Abstract
Reproductive physiology and immunology as scientific disciplines each have rich, largely independent histories. The physicians and philosophers of ancient Greece made remarkable observations and inferences to explain regeneration as well as illness and immunity. The scientific enlightenment of the renaissance and the technological advances of the past century have led to the explosion of knowledge that we are experiencing today. Breakthroughs in transplantation, immunology, and reproduction eventually culminated with Medawar’s discovery of acquired immunological tolerance, which helped to explain the transplantation success and failure. Medawar’s musings also keenly pointed out that the fetus apparently breaks these newly discovered rules, and with this, the field of reproductive immunology was launched. As a result of having stemmed from transplantation immunology, scientist still analogizes the fetus to a successful allograft. Although we now know of the fundamental differences between the two, this analogy remains a useful tool to understand how the fetus thrives despite its immunological disparity with the mother. Here, we review the history of reproductive immunology, and how major and minor histocompatibility antigens, blood group antigens, and tissue‐specific “self” antigens from the fetus and transplanted organs parallel and differ.
Keywords: AIRE, antigens, fetus, historical perspective, immunology, placenta, pregnancy
1. INTRODUCTION
Since Peter Medawar famously asked how the fetus manages to thrive despite its semi‐allogeneic relationship to the mother, 1 scientists have compared the fetus with a transplanted graft because of the paternal half of its genetic makeup and the intimate relationship between maternal and fetal tissues. Work of reproductive immunologists over the past 70 years has revealed that while transplanted tissues must overcome or evade a host immune system, viviparity and immunity co‐evolved rather than competed. Nonetheless, the pathophysiology of transplantation remains a useful comparator to understand how the fetus and maternal immune system coexist and further, how the fetus and placenta coopt maternal immune cells to promote implantation, placentation, and fetal growth.
Our grasp of the immunology of pregnancy has itself evolved, and questions were launched only after fundamental discoveries in each field occurred separately. In this article, we review the relationship between pregnancy and the maternal immune system by paralleling the histories of reproduction and of immunology, outlining how reproductive immunology emerged as a scientific field of its own (Figure 1). We discuss how the recent histories of reproduction and transplantation immunology are inextricably linked, and how fetal antigens both correspond and differ with those relevant to transplantation.
FIGURE 1.
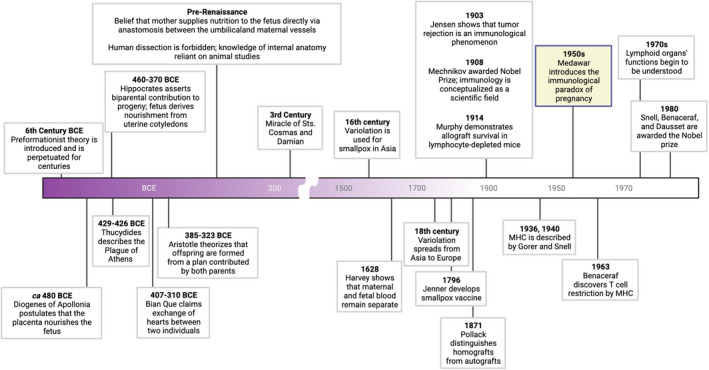
Timeline of seminal theories, observations, and discoveries in immunology and reproductive physiology that led to the development of reproductive immunology as a scientific field
2. EARLY PERCEPTIONS OF REPRODUCTION AND IMMUNOLOGY
Since ancient times, how animals reproduce has marveled scientists and philosophers. Philosophers and physicians of ancient Greece embraced preformationism, one of the earliest documented theories of how reproduction and embryological development occurrs. In this theory, animals reproduce via “animalcules” (or for humans, “homonculi”)—miniature versions of offspring that were thought to preexist in semen. According to Pythagoras (c. 570 – c. 495 BC), animalcules circulate throughout the male body to gather specific traits of the father to be passed on. The idea that the “seed” or “vitality” of the next generation came from the male dominated for much of ancient history; the mother’s womb was believed merely to serve as a vessel for growth of the tiny offspring.
Hippocrates' (460–370 BC) ideas on reproduction challenged this view: he asserted that two seeds are required for regeneration, one each from the male and the female. 2 He argued that only this could explain the resemblance of children to both parents. Later, Aristotle (385–323 BC) pointed out additional flaws of preformationism: first, the notion that animalcules circulate to gather characteristics of the father contradicts obvious anatomical and physiological differences between men and women. Second, if homunculi are preformed, they must be so ad infinitum; yet Aristotle believed that infinity only exists in theory. Instead, he offered another view—an idea that turned out to be fundamentally correct: he reasoned that offspring form according to a “plan” that both parents contributed. This plan is what we now know as the genetic code.
Meanwhile, the first documented observation of physiological immunity (from the Latin immunus, meaning “exempt from public service”) came from Thucydides' description of the Plague of Athens (429–426 BC). Interestingly, Thucydides was an Athenian general and historian who made a number of critical scientific observations. The “plague” he described was more likely smallpox than the bubonic plague; nonetheless, his observations proved foundational. He noticed that “… the disease did not attack the same person a second time, or at any rate not fatally.”. He also commented that some who recovered even believed that they would be protected from other diseases. Shrewdly, Thucydides thought this notion callow, unknowingly giving testimony to immunological specificity. 3
Some 2000 years later, the first intentional exposure to disease for preventative purposes—variolation—was documented. Variolation was used to prevent smallpox in 16th century China, India, and the Ottoman Empire; its origins remain obscure, however, and this practice may have been used for hundreds of years prior. 4 From its use in Asia, variolation spread into Europe and the United Kingdom, and became accepted medical protocol by the 18th century. Unfortunately, lack of standardization meant that it sometimes led to fulminant infection and death, although it still did so less commonly than natural infection. The scientific basis for variolation was not understood, but it set the stage for Jenner’s development of the vaccine using the cowpox virus. Pasteur and others then developed additional vaccines against other debilitating, life‐threatening diseases, and advanced the understanding of their effectiveness.
While protection from disease by the growing practice of vaccination was realized at the time, how it drove immunity was not. At the time, the major theoretical basis explain physiology, health, and illness was the four humors: blood, yellow bile, black bile, and phlegm. This idea dominated our understanding of physiology from ancient Greek times to well into the second millennium AD. The ancients thought the thymus to be the “seat of the soul” and was described by Galen as an “organ of mystery” that purified the nervous system 5 ; its function as the source of T cells was not comprehended until the 1970's. 6 Bone marrow was thought to nourish bone (Hippocrates, Galen) or consist of waste (Aristotle), 7 and the spleen to be the source of black bile. 8 Remarkably, the lymphatic system was correctly described to contain fluid, and both the spleen and lymph nodes to enlarge in pathological states. 9 Clearly, however, there was little understanding of the functions of these organs, and understandably, no awareness of immune cells.
Similarly, the embryological development and purpose of the placenta remained mysterious throughout most of written history. The Greek philosopher‐physicians thought the placenta was something of an alter ego or an external soul to the child. (Some communities retain similar beliefs today, which must continue to be respected when considering the use of the placenta for scientific purposes.) Diogenes of Apollonia (ca 480 BC) was the first in recorded history to postulate that the fetus derives nourishment from the placenta; others believed that nutrition was obtained from the amniotic fluid. 10 Hippocrates, on the other hand, held the more widely believed view that the fetus feeds and respires through suckling of uterine cotyledons; this theory may have included the belief that the umbilical vessels were connected to the uterus and in turn, the breasts.
Until the Renaissance, both church and government forbade dissection of cadavers, which hindered progress in human medicine for millennia. Physicians relied instead on dissection of animals to understand internal organs, and thus sometimes failed to appreciate major differences between the reproductive systems of animals and humans. This is infamously depicted in da Vinci’s depiction of the fetus in the womb, which, reminiscent of Hippocrates’ opinions, shows a human fetus with a bovine cotyledonary placenta —presumably the only resource available to him (Figure 2). 11
FIGURE 2.
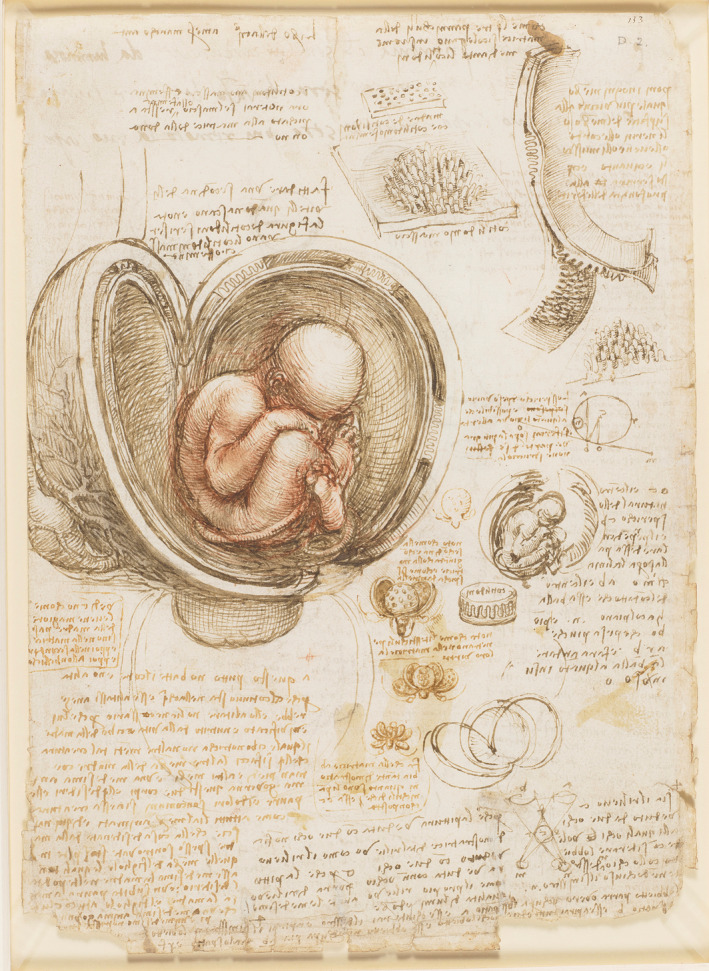
Fetus in the Womb, by Leonardo da Vinci. Da Vinci depicts a cotyledonary placenta characteristic of ruminants in the central drawing as well as in smaller studies to the lower and upper right. Used with permission, Royal Collection Trust/© Her Majesty Queen Elizabeth II 2022
Interestingly, da Vinci correctly concluded from these dissections that fetal and maternal vessels remain separate—an issue that contradicted centuries of belief that they fuse, and one that would not be resolved for another 100 years. Using the scientific method for the first time, J.C. Arantius (1530–1589) and William Harvey (1578–1657) showed, independently, that maternal and fetal blood remain separate—first by exsanguination of dogs, and then by perfusion of the human placenta. 12 We now understand from an immunological standpoint that intermixing of maternal and fetal blood would precipitate a significant and dangerous immune response in some patients.
Because of the disseminated nature of the immune system, the lack of appropriate technology, and the inability to link distinct organs with immunologic function, advances in gross anatomy and microscopy had a lower impact on immunologic discovery than in other areas. Thus, immunology remained undesignated as a scientific field until Mechnikov received the Nobel Prize in 1908 for his observations on phagocytes. 13 Nonetheless, physicians long savored the idea that grafting and transplantation could save life and limb. The Chinese physician Bian Que (407–310 B.C.) is said to have cured two patients, one of low “intellectual ability” and one of low “willpower”, by exchanging hearts between the two, and legend tells that the two men woke up “as good as new” (Bian Que is also credited to have invented anesthesia). Later, the Catholic Sts. Cosmas and Damien posthumously appeared to a church verger and replaced his cancerous leg with that of a deceased Moor, a “miracle” that inspired numerous Gothic‐ and Renaissance‐era paintings (Figure 3).
FIGURE 3.

Verger’s dream: Saints Cosmas and Damian performing a miraculous cure by transplantation of a leg. Oil on wood painting attributed to the Master of Los Balbases, ca. 1495. Wellcome Collection; Public Domain
These accounts may or may not have been actual attempts at transplantation. Regardless, neither physicians nor laymen could have been aware of why these or similar operations would have invariably failed. But because the stakes were high, surgeons persisted. What must have been persistent failure of homografts—grafts between two individuals—was either overlooked or disregarded until the late in the 19th century. In 1871, George Pollack (St. George’s Hospital in London) made the seminal observation that in the same patient, autografts could be successful but homografts would fail. Unfortunately, Pollack’s report escaped noticed by the scientific community—as did other important observations. In 1903, Carl Jensen, a veterinary surgeon at the Royal Veterinary and Agricultural College in Denmark, reported that failure of tumors transplanted between mice is an immunological phenomenon. This conclusion was discounted, however, as no antibody was evident; at the time, this was the hallmark of immunity. 14 Further, lymphocytes were thought to be stationary—counter to the idea that they could infiltrate tissues. Eventually, however, the role of lymphocytes was brought to light by James B. Murphy (Rockefeller Institute), who showed that allogeneic tumors survived indefinitely in lymphocyte‐depleted mice. 15 In the 1930's, Leo Loeb further showed that the rate at which homografts disappeared correlated with the genetic distance between the donor and recipient. 14 Under Loeb’s influence, Brown, and Padgett showed that identical twins accepted each other’s skin grafts. 14
Clarence Little, who founded the Jackson Laboratory, promoted the idea that if sufficiently inbred, grafts between mice could be interchanged. 16 Soon afterward, Peter Gorer and George Snell solidified this idea using congenic mice, narrowing down the major histocompatibility complex (MHC) genes as the locus responsible for inter‐strain graft failure in congenic mice. 17 , 18 Experiments by Baruj Benaceraf revealed that T cells are restricted by the MHC, finally explaining involvement of lymphocytes in graft rejection. 19 This was followed by Jean Dausset’s discovery of the human MHC locus (human leukocyte antigens, HLA). 20 Together, Snell, Benaceraf and Dausset received the Nobel prize “for their discoveries concerning genetically determined structures on the cell surface that regulated immunological reactions.”
The discovery of the MHC coincided with those of acquired immunity and transplantation tolerance by Peter Medawar. Medawar was trained as a zoologist but was commissioned by the Medical Research Council in London to study why skin grafts so often fail, in the hopes for improved treatment of World War II burn victims. 21 , 22 Prompted by a colleague and Brown and Padgett’s discovery about transplantation in twins, Medawar tested the idea that monozygotic and dizygotic cattle twins could be distinguished by skin grafting. The results were startling, however: twin cattle who were clearly dizygotic as assessed by phenotyping usually accepted each other’s grafts—even when of opposite sex. 23
How could this be explained? Medawar ingeniously considered prior findings by Owen that dizygotic twin cattle share circulating blood via chorionic vascular anastomosis. 24 He further looked to Burnet and Fenner’s theory that animals can acquire tolerance to each other’s antigens if exposed early enough, during embryonic life. 25 Reflecting on these contributions, Medawar and colleagues reasoned that sharing of blood must also mean sharing of antigens, and further, that there must be a period during fetal life in which immunological tolerance to foreign antigens can be acquired. He tested the idea directly by inoculating mouse fetuses with genetically dissimilar cells, then transplanting tissue from the donor. This celebrated experiment confirmed the hypothesis and laid the foundation for the sub‐discipline of immunological tolerance to antigens. 26
Medawar went on to contribute significantly to the science of transplantation, immune tolerance, cancer immunology, and senescence and ageing. He also had much to say about pregnancy, fetal cells and antigens, and their similarity to cancer cells. In both his musings and his science, he launched the field of reproductive immunology, wondering, after having discovered that mature individuals could not sustain a graft without prior actively acquired tolerance, how, during pregnancy, the mother can tolerate the fetus for a full nine months? 1 Medawar proposed three possibilities: the fetus could be sequestered from the maternal immune system; the mother could be immunologically suppressed; or the fetus could be antigenically immature—immunologically invisible to the mother.
All three mechanisms Medawar proposed to keep the fetus safe from the maternal immune system turn out to be true, but only partially. To understand how the fetus and maternal immune system, it remains useful to keep in mind the mechanisms of transplant rejection, and how the fetal‐placental unit differs. In the following section, we compare the basic mechanisms of homograft transplantation and failure, to implantation, and placentation, with a focus on antigens that mediate graft failure, and how these same antigens coming from the fetus are tolerated by the maternal immune system in pregnancy.
3. SELF, NON‐SELF, AND DANGER IN TRANSPLANTATION AND PREGNANCY
As the field of immunology grew, the Self/Non‐Self model of immunology dominated our understanding into the 1990's. We now know this model to be fundamentally flawed, as it asserts that the immune system is indiscriminately triggered by any non‐self entity. Janeway explained that the immune system distinguishes between “non‐infectious self” and “infectious non‐self” by recognizing conserved pathogen‐associated molecular patterns (PAMPS) borne by pathogens. 27 Matzinger expounded on this idea, pointing out that immune theory must also account for survival of both “harmful self” (e.g., cancer), and “harmless non‐self” (e.g., commensal bacteria, the fetus). 28 Matzinger’s Danger theory states that the immune system should, at least leave certain foreigners, such as the fetus, alone; at best, it should promote the establishment and growth of self, the fetus, and transplants. 29
3.1. Early processes in transplantation: inflammation
How does transplantation and pregnancy fit into these fundamental tenets of immunology? Both pregnancy and transplantation involve the entry of a foreigner past a first line of defense, for example, implantation of the embryo beneath the uterine epithelium. In transplantation, tissue damage is caused iatrogenically, unavoidably, and often extensively. Upon death or brain death of donors, organs hastily release an abundance of danger‐associated molecular patterns (DAMPs), such as high‐mobility group box 1, heat shock proteins, nucleic acids, extracellular adenosine triphosphate, uric acid, and reactive oxygen species. DAMPs ligate pattern recognition receptors (PRR)—the same receptors that recognize PAMPs—on epithelial, stromal, and resident immune cells. Ischemia and reperfusion of the organ further exacerbate DAMP release. 30 Extensive PRR ligation quickly triggers inflammation and, importantly, maturation of antigen presenting cells in the host, as well as passenger antigen presenting cells in the donated tissue.
In the absence of pathogens and foreign antigens, the DAMP‐initiated, “sterile” inflammatory response normally resolves with wound repair; exceptions may occur in cases of autoimmune disease. However, as explained below, various types of donor antigens, including blood group antigens, MHC, and minor histocompatibility antigens, dictate the ensuing adaptive response, and ultimately the outcome, of transplantation.
3.2. Inflammatory processes and introduction of antigens in pregnancy
Pregnancy in women—and all animals with invasive (hemochorial or endotheliochorial) placentation—unavoidably involves introduction of foreign material (sperm, seminal fluid), a barrier breach (implantation into uterine epithelium), and expression of foreign, paternally‐inherited antigens. In addition, the fetus and placenta express tissue‐specific antigens that the mature, gravid female lacks. From the start, however, the maternal immune reaction to fetal antigens differs fundamentally from that of transplanted organs.
Sperm lack MHC molecules and therefore cannot serve as a direct target of T cells. However, sperm cells do possess highly cell‐specific antigens that females mostly lack. Further, other cellular and soluble seminal fluid constituents contain both MHC and minor histocompatibility antigens. 31 , 32 In men, vasectomy, trauma, or infection are associated with tissue damage, breach of the blood testes barrier, production of DAMPs, and leakage of antigenic sperm proteins into the circulation. Despite these antigens belonging to self, they are perceived as foreign, and anti‐sperm antibodies develop and can trigger autoimmune‐mediated infertility. 33
Scientists have long held that sperm‐specific antigens are not only responsible for inciting anti‐sperm antibodies in males, but also have the potential to do so in females. Anti‐sperm antibody responses in women are associated with infertility, but this occurs in only rarely. 34 On the other hand, insemination represents the initial introduction of paternal (sperm) antigens into the female genital tract, and there produces a strong, but physiological, inflammatory response. This response likely serves multiple functions: to protect against incidental pathogens transferred during coitus, to clear excess sperm that does not reach the oviduct, and to prime the adaptive immune system for tolerance.
Directional motility allows sperm to vacate the vaginal canal quickly to avoid the pathogen‐averse acidic environment, and possibly also to avoid prolonged opportunity for an anti‐sperm immune response to develop. 35 Sperm reaching the uterus enter uterine glands where they rapidly recruit inflammatory cells and induce production of cytokines and prostaglandins. This reaction of endometrial tissue to sperm and seminal fluid is conserved across species, 36 attesting to its importance. Interestingly, sperm and/or seminal fluid components co‐opt the same pattern recognition receptors used by DAMPs and PAMPs to signal danger, including TLR2 and TLR4. 37 , 38 , 39 , 40 Expression of these receptors may be regulated by ovarian steroids, supporting a physiological role for inflammation‐induced insemination. Thus, the signals for activating these receptors arise not from tissue damage during coitus, but intrinsically from semen. Ultimately, excess sperm are phagocytosed neutrophils. 37
Studies in mice and women also show that sperm and seminal fluid also prompts an adaptive immune response, with influx of macrophages, dendritic cells, and effector (CD45RO+) CD8+ T cells. 41 This reaction, which is likely delayed relative to the rapidly induced innate response, may establish incipient events of antigen‐specific immune tolerance to gestational antigens. 42 Antigens in the seminal fluid can be presented by dendritic cells, 43 priming maternal T cells to differentiate into regulatory T cells (TReg), which are indispensable for tolerance to embryonic antigens. 44 , 45 Seminal fluid helps mediate these events, and although it is not required for successful pregnancy, data in women suggest that in vitro fertilization outcome is improved with exposure to seminal fluid around the time of embryo transfer. 46 Thus, despite the foreign antigens and inflammatory responses introduced during mating, the immunological events following coitus appear to promote tolerance, not immunization, to sperm‐associated antigens.
3.3. Implantation
Several days after fertilization, the semi‐allogeneic blastocyst positions itself in uterus for implantation. Trophoblast cells of the trophectoderm attach to and penetrate the epithelium, allowing the embryo to deeply invade the endometrium. This process generates prostaglandins, leukotrienes, histamines, cytokines, and chemokines, and vascular leakage—in other words, more inflammation. 47 , 48 , 49 This inflammatory reaction is beneficial and possibly required for implantation: use of anti‐inflammatory medications in women is associated with increased risk of miscarriage. 50 , 51 Conversely, mild endometrial wounding promotes implantation in women undergoing assisted reproduction technologies. 52
The endometrium responds to inflammation promoted by the implanting embryo in a highly distinct manner. Together with hormonal priming of the uterus, the implanting embryo triggers endometrial decidualization, in which maternal macrophages and dendritic cells immune cells play a compulsory role. Macrophages, which comprise more than 20% of cells at the implantation site, 53 likely serve to rapidly clear and prevent excessive release of danger signals from maternal cells that die in the process. 54 , 55 Further, macrophages of the M2 phenotype may promote decidualization through regulation of the Wnt/β‐catenin pathway. 56 Dendritic cells are also required for implantation, possibly by promoting angiogenesis through sFlt‐1 and TGF‐β production. 57
Finally, the inflammatory process of decidualization negatively feeds back by ultimately shutting down implantation‐associated inflammation, thereafter driving an anti‐inflammatory profile. 58 This is critical, as excessive inflammation damages the embryo and causes implantation failure. 59 This anti‐inflammatory state appears to dominate pregnancy following the implantation period until partrurition. 60
4. ANTIGENS IN TRANSPLANTATION AND PREGNANCY
Expression of fetus‐derived antigens drives our interest in the question of the fetal semi‐allograft; after all, only in the absence of foreign antigen are grafts accepted without immune suppression. The types of antigens carried by transplanted organs and cells determines the nature of the immune response, which can be mediated by antibodies or cytotoxic T cells, with assistance from helper T cells necessary for both. In pregnancy, the maternal immune system also responds via antibodies and T cells specific for fetal antigens, but in healthy pregnancy, with tolerizing rather than immunizing outcomes. As in transplantation, fetal‐placental antigens that alert the maternal immune system include blood group antigens, MHC antigens, and minor histocompatibility antigens. In addition, new lines of evidence suggest the importance of maternal tolerance to “former self” antigens—antigens whose expression is restricted to the fetus and placenta, that mother herself expressed through her own former existence as a fetus (Figure 4).
FIGURE 4.
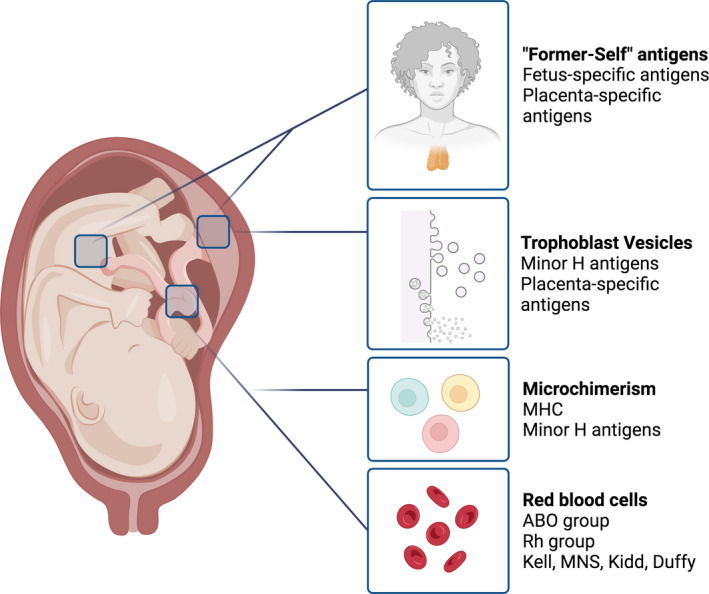
Fetal antigens include paternally‐inherited MHC and minor histocompatibility (H) antigens, blood group antigens, and fetus/placenta‐specific antigens that may be recognized as “former self” by the maternal immune system. These antigens access maternal blood and tissues through fetal microchimerism, placental shedding, and intermixing of maternal and fetal blood at childbirth
4.1. Blood group antigens
Hemolytic disease of the newborn (HDN) is perhaps the only definitive instance in which a fetal antigen can elicit a strong, dangerous maternal immune response that culminates in “fetal rejection.” Encoded by the RHD gene, Rh is critical to membrane structure in red blood cells, and because it is polymorphic, antigenic, and prominently expressed on fetal erythrocytes, is of considerable concern. In transplantation, RhD‐positive donor organs can cause delayed hemolysis, and in pregnancy, HDN—particularly in a second, but possibly in a first pregnancy.
In 1941, scientists first documented that erythroblastosis fetalis or HDN resulted from isoimmunization of RhD‐negative mothers by RhD‐positive fetuses, and subsequent passage of anti‐Rh antibodies across the placenta. These findings were facilitated by Erlich’s work, which established the role of antibodies in immune reactions. 61 Interestingly, this cause of HDN was realized nearly two decades before the function of T cells was known and Medawar introduced the immunological paradox of pregnancy. 62 Fortunately, as soon as the early 1960's, clinical treatment that essentially prevents the disease became available; Rh‐associated HDN remains, however, problematic in underdeveloped countries. 63 , 64
Unlike Rh antibodies, which occur only after transfusion or pregnancy, anti‐ABO group antibodies form at an early age in response widespread antigens in the environment similar to AB antigens. In transplantation, anti‐AB antibodies in incompatible ABO individuals trigger immediate and dangerous hyperacute rejection. Because these antibodies are IgM, they do not cross the placenta from mother to fetus. However, exposure to A or B antigens through transfusion fuels IgG production, which does cross the placenta and can cause mild HDN. This type of HDN is in general not risky for several reasons. First, AB‐mismatched transfusions and transplants are entirely preventable. Second, fetal red blood cells express lower amounts of surface AB than adult red blood cells, 65 limiting the number of antibodies that bind. Finally, the fetus expresses A/B antigens more widely, 66 effectively diluting the antibody targets.
While the blood group antigens MNS, Kidd, and Duffy rarely cause HDN, 66 Kell antigens serve as the third most common cause of blood group antigen‐associated newborn anemia. 67 Like Rh antibodies, anti‐Kell IgG antibodies are associated with prior transfusion and parity, and can cross the placenta. 68 , 69 , 70 Because Kell is expressed early on in erythropoiesis, anti‐Kell antibodies do not cause hemolysis but rather suppress growth of fetal erythroid precursors. 71 , 72 Relative incidence of this complication has risen since treatment for Rh incompatibility became available; fortunately, it remains rare. 73
4.2. MHC antigens
The highly polymorphic MHC class I and class II molecules are a substantial barrier to successful transplantation. Alloreactive T cells (those that recognize non‐self MHC) occur in individuals at very high rates—up 7% of all T cells—due to degeneracy and/or polyspecificity of T cell receptors for peptide–MHC complexes. 74 , 75 Because of this, failure to match donor and recipient MHC causes acute rejection of transplanted cells or tissues over days to weeks. In humans, clinicians attempt to match six MHC molecules: the class I molecules HLA‐A, ‐B, and ‐C; and the class II molecules HLA‐DP, ‐DQ, and ‐DR. Matching for all six antigens presents a challenge, but the relatively low number of antigens makes it feasible.
At first glance, paternally inherited MHC might seem problematic to pregnancy, particularly as the placenta is awash with maternal blood and lymphocytes for six or more months of pregnancy. JJ Van Rood first reported paternal MHC‐reactive antibodies in maternal serum in 1958, 76 and it is now known that as many as 30–40% of women develop antibodies to paternally inherited MHC. This proportion increases with parity and depends on paternal and maternal MHC haplotype. 77 , 78 Further, cytotoxic T cells against paternal MHC also arise during pregnancy, and can persist for years. 79
Display of fetal MHC molecules in the placenta was the among earliest questions to arise in reproductive immunology after their discovery and Medawar’s findings. The chorion functions as the placental interface between maternal and fetal tissues, and consists of an unbroken outer shell of trophoblast cells and underlying mesoderm. 80 Thus, Medawar’s fetal sequestration hypothesis is partially true: these layers establish an immunological barrier in two respects. First, potentially alloreactive fetal class I and class II MHC molecules reside entirely behind the trophoblast barrier. Second, trophoblast cells are equipped with immunosuppressive mechanisms: surface‐associated and secreted immunomodulatory mediators.
Faulk and others recognized early on that the syncytiotrophoblast, the layer of trophoblast that is bathed in maternal blood, lacks class I and class II MHC, thereby suggesting a mechanism by which the placenta escapes maternal immunosurveillance. 81 , 82 , 83 Because trophoblast cells (epigenetically) 84 , 85 , 86 , 87 repress expression and induction of polymorphic class I and class II MHC molecules, these cells can serve as neither a source of HLA antigens nor a target of maternal anti‐HLA responses in normal pregnancy. MHC repression in trophoblast cells is widely believed to be critical for immunological evasion. Surprisingly, two studies contradict this idea: in mice, targeted expression of paternal MHC in the placenta failed to affect fetal viability, despite inciting maternal alloreactivity. 88 , 89
While syncytiotrophoblast in the human placenta lacks MHC, extravillous trophoblast cells express certain MHC molecules that modulate activity of maternal immune cells for the benefit of fetal survival. Expression, regulation, and function of the unique trophoblast MHC has been extensively reviewed, 90 , 91 , 92 and can be summarized as follows:
Extravillous trophoblast cells express non‐polymorphic class Ib molecules, HLA‐E, ‐F, and ‐G, and the polymorphic class Ia molecule, HLA‐C.
Each of the trophoblast‐specific HLA molecules serve as ligands for receptors on uterine NK cells, and can inhibit their cytotoxic function.
Trophoblast HLAs and their alternatively spliced isoforms may also inhibit T‐cell proliferation and maturation of dendritic cells. 93 , 94
Trophoblast HLAs also stimulate uterine NK cell growth factors that promote spiral artery remodeling, which is critical for maternal vascular support of the growing placenta and fetus. 95
Imbalanced expression and genetic polymorphisms of class I and class II MHC expression by trophoblast cells, and their receptors by uterine leukocytes, have been implicated in pregnancy complications in women. 96 , 97 , 98 , 99 , 100 , 101 , 102
4.3. Minor histocompatibility antigens
A third hurdle to successful transplantation involves differences in minor histocompatibility (H) antigens between donor and recipient. Minor H antigens, which mediate chronic graft rejection, are peptides derived from normal self proteins that arise because of non‐synonymous genetic polymorphisms between individuals. If these polymorphisms result in peptides that can be presented by host class I and/or class II MHC molecules, cytotoxic and helper T cells can respond—particularly if PAMPS or DAMP danger signals are present.
In hematopoietic stem cell transplantation, recipients undergo conditioning radiation and/or chemotherapy, which deplete host hematopoietic cells. This reduces incidence of graft rejection and creates a niche for the donor cells. However, donor lymphocytes can still recognize host minor H antigens, which poses a significant risk for graft‐versus‐host disease. Although immune reactions to minor antigens are weaker and slower than alloresponses against MHC, they often necessitate long‐term immunosuppression in recipients.
The “male” antigens encoded on the Y chromosome are classic examples of minor H antigens, and cause rejection of male to female grafts in an otherwise matched transplantation scenario. However, most minor antigens are autosomally encoded: there are more than 100 known minor antigens, and likely hundreds or even thousands yet undiscovered 103 —far too many to match in the clinic.
Minor H antigens are expressed ubiquitously, including in the placenta. 104 Unlike MHC, minor antigens are abundantly expressed in the human placenta, including in the syncytiotrophoblast and extravillous trophoblast. Studies in mice revealed that both class I‐ and class II‐restricted minor H antigens expressed by the fetus, including male antigens, can escape the into the maternal circulation and prime maternal T cells. 105 , 106 This occurs through cross‐presentation of antigens that escape into the maternal circulation by maternal antigen presenting cells, 107 in much the same way donor antigens are cross‐presented by host antigen presenting cells in transplantation. These studies have been corroborated in women; such as maternal anti‐MHC‐specific T cells, minor H antigen‐specific T cells can persist as memory cells in vivo for years following pregnancy. 108 , 109 , 110 , 111
4.4. How do fetal antigens access the maternal immune system?
The hemochorial arrangement of the human (and model rodent) placenta facilitates antigen access to the maternal immune system, particularly when maternal blood flow to the placenta is established. Antigens gain access to maternal antigen presenting cells in lymphoid organs as they are released from the placenta into the maternal circulation. There are at least three mechanisms by which fetal antigens access the maternal lymphoid system: by direct expression and release from trophoblast, through release via extracellular vesicles, and via fetal microchimerism. A fourth possibility exists and warrants further exploration: through genetic alteration of maternal cells to express fetally‐derived genes.
The syncytiotrophoblast, which is inundated with maternal blood for two‐thirds of pregnancy, provides an exceptionally large surface area 112 from which fetal antigens are expressed and can escape directly into maternal circulation. This cell layer also releases large quantities of extracellular vesicles that carry minor antigens together with known modulators of maternal immune cells such as PDL1, FasL, and TRAIL. 104 , 113 , 114 , 115 , 116 , 117 These modulators, whether associated with trophoblast cells or the extracellular vesicles they release, likely modulate maternal immunoreactivity toward the fetus, and theoretically have the potential to reach any vascularized organ in the mother.
Although in vivo information remains sparse on how placental vesicles modulate the maternal immune system, they do access maternal lung macrophages, which can serve as antigen presenting cells. 118 Surprisingly, vesicles seem to access lymphoid organs only rarely. 118 , 119 Since fetal antigens are presented to and detected by T cells within lymphoid organs, it is possible that free, rather than vesicle‐associated, antigens are important in priming maternal T cells. 120
Fetal cells also actively traffic into and lodge within the mother during pregnancy, and persist there for many years—even decades. 121 Even as early as the first trimester, microchimeric fetal cells can transfer into maternal blood across all of pregnancy. Fetal microchimerism is often cited to provide fetal antigen that elicits maternal T cell and antibody responses. 111 , 122 , 123 It seems likely that this is true, particularly in the case of alerting maternal immune cells to paternally‐inherited fetal MHC, 124 which the trophoblast lacks.
Another possible source of fetal antigen arises from extracellular vesicles that carry fetal genetic material. Lotvall et al. 125 showed that exosomes can transfer and express mRNA from donor cells to targets via exosomes. Further, studies using model proteins and reporters demonstrated that vesicles could transfer mRNA between oligodendrocytes and neurons 126 ; between immune cells and Purkinje neurons 127 ; between tumor and host cells; 128 , 129 and between individual tumor cells. 129 In these studies, exosomes from Cre‐expressing cells carry Cre mRNA into reporter target cells, which then translated the message into functional protein. As a result, Cre recombinase induced reporter gene expression. Placental extracellular vesicles from Cre‐expressing fetuses similarly induced genetic recombination in dendritic cells in vitro, revealing horizontal genetic transfer and expression of functional fetal protein in target cells. 118 Thus, placental vesicles could mediate exchange of functional genetic material between the mother and fetus, causing expression of fetal proteins in maternal cells.
5. “FORMER MATERNAL SELF”: TISSUE‐SPECIFIC FETAL‐PLACENTAL ANTIGENS
5.1. A paradigm shift in our understanding of immune tolerance
The prevailing view of immune tolerance in pregnancy has been that peripheral mechanisms dominate, wherein mature T cells encounter paternal and paternally‐inherited fetal antigens in lymphoid tissues and the reproductive tract as they are introduced or expressed. 130 Virtually all focus has been on paternally‐inherited fetal antigens—those to which the mother is exposed for the first time—including MHC, minor antigens, and blood group antigens. However, a key principle in immunology is that tolerance to tissue‐specific antigens is indispensable: without self‐tolerance, lymphocytes attack self‐tissues, shutting down organ function as a result of devastating autoimmune disease.
Prior to the early 2000's, immunological dogma stated that tolerance to tissue‐specific antigens is established by peripheral tolerance mechanisms. Many immunologists believed that central tolerance, which occurs during T‐cell development in the thymus and requires antigen expression therein, directs tolerance only to ubiquitous self‐antigens. This opinion presumed that tissue‐specific antigens were unlikely to be expressed in the thymus.
Around the turn of the century, several discoveries changed this perception dramatically. First, early studies of targeted genes and proteins, and later global RNA expression strategies, revealed that medullary thymic epithelial cells, which were already known to mediate negative selection of self‐reactive T cells, promiscuously express thousands of antigens otherwise expressed in only a few other tissues. 131 , 132 , 133 Second, genetic mapping studies revealed that mutations in the AIRE gene (autoimmune regulator) are solely responsible for causing autoimmune polyglandular syndrome type 1 (APS‐1) in humans, a disease in which patients suffer hypoparathyroidism, adrenal insufficiency, and/or chronic mucocutaneous candidiasis. 134 , 135 Third, targeted deletion of AIRE in mice mimicked APS‐1, and showed that as a transcription factor, AIRE regulates expression of tissue‐specific antigens in medullary thymic epithelial cells (mTEC). 136 In fact, genes expressed in medullary thymic epithelial cells represent nearly every organ system in the body. 132 , 133
AIRE induces expression of tissue‐specific genes in mTEC by targeting transcriptionally repressed genes. 137 , 138 These cells then process and present them directly to developing T cells; alternatively, local dendritic cells may phagocytose mTEC‐expressed antigens and cross‐present them to the T cells. 139 These mechanisms promote deletion of autoreactive T cells 140 , 141 and production of antigen‐specific regulatory T cells. 142 , 143 , 144 Altogether, the discovery of promiscuous gene expression by mTEC, AIRE, and the mechanisms by which they induces T‐cell tolerance resulted in a paradigm shift in our understanding of how the immune system tolerates self.
5.2. Is there a role for AIRE in maternal tolerance to fetal antigens?
More than 60% of women suffering from APS‐1 are affected by primary infertility or fetal loss. 145 Further, representation and regulation by AIRE in thymic medullary epithelial cells includes fetus and placenta‐specific antigens (Petroff, Grzesiak & Ahn, unpublished). 131 , 132 , 133 , 146 The ovary and placenta are likely primary targets of autoimmunity in women with APS‐1, as evidenced by the presence of anti‐gonadal and anti‐placental antibodies. 147 , 148 , 149 Studies using AIRE‐deficient mice have also revealed profound female infertility or subfertility. These mice undergo age‐dependent autoimmune‐mediated depletion of ovarian follicular reserves 136 , 147 , 150 Prior to follicular loss, young females exhibit peri‐implantation loss, characterized by small implantation sites that disappear by mid‐gestation. 151 Mice in which AIRE was eliminated just prior to pregnancy also experienced subfertility; this study further suggested that extrathymic AIRE‐expressing cells mediates tolerance to fetal antigens. 152 Finally, embryonic grafts transplanted into AIRE‐deficient mice become surrounded by lymphocytes, suggesting that these mice fail to tolerate embryonic tissue and/or placental tissue (Warren and Petroff, unpublished).
Thus, the discovery of AIRE and its regulation of tissue‐specific antigens reminds us that fetal antigenicity may not be dictated solely by paternal origin, but that we must also consider maternal tolerance to fetus‐ and placenta‐specific antigens independent of their parental origin. Paradoxically, these are antigens that were, at one time, expressed by the mother herself, in her own fetal/neonatal life. In a landmark study that confirmed a long‐suspected notion, AIRE‐mediated tolerance was shown to be established during neonatal life. 153 This raises the intriguing possibility that females establish tolerance to fetal‐placental antigens that will reveal themselves again later in life, when pregnancy occurs and her own fetus begins to express them.
5.3. Other evidence supporting maternal‐fetal central tolerance.
Despite increasing evidence of regulation of fetal antigens by AIRE, we know little about mechanisms of central tolerance in pregnancy. Additional conspicuous links between pregnancy and the thymus exist. Some evidence suggests that regulatory T cells, which that are critical for maternal‐fetal tolerance, 45 , 154 , 155 , 156 , 157 originate from the thymus and replicate in the periphery. 158 Under the influence of progesterone, the thymus involutes dramatically in pregnancy. In mice, more than 70% of thymic mass and 95% of cellularity disappears by the second half of pregnancy, rebounding only after lactation. 146 , 159 This may occur in all mammals, including women, which undergo altered thymic output during pregnancy. 160 , 161 , 162 , 163 The nuclear progesterone receptor is dramatically upregulated in cortical thymic epithelial cells and mediates thymic involution, likely by signaling changes in thymocyte trafficking and lymphopoiesis. 146 , 164 , 165 Further, prevention of thymic involution by cell‐specific deletion of progesterone receptor may compromise allogeneic pregnancy, 146 , 165 suggesting a possible role in tolerance to paternally inherited antigens.
6. FUTURE DIRECTIONS
A number of clinical syndromes, such as preterm birth and preeclampsia, involve high levels of inflammation and immune dysregulation. As indicated, blood group incompatibility, particularly of RHD, causes true “fetal allograft rejection”. However, less certainty is placed on whether other obstetrical syndromes are mediated by antigen incompatibility; it is clear that co‐evolution of the reproductive and immune systems required that fetuses avoid the expression of highly antigenic proteins.
A classical approach to determining the necessity of a given factor for a pathophysiological process is ablation/replacement, and this approach has been used extensively in reproductive immunology. One firm requirement for allogeneic pregnancy appears to be regulatory T (Treg)cells, which has been shown by a number of studies independently. Moreover, successive pregnancies cause antigen‐specific Treg cells to expand more rapidly and to a greater degree than in first pregnancies, suggesting that these cells, such as conventional T cells, possess memory 166 T cells also expand during pregnancy in women, and reduced expansion is associated with adverse pregnancy outcome.
While a number of studies have confirmed that Treg cells are critical for pregnancy, little is known about their mechanism of action; important questions remain about which effector cells are controlled by pregnancy‐induced Treg, and whether antigen‐specificity and/or cell–cell contact is required for their function during pregnancy. Indeed, there is a need for identifying the source of Treg; it is possible that those specific for paternally‐inherited fetal antigens differentiate peripherally, and those specific for “former‐self” fetal antigens originate in the thymus. Further, the target specificity of conventional and regulatory T cells that react in pregnancy remains an important unanswered question. 167
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
The authors appreciate all of the discussion and enthusiastic input from members of the lab, Morgan Collins, Geoffrey Grzesiak, and Kathryn Brittain. Figures 1 and 4 were created using BioRender. Grant support: NIH grants AI142172, AI152192, and HD100832.
Petroff MG, Nguyen SL, Ahn SH. Fetal‐placental antigens and the maternal immune system: Reproductive immunology comes of age. Immunol Rev. 2022;308:25‐39. doi: 10.1111/imr.13090
This article is part of a series of reviews covering Immunity at the Maternal/Fetal interface appearing in Volume 308 of Immunological Reviews.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320‐338. [Google Scholar]
- 2. Hopwood N, Flemming R, Kassell L. Reproduction: Antiquity to Today. Cambridge University Press; 2020. [Google Scholar]
- 3. Poole JC, Holladay AJ. Thucydides and the plague of Athens. Class Q. 1979;29(2):282‐300. [DOI] [PubMed] [Google Scholar]
- 4. Boylston A. The origins of inoculation. J R Soc Med. 2012;105(7):309‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobs MT, Frush DP, Donnelly LF. The right place at the wrong time: historical perspective of the relation of the thymus gland and pediatric radiology. Radiology. 1999;210(1):11‐16. [DOI] [PubMed] [Google Scholar]
- 6. Miller JFAP. The golden anniversary of the thymus. Nat Rev Immunol. 2011;11(7):489‐495. [DOI] [PubMed] [Google Scholar]
- 7. Cooper B. The origins of bone marrow as the seedbed of our blood: from antiquity to the time of Osler. Proc (Bayl Univ Med Cent). 2011;24(2):115‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dionigi R, Boni L, Rausei S, Rovera F, Dionigi G. History of splenectomy. Int J Surg. 2013;11(Suppl 1):S42‐S43. [DOI] [PubMed] [Google Scholar]
- 9. Crivellato E, Travan L, Ribatti D. The Hippocratic treatise “on glands”: the first document on lymphoid tissue and lymph nodes. Leukemia. 2007;21(4):591‐592. [DOI] [PubMed] [Google Scholar]
- 10. Huppertz B. Human placentation. Encyclopedia of Reproduction. Amsterdam, The Netherlands: Elsevier; 2018:431‐439. [Google Scholar]
- 11. Keele K, Pedretti C. RCIN 919102: The Fetus in the Womb; Sketches and Notes on Reproduction c.1511. Royal Trust Collection; 1979‐80. https://www.rct.uk/collection/919102/the‐fetus‐in‐the‐womb‐sketches‐and‐notes‐on‐reproduction. Accessed March 1, 2022. [Google Scholar]
- 12. de Witt F. An historical study on theories of the placenta to 1900. J Hist Med Allied Sci. 1959;XIV(7):360‐374. [DOI] [PubMed] [Google Scholar]
- 13. Kaufmann SHE. Immunology’s foundation: the 100‐year anniversary of the Nobel prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol. 2008;9(7):705‐712. [DOI] [PubMed] [Google Scholar]
- 14. Barker CF, Markmann JF. Historical overview of transplantation. Cold Spring Harb Perspect Med. 2013;3(4):a014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murphy JB. Heteroplastic tissue grafting effected through roentgen‐ray lymphoid destruction. JAMA. 1914;LXII(19):1459. [Google Scholar]
- 16. Little CC. The genetics of cancer in mice. Biol Rev Camb Philos Soc. 1947;22(4):315‐343. [DOI] [PubMed] [Google Scholar]
- 17. Gorer PA. The genetic and antigenic basis of tumour transplantation. J Pathol Bacteriol. 1937;44(3):691‐697. [Google Scholar]
- 18. Snell GD, Higgins GF. Alleles at the histocompatibility‐2 locus in the mouse as determined by tumor transplantation. Genetics. 1951;36(3):306‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region‐specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978;120(6):1809‐1812. [PubMed] [Google Scholar]
- 20. Dausset J. Iso‐leuco‐anticorps. Acta Haematol. 1958;20(1–4):156‐166. [DOI] [PubMed] [Google Scholar]
- 21. Medawar PB. The behaviour and fate of skin autografts and skin homografts in rabbits: a report to the war wounds Committee of the Medical Research Council. J Anat. 1944;78(Pt 5):176‐199. [PMC free article] [PubMed] [Google Scholar]
- 22. Simpson E. Reminiscences of sir Peter Medawar: in hope of antigen‐specific transplantation tolerance. Am J Transplant. 2004;4(12):1937‐1940. [DOI] [PubMed] [Google Scholar]
- 23. Anderson D, Billingham RE, Lampkin GH, Medawar PB. The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle. Heredity (Edinb). 1951;5(3):379‐397. [Google Scholar]
- 24. Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102(2651):400‐401. [DOI] [PubMed] [Google Scholar]
- 25. Burnet FM, Fenner F. The Production of Antibodies. New York, NY: Macmillan; 1949. [Google Scholar]
- 26. Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603‐606. [DOI] [PubMed] [Google Scholar]
- 27. Janeway CA Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13(1):11‐16. [DOI] [PubMed] [Google Scholar]
- 28. Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991‐1045. [DOI] [PubMed] [Google Scholar]
- 29. Bonney EA, Matzinger P. Much IDO about pregnancy. Nat Med. 1998;4(10):1128‐1129. [DOI] [PubMed] [Google Scholar]
- 30. Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. Transplantation and damage‐associated molecular patterns (DAMPs). Am J Transplant. 2016;16(12):3338‐3361. [DOI] [PubMed] [Google Scholar]
- 31. Hutter H, Dohr G. HLA expression on immature and mature human germ cells. J Reprod Immunol. 1998;38(2):101‐122. [DOI] [PubMed] [Google Scholar]
- 32. Fedder J. Nonsperm cells in human semen: with special reference to seminal leukocytes and their possible influence on fertility. Arch Androl. 1996;36(1):41‐65. [DOI] [PubMed] [Google Scholar]
- 33. Vickram AS, Dhama K, Chakraborty S, et al. Role of antisperm antibodies in infertility, pregnancy, and potential forContraceptive and antifertility vaccine designs: research progress and pioneering vision. Vaccines (Basel). 2019;7(3):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark GF, Schust DJ. Manifestations of immune tolerance in the human female reproductive tract. Front Immunol. 2013;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suarez SS. Gamete and zygote transport. In: Plant T, Zeleznich A, eds. Knobil and Neill’s Physiology of Reproduction. Elsevier; 2015:197‐232. [Google Scholar]
- 36. Katila T. Post‐mating inflammatory responses of the uterus. Reprod Domest Anim. 2012;47(Suppl 5):31‐41. [DOI] [PubMed] [Google Scholar]
- 37. Akthar I, Marey MA, Kim Y, Shimada M, Suarez SS, Miyamoto A. Sperm interaction with the uterine innate immune system: toll‐like receptor 2 (TLR2) is a main sensor in cattle. Reprod Fertil Dev. 2021;34(2):139‐148. [DOI] [PubMed] [Google Scholar]
- 38. Cheng X, Zhang Y, Ma J, et al. NLRP3 promotes endometrial receptivity by inducing epithelial‐mesenchymal transition of the endometrial epithelium. Mol Hum Reprod. 2021;27(11):1‐13. doi: 10.1093/molehr/gaab056 [DOI] [PubMed] [Google Scholar]
- 39. Schjenken JE, Sharkey DJ, Green ES, et al. Sperm modulate uterine immune parameters relevant to embryo implantation and reproductive success in mice. Commun Biol. 2021;4(1):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Akthar I, Suarez SS, Morillo VA, et al. Sperm enter glands of preovulatory bovine endometrial explants and initiate inflammation. J Reprod Fertil. 2020;159(2):181‐192. [DOI] [PubMed] [Google Scholar]
- 41. Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell‐Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445‐2454. [DOI] [PubMed] [Google Scholar]
- 42. Robertson SA, Sharkey DJ. Seminal fluid and fertility in women. Fertil Steril. 2016;106(3):511‐519. [DOI] [PubMed] [Google Scholar]
- 43. Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross‐presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182(12):8080‐8093. [DOI] [PubMed] [Google Scholar]
- 44. Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19‐mediated recruitment. Biol Reprod. 2011;85(2):397‐408. [DOI] [PubMed] [Google Scholar]
- 45. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266‐271. [DOI] [PubMed] [Google Scholar]
- 46. Crawford G, Ray A, Gudi A, Shah A, Homburg R. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: review of the literature and meta‐analysis. Hum Reprod Update. 2015;21(2):275‐284. [DOI] [PubMed] [Google Scholar]
- 47. Lim H, Paria BC, Das SK, et al. Multiple female reproductive failures in cyclooxygenase 2‐deficient mice. Cell. 1997;91(2):197‐208. [DOI] [PubMed] [Google Scholar]
- 48. McMaster MT, Dey SK, Andrews GK. Association of monocytes and neutrophils with early events of blastocyst implantation in mice. J Reprod Fertil. 1993;99(2):561‐569. [DOI] [PubMed] [Google Scholar]
- 49. Kover K, Liang L, Andrews GK, Dey SK. Differential expression and regulation of cytokine genes in the mouse uterus. Endocrinology. 1995;136(4):1666‐1673. [DOI] [PubMed] [Google Scholar]
- 50. Li DK, Liu L, Odouli R. Exposure to non‐steroidal anti‐inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ. 2003;327(7411):368‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li DK, Ferber JR, Odouli R, Quesenberry C. Use of nonsteroidal antiinflammatory drugs during pregnancy and the risk of miscarriage. Am J Obstet Gynecol. 2018;219(3):275.e1‐275.e8. [DOI] [PubMed] [Google Scholar]
- 52. Potdar N, Gelbaya T, Nardo LG. Endometrial injury to overcome recurrent embryo implantation failure: a systematic review and meta‐analysis. Reprod Biomed Online. 2012;25(6):561‐571. [DOI] [PubMed] [Google Scholar]
- 53. Bulmer JN, Morrison L, Smith JC. Expression of class II MHC gene products by macrophages in human uteroplacental tissue. Immunology. 1988;63(4):707‐714. [PMC free article] [PubMed] [Google Scholar]
- 54. Piacentini M, Autuori F. Immunohistochemical localization of tissue transglutaminase and Bcl‐2 in rat uterine tissues during embryo implantation and post‐partum involution. Differentiation. 1994;57(1):51‐61. [DOI] [PubMed] [Google Scholar]
- 55. Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51(4):275‐282. [DOI] [PubMed] [Google Scholar]
- 56. Ono Y, Yoshino O, Hiraoka T, et al. CD206+ M2‐like macrophages are essential for successful implantation. Front Immunol. 2020;11:557184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Plaks V, Birnberg T, Berkutzki T, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118(12):3954‐3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci U S A. 2017;114(32):E6566‐E6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril. 2004;82(4):799‐804. [DOI] [PubMed] [Google Scholar]
- 60. Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17(8):469‐482. [DOI] [PubMed] [Google Scholar]
- 61. Valent P, Groner B, Schumacher U, et al. Paul Ehrlich (1854–1915) and his contributions to the foundation and birth of translational medicine. J Innate Immun. 2016;8(2):111‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Levine P, Burnham L, Katzin EM, Vogel P. The role of ISO‐immunization in the pathogenesis of erythroblastosis fetalis. Rhesus Haemolytic Disease. Springer; 1941:61‐73. [Google Scholar]
- 63. Mollison PL, Hughes‐Jones NC, Lindsay M, Wessely J. Suppression of primary rh immunization by passively‐administered antibody. Exp Vol Vox Sang. 1969;16(4–5):421‐439. [PubMed] [Google Scholar]
- 64. Pegoraro V, Urbinati D, Visser GHA, et al. Hemolytic disease of the fetus and newborn due to rh(D) incompatibility: a preventable disease that still produces significant morbidity and mortality in children. PLoS One. 2020;15(7):e0235807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Habibi B, Bretagne M, Bretagne Y, Forestier F, Daffos F. Blood group antigens on fetal red cells obtained by umbilical vein puncture under ultrasound guidance: a rapid hemagglutination test to check for contamination with maternal blood. Pediatr Res. 1986;20(11):1082‐1084. [DOI] [PubMed] [Google Scholar]
- 66. Dean L. Blood Groups and Red Cell Antigens. Bethesda, Maryland: National Center for Biotechnology Information; 2005. [Google Scholar]
- 67. Sun JB. The prenatal intervention of pregnancy complicated with anti‐Kell isoimmunization: a review. J Matern Fetal Neonatal Med. 2021;34(17):2893‐2899. [DOI] [PubMed] [Google Scholar]
- 68. Dajak S, Culić S, Stefanović V, Lukačević J. Relationship between previous maternal transfusions and haemolytic disease of the foetus and newborn mediated by non‐RhD antibodies. Blood Transfus. 2013;11(4):528‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Koelewijn JM, Vrijkotte TGM, de Haas M, van der Schoot CE, Bonsel GJ. Risk factors for the presence of non‐rhesus D red blood cell antibodies in pregnancy. BJOG. 2009;116(5):655‐664. [DOI] [PubMed] [Google Scholar]
- 70. Grant SR, Kilby MD, Meer L, Weaver JB, Gabra GS, Whittle MJ. The outcome of pregnancy in Kell alloimmunisation. BJOG. 2000;107(4):481‐485. [DOI] [PubMed] [Google Scholar]
- 71. Vaughan JI, Warwick R, Letsky E, Nicolini U, Rodeck CH, Fisk NM. Erythropoietic suppression in fetal anemia because of Kell alloimmunization. Am J Obstet Gynecol. 1994;171(1):247‐252. [DOI] [PubMed] [Google Scholar]
- 72. Vaughan JI, Manning M, Warwick RM, Letsky EA, Murray NA, Roberts IA. Inhibition of erythroid progenitor cells by anti‐Kell antibodies in fetal alloimmune anemia. N Engl J Med. 1998;338(12):798‐803. [DOI] [PubMed] [Google Scholar]
- 73. Goldman M, Lane D, Webert K, Fallis R. The prevalence of anti‐K in Canadian prenatal patients. Transfusion. 2015;55(6 Pt 2):1486‐1491. [DOI] [PubMed] [Google Scholar]
- 74. Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166(2):973‐981. [DOI] [PubMed] [Google Scholar]
- 75. Felix NJ, Allen PM. Specificity of T‐cell alloreactivity. Nat Rev Immunol. 2007;7(12):942‐953. [DOI] [PubMed] [Google Scholar]
- 76. van Rood JJ, Eernisse JG, van Leeuwen A. Leucocyte antibodies in sera from pregnant women. Nature. 1958;181:1735‐1736. [DOI] [PubMed] [Google Scholar]
- 77. Regan L, Braude PR, Hill DP. A prospective study of the incidence, time of appearance and significance of anti‐paternal lymphocytotoxic antibodies in human pregnancy. Hum Reprod. 1991;6(2):294‐298. [DOI] [PubMed] [Google Scholar]
- 78. Hönger G, Fornaro I, Granado C, Tiercy JM, Hösli I, Schaub S. Frequency and determinants of pregnancy‐induced child‐specific sensitization. Am J Transplant. 2013;13(3):746‐753. [DOI] [PubMed] [Google Scholar]
- 79. Bouma GJ, Van Caubergh P, Van Bree SP, et al. Pregnancy can induce priming of cytotoxic T lymphocytes specific for paternal HLA antigens that is associated with antibody formation. Transplantation. 1996;62(5):672‐678. [DOI] [PubMed] [Google Scholar]
- 80. Burton GJ, Jauniaux E. The cytotrophoblastic shell and complications of pregnancy. Placenta. 2017;60:134‐139. [DOI] [PubMed] [Google Scholar]
- 81. Faulk WP, Temple A. Distribution of β2 microglobulin and HLA in chorionic villi of human placentae. Nature. 1976;262(5571):799‐802. [DOI] [PubMed] [Google Scholar]
- 82. Sutton L, Mason DY, Redman CW. HLA‐DR positive cells in the human placenta. Immunology. 1983;49(1):103‐112. [PMC free article] [PubMed] [Google Scholar]
- 83. Sunderland CA, Naiem M, Mason DY, Redman CWG, Stirrat GM. The expression of major histocompatibility antigens by human chorionic villi. J Reprod Immunol. 1981;3:323‐331. [DOI] [PubMed] [Google Scholar]
- 84. Hunt JS, Andrews GK, Wood GS. Normal trophoblasts resist induction of class I HLA. J Immunol. 1987;138(8):2481‐2487. [PubMed] [Google Scholar]
- 85. Peyman JA, Hammond GL. Localization of IFN‐gamma receptor in first trimester placenta to trophoblasts but lack of stimulation of HLA‐DRA, ‐DRB, or invariant chain mRNA expression by IFN‐gamma. J Immunol. 1992;149(8):2675‐2680. [PubMed] [Google Scholar]
- 86. Holtz R, Choi JC, Petroff MG, Piskurich JF, Murphy SP. Class II transactivator (CIITA) promoter methylation does not correlate with silencing of CIITA transcription in trophoblasts. Biol Reprod. 2003;69(3):915‐924. doi: 10.1095/biolreprod.103.017103 [DOI] [PubMed] [Google Scholar]
- 87. Choi JC, Holtz R, Murphy SP. Histone deacetylases inhibit IFN‐gamma‐inducible gene expression in mouse trophoblast cells. J Immunol. 2009;182(10):6307‐6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shomer B, Toder V, Egorov I, Ehrlich R. Expression of allogeneic MHC Class I antigens by transgenic mouse trophoblast does not interfere with the normal course of pregnancy. Transgenic Res. 1998;7(5):343‐356. [DOI] [PubMed] [Google Scholar]
- 89. Aït‐Azzouzene D, Caucheteux S, Tchang F, et al. Transgenic major histocompatibility complex class I antigen expressed in mouse trophoblast affects maternal immature B cells. Biol Reprod. 2001;65(2):337‐344. [DOI] [PubMed] [Google Scholar]
- 90. Papúchová H, Meissner TB, Li Q, Strominger JL, Tilburgs T. The dual role of HLA‐C in tolerance and immunity at the maternal‐fetal interface. Front Immunol. 2019;10:2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xu X, Zhou Y, Wei H. Roles of HLA‐G in the maternal‐fetal immune microenvironment. Front Immunol. 2020;11:1‐11. doi: 10.3389/fimmu.2020.592010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tersigni C, Meli F, Neri C, et al. Role of human leukocyte antigens at the feto‐maternal interface in normal and pathological pregnancy: an update. Int J Mol Sci. 2020;21(13):4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Le Bouteiller P. HLA‐G in human early pregnancy: control of uterine immune cell activation and likely vascular remodeling. Biom J. 2015;38(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 94. Carosella ED, Gregori S, LeMaoult J. The tolerogenic interplay(s) among HLA‐G, myeloid APCs, and regulatory cells. Blood. 2011;118(25):6499‐6505. [DOI] [PubMed] [Google Scholar]
- 95. Nilsson LL, Hviid TVF. HLA class Ib‐receptor interactions during embryo implantation and early pregnancy. Hum Reprod Update. 2022;2:435‐454. doi: 10.1093/humupd/dmac007 [DOI] [PubMed] [Google Scholar]
- 96. Persson G, Stæhr CS, Klok FS, Lebech M, Hviid TVF. Evidence for a shift in placental HLA‐G allelic dominance and the HLA‐G isoform profile during a healthy pregnancy and preeclampsia. Biol Reprod. 2021;105(4):846‐858. [DOI] [PubMed] [Google Scholar]
- 97. Jacobsen DP, Lekva T, Moe K, et al. Pregnancy and postpartum levels of circulating maternal sHLA‐G in preeclampsia. J Reprod Immunol. 2021;143(103249):103249. [DOI] [PubMed] [Google Scholar]
- 98. Craenmehr MHC, Nederlof I, Cao M, et al. Increased HLA‐G expression in term placenta of women with a history of recurrent miscarriage despite their genetic predisposition to decreased HLA‐G levels. Int J Mol Sci. 2019;20(3):625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tersigni C, Redman CW, Dragovic R, et al. HLA‐DR is aberrantly expressed at feto‐maternal interface in pre‐eclampsia. J Reprod Immunol. 2018:48‐52. doi: 10.1016/j.jri.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 100. Moffett A, Colucci F. Co‐evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol Rev. 2015;267(1):283‐297. [DOI] [PubMed] [Google Scholar]
- 101. Xiong S, Sharkey AM, Kennedy PR, et al. Maternal uterine NK cell‐activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123(10):4264‐4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hiby SE, Apps R, Sharkey AM, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA‐C2. J Clin Invest. 2010;120(11):4102‐4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Summers C, Sheth VS, Bleakley M. Minor histocompatibility antigen‐specific T cells. Front Pediatr. 2020;8:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Holland OJ, Linscheid C, Hodes HC, et al. Minor histocompatibility antigens are expressed in syncytiotrophoblast and trophoblast debris: implications for maternal alloreactivity to the fetus. Am J Pathol. 2012;180(1):256‐266. doi: 10.1016/j.ajpath.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jiang SP, Vacchio MS. Multiple mechanisms of peripheral T cell tolerance to the fetal “allograft”. J Immunol. 1998;160(7):3086‐3090. [PubMed] [Google Scholar]
- 106. Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270(5236):630‐633. [DOI] [PubMed] [Google Scholar]
- 107. Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399‐1411. doi: 10.1172/JCI28214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. James E, Chai JG, Dewchand H, Macchiarulo E, Dazzi F, Simpson E. Multiparity induces priming to male‐specific minor histocompatibility antigen, HY, in mice and humans. Blood. 2003;102(1):388‐393. [DOI] [PubMed] [Google Scholar]
- 109. van Halteren AG, Jankowska‐Gan E, Joosten A, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114(11):2263‐2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PAH. Fetal‐specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J Immunol. 2012;189(2):1072‐1080. [DOI] [PubMed] [Google Scholar]
- 111. Piper KP, McLarnon A, Arrazi J, et al. Functional HY‐specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod. 2007;76(1):96‐101. [DOI] [PubMed] [Google Scholar]
- 112. Benirschke K, Burton GJ, Baergen RN. Pathology of the Human Placenta. 6th ed. Berlin, Germany: Springer‐Verlag; 2012. [Google Scholar]
- 113. Alam SMK, Jasti S, Kshirsagar SK, et al. Trophoblast glycoprotein (TPGB/5T4) in human placenta: expression, regulation, and presence in extracellular microvesicles and exosomes. Reprod Sci. 2018;25(2):185‐197. doi: 10.1177/1933719117707053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Linscheid C, Petroff MG. Minor histocompatibility antigens and the maternal immune response to the fetus during pregnancy. Am J Reprod Immunol. 2013;69(4):304‐314. doi: 10.1111/aji.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jasti S, Farahbakhsh M, Nguyen S, Petroff BK, Petroff MG. Immune response to a model shared placenta/tumor‐associated antigen reduces cancer risk in parous mice. Biol Reprod. 2017;96(1):134‐144. doi: 10.1095/biolreprod.116.144907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kshirsagar SK, Alam SM, Jasti S, et al. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012;33(12):982‐990. doi: 10.1016/j.placenta.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stenqvist AC, Nagaeva O, Baranov V, Mincheva‐Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome‐mediated immune privilege of the fetus. J Immunol. 2013;191:5515‐5523. doi: 10.4049/jimmunol.1301885 [DOI] [PubMed] [Google Scholar]
- 118. Nguyen SL, Ahn SH, Greenberg JW, et al. Integrins mediate placental extracellular vesicle trafficking to lung and liver in vivo. Sci Rep. 2021;11:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tong M, Chen Q, James JL, Wise MR, Stone PR, Chamley LW. In vivo targets of human placental micro‐vesicles vary with exposure time and pregnancy. Reproduction. 2017;153:835‐845. doi: 10.1530/REP-16-0615 [DOI] [PubMed] [Google Scholar]
- 120. Tay CS, Tagliani E, Collins MK, Erlebacher A. Cis‐acting pathways selectively enforce the non‐immunogenicity of shed placental antigen for maternal CD8 T cells. PLoS One. 2013;8:e84064. doi: 10.1371/journal.pone.0084064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, Demaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93:705‐708. doi: 10.1073/pnas.93.2.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cai J, Lee J, Jankowska‐Gan E, et al. Minor H antigen HA‐1‐specific regulator and effector CD8+ T cells, and HA‐1 microchimerism, in allograft tolerance. J Exp Med. 2004;199:1017‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kieffer TEC, Laskewitz A, Scherjon SA, Faas MM, Prins JR. Memory T cells in pregnancy. Front Immunol. 2019;10:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yan Z, Aydelotte T, Gadi VK, Guthrie KA, Nelson JL. Acquisition of the rheumatoid arthritis HLA shared epitope through microchimerism. Arthritis Rheum. 2011;63(3):640‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654‐659. [DOI] [PubMed] [Google Scholar]
- 126. Frühbeis C, Fröhlich D, Kuo WP, et al. Neurotransmitter‐triggered transfer of exosomes mediates oligodendrocyte‐neuron communication. PLoS Biol. 2013;11(7):e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ridder K, Keller S, Dams M, et al. Extracellular vesicle‐mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12:e1001874. doi: 10.1371/journal.pbio.1001874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ridder K, Sevko A, Heide J, et al. Extracellular vesicle‐mediated transfer of functional RNA in the tumor microenvironment. Onco Targets Ther. 2015;19:e1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zomer A, Maynard C, Verweij FJ, et al. In vivo imaging reveals extracellular vesicle‐mediated phenocopying of metastatic behavior. Cell. 2015;161:1046‐1057. doi: 10.1016/j.cell.2015.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Robertson SA, Petroff MG, Hunt JS. Immunology of pregnancy. Knobil and Neill's Physiology of Reproduction. Vol 2. Cambridge, MA: Academic Press; 2015:1835‐1874. [Google Scholar]
- 131. Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032‐1039. [DOI] [PubMed] [Google Scholar]
- 132. Derbinski J, Gabler J, Brors B, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Immunol. 2005;202(1):33‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sansom SN, Shikama‐Dorn N, Zhanybekova S, et al. Population and single‐cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self‐antigen expression in thymic epithelia. Genome Res. 2014;24(12):1918‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Aaltonen J, Björses P, Perheentupa J, et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD‐type zinc‐finger domains. Nat Genet. 1997;17:399‐403. doi: 10.1038/ng1297-399 [DOI] [PubMed] [Google Scholar]
- 135. Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17(4):393‐398. [DOI] [PubMed] [Google Scholar]
- 136. Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395‐1401. doi: 10.1126/science.1075958 [DOI] [PubMed] [Google Scholar]
- 137. Org T, Rebane A, Kisand K, et al. AIRE activated tissue specific genes have histone modifications associated with inactive chromatin. Hum Mol Genet. 2009;18(24):4699‐4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Org T, Chignola F, Hetenyi C, et al. The autoimmune regulator PHD finger binds to non‐methylated histone H3K4 to activate gene expression. EMBO rep. 2008;9(4):370‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hubert FX, Kinkel SA, Davey GM, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118(9):2462‐2472. [DOI] [PubMed] [Google Scholar]
- 140. Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ‐specific T cells. Nat Immunol. 2003;4(4):350‐354. [DOI] [PubMed] [Google Scholar]
- 141. DeVoss JJ, Shum AK, Johannes KPA, et al. Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular syndrome type 1. J Immunol. 2008;181(6):4072‐4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Aschenbrenner K, D’Cruz LM, Vollmann EH, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8(4):351‐358. [DOI] [PubMed] [Google Scholar]
- 143. Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self‐tolerance. Science. 2015;348(6234):589‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, Savage PA. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity. 2016;44(5):1102‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Perheentupa J. Autoimmune polyendocrinopathy‐candidiasis‐ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91(8):2843‐2850. [DOI] [PubMed] [Google Scholar]
- 146. Ahn SH, Nguyen SL, Kim TH, et al. Nuclear progesterone receptor expressed by the cortical thymic epithelial cells dictates thymus involution in murine pregnancy. Front Endocri. 2022:1‐13. doi: 10.3389/fendo.2022.846226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jasti S, Warren BD, McGinnis LK, Kinsey WH, Petroff BK, Petroff MG. The autoimmune regulator prevents premature reproductive senescence in female mice. Biol Reprod. 2012;86(4):110. doi: 10.1095/biolreprod.111.097501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Warren BD, Kinsey WK, McGinnis LK, et al. Ovarian autoimmune disease: clinical concepts and animal models. Cell Mol Immunol. 2014;11(6):510‐521. doi: 10.1038/cmi.2014.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Chen S, Sawicka J, Betterle C, et al. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison’s disease, and premature ovarian failure. J Clin Endocrinol Metab. 1996;81(5):1871‐1876. [DOI] [PubMed] [Google Scholar]
- 150. Ramsey C, Winqvist O, Puhakka L, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397‐409. doi: 10.1093/hmg/11.4.397 [DOI] [PubMed] [Google Scholar]
- 151. Warren BD, Ahn SH, McGinnis LK, et al. Autoimmune regulator is required in female mice for optimal embryonic development and implantation. Biol Reprod. 2019;100(6):1492‐1504. doi: 10.1093/biolre/ioz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gillis‐Buck E, Miller H, Sirota M, et al. Extrathymic Aire‐expressing cells support maternal‐fetal tolerance. Sci Immunol. 2021;6(61):eabf1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Guerau‐de‐Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206(6):1245‐1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kahn DA, Baltimore D. Pregnancy induces a fetal antigen‐specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107(20):9299‐9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zenclussen AC, Gerlof K, Zenclussen ML, et al. Abnormal T‐cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy‐induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Darrasse‐Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102(1):106‐109. [DOI] [PubMed] [Google Scholar]
- 157. Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T‐regulatory cell transcription factor Foxp3 in endometrial tissue. Mol hum Reprod. 2006;12:301‐308. [DOI] [PubMed] [Google Scholar]
- 158. Moldenhauer LM, Hope CM, Green ES, et al. Thymus‐derived regulatory T cells exhibit Foxp3 epigenetic modification and phenotype attenuation after mating in mice. J Immunol. 2019;203:647‐657. doi: 10.4049/jimmunol.1900084 [DOI] [PubMed] [Google Scholar]
- 159. Zoller AL, Schnell FJ, Kersh GJ. Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology. 2007;121:207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Clarke AG, Kendall MD. The thymus in pregnancy: the interplay of neural, endocrine and immune functions. Immunol Today. 1994;15(11):545‐551. [DOI] [PubMed] [Google Scholar]
- 161. Brouha L, Collin R. Equilibre hormonal et gravidite. Ann Physiol Physicochim Biol. 1935;11:773. [Google Scholar]
- 162. Hammar JA. Die Menschen Thymus in Gesundheit und Krankenheit Teil II: Das Organ unter anormalen Korerverhaltnissen. Ztschr F Mikr‐Anat Forsch. 1926;16:733. [Google Scholar]
- 163. Wagner MI, Mai C, Schmitt E, et al. The role of recent thymic emigrant‐regulatory T‐cell (RTE‐Treg) differentiation during pregnancy. Immunol Cell Biol. 2015;93(10):858‐867. [DOI] [PubMed] [Google Scholar]
- 164. Laan M, Haljasorg U, Kisand K, Salumets A, Peterson P. Pregnancy‐induced thymic involution is associated with suppression of chemokines essential for T‐lymphoid progenitor homing. Eur J Immunol. 2016;46(8):2008‐2017. [DOI] [PubMed] [Google Scholar]
- 165. Tibbetts TA, DeMayo F, Rich S, Conneely OM, O’Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci U S A. 1999;96(21):12021‐12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol. 2016;16(2):90‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ahn SH, Nguyen SL, Petroff MG. Exploring the origin and antigenic specificity of maternal regulatory T cells in pregnancy. Front Immunol. 2020;11:1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


