CoV-2 effectiveness in vivo.
Material & methods
Convalescent plasma collection
COVID-19 convalescent plasma (CCP) was collected by licensed Russian blood service organizations in accordance with current “Rules for the procurement, storage, transportation and clinical use of blood and its components” and the decree of 1 April 2020, no. 325, “On the introduction of technology for the use of fresh frozen plasma from convalescent donors COVID-19”, operating in Russia [31–33]. According to the Russian federal law of 20 July 2012, no. 125-FZ, “About donorship of blood and its components” (as amended on 11 June 2021), all donors gave their written informed consent to the donation. In addition to the major plasma requirements, the CCP had to meet the following inclusion criteria: prior diagnosis of COVID-19 documented by a laboratory test, absence of symptoms at the time of donation, at least 30 days passed since all symptoms disappeared and a negative result for SARS-CoV-2 testing using a real-time PCR test of oropharyngeal samples. All donations were screened for transfusion-transmitted infections using nucleic acid testing (HIV-1, HIV-2, HBV and HCV) and infection markers using ELISA (HIV-1, HIV-2, HBV, HCV and syphilis) according to national guidelines [34].
Virus neutralization test
Identification of the neutralizing antibody titer in CCP and anti-COVID-19 hyperimmune immunoglobulin (COVID-globulin) batches was conducted using a virus neutralization test on Vero E6 cells using a SARS-CoV-2 virus isolate (hCoV-19/Russia/Moscow_PMVL-1/2020) provided by the Department of the Russian State Collection of Viruses. The SARS-CoV-2-containing solution (100 plaque-forming units dose) was prepared in Dulbecco's modified Eagle medium with 2% inactivated fetal bovine serum and mixed with an equal volume of the tested samples (twofold serial dilutions from 1/20 to 1/1280). A serum batch with a known virus neutralization test titer (1/80 and 1/1280) was used as a control. The mixtures containing the virus and CCP and COVID-globulin samples were incubated for 60 min at 37°C and then introduced into SARS-CoV-sensitive Vero E6 cells. The specific virus-neutralizing activity of COVID-globulin was determined by measuring its ability to prevent cytopathic effects.
Enzyme-linked immunosorbent assay
Anti-SARS-CoV-2 IgG levels were determined using the anti-SARS-CoV-2 IgG ELISA Kit (Euroimmun AG, Luebeck, Germany) against structural S1 protein (Euroimmun ELISA). The determinations were carried out according to the manufacturer's instructions using plasma samples diluted in sample buffer at a ratio of 1:101. Each sample was analyzed in triplicate. Results were reported as the cutoff index, which is based on the ratio of specimen absorbance to calibrator absorbance. The assay was interpreted as positive when the ratio was ≥1.1. A larger ratio represents higher antibody levels.
Process development
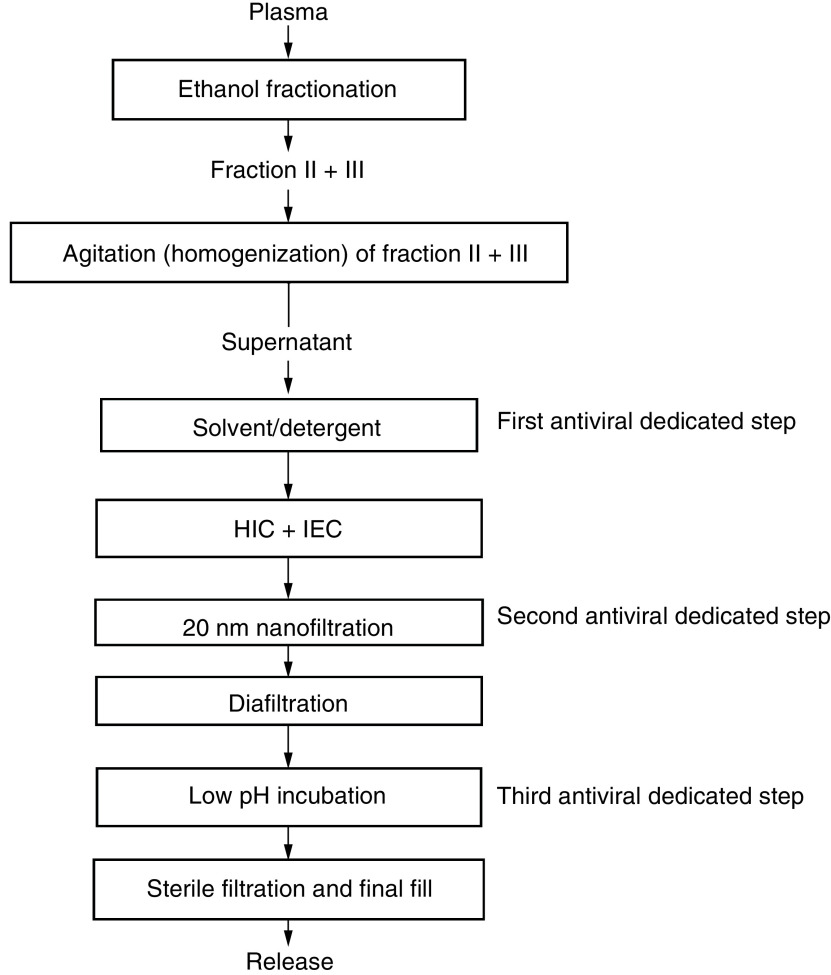
A block flow chart of the COVID-globulin manufacturing process based on the standard Cohn method is presented in Figure 1. Chromatographic purification methods on consistently used hydrophobic, anion and cation exchange media, as well as pathogen removal and inactivation steps, were included. The manufacturing process of COVID-globulin contains the following three dedicated steps of pathogen inactivation: solvent/detergent treatment, nanofiltration (20 nm antiviral filtration) and low-pH incubation. In compliance with current international guidelines, the steps have been separately validated in a series of in vitro experiments for their ability to inactivate or remove enveloped and nonenveloped viruses. The final protein concentration was obtained using tangential flow filtration followed by sterile filtration.
Figure 1. Block flow diagram of the anti-COVID-19 hyperimmune immunoglobulin production process.
HIC: Hydrophobic interaction chromatography; IEC: Ion exchange chromatography.
Product characterization
Analyses were carried out as described in the State Pharmacopoeia of the Russian Federation and the European Pharmacopoeia [34,35].
Assessment of nAbs in COVID-globulin lots
An in-house standard was used to determine the quantity of SARS-CoV-2 antibodies in COVID-globulin lots in conventional units. The specific activity titer of the in-house standard was assessed using a virus neutralization test and expressed in conventional units, where one anti-SARS-CoV-2 unit (ACU/ml) is equal to the dilution factor of the sample tested in the neutralization test. Validation studies were conducted that confirmed the possibility of using an in-house standard for the quantitative assessment of neutralizing antibodies in COVID-globulin lots using the Euroimmun ELISA assay.
COVID-globulin preclinical evaluation
Preclinical evaluation of COVID-globulin was performed in strict accordance with national and international guidelines [36–39]. In addition, the protective efficacy of COVID-globulin was studied in a COVID-19 infection lethal model. All animal experiments were performed in accordance with the ethical principles established by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (adopted in Strasbourg on 18 March 1986 and confirmed in Strasbourg on 15 June 2006).
In vivo toxicity assessment
The aim of the toxicity assessment of COVID-globulin was to determine its impact on the organs and systems of laboratory animals in comparison with 0.9% sodium chloride solution. Toxicity testing was carried out in Wistar rats and Chinchilla rabbits in strict accordance with national and international guidelines [36,37]. Equal numbers of male and female Wistar rats (150–240 g, 6–9 weeks old) and Chinchilla rabbits (1.8–2.2 kg, 10–12 weeks old) were utilized. Animals were used after a period of acclimatization. Rats and rabbits were divided into two groups of 30 and 12 animals, respectively. COVID-globulin was administered intravenously to rats and rabbits at a dosage of 2 ml and 20 ml (1.0 g/kg), respectively, which corresponded to the maximum possible volume for each species. Control group animals were administered an equal volume of saline. All groups were observed daily for behavioral and physical changes for 14 days and were sacrificed after 24 h, 7 days and 14 days after injection. Blood was collected for hematological and biochemical analyses. Brain, heart, liver, lung, spleen, kidney, adrenal gland, thymus, ovary, testis and lymph node (inguinal and mesenteric) samples were collected for histopathological analysis.
In vivo thrombogenic potential assessment
Thrombogenic potential of the COVID-globulin preparation was assessed according to the method of Wessler et al. [40] using Chinchilla rabbits (weight: 2.0–2.5 kg) as the model of venous stasis.
Rabbits were divided into four groups of three rabbits each. Rabbits in the first and second groups were administered two different batches of COVID-globulin into the carotid artery at a dosage of 0.1 g/kg. Rabbits in the positive control group were administered human blood plasma (thrombus formation in 100% cases of administration in a volume of 2.5 ml), whereas those in the negative control group were administered 0.9% sodium chloride solution (no thrombus formation in 100% cases of administration in a volume of 5.0 ml).
Assessment of the protective efficacy of COVID-globulin in an immunodeficiency lethal animal model
The analysis was carried out in Syrian hamsters treated with dexamethasone and cyclophosphamide within 7 days before inoculation and every 3 days thereafter.
For the lethal dose 50 evaluation, the animals were infected intranasally with 101, 102, 103 and 104 median tissue culture infectious doses of SARS-CoV-2 in 100 μl of phosphate-buffered saline. Control group animals received 100 μl of phosphate-buffered saline. For the assessment of the protective efficacy of COVID-globulin, the animals were randomly divided into five groups, as shown in Table 1. The animals in these groups were intranasally inoculated with 101 median tissue culture infectious dose of SARS-CoV-2 in 100 μl of PBS. The COVID-globulin was administered to the animals intraperitoneally at a dose limited by the recommended volume of administration (1.0 ml per animal), 24 h before infection (group 1), 2 h after infection (group 2) and 48 h after infection (group 3). Control-infected group 4 animals were administered a 10% intravenous immunoglobulin solution (in 1.0 ml) 24 h before infection. Control-infected group 5 animals were administered an equivalent volume of saline (1.0 ml) 2 h after infection intraperitoneally. All the animals were observed daily throughout the study. The body weight and health status of the animals were recorded daily for 7 days after infection and then every 3 days until the end of the study. Lungs taken from euthanized animals were subjected to macroscopic, histological and viral load studies.
Table 1. Characterization of animal groups in the protective efficacy assay.
| n of group | n of animals | Infection dose | Preparation dose | Time of injection preparation |
|---|---|---|---|---|
| 1 | 12 | 101 median tissue culture infectious dose in 100 μl intranasal | 1.0 ml anti-COVID-19 hyperimmune immunoglobulin | 24 h before infection |
| 2 | 12 | 2 h after infection | ||
| 3 | 12 | 48 h after infection | ||
| 4 | 12 | 1.0 ml normal intravenous immunoglobulin | 24 h before infection | |
| 5 | 12 | 1.0 ml placebo | 2 h after infection |
Viral load assay in Vero cells
Viral load within the lungs was determined by titration of 10% lung tissue homogenate in Vero cells. Serial tenfold dilutions of the testing material were prepared in Dulbecco's modified Eagle medium supplemented with 2% fetal bovine serum and 100 μl of each lung tissue homogenate was added to Vero E6 cells in 96-well plates in six repetitions. Cells were incubated for 96 h at 37°C in the presence of 5% CO2 and the cytopathic effect was visually evaluated. The median tissue culture infectious dose was calculated using the Reed and Muench method.
Histopathological examination
The organs and tissues of the rats were fixed in formalin, followed by rinsing, passage through alcohols and embedding in paraffin. Sections of 4–5 μm thick were made from paraffin blocks, stained with hematoxylin and eosin and examined by light microscopy at a magnification of 100×.
Fragments of the hamsters' lungs were fixed in buffered formalin, embedded in paraffin blocks, cut 3 μm thick, stained with Mayer's hematoxylin and examined by light microscopy at a magnification of 200×.
Hematological & biochemical parameters
To study the effect of the COVID-globulin on hematological and biochemical parameters, the animals were deprived of food 16 h before blood sampling; water remained in sufficient quantity. Blood was taken from the tail (rat) or ear (rabbit) vein. For the assessment of hematological parameters, blood was collected in a volume of 1 ml in tubes with sodium citrate solution and analyzed with automatic hematological analyzer GEMA 8-01-'ASTRA’ (SPC Astra, Russian Federation). Biochemical parameters were analyzed in serum using a semiautomatic biochemical analyzer, Clima MC-15 (RAL, Spain).
Statistical analysis
Statistical processing of the research results was carried out using Microsoft Excel and Statistica 10.0 (StatSoft, Inc., OK, USA). D'Agostino–Pearson, Shapiro–Wilk and Kolmogorov–Smirnov tests were used to determine the normal distribution of the data. To compare unconnected samples, the Student's t-test or the Mann–Whitney test was used, depending on the normality of the data. The log-rank test was used to compare the survival curves.
Results
Convalescent plasma collection
During the investigations, correlation analysis of the virus neutralization test (VNT) titer and ELISA results based on comparative measurements of 1499 COVID-19 convalescent plasma individual samples and 31 minipools showed that the correlation was no more than 0.84 in the samples. However, this is insufficient for the use of ELISA instead of a VNT. Since the project aim was to replace a VNT with ELISA for plasma selection, the receiver operating characteristic analysis method was successfully applied. Based on the receiver operating characteristic analysis results, the cutoff index of 2.0 was measured using Euroimmun ELISA, which was correlated with the VNT titer more than 1:20 with a specificity of 0.95, sensitivity of 0.81 and receiver operating characteristic area under the curve of 0.95. Overall, the correlation in minipools was 0.95 using Euroimmun ELISA, which allows the replacement of VNT with ELISA to measure the neutralizing activity in plasma pools and the final product.
To assess the quantity of anti-SARS-CoV-2 neutralizing antibodies (nAbs), conventional units of neutralizing activity (ACU/ml) were introduced, denoting a dilution in the titer value. For example, COVID-19 convalescent plasma neutralizing activity of 20 ACU/ml corresponds to the 1:20 VNT titer.
Process development
The manufacturing procedure of the COVID-globulin was based on standard ethanol fractionation with the inclusion of chromatographic purification steps and three pathogen removal and inactivation steps (solvent/detergent treatment, 20 nm virus filtration [nanofiltration] and low-pH incubation). The results of the validation process of the viral removal and inactivation steps are presented in Table 2. Commonly used techniques such as chromatography, tangential flow filtration, depth, sterile and nanofiltration are key components of the production of pure and safe immunoglobulins with 98% of the product having a high yield.
Table 2. Virus reduction efficacy of the manufacturing process of anti-COVID-19 hyperimmune immunoglobulin.
| Virus inactivation/removal step | Virus reduction factor, log10 | |||||||
|---|---|---|---|---|---|---|---|---|
| Enveloped viruses | Nonenveloped viruses | |||||||
| DHBV | HIV-1 | HCV | HBV | BVDV | B19V | PPV | EMCV | |
| Solvent/detergent treatment | ≥5.0 | ≥6.0 | – | – | ≥4.0 | – | – | – |
| Ethanol fractionation | – | – | ≥4.0 | ≥5.0 | – | ≥4.0 | – | – |
| Low-pH incubation | ≥5.0 | ≥4.0 | – | – | ≥5.5 | – | – | – |
| Chromatography | – | – | ≥2.0 | ≥3.5 | – | ≥3.5 | – | – |
| Nanofiltration (20 nm) | – | ≥4.0 | ≥4.0 | ≥4.0 | ≥5.0 | ≥4.0 | ≥5.0 | ≥5.5 |
| Global reduction factor | ≥10.0 | ≥14.0 | ≥10.0 | ≥12.5 | ≥14.5 | ≥11.5 | ≥5.0 | ≥5.5 |
B19V: Human parvovirus B19; BVDV: Bovine viral diarrhea virus; DHBV: Duck hepatitis B virus; EMCV: Encephalomyocarditis virus; PPV: Porcine parvovirus.
Product characterization
The quality control test results for the first ten batches are presented in Table 3. The proprietary COVID-globulin meets the criteria of the European Pharmacopoeia for 10% human hyperimmune globulin solution.
Table 3. Characteristics of anti-COVID-19 hyperimmune immunoglobulin.
| Characteristic | Specification† | Average of 10 batches‡ |
|---|---|---|
| Transparency | Optical density of not more than 0.05 | Meet criteria |
| Color intensity | Optical density of not more than 0.05 | Meet criteria |
| pH | 4.5–5.5 | 4.82 ± 0.12 |
| Protein concentration | 80–120 mg/ml | 98.1 ± 6.5 mg/ml |
| Purity | ≥95% of the total protein content | 97.6 ± 0.7% |
| Distribution of molecular size of IgG | ≤3% polymeric ≥90% monomeric/dimeric IgG |
0.17 ± 0.08% 99.82 ± 0.08% |
| IgA content | <1 mg/ml | 0.18 ± 0.05 mg/ml |
| Heat stability | No jelling | Meet criteria |
| Glycine content | 21.0–29.0 mg/ml | Meet criteria |
| Virus inactivating agents | Tween-80 ≤1.0 μg/ml Tri-n-butylphosphate ≤5.0 μg/ml |
≤1.0 μg/ml ≤5.0 μg/ml |
| Osmolality | ≥240 mOsmol/kg | 344.0 ± 19.4 mOsmol/kg |
| Sterility | Should be sterile | Meet criteria |
| Pyrogens | Should be nonpyrogenic | Meet criteria |
| Abnormal toxicity | Should be nontoxic | Meet criteria |
| Anti-COVID-19 IgG (concentration factor) | ≥6 × starting plasma pool | (9.4 ± 1.4) × starting plasma pool |
| Anticomplementary activity | <1 CH50/mg IgG | <1 CH50/mg IgG |
| Hemagglutinins anti-A and anti-B | 1/64 diluted solution shows no agglutination | 1/64 diluted solution shows no agglutination |
| Anti-D | Anti-D content no more than that of the positive standard sample | Anti-D content less than that of a positive standard sample |
| IgG subclass distribution | IgG distribution corresponding to normal human serum | IgG1 – 62.0 ± 4.0 IgG2 – 26.3 ± 4.8 IgG3 – 7.7 ± 2.0 IgG4 – 4.0 ± 0.7 |
| Fc function | ≥60% of intravenous immunoglobulin reference preparation | 134.6 ± 5.5% |
| PKA activity | ≤35 IU/ml | 4.5 ± 0.3 IU/ml |
| Thrombogenic potential | No thrombus formation should be observed | Meet criteria |
| Yield | 4.25 ± 0.24 g IgG/l plasma |
The characteristics of anti-COVID-19 hyperimmune immunoglobulin satisfy the requirements of the European Pharmacopoeia and State Pharmacopoeia of the Russian Federation.
One batch produced from 350 l of human plasma.
CH50: Complement, total.
Assessment of COVID-globulin nAbs
The neutralizing activity titer of the in-house standard was 1/320 in the VNT and 320 ACU/ml, where 1 ACU was equal to the dilution factor of the VNT titer. As the correlation between VNT and ELISA was 0.95, quantitative ELISA, with the in-house standard in ACU/ml, was used to assess the quantity of anti-SARS-CoV-2 nAbs in plasma pools and COVID-globulin lots. On average, the concentration of anti-SARS-CoV-2 nAbs in the final product was 9.4-fold higher than that in the original plasma pool across ten manufacturing batches (Table 3). The neutralizing activity of the VNT titer of the first ten batches was also correlated with the quantitative ELISA anti-SARS-CoV-2 nAb levels.
COVID-globulin preclinical evaluation
Toxicity testing showed no changes in behavior or mortality in any of the groups of rats or rabbits. There were no significant differences in body weight between the groups. Food and water intake was not significantly different during the 14-day follow-up period after the infusion. Histopathological examination of the organs of the rats treated with COVID-globulin showed no signs of necrosis, inflammation or atypia or any significant pathological changes (Supplementary Figure 1). In both the experimental and control groups, at all periods of animal withdrawal from the experiment, the lobular and beam structure of the liver was preserved. Hepatocytes were large, with slightly eosinophilic and light cytoplasm and rounded, euchromic nuclei. The kidneys were covered with a thin capsule of collagen fibers. The cortical substance was represented by numerous, small, normocellular renal glomeruli and tubules lined with cuboidal epithelium. Collecting tubules lined with cuboidal epithelium with light cytoplasm were found in the medulla. The walls of arterioles did not change as a result of exposure and remained of a typical structure.
Hematological and biochemical analyses did not show statistically significant differences in most parameters between the experimental and control groups (Supplementary Table 1). Administration of COVID-globulin to rabbits did not activate blood clot formation. Administration of 0.9% sodium chloride solution to rabbits confirmed the absence of spontaneous thrombus formations, whereas administration of human blood plasma caused thrombus formations.
Protective efficacy of COVID-globulin in the immunodeficiency lethal animal model
The lethal dose 50 was not determined because all the animals in all experimental groups died before the end of the experiment. Thus, lethal dose 50 was lower than the 101 median tissue culture infectious dose of SARS-CoV-2. For protective efficacy assessment, the authors used 101 median tissue culture infectious dose of SARS-CoV-2 in accordance with Good Laboratory Practice guidelines [39]. The weight and health status of the animals in the COVID-globulin groups (groups 1–3) were stable throughout the study, while the weight and health status of the intravenous immunoglobulin-treated and placebo-treated animals deteriorated over time (Figure 2B) and they lost 30% of their weight (Figure 2A).
Figure 2. Animal weight and health status.
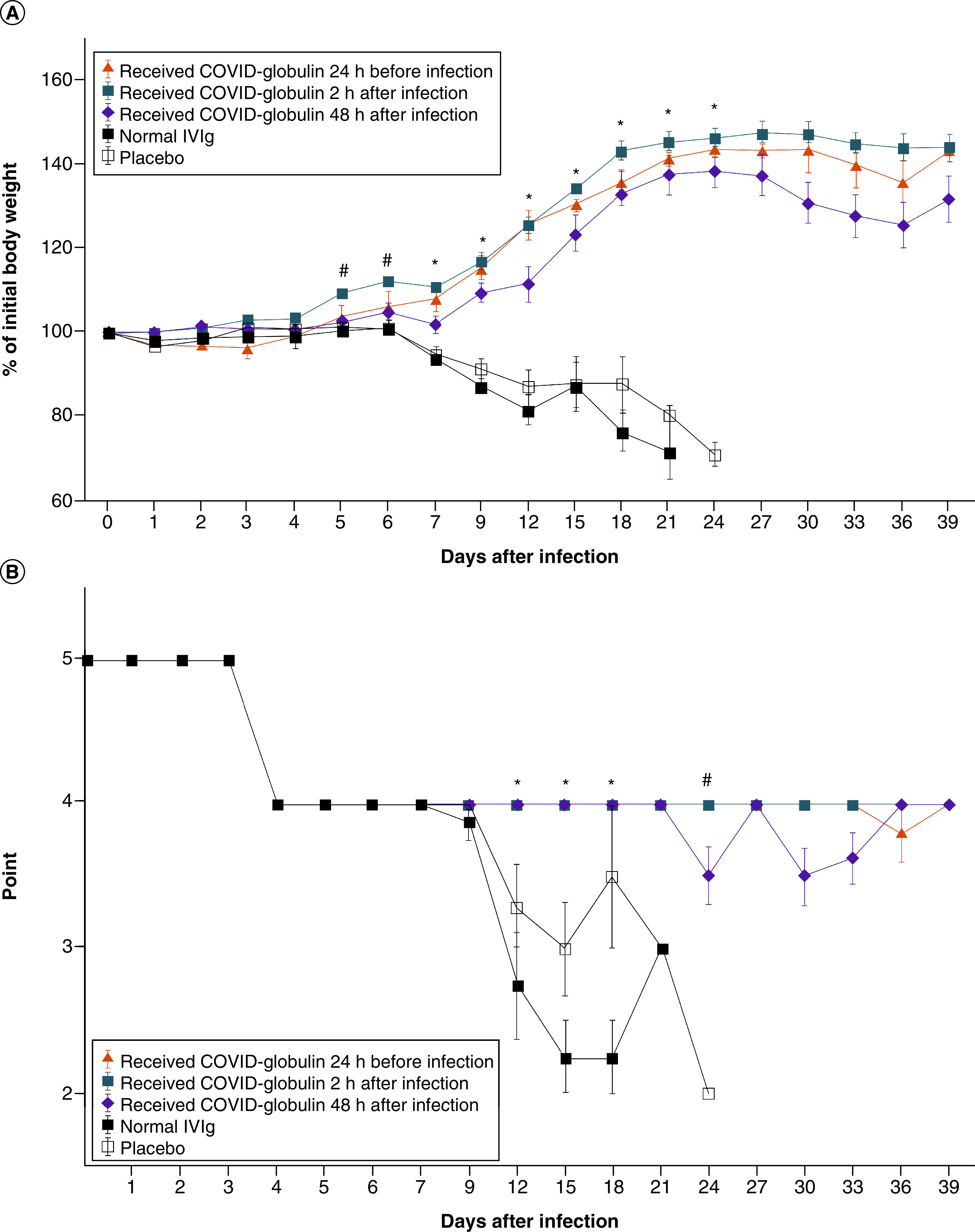
(A) Change in the body weight of laboratory animals in the different groups after infection. Body weights of the hamsters treated with COVID-globulin, normal IVIG and placebo measured at the indicated days after inoculation with SARS-CoV-2. Group 1 received COVID-globulin 24 h before infection; group 2 received COVID-globulin 2 h after infection; group 3 received COVID-globulin 48 h after infection; group 4 was treated with normal IVIG; group 5 was treated with placebo. Data are presented as mean percentages of the starting weight (± standard error [SE]). *significant difference compared with the IVIG- and placebo-treated groups, p < 0.05; #significant difference in body weight in group 3 compared with that of the normal IVIG- and placebo-treated groups, p < 0.05. (B) Change in the health status of laboratory animals after injection. Health status of animals was assessed on a 5-point scale, where 5: healthy animals; 4: active, reaction to stimuli is normal, flattened ears or tousled coat; 3: weak activity, reaction to stimuli is weak, changes in behavior, aggression or apathy; 2: no response to stimuli, refusal of food and water; 1: death of an animal. Data are presented as the mean average point (± SE). *significant difference compared with the normal IVIG- and placebo-treated groups, p < 0.05; #significant difference in the health status of group 3 compared with that of the normal IVIG- and placebo-treated groups, p < 0.05.
COVID-globulin: Anti-COVID-19 hyperimmune immunoglobulin; IVIG: Intravenous immunoglobulin.
The timing of drug administration was selected considering the pharmacokinetic parameters of the COVID-globulin. The maximum concentration of antibodies in Syrian hamsters' blood was detected within 24 h after administration of the COVID-globulin. The design of the study imitated three variants of events: 24 h before infection – prophylactic administration (at the time of the infection, maximum antibodies in the blood are reached), 2 h after infection – extra prophylactic administration (as soon as possible after infection but before the onset of clinical signs) and 48 h after infection – therapeutic administration (during the course of infection).
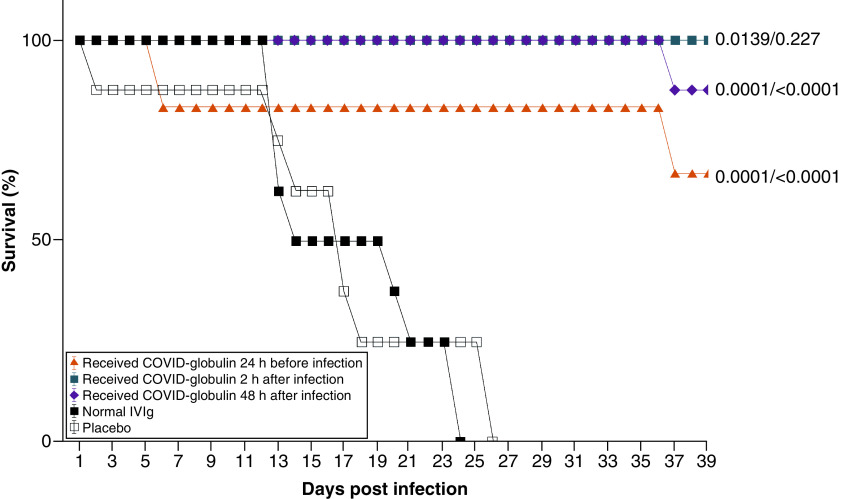
The animal survival rate was 66.6% in group 1, 100% in group 2 and 87.5% in group 3, and all the animals in groups 4 and 5 had died by day 26 (Figure 3). Statistical analysis (log-rank test) showed that the survival of the animals in the COVID-globulin groups after infection with SARS-CoV-2 at a dose of 101 median tissue culture infectious dose was significantly higher than that of the control groups, which indicates the effectiveness of the COVID-globulin.
Figure 3. Survival rate of Syrian hamsters.
Survival was evaluated in animals treated with COVID-globulin, normal intravenous immunoglobulin and placebo at the indicated days after inoculation with SARS-CoV-2. Group 1 received COVID-globulin 24 h before infection; group 2 received COVID-globulin 2 h after infection; group 3 received COVID-globulin 48 h after infection; group 4 was treated with normal intravenous immunoglobulin; group 5 was treated with placebo.
COVID-globulin: Anti-COVID-19 hyperimmune immunoglobulin; IVIG: Intravenous immunoglobulin.
Macroscopic analysis of the lungs showed extensive lesions in all the animals in the control groups. Two out of the four animals in groups 1 and 2 (COVID-globulin administered 24 h before and 2 h after infection) showed significantly lower levels of lung damage (Supplementary Figure 2). Histological examination of lung tissues (Supplementary Figure 3) showed no pronounced pathological changes in the pulmonary parenchyma in experimental groups 1–3 compared with the pathological changes observed in the healthy animals (groups 4 and 5). Focal inflammatory cell infiltration in the interstitial and alveolar cavities was prominent in groups 4 and 5. The predominant cell type infiltrating the lungs after SARS-CoV-2 infection in both control groups was lymphocytes. In addition, the interalveolar septa contained similar levels of eosinophil and mast cell infiltration. Severe lesions were characterized by lymphoid tissue hyperplasia, influx of macrophages into the tissue and mild interstitial pneumonia with expansion of alveolar septa by edema fluid, with few strands of fibrin and low numbers of leukocytes.
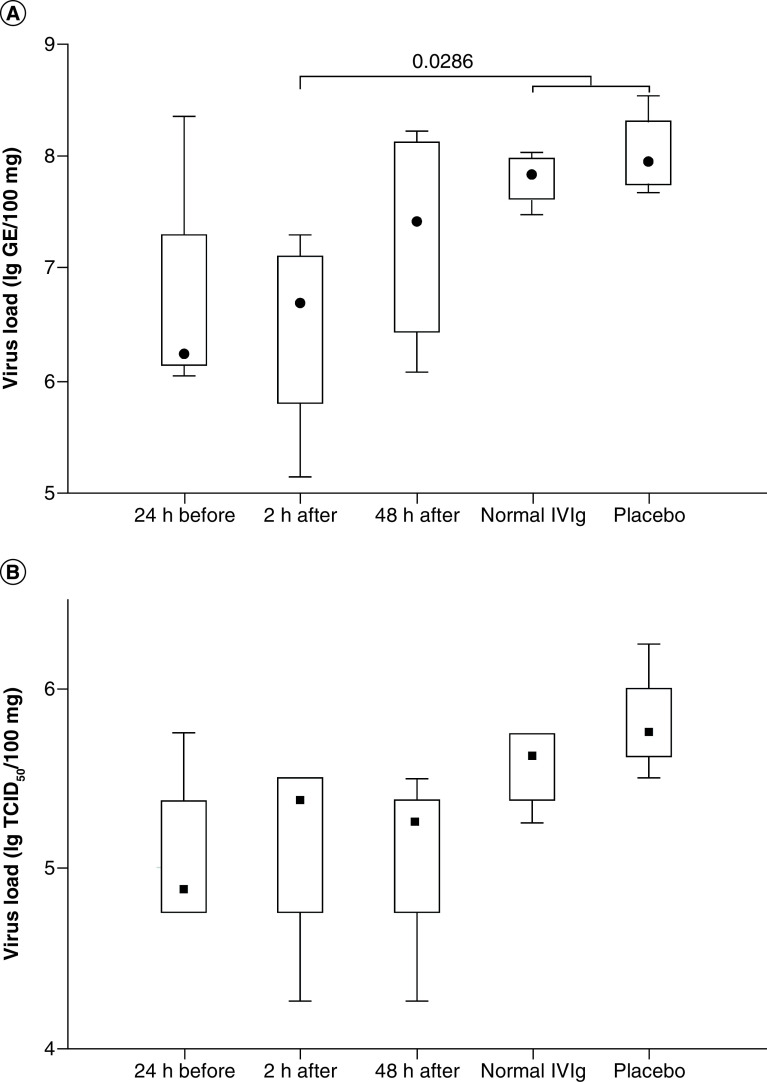
Viral load studies showed no significant difference in the viral titers of lung tissues at 7 days postinfection between the COVID-globulin-treated and control groups using titration in Vero cells (Figure 4B). Real-time PCR analysis showed a significant decrease in the viral load of group 2 animals (treated with COVID-globulin 2 h postinfection) compared with that of animals in control groups 4 and 5 (p = 0.0286; Mann–Whitney test) (Figure 4A).
Figure 4. Median tissue culture infectious dose from lung homogenates and quantification of SARS-CoV-2 RNA copies per 100 mg lung tissue.
The study of viral load was carried out by real-time PCR (A) and using titration in Vero cells (B). Group 1 received COVID-globulin 24 h before infection; group 2 received COVID-globulin 2 h after infection; group 3 received COVID-globulin 48 h after infection; group 4 was treated with normal intravenous immunoglobulin; group 5 was treated with placebo.
COVID-globulin: Anti-COVID-19 hyperimmune immunoglobulin; IVIG: Intravenous immunoglobulin.
Discussion
In this study, it was initially thought that for the development of a specific anti-COVID immunoglobulin it was necessary to select CCP with a high level of nAbs. This would demonstrate significant effectiveness and reduce the risk of the product containing nonneutralizing or subneutralizing antibodies, which could theoretically lead to an antibody-dependent enhancement of the viral infection. The Russian Federation's Ministry of Health recommends a minimum VNT titer target of 1:80 for CCP [33]. However, considering the fact that antibody concentration within the final product typically increases by 5- to 20-fold compared with that in the initial pooled material for the specific immunoglobulins, it is advisable to use plasma with an nAb VNT titer of at least 1:20.
VNT analysis is the gold standard for nAb concentration assessments. However, it is a biological assay that typically takes 5 to 7 days to perform and requires specialist equipment in a certified laboratory for biosafety. This makes VNT testing a difficult step to include in large-scale production for routine testing [41–43]. It is preferable that the testing method be simple and cost-efficient and correlate with the results of VNT testing. ELISA is a possible solution when considering utilizing such a step when thousands of plasma samples are to be tested. Receiver performance analyses were performed to develop criteria for Euroimmun ELISA kits to select CCPs with a VNT titer of at least 1:20 with a sensitivity of 95% and a specificity of 85%. Euroimmun ELISA assay results showed a high correlation with the VNT results in plasma minipools: 0.95 for Euroimmun ELISA assays, which made it possible to replace the VNT to assess the neutralizing activity of the finished product in conventional units of neutralizing activity (ACU/ml; e.g., a CCP neutralizing activity of 20 ACU/ml corresponds to the 1:20 VNT titer). The similar results of anti-SARS-CoV-2 nAb levels in the initial batches of anti-COVID-19 hyperimmune immunoglobulin (COVID-globulin) assessed via VNT and ELISA confirmed the high correlation between the methods and allowed the application of conventional units of neutralizing activity ACU/ml for the final product.
Previously described mixed immunoglobulin IgG-IgA-IgM preparation cases [19,29] are better suited for small-scale, single-product manufacturing. However, the majority of plasma-derived products on large-scale manufacturing utilize the ethanol Cohn fractionation method. This suggests, in regard to COVID-globulin manufacturing, the Cohn fractionation method could be more applicable for large-scale production. Also, the ethanol method for basic fractionation was employed because local facilities were already in place. The use of the developed production method makes it possible to obtain a product with a relatively high yield.
In this study, CCP was used as a source to produce COVID-globulin. One of the most important properties of hyperimmune intravenous immunoglobulin is the presence of specific nAbs in concentration not lower than 1:20 VNT than convalescent plasma or plasma from vaccine recipient were used. For instance, using the same manufacturing process, normal intravenous immunoglobulin and immunoglobulin against HBV can be produced (for anti-HBV, hyperimmune intravenous immunoglobulin from the plasma of donors vaccinated according to a special scheme to achieve high specific activity of preparation). Concerning SARS-CoV-2, the advantage will be the presence in the starting plasma pool of nAbs specific to various circulating variants of the virus.
The manufacturing process for COVID-globulin is designed to produce a high-quality product that meets all intravenous immunoglobulin requirements for intravenous 10% IgG preparations. The IgG subclass distribution was not substantially affected by the entire process. Furthermore, all the lots tested had a high level of Fc function 134.6 ± 5.5% and exceeded the minimal requirements of the European Pharmacopoeia – namely, to achieve at least 60% of the potency of the European Pharmacopoeia reference standard. The COVID-globulin contains 100 ± 10 mg/ml protein, of which 95% is human IgG. More than 99% of the IgG is available in monomeric and dimeric forms. The product showed low anticardiolipin antibodies (<1 CH50/mg IgG) and PKA activity (<4.47 IU/ml). Safety studies of COVID-globulin undertaken on animals demonstrated no signs of toxicity and thrombogenic activity. The level of anti-SARS-CoV-2 nAbs increased by approximately ninefold in the final product compared with that in the starting plasma pools. Such a rise in nAb activity indicates that patients treated with COVID-globulin would receive higher nAb levels compared with those receiving an equivalent volume of convalescent plasma. Furthermore, the use of conventional units of specific activity will allow for the standardization of nAb dosage during treatment.
The advantages of using concentrated immunoglobulins for the treatment of COVID-19 includes a high and precise quantity of nAbs and, more importantly, a variety of nAbs. Highly concentrated immunoglobulins for intravenous administration can provide a wider range of antiviral activity by attacking various viral epitopes and activating various cellular mechanisms, and nAbs against all circulating virus strains are always present in the final product. The latest results of the ITAC (INSIGHT 013) Study Group indicated insufficient efficacy of hyperimmune intravenous immunoglobulin in immunocompetent patients hospitalized with COVID-19. In contrast, the review of case reports and case series of the clinical experiences of 238 COVID-19 patients with primary and secondary immunosuppression who were treated with specific nAbs via CCP transfusion provided evidence suggesting a mortality benefit and rapid clinical improvement in COVID-19 in immunocompromised patients unable to develop effective humoral response against SARS-CoV-2 [44,45]. The administration of CCP, or preferably anti-SARS-CoV-2 intravenous IgG, in case to maintain effective antibody levels apparently could be a long-term, ‘chronic' therapy against COVID-19 for immune-deficient and immune-suppressed patients, especially for bridging the period when the immune system cannot itself produce the antibodies needed for viral clearance [46].
Thus, the data presented in this article describe a successful approach to the selection of suitable raw materials (plasma) and production technology for COVID-globulin that meets the general specifications of immunoglobulins and leads to a pure, safe and effective IgG preparation for intravenous administration.
This study demonstrates the effectiveness of COVID-globulin within preclinical trials, the results of which reliably showed a reduction in lung inflammation, and protective effects in a lethal model, in immunosuppressed hamsters. Evidence of lesion resolution was observed after COVID-globulin administration, with a decrease in alveolar cellular exudate and an absence of hyperplasia. Despite the fact that only one action mechanism of antibodies on the virus is involved (direct neutralization of the virus) in the hamster model, a positive effect of COVID-globulin administration was observed. Thus, it can be hypothesized that the use of COVID-globulin against COVID-19 in humans will be effective, which should be confirmed in clinical trials studying its effectiveness [47].
Summary points.
The results of several studies support the beneficial effect of COVID-19 convalescent plasma in the treatment of COVID-19 patients at various disease stages.
Hyperimmune immunoglobulins have long been proven effective against bacterial and viral infections.
The manufacturing procedure for anti-COVID-19 hyperimmune immunoglobulin was based on standard ethanol fractionation with the inclusion of chromatographic purification steps and three pathogen removal and inactivation steps (solvent/detergent treatment, 20 nm virus filtration [nanofiltration] and low-pH incubation).
The level of anti-SARS-CoV-2 neutralizing antibodies (nAbs) increased by approximately ninefold in the final product compared with that in the starting plasma pools.
The advantages of using concentrated immunoglobulin for the treatment of COVID-19 includes a high and precise quantity of nAbs and, more importantly, a variety of nAbs.
Safety studies in animals showed no signs of acute/chronic toxicity or allergenic or thrombogenic properties.
The final product, 10% immunoglobulin, is highly purified and meets all intravenous immunoglobulin requirements.
Anti-COVID-19 hyperimmune immunoglobulin demonstrates protective effects against SARS-CoV-2 in immunosuppressed hamsters.
Supplementary Material
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/imt-2022-0015
Author contributions
M Razumikhin conceived the project. T Smolyanova designed the study. A Nikolaeva, E Sakanyan and E Orlova were involved in the organization, coordination, conduct and support of the study. A Ivanov coordinated the study. N Zubkova and I Efimova organized validation studies of manufacturing process pathogen inactivation steps. O Belyakova, N Selezneva, T Vyaznikova, A Perevozchikov and A Sokolova performed research and did the statistical analysis and interpretation of the data. I Dolzhikova and D Logunov performed the protective efficacy investigation on the Syrian hamster mode and provided visualized data. A Nikolaeva and O Belyakova wrote the manuscript. M Razumikhin and T Smolyanova edited the manuscript, and all authors reviewed the manuscript before submission. All authors approved the manuscript and M Razumikhin had the final decision to submit for publication. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments
The authors would like to thank the employees of JSC NPO Microgen JSC Nacimbio, the Moscow health department and Russian Blood Service organizations for the COVID-19 convalescent plasma supply, and the Federal State Budget Institution “National Research Centre for Epidemiology and Microbiology Named after Honorary Academician N F Gamaleya” of the Ministry of Health of the Russian Federation for performing the COVID-globulin protective efficacy investigation on the Syrian hamster model. All authors have read the journal's policy on conflicts of interest and the authorship agreement.
Ethical conduct of research
The authors state that they have followed the principles outlined in the Declaration of Helsinki for all animal experimental investigations.
Footnotes
Financial & competing interests disclosure
This work was sponsored and performed by JSC Nacimbio. The employees of Nacimbio, M Razumikhin and T Smolyanova, participated in and contributed to the study. However, the company Nacimbio as a funding source had no direct role in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
This manuscript was prepared with the help of Editage Publication Support. Writing assistance was sponsored by JSC Nacimbio.
References
Papers of special note have been highlighted as: • of interest
- 1.Worldometer. COVID-19 coronavirus pandemic. www.worldometers.info/coronavirus/
- 2.Marano G, Vaglio S, Pupella S et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 14(2), 152–157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mupapa K, Massamba M, Kibadi K et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J. Infect. Dis. 179(1), 18–23 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Focosi D, Franchini M, Pirofski LA et al. COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors. Viruses 13(8), 1594 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provided the analysis of potential beneficial and detrimental factors of COVID-19 convalescent plasma.
- 5.Yuwono Soeroto A, Purwiga A, Alam A, Prasetya D. Plasma convalescent decrease mortality in COVID-19 patients: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 25(14), 4841–4853 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Zhang C, Zhou X et al. Convalescent plasma is of limited clinical benefit in critically ill patients with coronavirus disease-2019: a cohort study. J. Transl. Med. 19(1), 365 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Therapeutics and COVID-19: Living Guideline. Geneva, Switzerland: (2022). [PubMed] [Google Scholar]
- 8.Salazar E, Christensen PA, Graviss EA et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am. J. Pathol. 190(11), 2290–2303 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klassen SA, Senefeld JW, Johnson PW et al. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clin. Proc. 96(5), 1262–1275 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piechotta V, Iannizzi C, Chai KL et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst. Rev. 5(5), CD013600 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bégin P, Callum J, Jamula E et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat. Med. 27(11), 2012–2024 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Indicates the positive effects of treatment with convalescent plasma.
- 12.Focosi D, Farrugia A. The art of the possible in approaching efficacy trials for COVID19 convalescent plasma. Int. J. Infect. Dis. 102, 244–246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuccori M, Ferraro S, Convertino I et al. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline. mAbs 12(1), 1854149 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA. Blood and Blood Products. Silver Spring, MD, USA: (2021). [Google Scholar]
- 15.Cagdas D. Convalescent plasma and hyperimmune globulin therapy in COVID-19. Expert Rev. Clin. Immunol. 17(4), 309–316 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Focosi D, Tuccori M, Franchini M. The road towards polyclonal anti-SARS-CoV-2 immunoglobulins (hyperimmune serum) for passive immunization in COVID-19. Life 11(2), 144 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchacher A, Iberer G. Purification of intravenous immunoglobulin G from human plasma – aspects of yield and virus safety. Biotechnol. J. 1(2), 148–163 (2006). [DOI] [PubMed] [Google Scholar]
- 18.de Alwis R, Chen S, Gan ES, Ooi EE. Impact of immune enhancement on COVID-19 polyclonal hyperimmune globulin therapy and vaccine development. EBiomedicine 55, 102768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Indicates the possible mechanism of action of hyperimmune immunoglobulins.
- 19.Rojas-Jiménez G, Solano D, Segura Á et al. In vitro characterization of anti-SARS-CoV-2 intravenous immunoglobulins (IVIg) produced from plasma of donors immunized with the BNT162b2 vaccine and its comparison with a similar formulation produced from plasma of COVID-19 convalescent donors. Front. Med. Technol. 3, 772275 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda. CoVIg-19 Plasma Alliance. www.takeda.com/covid-19-information-center/covig-19-plasma-alliance/
- 21.Vandeberg P, Cruz M, Diez JM et al. Production of anti-SARS-CoV-2 hyperimmune globulin from convalescent plasma. Transfus. 61(6), 1705–1709 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kedrion. COVID-19: plasma and hyperimmune immunoglobulins, ongoing activities. www.kedrion.com/covid-19-plasma-and-hyperimmune-immunoglobulins-ongoing-activities-2/
- 23.Kamada. Kamada announces top-line results from its phase 1/2 clinical trial of its plasma-derived hyperimmune globulin (IgG) treatment for coronavirus disease (COVID-19). www.kamada.com/news/kamada-announces-top-line-results-from-its-phase-1-2-clinical-trial-of-its-plasma-derived-hyperimmune-globulin-igg-treatment-for-coronavirus-disease-covid-19/
- 24.ITAC (INSIGHT 013) Study Group. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial. Lancet 399(10324), 530–540 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali S, Uddin SM, Shalim E et al. Hyperimmune anti-COVID-19 IVIG (C-IVIG) treatment in severe and critical COVID-19 patients: a phase I/II randomized control trial. EClinicalMedicine 36, 100926 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkkinen J, Rahola A, von Bonsdorff L, Tölö H, Törmä E. A modified caprylic acid method for manufacturing immunoglobulin G from human plasma with high yield and efficient virus clearance. Vox Sang. 90(2), 97–104 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Buchacher A, Iberer G. Purification of intravenous immunoglobulin G from human plasma – aspects of yield and virus safety. Biotechnol. J. 1(2), 148–163 (2006). [DOI] [PubMed] [Google Scholar]
- 28.El-Ekiaby M, Vargas M, Sayed M et al. Minipool caprylic acid fractionation of plasma using disposable equipment: a practical method to enhance immunoglobulin supply in developing countries. PLoS Negl. Trop. Dis. 9(2), e0003501 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali S, Uddin SM, Ali A et al. Production of hyperimmune anti-SARS-CoV-2 intravenous immunoglobulin from pooled COVID-19 convalescent plasma. Immunother .13(5), 397–407 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vargas M, Segura Á, Wu YW et al. Human plasma-derived immunoglobulin G fractionated by an aqueous two-phase system, caprylic acid precipitation, and membrane chromatography has a high purity level and is free of detectable in vitro thrombogenic activity. Vox Sang. 108(2), 169–177 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Decree of the government of the Russian Federation of June. On Approval of the Rules for the Procurement, Storage, Transportation and Clinical Use of Donor Blood and Its Components and Invalidation of Some Bodies of the Russian Federation. (2019). [Google Scholar]
- 32.Order of the Ministry of Health of the Russian Federation. On Approval of the Procedure for Donors to Undergo a Medical Examination and a List of Medical Contraindications (Temporary and Permanent) for Donating Blood and (or) Its Components and the Timing of Withdrawal to Which a Person Is Subject in the Presence of Temporary Medical Indications, from Donation of Blood and (or) Its Components. (2020). [Google Scholar]
- 33.Ministry of Health of the Russian Federation. Prevention, Diagnosis and Treatment of New Coronavirus Infection (COVID-19). (2022). [Google Scholar]
- 34.State Pharmacopoeia of the Russian Federation (14th Edition). http://femb.ru/femb/pharmacopea.php [Google Scholar]; • Provided requirements for the quality of intravenous immunoglobulin.
- 35.European Pharmacopoeia (Ph. Eur.) (10th Edition). www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition [Google Scholar]; • Provided requirements for the quality of intravenous immunoglobulin.
- 36.Guidelines for Conducting Preclinical Trials of Medicines. Mironov AN (Ed.). Grif and K, Moscow, Russia: (2012). [Google Scholar]; • Provided the methodology of preclinical evaluation of immunoglobulin.
- 37.EMA. ICH Guidelines S6 (R1) – Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals. Amsterdam, The Netherlands: (2011).2011). [Google Scholar]; • Provided the methodology of preclinical evaluation of immunoglobulin.
- 38.Regulation of the chief state sanitary doctor of the Russian Federation №51 2.2.1.3218-14. Sanitary and epidemiological requirements for equipment, equipment and maintenance of experimental biological clinics (vivariums) (2014). [Google Scholar]
- 39.GOST 33044-2014 Principles of good laboratory practice. https://docs.cntd.ru/document/1200115791
- 40.Wessler S, Reiner L, Freiman DG, Reimer SM, Lertzman M. Serum-induced thrombosis. Studies of its induction, and evolution under controlled conditions in vivo. Circulation 20, 864–874 (1959). [DOI] [PubMed] [Google Scholar]; • Provided the methodology of preclinical evaluation of immunoglobulin.
- 41.Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Clinical performance of different SARS-CoV-2 IgG antibody tests. J. Med. Virol. 92(10), 2243–2247 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perera RA, Mok CK, Tsang OT et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill. 25(16), 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weidner L, Gänsdorfer S, Unterweger S et al. Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays. J. Clin. Virol. 129, 104540 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karaolidou F, Loutsidi N-E, Mellios Z et al. Convalescent plasma therapy in an immunocompromised patient with multiple COVID-19 flares: a case report. Respir. Case Rep. 9, e0858 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senefeld JW, Klassen SA, Ford SK et al. Use of convalescent plasma in COVID-19 patients with immunosuppression. Transfus 61, 2503–2511 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rnjak D, Ravlic S, Sola AM et al. COVID-19 convalescent plasma as long-term therapy in immunodeficient patients? Transfus. Clin. et Biolog. 28(3), 264–270 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US National Library of Medicine. Clinical study in the treatment of patients with moderate course of COVID-19 (2021). https://clinicaltrials.gov/ct2/show/NCT04842435?term=04842435&draw=2&rank=1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.