Abstract
Objective
To evaluate whether the results of a quasi‐randomized study, comparing dialectical behavior therapy for binge‐eating disorder (DBT‐BED) and an intensive, outpatient cognitive behavior therapy (CBT+) in individuals with BED, would be replicated in a nonrandomized study with patients who more closely resemble everyday clinical practice.
Method
Patients with (subthreshold) BED (N = 175) started one of two group treatments: DBT‐BED (n = 42) or CBT+ (n = 133), at a community eating disorder service. Measures of eating disorder pathology, emotion regulation, and general psychopathology were examined at end of treatment (EOT) and at 6‐month follow‐up using generalized linear models with multiple imputation.
Results
Both treatments lead to substantial decreases on primary and secondary measures. Statistically significant, medium‐size differences between groups were limited to global eating disorder psychopathology (d = −.62; 95% CI = .231, .949) at EOT and depressive symptoms at follow‐up (d = −.45; 95% CI = .149, 6.965), favoring CBT+. Dropout of treatment included 15.0% from CBT+ and 19.0% from DBT‐BED (difference nonsignificant).
Discussion
Decreases in global eating disorder psychopathology were achieved faster with CBT+. Overall, improvements in DBT‐BED were comparable to those observed in CBT+. Findings of the original trial, favoring CBT+ on the number of OBE episodes, emotional dysregulation and self‐esteem at EOT, and on eating disorder psychopathology and self‐esteem at follow‐up, were not replicated. With similar rates of treatment dropout and about half of the therapy time used in CBT+, DBT‐BED can be considered a relevant treatment for BED in everyday clinical practice.
Public Significance
In this effectiveness study, dialectical behavior therapy (DBT) resulted in clinically relevant improvements in individuals with binge eating disorder. Changes were broadly comparable to those of cognitive behavior therapy (CBT), the current treatment of choice. Although CBT resulted in decreases in eating disorder psychopathology faster, there was a trend toward relapse in CBT at 6‐month follow‐up. Therefore, the less costly DBT‐program can be considered a relevant treatment in clinical practice.
Keywords: binge‐eating disorder, cognitive behavior therapy, dialectical behavior therapy, effectiveness, group therapy
1. INTRODUCTION
Dialectical behavior therapy (DBT; Linehan, 1993) is one of the leading treatments for individuals with borderline personality disorder and ongoing self‐harm or suicidal behaviors (NICE, 2017). DBT conceptualizes self‐injury as a functional, although maladaptive, way to cope with painful emotional states. Based on the affect regulation model (Hawkins & Clement, 1984; Telch et al., 2001) and supported by accumulating empirical evidence linking affect and binge eating (e.g. Abraham & Beumont, 1982; Arnow et al., 1992; Berg et al., 2015, 2017; Haedt‐Matt & Keel, 2011; Leehr et al., 2015; Schaefer et al., 2020; Vanderlinden et al., 2004), DBT has been adapted for binge‐type eating disorders including bulimia nervosa (BN) and binge‐eating disorder (BED). This therapy (DBT‐BED) teaches adaptive emotion regulation skills in order to replace binge eating as a way of coping with negative affect (Safer et al., 2009; Telch et al., 2001).
In a systematic review, Linardon, Fairburn, et al. (2017) evaluated seven randomized controlled trials of DBT‐BED. While most studies compared DBT to a waitlist or a nonspecific supportive psychotherapy, one study (Chen et al., 2017) directly compared DBT‐BED to cognitive behavior therapy (CBT), the current treatment of choice for BED recommended by practice guidelines (e.g., Hay et al., 2014; NICE, 2017). Both DBT and CBT were helpful in reducing objective binge eating (OBE) episodes in a mixed BN and BED sample of early weak‐responders to guided self‐help CBT (n = 67). No differences were found between the two treatments at the end of treatment (EOT), 6‐month follow‐up, or 12‐month follow‐up. Taken together, these data indicate that DBT‐based treatments may be a relevant treatment for both BN and BED.
However, these highly controlled studies have mostly been conducted in research settings and delivered by the developers of the treatment. In addition, patients who agree to randomization may not be representative of patients in “real life” treatment settings. Thus, whether findings from these efficacy studies generalize to everyday clinical practice has been questioned (Hans & Hiller, 2013; Stewart & Chambless, 2009). In line with this, the importance of conducting effectiveness studies, in which “real life” patients are not randomized to different conditions, has been stressed (e.g. Hans & Hiller, 2013; Linardon et al., 2018; Seligman, 1995).
To date, several effectiveness studies have evaluated the feasibility of group‐based DBT‐BED (Blood et al., 2020; Chen et al., 2008; Erb et al., 2013; Klein et al., 2012; Mushquash & McMahan, 2015; Telch et al., 2000). All found significant improvements in binge eating at the EOT that were maintained during 6‐ to 12‐month follow‐up (Chen et al., 2008; Erb et al., 2013; Telch et al., 2000). One study included 56 adults with BED (Blood et al., 2020). However, most sample sizes were small, ranging from 3 to 11 treatment completers (Erb et al., 2013 and Telch et al., 2000, respectively), and none of these studies included a comparison group. One other study (Lammers et al., 2020, 2021) combined both efficacy elements (quasi‐randomization, training, and monitoring of therapists, and use of a treatment manual) and effectiveness elements (clinically representative setting and therapists, few exclusion criteria). DBT‐BED was compared to a more intensive outpatient group CBT program (CBT+) in 74 individuals with BED, combined with high levels of emotional eating and obesity. At EOT, CBT+ performed better on the number of OBE episodes, emotional dysregulation, and self‐esteem. At 6‐month follow‐up, the only retained difference was for self‐esteem, although eating disorder psychopathology scores were lower in the CBT+ group. Concurrently, the less costly DBT‐BED program lead to robust improvements, without significant differences between the groups on one important primary measure (the number of OBE episodes) at follow‐up. This may warrant a closer look into the effectiveness of DBT‐BED.
The present study aims to evaluate whether the results of the quasi‐randomized study (Lammers et al., 2020) would be replicated in patients with BED who were treated at the same center over the same period but were not eligible for that treatment trial. The key differences are in the present study patients are not randomized to treatment, inclusion and exclusion criteria are less restrictive, and those with subthreshold BED, lower levels of emotional eating, and BMI below 30 are now included. Thus, the current study more closely reflects everyday clinical practice. Unlike most effectiveness studies, we include a large sample and unlike the study by Blood et al. (2020) we include a CBT‐comparison group. In line with Lammers et al. (2020), we expect to find that CBT+ outperforms DBT‐BED on eating disorder specific measures, emotional dysregulation, and self‐esteem.
2. METHODS
2.1. Study design
This is an open, quasi‐experimental study with two arms: an intensive outpatient CBT‐program (CBT+) and DBT‐BED. All study participants provided written informed consent, allowing us to use their routinely gathered data anonymously for scientific purposes. Whether patients started in CBT+ or DBT‐BED was the result of a nonstandardized decision making process in which both therapist/team variables (e.g., ideas about which treatment fits, which case conceptualization best), availability and, ultimately, patient preference (e.g., for a certain treatment day, treatment intensity or treatment content) played a part. Participants were assessed on the first and last day of treatment as well as 6 months after the EOT. All participants reported for intake between October 2011 and December 2016, and started treatment between the beginning of 2012 and the beginning of 2017.
2.2. Participants
Participants were referred to a Dutch community eating disorder service by their general practitioner or other clinician. After a telephone screen, a licensed psychologist or psychiatrist conducted a clinical interview, resulting in a case formulation, and a DSM‐5 classification. Both the formulation and the classification were discussed and ascertained in a multidisciplinary team. Individuals with BED (DSM‐5; American Psychiatric Association, 2013) or with subthreshold BED (those with BED of low frequency and those with subjective binge eating episodes) were eligible for participation in either the DBT‐BED group or the CBT+ group. Exclusion criteria for participation were ascertained in the multidisciplinary team: concurrent treatment for BED or for weight problems (but those who had previously undergone bariatric surgery were included), comorbid psychiatric conditions that require immediate attention (e.g. suicidality and acute psychosis), medical conditions that preclude treatment of the eating disorder, conditions that warrant individual rather than group treatment (e.g. intellectual disability, pregnancy), and age below 16. Some patients chose to participate in a quasi‐randomized study running at the center over the same period (Lammers et al., 2020) and were excluded from the present study, as were those that reported occasional purging behavior. Figure 1 shows the Consort flow diagram.
FIGURE 1.
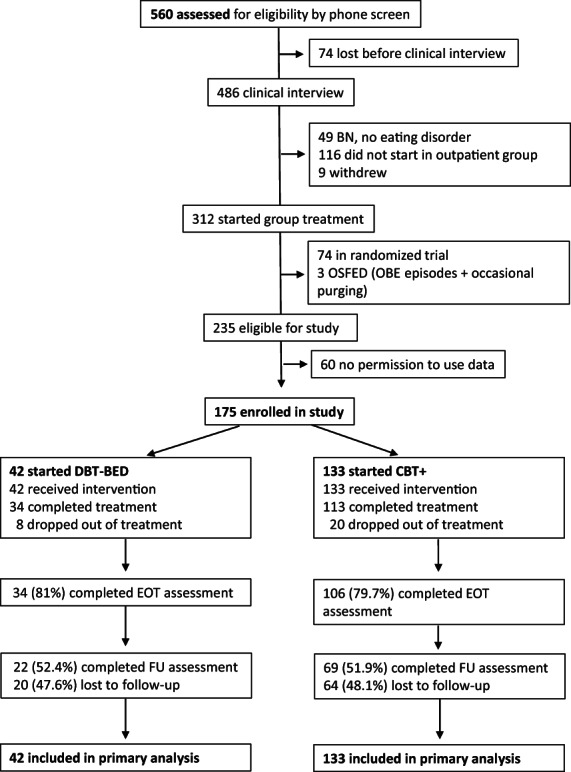
The consort flow diagram
2.3. Treatment
2.3.1. Dialectical behavior therapy for binge eating disorder
A session‐by‐session, prepublication version of the DBT‐BED protocol (Safer et al., 2009) was used (courtesy of C. Telch and D. Safer). The main objective of DBT‐BED is to help patients replace binge eating, as a way of coping with negative affect, by adequate emotion regulation skills. Over the course of 20 weeks, 20 sessions of 2 h each are delivered in a closed group format; all patients (maximum of nine) start and end together. The first two sessions are devoted to the rationale and goals of therapy and to commitment to change. Both eating disorder specific diary cards and chain analyses, and the concept of therapy‐interfering behavior is introduced. In sessions 3–18, adaptive emotion regulation skills are taught over three modules: mindfulness, emotion regulation, and distress tolerance. No specific attention is paid to eating patterns other than education on a balanced eating pattern and regular physical exercise. The two final sessions focus on the review of learned skills and on relapse prevention. In addition, by weekly weighing, patients monitor the consequences of (changes in) their eating behavior. Two trained psychologists/psychotherapists led each treatment‐cycle of 20 weeks. Patients were excluded from treatment if they missed more than two sessions. At 6‐month follow‐up, progress was reviewed and skills were refreshed in a single group session. In total, 13 DBT‐BED groups were run.
2.3.2. Intensive outpatient CBT+
We used an adapted version of CBT in group format of the manual developed by Fairburn et al. (1993; the protocol is available from M.L.). This intensive program had been treatment as usual (TAU) at the center when the present study was initiated. The main objective is to help patients regain control over eating and to diminish the influence of shape and weight on self‐esteem. Over the course of 20 consecutive weeks, 20 days of therapy are delivered in a half‐open group format; every 10th week new patients enter the group with a maximum group total of nine patients. Each day comprises weighing and three modules of 75 min each with a different focus: (1) discuss self‐monitoring of eating behavior, (2) challenge thoughts and conduct behavioral experiments, and (3) increase body‐awareness and promote regular exercise. By discussing how to deal differently with triggers, some attention is paid to emotion regulation. Each treatment‐cycle was led by a team consisting of a psychologist, a psychiatric nurse, and a psychomotor therapist. The latter is trained to systematically use body experiences and physical activities to achieve specific therapeutic goals. This approach has been appreciated as providing added value in the treatment for eating disorders in the Netherlands (e.g. to improve body‐image and to decrease avoidance of regular exercise; Alliantie kwaliteit in de geestelijke gezondheidszorg GGZ, 2017) and is easily integrated within the CBT‐model (Probst et al., 2010). In the present protocol, the therapist offers practice within the therapy session of what is usually only given as homework between sessions (e.g. look at the whole body instead of at parts of the body, go for a swim). If patients missed more than 2 days, they were excluded from the program. In addition, patients and their partners were encouraged to participate in six supportive group meetings of 90 min each. After treatment, six monthly group sessions were offered to consolidate the changes made and to deal with setbacks. Six months after treatment, progress was reviewed in one individual follow‐up session.
2.3.3. Therapist training
All therapists were well trained and experienced in eating disorder specific CBT, as this is TAU at the center. Because a randomized study was running at the center at the same time, procedures to optimize treatment adherence were conducted including the training of DBT‐BED therapists. This training was initially provided by an independent senior psychologist, well trained in this specific protocol. Later on, the initially trained therapists trained the co‐therapist. Monthly supervision was provided by a leading expert in DBT. For the CBT+ therapists, peer‐consultation was ensured. Each therapist was assigned to a single treatment to avoid content or procedural overlap. Sessions were audio‐recorded and rated for treatment adherence by five masters‐level students in psychology. Treatment integrity was only assessed after the completion of the data collection so there was no feedback to the therapists during the study. For more details, see Lammers et al. (2020).
2.3.4. Assessment
All assessment instruments were administered by a research assistant, aware of the treatment condition that patients were in. All the psychopathology measures were self‐report questionnaires, assessed at the start of treatment, at the EOT and 6 months after treatment. Demographic information and height were collected at baseline only. Patients' weight was measured on a balanced scale wearing clothes but no shoes. BMI was computed by dividing bodyweight in kilograms by height in squared meters (kg/m2).
2.3.5. Primary outcome measures
The frequency of OBE episodes and the global level of eating disorder psychopathology over the past 28 days, were measured using the Dutch version of the Eating Disorder Examination Questionnaire (EDE‐Q: Fairburn & Beglin, 2008). The EDE‐Q consists of four subscales (dietary restraint, eating concern, shape concern, and weight concern) out of which a global score can be calculated. One separate item assesses the amount of OBE's. Higher scores indicate greater severity. The global score is valid (Aardoom et al., 2012) and the EDE‐Q has acceptable to high internal consistency and test–retest reliability (Berg et al., 2012). The internal consistency of the EDE‐Q global score at baseline was good (α = .88) in the present sample.
2.3.6. Secondary outcome measures
The 13‐item subscale “emotional eating” of the Dutch Eating Behavior Questionnaire (DEBQ: Van Strien et al., 1986) was used to assess the desire to eat in response to negative emotions. This subscale has good internal consistency and factorial validity (e.g. Barrada et al., 2016); both the reliability and validity of the DEBQ are rated as good (enough) (COTAN, 2013). Higher scores indicate higher levels of emotional eating. The internal consistency of the DEBQ emotional eating score at baseline was excellent (α = .910) in the present sample.
In order to measure the tendency toward poor impulse regulation and mood intolerance, the 8‐item subscale “emotional dysregulation” of the Eating Disorder Inventory (EDI‐3, Garner & Van Strien, 2015) was used. The EDI‐3 is a reliable and valid instrument and can be used in eating disorder patients (Clausen et al., 2011; Lehmann et al., 2013; Segura‐Garcia et al., 2015). Higher scores indicate more dysregulation in emotion. The internal consistency of the EDI‐3 emotional dysregulation score at baseline was questionable (α = .70) in the present sample.
General psychopathology was assessed by using the Dutch version of the Symptom Checklist 90 (SCL‐90) (Arrindel & Ettema, 2003). The total score of this 90‐item questionnaire reflects physical and psychological symptoms experienced over the past week. Higher scores indicate more psychopathology. The reliability and validity of the SCL‐90 are good (Arrindel & Ettema, 2003). The internal consistency at baseline was excellent (α = .97) in the present sample.
The level of depressive symptoms was assessed using the total score of the Beck Depression Inventory‐II (BDI‐II) (Beck et al., 1996). The BDI‐II consists of 21 questions about the severity of depressive symptoms in the past week. Higher scores indicate more depressive symptoms. The reliability and validity of the BDI‐II are good (Beck et al., 1996). The internal consistency at baseline was good (α = .89) in the present sample.
Self‐esteem was assessed with the six‐item EDI‐3 subscale “low self‐esteem” (Garner & Van Strien, 2015). Higher scores indicate lower self‐esteem. The internal consistency at baseline was good (α = .87) in the present sample.
2.3.7. Dropout
Patients that stopped before the EOT were considered a dropout. When staff and patient mutually agreed that treatment goals were achieved before the 20‐week period ended and treatment, as a consequence, was terminated, this was considered as completion of treatment instead of dropout.
2.3.8. Power and sample size
We assessed and followed up all the patients who reported for intake between October 2011 and the end of 2016, and who met inclusion–exclusion criteria. No a priori power analysis was conducted.
2.3.9. Statistical analyses
All analyses were conducted using SPSS Version 25 (IBM Corp, 2017). Baseline demographic and clinical characteristics were summarized separately for the CBT and DBT treatment groups. For continuous measures, 95% confidence intervals (CI) of the difference between groups were calculated, along with effect size information based upon Cohen's d. For categorical measures, effect size information was calculated based upon the phi coefficient.
A logistic regression analysis was performed using baseline demographics (age, gender, nationality, and education) and clinical (BMI, BED diagnosis, lifetime vomiting, lifetime laxatives, lifetime diuretics, lifetime excessive exercise, OBE episodes, EDE‐Q Global, DEBQ Emotional Eating, SCL‐90 total, BDI‐II total, EDI‐3 Low Self‐Esteem, and EDI‐3 Emotional Dysregulation) characteristics to predict treatment assignment. Duration of illness and living situation were not included in the propensity analysis due to the amount of missing data (23/175 and 22/175, respectively). The predicted probability of receiving CBT, referred to as a propensity score, was derived for each study participant from this model. The propensity score was used as a covariate in all outcome analyses to control for pretreatment differences among the nonrandomized groups.
Generalized linear models were used to compare treatment groups on primary and secondary measures of outcome at baseline, EOT, and follow‐up. Primary measures of outcome used to evaluate effectiveness included OBE episodes and EDE‐Q Global scores. Secondary measures of outcome included DEBQ Emotional Eating, EDI‐3 Emotional Dysregulation, SCL‐90 total score, BDI‐II total score, and EDI‐3 Low Self‐Esteem. A negative binomial model with log link (appropriate for count data) was used for OBE episodes. A normal distribution with log link was used for symmetrically distributed measures (EDE‐Q Global, DEBQ Emotional Eating, and EDI‐3 Low Self‐Esteem), while a gamma distribution with log link was used for the remaining outcome measures (BDI‐II, EDI‐3 Emotional Dysregulation, and SCL‐90 total). Models included a random intercept and fixed effects for treatment, study visit, treatment‐by‐visit interaction, and propensity score. Treatment outcome at each postbaseline visit was compared by calculating the 95% CI of the difference between treatments and corresponding effect size. CIs that do not contain zero were considered evidence of a significant difference in outcome between treatments. Given that treatment for both CBT and DBT was delivered in group settings, preliminary models were run nesting participants within therapists within therapeutic groups. As no significant variation attributable to therapist or therapeutic group was found, subsequent analyses were conducted without nesting. Effect sizes between treatments were calculated using both Cohen's (Cohen, 1988) d and the success rate difference (SRD; Kraemer & Kupfer, 2006). Cohen's d values were calculated from covariate‐adjusted estimated marginal means; Cohen uses values of .2, .5, and .8 to characterize “small,” “medium,” and “large” differences between groups, respectively. SRD values, which can range from −1 to +1, represent the probability that a randomly selected case from one treatment will have an outcome preferable to a randomly selected case from another treatment.
All outcome analyses were based upon the intention‐to‐treat principle (McCoy, 2017). Multiple imputation using fully conditional Markov chain Monte Carlo (Schafer, 1987) modeling was used to impute missing data at baseline, EOT, and follow‐up. The final analyses were based upon the pooled results of 20 separate imputation sets. Sensitivity analyses were conducted using maximum likelihood (ML) imputation. As results were generally consistent across sensitivity methods, the final results for multiple imputation are presented.
Clinically meaningful change was operationalized as proposed by Jacobson & Truax (1991). We calculated the percentage of patients on the EDE‐Q Global score that shifted from being closer to the mean of the dysfunctional group (current sample: mean = 3.40; SD = 1.00) to being closer to the mean of a functional group (i.e. a normative nonstudents sample of males and females from the UK: mean = 1.92; SD = 1.42 (Carey et al., 2019).
3. RESULTS
3.1. Study participants
A total of 175 participants started treatment: 133 in CBT+ and 42 in DBT‐BED, 84% had full BED diagnosis and 16% subthreshold BED. Participants included 156 (89.1%) women and 19 (10.9%) men, with an average age of 34.9 years (SD = 10.9, range = 17–69) and an average duration of illness of 17.3 years (valid N = 152, SD = 11.3, range = .6–55.0). BMI of participants averaged 42.3 (SD = 7.6, range = 23.9–68.1). More than half of the participants (valid N = 153, n = 84 [55%]) lived with a partner/spouse. Table 1 presents baseline demographic and clinical characteristics separately by treatment. There were no significant differences between this study's sample and Lammers et al. (2020) except for BMI and BDI‐II scores. Both were significantly higher in the present study, with small effect sizes (BMI: current study M = 42.3, SD = 7.6; randomized study: M = 39.9, SD = 5.6; 95% CI = −4.10 to −.68; d = .251; BDI‐II: current study M = 23.56, SD = 11.13; randomized study: M = 20.77, SD = 9.26; 95% CI = −5.49 − −.10; d = .344).
TABLE 1.
Baseline demographic and clinical characteristics
| Variable | CBT (N = 133) | DBT (N = 42) | 95% CI | Effect size a |
|---|---|---|---|---|
| Age (mean, SD) | 33.46 (10.75) | 39.40 (10.22) | 2.23, 9.66 | d = .559 |
| Female (n, %) | 118 (88.7%) | 38 (90.5%) | – | Φ = .024 |
| Dutch nationality (n, %) | 128 (96.2%) | 41 (97.6%) | – | Φ = .165 |
| University education (n, %) | 12 (9.0%) | 2 (4.8%) | – | Φ = .210 |
| Living with partner/spouse b (n, %) | 62 (52.5%) | 22 (62.9%) | – | Φ = .206 |
| BMI (mean, SD) | 42.10 (7.74) | 42.77 (7.36) | −2.01, 3.34 | d = .088 |
| Full BED diagnosis (n, %) | 111 (83.5%) | 36 (85.7%) | – | Φ = .026 |
| Duration of illness (mean, SD) c | 15.89 (10.31) | 21.34 (13.13) | 1.35, 9.55 | d = .492 |
| Lifetime vomiting (n, %) | 25 (18.8%) | 9 (21.4%) | – | Φ = .146 |
| Lifetime laxatives (n, %) | 14 (10.5%) | 5 (11.9%) | – | Φ = .083 |
| Lifetime diuretics (n, %) | 3 (2.3%) | 1 (2.4%) | – | Φ = .004 |
| Lifetime excessive exercise (n, %) | 93 (69.9%) | 25 (59.5%) | – | Φ = .153 |
| OBE episodes (mean, SD) | 7.06 (7.93) | 6.52 (9.33) | −3.43, 2.36 | d = −.056 |
| EDE‐Q Global (mean, SD) | 3.42 (.96) | 3.33 (1.11) | −.45, .25 | d = −.031 |
| DEBQ emotional eating (mean, SD) | 3.89 (.60) | 4.03 (.69) | −.08, .36 | d = .231 |
| SCL‐90 total (mean, SD) | 192.26 (53.04) | 200.26 (63.47) | −11.46, 27.46 | d = .126 |
| BDI‐II total (mean, SD) | 23.78 (11.15) | 22.88 (11.16) | −4.80, 3.00 | d = −.081 |
| EDI‐3 Low self‐esteem (mean, SD) | 12.17 (5.59) | 12.33 (5.37) | −1.77, 2.10 | d = .029 |
| EDI‐3 Emotional dysregulation (mean, SD) | 6.18 (4.34) | 6.18 (4.57) | −1.54, 1.53 | d = .001 |
Abbreviation: CI, confidence interval.
Cohen's d for continuous measures and phi coefficient for categorical measures.
N = 153 (CBT = 118; DBT = 35).
N = 152 (CBT = 114; DBT = 38).
3.2. Study retention
A total of 28 (16.0%) participants dropped out of the treatment during the course of the trial, including 20 (15.0%) from CBT+ and 8 (19.0%) from DBT‐BED (Fisher's Exact p = .63). Two participants (1.5%) in the CBT+ group and none in the DBT‐BED group discontinued treatment before 20 weeks because they had achieved treatment goals. Of the 175 participants that started treatment, 140 (80%) completed EOT assessments and 91 (52%) completed follow‐up assessments. Assessment completion rates for CBT+ and DBT‐BED were 51.9% versus 52.4% (Fisher's Exact p = 1.00) at follow‐up. There were no differences between dropout and completers on gender, age, duration of illness, living situation, BMI as well as all primary and secondary outcome measures.
3.3. Primary outcomes
Adjusted mean scores on primary measures of outcome for CBT+ and DBT‐BED groups at EOT and follow‐up are presented in Table 2. The CBT+ group experienced greater reductions in EDE‐Q Global score at EOT (95% CI = .231–.949), with a medium effect size (d = −.62; SRD = −.35). Results of sensitivity analyses using ML imputation produced comparable results. Based upon pairwise 95% CIs, EDE‐Q Global scores in both groups decreased significantly between baseline and EOT (95% CI CBT = 1.12–1.56; 95% CI DBT = .35–1.09) and between baseline and follow‐up (95% CI CBT = .93–1.41; 95% CI DBT = .74–1.66). In DBT‐BED, the decrease in scores between EOT and follow‐up was also significant (95% CI = .15–.80). Table 3 presents the percentage of participants who completed the EDE‐Q that shifted from a dysfunctional level at baseline to a functional level at EOT and follow‐up (cutoff EDE‐Q score = 2.66; Jacobson & Truax, 1991). We found a significant difference at EOT (Fisher's exact p = .03) favoring CBT+ (55.6%) over DBT‐BED (35.7%). This difference between treatments at follow‐up was not significant (CBT = 59.4%; DBT = 52.4%, Fisher's exact p = .48).
TABLE 2.
CBT+ versus DBT‐BED comparison of treatment outcome
| Outcome | Group | N | Study visit (adjusted mean, SE) | CBT+ versus DBT‐BED | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EOT | FU | ||||||||||
| BL | EOT | FU | 95% CI | d a | SRD a | 95% CI | d a | SRD a | |||
| EDE‐Q global b | CBT | 133 | 3.44 (.09) | 2.09 (.08) | 2.27 (.08) | .231, .949 | −.62 | −.347 | −.405, .295 | .06 | .034 |
| DBT | 42 | 3.40 (.17) | 2.68 (.16) | 2.21 (.15) | |||||||
| OBE episodes b | CBT | 133 | 5.48 (.61) | .90 (.11) | 1.15 (.14) | −.231, 1.079 | −.19 | −.106 | −.512, .833 | −.09 | −.050 |
| DBT | 42 | 5.18 (1.09) | 1.32 (.030) | 1.31 (.30) | |||||||
| DEBQ emotional eating c | CBT | 133 | 3.93 (.06) | 2.90 (.06) | 2.86 (.06) | −.142, .371 | −.16 | −.090 | −.257, .252 | .00 | .000 |
| DBT | 42 | 3.91 (.12) | 3.01 (.11) | 2.86 (.11) | |||||||
| EDI‐3 emotional dysregulation c | CBT | 133 | 4.94 (.39) | 3.53 (.28) | 3.73 (.29) | −.770, 1.986 | −.18 | −.101 | −.701, 2.296 | −.22 | −.123 |
| DBT | 42 | 5.25 (.78) | 4.14 (.62) | 4.53 (.67) | |||||||
| SCL‐90 c | CBT | 133 | 189.6 (4.59) | 150.1 (3.64) | 152.1 (3.68) | −4.049, 29.986 | −.30 | −.168 | −7.650, 26.207 | −.21 | −.118 |
| DBT | 42 | 190.0 (8.73) | 163.1 (7.49) | 161.4 (7.41) | |||||||
| BDI‐II c | CBT | 133 | 21.87 (1.30) | 10.28 (.61) | 10.38 (.62) | −.980, 5.194 | −.28 | −.157 | .149, 6.965 | −.45 | −.252 |
| DBT | 42 | 22.22 (2.49) | 12.39 (1.39) | 13.94 (1.56) | |||||||
| EDI‐3 low self‐esteem c | CBT | 133 | 12.38 (.49) | 8.48 (.41) | 8.74 (.41) | −.209, 3.576 | −.35 | −.196 | −.674, 3.087 | −.25 | −.140 |
| DBT | 42 | 12.36 (.93) | 10.17 (.83) | 9.94 (.82) | |||||||
Abbreviations: BL, baseline; EOT, end of treatment; FU, follow‐up; CI, confidence interval; d, Cohen's d; SRD, success rate difference.
Positive values indicate the estimate for CBT+ is higher than the estimate for DBT‐BED; negative values indicate the estimate for DBT‐BED is higher than the estimate for CBT+.
Primary outcome measure.
Secondary outcome measure.
TABLE 3.
Percentage of participants that went from above to below the cutoff of 2.66 on the EDE‐Q global score
| CBT+ | DBT‐BED | Fisher's exact p | |
|---|---|---|---|
| EOT | (74/133) 55.6% | (15/42) 35.7% | .033 |
| Follow‐up | (79/133) 59.4% | (22/42) 52.4% | .475 |
There were no differences between treatments at any time point regarding OBE episode frequency. Scores in both groups decreased significantly between baseline and EOT (95% CI CBT = 3.26–5.91; 95% CI DBT = 1.60–6.13), and between baseline and follow‐up (95% CI CBT = 3.06–5.61; 95% CI DBT = 1.64–6.12).
3.4. Secondary outcomes
Results of secondary outcome analyses are presented in Table 2. Small effects are observed in favor of CBT+ on all secondary measures at EOT and on all but DEBQ Emotional Eating (no difference) at follow‐up. However, the only difference in secondary outcome measures that reached significance was for BDI‐II. The CBT+ group had significantly lower scores on the BDI‐II at follow‐up (95% CI = .15–6.97) with a medium effect size (d = −.45, SRD = −.25). Results of sensitivity analyses confirmed these findings. BDI‐II scores in both groups decreased significantly between baseline and EOT (95% CI CBT = 8.97–14.20; 95% CI DBT = 5.05–14.62) and between baseline and follow‐up (95% CI CBT = 8.94–14.04; 95% CI DBT = 3.56–13.02), but not between EOT and follow‐up (Figure 2).
FIGURE 2.
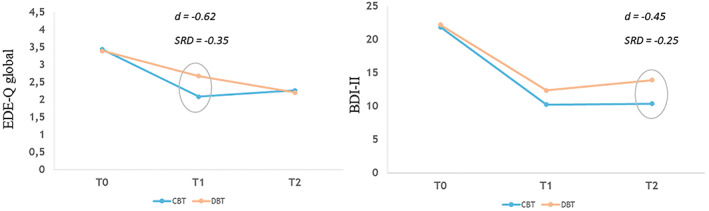
EDE‐Q global and BDI‐II scores for the CBT+ and DBT‐BED groups
3.5. Treatment adherence
Mean session integrity for DBT‐BED and CBT+ was 79.1% (SD = 15.0) and 63.5% (SD = 24.1), respectively, with a statistically significant difference in favor of DBT (95% CI = 7.82–23.38). Interrater reliability was established by five raters rating four tapes independently. The average kappa coefficient across raters and tapes was .63 (95% CI = .476–.780) suggesting good agreement.
4. DISCUSSION
This study evaluated whether the results of a quasi‐randomized treatment trial comparing DBT‐BED and CBT+ in individuals with BED (Lammers et al., 2020), would be replicated in patients who were not included in that study, but were treated at the same center over the same period. This group more closely represents everyday clinical practice, including those with both BED and subthreshold BED, BMI below 30, and lower scores on emotional eating. Overall, findings are relatively similar: both DBT‐BED and CBT+ lead to significant improvements in outcomes. However, decreases in global eating disorder psychopathology were achieved faster with CBT+ and the CBT+ group showed less depressive symptomatology at 6‐month follow‐up. Our earlier findings were not replicated: there were neither any significant differences between the groups in the number of OBE episodes, emotional dysregulation and self‐esteem at EOT, nor in eating disorder psychopathology and self‐esteem at 6‐month follow‐up.
Effect sizes indicate that, like in Lammers et al. (2020), small to medium differences on primary measures were in favor of CBT+ at EOT. In contrast to our earlier study, these differences were not retained at follow‐up. The only statistically significant difference was on global eating disorder psychopathology at EOT. The present findings are more in line with Chen et al. (2017). They found no differences between DBT and CBT regarding the number of OBE days and EDE global score from EOT up to 12‐month follow‐up. Interestingly, the CBT+ group in the present study showed a trend toward relapse on eating disorder psychopathology between EOT and 6‐month follow‐up; in contrast, the DBT‐BED group continued to improve. Longer term follow‐up data are needed to see how this trend evolves.
On secondary measures, effect sizes indicate that overall differences between the two treatments favored CBT+; however, in contrast to Lammers et al. (2020), most differences were statistically nonsignificant and small. The only significant and medium difference was on depressive symptoms at follow‐up (d = .45). Concurrently, within both groups, BDI‐II scores dropped significantly from moderate levels at baseline to minimal/mild levels at EOT and follow‐up. Based on these findings, we may conclude that both CBT+ and DBT‐BED lead to substantial decreases in depressive symptoms with the CBT+ group showing lower scores at follow‐up. These findings are broadly in line with Lammers et al. (2020) and in keeping with studies showing that eating disorder specific CBT reduces depressive symptoms (Fairburn et al., 2015; Turner et al., 2016). Concurrently, these findings positively contrast with studies showing limited levels of change in mood in DBT‐BED treatment groups (Blood et al., 2020; Safer et al., 2010; Telch et al., 2000).
Dropout rates were comparable in CBT+ and DBT‐BED (19% vs. 15%; Fisher's Exact p = .63). Dropout from CBT+ was higher than in Lammers et al. (2020; 6%) but still relatively low when compared to other controlled CBT‐treatment studies (e.g., 11.7%–37%, Chen et al., 2017; Peterson et al., 2009). In DBT‐BED, dropout rates were high when compared to the efficacy study of Safer et al. (2010; 4%), but comparable to studies conducted in everyday clinical practice (16.1% and 17.1%, Blood et al., 2020; Lammers et al., 2020).
In contrast to our controlled study (Lammers et al., 2020), where CBT+ favored DBT‐BED on emotional dysregulation at the EOT, no differential treatment effects were found on measures assessing emotion regulation, an hypothesized maintenance mechanism in DBT‐BED. The current findings are in line with studies that compared emotion regulation‐based treatments for binge‐type eating disorders, to a supportive control group (Safer et al., 2010) and CBT (Peterson et al., 2020; Wonderlich et al., 2014). This suggests that improvements in emotion regulation in this study might be attributable to therapeutic elements shared across various treatments and not to the specific emotion regulation strategies taught in DBT‐BED. The current study highlights the need to further understand the underlying mechanisms of both CBT and BED. Early symptom change was found to consistently mediate better treatment outcomes in CBT (Linardon, de la Piedad Garcia & Brennan, 2017) and, in one study, also in DBT‐BED (Safer & Joyce, 2011). In CBT, reductions in weight concern (Dingemans et al., 2007) and dietary restraint (Linardon, de la Piedad Garcia & Brennan, 2017) mediated treatment outcome for binge‐eating, as did regular eating (Sivyer et al., 2020) in nonunderweight eating disorders. Integrating related elements of CBT (e.g. regular eating, decrease body‐checking, and body‐avoidance) into DBT‐BED may further improve outcome.
Also, subgroups may profit more from one treatment than the other. However, most tested moderator variables did not affect cognitive‐behavioral treatment outcome relative to other treatments while some produced conflicting findings (Linardon, de la Piedad Garcia & Brennan, 2017). To BED patients who report greater difficulties in areas that are central to CBT treatment models (i.e. dietary restraint and overvaluation of shape and weight; Fairburn, 2008), CBT may offer incremental benefit over DBT‐BED while patients with distinct emotion regulation problems may profit more from DBT‐BED. Future research should address these issues.
The major difference between this study and the study described in Lammers et al. (2020) is the way assignments to the two treatments were made. In the controlled study, patients were randomized, whereas in this study (with less differences in outcome between CBT+ and DBT‐BED) multiple factors were involved in group assignment, including the patients' preference. These touches upon the possible role of the patients' active involvement in treatment choice. Shared decision making (e.g. Adams & Drake, 2006) may provide a framework to explore this in the future. To date, research on shared decision making in eating disorders is very limited (Jansingh et al., 2020).
This study has several limitations. First, we did not control for treatment dosage. DBT‐BED contained less face‐to‐face contact time per treatment day (2 h per week versus 3.75 h per week), offered only one follow‐up session (vs. six for some in the CBT+ group) and no group meetings to patients with a partner. This may have disadvantaged the DBT‐BED group and limits the reach of our conclusions about the observed differences in outcome. Second, the combination of the large CBT sample (n = 133) and the smaller DBT‐BED sample (n = 42) provides adequate power to detect large differences between groups, but limits the ability to detect small or moderate differences. Third, we did not record whether patients in this study had received any prior psychological treatment. This might have helped to provide some insight into whether DBT‐BED could be a viable option for patients who have not sufficiently improved with other treatment approaches. Also, because of the controlled study, therapists were supervised and sessions were audiotaped. This may have influenced treatment adherence (allegedly in a positive way) and may therefore not be completely representative for “everyday clinical practice.” However, treatment integrity per se was only assessed after the completion of the data collection. There was no regular feedback during treatment.
Despite the limitations, the present study has several strengths. To our knowledge this is the first study that evaluates the effectiveness of DBT‐BED in direct comparison to an active CBT control condition in everyday clinical practice. With follow‐up until 6 months after treatment, conclusions can be drawn about the medium‐long term effectiveness of the interventions. Also, compared to most other DBT‐BED effectiveness studies, this study had a relatively large sample size. Another strength is the use of a manualized treatment (DBT‐BED) by clinicians who did not develop the manual.
In conclusion, although decreases in global eating disorder psychopathology were achieved faster with CBT+ and the CBT+ group showed less depressive symptomatology at 6‐month follow‐up, the less costly DBT‐BED program lead to robust improvements, without significant differences between the groups on primary measures at follow‐up. Findings from the original study (Lammers et al., 2020), favoring CBT+ more distinctively, were not replicated. With similar rates of treatment dropout and about half of the therapy time used in CBT+, DBT‐BED can be considered a relevant treatment for BED in everyday clinical practice. Future research should include both dose‐matched comparisons between CBT and DBT‐BED in everyday clinical practice, and longer term follow‐up to see how trends evolve over time. It could also include the use of shared decision making and the identification of mediator and moderator variables, preferably only for those with BED who show weak initial response to effective and less intensive treatments like CBT‐guided self‐help. This could inform a more effective use of limited resources.
AUTHOR CONTRIBUTIONS
Mirjam W. Lammers: Conceptualization; data curation; investigation; methodology; visualization; writing – original draft; writing – review and editing. Maartje Vroling: Conceptualization; data curation; investigation; methodology; supervision. Ross D Crosby: Conceptualization; formal analysis; writing – original draft; writing – review and editing. Tatjana Van Strien: Conceptualization; supervision; writing – review and editing.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
CONFLICT OF INTEREST
The authors declare that they have no competing interests other than TvS receiving royalties from the DEBQ and the EDI‐3 and RDC being a paid statistical consultant for Health Outcomes Solutions, Winter Park, FL, USA.
ACKNOWLEDGMENTS
The authors wish to thank all therapists and support staff at Amarum who contributed to collecting and entering the data.
Lammers, M. W. , Vroling, M. S. , Crosby, R. D. , & van Strien, T. (2022). Dialectical behavior therapy compared to cognitive behavior therapy in binge‐eating disorder: An effectiveness study with 6‐month follow‐up. International Journal of Eating Disorders, 55(7), 902–913. 10.1002/eat.23750
Action Editor: Tracey Wade
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request
REFERENCES
- Aardoom, J. J. , Dingemans, A. E. , Landt, M. , & Van Furth, E. F. (2012). Norms and discriminative validity of the eating disorder examination questionnaire (EDE‐Q). Eating Behaviors, 13, 305–309. 10.1016/j.eatbeh.2012.09.002 [DOI] [PubMed] [Google Scholar]
- Abraham, S. F. , & Beumont, P. J. V. (1982). How patients describe bulimia or binge eating. Psychological Medicine, 12, 625–635. 10.1017/S0033291700055732 [DOI] [PubMed] [Google Scholar]
- Adams, J. R. , & Drake, R. E. (2006). Shared decision‐making and evidence‐based practice. Community Mental Health Journal, 42, 87–105. 10.1007/s10597-005-9005-8 [DOI] [PubMed] [Google Scholar]
- Alliantie Kwaliteit in de Geestelijke Gezondheidszorg; Akwa GGZ (2017). Zorgstandaard eetstoornissen [Standard of care for eating disorders]. Retrieved from https://www.ggzstandaarden.nl/zorgstandaarden/eetstoornissen.
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing. [Google Scholar]
- Arnow, B. , Kenardy, J. , & Agras, W. S. (1992). Binge eating among the obese: A descriptive study. Journal of Behavioral Medicine, 15, 155–170. 10.1007/BF00848323 [DOI] [PubMed] [Google Scholar]
- Arrindell, W. A. , & Ettema, J. H. M. (2003). SCL‐90, symptom checklist. Handleiding bij een multidimensionele psychopathologie‐indicator [manual for a multidimensional psychopathology indicator]. Swets Test Publishers. [Google Scholar]
- Barrada, J. R. , Van Strien, T. , & Cebolla, A. (2016). Internal structure and measurement invariance of the Dutch eating behavior questionnaire (DEBQ) in a (nearly) representative Dutch community sample. European Eating Disorders Review, 24, 503–509. 10.1002/erv.2448 [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. K. (1996). Beck depression inventory‐II (BDI‐II). Harcourt Assessment Inc. [Google Scholar]
- Berg, K. C. , Cao, L. , Crosby, R. D. , Engel, S. G. , Peterson, C. B. , Crow, S. J. , Le Grange, D. , Mitchell, J. E. , Lavender, J. M. , Durkin, N. , & Wonderlich, S. A. (2017). Negative affect and binge eating: Reconciling differences between two analytic approaches in ecological momentary assessment research. International Journal of Eating Disorders, 50, 1222–1230. 10.1002/eat.22770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, K. C. , Crosby, R. D. , Cao, L. , Crow, S. J. , Engel, S. G. , Wonderlich, S. A. , & Peterson, C. B. (2015). Negative affect prior to and following overeating‐only, loss of control eating‐only, and binge eating episodes in obese adults. International Journal of Eating Disorders, 48, 641–653. 10.1002/eat.22401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, K. C. , Peterson, C. B. , Frazier, P. , & Crow, S. J. (2012). Psychometric evaluation of the eating disorder examination and eating disorder examination‐questionnaire: A systematic review of the literature. International Journal of Eating Disorders, 45, 428–438. 10.1002/eat.20931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood, L. , Adams, G. , Turner, H. , & Waller, G. (2020). Group dialectical behavioral therapy for binge‐eating disorder: Outcomes from a community case series. International Journal of Eating Disorders, 53, 1863–1867. 10.1002/eat.23377 [DOI] [PubMed] [Google Scholar]
- Carey, M. , Kupeli, N. , Knight, R. , Troop, N. A. , Jenkinson, P. M. , & Preston, C. (2019). Eating disorder examination questionnaire (EDE‐Q): Norms and psychometric properties in UKfemales and males. Psychological Assessment, 31, 839–850. 10.1037/pas0000703 [DOI] [PubMed] [Google Scholar]
- Chen, E. Y. , Cacioppo, J. , Fettich, K. , Gallop, R. , McCloskey, M. S. , Olino, T. , & Zeffiro, T. A. (2017). An adaptive randomized trial of dialectical behaviour therapy and cognitive behavior therapy for binge‐eating. Psychological Medicine, 47, 703–717. 10.1017/S0033291716002543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, E. Y. , Matthews, L. , Allen, C. , Kuo, J. R. , & Linehan, M. M. (2008). Dialectical behavior therapy for clients with binge‐eating disorder or bulimia nervosa and borderline personality disorder. International Journal of Eating Disorders, 41, 505–512. 10.1002/eat.20522 [DOI] [PubMed] [Google Scholar]
- Clausen, L. , Rosenvinge, J. H. , Friborg, O. , & Rokkedal, K. (2011). Validating the eating disorder Inventory‐3 (EDI‐3): A comparison between 561 female eating disorders patients and 878 females from the general population. Journal of Psychopathology and Behavioral Assessment, 33, 101–110. 10.1007/s10862-010-9207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Erlbaum. [Google Scholar]
- COTAN (2013). Beoordeling Nederlandse Vragenlijst voor Eetgedrag, NVE [Review Dutch Eating Behaviour Questionnaire, DEBQ]. http://www.cotandocumentatie.nl/test_details.php?id=848. Accessed 20 Feb 2017.
- Dingemans, A. E. , Spinhoven, P. , & van Furth, E. F. (2007). Predictors and mediators of treatment outcome in patients with binge eating disorder. Behaviour Research and Therapy, 45, 2551–2562. 10.1016/j.brat.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Erb, S. , Farmer, A. , & Mehlenbeck, R. (2013). A condensed dialectical behavior therapy skills group for binge eating disorder: Overcoming winter challenges. Journal of Cognitive Psychotherapy, 27, 338–358. 10.1891/0889-8391.27.4.338 [DOI] [PubMed] [Google Scholar]
- Fairburn, C. G. (2008). Cognitive behavior therapy and eating disorders. Guilford Press. [Google Scholar]
- Fairburn, C. G. , Bailey‐Straebler, S. , Basden, S. , Doll, H. A. , Jones, R. , Murphy, R. , O'Connor, M. E. , & Cooper, Z. (2015). A transdiagnostic comparison of enhanced cognitive behaviour therapy (CBT‐E) and interpersonal psychotherapy in the treatment of eating disorders. Behaviour Research and Therapy, 70, 64–71. 10.1016/j.brat.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn, C. G. , & Beglin, S. J. (2008). Eating disorder examination questionnaire (EDEQ 6.0). In Fairburn C. (Ed.), Cognitive behavior therapy and eating disorders (pp. 309–313). Guilford Press. [Google Scholar]
- Fairburn, C. G. , Marcus, M. D. , & Wilson, G. T. (1993). Cognitive‐behavioral therapy for binge eating and bulimia nervosa: A comprehensive treatment manual. In Fairburn C. G. & Wilson G. T. (Eds.), Binge eating: Nature, assessment, and treatment (pp. 361–404). Guilford Press. [Google Scholar]
- Garner, D. M. , & Van Strien, T. (2015). EDI‐3. Inventarisatie van eetstoornissymptomen. Handleiding [EDI‐3. Inventory of eating disorder symptoms. Manual]. Hogrefe. [Google Scholar]
- Haedt‐Matt, A. A. , & Keel, P. K. (2011). Revisiting the affect regulation model of binge eating: A meta‐analysis of studies using ecological momentary assessment. Psychological Bulletin, 137, 660–681. 10.1037/a0023660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans, E. , & Hiller, W. (2013). Effectiveness of and dropout from outpatient cognitive behavioral therapy for adult unipolar depression: A meta‐analysis of nonrandomized effectiveness studies. Journal of Consulting and Clinical Psychology, 81, 75–88. 10.1037/a0031080 [DOI] [PubMed] [Google Scholar]
- Hawkins, R. C. , & Clement, P. F. (1984). Binge eating: Measurement problems and a conceptual model. In Hawkins R. C., Fremouw W. J., & Clement P. F. (Eds.), The binge purge syndrome: Diagnosis, treatment, and research (pp. 229–251). Springer. [Google Scholar]
- Hay, P. , Chinn, D. , Forbes, D. , Madden, S. , Newton, R. , Sugenor, L. , Touyz, S. , & Ward, W. (2014). Royal Australian and New Zealand college of psychiatrists: Clinical practice guidelines for the treatment of eating disorders. Australian and New Zealand Journal of Psychiatry, 48, 977–1008. 10.1177/0004867414555814 [DOI] [PubMed] [Google Scholar]
- IBM Corp . (2017). SPSS statistics for windows, version 25.0. IBM Corp. [Google Scholar]
- Jacobson, N. S. , & Truax, P. (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59, 12–19. 10.1037/0022-006X.59.1.12 [DOI] [PubMed] [Google Scholar]
- Jansingh, A. , Danner, U. N. , Hoek, H. W. , & Van Elburg, A. A. (2020). Developments in the psychological treatment of anorexia nervosa and their implications for daily practice. Current Opinion in Psychiatry, 33, 534–541. 10.1097/YCO.0000000000000642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, A. S. , Skinner, J. B. , & Hawley, K. M. (2012). Adapted group‐based dialectical behaviour therapy for binge eating in a practicing clinic: Clinical outcomes and attrition. European Eating Disorders Review, 20, 148–153. 10.1002/erv.2165 [DOI] [PubMed] [Google Scholar]
- Kraemer, H. C. , & Kupfer, D. J. (2006). Size of treatment effects and their importance to clinical research and practice. Biological Psychiatry, 59, 990–996. 10.1016/j.biopsych.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Lammers, M. W. , Vroling, M. S. , Crosby, R. D. , & Van Strien, T. (2020). Dialectical behavior therapy adapted for binge eating compared to cognitive behavior therapy in obese adults with binge eating disorder: A controlled study. Journal of Eating Disorders, 8, 27. 10.1186/s40337-020-00299-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers, M. W. , Vroling, M. S. , Crosby, R. D. , & Van Strien, T. (2021). Correction to: Dialectical behavior therapy adapted for binge eating compared to cognitive behavior therapy in obese adults with binge eating disorder: A controlled study. Journal of Eating Disorders, 9, 165. 10.1186/s40337-021-00515-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehr, E. J. , Krohmer, K. , Schag, K. , Dresler, T. , Zipfel, S. , & Giel, K. E. (2015). Emotion regulation model in binge eating disorder and obesity: A systematic review. Neuroscience and Biobehavioral Reviews, 49, 125–134. 10.1016/j.neubiorev.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Lehmann, V. , Ouwens, M. A. , Braeken, J. , Danner, U. N. , Van Elburg, A. A. , Bekker, M. H. J. , Breurkens, A. , & Van Strien, T. (2013). Psychometric properties of the Dutch version of the eating disorder inventory‐3. SAGE Open, 1–7. 10.1177/2158244013508415 [DOI] [Google Scholar]
- Linardon, J. , de la Piedad Garcia, X. , & Brennan, L. (2017). Predictors, moderators, and mediators of treatment outcome following manualised cognitive‐behavioural therapy for eating disorders: A systematic review. European Eating Disorders Review, 25, 3–12. 10.1002/erv.2492 [DOI] [PubMed] [Google Scholar]
- Linardon, J. , Fairburn, C. G. , Fitzsimmons‐Craft, E. E. , Wilfley, D. E. , & Brennan, L. (2017). The empirical status of the third‐wave behaviour therapies for the treatment of eating disorders: A systematic review. Clinical Psychology Review, 58, 125–140. 10.1016/j.cpr.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Linardon, J. , Messer, M. , & Fuller‐Tyszkiewicz, M. (2018). Meta‐analysis of the effects of cognitive‐behavioral therapy for binge‐eating–type disorders on abstinence rates in nonrandomized effectiveness studies: Comparable outcomes to randomized, controlled trials? International Journal of Eating Disorders, 51, 1303–1311. 10.1002/eat.22986 [DOI] [PubMed] [Google Scholar]
- Linehan, M. M. (1993). Cognitive‐behavioral treatment of borderline personality disorder. Guilford Press. [Google Scholar]
- McCoy, C. E. (2017). Understanding the intention‐to‐treat principle in randomized controlled trials. The Western Journal of Emergency Medicine, 18, 1075–1078. 10.5811/westjem.2017.8.35985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushquash, A. R. , & McMahan, M. (2015). Dialectical behavior therapy skills training reduces binge eating among patients seeking weight‐management services: Preliminary evidence. Eating and Weight Disorders, 20, 415–418. 10.1007/s40519-015-0177-0 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence [NICE] (2017). Eating disorders: recognition and treatment. https://www.nice.org.uk/guidance/ng69/chapter/Recommendations#treating-binge-eating-disorder [PubMed]
- Peterson, C. B. , Engel, S. G. , Crosby, R. D. , Strauman, T. , Smith, T. L. , Klein, M. , Crow, S. J. , Mitchel, J. E. , Erickson, A. , Coa, L. , Biorlie, K. , & Wonderlich, S. A. (2020). Comparing integrative cognitive‐affective therapy and guided self‐help cognitive‐behavioral therapy to treat binge‐eating disorder using standard and naturalistic momentary outcome measures: A randomized controlled trial. International Journal of Eating Disorders, 53, 1418–1427. 10.1002/eat.23324 [DOI] [PubMed] [Google Scholar]
- Peterson, C. B. , Mitchell, J. E. , Crow, S. J. , Crosby, R. D. , & Wonderlich, S. A. (2009). The efficacy of self‐help group treatment and therapist‐led group treatment for binge eating disorder. American Journal of Psychiatry, 166, 1347–1354. 10.1176/appi.ajp.2009.09030345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst, M. , Knapen, J. , Poot, G. , & Vancampfort, D. (2010). Psychomotor therapy and psychiatry: What is in a name. Open Complementary Medicine Journal, 2, 105–113. 10.2174/1876391X01002010105 [DOI] [Google Scholar]
- Safer, D. L. , & Joyce, E. E. (2011). Does rapid response to two group psychotherapies for binge eating disorder predict abstinence? Behaviour Research and Therapy, 49, 339–345. 10.1016/j.brat.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer, D. L. , Robinson, A. H. , & Jo, B. (2010). Outcome from a randomized controlled trial of group therapy for binge eating disorder: Comparing dialectical behavior therapy adapted for binge eating to an active comparison group therapy. Behavior Therapy, 41, 106–120. 10.1016/j.beth.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer, D. L. , Telch, C. F. , & Chen, E. Y. (2009). Dialectical behavior therapy for binge eating and bulimia. Guilford Press. [Google Scholar]
- Schaefer, L. M. , Smith, K. E. , Anderson, L. M. , Cao, L. , Peterson, C. , Engel, S. , Crosby, R. D. , & Wonderlich, S. A. (2020). The role of affect in the maintenance of binge‐eating disorder: Evidence from an ecological momentary assessment study. Journal of Abnormal Psychology, 129, 387–396. 10.1037/abn0000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, J. L. (1987). Analysis of incomplete multivariate data. Chapman & Hall. [Google Scholar]
- Segura‐García, C. , Aloi, M. , Rania, M. , Ciambrone, P. , Palmieri, A. , Pugliese, V. , Moruno, J. J. R. , & De Fazio, P. (2015). Ability of EDI‐2 and EDI‐3 to correctly identify patients and subjects at risk for eating disorders. Eating Behaviors, 19, 20–23. 10.1016/j.eatbeh.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Seligman, M. E. (1995). The effectiveness of psychotherapy: The consumer reports study. American Psychologist, 50, 965–974. 10.1037/0003-066X.50.12.965 [DOI] [PubMed] [Google Scholar]
- Sivyer, K. , Allen, E. , Cooper, Z. , Bailey‐Straebler, S. , O'Connor, M. E. , Fairburn, C. G. , & Murphy, R. (2020). Mediators of change in cognitive behavior therapy and interpersonal psychotherapy for eating disorders: A secondary analysis of a transdiagnostic randomized controlled trial. International Journal of Eating Disorders, 53, 1928–1940. 10.1002/eat.23390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, R. E. , & Chambless, D. L. (2009). Cognitive–behavioral therapy for adult anxiety disorders in clinical practice: A meta‐analysis of effectiveness studies. Journal of Consulting and Clinical Psychology, 77, 595–606. 10.1037/a0016032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telch, C. E. , Agras, W. S. , & Linehan, M. M. (2000). Group dialectical behavior therapy for binge‐eating disorder: A preliminary, uncontrolled trial. Behavior Therapy, 31, 569–582. 10.1016/S0005-7894(00)80031-3 [DOI] [Google Scholar]
- Telch, C. F. , Agras, W. S. , & Linehan, M. M. (2001). Dialectical behavior therapy for binge eating disorder. Journal of Consulting and Clinical Psychology, 69, 1061–1065. 10.1037//0022-006X.69.6.1061 [DOI] [PubMed] [Google Scholar]
- Turner, H. , Marshall, E. , Wood, F. , Stopa, L. , & Waller, G. (2016). CBT for eating disorders: The impact of early changes in eating pathology on later changes in personality pathology, anxiety and depression. Behaviour Research and Therapy, 77, 1–6. 10.1016/j.brat.2015.11.011 [DOI] [PubMed] [Google Scholar]
- Van Strien, T. , Frijters, J. , Bergers, G. , & Defares, P. (1986). The Dutch eating behavior questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders, 5, 295–315. [DOI] [Google Scholar]
- Vanderlinden, J. , Grave, R. D. , Fernandez, F. , Vandereycken, W. , Pieters, G. , & Noorduin, C. (2004). Which factors do provoke binge eating? An exploratory study in eating disorder patients. Eating and Weight Disorders, 9, 300–305. 10.1007/BF03325086 [DOI] [PubMed] [Google Scholar]
- Wonderlich, S. A. , Peterson, C. B. , Crosby, R. D. , Smith, T. L. , Klein, M. H. , Mitchell, J. E. , & Crow, S. J. (2014). A randomized controlled comparison of integrative cognitive affective therapy (ICAT) and enhanced cognitive‐behavioral therapy (CBT‐E) for bulimia nervosa. Psychological Medicine, 44, 543–552. 10.1017/S0033291713001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request


