Abstract
The incidence of non‐melanoma skin cancer is on the rise and melanoma is among the most common cancers in the United States. Establishing an early diagnosis is essential for improving the prognosis of patients with skin cancer. High‐resolution non‐invasive imaging techniques may represent key tools for helping to identify and monitor early signs of skin cancer in seemingly healthy skin. Cumulative lifetime sun exposure leads to photoaging and photocarcinogenenis and the reaction of the skin to this solar‐induced damage is balanced between the DNA repair and photoprotection defence mechanisms of melanocytes and keratinocytes. In the first part of this article we provide an overview of these defence mechanisms and of the photoaging process, and discuss how non‐invasive imaging can be used to evaluate these changes. We then propose a model in which skin aging manifestations can be classified according to subject‐specific sun‐damage reaction profiles observed by reflectance confocal microscopy (RCM) and optical coherence tomography (OCT). These photoaging profiles include an atrophic phenotype characterized by actinic keratosis, and a hypertrophic phenotype characterized by hyperplastic pigmented skin. According to our model, these phenotypes may be predictive of predispositions to different types of skin cancer: squamous cell carcinoma for the atrophic phenotype and lentigo maligna and freckles for the hypertrophic phenotype. In addition to RCM and OCT, dermoscopy is another non‐invasive technique that has improved the diagnosis of skin cancer. In the second part of this article, we describe how the YouDermoscopy™ application can improve skills and thus enhance the dermoscopic recognition of sun‐induced skin tumours, and then show how this training tool enables its users to collaborate with dermatologists worldwide to obtain second opinions for the diagnosis of ambiguous lesions. Altogether, RCM, OCT and dermoscopy are valuable tools that can contribute significantly to improving the early diagnosis of precancerous and cancerous lesions.
Keywords: confocal microscopy, dermoscopy, diagnosis, melanoma, actinic keratosis
Introduction
The field of dermatology has greatly benefited from non‐invasive imaging techniques, which are especially useful in the diagnosis of sun‐induced skin lesions, in particular malignant lesions. Indeed, the incidence of non‐melanoma skin cancer (NMSC) has increased recently. 1 In particular, cutaneous squamous cell carcinoma (cSCC) is one of the most common cancers in the United States. 2 Although cSCC is not often fatal, it can cause significant morbidity: most cSCCs are located in the head‐and‐neck region and the extensive excision required to treat the advanced stage of the disease can cause disfigurement. The cutaneous melanoma incidence rate is about one‐tenth of that of NMSC; however, its mortality rate is approximately eight‐fold higher than that of NMSC (reviewed in Ref. [3]). In the United States, melanoma is still the fifth most common cancer in men and the sixth most common cancer in women. 4 However, the survival rates for both melanoma and NMSC are high when these malignancies are detected early and treated appropriately. For this reason, the diagnosis of precancerous lesions, such as actinic keratosis (AK) is important to allow for more targeted patient monitoring. It is estimated that about 28%–50% of fair‐skinned individuals over 60 years of age are affected by AK, 5 and its incidence continues to increase worldwide. However, despite the very high prevalence of AK in some countries, its importance to public health is often underestimated. 6
Visual examination of the skin by clinicians is sometimes not sufficient to discriminate between benign and malignant lesions, and to establish a clinical diagnosis. In particular, early diagnosis of lentigo maligna (LM) is often difficult, even for experienced dermatologists, with the differential diagnoses including solar lentigo, early‐stage seborrheic keratosis, lichen planus‐like keratosis, pigmented actinic keratosis (PAK) and melanocytic nevus. 7
Although skin biopsy with histopathology remains the gold standard for differential diagnosis, it is invasive, can be painful and cannot be repeated in case of several suspicious lesions. Non‐invasive techniques are especially useful for sensitive areas such as the face and neck, and allow exploration of a wider skin area. They also allow an area of skin to be monitored over time, unlike conventional histopathology in which removed tissue cannot be subsequently analysed. 8 Various medical imaging tools are available for non‐invasive in‐depth skin examinations: dermoscopy, reflectance confocal microscopy (RCM), 9 cross‐polarized light and fluorescence photography, optical coherence tomography (OCT), and high‐frequency ultrasound. 6
However, it would also be useful to be able to predict the risk of developing melanoma or cSCC after a lifetime of solar exposure: are there early signs that could be observed in healthy skin with the help of non‐invasive imaging techniques? Do the signs of photoaging reflect skin reactions to solar exposure? Can we determine the degree of skin cancer predisposition according to observed patterns of sun‐damage reactions and photoaging signs?
In the first part of this article, we provide a brief overview of skin defence mechanisms against solar damage and photocarcinogenesis, and of the photoaging processes. We then describe the signs of photoaging that can be observed using non‐invasive imaging techniques, and discuss the potential links between skin reactions to solar exposure, signs of photoaging, and skin cancer lesions, and examine how non‐invasive imaging could be of interest for exploring these interactions. In the second part of this review, we will demonstrate how increasing knowledge of dermoscopy via an application could help improve the accuracy and timely diagnosis of sun‐induced skin tumours.
Non‐invasive investigations of skin defence mechanisms against photocarcinogenesis and photoaging induced skin tumours
Photocarcinogenesis and skin defence mechanisms against solar exposure
Excess exposure to solar UV‐B radiation is a key risk factor for photoaging and photocarcinogenesis. In particular, sun exposure is associated with approximately 65% of melanoma cases, and 90% of NMSC cases, including basal cell carcinoma (BCC) and cSCC. These skin cancers arise from photocarcinogenesis of either melanocytes (melanoma) or keratinocytes (BCC and cSCC). The mutagenesis process is triggered by UV light reaching the skin and generating a cascade of damage. Pyrimidine dimers are good markers of the quantity of this damage. Significant mutations lead to oncogene activation and tumour suppressor gene inactivation. Different molecular changes characterize the three major types of skin cancer: p53 gene inactivation is found in cSCC, patched gene mutations in BCC and p16 gene inactivation in melanoma. 10 These mutations result in the survival and proliferation of cells harbouring DNA damage. Moreover, inflammatory responses within the tumour microenvironment also contribute significantly to skin tumorigenesis. 1
In the absence of defence mechanisms, as in patients with xeroderma pigmentosum who harbour various DNA excision repair defects, skin cancers develop very early in life. According to clinical studies from the National Institutes of Health, patients under the age of 20 years with xeroderma pigmentosum have a nearly 10 000‐fold higher risk of skin cancer than those without the disease, which underlines the importance of DNA repair in cancer prevention in the general population. 11
Endogenous skin protection processes include both direct mechanisms of DNA repair and indirect mechanisms of photoprotection. To maintain genomic stability, endogenous DNA repair processes provide an effective response to DNA lesions. Nucleotide excision repair involves two main mechanisms: global genome repair and transcription‐coupled repair. 10 However, some lesions can escape these repair mechanisms. Skin photoprotection involves pigmentation – which absorbs the light and protects the nucleus – and hyperplasia of the epidermis. Indeed, UV‐induced tanning provides the equivalent of a sun protection factor (SPF) of 3–4 together with epidermal hyperplasia. 12 Melanin limits the extent of UV penetration through the epidermal layers and scavenges the reactive oxygen radicals that may lead to oxidative DNA damage. 13 The photoprotective role of melanin has been demonstrated by studies measuring the levels of cyclobutane pyrimidine dimers and 6‐4 photoproducts in the epidermis. 14 , 15
Moreover, the UV‐induced activation of the epidermal growth factor receptor increases keratinocyte proliferation and suppresses apoptosis, leading to epidermal hyperplasia. 16 Epidermal hyperplasia mechanically protects the basal layer of the skin from UV radiation by increasing the thickness of the cellular barrier.
Photoaging mechanisms
Whereas intrinsic chronoaging is mostly characterized by the reduced proliferation and metabolism of skin cells, photoaging is characterized by hyperplasia of the epidermis and dyskeratosis, as well as by increases in the activity and number of melanocytes (Fig. 1).
Figure 1.
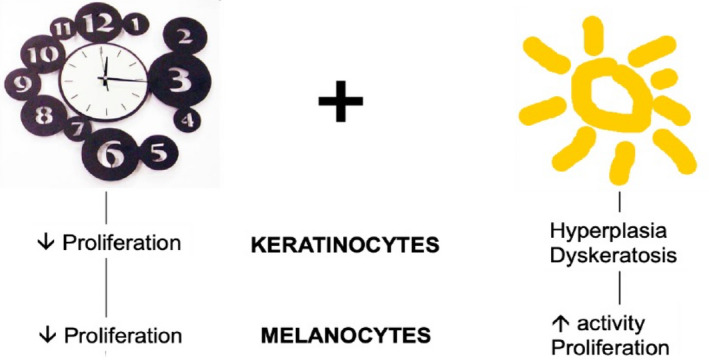
Chronoaging and photoaging induce different types of changes in keratinocytes and melanocytes. [Colour figure can be viewed at wileyonlinelibrary.com]
Photoaging results from the combination of mechanisms of UV‐induced damage and the defensive reactions of melanocytes and keratinocytes, which add together to produce signs of aging on the skin. On the one hand, the cellular processes leading to carcinogenesis produce subclinical irregular keratinocyte and melanocyte proliferation before manifesting clinically as dyskeratosis and melanocyte hyperplasia. The deleterious effects of sustained solar exposure can potentially further progress to either AK and cSCC or melanoma, depending on the cell type involved. On the other hand, skin protection mechanisms manifest as keratinocyte hyperplasia and increased melanin production, progressing to clinically detectable elongation of cristae and mottled pigmentation. These graded skin reactions to solar exposure (keratinocyte hyperplasia and dyskeratosis, pigmentation and melanocyte hyperplasia) can coexist and interact to generate a range of benign to malignant cutaneous lesions, as described in Fig. 2. As a result, the signs of photoaging range from skin roughness and scales, and macules and permanent pigmentation, to solar lentigo, freckles, red macules and finally actinic keratosis, pigmented actinic keratosis and lentigo maligna.
Figure 2.
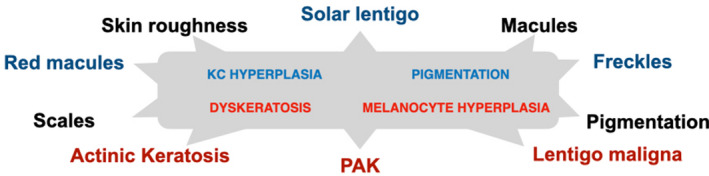
Signs of photoaging of the skin (in lowercase) range from benign (black) to intermediate (blue) and to premalignant or malignant (red), and result from keratinocyte and melanocyte reactions to UV exposure (in the grey box): damage mechanisms (uppercase red) and protection mechanisms (uppercase blue) can have an additive effect. For instance, solar lentigo results from a combination of keratinocyte hyperplasia and pigmentation, whereas pigmented actinic keratosis (PAK) results from a combination of dyskeratosis and melanocyte hyperplasia.
Solar lentigo is a benign macular skin lesion with uniform hyperpigmentation. It is usually flat with a well‐defined border and appears darker than freckles (ephelides). Characteristic findings include solar elastosis, elongated rete ridges and a normal number of melanocytes producing increased amounts of melanin. AK lesions form erythematous, squamous, crusty and keratotic papules, resulting from the proliferation of atypical keratinocytes. These lesions can remain limited to the epidermis; however, they may progress to cSCC in situ, with full‐thickness involvement of the epidermis, or to cSCC, with the presence of malignant cells in the basement membrane and the dermis and the potential to invade the lymph nodes and adjacent vital structures. 17 cSCC presents clinically as a shallow ulcer with elevated margins, often covered by a plaque. Skin surface changes may include scaling, deep ulceration, crusting and cutaneous horn.
Pigmented actinic keratosis, which presents as a rough small macule that is either pink, red or brown, is also a precursor of cSCC. Finally, the premalignant melanocytic neoplasm LM and the malignant form lentigo maligna melanoma (LMM) present an asymmetric aspect based on irregular contours and uneven pigmentation. 18 They arise when melanocytes proliferate anarchically.
The proportion of these different signs present on the skin depends on the predominant process, shifting towards either carcinogenesis or skin protection, and on the predominant cell type involved.
Skin aging features identified by in vivo high‐resolution imaging
Among the non‐invasive imaging methods available, some offer high‐definition in vivo resolutions approaching those of histopathology. In particular, RCM and OCT have shown excellent correlations with histopathology in the diagnosis of skin cancers 19 and inflammatory skin diseases (reviewed in Ref. [20]). Both techniques are complementary: RCM enables cells to be visualized with a very high resolution, but cannot be used to examine cells deeper within the skin (i.e. cells below 250 μm, which usually correlates to the level of the papillary dermis or the upper reticular dermis); whereas OCT can be used to visualize cell architecture and the stroma, but cannot be used for cytology.
Reflectance confocal microscopy can detect epidermal and dermal changes occurring in aging skin with a level of detail similar to that of histopathology. 21 , 22 It provides horizontal sections of the epidermis and upper dermis at cellular‐level resolution. As melanin provides a source of contrast, the diagnosis of melanoma and pigmented lesions represents one of the best indications of RCM.
When the skin is young, the spinous and granular layers are characterized on RCM images by regular polygonal keratinocytes designated as the ‘honeycomb pattern’, bright keratinocyte intercellular connections with a dark cytoplasm and nuclei, a dermal‐epithelial junction with regular architecture, and thin reticulated collagen fibres.
With aging, RCM reveals more irregularly shaped keratinocytes (mild dyskeratosis, with cells of variable size and shape, and a cell border that is sometimes poorly defined or preserved) and areas with irregularly distributed pigmentation (mottled pigmentation, corresponding to the presence of clustered bright keratinocytes in the honeycomb pattern). 21 , 23 At the dermal‐epithelial junction, polycyclic papillary contours have been found to be present in more than half of subjects aged over 45 years, showing elongation of the epidermis and hyperplasia. 21 However, reticular dermal changes cannot be assessed by RCM, because of the limited depth of laser penetration, thus the extent of solar elastosis in deeper dermal layers is concealed. Collagen alterations can be observed by both RCM (loss of thin collagen fibres and the presence of collagen clods) 23 and OCT (disruption of collagen fibres). 20
As regards photoaged phenotypes, there is no single morphotype and it is not possible to determine which subjects have received more solar exposure during their lifetime on the basis of pictures of their skin. Clinically, two photoaging phenotypes have been described, either atrophic or hypertrophic. 24 Non‐invasive imaging allows these two main photoaging phenotypes to be described more thoroughly by examining seemingly healthy areas of the skin of subjects with extensive cumulative solar exposure.
The atrophic phenotype is characterized by dyskeratosis visible by RCM, and by a very thin epidermis visible by OCT, revealing epidermal atrophy (Fig. 3). Lesions of AK may be present and reveal more marked dyskeratosis and epidermal profile alterations, with a heterogeneous thickness.
Figure 3.
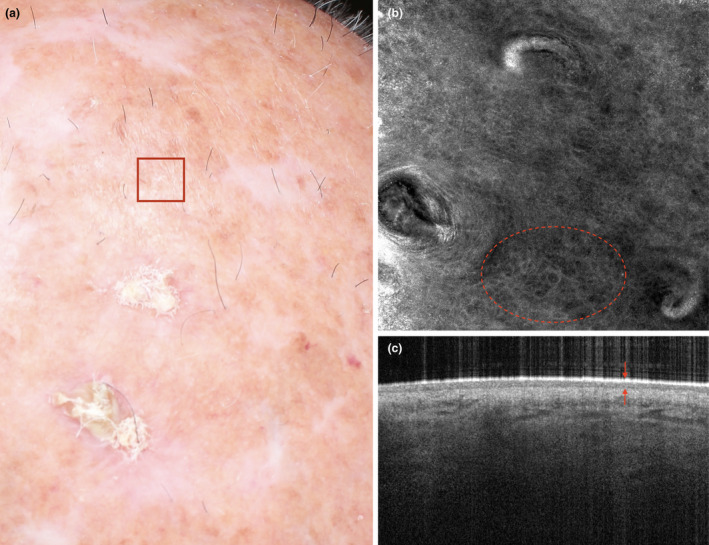
A subject with photoaging characterized by actinic keratosis and field cancerization (a). Reflectance confocal microscopy (b) reveals that irregularly shaped keratinocytes (dyskeratosis) are present in the epidermis (dashed circle). Optical coherence tomography (c) reveals the presence of a thin epidermis (arrows) upon altered collagen fibres. The overall picture is characteristic of an atrophic photoaging phenotype. [Colour figure can be viewed at wileyonlinelibrary.com]
The hypertrophic phenotype is characterized by pigmentation and solar lentigo, and by a thickened epidermis (Fig. 4). Solar lentigo is characterized by changes mainly located in the dermal‐epidermal junction: in the papillary dermis and rete ridges. These alterations can be observed by RCM as marked, bright, polycyclic, papillary contours that correspond to the increased density of the dermal papillae and elongation of the cristae. By OCT, these changes can be observed as elongated cristae (a thickened rete ridge) and pigmentation. Mottled dyspigmentation is commonly observed and corresponds to the irregular distribution of melanocytes and the heterogeneous distribution of melanosomes within keratinocytes.
Figure 4.
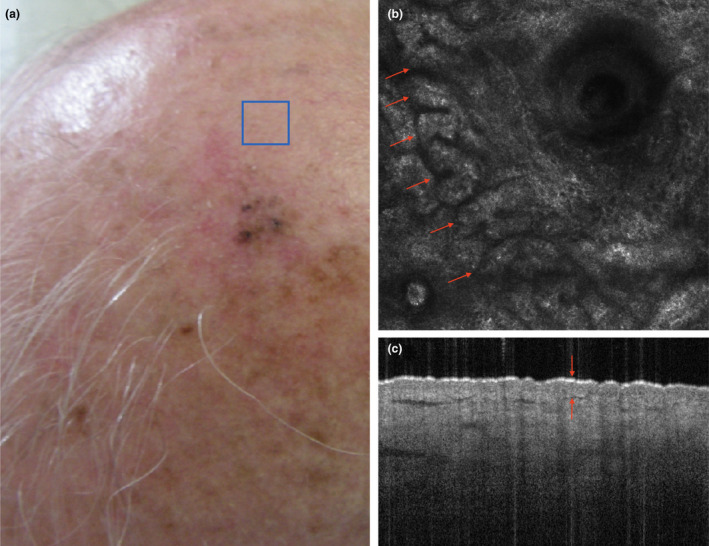
A subject with photoaging characterized by pigmentation and solar lentigines (a). Reflectance confocal microscopy (b) reveals that elongated epidermal cords forming polycyclic papillary contours (arrows), corresponding to the elongation and anastomosis of the rete ridge, are present at the dermal‐epithelial junction. Optical coherence tomography (c) reveals a thickened epidermis (arrows) upon dense and compact (elastotic) collagen fibres. The overall picture is characteristic of a hypertrophic/hyperplastic photoaging phenotype. [Colour figure can be viewed at wileyonlinelibrary.com]
Do photoaging signs reflect skin reactions to solar exposure?
The existence of two different photoaging phenotypes could suggest that skin aging manifestations correspond to subject‐specific sun‐damage reaction profiles. We thus propose a model in which skin aging manifestations are classified as corresponding to one of the two photodamage profiles resulting from a balance between the damage and protection mechanisms of keratinocytes and melanocytes (Fig. 5).
Figure 5.
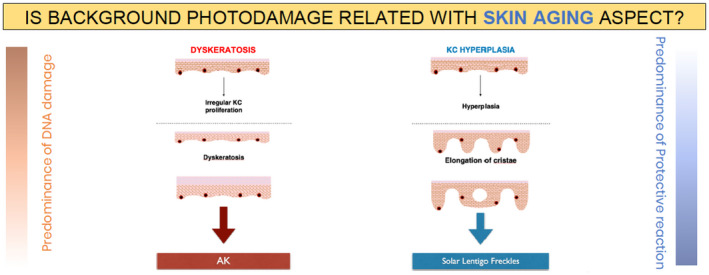
Hypothesis 1: Skin aging manifestations (actinic keratosis [AK] or solar lentigo freckles) correspond to subject‐specific sun‐damage reaction profiles (dyskeratosis or keratinocyte [KC] hyperplasia, respectively).
According to this hypothesis, individuals with AK would have a poor degree of protection and consequently have high levels of DNA damage, leading to abnormal keratinocytes (dyskeratosis) and keratinocyte apoptosis (epidermal atrophy), and resulting in the development of AK lesions. Support for this hypothesis comes from multivariate analyses of epidemiological data showing that older age, fair skin, severe baldness, skin wrinkling and a strong tendency for sunburn – all factors associated with sun susceptibility – are significantly associated with extensive actinic damage (more than 10 AK lesions). 5 Moreover, the keratinocyte morphology observed with RCM is homogeneous on sun‐damaged epidermis, regardless of the presence of visible AK lesions; an observation that favours of the presence of subclinical AKs occurring in photodamaged skin. Subclinical AKs are estimated to occur up to 10 times more often than visible AKs. 25 Different degrees of keratinocyte atypia are present in AK, showing a progressive change. 26 Fully developed AKs also display parakeratosis and epidermal atrophy, with hyperplasia being present on the edges of the lesion in some cases. 26
By contrast, in individuals with solar lentigines, the response to solar overexposure would be predominantly mediated by pigmentation reactions and increased keratinocyte proliferation as part of the protective mechanism to reduce the level of DNA damage. Indeed, melanin has light‐absorbing properties, and the thickening of the skin protects the basal layer from solar radiation. However, this protective mechanism can also lead to the formation of solar lentigines, as a ‘side effect’.
Two phenotypes of skin photoaging can thus be defined: an AK skin type, characterized by atrophic thinner skin, scales and redness (i.e. the atrophic phenotype), and a hyperplastic pigmented skin type, characterized by tanning, permanent tanning, solar lentigines and hyperplasia (i.e. the hypertrophic phenotype). In our clinical practice, we have indeed observed individuals with photoaging presenting with either the AK skin type (the atrophic phenotype) or the hyperplastic pigmented skin type (the hypertrophic phenotype), or a mixture of features from both phenotypes. Interestingly, in individuals presenting with the visible signs of the atrophic phenotype (i.e. AK) or the hypertrophic phenotype (i.e. solar lentigo/freckles), these visible characteristics are associated with subclinical signs observable by RCM and OCT.
A study is currently underway to test the validity of our model in three groups of patients: those presenting AK (Fig. 6), those presenting solar lentigo and a complete absence of AK (Fig. 7), and those presenting a mixed phenotype with both AK and solar lentigo (Fig. 8). In this study, the frequency of subclinical signs observable on apparently normal skin by RCM and OCT, and indicative of dyskeratosis, dyspigmentation or keratinocyte hyperplasia, will be recorded: irregular keratinocytes (revealing dyskeratosis), mottled pigmentation (revealing dyspigmentation) and polycyclic papillary contours (associated with solar lentigo).
Figure 6.
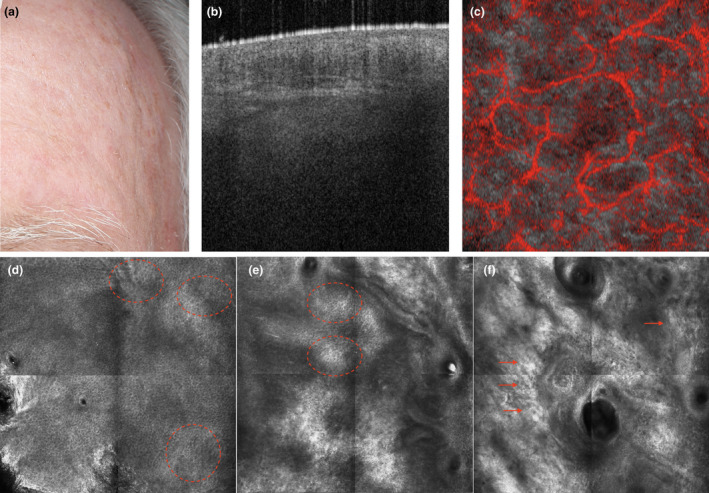
The atrophic phenotype: a subject with clinically visible actinic keratosis lesions suggesting a low level of ‘protective reaction capability’ and a high level of ‘DNA damage susceptibility’ (a). Dynamic optical coherence tomography imaging on apparently healthy skin shows an atrophic epidermis with altered collagen and an expanded vascular plexus (b and c, respectively). The corresponding reflectance confocal microscopy (RCM) images reveal dyskeratotic keratinocytes in the epidermis (dashed circles) and fragmented collagen fibres in the upper dermis (arrows) (representative RCM images of the epidermis (d), junction (e) and upper dermis (f)). [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 7.
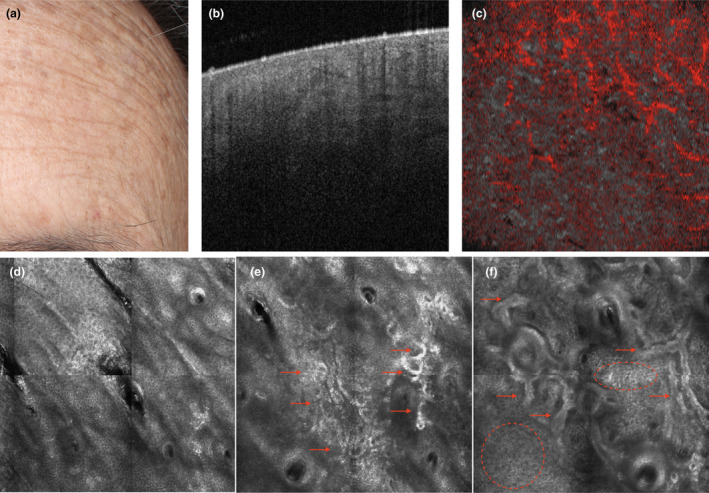
The hypertrophic phenotype: a subject with clinically visible solar lentigines and freckles suggesting a high level of ‘protective reaction capability’ and a low level of ‘DNA damage susceptibility’ (a). Dynamic optical coherence tomography imaging on apparently healthy skin shows a thickened epidermis with dense collagen in clods and a diminished vascular plexus (b and c, respectively). The corresponding reflectance confocal microscopy (RCM) images reveal polycyclic papillary contours and epidermal cords (dashed circles), corresponding to an elongated rete ridge, as well as amorphous collagen and curled fibres (arrows), corresponding to elastosis in the upper dermis (representative RCM images of the epidermis (d), junction (e) and upper dermis (f)). [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 8.
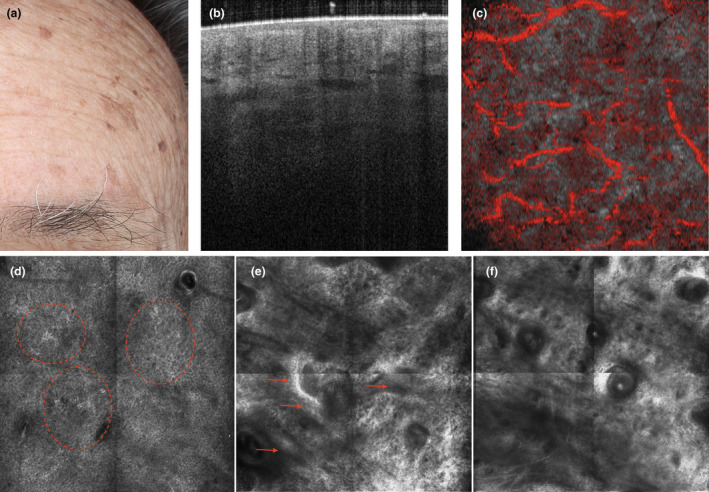
The mixed phenotype: a subject with clinically visible actinic keratosis, solar lentigines and freckles suggesting both ‘protective reaction capability’ and ‘DNA damage susceptibility’ (a). Dynamic optical coherence tomography imaging on apparently healthy skin shows an irregular epidermis with altered collagen and an irregular vascular plexus (b and c, respectively). The corresponding reflectance confocal microscopy images reveal dyskeratotic keratinocytes (dashed circles), as well as mottled pigmentation and epidermal cords (arrows), corresponding to elongation of rete ridge, with amorphous elastotic collagen in the upper dermis (d), junction (e) and upper dermis (f). [Colour figure can be viewed at wileyonlinelibrary.com]
Do different skin cancer predispositions correspond to specific patterns of sun‐damage reactions and thus photoaging signs?
Is background photodamage related to skin cancer development?
Actinic keratosis is one of the most common skin malignancies and was previously classified as in situ cSCC. With reported progression rates of up to 20% over 10 years, 27 AK represents a spectrum along the continuum to invasive cancer. Case studies show evidence of morphologic and biologic progression from AK to cSCC, with cSCC developing on preexisting large AK lesions. 28 Clinical and subclinical AK lesions occurring in photodamaged skin are referred to as the ‘cancerization field’ or ‘cancer field’. In this cancer field, some atypical cells have the potential to progress to invasive malignancy. 17 , 25 Cancer fields are thus normal‐appearing premalignant areas with several subclinical abnormalities: they are most frequent in photo‐exposed skin and around AK lesions, and can form the basis for new neoplastic lesions. 17 Thus, early diagnosis and treatment to clear actinically damaged sites diminishes the risk of invasive cSCC. 29 RCM and OCT imaging can be used to detect cellular and histological changes characteristic of subclinical lesions, allowing these ‘invisible’ lesions to be identified early. 27 , 30
Similarly, LM and the proliferation of dendritic melanocyte cells could be seen as a step along the continuum to LMM. Drawing a parallel with the use of the term ‘cancerization field’ for areas with AK lesions, the term ‘melaninization field’ can be coined to describe photodamaged skin areas with diffuse solar lentigines and freckles accompanying LM.
If background photodamage is indeed related to skin cancer development, patients with cSCC would then be more prone to present associated subclinical AK (Fig. 9 panel a), whereas patients with LM would be more prone to show diffuse solar lentigo and freckles in an area with LM (Fig. 9 panel b).
Figure 9.
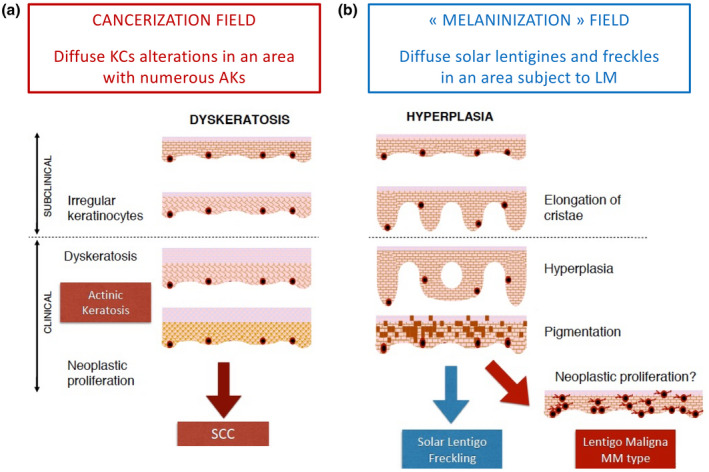
Hypothesis 2. (a) If background photodamage is related to skin cancer development, patients with squamous cell carcinoma (SCC) would also present a cancerization field, characterized by subclinical irregular keratinocytes, as well as clinically visible AK lesions, susceptible to undergoing neoplastic changes. (b) If background photodamage is related to skin cancer development, patients with lentigo maligna (LM) also present a ‘melaninization field’, characterized by subclinical epidermal hyperplasia (elongation of cristae on RCM images), as well as diffuse visible solar lentigo and freckles susceptible to undergoing neoplastic changes in an area with LM. [Colour figure can be viewed at wileyonlinelibrary.com]
Identifying different patterns of skin photoaging according to the characteristics observed by non‐invasive imaging could eventually be used to predict the potential for evolution towards carcinogenesis. In order to test this hypothesis, a study has been initiated using RCM and OCT to assess of the frequency of irregular keratinocytes, mottled pigmentation and polycyclic papillary contours in the normal skin of patients with cSCC or LM.
High‐resolution imaging of seemingly healthy skin allows the detection of early signs of abnormalities, which could progress into malignancies. It is especially relevant because some of these lesions can be misdiagnosed and treated with laser or cryotherapy for cosmetic reasons without being excised and analysed by histopathology. This can be the case for melanoma clinically mimicking seborrheic keratosis. 31 Laser treatment of freckles by non‐dermatologists leads to depigmentation of potentially cancerous lesions and can delay the diagnosis of melanoma. Furthermore, in patients presenting numerous pigmented lesions, LM lesions could be overlooked. In this context, it is of the utmost importance to study the skin of the patient thoroughly: RCM imaging is a valuable tool for improving the accuracy of these evaluations.
Improving skills for the dermoscopic recognition of sun‐induced skin tumours using the Youdermoscopy™ application
In addition to RCM and OCT, dermoscopy is another non‐invasive technique that has improved the diagnosis of skin cancer. 32 Today, dermoscopy is the mainstay of diagnosis of pigmented skin lesions, especially for dermatologists in private practice. Its usefulness has been demonstrated in meta‐analyses 33 and it is used by dermatologists and primary care physicians alike. 34 In particular, it is a helpful and relatively inexpensive tool for detecting melanoma in local general practice settings. 35 However, some lesions can be difficult to discriminate between, even for dermatologists. In particular, distinguishing which flat pigmented facial lesions are pigmented AK lesions and which are LM can represent a diagnostic challenge. 36 Hence the need for continuous training and, in cases of ambiguous lesions, the possibility of seeking a second opinion.
There are two teaching methods for the dermoscopic diagnosis of pigmented skin tumours, namely the verbal‐based analytic approach and the more visual global heuristic method. The YouDermoscopy™ application relies on the heuristic ‘elephant approach’, according to which ‘a picture is worth a thousand words’ when one needs to explain what an ‘elephant’ or an LM looks like.
However, one concern over using the heuristic approach during dermoscopy training is that it is assumed that trainees would have to have acquired extensive expertise on the many different clinical presentations of skin tumours before being able to apply this approach. However, the results of a study evaluating the two methods of short‐term training for dermoscopy appear to challenge this assumption. In this study, 57 medical students in the last year of the curriculum (dermoscopy novices) were given a 1‐h lecture using either the verbal analytic or the visual heuristic method for the dermoscopic diagnosis of pigmented skin tumours. Students were shown the same batch of 50 lesions (melanomas, BCCs, nevi, seborrheic keratoses, benign vascular tumours, dermatofibromas, etc.) and asked to diagnose them and rate their potential for malignancy before and after the 1‐h lecture. Diagnostic accuracy and the percentage of correct diagnoses increased by +0.21 and +32.9%, respectively, after heuristic teaching, and +0.19 and +35.7%, respectively, after analytic teaching (P for all <0.001). There was no difference between the two groups in terms of diagnostic accuracy (P = 0.585), or in terms of the percentage of correct diagnoses (P = 0.298), showing that both methods were equally effective in the same timeframe. Very importantly, short‐term dermoscopy training for medical students using either method led to significant improvements in their diagnostic abilities. 37
Youdermoscopy™ for continuous medical education
The YouDermoscopy™ free application proposes more than 1000 training cases, in the form of dermoscopic images of pigmented skin tumours. It has been translated into 12 languages and downloaded in more than 150 countries all over the world. Since 2016, there have been more than 40 000 downloads and 26 000 registered users. The top 10 countries in which the application is used are, in decreasing order: Italy, Brazil, Russia, Spain, the US, the UK, Poland, France, Sweden and Mexico.
Youdermoscopy™ uses a game format in which players are shown dermoscopic images of lesions displayed on their screen for a few seconds and are then asked to select one diagnosis out of eight different propositions: ‘BCC’, ‘solar lentigo and seborrheic keratitis’, ‘melanoma’, ‘SCC in situ and invasive’, ‘dermatofibroma’, ‘vascular lesion’, ‘nevus’ or ‘other’. When the answer given is correct, it is highlighted in green; when it is incorrect, it appears in red and the correct answer simultaneously appears highlighted in green (Fig. 10).
Figure 10.
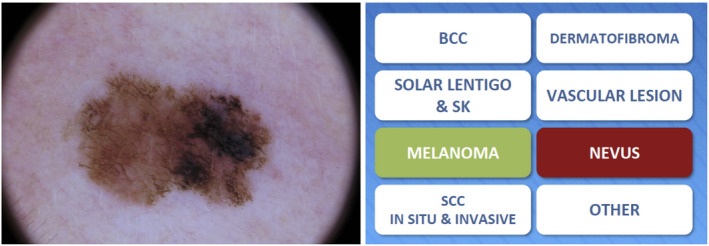
The Youdermoscopy™ game format: users are shown a dermoscopic image of a pigmented skin lesion, and provided with a choice of eight possible diagnoses: the correct answer (MELANOMA) is highlighted in green, whereas the user's incorrect answer (NEVUS) is highlighted in red. [Colour figure can be viewed at wileyonlinelibrary.com]
The YouDermoscopy™ game format is made up of levels: one level is made of eight sessions presenting eight pictures, and 75% of the 64 lesions have to be correctly recognized to proceed to the next level. If an insufficient number of correct answers is obtained, the user has to repeat the level before being allowed to progress. By passing levels, the players increase their national and international ranking, which can motivate them to play again. New features are currently being developed, such as ‘Bonus research’, ‘New levels each month’ and ‘live webinars’, in order to maintain user interest in the application.
Youdermoscopy™ for obtaining second opinions from the community of users
Another value‐added functionality of YouDermoscopy™ is the possibility to ‘Play Live’, which allows all registered users to submit a dermoscopy picture (and potentially a clinical picture) of a patient case, together with anonymized data for the lesion location, and the sex, age and country of origin of the patient. This function also creates the possibility for users to ask the community for a second opinion (Fig. 11 panel a).
Figure 11.
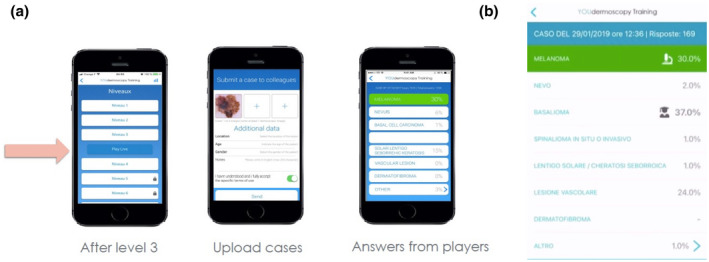
Youdermoscopy™ ‘Play Live’: players who have completed the first three levels of the game format can submit a dermoscopic image of a pigmented skin lesion, together with data on the lesion location, and the sex, age and country of the patient (a), allowing them to ask the community for a second opinion. The community then votes for the most likely diagnosis, shown as a distribution of percentages (b). A group of experts also provides their opinion, with their vote being indicated by the icon of a scholar. The histopathological diagnosis is also provided – whenever it is available – and is indicated by the icon of a microscope and highlighted in green. In the example provided here: Case submitted on the 29/01/2019, 169 Answers: Melanoma 30.0%, Nevus 2.0%, Basal Cell Carcinoma 37.0%, Squamous Cell Carcinoma in situ or invasive 1%, Solar lentigo/seborrheic keratitis 1.0%, Vascular lesion 24.0%, Dermatofibroma None, and Other 1%. [Colour figure can be viewed at wileyonlinelibrary.com]
In order to access the ‘Play Live’ option, the player must have completed the three first levels of the game format. More than 6000 cases were uploaded as of June 25th, 2021.
Once an image of a case has been uploaded, the community of players are asked to provide their opinion (i.e. vote) on the most likely diagnosis. After voting, the player can see the votes of the other players as a distribution of percentages (Fig. 11 panel b). In addition to this ‘democratic’ vote, YouDermoscopy™ also displays the ‘oligarchic’ vote of a group of experts, which is composed of the members of the international board, together with the 100 players who were ranked first in the training score of the application during the previous month. Each month, this selection of 100 players is updated, depending on the new scores obtained. In some cases, a histopathological diagnosis is also provided, featuring the ‘word of the king’, so to speak.
This constantly incremented collection of cases represents a vast opportunity for learning. Moreover, analysis of the results obtained by players has highlighted a panel of ‘difficult lesions’, for which the majority of players gave the wrong answer. These difficult lesions represent approximately 1% of the cases encountered in clinical practice. In addition, the application data revealed that the most difficult differential diagnosis is not between nevus and melanoma, but rather between seborrheic keratosis (or in some cases dermatofibroma) and melanoma. Above all, these data showed that even the majority of voters and/or experts can be misled by ambiguous dermoscopy images. This finding reinforces the need for biopsy and histopathological analysis or RCM imaging whenever the morphology of a lesion is not clear‐cut.
Youdermoscopy™ another way to improve the diagnosis of skin cancers
The YouDermoscopy™ application is a very straightforward and educational tool for dermoscopy training. It allows users to learn by playing, and gamification has been proven to be a performant way of learning. Combining competition with collaboration is particularly effective for fostering behavioural learning outcomes, as shown by a meta‐analysis on the effects of gamification on cognitive, motivational and behavioural learning outcomes. 38
Furthermore, practice is the key to success for sharpening the ability of dermoscopy users to discriminate between skin lesions and establish an accurate diagnosis: a picture is worth a 1000 words in the achievement of these training goals. Finally, the opportunity to obtain several opinions on a single lesion is a valuable component of the YouDermoscopy™ application, which can be of help to all clinicians. Future development of the application, including the incorporation of artificial intelligence, will enhance its training effectiveness.
Conclusion
Early detection of lesions is essential for improving the prognosis of skin cancer patients. The detection of precancerous lesions according to the profile of the patients observed by RCM and OCT may be an effective strategy for early diagnosis. According to the model proposed in this article, different patterns of skin photoaging may allow prediction of the potential of lesions to evolve towards different types of skin cancer. This theoretical model needs to be tested and RCM and OCT imaging studies are already underway. Refining our knowledge on the susceptibility of various types of skin lesion to cancerization could offer valuable help to clinicians for more specific monitoring of their patients. Another way of detecting lesions early is to improve clinical practice by increasing the skills of dermatologists. Wider dissemination of YouDermoscopy™ in countries where it is not yet widely used could increase the level of knowledge of the international dermatology community on the use of dermoscopy for the differential diagnosis of pigmented lesions, and ultimately provide another strategy for improving the early detection and prompt treatment of skin cancer.
Acknowledgements
The authors thank Françoise Nourrit‐Poirette, PhD, and Marielle Romet, PhD (Synergy Pharm – Santé Active Edition) for medical writing and Emma Pilling, PhD (Synergy Pharm – Santé Active Edition), for English editing assistance funded by Laboratoires Dermatologiques Avène – Pierre Fabre Dermo‐Cosmétique. Open Access Funding provided by Universita degli Studi di Roma La Sapienza within the CRUI‐CARE. [Correction added on 22 July 2022, after first online publication: CRUI‐CARE funding statement has been added.]
Funding sources
Medical writing assistance was funded by Laboratoires Dermatologiques Avène–Pierre Fabre Dermo‐Cosmétique.
Conflicts of interest
GP and GA: None.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1. Kim Y, He YY. Ultraviolet radiation‐induced non‐melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis 2014; 1: 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howell JY, Ramsey ML. Squamous Cell Skin Cancer. StatPearls, Treasure Island (FL), 2021. [Google Scholar]
- 3. Liu‐Smith F, Jia J, Zheng Y. UV‐Induced Molecular Signaling Differences in Melanoma and Non‐melanoma Skin Cancer. Adv Exp Med Biol 2017; 996: 27–40. [DOI] [PubMed] [Google Scholar]
- 4. Carr S, Smith C, Wernberg J. Epidemiology and Risk Factors of Melanoma. Surg Clin North Am 2020; 100: 1–12. [DOI] [PubMed] [Google Scholar]
- 5. Flohil SC, van der Leest RJ, Dowlatshahi EA et al. Prevalence of actinic keratosis and its risk factors in the general population: the Rotterdam Study. J Invest Dermatol 2013; 133: 1971–1978. [DOI] [PubMed] [Google Scholar]
- 6. Malvehy J, Pellacani G. Dermoscopy, confocal microscopy and other non‐invasive tools for the diagnosis of non‐melanoma skin cancers and other skin conditions. Acta Derm Venereol 2017; (Suppl 218): 22–30. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka M, Sawada M, Kobayashi K. Key points in dermoscopic differentiation between lentigo maligna and solar lentigo. J Dermatol 2011; 38: 53–58. [DOI] [PubMed] [Google Scholar]
- 8. Pellacani G, Longo C. Reflectance confocal microscopy: a crucial role for actinic keratosis treatment monitoring. J Eur Acad Dermatol Venereol 2018; 32: 1055. [DOI] [PubMed] [Google Scholar]
- 9. Farnetani F, Manfredini M, Chester J et al. Reflectance confocal microscopy in the diagnosis of pigmented macules of the face: differential diagnosis and margin definition. Photochem Photobiol Sci 2019; 18: 963–969. [DOI] [PubMed] [Google Scholar]
- 10. Cleaver JE, Crowley E. UV damage, DNA repair and skin carcinogenesis. Front Biosci 2002; 7: d1024–d1043. [DOI] [PubMed] [Google Scholar]
- 11. DiGiovanna JJ, Kraemer KH. Shining a light on xeroderma pigmentosum. J Invest Dermatol 2012; 132: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohania D, Chandel S, Kumar P et al. Ultraviolet radiations: skin defense‐damage mechanism. Adv Exp Med Biol 2017; 996: 71–87. [DOI] [PubMed] [Google Scholar]
- 13. Kadekaro AL, Kavanagh RJ, Wakamatsu K et al. Cutaneous photobiology. The melanocyte vs. the sun: who will win the final round? Pigment Cell Res 2003; 16: 434–447. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi N, Nakagawa A, Muramatsu T et al. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J Invest Dermatol 1998; 110: 806–810. [DOI] [PubMed] [Google Scholar]
- 15. Smit NP, Vink AA, Kolb RM et al. Melanin offers protection against induction of cyclobutane pyrimidine dimers and 6‐4 photoproducts by UVB in cultured human melanocytes. Photochem Photobiol 2001; 74: 424–430. [DOI] [PubMed] [Google Scholar]
- 16. El‐Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis 2006; 27: 225–231. [DOI] [PubMed] [Google Scholar]
- 17. Reygagne P, Rostain G. Beyond actinic keratoses: Field cancerization of the skin. Ann Dermatol Venereol 2018; 145: 587–592. [DOI] [PubMed] [Google Scholar]
- 18. Goorochurn R, Viennet C, Granger C et al. Biological processes in solar lentigo: insights brought by experimental models. Exp Dermatol 2016; 25: 174–177. [DOI] [PubMed] [Google Scholar]
- 19. Guida S, Longo C, Casari A et al. Update on the use of confocal microscopy in melanoma and non‐melanoma skin cancer. G Ital Dermatol Venereol 2015; 150: 547–563. [PubMed] [Google Scholar]
- 20. Ciardo S, Pezzini C, Guida S et al. A plea for standardization of confocal microscopy and optical coherence tomography parameters to evaluate physiological and para‐physiological skin conditions in cosmetic science. Exp Dermatol 2021; 30: 911–922. [DOI] [PubMed] [Google Scholar]
- 21. Longo C, Casari A, Beretti F, Cesinaro AM, Pellacani G. Skin aging: in vivo microscopic assessment of epidermal and dermal changes by means of confocal microscopy. J Am Acad Dermatol 2013; 68: e73–e82. [DOI] [PubMed] [Google Scholar]
- 22. Guida S, Pellacani G, Ciardo S, Longo C. Reflectance Confocal Microscopy of Aging Skin and Skin Cancer. Dermatol Pract Concept 2021; 11: e2021068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wurm EM, Longo C, Curchin C et al. In vivo assessment of chronological ageing and photoageing in forearm skin using reflectance confocal microscopy. Br J Dermatol 2012; 167: 270–279. [DOI] [PubMed] [Google Scholar]
- 24. Sachs DL, Varani J, Chubb H et al. Atrophic and hypertrophic photoaging: Clinical, histologic, and molecular features of 2 distinct phenotypes of photoaged skin. J Am Acad Dermatol 2019; 81: 480–488. [DOI] [PubMed] [Google Scholar]
- 25. Filosa A, Filosa G. Actinic keratosis and squamous cell carcinoma: clinical and pathological features. G Ital Dermatol Venereol 2015; 150: 379–384. [PubMed] [Google Scholar]
- 26. Pellacani G, Ulrich M, Casari A et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol 2015; 29: 2216–2221. [DOI] [PubMed] [Google Scholar]
- 27. Ulrich M, Maltusch A, Rowert‐Huber J et al. Actinic keratoses: non‐invasive diagnosis for field cancerisation. Br J Dermatol 2007; 156(Suppl 3): 13–17. [DOI] [PubMed] [Google Scholar]
- 28. Ruini C, Witkowski AM, Cesinaro A, Teixeira De Carvalho N, Pellacani G. From actinic keratosis to squamous cell carcinoma: evidence of morphologic and biologic progression. J Am Acad Dermatol 2015; 72: S8–S10. [DOI] [PubMed] [Google Scholar]
- 29. Rigel DS, Stein Gold LF. The importance of early diagnosis and treatment of actinic keratosis. J Am Acad Dermatol 2013; 68: S20–S27. [DOI] [PubMed] [Google Scholar]
- 30. Fernandez Figueras MT. From actinic keratosis to squamous cell carcinoma: pathophysiology revisited. J Eur Acad Dermatol Venereol 2017; 31(Suppl 2): 5–7. [DOI] [PubMed] [Google Scholar]
- 31. Izikson L, Sober AJ, Mihm MC Jr, Zembowicz A. Prevalence of melanoma clinically resembling seborrheic keratosis: analysis of 9204 cases. Arch Dermatol 2002; 138: 1562–1566. [DOI] [PubMed] [Google Scholar]
- 32. Reggiani C, Manfredini M, Mandel VD et al. Update on non‐invasive imaging techniques in early diagnosis of non‐melanoma skin cancer. G Ital Dermatol Venereol 2015; 150: 393–405. [PubMed] [Google Scholar]
- 33. Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta‐analysis of studies performed in a clinical setting. Br J Dermatol 2008; 159: 669–676. [DOI] [PubMed] [Google Scholar]
- 34. Tschandl P, Rosendahl C, Kittler H. Dermatoscopy of flat pigmented facial lesions. J Eur Acad Dermatol Venereol 2015; 29: 120–127. [DOI] [PubMed] [Google Scholar]
- 35. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician 2012; 58: e372–e748. [PMC free article] [PubMed] [Google Scholar]
- 36. Akay BN, Kocyigit P, Heper AO, Erdem C. Dermatoscopy of flat pigmented facial lesions: diagnostic challenge between pigmented actinic keratosis and lentigo maligna. Br J Dermatol 2010; 163: 1212–1217. [DOI] [PubMed] [Google Scholar]
- 37. Tschandl P, Kittler H, Schmid K, Zalaudek I, Argenziano G. Teaching dermatoscopy of pigmented skin tumours to novices: comparison of analytic vs. heuristic approach. J Eur Acad Dermatol Venereol 2015; 29: 1198–1204. [DOI] [PubMed] [Google Scholar]
- 38. Sailer M, Homner L. The Gamification of Learning: a Meta‐analysis. Educ Psychol Rev 2019; 32: 77–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


