Abstract
Objectives
Hair loss and reduction in hair volume are hallmarks of hair disorders, such as telogen effluvium, or male or female pattern hair loss, and hair ageing, which can cause severe distress in both men and women. Common anti‐hair loss drugs carry some side effects; therefore, novel, safer approaches targeting milder phenotypes are highly advocated. In this context, we investigated an extract of the alpine plant Edelweiss, Leontopodium alpinum var. Helvetia, for its ability to modulate hair follicle (HF) growth ex vivo and inhibit hair loss while increasing hair regeneration in vivo.
Methods
Human amputated HFs were microdissected from three donors, two women and one man, and cultured ex vivo for 6 days. After treatment with 0.001% Edelweiss extract (EWDE), we investigated hair shaft production and anagen/catagen conversion, and measured known parameters associated with hair growth, that is hair matrix keratinocyte proliferation and apoptosis, dermal papilla inductivity, and growth factors, by quantitative (immuno)histomorphometry. To assess the anti‐hair loss potential of the alpine plant compound, we performed a randomized, placebo‐controlled human study enrolling Caucasian women and men, aged 18 to 65 years, with normal hair loss. After 5 months’ daily use of an extract containing leave‐on serum, we analysed hair density and anagen‐to‐catagen/telogen ratio by the Trichogram analysis.
Results
Our results revealed a significant prolongation in the anagen phase in HFs treated with 0.001% Edelweiss, as indicated by an increase in HFs remaining in anagen and a significant decrease in hair cycle score. In line with this effect, EWDE significantly stimulated hair matrix (HM) keratinocyte proliferation, and dermal papilla inductivity, as shown by a significant up‐regulation of versican expression and alkaline phosphatase activity, and a tendential increase in FGF7 immunoreactivity in the dermal papilla of all HFs or only anagen VI HFs. Corroborating the ex vivo results, we observed a significant increase in growing hair shaft numbers (hair density) after treatment with Edelweiss extract formulation, and a tendential up‐regulation in the anagen‐to‐catagen/telogen ratio.
Conclusions
We show here, through several lines of evidence, that the selected extract of the alpine plant Leontopodium alpinum var Helvetia (Edelweiss) inhibits premature catagen induction, possibly by stimulating dermal papilla inductivity. It is therefore worth exploiting this extract clinically as an anti‐hair loss agent, both for preventing ageing‐associated hair shedding and as an adjuvant therapy for hair loss disorders.
Keywords: anagen phase, anti‐hair loss, hair follicle, hair growth, Leontopodium alpinum, versican
An extract of the alpine plant Edelweiss prolongs anagen phase and increases matrix keratinocyte proliferation in human hair follicles. The extract enhances hair density for fuller hair in Caucasian volunteers.
Résumé
Objectifs
La perte de cheveux et la réduction du volume des cheveux sont caractéristiques des troubles capillaires, tels que l'effluvium télogène, ou la calvitie chez l'homme ou la femme, et le vieillissement des cheveux, qui peuvent causer une certaine détresse chez les hommes et les femmes. Les médicaments courants contre la chute des cheveux ont des effets secondaires, par conséquent, de nouvelles approches plus sûres ciblant des phénotypes légers sont fortement recommandées. Dans ce contexte, nous avons étudié un extrait de la plante alpine Edelweiss, Leontopodium alpinum var. Helvetia, pour sa capacité à stimuler la croissance du follicule pileux (HF) ex vivo et à inhiber la chute des cheveux tout en augmentant la régénération des fibres capillaires in vivo.
Méthodes
Les follicules pileux (HF) humains prélevés ont été microdisséqués chez trois donneurs, deux femmes et un homme, et cultivés ex vivo pendant 6 jours. Après le traitement avec l’extrait d’Edelweiss à 0,001 % (EWDE), nous avons étudié la production de fibre capillaire et la conversion anagène/catagène, ainsi que mesuré les paramètres connus associés à la croissance des cheveux, à savoir, la prolifération des kératinocytes dans la matrice capillaire et l’apoptose, l’induction des papilles dermiques, et des facteurs de croissance, par (immuno‐)histomorphométrie quantitative. Pour évaluer le potentiel des propriétés anti‐chute du cheveu de l’extrait de plante alpine, nous avons réalisé une étude clinique aléatoire avec placebo, sur des femmes et des hommes de type caucasien âgés de 18 à 65 ans présentant une perte de cheveux normale. Après cinq mois d’utilisation quotidienne d’un sérum sans rinçage contenant l’extrait de plante, nous avons analysé la densité capillaire et le rapport anagène à catagène/télogène par trichogramme.
Résultats
Nos résultats ont révélé une prolongation significative de la phase anagène dans les HF traités avec 0,001% d’Edelweiss, comme l’indique une augmentation des HF restant en phase anagène et une diminution significative du « hair cycle score ». En ligne avec cet effet, EWDE a stimulé de façon significative la matrice du cheveux (HM), la prolifération des kératinocytes, et l’induction de la papille dermique, comme le montre une augmentation significative de l’expression du versican et de l’activité de la phosphatase alcaline, et une augmentation tendancielle de l’immunoréactivité FGF7 dans la papille dermique de tous les HF ou seulement des HF anagènes VI. Corroborant les résultats ex vivo, nous avons observé une augmentation significative du nombre de fibres capillaires (densité de cheveux) après le traitement avec la formulation d’extrait d’Edelweiss, et une augmentation tendancielle dans le rapport anagène à catagène/télogène.
Conclusions
Nous montrons ici, à travers plusieurs éléments de preuve, que l’extrait sélectionné de la plante alpine Leontopodium alpinum var Helvetia (Edelweiss) inhibe l’induction prématurée de la phase catagène, en stimulant la papille dermique. Il est donc possible d’utiliser cet extrait comme un agent anti‐chute, à la fois pour prévenir la chute des cheveux associée au vieillissement mais aussi comme une thérapie complémentaire pour les troubles liés à la perte des cheveux.
INTRODUCTION
The impact of reduced hair density and volume is currently underestimated [1] and can cause significant psychological distress in affected individuals, particularly women [2]. Clinically visible changes result mainly from increased hair shedding and/or hair thinning due to hair follicle miniaturization, and are associated both with cosmetically relevant forms of hair follicle disorder, such as telogen effluvium [3, 4], or male or female pattern alopecia, and with ageing [5, 6, 7, 8].
Hair growth and hair shedding are tightly regulated processes. The hair growth cycle has three main distinct phases: anagen (growth phase), catagen (regression phase), and telogen (resting phase) [9, 10].
During telogen, hair follicle stem cells, residing in the bulge area, and dermal papilla fibroblasts cross‐communicate to initiate anagen. The bulge stem cells start proliferating and differentiating in progenitors that generate what are known as transient amplifying cells located in the hair matrix. These cells proliferate considerably during the anagen VI phase and differentiate to stimulate the hair shaft, thus supporting its growth and elongation. Catagen development, on the contrary, is characterized by inhibited proliferation and induction of apoptosis in the hair matrix [11, 12, 13, 14]. At the end of catagen, the hair follicle again enters telogen, and the old hair is shed at the beginning of the next anagen phase when it is pushed out by the next growing hair shaft [15].
On the molecular level, each phase is characterised by the spatiotemporal expression and activity of specific growth factors regulating the length of each phase and the transition from one phase to another [16, 17, 18], among which are the hair growth promoters FGF‐7 and IGF‐1 [19, 20, 21, 22, 23, 24]. Additionally, hair regeneration, or maintenance of anagen, is dependent on dermal papilla inductivity [25, 26]. The latter is sustained by the expression of the extracellular matrix protein, versican and the expression and activity of alkaline phosphatase in the hair follicle dermal papilla [27, 28, 29, 30]. Notably, dermal papilla inductivity is linked to the production of Wnt molecules that stimulate the proliferation of hair matrix keratinocytes [27, 31, 32]. Importantly, DP inductivity is decreased in androgenetic alopecia and telogen effluvium [33].
Hair growth control is orchestrated by several mechanisms, including neuroendocrine factors [16]. Along those lines, it has been shown, for example, that thyroid hormones, testosterone, prolactin and caffeine regulate hair follicle growth ex vivo [34, 35, 36, 37]. One of the main causes of tissue ageing, for example in skin, is oxidative stress and inflammation [38, 39, 40, 41]. Oxidative stress and reactive oxygen species formation are triggered by factors such as solar irradiation and air pollution [42]. Few studies have proposed oxidative stress as a cause of impaired hair growth [43]. However, the activation of Nrf2 has been shown, recently, to attenuate oxidative stress‐induced signs of hair growth inhibition, such as lipid peroxidation, premature catagen induction and reduced cell proliferation, ex vivo [43]. Therefore, antioxidants such as vitamins or plant extracts, usually rich in antioxidative molecules such as phenols and flavonoids, may provide an interesting solution to counteract hair loss [44]. One of the main approaches known to promote hair growth is caffeine, which also possesses antioxidative properties. Caffeine has been shown to counteract the negative effects of testosterone ex vivo by maintaining the proliferation of hair matrix keratinocytes, and the release of the anagen‐associated growth factor IGF‐1, thus inhibiting premature catagen development [45]. Similar effects have also been found for corticotrophin‐releasing hormone along the hypothalamic–pituitary–adrenal (HPA) axis and the non‐HPA axis, such as decreased hair matrix keratinocyte proliferation and negative modulation of hair follicle growth‐regulating growth factors. These inhibitory effects on hair follicle growth were rescued by caffeine in a significant and dose‐dependent manner [46].
Drug‐like solutions such as finasteride, dutasteride and minoxidil [47, 48, 49] are available to treat hair loss. The active principle of finasteride and dutasteride is to inhibit the enzyme type II 5 alpha‐reductase, which converts testosterone into its active form dihydrotestosterone. These treatments are mainly prescribed for androgenetic alopecia, to improve dermal papilla inductivity. Minoxidil is a somewhat less invasive treatment for topical application and has been known for decades, but many patients report scalp irritation on use [50]. Therefore, the industry is looking for milder, less aggressive solutions.
Edelweiss, Leontopodium alpinum, is an alpine plant that grows at high altitudes under harsh environmental conditions. It contains high amounts of flavonoids and leontopodic acid and has been shown to have antibacterial, antioxidative and DNA‐protective potential, as well as anti‐inflammatory efficacy [51, 52, 53]. Moreover, it was shown to have anti‐ageing effects in skin in a recent clinical study [54]. In view of the plant's published activity profile, it is reasonable to consider whether it might have a beneficial effect on the hair follicle in general and hair ageing more specifically. Hence, this study was designed to evaluate an extract of Leontopodium alpinum, var Helvetia, for its ability to modulate human hair follicle growth ex vivo and to achieve fuller hair in vivo.
MATERIALS AND METHODS
Donor material and information
This study and the acquisition of hair follicles were conducted according to Declaration of Helsinki principles. Hair follicle specimens were obtained after informed written patient consent and ethics committee approval (University of Muenster 2015‐602‐f‐S) from healthy donors, two women (51 and 40 years old) and one man (34 years old).
Leontopodium alpinum extract preparation
Edelweiss (Leontopodium alpinum, var. Helvetia) extract (EWDE), batch CM‐19.20a, was prepared from the leaves and flowers of the plant. It is a standardized EtOH/water (60/40w/w) extract. For ex vivo studies, we used a lyophilized dry extract (EWDE), and for clinical studies, a water‐based product containing 2% dry extract. All concentrations mentioned in this publication are based on dry extract content.
Hair follicle organ culture and treatment
Human amputated hair follicles (HFs) were microdissected from follicular unit extract (FUE) or scalp skin as previously described [11, 55]. HFs were cultured at 37°C with 5% CO2 in a minimal media of William's E media (Gibco, Life Technologies) supplemented with 2 mM of L‐Glutamine (Gibco), 10 ng/ml hydrocortisone (Sigma‐Aldrich), 10 μg/ml insulin (Sigma‐Aldrich), and 1% penicillin and streptomycin mix (Gibco) to make William's complete media (WCM). After quality control, growing anagen VI HFs only were allocated randomly to the different experimental groups. At 24 h after isolation, WCM was replaced, and HFs were treated with the vehicle (WCM) or Edelweiss extract (EWDE) at 0.001% until Day 6 of culture. The culture medium was replaced every second day (Day 3 and Day 5 of culture). HFs were then embedded in a cryomatrix (Fisher Scientific) and stored at −80°C.
Frozen hair sample processing
Snap frozen hair follicle samples embedded in OCT matrix were sectioned with a cryostat (Leica), and 6‐μm sections were collected. Consecutive sections of each amputated HF were collected, and slides were stored at −80°C.
Immunofluorescence
To stain apoptotic and proliferating cells in the hair matrix, a Ki‐67/TUNEL double section was performed using the ApopTag® Fluorescein In Situ Apoptosis Detection Kit (Merck Millipore) in combination with a Ki‐67 staining as described previously [11]. Cryosections were fixed with 4% paraformaldehyde (PFA) in PBS and incubated overnight at 4°C with a mouse anti‐Ki‐67 antibody (1:800 in PBS; Cell Signalling Technology) after the TdT enzyme step. Ki‐67 primary antibody was detected with a secondary antibody, goat anti‐mouse IgG rhodamine (1:200; Jackson ImmunoResearch), incubated for 45 min at RT after the fluorescent‐labelled anti‐digoxigenin step of the ApopTag® kit. Counterstaining with DAPI was performed to visualize nuclei. Images were taken using a Keyence fluorescence microscope (BZ9100; Osaka, Japan), maintaining a constant set exposure time throughout imaging for further analysis [31, 32].
For versican expression, cryosections were fixed in PFA at +4°C and permeabilized by incubation with 0.1% Triton X‐100 for 30 min at RT followed by a blocking step in 3% BSA for 45 min at RT. Mouse anti‐human versican (1/400 in 3% BSA, DSHB) was applied overnight at 4°C and detected with a secondary antibody, goat anti‐mouse Alexa Fluor 488 (1/400 in 3% BSA, Invitrogen). Counterstaining with DAPI was performed to visualize nuclei [55].
For IGF‐1 [32] and FGF‐7 [55] expression, cryosections were fixed in acetone at −20°C and pre‐incubated with 2% normal goat serum in TNB buffer (Tris‐HCl + NaCl + Casein) or 10% NGS for 30 min at room temperature (RT) prior to overnight incubation at 4°C with a rabbit anti‐human IGF‐1 primary antibody (1/200 in 2% NGS, Abcam) or a rabbit anti‐human FGF‐7 primary antibody (1/150 in 10% NGS, Abcam). Secondary antibody incubation was performed at RT for 45 min using a goat anti‐rabbit Alexa Fluor 488 (1/400 in 2% NGS (IGF‐1) or 1/750 in 10% NGS (FGF‐7), Invitrogen). Counterstaining with DAPI was performed to visualize nuclei.
Histochemistry
For the histochemical visualization of melanin, the Masson–Fontana staining was performed as previously described [56].
Alkaline phosphatase in situ activity
Alkaline phosphatase activity was detected in situ using the Vector® Blue Substrate Kit and alkaline phosphatase (AP) (SK5300, Biozol) according to the manufacturer's instructions, and using levamisole as a negative control [26].
Hair cycle staging and scoring
Hair cycle staging was performed at the end of the culture, using the Masson–Fontana histochemistry and Ki‐67/TUNEL immunostaining, and determined according to established parameters from Langan et al [11, 55]. A standardized score was applied to the method developed by Kloepper et al., attributing a score of 100 to anagen hair follicles, 200 to early catagen follicles, 300 to mid‐catagen follicles and 400 to late catagen follicles [12]. Thus, the lower the score, the more established the HFs were in anagen, and the higher the score, the more they progressed into catagen.
Hair follicle elongation/Hair shaft production
To determine hair follicle length, each HF was measured from the end of the connective tissue sheath to the end of the distal outer root sheath at different time points (Day 0, Day 1, Day 3, Day 5 and Day 6), using a digital light microscope at 50X magnification (VHX900; Keyence Corporation, Osaka, Japan) and affiliated software [11, 55].
Clinical study
A randomized, placebo‐controlled, single‐centre study was conducted. All volunteers were informed of the study protocol and purpose and signed an informed consent form. The study was approved by the Reading Independent Ethics Committee (RIEC), Woodley, England, Ref.240720‐1 on 31 July 2020.
General inclusion criteria were applied: age between 18 and 65 years, and healthy Caucasian women and men with hair loss. Volunteers were included when having more than 90 hairs lost in a combined combing and wash test. Volunteers were required to keep their normal diet throughout the study.
General exclusion criteria were applied: known allergy or hypersensitivity to the used products, scalp diseases such as psoriasis and dermatitis, pregnancy or planning to become pregnant, use of medication known to cause hair loss and use of anti‐hair loss products.
Clinical study formulations: The study formulations consisted of a leave‐on serum containing 3% of a product containing 2% of the Edelweiss dry extract (ALPAFLOR® EDELWEISS CB, DSM Nutritional Products), and a base formulation without the product containing the extract (placebo). The formulations and their ingredients are listed in Table 1. The formulations' safety was assessed and approved by Eurofins in safety review R20 0103_V3 and complied with Cosmetic Regulation (EC) No. 1223/2009. Adverse events were documented and reported.
TABLE 1.
Formulations used in the clinical study
| Formulation | 3% Edelweiss CB | Placebo | |
|---|---|---|---|
| Ingredients | INCI | % w/w | % w/w |
| Water dem. | Aqua | 61.70 | 64.85 |
| Cosphaderm Pentiol natural | Pentylene glycol | 5 | 5 |
| Cosmedia® Ultragel 300 | Polyquaternium‐37 | 0.30 | 0.15 |
| Ethanol abs. | Alcohol | 30 | 30 |
| ALPAFLOR® EDELWEISS CB | Leontopodium alpinum flower/leaf extract, glycerine, aqua, citric acid | 3.00 | – |
Volunteers: We recruited 30 Caucasian volunteers per study group, consisting of both healthy women and men with hair loss—placebo group: 25 women and 5 men, age 19 to 62 years (45 ± 12); and Edelweiss group: 28 women and 2 men, age 18 to 63 years (44 ± 13). The volunteers were randomly assigned to the two groups using the RAND function in Microsoft Excel.
Product application: The volunteers applied 4 ml of the serum daily, at night (before going to sleep). To do this, they opened three lines over the length of the scalp, applied the product and gently massaged it with their fingertips to aid absorption without rinsing afterwards. This was a full‐head study to avoid cross‐contamination between placebo and active formulation.
General outline of the study: The study duration was 150 days. Volunteers came to the study site at the beginning of the study and at the end of the study. They did not wash or comb their hair before each visit for 48 h and 24 h, respectively. On each visit, hair density was analysed by TrichoScan® (Tricholog GmbH, Freiburg, Germany). To do this, a small area in the volunteer's scalp was shaved and a picture of this zone was taken immediately after shaving. To count the hairs, the software included in TrichoScan® was used but a manual verification was also performed when analysing the images. We used a cap with which we marked the place on the head to always analyse at the same area.
Finally, the anagen‐to‐catagen/telogen ratio was analysed using a Trichogram. In this method, about 20–25 hairs from each volunteer were plucked at each time point. The hairs' growth phase was analysed microscopically to see the state of the root. After this analysis, the anagen/telogen ratio was calculated.
RESULTS
Edelweiss extract prolongs anagen phase ex vivo
We first investigated whether the Edelweiss extract was able to modulate hair growth ex vivo. To this end, hair shaft production was measured using bright‐field images, and microscopic hair cycle staging and scoring was performed by employing Ki‐67/TUNEL immunostaining and the Masson–Fontana histochemistry [30].
Although we did not observe an increase in hair follicle elongation in the Edelweiss extract compared with the vehicle (Figure S1a,b), significantly more anagen HFs were detected after treatment (Figure 1a). The anagen‐prolonging effect of EWDE was also demonstrated by a significant decrease in hair cycle scoring (p < 0.05) (Figure 1b).
FIGURE 1.
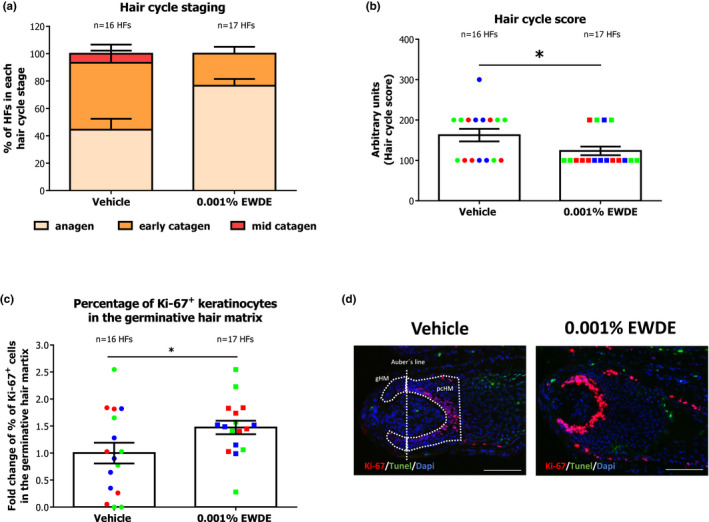
Edelweiss extract (EWDE) prolongs anagen phase in human hair follicles. (a) Quantitative analysis of anagen and catagen HFs (Hair cycle staging) in vehicle‐ and EWDE‐treated hair follicles after organ culture using Ki‐67/TUNEL immunostaining and the Masson–Fontana histochemistry (11). Mean ± SEM, n = 16–17 HFs from three donors (independent experiments). (b) Arbitrary units were assigned to anagen (100), early catagen (200), and mid‐catagen (300) to calculate the hair cycle score in vehicle‐ and EWDE‐treated hair follicles (11). Mean ± SEM, n = 16–17 HFs from three donors (independent experiments, indicated with dots of different colours); unpaired Student's t test, *p = 0.0441. (c) Quantification of Ki‐67+ keratinocytes in the germinative hair matrix of vehicle‐ and EWDE‐treated hair follicles. Mean ± SEM, n = 16–17 HFs from three donors (independent experiments indicated with dots of different colours); unpaired Student's t test, *p = 0.0401. (d) Representative images of Ki‐67/TUNEL immunostaining to assess hair matrix keratinocyte proliferation and apoptosis. Positive cells in the germinative hair matrix below the Auber's line were counted for proliferation and in the germinative and precortical hair matrix for apoptosis. Blue and green represent hair follicles from the female donors, and red represents hair follicles from the male donor. HFs, hair follicles; gHM, germinative hair matrix; and pcHM, precortical hair matrix. Scale bar: 100 μm [Colour figure can be viewed at wileyonlinelibrary.com]
Our data therefore indicate that Edelweiss extract inhibits premature catagen development ex vivo, highlighting its ability to possibly suppress hair loss in vivo.
Edelweiss extract significantly stimulates hair matrix keratinocyte proliferation
To confirm the effect of the Edelweiss extract on inhibiting catagen development, we analysed the percentage of proliferative and apoptotic hair matrix keratinocytes in all HFs at the end of the culture (anagen + catagen) [11, 55]. While no change was detected in the number of TUNEL+ cells in the hair matrix (data not shown), Edelweiss‐treated hair follicles displayed 1.5‐fold more proliferating keratinocytes than vehicle‐treated follicles (p < 0.05 EWDE vs vehicle) as assessed by quantitative (immuno)histomorphometry for Ki‐67+ cells in the germinative hair matrix (Figure 1c, d).
Edelweiss extract stimulates dermal papilla inductivity ex vivo
To investigate possible mechanisms of action in EWDE extract, we quantified the expression of markers associated with dermal papilla inductivity (versican and alkaline phosphatase) [25] and anagen‐associated growth factors (FGF‐7 and IGF‐1) [23, 57] in all HFs and in HFs remaining in anagen VI at the end of the culture period.
Quantitative (immuno)histomorphometry for versican expression revealed a significant 1.4‐fold increase in the dermal papilla of hair follicles treated with the Edelweiss extract compared to follicles treated with the vehicle (p < 0.01) (Figure 2a). Interestingly, this effect was more prominent when only HFs from the female donors were pooled together (Figure 2b), even when only anagen VI HFs were analysed (Figure 3a, b). Along this line, alkaline phosphatase activity was also increased significantly by EWDE treatment, albeit only in hair follicles from two donors, namely those deriving from female donors (Figure 2c, d). This effect was even more prominent when only anagen HFs were evaluated after the culture (Figure 3c, d).
FIGURE 2.
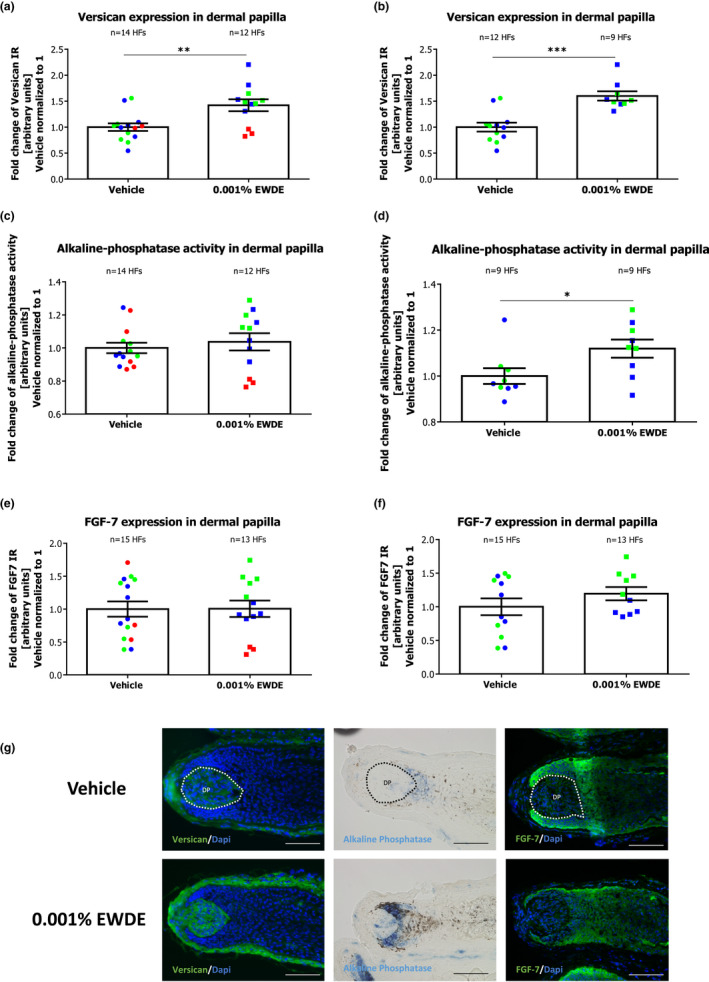
Effect of EWDE on dermal papilla inductivity on all hair follicles. (a) Versican expression was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs. Mean ± SEM, n = 12–14 HFs from three donors (independent experiments); unpaired Student's t test, **p = 0.0039. (b) Versican expression was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs from female donors. Mean ± SEM, n = 9–12 HFs from two female donors (independent experiments); Mann–Whitney test, ***p = 0.0009. (c) Alkaline phosphatase activity was measured in dermal papilla of Vehicle‐ and EWDE‐treated HFs. Mean ± SEM, n = 12–14 HFs from three donors (independent experiments); Unpaired Student's t test, n.s. (d) Alkaline phosphatase activity was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs from female donors. Mean ± SEM, n = 9 HFs from two female donors (independent experiments); Mann–Whitney test, *p = 0.0399. (e) FGF‐7 expression was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs. Mean ± SEM, n = 12–14 HFs from three donors (independent experiments); unpaired Student's t test, n.s. (f) FGF‐7 expression was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs from female donors. Mean ± SEM, n = 9 HFs from two female donors (independent experiments); unpaired Student's t test, n.s. (g) Representative pictures of versican, alkaline phosphatase and FGF‐7 immunostaining in vehicle‐ and EWDE‐treated HFs. Immunoreactivity was quantified in dermal papilla (demarcated areas). Blue and green dots represent hair follicles from female donors, and red dots represent hair follicles from the male donor. HFs, hair follicles; DP, dermal papilla; and n.s., not significant. Scale bar: 100 μm [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
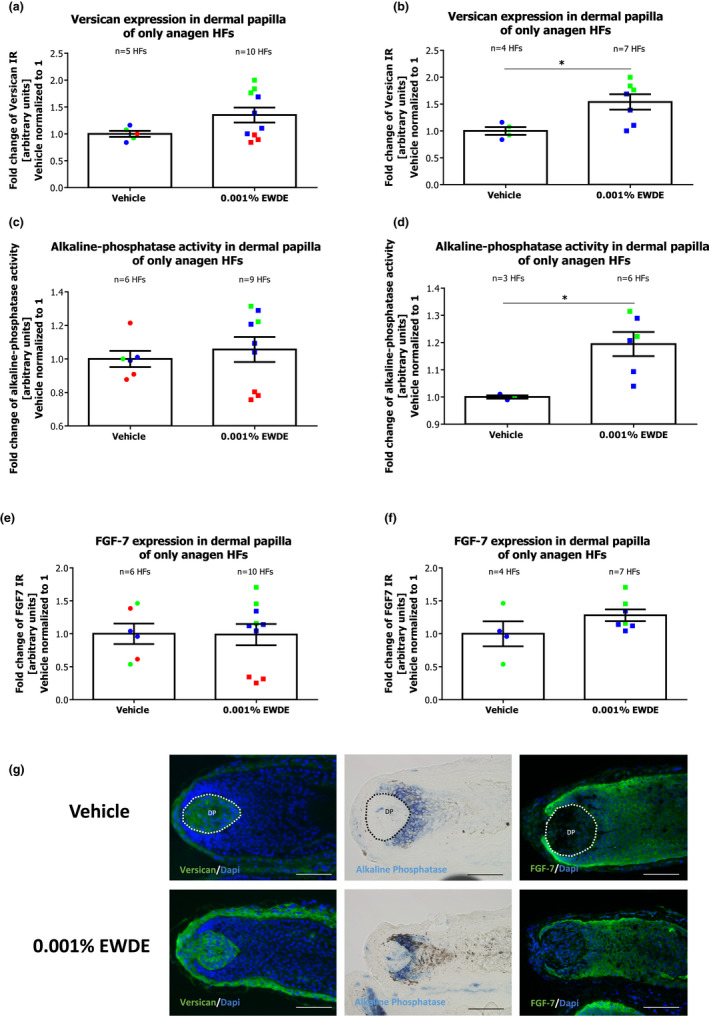
Effect of EWDE on dermal papilla inductivity in hair follicles remaining in anagen after the culture. (a) Versican expression was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs. Mean ± SEM, n = 5–10 anagen HFs from three donors (independent experiments); Mann–Whitney test, n.s. (b) Versican expression was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs from female donors. Mean ± SEM, n = 6–7 HFs from two female donors (independent experiments); Mann–Whitney test, *p = 0.0424. (c) Alkaline phosphatase activity was measured in dermal papilla of vehicle‐ and EWDE‐treated anagen HFs. Mean ± SEM, n = 6–9 HFs from three donors (independent experiments); Mann–Whitney test, n.s. (d) Alkaline phosphatase activity was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs from female donors. Mean ± SEM, n = 3–6 anagen HFs from two female donors (independent experiments); Mann–Whitney test, *p = 0.0238. (e) FGF‐7 expression was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs. Mean ± SEM, n = 6–10 HFs from three donors (independent experiments); Mann–Whitney test, n.s. (f) FGF‐7 expression was measured in dermal papilla of vehicle‐ and EWDE‐treated HFs from female donors. Mean ± SEM, n = 4–7 HFs from two female donors (independent experiments); Mann–Whitney test, n.s. (g) Representative pictures of versican, alkaline phosphatase and FGF‐7 immunostaining in vehicle‐ and EWDE‐treated HFs. Immunoreactivity was quantified in dermal papilla (demarcated areas). Blue and green dots represent hair follicles from female donors, and red dots represent hair follicles from the male donor. HFs, hair follicles; DP, dermal papilla; and n.s., not significant. Scale bar: 100 μm [Colour figure can be viewed at wileyonlinelibrary.com]
Although FGF‐7 was not affected in the dermal papilla of anagen and catagen HFs from the analysed donors (Figure 2e), a slight increase in FGF‐7 was detected in female donors' HFs (Figure 2f), even when only anagen HFs were analysed (Figure 3e,f). Instead, FGF‐7 expression in the outer root sheath remained unchanged after EWDE treatment (Figure S2). The expression of IGF‐1 in the dermal papilla or in the outer root sheath was not affected by EWDE treatment in any of the analysed donors (Figure S3).
Taken together, our data support the hypothesis that the anagen‐prolonging effect of EWDE derives from the stimulation of DP activity, at least in selected donors, which eventually leads to the maintenance of hair matrix keratinocyte proliferation.
Edelweiss extract significantly increases hair density in our study population
To transfer our ex vivo findings to human subjects, we conducted a clinical study as described in Material and Methods. Hair density at the beginning and end of the study was investigated using TrichoScan® and displayed a significant increase of +22 hair/cm2 (approximately 13 200 new hairs for the entire scalp) in the group using the Edelweiss formulation compared with the group using the placebo formulation (no increase) (Figure 4a, b). Using ANOVA and the post hoc Tukey analysis, the difference between active formulation and placebo was highly significant (p < 0.001). This increase in hairs was visible on macroscopic images of a selected volunteer using the active formulation—more hairs could be seen after 150 days of treatment than at the beginning of the study (Figure 4b).
FIGURE 4.
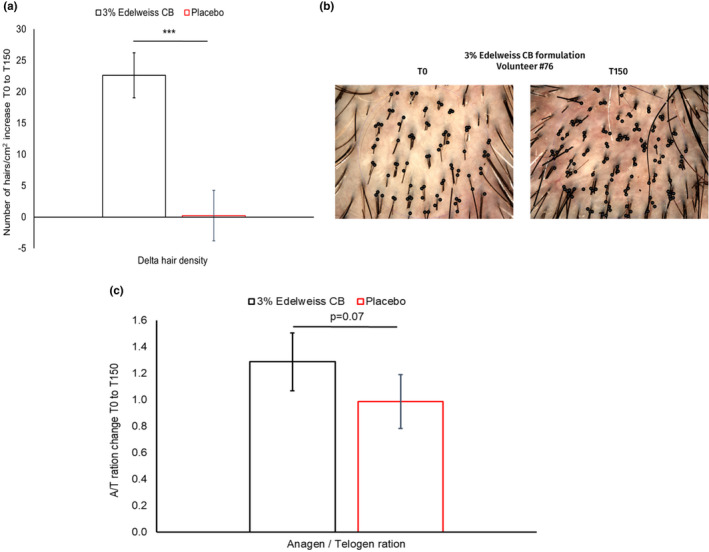
Effect of EWDE on hair density and anagen‐to‐catagen/telogen ratio in vivo. (a) Hair density was measured by Trichogram® from baseline to T150 and compared between the group using the 3% Edelweiss CB formulation (black bar) and the group using the placebo formulation (red bar). ***p < 0.001 vs placebo by ANOVA and post hoc Tukey’s test. Error bars represent the standard error of the mean (n = 30 per group). (b) Microimages of scalp showing hairs (black dots) at the baseline T0 and at the end of the study T150 of one volunteer, #76, using the active formulation with Edelweiss. (c) Hair follicles were analysed using TrichoScan®. The result was a comparison between the group using the 3% Edelweiss formulation (black bar) and the group using the placebo formulation (red bar). Error bars represent the standard error of the mean. The Mann–Whitney U test was used for statistics (n = 30 per group) [Colour figure can be viewed at wileyonlinelibrary.com]
Edelweiss extract increases anagen/catagen‐to‐telogen ratio
Using Trichogram® analysis, the hair cycle stage of HFs at the beginning and end of the study (Day 150) was determined and shown as an anagen‐to‐catagen/telogen ratio. The Edelweiss group displayed a strong trend towards an increased anagen‐to‐catagen/telogen ratio compared with the placebo group (p = 0.07), which was normalized to 1 (Figure 4c).
Taken together, these data suggest that an extract of Leontopodium alpinum has a beneficial effect in hair loss treatments and treatments to counteract signs of hair ageing.
DISCUSSION
Hair loss is a common sign of ageing in both men and women and can lead to psychological distress in affected individuals. The cosmetics industry in particular is looking for natural, biodegradable, but mild and effective treatments.
Our study focused on investigating the potential of an extract of the alpine plant Leontopodium alpinum (Edelweiss) to inhibit excessive hair shedding. Our analysis revealed that the extract significantly maintains HFs longer in anagen ex vivo. We can also confirm that the ex vivo data attained clinical significance, as a topical formulation containing the edelweiss extract increased hair density and showed a strong trend towards improved anagen/catagen‐to‐telogen ratio in subjects experiencing hair loss.
Our data suggest that EWDE prolongs the anagen phase by stimulating dermal papilla inductivity, as indicated by an up‐regulation in versican and alkaline phosphatase activity. Dermal papilla inductivity, especially alkaline phosphatase expression/activity, correlates with the stimulation of the Wnt pathway [27, 31]. This, in turn, has been shown to stimulate the expression of FGF7 in mice [58], just as in the case of HF treatment with EWDE. Therefore, it is conceivable that EWDE may maintain hair matrix keratinocyte proliferation by stimulating Wnt signalling and FGF7 production in dermal papilla fibroblasts. Interestingly, and further supporting this hypothesis, a recent publication at the Society of Investigative Dermatology (SID) annual meeting showed that EWDE was able to induce the Wnt/b‐catenin pathway in a luciferase‐based reporter assay using transfected human hair follicle dermal papilla cells [59].
However, it must be acknowledged that stimulation of DP inductivity was only observed in 2 of 3 donors analysed in this study. Therefore, there may be inter‐individual variations in EWDE's mechanism of action leading to anagen prolongation. Interestingly, only female donors responded with an increased expression of dermal papilla inductivity markers. Thus, it is conceivable that EWDE may trigger different mechanistic responses or target different cell populations in female and male hair follicles. However, follow‐up studies with significantly more female and male donors are required to clarify this hypothesis. Despite this, no gender‐dependent variation has been seen in the overall effect of EWDE in female and male hair follicles, as the ex vivo treatment culminates in anagen prolongation in all donors analysed.
In line with the ex vivo results, our pilot clinical trial confirmed that EWDE inhibits hair loss in female and male subjects. Interestingly, our findings regarding an increase in hair density of 22 new hairs per cm2 (Figure 4a) are comparable with others who found an increase of 24 hairs per cm2 in 6 months with a 5% minoxidil formulation over 6 months [60].
The formulation containing EWDE, which was used in this study, is an alcohol‐based leave‐on serum for topical application on the scalp. Compared with orally administered drugs, the active principle is applied in a targeted manner to the site where its efficacy can unfold. Therefore, treatments using topical applications are considered mild and safe to use.
Despite the low number of donors, or subjects, our results provide evidence that an extract of Edelweiss, Leontopodium alpinum var. Helvetia, was able to support hair follicle growth both ex vivo and in vivo. Therefore, topical application of EWDE extract may be used by individuals looking for fuller hair. In addition, EWDE may be employed as an adjuvant strategy for hair loss disorders, or as an alternative to orally administered solutions, for those preferring milder, safer approaches.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
We are grateful to the hair follicle donors and the clinical study volunteers. We would like to acknowledge the valuable discussions with our colleagues at DSM Nutritional Products and Monasterium Laboratory who helped to advance this work. The Edelweiss extract mentioned in this study is marketed as ALPAFLOR® EDELWEISS CB by DSM Nutritional Products. This study was supported by DSM Nutritional Products and executed either at Monasterium Laboratory, a CRO performing dermatological research projects, or at Centro de Tecnologia Capilar, Barcelona, Spain. RC, EM and MG are employees of DSM Nutritional Products; ALR, JE and MB are employees of Monasterium Laboratory; and AFB is an employee of Centro de Tecnologia Capilar.
Campiche R, Le Riche A, Edelkamp J, Botello AF, Martin E, Gempeler M, et al. An extract of Leontopodium alpinum inhibits catagen development ex vivo and increases hair density in vivo. Int J Cosmet Sci. 2022;44:363–376. doi: 10.1111/ics.12783
REFERENCES
- 1. Calvo Peretti M, Caballero Uribe N, Regnier A, Trueb RM. Look at your hair the way you look at your face: concept of total facial skin and hair care. Skin Appendage Disord. 2020;6(2):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dhami L. Psychology of hair loss patients and importance of counseling. Indian J Plast Surg. 2021;54(4):411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liyanage D, Sinclair R. Telogen effluvium. Cosmetics. 2016;3(2):13. [Google Scholar]
- 4. Telogen MS. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trueb RM. Aging of hair. J Cosmet Dermatol. 2005;4(2):60–72. [DOI] [PubMed] [Google Scholar]
- 6. Van Neste D, Tobin DJ. Hair cycle and hair pigmentation: dynamic interactions and changes associated with aging. Micron. 2004;35(3):193–200. [DOI] [PubMed] [Google Scholar]
- 7. Seiberg M. Age‐induced hair greying ‐ the multiple effects of oxidative stress. Int J Cosmet Sci. 2013;35(6):532–8. [DOI] [PubMed] [Google Scholar]
- 8. Peters EM, Imfeld D, Gräub R. Graying of the human hair follicle. J Cosmet Sci. 2011;62:121–5. [PubMed] [Google Scholar]
- 9. Paus R, Foitzik K. In search of the “hair cycle clock”: a guided tour. Differentiation. 2004;72:489–511. [DOI] [PubMed] [Google Scholar]
- 10. Schneider MR, Schmidt‐Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19(3):R132–42. [DOI] [PubMed] [Google Scholar]
- 11. Langan EA, Philpott MP, Kloepper JE, Paus R. Human hair follicle organ culture: theory, application and perspectives. Exp Dermatol. 2015;24(12):903–11. [DOI] [PubMed] [Google Scholar]
- 12. Kloepper JE, Sugawara K, Al‐Nuaimi Y, Gaspar E, van Beek N, Paus R. Methods in hair research: how to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp Dermatol. 2010;19(3):305–12. [DOI] [PubMed] [Google Scholar]
- 13. Geyfman M, Plikus MV, Treffeisen E, Andersen B, Paus R. Resting no more: re‐defining telogen, the maintenance stage of the hair growth cycle. Biol Rev Camb Philos Soc. 2015;90(4):1179–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oh JW, Kloepper J, Langan EA, Kim Y, Yeo J, Kim MJ, et al. A guide to studying human hair follicle cycling in vivo. J Invest Dermatol. 2016;136(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins CA, Westgate GE, Jahoda CA. From telogen to exogen: mechanisms underlying formation and subsequent loss of the hair club fiber. J Invest Dermatol. 2009;129(9):2100–8. [DOI] [PubMed] [Google Scholar]
- 16. Paus R. Frontiers in the (neuro‐)endocrine controls of hair growth. J Investig Dermatol Symp Proc. 2007;12(2):20–2. [DOI] [PubMed] [Google Scholar]
- 17. Philpott MP, Sanders D, Kealey T. Cultured human hair follicles and growth factors. J Invest Dermatol. 1995;104(5):44S–5S. [DOI] [PubMed] [Google Scholar]
- 18. Philpott MP, Sanders D, Westgate GE, Kealey T. Human hair growth in vitro: a model for the study of hair follicle biology. J Dermatol Sci. 1994;7:S55–72. [DOI] [PubMed] [Google Scholar]
- 19. Alam M, Bertolini M, Gherardini J, Keren A, Ponce L, Cheret J, et al. An osteopontin‐derived peptide inhibits human hair growth at least in part by decreasing fibroblast growth factor‐7 production in outer root sheath keratinocytes. Br J Dermatol. 2020;182(6):1404–14. [DOI] [PubMed] [Google Scholar]
- 20. Trueb RM. Further Clinical Evidence for the Effect of IGF‐1 on Hair Growth and Alopecia. Skin Appendage Disord. 2018;4(2):90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hwang KA, Hwang YL, Lee MH, Kim NR, Roh SS, Lee Y, et al. Adenosine stimulates growth of dermal papilla and lengthens the anagen phase by increasing the cysteine level via fibroblast growth factors 2 and 7 in an organ culture of mouse vibrissae hair follicles. Int J Mol Med. 2012;29(2):195–201. [DOI] [PubMed] [Google Scholar]
- 22. Rosenquist TA, Martin GR. Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 1996;205(4):379–86. [DOI] [PubMed] [Google Scholar]
- 23. Philpott MP, Sanders DA, Kealey T. Effects of insulin and insulin‐like growth factors on cultured human hair follicles: IGF‐I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Investig Dermatol. 1994;102(6):857–61. [DOI] [PubMed] [Google Scholar]
- 24. Weger N, Schlake T. Igf‐I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005;125(5):873–82. [DOI] [PubMed] [Google Scholar]
- 25. Taghiabadi E, Nilforoushzadeh MA, Aghdami N. Maintaining hair inductivity in human dermal papilla cells: a review of effective methods. Skin Pharmacol Physiol. 2020;33(5):280–92. [DOI] [PubMed] [Google Scholar]
- 26. Platt CI, Cheret J, Paus R. Towards developing an organotypic model for the preclinical study and manipulation of human hair matrix‐dermal papilla interactions. Arch Dermatol Res. 2021. doi: 10.1007/s00403-020-02178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kishimoto J, Soma T, Burgeson RE, Hibino T. Versican expression by dermal papilla‐regenerated hair follicles – a promising tool for hair‐regrowth products. Int J Cosmet Sci. 2004;26:165–6. [Google Scholar]
- 28. Soma T, Tajima M, Kishimoto J. Hair cycle‐specific expression of versican in human hair follicles. J Dermatol Sci. 2005;39(3):147–54. [DOI] [PubMed] [Google Scholar]
- 29. Kwack MH, Jang YJ, Won GH, Kim MK, Kim JC, Sung YK. Overexpression of alkaline phosphatase improves the hair‐inductive capacity of cultured human dermal papilla spheres. J Dermatol Sci. 2019;95(3):126–9. [DOI] [PubMed] [Google Scholar]
- 30. Kopf AW, Orentreich N. Alkaline phosphatase in alopecia areata. AMA Arch Derm. 1957;76(3):288–95. [DOI] [PubMed] [Google Scholar]
- 31. Hawkshaw NJ, Hardman JA, Haslam IS, Shahmalak A, Gilhar A, Lim X, et al. Identifying novel strategies for treating human hair loss disorders: Cyclosporine A suppresses the Wnt inhibitor, SFRP1, in the dermal papilla of human scalp hair follicles. PLoS Biol. 2018;16(5):e2003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bertolini M, Cheret J, Pinto D, Hawkshaw N, Ponce L, Erdmann H, et al. A novel nondrug SFRP1 antagonist inhibits catagen development in human hair follicles ex vivo. Br J Dermatol. 2021;184(2):371–3. [DOI] [PubMed] [Google Scholar]
- 33. Kubanov AA, Gallyamova YA, Korableva OA, Kalinina PA. The role of the VEGF, KGF, EGF, and TGF‐β1 growth factors in the pathogenesis of telogen effluvium in women. Biomed Pharmacol J. 2017;10(1):191–8. [Google Scholar]
- 34. van Beek N, Bodo E, Kromminga A, Gaspar E, Meyer K, Zmijewski MA, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93(11):4381–8. [DOI] [PubMed] [Google Scholar]
- 35. Olah A, Gherardini J, Bertolini M, Cheret J, Ponce L, Kloepper J, et al. The thyroid hormone analogue KB2115 (eprotirome) prolongs human hair growth (anagen) ex vivo. J Invest Dermatol. 2016;136(8):1711–4. [DOI] [PubMed] [Google Scholar]
- 36. Langan EA, Ramot Y, Hanning A, Poeggeler B, Biro T, Gaspar E, et al. Thyrotropin‐releasing hormone and oestrogen differentially regulate prolactin and prolactin receptor expression in female human skin and hair follicles in vitro. Br J Dermatol. 2010;162(5):1127–31. [DOI] [PubMed] [Google Scholar]
- 37. Gaspar E, Hardenbicker C, Bodo E, Wenzel B, Ramot Y, Funk W, et al. Thyrotropin releasing hormone (TRH): a new player in human hair‐growth control. FASEB J. 2010;24(2):393–403. [DOI] [PubMed] [Google Scholar]
- 38. Giacomoni PU, Rein G. Factors of skin ageing share common mechanisms. Biogerontology. 2001;2:219–29. [DOI] [PubMed] [Google Scholar]
- 39. Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29. [DOI] [PubMed] [Google Scholar]
- 40. Pilkington SM, Bulfone‐Paus S, Griffiths CEM, Watson REB. Inflammaging and the skin. J Invest Dermatol. 2021;141(4S):1087–95. [DOI] [PubMed] [Google Scholar]
- 41. Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–61. [DOI] [PubMed] [Google Scholar]
- 43. Haslam IS, Jadkauskaite L, Szabo IL, Staege S, Hesebeck‐Brinckmann J, Jenkins G, et al. Oxidative damage control in a human (mini‐) organ: Nrf2 activation protects against oxidative stress‐induced hair growth inhibition. J Invest Dermatol. 2017;137(2):295–304. [DOI] [PubMed] [Google Scholar]
- 44. Bassino E, Gasparri F, Munaron L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: a review. Int J Mol Sci. 2020;21(2):523. doi: 10.3390/ijms21020523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fischer TW, Herczeg‐Lisztes E, Funk W, Zillikens D, Biro T, Paus R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor‐beta2/insulin‐like growth factor‐1‐mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br J Dermatol. 2014;171(5):1031–43. [DOI] [PubMed] [Google Scholar]
- 46. Fischer TW, Bergmann A, Kruse N, Kleszczynski K, Skobowiat C, Slominski AT, et al. New effects of caffeine on corticotropin‐releasing hormone (CRH)‐induced stress along the intrafollicular classical hypothalamic‐pituitary‐adrenal (HPA) axis (CRH‐R1/2, IP3 ‐R, ACTH, MC‐R2) and the neurogenic non‐HPA axis (substance P, p75[NTR] and TrkA) in ex vivo human male androgenetic scalp hair follicles. Br J Dermatol. 2021;184(1):96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gubelin Harcha W, Barboza Martinez J, Tsai TF, Katsuoka K, Kawashima M, Tsuboi R, et al. A randomized, active‐ and placebo‐controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014;70(3):489–98 e3. [DOI] [PubMed] [Google Scholar]
- 48. Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta‐analysis. J Am Acad Dermatol. 2017;77(1):136–41. [DOI] [PubMed] [Google Scholar]
- 49. Goren A, Naccarato T. Minoxidil in the treatment of androgenetic alopecia. Dermatol Ther. 2018;31(5):e12686. [DOI] [PubMed] [Google Scholar]
- 50. Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6(2):130–6. [DOI] [PubMed] [Google Scholar]
- 51. Schwaiger S, Cervellati R, Seger C, Ellmerer EP, About N, Renimel I, et al. Leontopodic acid – a novel highly substituted glucaric acid derivative from Edelweiss (Leontopodium alpinum Cass.) and its antioxidative and DNA protecting properties. Tetrahedron. 2005;61:4621–30. [Google Scholar]
- 52. Lulli D, Alla P, Maurelli R, Elena D, Giovanna P, Vladimir K, et al. Anti‐inflammatory effects of concentrated ethanol extracts of Edelweiss (Leontopodium alpinum Cass.) callus cultures towards human keratinocytes and endothelial cells. Mediators Inflamm. 2012;2012:498373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dobner MJ, Schwaiger S, Jenewein IH, Stuppner H. Antibacterial activity of Leontopodium alpinum (Edelweiss). J Ethnopharmacol. 2003;89(2–3):301–3. [DOI] [PubMed] [Google Scholar]
- 54. Cho WK, Kim HI, Kim SY, Seo HH, Song J, Kim J, et al. Anti‐aging effects of Leontopodium alpinum (Edelweiss) callus culture extract through transcriptome profiling. Genes (Basel). 2020;11(2):230. doi: 10.3390/genes11020230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Edelkamp J, Gherardini J, Bertolini M. Methods to study human hair follicle growth ex vivo: human microdissected hair follicle and human full thickness skin organ culture. Methods Mol Biol. 2020;2154:105–19. [DOI] [PubMed] [Google Scholar]
- 56. Bodo E, Tobin DJ, Kamenisch Y, Biro T, Berneburg M, Funk W, et al. Dissecting the impact of chemotherapy on the human hair follicle: a pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy. Am J Pathol. 2007;171(4):1153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peus D, Pittelkow MR. Growth factors in hair organ development and the hair growth cycle. Dermatol Clin. 1996;14(4):559–72. [DOI] [PubMed] [Google Scholar]
- 58. Enshell‐Seijffers D, Lindon C, Kashiwagi M, Morgan BA. beta‐catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18(4):633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leveque M, Mas C, Haure M, Lejeune O, Duplan H, Castex‐Rizzi N, et al. 601 Hair growth properties of Cinchona succirubra extract, Leontopodium alpinum extract and manganese PCA in human hair follicle dermal papilla cells. J Investig Dermatol. 2021;141(5):S104. [Google Scholar]
- 60. Liu Y, Jiang LL, Liu F, Qu Q, Fan ZX, Guo Z, et al. Comparison of low‐level light therapy and combination therapy of 5% minoxidil in the treatment of female pattern hair loss. Lasers Med Sci. 2021;36(5):1085–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


