Abstract
Natural microbial sensing circuits can be rewired into new gene networks to build living sensors that detect and respond to disease-associated biomolecules. However, synthetic living sensors, once ingested, are cleared from the gastrointestinal (GI) tract within 48 hours; retaining devices in the intestinal lumen is prone to intestinal blockage or device migration. To localize synthetic microbes and safely extend their residence in the GI tract for health monitoring and sustained drug release, an ingestible magnetic hydrogel carrier is developed to transport diagnostic microbes to specific intestinal sites. The magnetic living hydrogel is localized and retained by attaching a magnet to the abdominal skin, resisting the peristaltic waves in the intestine. The device retention is validated in a human intestinal phantom and an in vivo rodent model, showing that the ingestible hydrogel maintains the integrated living bacteria for up to seven days, which allows the detection of heme for GI bleeding in the harsh environment of the gut. The retention of microelectronics is also demonstrated by incorporating a temperature sensor into the magnetic hydrogel carrier.
Keywords: ingestible materials, magnetic hydrogels, synthetic biology, intestinal retention, blood sensor
Graphical Abstract
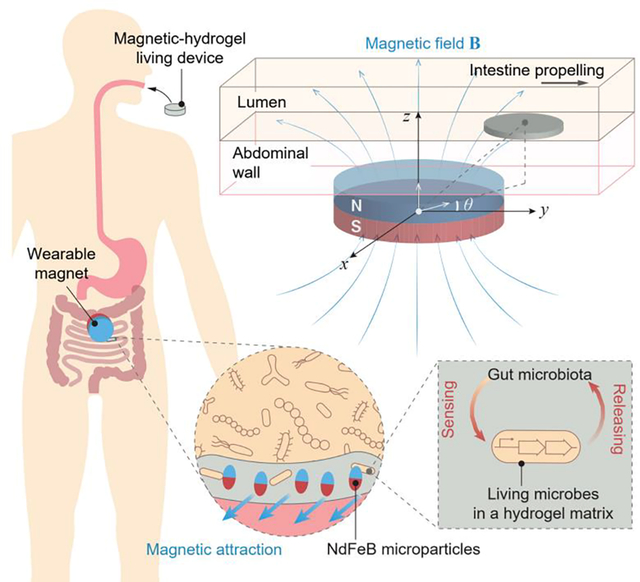
An ingestible hydrogel device is localized and retained in the intestine by attaching a magnet to the abdominal skin, and the living microbial sensors integrated in the hydrogel device can detect GI bleeding in a rodent model.
1. Introduction
Over the past few decades, technologies based on electronics and living bacteria have been developed for orally administered diagnostics and therapeutics.[1–8] The service life of existing ingestible devices is limited by the gastrointestinal (GI) transit time, i.e., the time it takes for food to move through the digestive system.[9, 10] In the human body, the GI transit time usually ranges from 6 to 48 h.[2, 11, 12] However, for most GI applications, such as long-lasting biosignal collection,[9] prolonged drug delivery[13, 14] and sustained diet control,[15, 16] longer retention times are needed. The effective diagnosis and treatment of GI diseases may also require ingestible devices to be positioned at a specific location in the gut. Such positioning would be desirable for the detection and treatment of localized diseases such as Crohn’s disease, characterized by patchy inflammation along the GI tract.[17, 18]
Although several strategies have been proposed to achieve localization in the GI tract, they all have limitations for intestinal retention. For example, ingestible devices have been developed to reside in the GI tract by floating, unfolding, or swelling;[19, 20] these can be applied to the large-volume gastric cavity but not to tubular structures (e.g., small and large intestines). There are high risks of intestinal blockage caused by the swelling hydrogels and of intestinal perforation caused by stiff or sharp pieces in the intestine.[2, 9, 21] Intestinal peristalsis may also induce the migration of unfolding structures, shortening their service life.[22] Moreover, intestinal mucus adhesion fails to anchor the materials and devices for more than 4 hours due to the rapid turnover of the mucus layer.[23–25] Finally, biological scaffolds that are continuously generated by engineered probiotics can persist in the intestine but may have side effects, including the irreversible alteration of the native gut microbiota.[26]
Magnetic materials have been of special interest for GI surgery because the magnetic force can be exerted over a distance.[27–29] Because of the constrained and tortuous architectures of the intestines, magnet-assisted spatial control is a useful feature for the diagnosis, evaluation, and management of localized pathologies, such as inflammation, ulcers, polyps, and tumors.[29, 30] A number of magnet-assisted technologies have been utilized in the GI tract for spatial control, including the placement of enteral tubes,[31] the navigation of devices (e.g., capsule endoscopy and micromotors),[30, 32] and the manipulation of surgical instruments (e.g., micro-snares, baskets, and forceps).[33] However, the existing surgical procedures require sophisticated medical equipment for spatial steering (e.g., X-ray emitter, detector, and robotic arm), and subjects under anesthesia.[31–34] They are applicable only for temporary use because the surgical interventions prevent the free movement of the subjects. In order to have prolonged control over the magnetic materials in the GI tract and permit normal movement of the subject in daily life, we designed a wearable magnet that can allow both spatial localization and temporal retention of ingested magnetic materials.
Here, we built a magnetic hydrogel retentive system, consisting of an ingestible, magnetic hydrogel carrying diagnostic microbes that could be localized by a wearable neodymium magnet, for long-term disease diagnosis in the intestine (Figure 1a). To prevent dislocation of the magnetic living hydrogel due to peristaltic waves and intestinal fluid flow, we optimized attractive magnetic force between the magnetic hydrogel in the intestine and the magnet by tuning the magnet dimensions (Figure 1d). Since the ingestible hydrogel is soft and flexible, it conforms to the intestinal surface, minimizing tissue damage to the intestinal lining. The mechanical robustness of the ingestible hydrogel helps maintain its structural integrity during intestinal segmentation (Figure 1d,e). The wearable magnet is lightweight and portable, and causes minimal tissue compression. Most importantly, the magnetic hydrogel is sufficiently retained by the wearable magnet in both rodent and human models based on our theoretical calculation. We validated the capability of magnet-assisted retention and localization in a human-scale GI tract phantom and in free-moving rodents (for at least a week) without causing any obstruction. Because the ingestible hydrogels are biocompatible, the living bacteria integrated into the soft hydrogel matrix were functional for 7 days in vitro (Figure 1c). Bleeding was detected by these biosensing bacteria in the mouse intestine. In contrast to other systems (e.g., magnetically driven position control) which require high-precision medical equipment and comprehensive procedures, the magnetic living hydrogel permits the prolonged monitoring and modulation of the digestive system and adjacent organs and constitutes a non-invasive, accessible platform for the intestinal localization and retention of bacterial biosensors and electronic sensors.
Figure 1.
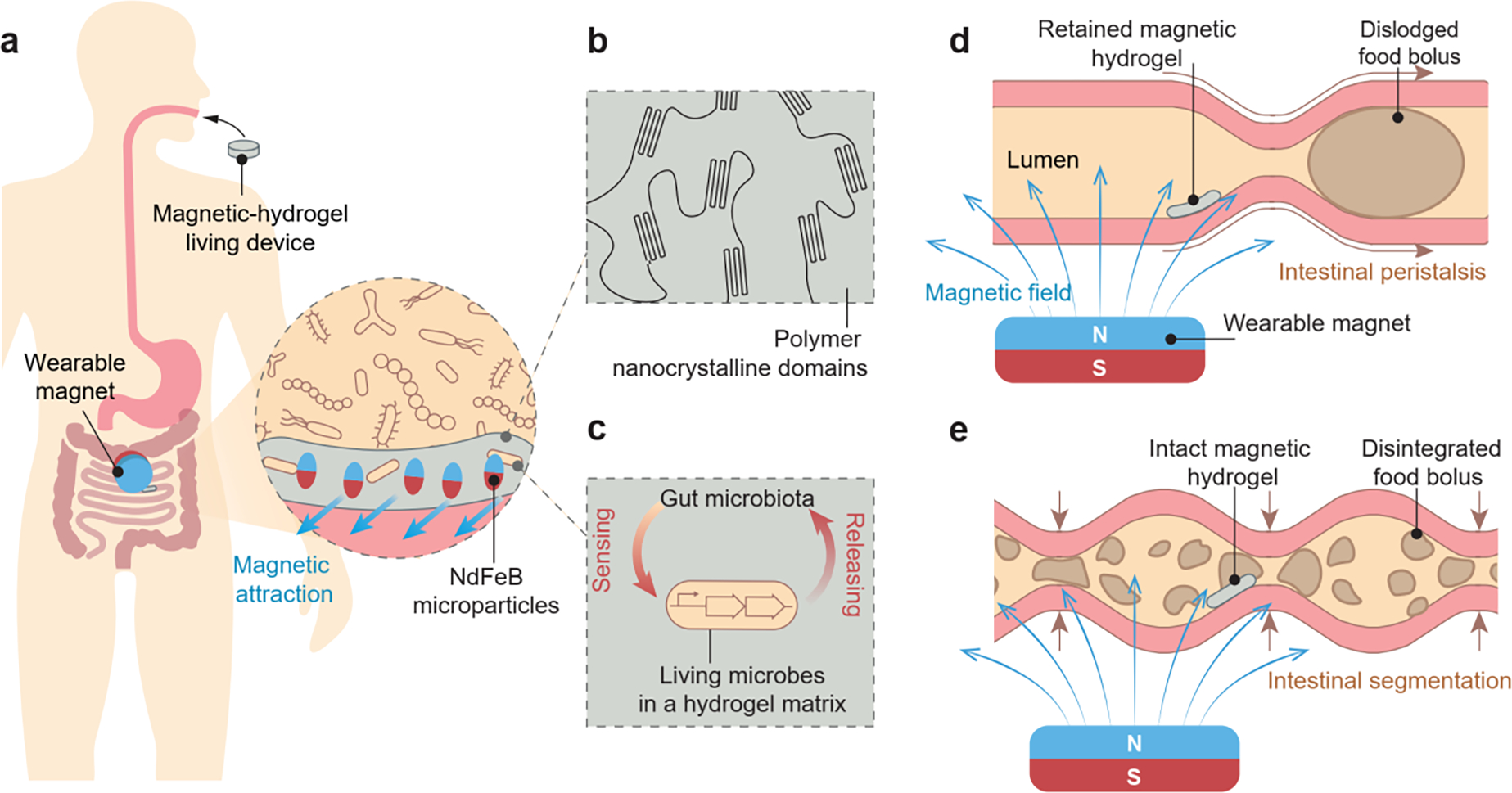
Design and mechanism of the magnetic living hydrogels localized and retained in the intestine. a) The magnetic living hydrogel is orally administrated and retained in the intestine by a magnet worn on the abdominal skin. Inset: magnetized NdFeB microparticles incorporated in the hydrogel matrix provide the attractive magnetic force between the magnetic hydrogel and wearable magnet for intestinal localization and retention. b) The hydrogel matrix is physically crosslinked by nanocrystalline domains and reinforced by NdFeB microparticles, making it mechanically tough. c) Living synthetic microbes encapsulated in the hydrogel matrix interact with the gut microbiota when the magnetic hydrogel is retained in the gut, enabling biological functions such as sensing and releasing. d) Intestinal peristalsis propels a food bolus along the intestinal lumen, while the magnetic hydrogel can be retained due to the magnetic attraction with the wearable magnet. e) Intestinal segmentation breaks a food bolus into fragments in the intestinal lumen, while the magnetic hydrogel can remain intact due to its mechanical toughness.
2. Results and Discussion
Design and fabrication of magnetic living hydrogels
Neodymium-iron-boron (NdFeB) magnets are hard ferromagnets that can retain high remanent magnetization after being magnetized.[28] Magnetic hydrogels for oral administration and intestinal retention were fabricated by incorporating NdFeB microparticles into a polyvinyl alcohol (PVA) hydrogel matrix (Figure 1a,b). First, these NdFeB microparticles (5 μm in average diameter) were coated with a nanolayer of silica shell (10 nm in thickness) to protect the alloys from corrosion in the hydrated environment.[28] Overnight cultures of engineered bacteria were centrifuged and resuspended in an equal volume of 10 wt% PVA solution. Then, NdFeB microparticles with volume fractions ranging from 0 to 21% were mixed with the bacteria-containing PVA solution and poured into a mold. The mixture in the mold was subjected to a strong impulse magnetic field (2.5 T) to magnetize the NdFeB microparticles along the applied field direction. This step of magnetization generated a uniform magnetic moment across the bulk mixture.[27] The PVA-NdFeB-bacteria mixture in the mold was then exposed to the temperature of −20 °C for 24 h, which promotes the phase separation of polymers in water and the formation of nanocrystalline domains in the PVA solution, resulting in a hydrogel matrix (Figure 1b).[35]
The magnetic hydrogels are crosslinked by PVA nanocrystalline domains.[35, 36] Increasing the volume fraction of NdFeB microparticles in the nanocrystalline hydrogel further contributes to a higher Young’s modulus (Figure S1a), higher toughness (Figure S1b), and higher magnetization (Figure S1e) of the magnetic hydrogel. We determined the amount of NdFeB microparticles in the magnetic hydrogel to be 12 vol%. The hydrogel with 12 vol% NdFeB exhibits a low Young’s modulus of 21 kPa, a high toughness of ~ 280 kJ/m3, and a high magnetization of 6×104 A/m (Figure S1a–e). The low Young’s modulus of the magnetic hydrogel drastically alleviates the stress concentration on the intestinal wall, as manifested by the finite element simulations (Figure S2d,e). Low mechanical stiffness of hydrogels is also indispensable for maintaining the viability of encapsulated bacteria, especially in the long-term (Figure S3). The high toughness of the magnetic hydrogel ensures the structural integrity when the hydrogel is subject to intestinal peristalsis and segmentation (Figure 1f). In addition, the high magnetization enables effective retention of the hydrogel and its localization in spite of intestinal motility (Figure S1e).
Model for retention of magnetic hydrogels
A large wearable magnet can produce a strong magnetic field favorable for the retention and localization of magnetic hydrogels, but may cause a series of side effects including tissue damage due to the magnetic attraction force and overload caused by a heavy magnet being worn on the body. We developed a model to rationally select the wearable magnet with optimized dimensions. The model included a disc-shaped wearable magnet and a disc-shaped magnetic hydrogel residing in a lumen with intestinal peristalsis (Figure 2a). The intestinal peristalsis in the lumen propels the hydrogel to move forward, and the friction between the hydrogel and the lumen wall resists the hydrogel’s motion (Figure 2a,b). The intestinal propelling force is denoted as Fpropel, and the friction force as Ffriction. The magnetic force between the wearable magnet and the NdFeB microparticles in the hydrogel may propel or retain the magnetic hydrogel, depending on the direction of the magnetic force (Figure 2b).
Figure 2.
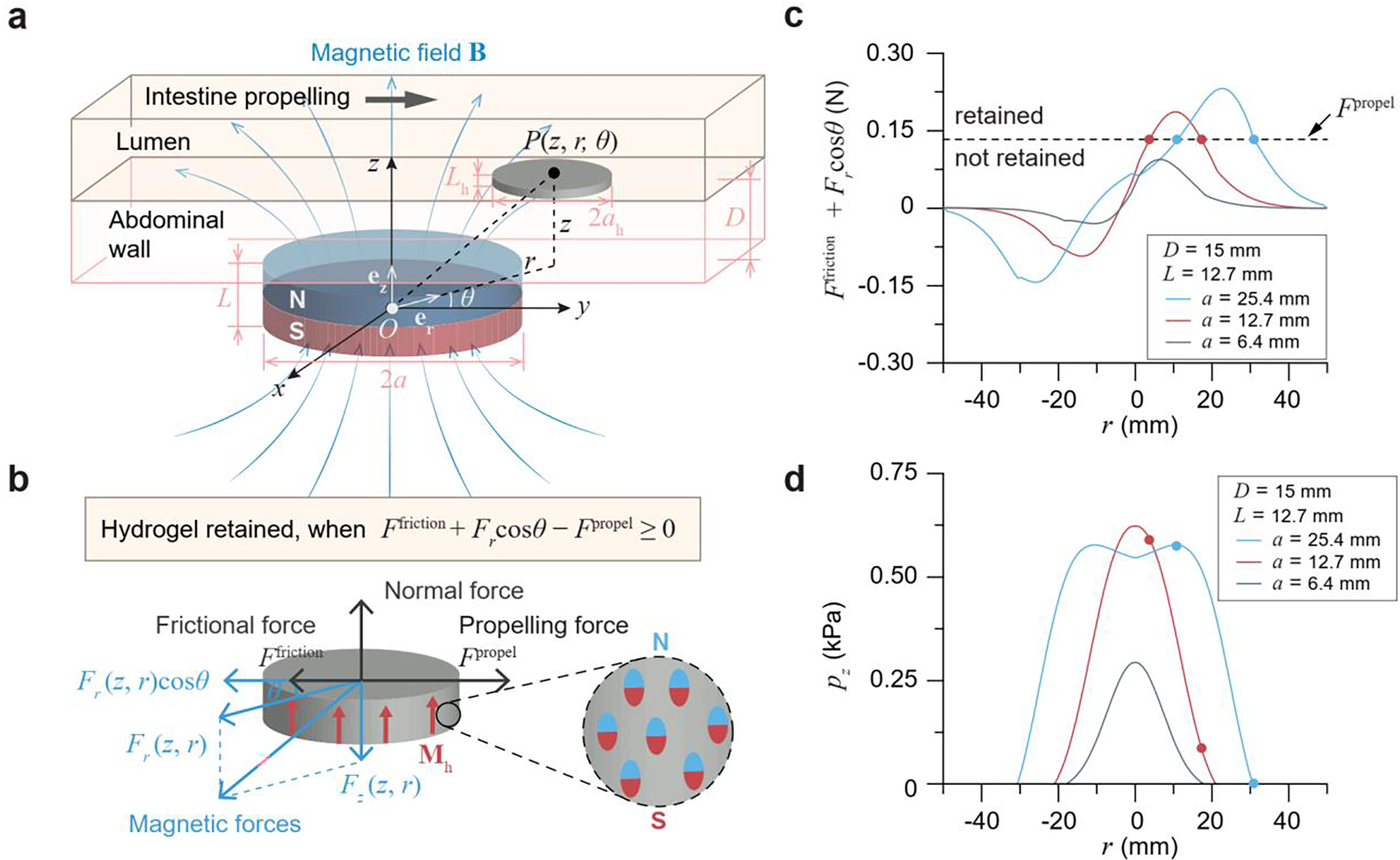
Model for intestinal retention and localization of the magnetic living hydrogel. a) Schematic illustration of a disc-shaped magnetic hydrogel (radius ah and thickness Lh) residing in a lumen under a magnetic field B generated by a disc-shaped magnet (radius a and thickness L). The vertical distance between the lumen and the magnet is D. b) Free body diagram of the magnetic hydrogel in the intestinal lumen, when it is moving along the intestinal propelling direction. The magnetization of the hydrogel Mh is uniform and along the axial direction of the hydrogel disc. c) Net retarding force (Ffriction +Fr cos θ) of a magnetic hydrogel (radius ah 10 mm, thickness Lh 1 mm) at a vertical distance of 15 mm away from a magnet (radius a 6.4, 12.7, or 25.4 mm, thickness L 12.7 mm). The magnetic hydrogel can be retained when the net retarding force is higher than the propelling force Fpropel. d) Normal pressure pz applied by a magnetic hydrogel (radius ah 10 mm, thickness Lh 1 mm) on the intestinal wall at a vertical distance of 15 mm away from a magnet (radius a 6.4, 12.7, or 25.4 mm, thickness L 12.7 mm). Solid circles on each curve in c-d indicate the stably retained locations for the magnetic hydrogel, in which the retarding force (Ffriction +Fr cos θ) equals the propelling force Fpropel.
We defined a cylindrical coordinate system with the center of the disc-shaped wearable magnet as the origin O, the radial direction of the magnet as the r-axis, and the axial direction of the magnet as the z-axis (Figure 2a). In addition, the point P(r, z, θ) refers to the center of the magnetic hydrogel, where θ is the angle between the intestinal propelling direction and the projection of OP line segment on the upper surface of the magnet. (Figure 2a). We next calculated the magnetic force applied on the magnetic hydrogel. The magnetic field B around the disc-shaped wearable magnet can be expressed as B = Brer + Bzez, where er and ez are unit vectors in the direction of the r-axis and z-axis, respectively, and Br and Bz are expressed in Supporting Information.[37] The axial direction of the disc-shaped magnetic hydrogel is assumed to be parallel with the axial direction of the wearable magnet. The vertical distance between the adjacent surfaces of the magnet and the hydrogel is D. The magnetic hydrogel is assumed to be uniformly magnetized with the remanent magnetization Mh formed along the axial direction of the hydrogel (Figure 2b). We also assumed that the magnetization of the magnetic hydrogel is unaffected by the applied magnetic field B due to the high coercivity of the NdFeB microparticles in the hydrogel.[28] Given the magnetic field applied by the magnet and the magnetization of the hydrogel, we calculated the magnetic force applied on the hydrogel as
| 1 |
where V is the volume of the hydrogel and . Specifically, the magnetic force acting on the hydrogel can be decomposed into the force component along the z direction Fz and the force component along the r direction Fr, namely, Fmagnetic = −Frer −Fzez, where Fr and Fz are both positive because the magnetic force is attractive between the magnet and hydrogel (Figure 2b). The hydrogel can be effectively retained if the forces along r direction satisfy the criterion:
| 2 |
θ ranges from 0 to π/2 when the magnetic hydrogel is on the downstream side of the magnet, and from π/2 to π when the magnetic hydrogel is on the upstream side of the magnet (Figure 2a). In the current model, we further assumed the longitudinal axes of the lumen, the magnetic hydrogel, and the magnet are in the same plane, so that θ = 0 when the magnetic hydrogel is on the downstream side of the magnet, and θ = π when the magnetic hydrogel is on the upstream side of the magnet (Figure 2a,b).
Based on the calculated intestinal propelling force, friction force, and magnetic force (see details in Supporting Information), we applied this criterion to a human model, where the vertical distance between the adjacent surfaces of the magnetic hydrogel and the magnet is 15 mm (Figure 2c). We fixed the thickness (L) of the magnet to be 12.7 mm and varied its radius (a) from 6.4 to 25.4 mm. We found that, when the radius of the magnet is greater than 9 mm, there exists a region in the lumen where the hydrogel can be retained. For example, when the radius of the magnet is 12.7 mm or 25.4 mm, the magnetic hydrogel can be successfully retained on the downstream side of the magnet (Figure 2c). The solid circles indicate the stably retained locations for the magnetic hydrogels, at which Ffriction + Frcosθ – Fpropel = 0 with θ = 0. To the contrary, the magnetic hydrogel can pass through the lumen without retention when the radius of the magnet is 6.4 mm (Figure 2c). In addition, as can be seen in Figure 2d, the normal pressure applied by the retained magnetic hydrogel on the intestinal wall is mild (pz < 0.6 kPa) at any retained locations around the magnets (a = 25.4 or 12.7 mm, L = 12.7 mm). Therefore, we selected the magnet with a radius of 25.4 mm and a thickness of 12.7 mm, due to the portable weight of the magnet (193 g), mild normal pressure applied on the intestinal tissue (0 – 0.6 kPa),[32] and capability of retaining the ingestible hydrogel (Figure 2c,d and S4).
In vitro retention of magnetic living hydrogels
Next, we used transparent plastic tubes with flowing liquids to replicate the geometries and dimensions of mouse and human small intestines, and investigated the in vitro retention and positioning of magnetic living hydrogels based on remote magnetic attraction. In human intestines, the luminal chyme movement is driven by waves of contraction propagating along the intestinal wall.[38] To model the propelling force, fluid flow at different flow rates and viscosities were generated in the tube by a peristaltic pump, which applied a shear stress of 0–10 kPa to the trapped contents close to the wall. As illustrated in Figure 3a,c, the fluid flow drove the motion of the suspended magnetic hydrogel so that it travelled through the whole conduit. In contrast, a magnet placed beneath the tube at a distance (2 mm for a mouse size and 15 mm for a human size) arrested the smooth movement of the magnetic hydrogel in the tube and kept the hydrogel static for 24 h afterward (Figure 3b,d and Video S1). The magnetic force was able to resist a maximum shear stress of 10 kPa in tubes having the same size as the mouse or human small intestines (Figure 3e,f, S5a and Video S1). When the magnet was removed, the originally retained hydrogel moved forward through the tube (Figure S5b).
Figure 3.
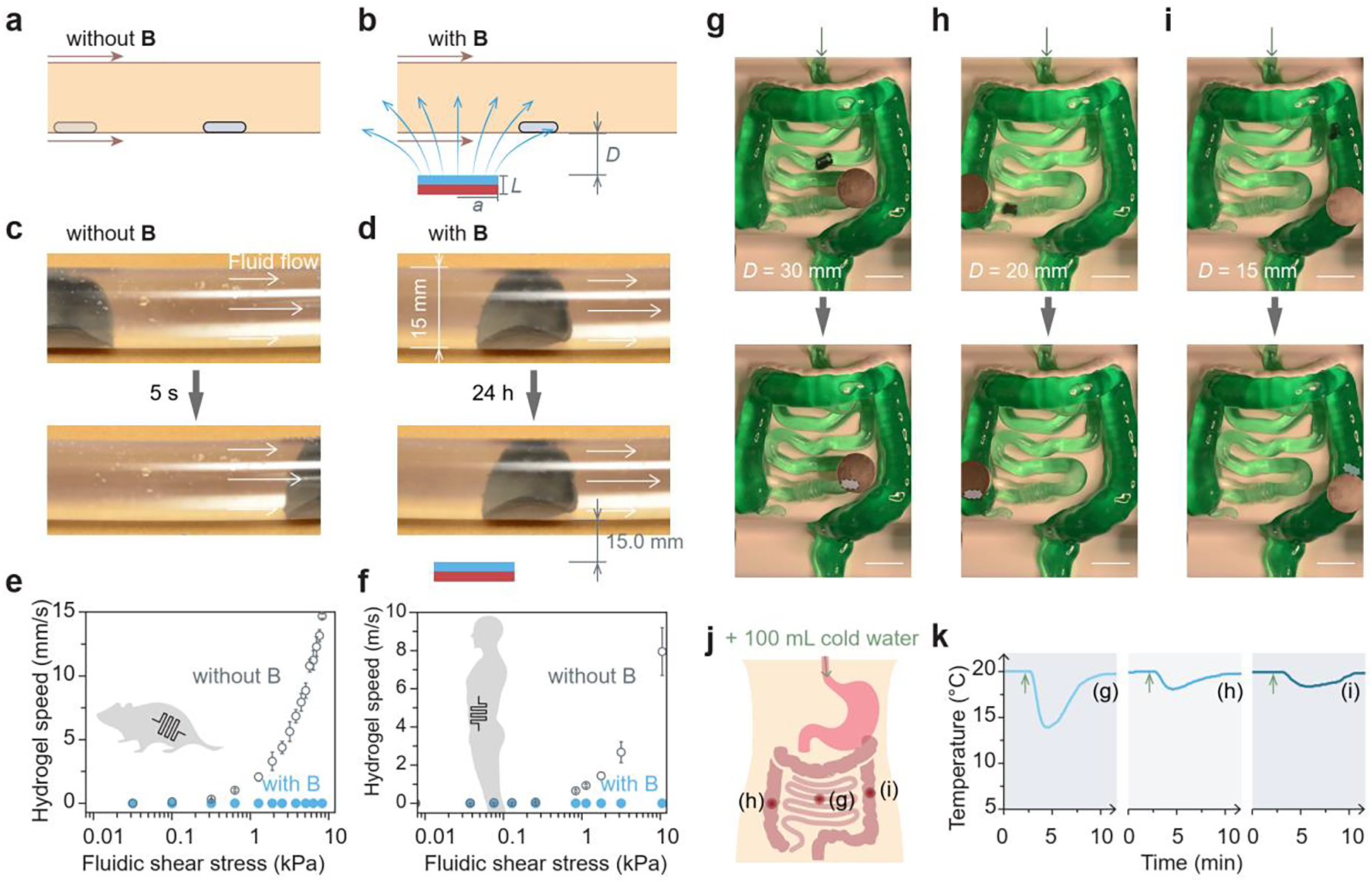
In vitro validation of retention and localization of the magnetic living hydrogel. a) Schematic illustration of the magnetic hydrogel movement propelled by intestinal peristalsis without any external magnet. Magnetic field, B. b) Schematic illustration of the magnetic hydrogel retention by the magnetic attraction force. c) Movement of a magnetic hydrogel (radius 10 mm, thickness 1 mm) in a transparent plastic tube (diameter 15 mm) driven by the fluidic flow without any external magnet. d) Retention of a magnetic hydrogel in a transparent plastic tube (diameter 15 mm) for 24 h with an external magnet (radius 25.4 mm, thickness 12.7 mm) placed 15 mm beneath the tube. e) Moving speed of a magnetic hydrogel (radius 1 mm, thickness 1 mm) against different fluidic shear stresses in a tube with dimensions similar to those of mouse intestines, with and without an external magnet (radius 5.6 mm, thickness 1.6 mm) at a vertical distance of 2 mm. f) Moving speed of a magnetic hydrogel (radius 10 mm, thickness 1 mm) against different fluidic shear stresses in a tube with dimensions similar to those of human intestines, with and without an external magnet (radius 25.4 mm, thickness 12.7 mm) at a vertical distance of 15 mm. g-i) Localization and retention of an integrated system consisting of a magnetic hydrogel (radius 10 mm, thickness 1 mm) and a miniature temperature sensor in a silicone phantom mimicking the human intestine, and the integrated system was pinned at the (g) small intestine, (h) ascending colon, and (i) descending colon by an external magnet (radius 25.4 mm, thickness 12.7 mm) placed 15–30 mm above the phantom. Scale bars in (g)-(i): 50 mm. j) Schematic illustration of oral intake of cold water to the GI tract and three locations (g)-(i) where the temperature was probed after water intake. k) Temperature variations after cold water intake measured by a miniature temperature sensor when the integrated system was retained at the three locations in the GI tract.
To illustrate the potential utility of the magnetic hydrogel in the clinical setting, we examined the localization and retention of the ingestible magnetic hydrogel with a more clinically relevant model. We used a life-sized, silicone phantom that replicates the anatomy of human intestines, including the small and large intestine. As seen in Figure 3g, the tubular structure of the phantom is continuous and tortuous, containing several acute-angled corners, and its inlet and outlet are connected to a peristaltic pump with a flow flux of 3400 mL/min. The magnetic hydrogel alone automatically passed through the artificial intestine without any navigation (Figure S6a and Video S2). When a magnet was placed on a transparent acrylic sheet right above the winding intestine phantom (distance 15–30 mm), the magnetic hydrogel was retained at several different locations, such as the small intestine, ascending colon, and descending colon (Figure S6b and Video S3). When the magnetic hydrogel encapsulated a miniature temperature sensor (6 mm in diameter and 17 mm in length), the hydrogel piece was effectively pinned by the magnet placed above at several different locations corresponding to the small intestine (Figure 3g), the ascending colon (Figure 3h), and the descending colon (Figure 3i) in the phantom. Since the overall diameter of the electronic sensor-encapsulated magnetic hydrogel was around 8 mm, smaller than the inner diameters of artificial intestines (15 mm for small intestine, 20–43 mm for large intestine), it did not obstruct fluid flow. Furthermore, the strong static magnetic field imposed by the wearable magnet did not affect the temperature sensing and recording functions of the microelectronics. When we infused 100 mL of cold water (0 °C) through the inlet of the tubular phantom to replicate water drinking, temperature variations at different locations were recorded (points (g)–(h) in Figure 3j). Figure 3k indicates that the intake of cold drinks induced temperature variations, and the magnitude and delay time of these variations depended on the locations in the GI tract. When the integrated sensor was close to the inlet (g), the temperature decrease was swift and sharp, whereas there were gradual and gentle temperature fluctuations in the downstream regions, as seen in (h) and (i).
In vitro biocompatibility of magnetic living hydrogels
Interfacing magnetic hydrogels with the human body requires assessments of chemical corrosion and cytotoxicity. We further investigated the corrosion of embedded NdFeB alloys in magnetic hydrogels by element analysis of the leaching solution. The as-prepared magnetic hydrogels were incubated in simulated gastric fluids (pH 1.2) at 37 °C for 1 day and simulated intestinal fluids (pH 6.8) at 37 °C for 10 days to allow the solvent to extract corroded metal ions, including Nd(III) and Fe(III). Element concentrations in these aqueous solutions were obtained by inductively coupled plasma mass spectrometry, from which we calculated the quantity of neodymium and iron ions that were corroded and dissolved from 1 g of magnetic hydrogel in 1 mL of fluids. The lower limits of toxicity, calculated by oral LD50 (i.e., 0.2 g/kg for FeCl3 and 3.7 g/kg for NdCl3) times an average human weight (i.e., 62 kg), were orders of magnitude higher than the amounts of corroded ions [Nd(III) and Fe(III)] from 1 g of magnetic hydrogel over 10 days (Figure 4a), suggesting that the presence of silica shells on the NdFeB particles would help to minimize direct exposure of the alloys to intestinal environments with neutral pH and consequently limit corrosion. The magnetic hydrogels were more severely oxidized and corroded in acidic solution, when they were incubated with simulated gastric fluid (pH 1.2) for 4 h, but the concentrations of corroded metal ions were still much lower than the limits of oral doses that induce toxicity (Figure 4a).
Figure 4.
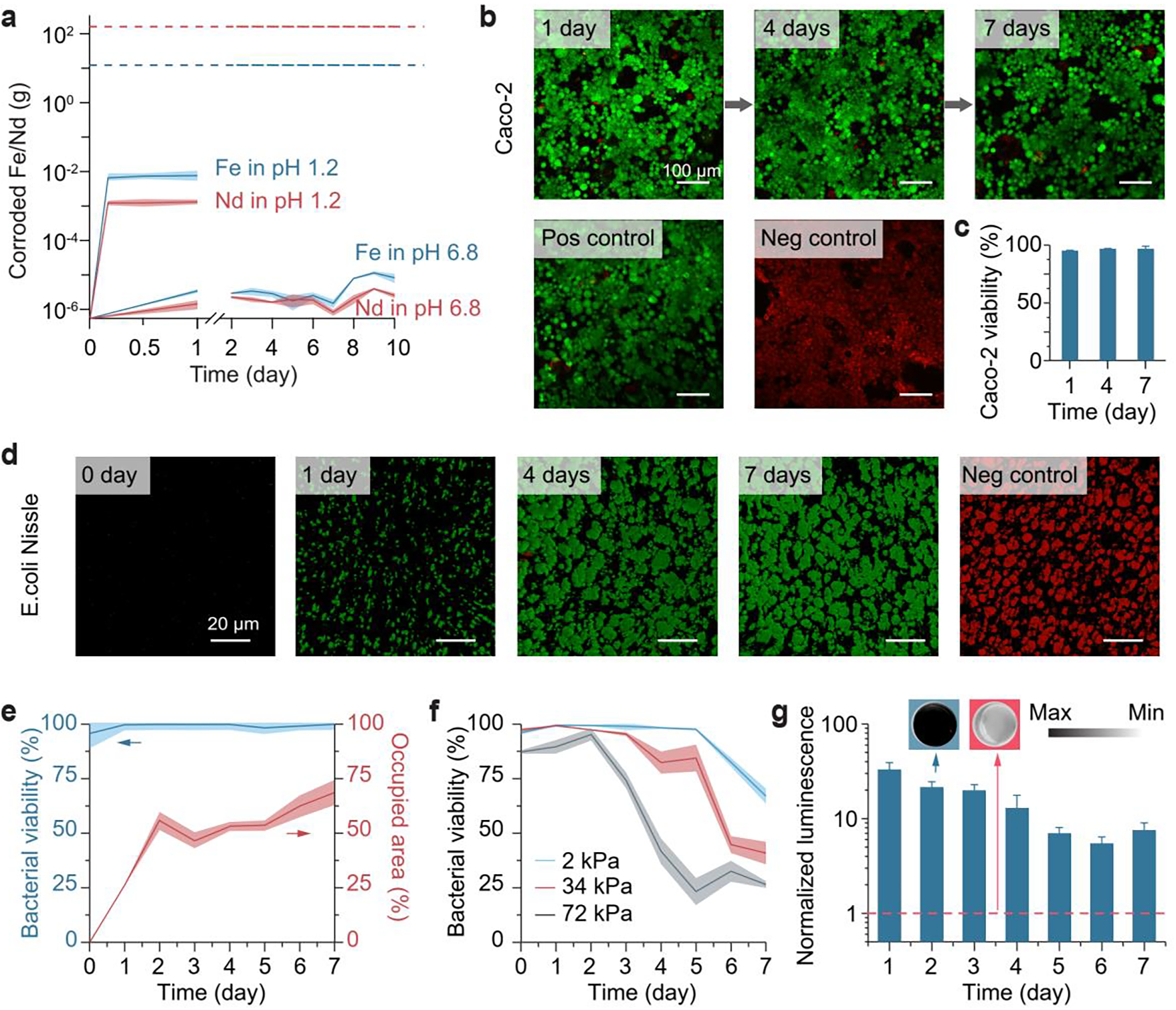
Biocompatibility and functionality of the magnetic living hydrogel. a) Chemical element analysis of the corroded metal ions from the magnetic hydrogel in simulated gastric fluid (pH 1.2) over 24 h and in simulated intestinal fluid (pH 6.8) over 10 days. The values correspond to the corroded metal masses from 1 g of the magnetic hydrogel, and represent the mean ± s.d. (n = 4). Dashed lines represent maximum oral doses having no significant toxic effect. b) Biocompatibility of the magnetic hydrogel in a live/dead assay of Caco-2 cells, using a hydrogel-conditioned medium (conditioning time: 1, 4, 7 days) for cell culture. After culture, the Caco-2 cells were stained with calcein-AM (green) and ethidium homodimer-1 (red) to visualize the viable and dead cells, respectively. Scale bars: 100 μm. c) Caco-2 cell viability using a hydrogel-conditioned medium with varied conditioning time. The values represent the mean ± s.d. (n = 3). d) Encapsulation and visualization of engineered bacteria (E. coli Nissle 1917) in the magnetic hydrogel for 1–7 days. The encapsulated bacteria were stained with SYTO 9 dye (green) and propidium iodide (red) to visualize the viable and dead bacteria, respectively. Scale bars: 20 μm. e) Bacterial viability and area occupied by viable bacteria (E. coli Nissle 1917) in hydrogels over time. The values represent the mean ± s.d. (n = 3). f) Bacterial viability (E. coli DH5α) in hydrogels with varied Young’s modulus over time. The values represent the mean ± s.d. (n = 3). g) Blood-sensing ability of the magnetic living hydrogels that encapsulated engineered bacteria. The values represent the mean ± s.d. (n = 3). Insets: typical images of the luminescent hydrogel (left, blue background) and non-luminescent hydrogel (right, red background; negative control). Dashed red line represents values obtained with the negative control.
We then analyzed the cytotoxicity of magnetic hydrogels using the Caco-2 cell line, which has been widely used as a model of the epithelial barrier on the intestine.[39] Similar to the trace element analysis mentioned above, leaching experiments were used to collect the chemicals released from magnetic hydrogels in fresh culture media for 1–7 days. Caco-2 cells were then exposed to the culture media containing the released compounds and left for 2 days, and cell viability was analyzed by using live/dead cell stains. As shown in Figure 4b,c and S7, there was no significant difference in viability for cells kept in the pristine culture medium (positive control), and the media which had been exposed to the magnetic hydrogels and pure PVA hydrogels for 1–7 days.
To determine whether the magnetic hydrogels could maintain living bacteria, as well as to test their in vitro functionality, we used a strain of engineered Escherichia coli (E. coli) Nissle 1917 as a bacterial blood sensor. These probiotic bacteria have been genetically engineered with the gene of PL(HrtO)-luxCDABE with HrtR.[12] When they are physically entrapped in the magnetic hydrogel, they express bioluminescence when extracellular heme is present in the surrounding medium. As demonstrated in Figure 4d,e, bacterial viability in the hydrogel was maintained above 95% for a week after day 0, and the colony size and density of viable bacteria continuously grew post-encapsulation. A few factors contributed to this lasting survival and growth: the biocompatibility of the chemical components and the fabrication process; chemical permeability, which allowed mass transport of nutrient supplies and metabolic products (Figure S1f); and mechanical softness, which permitted bacterial growth and division within the hydrogel matrix (Figure 4f; Figure S3).[40, 41] We then validated the biosensing function of the bacteria encapsulated in the magnetic hydrogels. As shown in Figure 4g, visible luminescence production in the hydrogels was induced by 0.1 vol% of whole horse blood in vitro. The induction tests were conducted at different time points of hydrogel incubation, during which the normalized luminescence gradually decreased from 33 after 24-h incubation to 7.5 after 7 days (Figure 4g).
In vivo retention and sensing ability of magnetic living hydrogels
After establishing the retention and positioning of the magnetic hydrogel in vitro, we evaluated the in vivo intestinal retentive ability of magnetic hydrogels in a mouse model (C57BL/6, Jackson Laboratory). Despite differences in size and physiology with the human body, mouse models have been frequently adopted to study the gut microbiota and the interactions of gut microbes with the host; in fact, mice share a similar GI transit time with humans.[42, 43] Magnetic living hydrogels (radius 1 mm, thickness 1 mm) were dispersed in a block of nutritious jelly (DietGel 76A, ClearH2O) and provided to mice after overnight fasting, so that mice would voluntarily consume the DietGel together with the magnetic hydrogels through oral administration without gavage or other invasive procedures (Figure S8). Owing to high radiographic contrast between magnetic hydrogels and mouse tissues, we employed X-ray microtomography (microCT) to visualize the 3D spatial position of the magnetic hydrogels in the GI tract over time. We observed that fasted mice ingested the magnetic hydrogels within approximately 1 hour after DietGel distribution. Mice with ingested magnetic hydrogels inside their bodies were randomly allocated into two groups: the control group without any treatment (n = 5) and the experimental group carrying a disc-shaped magnet (radius 11 mm, thickness 1.6 mm), which was adhered on the abdomen by skin adhesives (n = 5).
As illustrated in Figure 5a,c, the ingested magnetic hydrogel was completely cleared from the GI tract in the control group within 6 h. The magnetic hydrogel, in constrast, was magnetically retained in the GI tract for 7 days in the experimental group (Figure 5b,d and Video S4). After the removal of the magnet from the abdomen, the magnetic hydrogel was released rapidly and completed its passage through the remaining tract within 6 h (Figure S8 and Video S5). During its retention period, the magnetic hydrogel was able to resist the mechanical loads caused by intestinal motility and preserve its structural integrity. As indicated in the representative histological images of the small intestine cross-section with (Figure 5f) and without (Figure 5e) a magnetic hydrogel, intestinal tissues in contact with the magnetic hydrogel did not exhibit significant inflammatory responses, nor was the intestinal lumen blocked by the retained hydrogel. In addition, although the extracorporeal magnet exerted robust magnetic control of the ingestible hydrogel, no significant change in overall animal well-being, including body weight, movement, and food/water intake, was observed in the animals.
Figure 5.
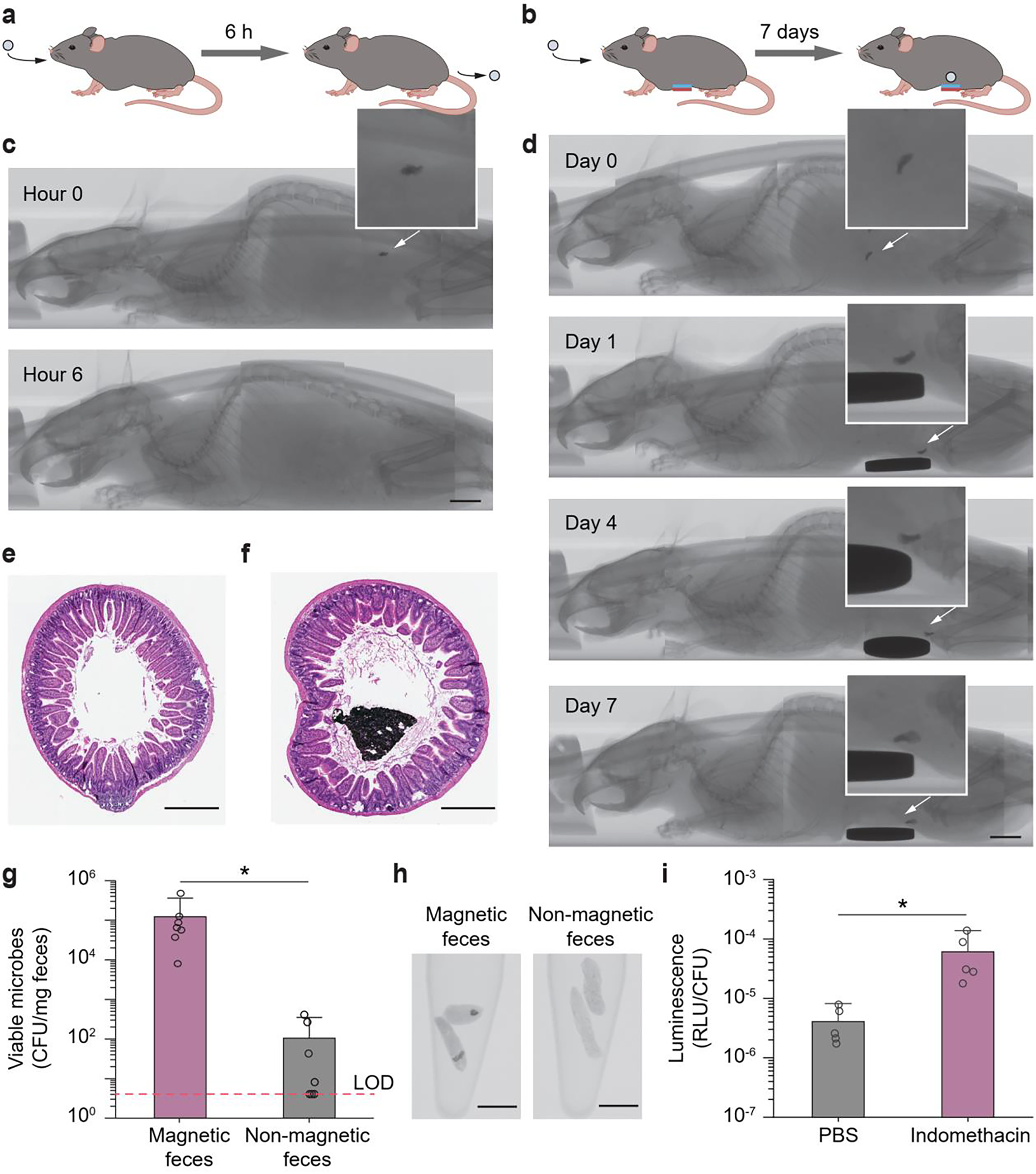
In vivo validation of magnet-assisted retention and in vivo heme sensing function of the magnetic living hydrogels. a) Illustration of an ingested magnetic living hydrogel passing through the whole GI tract of a mouse within ~ 6 h. b) Illustration of an ingested magnetic living hydrogel retained in the GI tract of a mouse for 7 days by an external magnet attached to the abdomen. c) CT images of a mouse with an ingested magnetic living hydrogel passing through the whole GI tract of a mouse within ~ 6 h. d) CT images of a mouse with an ingested magnetic living hydrogel retained in its intestine for 7 days by an extracorporeal magnet attached on the abdomen. Scale bars in c-d: 5 mm. e-f) Representative histological images stained with haematoxylin and eosin (H&E) for assessment of the small intestine cross-section without (e) and with (f) a magnetic hydrogel after 7-day intestinal retention. Scale bars: 500 μm. g) Numbers of viable microbes in fecal pellets collected from mice at 12 h post-administration of the hydrogel (containing blood-sensing E. coli Nissle 1917). The fecal samples with and without magnetic hydrogels exhibit significant differences in microbe numbers (*P < 0.05; Student’s t test; n = 7). Dashed line indicates the limit of detection (LOD) of the assay. h) CT images of fecal pellets with or without magnetic hydrogels. Scale bars: 5 mm. i) In vivo blood sensing performance. After the magnetic hydrogel (containing blood-sensing E. coli Nissle 1917) was retained in the intestine, mice were administered indomethacin (to induce gastrointestinal bleeding) or PBS. Normalized luminescence values of fecal pellets 24 h post-induction are significantly higher in mice administered indomethacin compared to control animals (*P < 0.05; Student’s t test; n = 5). RLU, relative luminescence units.
To demonstrate that magnetic hydrogels can serve as universal carriers for living sensors in vivo, we verified the biomarker detection in mice enabled by living bacteria encapsulated in the intestinal retentive hydrogel. We chose E. coli Nissle 1917 as a chassis for its excellent safety profile for long-term use and its incapacity to colonize the gut if swallowed.[44] Direct oral administration of E. coli Nissle 1917 resulted in viable cells in stools on the first day of administration (peak at the 6th hour), but bacteria were completely cleared in the subjects afterward.[12, 44] In general, the delivery of bacteria to the small intestine is challenging: colonization by the bacteria of the gut microbiota may cause drastic microbiome perturbation; while competition for nutrients and location with resident microbes may prevent the engrafted bacteria from colonizing, so that they lose their efficacy.[45] Constraining the location of the engineered bacteria by encapsulating them in the magnetic hydrogel offers a solution, because it not only allows long-term, localized residence of bacteria in the GI tract to overcome resistance to colonization but also reduces undesirable dispersion of the gut microbiota. We first fed the animals with the bacteria-encapsulating magnetic hydrogels. After 12 hours, we removed the magnet discs on the abdomen. Then, 1–6 hours after magnet removal, microbes were recovered from the stool samples. In the collected feces with no magnetic hydrogel, few viable bacteria (0–103 CFU/mg feces. CFU, colony-forming units) were detected (Figure 5g,h). In contrast, there were ~ 105 CFU of bacteria in 1 milligram of fecal samples containing magnetic hydrogels (Figure 5g,h). The difference in numbers of living bacteria recovered from collected fecal samples with and without magnetic hydrogels indicated that the hydrogel-encapsulated bacteria were contained in the hydrogel without significant alteration of the global gut microbiota. It also showed that high loads of bacteria can be retained, which would be useful for efficient therapeutic delivery and accurate biosensing.
To determine whether this system was responsive to GI bleeding elicited by indomethacin, we tested the bacterial blood sensor encapsulated in the ingested magnetic hydrogels. C57BL/6J mice carrying the magnetic living hydrogels were administered indomethacin (10 mg/kg) to induce GI bleeding in the experimental group (n = 5) and phosphate-buffered saline (PBS) in controls (n = 5). Twenty-four hours after indomethacin administration in the experimental group, the magnets on the abdomen were removed and fecal pellets in both groups were collected. We performed the CT scanning to confirm the presence of the magnetic hydrogels, luminescence analysis and CFU enumeration to calculate the bacterial luminescence, and guaiac test to validate the GI bleeding in the mice. As seen in Figure 5i, normalized luminescence values of fecal pellets were significantly higher in mice administered with indomethacin compared to those of control animals. The results revealed that the 24-h, localized colonization of the bacterial sensors in the mouse intestine allows effective detection of GI bleeding in vivo.
3. Conclusions
In summary, we introduced a magnetic hydrogel retentive system that combined an ingestible magnetic living hydrogel with a wearable magnet. This system relied on magnetic attraction to counteract the propelling effects of normal intestinal motility. Using theoretical calculations, we optimized the dimensions of the extracorporeal magnet for retention of the magnetic hydrogels applicable to either mice or humans. The retention and positioning of the magnetic hydrogel under the control of an extracorporeal magnet were verified by using a mouse-sized tube, a human-sized tube, a human-sized intestine phantom in vitro, and a mouse model in vivo. By incorporating genetically engineered heme-sensing bacteria into the ingestible hydrogel, we demonstrated that the device functioned to detect GI bleeding, as a proof of concept for biosensing in the gut. Engineering bacteria encapsulated in the protective magnetic hydrogel could thus perform diagnostic functions at localized sites in the gut.
The magnetic living hydrogels display a few advantages over other systems for tracking and treating conditions in the GI tract. The magnetic living materials are designed to have precise spatial localization, so their effects are also likely to be highly localized. When the hydrogel interacts with the intestinal tissues, alteration of the overall gut environment is likely to be minimized, because the bacteria are encapsulated. The mechanical compliance and biological compatibility of the ingestible magnetic hydrogel minimized mechanical and biological mismatch between the tissue and the device. The tissue-like softness of the device is likely to prevent it from causing tissue injury. Intestinal obstruction is unlikely, because the device is much smaller than the diameter of the intestinal lumen. The magnetic living hydrogels have several additional advantages. Compared to laparotomy and laparoscopic surgery, these ingestible hydrogels are non-invasive. Furthermore, they do not rely on medical professionals with complicated equipment and procedures.[29, 32, 33] We expect that the remote control achieved by wearing an extracorporeal magnet and ingesting hydrogels can reduce cost (i.e., < $50 for a set), simplify deployment (i.e., wearing and ingestion), and enable much wider applicability than the current methods,[32] which require patients to be anesthetized).
There is still room for improvement in the current magnetic hydrogel retentive system. First, the dimensional discrepancy between mouse and human may limit the generalization of the results obtained from mouse studies to clinical settings.[42] The distance between the two parts affects magnetic control: given that the magnetic field varies inversely with the third power of distance and that magnetic force varies inversely with the fourth power of distance,[28] validation in large animal species such as pigs and dogs will be required prior to translation to humans. Second, we have shown blood sensing and temperature recording in this work as a proof of concept to demonstrate several functionalities of the intestinal retentive hydrogels. Magnetic hydrogels can also be combined with other actuators, optics, or electronics[15, 27] to enable a range of biomedical applications for these intestinal retentive devices. For example, coupling with bioelectronics to transduce the detected values in real-time[12] and wiring them to next-step actuation would achieve a series of semi-autonomous or autonomous programs while the device is retained in the intestine.[8, 27] Ultimately, spatially localized and temporally retained hydrogel devices may be used to deliver drug-producing bacteria at targeted enteric sites, which can overcome the shortcomings of systemic treatment (e.g., frequent high-dose injections and side effects) and conventional oral administration (e.g., protein degradation in the acidic, protease-rich upper GI tract). Incorporating drug-producing bacteria in the intestinal retentive hydrogel could allow the requisite doses to be reduced by several orders of magnitudes, thus reducing systemic side effects.[46, 47]
4. Experimental Section
Bacterial strains:
Plasmid construction and DNA manipulations of bacteria were performed following standard molecular biology techniques. Genetics parts and plasmids used in this study are available from Addgene and the previous paper.[12] All plasmids were constructed by combining PCR fragments generated by Kapa HiFi Polymerase using Gibson assembly.[12] Routine cloning and plasmid propagation were performed in E. coli DH5α, and the gene circuits were transferred into probiotic E. coli Nissle 1917 for bacterial encapsulation.
Fabrication of magnetic living hydrogels:
NdFeB microparticles were coated with a thin shell of silica based on the condensation reaction of tetraethylorthosilicate (TEOS), which nucleated around the particles to form a cross-linked silica layer.[28] First, 40 g of NdFeB microparticles was dispersed in 1000 mL of ethanol with vigorously stirring to avoid sedimentation at 1500 rpm using a digital mixer (Cole-Parmer). Then, 60 mL of 29% ammonium hydroxide was slowly added to the mixture, followed by slow addition of 2 mL of TEOS. The mixture was stirred for 12 hours at room temperature and then washed with acetone multiple times after the reaction. The suspension was then vacuum-filtered to obtain the silica-coated microparticles.
Engineered bacteria were routinely cultured overnight at 37°C in Luria-Bertani (LB) media (Difco) supplemented with 50 μg/mL kanamycin. For bacterial encapsulation, overnight cultures of bacteria (E. coli Nissle 1917, 10 mL) were centrifuged at 5000g for 5 minutes, followed by supernatant removal. The remaining bacteria pellets were resuspended in 10 mL of 10 wt% PVA solution (Mw 146,000–186,000, 99+% hydrolyzed, Sigma-Aldrich) using a planetary mixer (AR-100, Thinky) at 2000 rpm for 2 min. The magnetic hydrogel was prepared by homogeneously mixing silica-coated NdFeB microparticles with an average size of 5 μm (MQFP-B 20441–089, Magnequench) into the bacteria-containing PVA solution using a planetary mixer (AR-100, Thinky) at 2000 rpm for 2 min. The mixture was then magnetized by impulse magnetic fields (about 2.5 T) generated by an impulse magnetizer (IM-10–30, ASC Scientific) to impart magnetic polarities to the NdFeB microparticles embedded in the uncured PVA solution. Afterward, the magnetized solution was exposed to −20 °C for 24 hours, resulting in a physically crosslinked hydrogel matrix. Magnetic hydrogels with different Young’s moduli in Figure 4f and S3 were fabricated by adjusting the freezing time from 2 h to 48 h. Bacteria (E. coli DH5α) were encapsulated in the magnetic hydrogels with different Young’s moduli.
Mechanical characterization:
Magnetic living hydrogels based on PVA and NdFeB with different particle concentrations were prepared and then swollen in phosphate-buffered saline (PBS) for 1 h. Subsequently, they were cut into dog bone–shaped specimens with known dimensions (width 4.7 mm, thickness 1.6 mm, gauge length 17 mm) for tensile testing. The specimens were tested on a mechanical testing machine (Z2.5, Zwick/Roell) with a 20-N load cell at a strain rate of 0.01 s−1. A nominal stress–stretch curve was plotted for each specimen, and the Young’s modulus and toughness were identified by the initial slope and the area under the stress strain curve, respectively.
Magnetic characterization:
The magnetic moment densities of magnetic hydrogels based on PVA and NdFeB with different particle concentrations were measured with a vibrating sample magnetometer (DMS 1660, ADE Technologies). Specimens were prepared from thin sheets of the hydrogels obtained from molding by cutting them into 6-mm circles using a biopsy punch (size 6, Miltex) to fit into the sample holder of the magnetometer. The remanent magnetization of the samples was measured when the applied external magnetic field was zero and then divided by the sample volume to obtain the magnetization or magnetic moment density.
Magnetic field measurement and shielding:
We used an AC/DC magnetic meter (PCE-MFM 3000) to measure the magnitude of the magnetic field near the magnet (DY08-N52, K&J Magnetics). A sheet of spring steel (thickness 2.6 mm, McMaster Carr) was attached to the magnet for shielding the magnetic field.
Diffusion coefficient measurement:
Diffusion coefficients of molecules within the magnetic hydrogels (12 vol% NdFeB) were determined using fluorescence recovery after photobleaching (FRAP). A series of fluorescent probes, that is, fluorescein isothiocyanate-dextran with molecular weights of 500 Da, 4 kDa, 10 kDa, and 70 kDa (FITC-dextran, Sigma-Aldrich) were dissolved in PBS at 5 mg/mL. The magnetic hydrogels (1 g) were added to the fluorescent solution (50 mL), yielding a final concentration of 5 mg/mL FITC-dextran in the hydrogels. FRAP measurement of diffusion coefficients was performed on a confocal microscope (SP 8, Leica) with a 5× magnification objective and a 488 nm laser. A circle (radius ω ~15 μm) was selected as the bleaching spot. 20 pre-bleach images were scanned at a low laser intensity (1%), then the bleaching spot was bleached with five iterations (~2.6 s in total) at 100% laser intensity, and followed by detection of the fluorescence recovery in the bleaching spot at low intensity (1%). For each experiment, we obtained a normalized fluorescence recovery curve and a fitting parameter, the characteristic diffusion time τD. The diffusion coefficient Dh was then calculated according to Dh = 0.22 ω2/τD.
Friction coefficient measurement:
Magnetic hydrogel samples (20 mm × 20 mm × 1 mm) were prepared with 12 vol% NdFeB microparticles. To quantify the friction coefficients, the torque required to shear the specimens at a prescribed shear rate of 1.0 s−1 under prescribed normal pressure (from 1.5 to 9 kPa) was measured from a rotational rheometer (AR-G2, TA Instruments) in normal force control mode with a 20-mm-diameter steel plate geometry. In the control group, deionized water was smeared between the hydrogel sample and steel plate before shearing the samples. In the experimental group, a piece of mucus-covered intestinal tissue (Sierra Medical Inc.) was attached to the steel plate and the mucus layer was brought in contact with the magnetic hydrogel. The friction coefficients were calculated following the previously reported protocol.[48]
Retention in a plastic tube:
A transparent polyvinyl chloride tube (McMaster Carr) with the inner diameter of 2 mm or 15 mm was filled with liquid (i.e., water, 8.90 × 10−4 Pa·s in viscosity; or glycerol, 1.4 Pa·s in viscosity) and a magnetic hydrogel. Two ends of the plastic tube were then connected to a soft rubber tube (Masterflex L/S), which was loaded into a peristaltic pump (Masterflex L/S) to drive the fluid flow in the tube. A magnet (D71-N52 or DY08-N52, K&J Magnetics) was placed beneath the tube at a distance. The shear stress at the wall is written as , where η is the fluid velocity, Q is the flow flux of the fluid, and rtube is the radius of the tube.
Retention and localization in a silicone phantom:
An intestine phantom model made of silicone (Trandomed 3D) was custom-designed and 3D-printed. This silicone phantom was filled with swollen polyacrylate particles (Waste Lock 770, M2 Polymer) and connected to a peristaltic pump (Masterflex L/S) to simulate the intestinal peristalsis. In the set of demonstrations, the retention and localization were controlled by a magnet (DY08-N52, K&J Magnetics) on a transparent acrylic sheet which was placed horizontally on top of the phantom at a closest distance of 15 mm.
Temperature sensing in a silicone phantom:
The magnetic hydrogel was glued to a temperature sensor (DST nanoT, Star-Oddi) using cyanoacrylate adhesives (Loctite 460 instant adhesive). The temperature sensor has a size of 6 mm in diameter and 17 mm in length, completely encapsulated in the magnetic hydrogel. The sensor contains a tiny silver-oxide battery, which is weakly magnetic. The retention and localization of the integrated device in the artificial intestine were controlled by the external magnet. The presence of the external magnet did not influence the temperature readings of the sensor, and the presence of the sensor did not affect the magnetic retention (retention of a magnetic hydrogel alone in Figure S6b; retention of a magnetic hydrogel and a temperature sensor in Figure 3g–i). 100 mL cold water (0 °C) was infused from the inlet of the phantom to replicate the water drinking. The temperature sensor was retrieved from the outlet of the phantom and the temperature over time was recorded at different spots in the phantom.
Chemical element analysis in leaching solutions:
Leaching test of magnetic hydrogels was performed by incubating 1 g of the magnetic hydrogel in 1 mL of simulated intestinal fluid (pH 6.8)[13] for 0–10 days or 1 mL of simulated gastric fluid (pH 1.2)[13] for 0–1 day. Leached elements in the solutions were identified and quantified by inductively coupled plasma mass spectrometry (ICP-MS). ICP-MS analysis was performed on an Agilent 7900 instrument in helium mode (4.5 mL/min flow rate). 2% HNO3 was used as a rinse solution, and 10 ppb terbium was included as an internal standard among all samples. Calibration standards were prepared by volumetric dilution of a multielement standard. Both calibration standards and experimental samples were diluted with 2% HNO3 to ensure the stability of elements before spectroscopic analysis. Data were collected and analyzed for the following elements of interest: 56Fe and 146Nd.
In vitro biocompatibility:
We conducted in vitro biocompatibility tests using a magnetic hydrogel-conditioned medium for Caco-2 (American Type Culture Collection) cell culture. There were no bacteria in the magnetic hydrogel for the biocompatibility test. To prepare the magnetic hydrogel-conditioned medium for in vitro cytotoxicity tests, we incubated 20 mg of the magnetic hydrogel in 1 mL of Dulbecco’s modified Eagle medium (DMEM, Life Technologies) at 37 °C for 1, 4, 7 days. Caco-2 cells were cultured in DMEM supplemented with 10% fetal bovine serum (Sigma-Aldrich), 1× non-essential amino acids solution (Life Technologies), 1× GlutaMAX (Life Technologies), and penicillin/streptomycin (Life Technologies), and plated in 96-well plates. The cells were then treated with the magnetic hydrogel-conditioned medium and incubated at 37 °C for 48 h in 5% CO2. Cells grown in pristine DMEM were used as a positive control. Cells grown in pristine DMEM and then treated with 70% ethanol for 30 min were used as a negative control. Cell viability was determined by using a live/dead viability/cytotoxicity kit for mammalian cells (Life Technologies). We used a confocal microscope (SP 8, Leica) to image live cells with excitation/emission at 495 nm/515 nm, and dead cells at 495 nm/635 nm. Live and dead cell numbers were counted using ImageJ, and the ratio of live cells to all cells in the images was calculated as Caco-2 cell viability.
Bacterial viability in hydrogels:
1 g of magnetic hydrogels containing living bacteria were incubated in 20 mL of fresh LB broth at 37°C for 1–7 days, and the media were changed every 12 h. To test the bacterial viability, the hydrogels were first washed with 0.85% NaCl solution and then stained using the live/dead BacLight bacterial viability kit. 15 min after staining, fluorescent imaging of samples (n = 3) was carried out using a confocal microscope (SP 8, Leica). Magnetic hydrogels treated with 70% ethanol for 1 h were used as a negative control. The occupied areas of green (Sg) and red colonies (Sr) in the images were obtained using ImageJ, and the bacterial viability equals the viable populations over all populations, that is, viability = Sg / (Sg + Sr) in Figure 4e,f.
In vitro blood sensing in hydrogels containing bacteria:
The heme-sensing ability of bacteria in the magnetic hydrogel was induced by 0.1 vol% defibrinated horse blood (Hemostat) in the culture media and incubated with shaking at 37°C for 24 hours. Luminescence of hydrogels was visualized via a ChemiDoc Imaging System (Bio-Rad), with an exposure time of 60 s. To quantify the luminescence, 0.1 g of magnetic hydrogels were homogenized in 1 mL of PBS with a 4 mm stainless steel bead using a TissueLyser II (Qiagen) at 30 Hz for 5 minutes. Samples were centrifuged at 1000g for 1 min to deposit large hydrogel debris. The supernatant was serially diluted in sterile PBS and spot plated on MacConkey agar supplemented with 50 μg/mL kanamycin. Colonies (CFU) were enumerated following overnight incubation at 37°C. On the other hand, we measured the luminescence in hydrogel homogenate in a BioTek Synergy H1 Hybrid Reader with an integration time of 1 second and a sensitivity of 150. The normalized luminescence of the magnetic hydrogel on different days was calculated as the ratio of the luminescence values (RLU/CFU; RLU, relative luminescence) of the magnetic hydrogel induced by 0.1 vol% blood to that of a non-induced magnetic hydrogel (a negative control) on the same day.
Retention in mouse intestine:
All mouse experiments were approved by the Committee on Animal Care at the Massachusetts Institute of Technology (protocol number: 0818-075-21). Specific-pathogen free (SPF), male C57BL/6J mice (8–10 weeks of age) were purchased from Jackson Labs and were housed and handled under conventional conditions. Mice were acclimated to the animal facility 1 week prior to the commencement of experiments. Magnetic hydrogels containing engineered bacteria were prepared and incubated in fresh LB broth for 24 h. Then, the hydrogels were cut into small particles (radius 1 mm, thickness 1 mm) by a razor blade and dispersed in a block of nutritious jelly (DietGel 76A, ClearH2O). The nutritious jelly was provided to mice after overnight fasting. After mice ingested the jelly and magnetic hydrogels, X-ray microtomography (microCT, Bruker) was used to visualize the 3D spatial position of magnetic hydrogels in the GI tract over time. Mice with magnetic hydrogels inside their bodies are allocated into two groups: the control group without any treatment (n = 5), while the experimental group carrying a disc-shaped magnet (D71–52, K&J Magnetics) adhered on the abdomen (n = 5). The disc-shaped magnet was attached to the shaved abdomen skin and adhered by a layer of Histoacryl flexible adhesive (B. Braun Medical Inc.), and then mice were singly housed to prevent magnet-induced physical harm. The magnetic hydrogel retention in the mouse intestine was monitored by microCT in the experimental group daily for 7 days. All animals were monitored clinically at least twice a day for any evidence of morbidity, including lethargy, inappetence, decreased fecal output, abdominal distension, and decreased body weight.
Blood sensing in mouse intestine:
To examine the blood sensing ability of magnetic hydrogels in vivo, we evaluated whether magnetic hydrogels containing engineered bacteria that retained in the intestine could detect upper GI bleeding elicited by oral indomethacin administration. Before indomethacin treatment, mice had taken the magnetic hydrogel containing bacteria and a small magnet disc was fixed on the abdomen skin. After 12-h retention of magnetic hydrogels in the intestine, we induced GI bleeding by indomethacin. Indomethacin (Sigma-Aldrich) solution was prepared by dissolving the compound in absolute ethanol to a concentration of 20 mg/mL. Immediately prior to mouse gavage, the indomethacin stock solution was diluted to 2 mg/mL in PBS, and the dilute indomethacin solution was administered to each animal in the treatment group (10 mg/kg). In the control group, mice were fed with 0.2 mL of PBS. In the following morning, gastrointestinal bleeding was confirmed by performing a guaiac test (Hemoccult, Beckman Coulter) on fecal pellets from each animal. Magnet discs on the abdomen were removed and fecal pellets were collected at 1–6 h after magnet removal. The presence of magnetic hydrogels in the fecal pellets was checked by CT scanning of the collected fecal samples. The feces with magnetic hydrogels were used for luminescence analysis and CFU enumeration, and luminescence values were normalized to CFU values and reported in RLU/CFU.
Histology analysis:
All mice were sacrificed for histopathological analysis after experiments. Small intestines with and without the magnetic hydrogels were isolated, gently cleaned with PBS, fixed in 10% formaldehyde for 24 h, embedded in paraffin, sectioned, and stained with haematoxylin and eosin. Standard H&E-stained sections for the segments of interest were examined.
Finite element simulations:
We built 3D finite element model to simulate the stress distribution in the human intestine when a magnetic hydrogel was conformed to the human intestine. The geometries of the human intestine and magnetic hydrogel device were imported into ABAQUS CAE using STEP files generated from a commercial 3D modelling software. The imported geometry was subsequently meshed using eight-node brick elements with reduced integration (ABAQUS element type C3D8R) for both the human intestine and the magnetic hydrogel device. We applied the normal pressure of 1 kPa to the in-plane surface of the hydrogel. The boundary conditions and geometrical dimensions were summarized in Figure S2c. To capture the non-linear elastic response of both the human intestine tissue[49] and magnetic hydrogel, we adopted incompressible Ogden model with the strain energy density of
| 3 |
where with J = λ1λ2λ3, μi and αi represents material parameters fitted from the experimentally measured stress versus stretch curve, N represents the order parameter which is set as 1 here. By fitting with the model, the material parameters for the human intestine were identified as μ1 = 4.75kPa and α1 =11.92, while that for the magnetic hydrogel were identified as μ1 = 4.38kPa and α1 =3.23 (Figure S2a,b). D1 was set as 0.01 to impose the incompressible condition of the human intestine and the magnetic hydrogel disc.
Data analysis and statistics:
All data were analyzed using Microsoft Excel and MATLAB (MathWorks). The computation of magnetic fields, forces, and pressures was implemented via MATLAB (MathWorks). As noted, error bars represent the standard deviation of at least three independent experiments. Significance between groups was determined using a two-sample, one-tailed Student’s t test assuming unequal variances.
Data availability:
The main data supporting the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding author upon reasonable request.
Code availability:
Acquisition and analysis code will be available on reasonable request.
Supplementary Material
Acknowledgements
X.L., Y.Y. and M.E.I. contributed equally to this work. The authors thank Tian Luo, Cheng Chang, Karen Pepper, Liu Wang, Sufeng Zhang, and Huanhuan Tian for helpful discussion; Mark Mimee for providing biosensor bacteria; and Milton Cornwall-Brady, Virginia Spanoudaki, David Bono, and Bogdan Fedeles for their training and assistance in operating the analytical instrumentations. This work is supported by MIT, the US National Science Foundation (NSF; EFMA-1935291, CCF-1521925 and DMR-1419807), Defense Threat Reduction Agency (DTRA; E2045481 via George Mason University, HDTRA1-15-1-0050, and HDTRA1-14-1-0007), Leona M. and Harry B. Helmsley Charitable Trust (3239), National Institutes of Health (NIH; 229825 via Massachusetts General Hospital, 4-R33-AI121669-04, 1R01HL153857-01), U.S. Army Medical Research and Material Command (W81XWH-16-1-0565, W81XWH-17-1-0159, and W81XWH-18-1-0513), Space and Naval Warfare Systems Center (N66001-13-C-4025), Defense Advanced Research Projects Agency (DARPA; 152304.5106735.0006 and HR0011-15-C-0084), Army Research Office (W911NF-17-2-0077, W911NF-13-D-0001 T.O. 8), American Heart Association (229460), Human Frontier Science Program (LT000595/2017-L), Singapore-MIT Alliance for Research and Technology (S.M.A.R.T.), and Pew Charitable Trusts (to M.E. Inda; 00030623).
Footnotes
Competing interests
T.K.L. is a co-founder of Senti Biosciences, Synlogic, Engine Biosciences, Tango Therapeutics, Corvium, BiomX, Eligo Biosciences, Bota.Bio, and Avendesora. T.K.L. also holds financial interests in nest.bio, Ampliphi, IndieBio, MedicusTek, Quark Biosciences, Personal Genomics, Thryve, Lexent Bio, MitoLab, Vulcan, Serotiny, and Avendesora. Other authors declare no competing interests.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Xinyue Liu, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Yueying Yang, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Maria Eugenia Inda, Synthetic Biology Group, Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.; Center for Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
Shaoting Lin, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Jingjing Wu, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Yoonho Kim, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Xiaoyu Chen, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Dacheng Ma, Synthetic Biology Group, Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.; Center for Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
Timothy K. Lu, Synthetic Biology Group, Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Center for Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
Xuanhe Zhao, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
References
- [1].Riglar DT, Silver PA, Nat. Rev. Microbiol. 2018, 16, 214. [DOI] [PubMed] [Google Scholar]
- [2].Steiger C, Abramson A, Nadeau P, Chandrakasan AP, Langer R, Traverso G, Nat. Rev. Mater. 2019, 4, 83. [Google Scholar]
- [3].Kalantar-Zadeh K, Berean KJ, Ha N, Chrimes AF, Xu K, Grando D, Ou JZ, Pillai N, Campbell JL, Brkljača R, Nat. Electron. 2018, 1, 79. [Google Scholar]
- [4].Riglar DT, Giessen TW, Baym M, Kerns SJ, Niederhuber MJ, Bronson RT, Kotula JW, Gerber GK, Way JC, Silver PA, Nat. Biotechnol. 2017, 35, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lim B, Zimmermann M, Barry NA, Goodman AL, Cell 2017, 169, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ, Nature 2014, 514, 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kotula JW, Kerns SJ, Shaket LA, Siraj L, Collins JJ, Way JC, Silver PA, Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mimee M, Citorik RJ, Lu TK, Adv. Drug Deliv. Rev. 2016, 105, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu X, Steiger C, Lin S, Parada GA, Liu J, Chan HF, Yuk H, Phan NV, Collins J, Tamang S, Traverso G, Zhao X, Nat. Commun. 2019, 10, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hillemeier C, Pediatrics 1995, 96, 997. [PubMed] [Google Scholar]
- [11].Bettinger CJ, Trends Biotechnol. 2015, 33, 575. [DOI] [PubMed] [Google Scholar]
- [12].Mimee M, Nadeau P, Hayward A, Carim S, Flanagan S, Jerger L, Collins J, McDonnell S, Swartwout R, Citorik RJ, Bulović V, Langer R, Traverso G, Chandrakasan AP, Lu TK, Science 2018, 360, 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang S, Bellinger AM, Glettig DL, Barman R, Lee Y-AL, Zhu J, Cleveland C, Montgomery VA, Gu L, Nash LD, Maitland DJ, Langer R, Traverso G, Nat. Mater. 2015, 14, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abramson A, Caffarel-Salvador E, Soares V, Minahan D, Tian RY, Lu X, Dellal D, Gao Y, Kim S, Wainer J, Collins J, Tamang S, Hayward A, Yoshitake T, Lee H-C, Fujimoto J, Fels J, Frederiksen MR, Rahbek U, Roxhed N, Langer R, Traverso G, Nat. Med. 2019, 25, 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Payne SC, Furness JB, Stebbing MJ, Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 89. [DOI] [PubMed] [Google Scholar]
- [16].Dumonceau J-M, Obes. Surg. 2008, 18, 1611. [DOI] [PubMed] [Google Scholar]
- [17].Cobrin GM, Abreu MT, Immunol. Rev. 2005, 206, 277. [DOI] [PubMed] [Google Scholar]
- [18].Landry BP, Tabor JJ, Engineering diagnostic and therapeutic gut bacteria, 2018. [DOI] [PubMed] [Google Scholar]
- [19].Moës AJ, Crit. Rev. Ther. Drug. 1993, 10, 143. [PubMed] [Google Scholar]
- [20].Hwang S-J, Park H, Park K, Crit. Rev. Ther. Drug. 1998, 15. [PubMed] [Google Scholar]
- [21].Sarmast AH, Showkat HI, Patloo AM, Parray FQ, Lone R, Wani KA, Br. J. Medical Pract. 2012, 5. [Google Scholar]
- [22].Park J-S, Jeong S, Lee DH, Clin. Endosc. 2015, 48, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Smart JD, Adv. Drug Deliv. Rev. 2005, 57, 1556. [DOI] [PubMed] [Google Scholar]
- [24].Yang SY, O’Cearbhaill ED, Sisk GC, Park KM, Cho WK, Villiger M, Bouma BE, Pomahac B, Karp JM, Nat. Commun. 2013, 4, 1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee Y, Deelman TE, Chen K, Lin DSY, Tavakkoli A, Karp JM, Nat. Mater. 2018, 17, 834. [DOI] [PubMed] [Google Scholar]
- [26].Praveschotinunt P, Duraj-Thatte AM, Gelfat I, Bahl F, Chou DB, Joshi NS, Nat. Commun. 2019, 10, 5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim Y, Yuk H, Zhao R, Chester SA, Zhao X, Nature 2018, 558, 274. [DOI] [PubMed] [Google Scholar]
- [28].Kim Y, Parada GA, Liu S, Zhao X, Sci. Robot. 2019, 4, eaax7329. [DOI] [PubMed] [Google Scholar]
- [29].Gottlieb KT, Banerjee S, Barth BA, Bhat YM, Chauhan SS, Konda V, Maple JT, Murad F, Pfau P, Pleskow D, Gastrointest. Endosc. 2013, 78, 561. [DOI] [PubMed] [Google Scholar]
- [30].Wu Z, Li L, Yang Y, Hu P, Li Y, Yang S-Y, Wang LV, Gao W, Sci. Robot. 2019, 4, eaax0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gabriel S, Ackermann R, J. Parenter. Enter. Nutr. 2004, 28, 119. [DOI] [PubMed] [Google Scholar]
- [32].Laulicht B, Gidmark NJ, Tripathi A, Mathiowitz E, Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yim S, Gultepe E, Gracias DH, Sitti M, IEEE. Trans. Biomed. Eng. 2014, 61, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hu C, Meng MQH, Mandal M, International Journal of Information Acquisition 2005, 02, 23. [Google Scholar]
- [35].Lin S, Liu J, Liu X, Zhao X, Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lin S, Liu X, Liu J, Yuk H, Loh H-C, Parada GA, Settens C, Song J, Masic A, McKinley GH, Sci. Adv. 2019, 5, eaau8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].K&J Magnetics I, Demagnetization (BH) Curves for Neodymium Magnets, https://www.kjmagnetics.com/bhcurves.asp, accessed.
- [38].Avvari RK, in Digestive System-Recent Advances, IntechOpen; 2019. [Google Scholar]
- [39].Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F, Cell Biology and Toxicology 2005, 21, 1. [DOI] [PubMed] [Google Scholar]
- [40].Liu X, Yuk H, Lin S, Parada GA, Tang T-C, Tham E, de la Fuente-Nunez C, Lu TK, Zhao X, Adv. Mater. 2018, 30, 1704821. [DOI] [PubMed] [Google Scholar]
- [41].Liu X, Tang T-C, Tham E, Yuk H, Lin S, Lu TK, Zhao X, Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nguyen TLA, Vieira-Silva S, Liston A, Raes J, Dis. Models Mech. 2015, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hugenholtz F, de Vos WM, Cell. Mol. Life Sci. 2018, 75, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Isabella VM, Ha BN, Castillo MJ, Lubkowicz DJ, Rowe SE, Millet YA, Anderson CL, Li N, Fisher AB, West KA, Reeder PJ, Momin MM, Bergeron CG, Guilmain SE, Miller PF, Kurtz CB, Falb D, Nat. Biotechnol. 2018, 36, 857. [DOI] [PubMed] [Google Scholar]
- [45].Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA, Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605. [DOI] [PubMed] [Google Scholar]
- [46].Hamady ZZR, Scott N, Farrar MD, Lodge JPA, Holland KT, Whitehead T, Carding SR, Gut 2010, 59, 461. [DOI] [PubMed] [Google Scholar]
- [47].Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E, Science 2000, 289, 1352. [DOI] [PubMed] [Google Scholar]
- [48].Parada GA, Yuk H, Liu X, Hsieh AJ, Zhao X, Adv. Healthc. Mater. 2017, 6, 1700520. [DOI] [PubMed] [Google Scholar]
- [49].Christensen MB, Oberg K, Wolchok JC, SpringerPlus 2015, 4, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The main data supporting the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding author upon reasonable request.


