Abstract
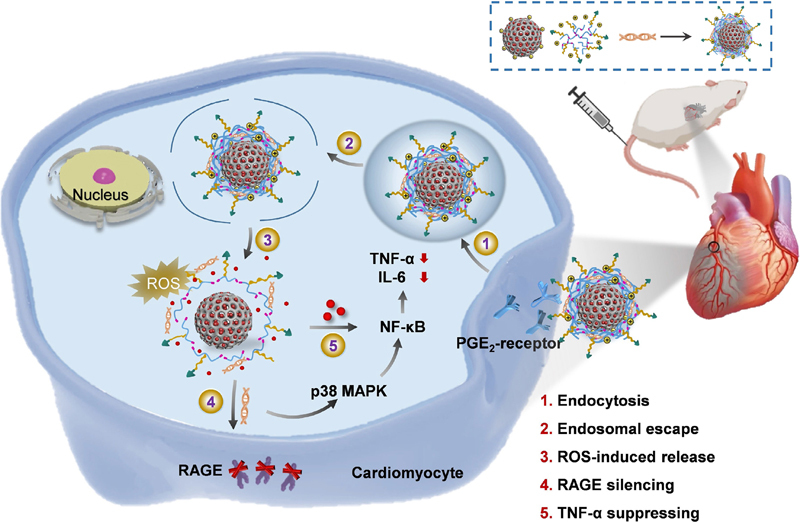
Myocardial ischemia reperfusion (IR) injury is closely related to the overwhelming inflammation in the myocardium. Herein, cardiomyocyte-targeted nanotherapeutics were developed for the reactive oxygen species (ROS)-ultrasensitive co-delivery of dexamethasone (Dex) and RAGE small interfering RNA (siRAGE) to attenuate myocardial inflammation. PPTP, a ROS-degradable polycation based on PGE2-modified, PEGylated, ditellurium-crosslinked polyethylenimine (PEI) was developed to surface-decorate the Dex-encapsulated mesoporous silica nanoparticles (MSNs), which simultaneously condensed siRAGE and gated the MSNs to prevent the Dex pre-leakage. Upon intravenous injection to IR-injured rats, the nanotherapeutics could be efficiently transported into the inflamed cardiomyocytes via PGE2-assisted recognition of over-expressed E-series of prostaglandin (EP) receptors on the cell membranes. Intracellularly, the over-produced ROS degraded PPTP into small segments, promoting the release of siRAGE and Dex to mediate effective RAGE silencing (72%) and cooperative antiinflammatory effect. As a consequence, the nanotherapeutics notably suppressed the myocardial fibrosis and apoptosis, ultimately recovering the systolic function. Therefore, the current nanotherapeutics represent an effective example for the co-delivery and on-demand release of nucleic acid and chemodrug payloads, and might find promising utilities toward the synergistic management of myocardial inflammation.
Electronic Supplementary Material
Supplementary material (experimental methods, RNA and primer sequences, 1H NMR spectra, FTIR spectrum, TEM images, zeta potential, drug loading content, RNA and drug release, cytotoxicity, etc.) is available in the online version of this article at 10.1007/s12274-022-4553-6.
Keywords: small interfering RNA (siRNA) delivery, reactive oxygen species (ROS) responsiveness, ditellurium-crosslinked polyethylenimine (PEI), myocardial ischemia reperfusion injury, anti-inflammation
Electronic Supplementary Material
Cardiomyocyte-targeted anti-inflammatory nanotherapeutics against myocardial ischemia reperfusion (IR) injury
Acknowledgements
We appreciate the funding support from the National Natural Science Foundation of China (No. 52033006 and 51873142), Suzhou Science and Technology Development Project (No. SYS2019072), Collaborative Innovation Center of Suzhou Nano Science & Technology, the 111 project, Suzhou Key Laboratory of Nanotechnology and Biomedicine, and Joint International Research Laboratory of Carbon-Based Functional Materials and Devices.
Contributor Information
Jing Yan, Email: jyan@suda.edu.cn.
Yong Ji, Email: jiyongmyp@163.com.
Lichen Yin, Email: lcyin@suda.edu.cn.
References
- [1].Li Y, Chen X, Jin R H, Chen L, Dang M, Cao H, Dong Y, Cai B L, Bai G, Gooding J, et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 2021;7:eabd6740. doi: 10.1126/sciadv.abd6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Huang K, Ozpinar E W, Su T, Tang J N, Shen D L, Qiao L, Hu S Q, Li Z H, Liang H X, Mathews K, et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 2020;12:eaat9683. doi: 10.1126/scitranslmed.aat9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhu D S, Li Z H, Huang K, Caranasos T G, Rossi J S, Cheng K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 2021;12:1412. doi: 10.1038/s41467-021-21682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Z H, Hu S Q, Huang K, Su T, Cores J, Cheng K. Targeted anti-IL-1β platelet microparticles for cardiac detoxing and repair. Sci. Adv. 2020;6:eaay0589. doi: 10.1126/sciadv.aay0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li Y, Chen X G, Li P, Xiao Q X, Kong X Q. CD47 antibody suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Am. J. Transl. Res. 2020;12:5908–5923. [PMC free article] [PubMed] [Google Scholar]
- [6].Li Z H, Zhu D S, Hui Q, Bi J N, Yu B J, Huang Z, Hu S Q, Wang Z Z, Caranasos T, Rossi J, et al. Injection of ROS-responsive hydrogel loaded with basic fibroblast growth factor into the pericardial cavity for heart repair. Adv. Funct. Mater. 2021;31:2004377. doi: 10.1002/adfm.202004377. [DOI] [Google Scholar]
- [7].Hausenloy D J, Yellon D M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- [8].Liu M R, Lutz H, Zhu D S, Huang K, Li Z H, Dinh P U C, Gao J Q, Zhang Y, Cheng K. Bispecific antibody inhalation therapy for redirecting stem cells from the lungs to repair heart injury. Adv. Sci. 2021;8:2002127. doi: 10.1002/advs.202002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Su T, Huang K, Ma H, Liang H X, Dinh P U, Chen J, Shen D L, Allen T A, Qiao L, Li Z H, et al. Platelet-inspired nanocells for targeted heart repair after ischemia/reperfusion injury. Adv. Funct. Mater. 2019;29:1803567. doi: 10.1002/adfm.201803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu C Y, Zhang Y H, Li R B, Zhou L Y, An T, Zhang R C, Zhai M, Huang Y, Yan K W, Dong Y H, et al. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat. Commun. 2018;9:29. doi: 10.1038/s41467-017-02280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tang Y Q, Zeng Z Y, He X, Wang T T, Ning X H, Feng X L. siRNA crosslinked nanoparticles for the treatment of inflammation-induced liver injury. Adv. Sci. 2017;4:1600228. doi: 10.1002/advs.201600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shen W W, Wang R J, Fan Q Q, Gao X, Wang H, Shen Y, Li Y W, Cheng Y Y. Natural polyphenol inspired polycatechols for efficient siRNA delivery. CCS Chem. 2020;2:146–157. doi: 10.31635/ccschem.020.201900084. [DOI] [Google Scholar]
- [13].Wang M, Alberti K, Varone A, Pouli D, Georgakoudi I, Xu Q B. Enhanced intracellular siRNA delivery using bioreducible lipid-like nanoparticles. Adv. Healthc. Mater. 2014;3:1398–1403. doi: 10.1002/adhm.201400039. [DOI] [PubMed] [Google Scholar]
- [14].Yang J D, Duan S Z, Ye H, Ge C L, Piao C X, Chen Y B, Lee M, Yin L C. Pro-peptide-reinforced, mucus-penetrating pulmonary siRNA delivery mitigates cytokine storm in pneumonia. Adv. Funct. Mater. 2021;31:2008960. doi: 10.1002/adfm.202008960. [DOI] [Google Scholar]
- [15].Hou M Y, Wu X J, Zhao Z Y, Deng Q R, Chen Y B, Yin L C. Endothelial cell-targeting, ROS-ultrasensitive drug/siRNA co-delivery nanocomplexes mitigate early-stage neutrophil recruitment for the anti-inflammatory treatment of myocardial ischemia reperfusion injury. Acta Biomater. 2022;143:344–355. doi: 10.1016/j.actbio.2022.02.018. [DOI] [PubMed] [Google Scholar]
- [16].Cai C D, Zhang X S, Li Y G, Liu X Z, Wang S, Lu M K, Yan X, Deng L F, Liu S, Wang F, et al. Self-healing hydrogel embodied with macrophage-regulation and responsive-genesilencing properties for synergistic prevention of peritendinous adhesion. Adv. Mater. 2022;34:2106564. doi: 10.1002/adma.202106564. [DOI] [PubMed] [Google Scholar]
- [17].Shen S Y, Zhang L, Li M R, Feng Z Z, Li H X, Xu X, Lin S Q, Li P, Zhang C, Xu X J, et al. Collaborative assembly-mediated siRNA delivery for relieving inflammation-induced insulin resistance. Nano Res. 2020;13:2958–2966. doi: 10.1007/s12274-020-2954-y. [DOI] [Google Scholar]
- [18].Hong J, Ku S H, Lee M S, Jeong J H, Mok H, Choi D, Kim S H. Cardiac RNAi therapy using RAGE siRNA/deoxycholic acid-modified polyethylenimine complexes for myocardial infarction. Biomaterials. 2014;35:7562–7573. doi: 10.1016/j.biomaterials.2014.05.025. [DOI] [PubMed] [Google Scholar]
- [19].Liang Q J, Li F F, Li Y J, Liu Y, Lan M, Wu S H, Wu X J, Ji Y, Zhang R J, Yin L C. Self-assisted membranepenetrating helical polypeptides mediate anti-inflammatory RNAi against myocardial ischemic reperfusion (IR) injury. Biomater. Sci. 2019;7:3717–3728. doi: 10.1039/C9BM00719A. [DOI] [PubMed] [Google Scholar]
- [20].Piao C X, Zhuang C Y, Choi M, Ha J, Lee M. A RAGE-antagonist peptide potentiates polymeric micelle-mediated intracellular delivery of plasmid DNA for acute lung injury gene therapy. Nanoscale. 2020;12:13606–13617. doi: 10.1039/D0NR01367F. [DOI] [PubMed] [Google Scholar]
- [21].Dhumal D, Lan W J, Ding L, Jiang Y F, Lyu Z, Laurini E, Marson D, Tintaru A, Dusetti N, Giorgio S, et al. An ionizable supramolecular dendrimer nanosystem for effective siRNA delivery with a favorable safety profile. Nano Res. 2021;14:2247–2254. doi: 10.1007/s12274-020-3216-8. [DOI] [Google Scholar]
- [22].Shen K, Sun G D, Chan L, He L Z, Li X W, Yang S X, Wang B C, Zhang H, Huang J R, Chang M M, et al. Anti-inflammatory nanotherapeutics by targeting matrix metalloproteinases for immunotherapy of spinal cord injury. Small. 2021;17:2102102. doi: 10.1002/smll.202102102. [DOI] [PubMed] [Google Scholar]
- [23].Rinoldi C, Zargarian S S, Nakielski P, Li X R, Liguori A, Petronella F, Presutti D, Wang Q S, Costantini M, De Sio L, et al. Nanotechnology-assisted RNA delivery: From nucleic acid therapeutics to COVID-19 vaccines. Small Methods. 2021;5:2100402. doi: 10.1002/smtd.202100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nie J J, Qiao B K, Duan S, Xu C, Chen B Y, Hao W J, Yu B R, Li Y L, Du J, Xu F J. Unlockable nanocomplexes with self-accelerating nucleic acid release for effective staged gene therapy of cardiovascular diseases. Adv. Mater. 2018;30:1801570. doi: 10.1002/adma.201801570. [DOI] [PubMed] [Google Scholar]
- [25].Hao K, Guo Z P, Lin L, Sun P J, Li Y H, Tian H Y, Chen X S. Covalent organic framework nanoparticles for anti-tumor gene therapy. Sci. China Chem. 2021;64:1235–1241. doi: 10.1007/s11426-021-9998-x. [DOI] [Google Scholar]
- [26].Xu C F, Lu Z D, Luo Y L, Liu Y, Cao Z T, Shen S, Li H J, Liu J, Chen K G, Chen Z Y, et al. Targeting of NLRP3 inflammasome with gene editing for the amelioration of inflammatory diseases. Nat. Commun. 2018;9:4092. doi: 10.1038/s41467-018-06522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weng Y H, Xiao H H, Zhang J C, Liang X J, Huang Y Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol. Adv. 2019;37:801–825. doi: 10.1016/j.biotechadv.2019.04.012. [DOI] [PubMed] [Google Scholar]
- [28].Ge C L, Yang J D, Duan S Z, Liu Y, Meng F H, Yin L C. Fluorinated α-helical polypeptides synchronize mucus permeation and cell penetration toward highly efficient pulmonary siRNA delivery against acute lung injury. Nano Lett. 2020;20:1738–1746. doi: 10.1021/acs.nanolett.9b04957. [DOI] [PubMed] [Google Scholar]
- [29].Hu B, Li B, Li K, Liu Y Y, Li C H, Zheng L L, Zhang M J, Yang T R, Guo S, Dong X Y, et al. Thermostable ionizable lipid-like nanoparticle (iLAND) for RNAi treatment of hyperlipidemia. Sci. Adv. 2022;8:eab–1418. doi: 10.1126/sciadv.abm1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang C, Liu Q, Zhang Z Z, Wang Y, Zheng Y D, Hao J L, Zhao X Z, Liu Y, Shi L Q. Tumor targeted delivery of siRNA by a nano-scale quaternary polyplex for cancer treatment. Chem. Eng. J. 2021;425:130590. doi: 10.1016/j.cej.2021.130590. [DOI] [Google Scholar]
- [31].Ye L, Liu H M, Fei X, Ma D, He X Z, Tang Q Y, Zhao X, Zou H B, Chen X J, Kong X M, et al. Enhanced endosomal escape of dendrigraft poly-L-lysine polymers for the efficient gene therapy of breast cancer. Nano Res. 2022;15:1135–1144. doi: 10.1007/s12274-021-3616-4. [DOI] [Google Scholar]
- [32].Liu Y, Yin L C. α-Amino acid N-carboxyanhydride (NCA)-derived synthetic polypeptides for nucleic acids delivery. Adv. Drug Deliv. Rev. 2021;171:139–163. doi: 10.1016/j.addr.2020.12.007. [DOI] [PubMed] [Google Scholar]
- [33].Liu X, Zhao Z Y, Wu F, Chen Y B, Yin L C. Tailoring hyperbranched poly(β-amino ester) as a robust and universal platform for cytosolic protein delivery. Adv. Mater. 2022;34:2108116. doi: 10.1002/adma.202108116. [DOI] [PubMed] [Google Scholar]
- [34].Wen L J, Peng Y, Wang K, Huang Z H, He S Y, Xiong R W, Wu L P, Zhang F T, Hu F Q. Regulation of pathological BBB restoration via nanostructured ROS-responsive glycolipid-like copolymer entrapping siVEGF for glioblastoma targeted therapeutics. Nano Res. 2022;15:1455–1465. doi: 10.1007/s12274-021-3686-3. [DOI] [Google Scholar]
- [35].Zheng M, Liu Y Y, Wang Y B, Zhang D Y, Zou Y, Ruan W M, Yin J L, Tao W, Park J B, Shi B Y. ROS-responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv. Mater. 2019;31:1903277. doi: 10.1002/adma.201903277. [DOI] [PubMed] [Google Scholar]
- [36].Wang J X, He X Y, Shen S, Cao Z Y, Yang X Z. ROSsensitive cross-linked polyethylenimine for red-light-activated siRNA therapy. ACS Appl. Mater. Interfaces. 2019;11:1855–1863. doi: 10.1021/acsami.8b18697. [DOI] [PubMed] [Google Scholar]
- [37].Zhang M J, Weng Y H, Cao Z Y, Guo S, Hu B, Lu M, Guo W S, Yang T R, Li C H, Yang X Z, et al. ROS-activatable siRNA-engineered polyplex for NIR-triggered synergistic cancer treatment. ACS Appl. Mater. Interfaces. 2020;12:32289–32300. doi: 10.1021/acsami.0c06614. [DOI] [PubMed] [Google Scholar]
- [38].Ye H, Zhou Y, Liu X, Chen Y B, Duan S Z, Zhu R Y, Liu Y, Yin L C. Recent advances on reactive oxygen species-responsive delivery and diagnosis system. Biomacromolecules. 2019;20:2441–2463. doi: 10.1021/acs.biomac.9b00628. [DOI] [PubMed] [Google Scholar]
- [39].Li F, Li T Y, Cao W, Wang L, Xu H P. Near-infrared light stimuli-responsive synergistic therapy nanoplatforms based on the coordination of tellurium-containing block polymer and cisplatin for cancer treatment. Biomaterials. 2017;133:208–218. doi: 10.1016/j.biomaterials.2017.04.032. [DOI] [PubMed] [Google Scholar]
- [40].Zhou W Q, Wang L, Li F, Zhang W N, Huang W, Huo F W, Xu H P. Selenium-containing polymer@metal-organic frameworks nanocomposites as an efficient multiresponsive drug delivery system. Adv. Funct. Mater. 2017;27:1605465. doi: 10.1002/adfm.201605465. [DOI] [Google Scholar]
- [41].Ji S B, Cao W, Yu Y, Xu H P. Dynamic diselenide bonds: Exchange reaction induced by visible light without catalysis. Angew. Chem., Int. Ed. 2014;53:6781–6785. doi: 10.1002/anie.201403442. [DOI] [PubMed] [Google Scholar]
- [42].Wang H, Zhang S, Lv J, Cheng Y Y. Design of polymers for siRNA delivery: Recent progress and challenges. View. 2021;2:20200026. doi: 10.1002/VIW.20200026. [DOI] [Google Scholar]
- [43].Wen Y T, Bai H Z, Zhu J L, Song X, Tang G P, Li J. A supramolecular platform for controlling and optimizing molecular architectures of siRNA targeted delivery vehicles. Sci. Adv. 2020;6:eabc2148. doi: 10.1126/sciadv.abc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhuang J, Gong H, Zhou J R, Zhang Q Z, Gao W W, Fang R H, Zhang L F. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci. Adv. 2020;6:eaaz6108. doi: 10.1126/sciadv.aaz6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yan J, Liu X, Wu F, Ge C L, Ye H, Chen X Y, Wei Y S, Zhou R X, Duan S Z, Zhu R Y, et al. Platelet pharmacytes for the hierarchical amplification of antitumor immunity in response to self-generated immune signals. Adv. Mater. 2022;34:2109517. doi: 10.1002/adma.202109517. [DOI] [PubMed] [Google Scholar]
- [46].Bellis A, Mauro C, Barbato E, Di Gioia G, Sorriento D, Trimarco B, Morisco C. The rationale of neprilysin inhibition in prevention of myocardial ischemia-reperfusion injury during ST-elevation myocardial infarction. Cells. 2020;9:2134. doi: 10.3390/cells9092134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hou M Y, Wei Y S, Zhao Z Y, Han W Q, Zhou R X, Zhou Y, Zheng Y R, Yin L C. Immuno-engineered nanodecoys for the multi-target anti-inflammatory treatment of autoimmune diseases. Adv. Mater. 2022;34:2108817. doi: 10.1002/adma.202108817. [DOI] [PubMed] [Google Scholar]
- [48].Sager H B, Dutta P, Dahlman J E, Hulsmans M, Courties G, Sun Y, Heidt T, Vinegoni C, Borodovsky A, Fitzgerald K, et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl. Med. 2016;8:342ra80. doi: 10.1126/scitranslmed.aaf1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang Y, Hou M Y, Duan S Z, Zhao Z Y, Wu X J, Chen Y B, Yin L C. Macrophage-targeting gene silencing orchestrates myocardial microenvironment remodeling toward the anti-inflammatory treatment of ischemia-reperfusion (IR) injury. Bioact. Mater. 2022;17:320–333. doi: 10.1016/j.bioactmat.2022.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yin N, Tan X Y, Liu H B, He F M, Ding N, Gou J X, Yin T, He H B, Zhang Y, Tang X. A novel indomethacin/methotrexate/MMP-9 siRNA in situ hydrogel with dual effects of anti-inflammatory activity and reversal of cartilage disruption for the synergistic treatment of rheumatoid arthritis. Nanoscale. 2020;12:8546–8562. doi: 10.1039/D0NR00454E. [DOI] [PubMed] [Google Scholar]
- [51].Wang Q, Jiang H, Li Y, Chen W F, Li H M, Peng K, Zhang Z R, Sun X. Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials. 2017;122:10–22. doi: 10.1016/j.biomaterials.2017.01.008. [DOI] [PubMed] [Google Scholar]
- [52].Jiang K Y, Weaver J D, Li Y J Y, Chen X J, Liang J P, Stabler C L. Local release of dexamethasone from macroporous scaffolds accelerates islet transplant engraftment by promotion of anti-inflammatory M2 macrophages. Biomaterials. 2017;114:71–81. doi: 10.1016/j.biomaterials.2016.11.004. [DOI] [PubMed] [Google Scholar]
- [53].Li X D, Wei Y S, Wu Y C, Yin L C. Hypoxia-induced proprotein therapy assisted by a self-catalyzed nanozymogen. Angew. Chem., Int. Ed. 2020;59:22544–22553. doi: 10.1002/anie.202004008. [DOI] [PubMed] [Google Scholar]
- [54].Sun P C, Scharnweber T, Wadhwani P, Rabe K S, Niemeyer C M. DNA-directed assembly of a cell-responsive biohybrid interface for cargo release. Small Methods. 2021;5:2001049. doi: 10.1002/smtd.202001049. [DOI] [PubMed] [Google Scholar]
- [55].Dong P, Hu J L, Yu S Y, Zhou Y Z, Shi T H, Zhao Y, Wang X Y, Liu X Q. A mitochondrial oxidative stress amplifier to overcome hypoxia resistance for enhanced photodynamic therapy. Small Methods. 2021;5:2100581. doi: 10.1002/smtd.202100581. [DOI] [PubMed] [Google Scholar]
- [56].Gan Q, Zhu J Y, Yuan Y, Liu H L, Qian J C, Li Y S, Liu C S. A dual-delivery system of pH-responsive chitosan-functionalized mesoporous silica nanoparticles bearing BMP-2 and dexamethasone for enhanced bone regeneration. J. Mater. Chem. B. 2015;3:2056–2066. doi: 10.1039/C4TB01897D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cardiomyocyte-targeted anti-inflammatory nanotherapeutics against myocardial ischemia reperfusion (IR) injury


