Abstract
Aims
The introduction of direct oral anticoagulants (DOACs) has broadened the treatment arsenal for nonvalvular atrial fibrillation, but observational studies on the benefit–risk balance of DOACs compared to vitamin K antagonists (VKAs) are needed. The aim of this study was to characterize the risk of major bleeding in DOAC users using longitudinal data collected from electronic health care databases from 4 different EU‐countries analysed with a common study protocol.
Methods
A cohort study was conducted among new users (≥18 years) of DOACs or VKAs with nonvalvular atrial fibrillation using data from the UK, Spain, Germany and Denmark. The incidence of major bleeding events (overall and by bleeding site) was compared between current use of DOACs and VKAs. Cox regression analysis was used to calculate hazard ratios and 95% confidence intervals (CI) and adjust for confounders.
Results/Conclusion
Overall, 251 719 patients were included across the 4 study cohorts (mean age ~75 years, % females between 41.3 and 54.3%), with overall hazard ratios of major bleeding risk for DOACs vs VKAs ranging between 0.84 (95% CI: 0.79–0.90) in Denmark and 1.13 (95% CI 1.02–1.25) in the UK. When stratifying according to the bleeding site, risk of gastrointestinal bleeding was increased by 48–67% in dabigatran users and 30–50% for rivaroxaban users compared to VKA users in all data sources except Denmark. Compared to VKAs, apixaban was not associated with an increased risk of gastrointestinal bleeding in all data sources and seemed to be associated with the lowest risk of major bleeding events compared to dabigatran and rivaroxaban.
Keywords: atrial fibrillation, cohort study, major bleeding, non‐vitamin K antagonist oral anticoagulants, pharmacoepidemiology, vitamin K antagonists
What is already known about this subject
The introduction of direct oral anticoagulants (DOACs) has broadened the treatment arsenal for nonvalvular atrial fibrillation, but observational studies on the benefit–risk balance of these drugs compared to traditional vitamin K antagonists (VKAs) are still needed.
What this study adds
This large multicountry database study using observational data from 4 EU‐countries involving over 250 000 patients showed a consistent pattern where current use of DOACs had a similar risk of major bleeding compared to current use of VKAs. When assessing individual bleeding sites, the risk of gastrointestinal bleeding was statistically significant increased by 48–67% in dabigatran users and 30–50% for rivaroxaban users compared to VKA users in all data sources except Denmark. In general, apixaban seemed to have a better risk profile for major bleeding compared to dabigatran and rivaroxaban in Denmark, Germany and Spain, while an increased risk of any stroke was seen in the UK. The reduction in bleeding risk for DOACs does not resemble reductions found in clinical trials. This could be at least be partially attributable to nonadherence and limited external validity of trials, reflecting real‐life settings.
1. INTRODUCTION
Nonvalvular atrial fibrillation (NVAF) is 1 of the most common cardiac rhythm disorders. The current prevalence is approximately 1.5–2.0% in the developed world and it has been estimated that 17.9 million people in Europe will be affected by the year 2060. 1 , 2 Patients with NVAF have a 5‐fold increased risk of ischaemic stroke and therefore are often prescribed oral anticoagulants. 3 , 4 Vitamin K‐antagonists (VKAs), most commonly warfarin, have been the cornerstone of treatment over recent decades. However, since the introduction of the direct oral anticoagulants (DOACs), such as dabigatran, rivaroxaban, apixaban and edoxaban, the treatment arsenal has broadened.
Clinical trials that were carried out for dabigatran (RELY), rivaroxaban (ROCKET‐AF), apixaban (ARISTOTLE) and edoxaban (ENGAGE AF‐TIMI 48) showed that these drugs are at least noninferior to warfarin in reducing the risk of stroke and systemic embolism. 5 , 6 , 7 , 8 In a meta‐analysis of these trials, it was shown that the risk of haemorrhagic stroke and intracranial bleeding was lower for DOACs compared to VKAs, but the risk of gastrointestinal (GI) bleeding was increased. 9 There is evidence that the increased risk for GI bleeding is restricted to dabigatran and rivaroxaban and not for apixaban. 10
Patients included in clinical trials have shown to be different from real‐life populations and therefore several observational studies have been conducted to assess the real‐world benefit–risk balance of DOACs compared to VKAs. 11 , 12 , 13 , 14 , 15 However, this balance remains inconclusive with respect to specific patient populations, such as those with older age, impaired renal function and other comorbidities. Also, there is not enough information available about the direct comparative effectiveness and safety within the class of DOACs as the bleeding risk seems to vary between the different drugs.
The aim of this study was to conduct a pharmacoepidemiological study using longitudinal data from 4 EU countries and a common study protocol to characterize the risk of major bleeding in DOAC users in a real‐world setting to help establish the effectiveness of existing and future risk minimization measures.
2. METHODS
2.1. Setting
We conducted a retrospective cohort study within 4 European health care databases: the Clinical Practice Research Datalink (CPRD) in the UK, Base de Datos para la Investigación Farmacoepidemiológica en Atencion Primaria (BIFAP) in Spain, the Allgemeine Ortskrankenkasse (AOK) Nordwest in Germany, and the Danish National Registers. Characteristics of these databases are summarized in Table 1. A common protocol approved by all centres involved and registered under European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) EU PAS register number 16014 was used (http://www.encepp.eu/).
TABLE 1.
Characteristics of the 4 electronic health care sources used
| CPRD | BIFAP | AOK NORDWEST | Denmark national registries | |
|---|---|---|---|---|
| Source population | 12.5 m | 7.5 m | 2.7 m | 5.5 m |
| Year(s) covered for this study | 2008–2015 | 2008–2015 | 2008–2015 | 2008–2015 |
| Type of database | General practice prescribing data | General practice database including data for prescriptions until 2010. From 2011 onwards information on dispensed drugs is also being included gradually | Claims database including data for dispensed and reimbursed drugs | Dispensing data |
| Data available since | 1987 | 2001 | 2007 | 1994 |
| Demographic variables available | ||||
| Date of registration | Yes | Yes | Yes | Yes |
| Date of transferring out | Yes | Yes | Yes | Yes |
| Date of birth | MM‐YY | MM‐YY | MM‐YY | MM‐YY |
| Sex | Yes | Yes | Yes | Yes |
| Drug information available | ||||
| Active international coding | BNF | ATC | ATC | ATC |
| Product coding | Product code | CNF | PZN | Varenr |
| Date of prescribing/dispensing | Yes | Yes |
Primary (ambulatory) care sector (GPs/specialists): Yes Secondary care sector (hospital): Yes (only for a few selected (expensive) compounds, but no DOAC/VKA prescriptions) |
Yes |
| Quantity prescribed/dispensed | Yes | Yes | Yes | Yes |
| Dosing regimen | Yes | Yes | No | No |
| Outcome information | ||||
| Outpatient primary care diagnosis | ICD‐9, ICD‐10 | ICPC‐2, ICD‐9 | ICD‐10‐GM (quarterly base) | Yes |
| Hospital discharge diagnosis | ICD‐9, ICD‐10 | Not systematically, as recorded by the GP | ICD‐10‐GM | ICD‐8, ICD‐9, ICD‐10 |
| Laboratory tests | Yes | Yes (as requested and recorded by GP) | No | No |
| Mortality | Yes | Yes (no cause of death) | No (incompletely recorded, e.g. no follow‐up for patients leaving the AOK) | Yes |
ATC, Anatomical Therapeutical Clinical; BNF, British National Formulary; CNF, Código Nacional de Fármaco; PZN, Pharma‐Zentral‐Nummer; ICD, International Classification of Disease; vitamin K antagonist; DOAC, direct oral anticoagulant; AOK, Allgemeine Ortskrankenkasse
2.2. Study population
The study population comprised all new DOAC or VKA users in the period 2008–2015, aged ≥18 years with a documented indication or diagnosis of NVAF. Patients were required to have at least 1 year of prior registration in the database of interest. The date of the first prescription of a DOAC or VKA defined cohort entry. New users were defined as patients initiating a DOAC or VKA during the study period without any use of these medicines for at least 12 months prior to cohort entry. Patients with a history of valvular atrial fibrillation on or before initiating DOAC or VKA were excluded. Each patient was followed until the outcome, the end of valid data collection, loss to follow‐up or death, whichever came first. Patients with the outcome on the cohort entry date were excluded from the analysis. To ensure that only patients having an indication of NVAF were included, the following methodology was applied in a hierarchical manner. First, we assessed whether there was a linked diagnosis of NVAF with the first prescription of DOAC or VKAs (BIFAP). If this was not possible, we evaluated whether a medical code for NVAF in a 3‐month time window around the index date was present in primary care (CPRD, BIFAP) or claims records (AOK Nordwest). In Denmark, we assessed a hospital admission for NVAF prior to index date plus 3 months following the index date.
2.3. Outcome definition
The primary outcome was the occurrence of a first major bleeding event, defined as symptomatic bleeding in a critical area or organ, as agreed by the International Society on Thrombosis and Haemostasis. 16 These include haemorrhagic stroke/intracranial bleeding, GI bleeding, other extracranial or unclassified bleeding, and traumatic intracranial bleeding. Outcomes were identified using Read, ICD‐9, ICD‐10(−GM) or ICPC‐2 codes, depending on data source (relevant codes available on http://www.encepp.eu/encepp/viewResource.htm?id=28664,). GI and intracranial bleeding events were analysed separately. Occurrence of any stroke (both ischaemic, haemorrhagic or unspecified stroke and transient ischaemic attacks) was evaluated as a secondary outcome.
2.4. Exposure definition
For each patient we assessed periods of current of DOAC or VKA use during follow‐up. A uniform, step‐wise approach for assessing exposure was used in all databases. The preferred method for calculating the individual prescription duration was by using information on the prescribed number of tablets and the dosage. When information on the prescribed number of tablets and or the dosage was not available, imputation was performed based on the most common practice used within each data source (e.g. most common instruction for the drug in question, median time prescriptions as assessed for individual patients). To assess periods of current use, treatment episodes of DOACs or VKAs were constructed allowing for a 30‐day permissible gap between the theoretical end date of 1 prescription and the date of the subsequent prescription. A treatment episode was defined as a series of subsequent prescriptions for DOACs or VKAs, independent of dose changes and constructed according to the method of Gardarsdottir et al. 17 If such a gap was >30 days, a new treatment episode was assumed. To facilitate exposure classification, a new row was created in case a patient switched from 1 type of oral anticoagulant to another within a treatment episode, which allows studying individual drugs. Longer periods of current or past use were split into 6‐month periods to allow for time‐dependent covariate updates (not possible in AOK Nordwest).
2.5. Potential confounders
Potential confounders considered in this study were based on literature review and are shown in Appendix A and described in detail elsewhere (http://www.encepp.eu/encepp/viewResource.htm?id=28664/). Laboratory values were used where possible to assess renal function. Sex, weight, body mass index, smoking status and alcohol status were assessed at cohort entry and considered constant throughout follow‐up. Age, comorbidities (measured in various time intervals prior to the start of follow‐up) and comedication (6 months before) were considered as time dependent confounders and their status was updated whenever the exposure status changed, or when exposure state exceeded 6 months at the start of each 6‐month interval.
2.6. Sensitivity analyses
A sensitivity analysis was performed where patients with other NVAF indications in a 3‐month window around cohort entry (e.g. knee/hip replacement surgery) were excluded. Also, the impact of varying the permissible gap between prescriptions (0, 60, 90 days) was studied. As the recording of outcomes was heterogeneous across data sources (hospital events, GP records, claims data), we performed analyses with comparable event types. In BIFAP, validation of both GI bleeding and stroke cases was performed. In CPRD, a sensitivity analysis was performed by restricting outcomes to hospitalized cases. In posthoc analyses, we assessed the impact on risk estimates of adjusting the primary outcome definition by excluding nonmajor bleeding codes, such as haematuria and epistaxis and we adjusted the stroke definition by excluding transient ischaemic attacks from the original definition.
2.7. Data analysis
Baseline characteristics were summarized as means and standard deviations or proportions where appropriate. Crude incidence rates of outcome events per 1,000 person years were calculated. Cox proportional hazard regression analysis was used to estimate the risk of study outcomes between current use of DOACs vs current use of VKAs, expressed as hazard ratios (HRs) with 95% confidence intervals (95% CI). Data were analysed using STATA or SAS software.
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries inhttp://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
Overall, we identified 39 129; 51 030; 88 742 and 72 818 patients starting oral anticoagulants in CPRD, BIFAP, AOK Nordwest and Denmark, respectively. The characteristics of the study populations are shown in Table 2. The proportion of female users was similar in CPRD, BIFAP and Denmark, but slightly higher in AOK. The mean age of the users was comparable across data sources (around 75 years), slightly younger in the Danish registers.
TABLE 2.
Baseline characteristics for users of DOACs and VKAs within the different data sources in the UK, Spain, Germany and Denmark
| UK (CPRD) | Spain (BIFAP) | Germany (AOK Nordwest) | Denmark (National Registries) | |||||
|---|---|---|---|---|---|---|---|---|
| DOAC (n = 5,852) | VKA (n = 33,277) | DOAC (n = 8775) | VKA (n = 42 255) | DOAC (n = 18 566) | VKA (n = 70,176) | DOAC (n = 28,113) | VKA (n = 44,705) | |
| Follow‐up, y (mean, SD) | 0.8 (0.7) | 2.7 (2.0) | 1.5 (1.1) | 2.6 (1.9) | # | # | 1.7 (1.2) | 3.7 (2.2) |
| Females | 44.0 | 43.3 | 46.4 | 47.8 | 54.3 | 51.9 | 46.4 | 41.3 |
| Age | ||||||||
| Mean age at index date (y, SD) | 74.8 (11.0) | 73.8 (10.4) | 75.6 (10.0) | 75.4 (10.8) | 74.8 (11.4) | 73.9 (9.6) | 73.4 (11.2) | 71.4 (11.2) |
| 18–55 y | 5.6 | 5.5 | 5.0 | 4.1 | 7.1 | 4.9 | 5.9 | 8.3 |
| 56–65 y | 12.9 | 13.8 | 12.5 | 11.0 | 12.2 | 11.8 | 16.3 | 18.6 |
| 66–75 y | 26.0 | 27.9 | 26.9 | 27.1 | 25.8 | 34.7 | 34.4 | 33.3 |
| 75+ y | 55.5 | 52.8 | 55.6 | 57.7 | 54.8 | 48.6 | 43.3 | 39.7 |
| Weight | ||||||||
| <50 kg | 1.1 | 0.7 | 2.0 | 2.0 | NA | NA | NA | NA |
| 50–100 kg | 28.1 | 28.7 | 39.1 | 45.2 | NA | NA | NA | NA |
| >100 kg | 6.2 | 7.0 | 3.1 | 4.1 | NA | NA | NA | NA |
| Missing | 64.6 | 63.6 | 56.9 | 49.7 | NA | NA | NA | NA |
| BMI | ||||||||
| Mean BMI at index date (SD) | 29.0 (6.3) | 29.5 (6.3) | 29.7 (5.1) | 30.2 (5.3) | NA | NA | NA | NA |
| <20 kg/m2 | 1.6 | 1.2 | 0.5 | 0.5 | NA | NA | NA | NA |
| 20–24.9 kg/m2 | 7.5 | 7.1 | 6.3 | 6.4 | NA | NA | NA | NA |
| 25–29.9 kg/m2 | 12.7 | 12.9 | 16.6 | 19.1 | NA | NA | NA | NA |
| 30–34.9 kg/m2 | 8.0 | 8.7 | 12.6 | 15.1 | NA | NA | NA | NA |
| ≥35 kg/m2 | 5.3 | 6.1 | 5.3 | 7.8 | NA | NA | NA | NA |
| Missing | 64.8 | 63.9 | 58.7 | 51.1 | NA | NA | NA | NA |
| Smoking status | ||||||||
| Never | 38.2 | 37.6 | 9.5 | 11.2 | NA | NA | NA | NA |
| Current | 11.2 | 11.1 | 35.9 | 41.6 | NA | NA | NA | NA |
| Ex | 50.4 | 51.0 | 4.3 | 8.1 | NA | NA | NA | NA |
| Missing | 0 | 0 | 50.4 | 39.0 | NA | NA | NA | NA |
| Alcohol | ||||||||
| Yes | 9.6 | 6.7 | 18.3 | 22.6 | 0.0 | 0.0 | 4.8 | 4.3 |
| Missing | 47.8 | 36.4 | ||||||
| Renal function | ||||||||
| Normal (>80 mL/min) | 16.6 | 12.8 | NA | NA | NA | NA | ||
| Normal – Mildly reduced (CrCl 50–80 mL/min) | 45.4 | 44.9 | 12.3* | 18.0* | NA | NA | NA | NA |
| Moderately reduced (CrCl 30–49 mL/min) | 20.0 | 22.1 | 4.5* | 6.4* | NA | NA | NA | NA |
| Severely reduced (CrCl 15–29 mL/min) | 0.6 | 1.4 | 0.2 | 0.5 | NA | NA | NA | NA |
| Very severely reduced (CrCl <15 mL/min) | 0.0 | 0.2 | 0.0 | 0.1 | NA | NA | NA | NA |
| Missing | 17.3 | 18.6 | 83.0 | 75.0 | NA | NA | NA | NA |
| History of disease | ||||||||
| Other cardiovascular disease (angina, myocardial infarction, coronary heart disease, aortic plaque, PAD) | 24.8 | 27.0 | 19.3 | 19.6 | 67.2 | 65.8 | 28.1 | 31.8 |
| Chronic kidney disease ** | n/a | n/a | 5.0 | 7.1 | 5.8 | 6.6 | 3.6 | 5.7 |
| Congestive heart failure | 9.6 | 11.8 | 10.2 | 11.9 | 42.8 | 42.3 | 13.7 | 17.2 |
| Deep vein thrombosis/pulmonary embolism | 2.2 | 3.2 | 1.5 | 2.4 | 8.1 | 7.2 | 2.9 | 5.1 |
| Diabetes mellitus | 18.4 | 17.7 | 21.8 | 25.3 | 43.1 | 42.8 | 11.9 | 13.1 |
| Hypertension | 4.6 | 5.1 | 4.6 | 5.4 | 86.0 | 85.5 | 21.2 | 22.0 |
| Hepatic impairment (moderate/severe) | 0,0 | 0.1 | 0.2 | 0.3 | 14.7 | 12.1 | 1.0 | 1.1 |
| Malignancy, including lymphoma and leukaemia and metastatic solid tumour, except malignant neoplasm of the skin | 2.0 | 2.2 | 0.9 | 1.2 | 18.5 | 17.7 | 3.2 | 3.7 |
| Major bleeding event | 32.2 | 29.5 | 15.7 | 15.4 | 33.7 | 24.5 | 19.3 | 17.5 |
| Peptic ulcer disease | 6.4 | 6.0 | 3.1 | 4.9 | 10.8 | 8.0 | 6.8 | 6.9 |
| Stroke/TIA | 21.1 | 17.4 | 11.8 | 11.0 | 25.2 | 20.6 | 19.4 | 17.5 |
| Thrombocytopenia | 0.0 | 0.1 | 0.2 | 8.0 | 1.4 | 1.5 | 0.1 | 0.1 |
| Drug use within 6 months prior to index date | ||||||||
| Antihypertensive drugs *** | 79.6 | 83.2 | 77.4 | 81.9 | 93.9 | 91.0 | 88.2 | 89.9 |
| Antidiabetic drugs (including insulin) | 12.7 | 13.0 | 18.3 | 20.6 | 20.5 | 20.9 | 12.4 | 12.9 |
| Antiplatelet drugs | 49.3 | 58.2 | 44.1 | 40.9 | 22.6 | 18.1 | 45.2 | 52.3 |
| Systemic glucocorticoids | 10.7 | 10.6 | 7.7 | 7.8 | 10.6 | 8.8 | 8.8 | 9.4 |
| NSAIDs | 10.8 | 12.4 | 28.7 | 26.2 | 26.3 | 23.9 | 16.0 | 16.5 |
| SSRIs | 8.3 | 6.4 | 9.2 | 8.7 | 4.7 | 3.1 | 8.1 | 8.0 |
VKA, vitamin K antagonist; DOAC, direct oral anticoagulant; BMI, body mass index; PAD, peripheral artery disease; TIA, transient ischaemic attack; CrCl, creatinine clearance; AOK, Allgemeine Ortskrankenkasse
Not possible due to quarterly updates.
Coding of renal function differs in the BIFAP database, where CrCl ≥ 60 mL/min is considered normal kidney function, and therefore there is no coding for 80 mL/min. Therefore, BIFAP category 30–49 mL/min was considered corresponding to category 30–59 mL/min in the other sources.
For those databases that do not have lab‐values available for renal function.
Antihypertensive drugs include angiotensin‐converting enzyme inhibitors, angiotensin‐II‐blockers, β‐blockers, calcium channel blockers, diuretics, doxazosine and moxonidin.
Figure 1 shows the incidence rates HRs for the occurrence of a first major bleeding event during follow‐up for current users of VKAs and current users of DOACs. Although there was some variation in incidence rates between data sources, fully adjusted HRs for current use of DOACs vs VKA were comparable between sources with estimates around unity in 3 of the 4 data sources, except for Denmark, where there was a statistically significant 16% lower risk of bleeding events. Generally, there was minimal impact of adjusting for confounding in all data sources. For individual DOACs, only rivaroxaban was associated with an increased risk of bleeding events (in both CPRD and AOK, adjusted HR around 1.27 [95% CI: 1.12–1.44] and 1.20 [95% CI: 1.13–1.27], respectively). Dabigatran and apixaban were not associated with an increased risk of any bleeding in any data source.
FIGURE 1.
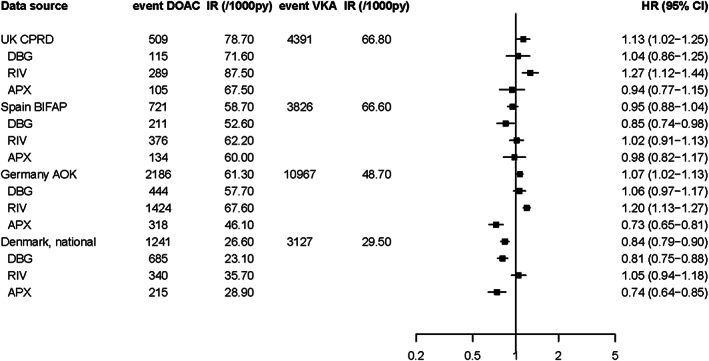
Risk of major bleeding in 4 European data sources for DOACs vs VKAs, overall and by DOAC type #. VKA, vitamin K antagonist; DOAC, direct oral anticoagulant; IR, incidence rate; PY, person‐years; HR, hazard ratio; CI, confidence interval; DBG, dabigatran; RIV, rivaroxaban; APX, apixaban. # HRs were adjusted for age, sex, body mass index, smoking, alcohol, hypertension, renal failure, history of stroke/transient ischaemic attack, deep venous thromboembolism/pulmonary embolism, malignancy, peptic ulcer disease, thrombocytopenia, moderate/severe hepatic impairment, history of major bleeding, use of antiplatelet drugs, NSAIDs, SSRIs, systemic glucocorticoids
When assessing bleeding site, current DOAC use was associated with an increased risk of GI bleeding events compared with current VKA use, which was found in 3 data sources with statistically significant increased risks of 26–40%. In the Danish National Registers, the HR was around unity (Figure 2A). These effects were mainly driven by dabigatran and rivaroxaban, as the HR for apixaban was around 1.10 in both BIFAP and CPRD, and protective in both AOK (adjusted HR 0.80, 95% CI: 0.66–0.96) and Denmark (adjusted HR 0.74, 95% CI: 0.60–0.92). The incidence of intracranial bleeding (Figure 2B) was low in all 4 data sources and point estimates were <1 in all, except for a statistically significant increased risk for rivaroxaban in CPRD (adjusted HR 2.37, 95% CI 1.19–4.71). When assessing (any) stroke events (Figure 2C) there was a statistically significant increased overall risk for DOACs vs VKA in CPRD with significantly increased risks for rivaroxaban (adjusted HR 1.78, 95% CI:1.29–2,44) and apixaban (adjusted HR 2.20, 95% CI: 1.45–3.35). In BIFAP (adjusted HRs 1.20–1.14), AOK (adjusted HRs 0.60–0.98) and Denmark (adjusted HRs 1.03–1.11), this effect was not found.
FIGURE 2.
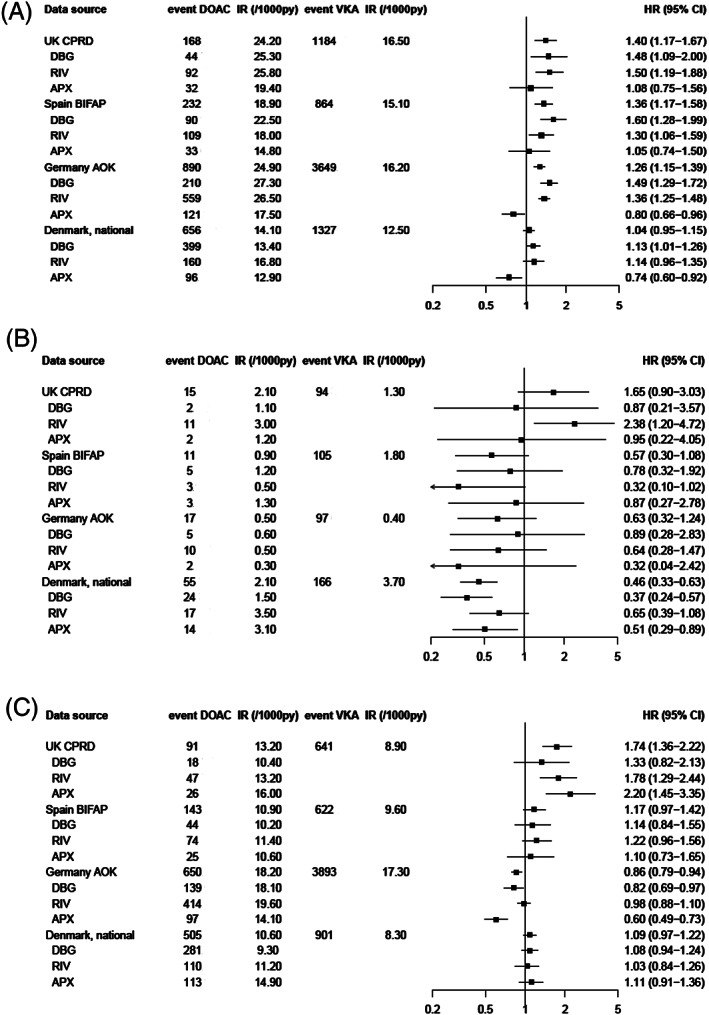
Risk of gastrointestinal bleeding# (a), intracranial bleeding# (B) and any stroke † (C) in 4 European data sources for DOACs vs VKAs, overall and by DOAC type. VKA, vitamin K antagonist; DOAC, direct oral anticoagulant; IR, incidence rate; PY, person‐years; HR, hazard ratio; CI, confidence interval; DBG, dabigatran; RIV, rivaroxaban; APX, apixaban. # HRs were adjusted for age, sex, body mass index, smoking, alcohol, hypertension, renal failure, history of stroke/transient ischaemic attack, deep venous thromboembolism/pulmonary embolism, malignancy, peptic ulcer disease, thrombocytopenia, moderate/severe hepatic impairment, history of major bleeding, use of antiplatelet drugs, NSAIDs, SSRIs, systemic glucocorticoids. † HRs are adjusted for age, sex, body mass index, smoking, alcohol, hypertension, renal failure, history of stroke/transient ischaemic attack, deep venous thromboembolism/pulmonary embolism, congestive heart failure, diabetes, other cardiovascular disease, renal failure
Several sensitivity analyses were performed to assess the robustness of our results. First, the impact of changing the permissible gap length between prescriptions was assessed in CPRD, BIFAP and Denmark. Using a stricter definition resulted in a marginal increase of bleeding risk in CPRD and decrease in BIFAP, giving stronger effects in both data sources. Being more lenient resulted in HRs going toward unity in CPRD and going toward 1.10 in BIFAP. Impact on estimates in Denmark was minimal compared to the main definition (data not shown). Second, restriction of our cohorts to patients not having any other indications than NVAF did not yield different results in all 4 data sources. Third, a posthoc redefinition of major bleeding where nonmajor bleeding events (e.g. haematuria) were excluded lead to the retention of GI and intracranial bleedings with risk estimates reflective of those results (data not shown). Fourth, as both AOK and the Danish National Registers use hospital admissions for assessment of outcomes, a sensitivity analysis was performed within CPRD using bleeding events from hospital admission data (HES) only. The adjusted HR for HES‐events in CPRD was 1.14 (95% CI 0.96–1.35), thus similar to the results obtained using all primary care records. Figures 3A (GI bleeding) and 3B (any stroke) show risk estimates when using comparable type of outcomes in all 4 data sources, i.e. hospital admissions (CPRD, AOK, Denmark) and/or validated events (BIFAP). In CPRD, the adjusted HR for GI bleeding events leading to hospital admission was similar to the original result. In BIFAP, the adjusted HR changed from 1.39 (95% CI: 1.21–1.60) to 1.13 (95% CI: 0.83–1.56), but results remained consistent to the primary analysis. For stroke, the magnitude of the adjusted HRs in both CPRD and BIFAP was lower compared to the main analysis, but statistically significant increased in CPRD (adjusted HR 1.42, 95% CI: 1.01–1.99) with rivaroxaban (adjusted HR 1.50, 95% CI: 0.96–2.33) and especially apixaban (adjusted HR 2.07, 95% CI: 1.13–3.82) driving this effect. In the other 3 data sources, no increased risks of stroke were observed.
FIGURE 3.
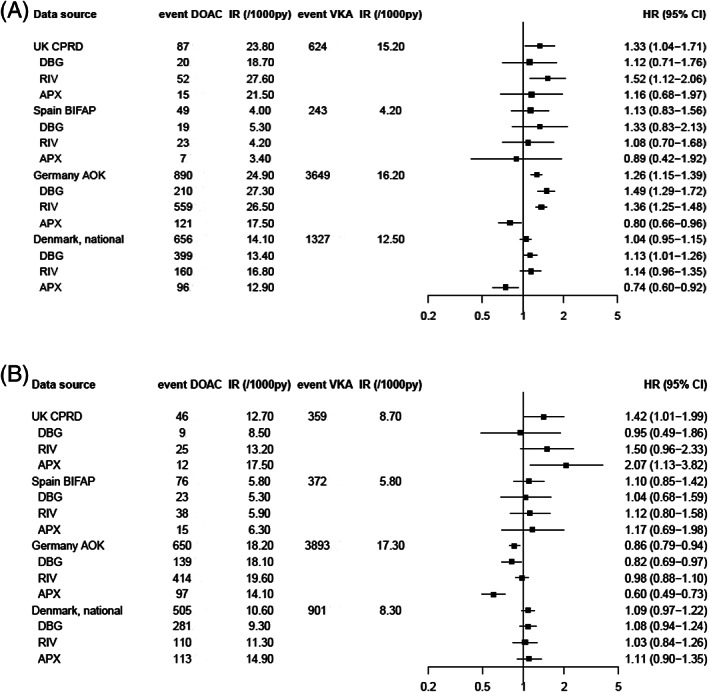
Risk of gastrointestinal bleeding# (a) and any stroke† (B) in 4 European data sources for DOACs vs VKAs, overall and by DOAC type, sensitivity analysis restricted to hospital admission (CPRD, AOK, Denmark)/validated (BIFAP) events only. VKA, vitamin K antagonist; DOAC, direct oral anticoagulant; IR, incidence rate; PY, person‐years; HR, hazard ratio; CI, confidence interval; DBG, dabigatran; RIV, rivaroxaban; APX, apixaban. # HRs were adjusted for age, sex, body mass index, smoking, alcohol, hypertension, renal failure, history of stroke/transient ischaemic attack, deep venous thromboembolism/pulmonary embolism, malignancy, peptic ulcer disease, thrombocytopenia, moderate/severe hepatic impairment, history of major bleeding, use of antiplatelet drugs, NSAIDs, SSRIs, systemic glucocorticoids. † HRs are adjusted for age, sex, body mass index, smoking, alcohol, hypertension, renal failure, history of stroke/transient ischaemic attack, deep venous thromboembolism/pulmonary embolism, congestive heart failure, diabetes, other cardiovascular disease, renal failure
4. DISCUSSION
This multicountry study across 4 European databases showed that the overall HRs of major bleeding risk for DOACs vs VKAs ranged between 0.84 (Denmark) and 1.13 (UK). When stratifying according to the type of bleeding site, the risk of GI bleeding was statistically significantly increased by 48–67% in users of dabigatran and 30–50% for rivaroxaban users compared to VKA users in all data sources except for Denmark. Compared to VKAs, apixaban was not associated with an increased risk of GI bleeding in all data sources and seemed to be associated with the lowest risk of major bleeding events compared to dabigatran and rivaroxaban vs VKAs. In contrast, the risk of intracranial bleeding was lower for DOACs compared to VKAs in all databases, except for rivaroxaban in CPRD, which showed a significantly higher risk compared to VKAs although the number of events was low.
When stratifying according to clinically relevant subgroups, we found no remarkable differences between males and females and a generally higher risk of major bleeding among DOAC users aged ≥75 years when compared to younger users, which seemed to be driven by the higher risk of rivaroxaban in CPRD. This finding could suggest that the benefit–risk balance of especially rivaroxaban might be different for an older patient population. As the incidence of AF increases with older age, this is important information. Due to low number of patients with extreme low or high weight and impaired renal function, we could not identify differences in benefit–risk balance for this subgroup of patients. From a clinical perspective, it seems reassuring that physicians do not seem to prescribe DOACs to such vulnerable patients in daily clinical practice at least for DOAC compounds with a substantial renal elimination (e.g. dabigatran).
Several observational studies have been carried out to address the safety and effectiveness of DOACs in the recent past. 14 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 Although these studies differ in study design, reporting of outcomes and comparisons, the results found are generally the same: DOACs are safe and effective alternatives to warfarin. Similar to our study a propensity‐weighted nationwide cohort study in Denmark showed that rivaroxaban is associated with higher risk of major bleeding. 14 This finding is not restricted to Europe: a study by Lip et al. in a US claims database also found that rivaroxaban had a worse safety profile than dabigatran and apixaban. 23 Similar observations have been made in other studies as well. 27 , 29 , 30 , 31 The difference found for rivaroxaban might be explained by selective prescribing of this drug to patients with higher stroke and bleeding risk. Although the analysis was corrected for confounding, unobserved confounding could still be present. However, for all analyses, there was no large difference between the unadjusted and adjusted estimates. A recent study by Vinogravova et al. found a lower risk of major bleeding for DOACs compared to warfarin in CPRD in a similar time period with especially apixaban being associated with a decreased risk of major bleeding. 32 When comparing their results to ours, point estimates for dabigatran and rivaroxaban are relatively comparable, but their estimate for apixaban (HR 0.75, 95% CI: 0.49–1.14) is not (HR 1.25, 95% CI: 0.90–1.74), although it can be argued that confidence intervals overlap. Differences in methodological approach (cohort selection strategy, first vs all treatment episodes, exclusion vs adjustment for prior history of outcome events) could be responsible. These types of discrepancies are not uncommon in database studies 33 and again stress the importance of transparency and sharing programming syntax to help researchers understand the decision‐making process underlying the creation of the final data analysis matrix.
Although the aim of this study was mainly to look at major bleeding outcomes, we also assessed the risk of any stroke. The reason for performing this analysis was that many stroke events tend to be classified with a generic rather than specific code in electronic healthcare databases. Results differed across databases, with statistically significant increased risks of stroke for DOAC vs VKAs in CPRD, but not in the other 3 data sources. The effect seemed to be present for rivaroxaban and apixaban. Restricting outcome to hospital admissions lowered the risk estimates, but pointed in the same direction. A previous study in CPRD with fewer stroke events did not show a statistically significant difference for DOACs vs VKAs (adjusted HR 1.38, 0.81–2.35). 34 The reason for this finding and the difference with respect to the other sources is not immediately obvious. For apixaban, its later introduction compared to dabigatran and rivaroxaban and its marketing as being potentially safer with respect to GI bleeding could be a factor, potentially leading to channelling bias with apixaban being prescribed to a more fragile patient population with an increased baseline bleeding risk. However, this is not reflected in the risks found for bleeding events for apixaban, which are generally lower compared to the other DOACs.
The strength of this study was that we combined several renowned electronic health care databases from 4 countries using a common protocol in a network of centres with experience in collaborating on harmonised study protocols. 35 The use of different types of data sources captured a broad spectrum of data with the option of harmonising similar types of outcome event for some important subgroups. We carefully conducted a variety of sensitivity analyses to assess the impact of changing definitions on the associations found in this study. A further strength is that we looked at all treatment periods with DOACs and VKAs, rather than first treatment episode only, as this reflects all 3 pillars of treatment adherence to anticoagulant therapy in daily clinical practice. 36 The finding that the reduction in bleeding risk for DOACs in our study does not resemble the reductions found in RCTs, could at least be partially attributable to nonadherence and to the limited external validity of RCTs reflecting real life. This finding suggests that looking further into the reasons for nonadherence in oral anticoagulant use is important for future research into risk–benefit assessment of DOACs under real‐life conditions. A recent study by Komen et al. using data from Sweden showed that nonpersistence and poor adherence to DOAC treatment were both associated with increased stroke risk. 37
There are also some limitations, either due to the observational nature of the data or inherent to the conduct of multicountry database studies. Residual confounding always remains a possibility, although there was just small impact of confounder adjustment on risk estimates. We performed a substantial number of different comparisons and sensitivity analyses, both prespecified and posthoc. The different data sources in our study required different approaches to define prescription length, which could have resulted in exposure misclassification. However, our sensitivity analyses where we changed the permissible gap, to account partially for this misclassification, did not lead to different HRs. We did not take into account adjustment for multiple testing, given the fact that the trade‐off between type I and type II errors can also lead to false securities by rejecting the null hypothesis for associations that are not null. 38 We did not have information on INR measurements, meaning that we do not know how well controlled the patients on VKA treatment were.
The data sources we used were diverse with respect to the type of outcome events measured, the coding systems used, and health care system in general. Our definition of the primary outcome based on the definition by the International Society on Thrombosis and Haemostasis, 16 but an exact replication was difficult as data on blood transfusions and blood loss is not available in electronic healthcare sources. Many outcome events that in daily clinical practice are unlikely to represent genuinely major bleeding events, such as epistaxis, haematuria and conjunctival bleeding, were included. Hospital admissions for such events are an indicator for severity, but our sensitivity analysis showed that the majority of events were than restricted to GI and intracranial sites and with HRs following the direction of the individual outcome events. Even for GI bleeding, the actual severity can be doubtful (i.e. nonsevere haemorrhoidal bleeding). However, it can be argued that even such complaints were serious enough for patients to visit a health care provider and, when restricting to hospital admission events, can be considered to be major from a clinical perspective. In our study we did not have opportunity to validate major bleeding events, except for GI bleeding and stroke in BIFAP. Manual review of patients' clinical profiles including GP free text comments in BIFAP showed a low positive predictive value (62%) to identify hospitalized or blood transfused patients with GI bleeding while the negative predictive value was high (95%). Ruigomez et al. found that the positive predictive value of using Read codes for assessment of major bleeding events was 70.4% for GI bleeding and 64.1% for urogenital bleeding as compared to 96.6% for intracranial bleeding, leading to overestimated incidence rates of major bleeding in the absence of validation using additional information. 39 Still, restricting CPRD‐analyses to hospital admissions only yielded similar risk estimates.
Despite identifying the same outcome events in all data sources, we cannot exclude some misclassification due to noncomparability of codes underlying the major bleeding events. The 4 data sources constitute a combination of primary care data, hospital admission data and claims data. Completeness of diagnoses from medical specialists in primary care data sources might be a concern. In the German AOK, the quarterly updates of comorbidities made time‐varying confounder assessment not possible. Differences in healthcare systems, access to and use of health care and differences in policies in prescribing DOACs both on a national or local level are all sources of variation that could impact comparability between the 4 data sources used in this study.
In conclusion, data from electronic health care databases in 4 European countries showed a consistent pattern where current use of DOACs had a similar risk of major bleeding compared to current use of VKAs. When stratifying according to the site of bleeding event, the risk of GI bleeding was statistically significant increased by 48–67% in dabigatran users and 30–50% for rivaroxaban users compared to VKA users in all data sources except Denmark. In general, apixaban seemed to have a better risk profile for major bleeding compared to dabigatran and rivaroxaban in Denmark, Germany and Spain, while an increased risk of any stroke was seen in the UK population. Reasons for nonadherence to DOAC treatment and its impact on bleeding risk should be explored further in future studies.
COMPETING INTERESTS
The research leading to these results was conducted as part of the activities of the PE & PV (Pharmacoepidemiology and Pharmacovigilance) Research Network which is a public academic partnership coordinated by the University of Utrecht, The Netherlands. The project has received support from the European Medicines Agency under the Framework service contract nr EMA/2015/27/PH. This document expresses the opinion of the authors of the paper, and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or 1 of its committees or working parties, or of the participating centres of the Research Network. O.H.K. has provided an educational lecture (nonproduct related) for Roche in May 2017.
M.An. reports research grants from AstraZeneca, H. Lundbeck & Mertz, Janssen, Merck Sharp & Dohme, Novartis, Nycomed, and Pfizer, received by the institutions at which he has been employed. M.An. has received fees for organizing and teaching pharmacoepidemiology courses at Medicademy, the Danish Association for the Pharmaceutical Industry. M.An.’s professorship is supported by a grant from the Novo Nordisk Foundation to the University of Copenhagen (NNF15SA0018404).
CONTRIBUTORS
P.S. wrote the paper. P.S. and H.v.d.H. participated in the design and data analysis of CPRD. C.H., E.M.M., D.M. and L.L.M. participated in the design, data analysis of BIFAP. M.R. and S.S. participated in the design, data analysis of AOK Nordwest database. A.H. participated in the data analysis of AOK Nordwest database. M.Aa., M.An. and M.d.B. participated in the design, data analysis of DNR data. O.H.K. and H.G. coordinated the whole project. All other authors participated in the conception and design, interpretation of data, and revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
APPENDIX A.
List of confounders assessed in cohort study with time window (list of relevant codes can be found in the online study protocol (http://www.encepp.eu/encepp/viewResource.htm?id=28664)
| Confounder | Time window |
|---|---|
| For major bleeding | |
| Comorbidities | |
| Major bleeding | Ever before index date ** |
| Hypertension | 6 months before index date |
| Stroke/TIA | Ever before index date |
| Deep venous thromboembolism/pulmonary embolism | Ever before index date |
| Alcohol | Ever before index date |
| Any malignancy, including lymphoma and leukaemia and metastatic solid tumour, except malignant neoplasm of the skin | 6 months before index date |
| Gastrointestinal ulcer | Ever before index date |
| Thrombocytopenia | 6 months before index date |
| Hepatic impairment | Ever before index date |
| For ischaemic stroke/TIA | |
| Stroke/TIA | Ever before index date |
| Deep venous thromboembolism/pulmonary embolism | Ever before index date |
| Hypertension | 6 months before index date |
| Congestive heart failure | Ever before index date |
| Diabetes ** | Ever before index date |
| Other cardiovascular disease—coronary heart disease (myocardial infarction/angina pectoris), atherosclerotic plaque, peripheral vascular disease | Ever before index date |
| Medication use | 6 months before index date |
| Nosteroidal anti‐inflammatory drugs | 6 months before index date |
| Selective serotonin reuptake inhibitors | 6 months before index date |
| Corticosteroids | 6 months before index date |
| Antiplatelet drugs | 6 months before index date |
| Antidiabetic drugs | 6 months before index date |
| Antihypertensive drugs | 6 months before index date |
| Diuretics | |
| Beta blocking agents | |
| Calcium channel blockers | |
| Angiotensin‐converting enzyme inhibitors | |
| Angiotensin II antagonists | |
| Moxonidin | |
| Doxazosin | |
index date is the first prescription for an oral anticoagulant;
besides a clinical diagnosis for diabetes a patient was also able to get marked as somebody with.
TIA, transient ischaemic attack
Souverein PC, van den Ham HA, Huerta C, et al. Comparing risk of major bleeding between users of different oral anticoagulants in patients with nonvalvular atrial fibrillation. Br J Clin Pharmacol. 2021;87:988–1000. 10.1111/bcp.14450
Major bleeding risk: DOACs vs VKA
On behalf of the EU PE&PV Research Network: Luisa Ibanez, Elena Ballarín, Mónica Sabaté, Xavier Vidal, Nicholas Moore, Cecile Droz, Régis Lassalle, Henriette Thisted Horsdal, Rolf Groenwold, Katrin Janhsen
This project was requested by Pharmacovigilance Risk Assessment Committee of the European Medicines Agency, which involves a member representing patients organizations nominated by the European Commission. During the actual study, patients were not invited to comment on the design and were not invited to contribute to the writing or editing of this document for readability or accuracy.
DATA AVAILABILITY STATEMENT
Sharing of data is not allowed due to regulations imposed by the data suppliers.
REFERENCES
- 1. Chugh SS, Roth GA, Gillum RF, Mensah GA. Global burden of atrial fibrillation in developed and developing nations. Glob Heart. 2014;9(1):113‐119. [DOI] [PubMed] [Google Scholar]
- 2. Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746‐2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjorck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population‐based study. Stroke. 2013;44(11):3103‐3108. [DOI] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893‐2962. [DOI] [PubMed] [Google Scholar]
- 5. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139‐1151. [DOI] [PubMed] [Google Scholar]
- 6. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093‐2104. [DOI] [PubMed] [Google Scholar]
- 7. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981‐992. [DOI] [PubMed] [Google Scholar]
- 8. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883‐891. [DOI] [PubMed] [Google Scholar]
- 9. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383(9921):955‐962. [DOI] [PubMed] [Google Scholar]
- 10. Miller CS, Dorreen A, Martel M, Huynh T, Barkun AN. Risk of gastrointestinal bleeding in patients taking non‐vitamin K antagonist Oral anticoagulants: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2017;15(11):1674‐1683. e3 [DOI] [PubMed] [Google Scholar]
- 11. Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131(2):157‐164. [DOI] [PubMed] [Google Scholar]
- 13. Larsen TB, Rasmussen LH, Skjoth F, et al. Efficacy and safety of dabigatran etexilate and warfarin in "real‐world" patients with atrial fibrillation: a prospective nationwide cohort study. J am Coll Cardiol. 2013;61(22):2264‐2273. [DOI] [PubMed] [Google Scholar]
- 14. Larsen TB, Skjoth F, Nielsen PB, Kjaeldgaard JN, Lip GY. Comparative effectiveness and safety of non‐vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smythe MA, Forman MJ, Bertran EA, Hoffman JL, Priziola JL, Koerber JM. Dabigatran versus warfarin major bleeding in practice: an observational comparison of patient characteristics, management and outcomes in atrial fibrillation patients. J Thromb Thrombolysis. 2015;40(3):280‐287. [DOI] [PubMed] [Google Scholar]
- 16. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3(4):692‐694. [DOI] [PubMed] [Google Scholar]
- 17. Gardarsdottir H, Souverein PC, Egberts TC, Heerdink ER. Construction of drug treatment episodes from drug‐dispensing histories is influenced by the gap length. J Clin Epidemiol. 2010;63(4):422‐427. [DOI] [PubMed] [Google Scholar]
- 18. Carmo J, Moscoso Costa F, Ferreira J, Mendes M. Dabigatran in real‐world atrial fibrillation. Meta‐analysis of observational comparison studies with vitamin K antagonists. Thromb Haemost. 2016;116:754‐763. [DOI] [PubMed] [Google Scholar]
- 19. Forslund T, Wettermark B, Andersen M, Hjemdahl P. Stroke and bleeding with non‐vitamin K antagonist oral anticoagulant or warfarin treatment in patients with non‐valvular atrial fibrillation: a population‐based cohort study. Europace. 2018;20(3):420‐428. [DOI] [PubMed] [Google Scholar]
- 20. Gorst‐Rasmussen A, Lip GY, Bjerregaard Larsen T. Rivaroxaban versus warfarin and dabigatran in atrial fibrillation: comparative effectiveness and safety in Danish routine care. Pharmacoepidemiol Drug Saf. 2016;25(11):1236‐1244. [DOI] [PubMed] [Google Scholar]
- 21. Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for Nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176(11):1662‐1671. [DOI] [PubMed] [Google Scholar]
- 22. Hohnloser SH, Basic E, Hohmann C, Nabauer M. Effectiveness and safety of non‐vitamin K Oral anticoagulants in comparison to Phenprocoumon: data from 61,000 patients with atrial fibrillation. Thromb Haemost. 2018;118:526‐538. [DOI] [PubMed] [Google Scholar]
- 23. Lip GY, Keshishian A, Kamble S, et al. Real‐world comparison of major bleeding risk among non‐valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. A propensity score matched analysis. Thromb Haemost. 2016;116:975‐986. [DOI] [PubMed] [Google Scholar]
- 24. Maura G, Blotiere PO, Bouillon K, et al. Comparison of the short‐term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity‐matched cohort study. Circulation. 2015;132(13):1252‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ujeyl M, Koster I, Wille H, et al. Comparative risks of bleeding, ischemic stroke and mortality with direct oral anticoagulants versus phenprocoumon in patients with atrial fibrillation. Eur J Clin Pharmacol. 2018;74(10):1317‐1325. [DOI] [PubMed] [Google Scholar]
- 26. Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and Apixaban versus warfarin in Nonvalvular atrial fibrillation. J am Heart Assoc. 2016;5:e003725. 10.1161/JAHA.116.003725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graham DJ, Baro E, Zhang R, et al. Comparative stroke, bleeding, and mortality risks in older Medicare patients treated with Oral anticoagulants for Nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596‐604, e11. [DOI] [PubMed] [Google Scholar]
- 28. Chan YH, Kuo CT, Yeh YH, et al. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with Nonvalvular atrial fibrillation. J am Coll Cardiol. 2016;68(13):1389‐1401. [DOI] [PubMed] [Google Scholar]
- 29. Tepper PG, Mardekian J, Masseria C, et al. Real‐world comparison of bleeding risks among non‐valvular atrial fibrillation patients prescribed apixaban, dabigatran, or rivaroxaban. PLoS ONE. 2018;13(11):e0205989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hernandez I, Zhang Y, Saba S. Comparison of the effectiveness and safety of Apixaban, dabigatran, rivaroxaban, and warfarin in newly diagnosed atrial fibrillation. Am J Cardiol. 2017;120(10):1813‐1819. [DOI] [PubMed] [Google Scholar]
- 31. Fralick M, Colacci M, Schneeweiss S, Huybrechts KF, Lin KJ, Gagne JJ. Effectiveness and safety of Apixaban compared with rivaroxaban for patients with atrial fibrillation in routine practice: a cohort study. Ann Intern Med. 2020;172(7):463–473. [DOI] [PubMed] [Google Scholar]
- 32. Vinogradova Y, Coupland C, Hill T, Hippisley‐Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ. 2018;362:k2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Vries F, de Vries C, Cooper C, Leufkens B, van Staa TP. Reanalysis of two studies with contrasting results on the association between statin use and fracture risk: the general practice research database. Int J Epidemiol. 2006;35(5):1301‐1308. [DOI] [PubMed] [Google Scholar]
- 34. Gieling EM, van den Ham HA, van Onzenoort H, et al. Risk of major bleeding and stroke associated with the use of vitamin K antagonists, nonvitamin K antagonist oral anticoagulants and aspirin in patients with atrial fibrillation: a cohort study. Br J Clin Pharmacol. 2017;83(8):1844‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klungel OH, Kurz X, de Groot MC, et al. Multi‐Centre, multi‐database studies with common protocols: lessons learnt from the IMI PROTECT project. Pharmacoepidemiol Drug Saf. 2016;25(Suppl 1):156‐165. [DOI] [PubMed] [Google Scholar]
- 36. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Komen JJ, Heerdink ER, Klungel OH, Mantel‐Teeuwisse AK, Forslund T, Wettermark B, Hjemdahl P. Long‐term persistence and adherence with non‐vitamin K oral anticoagulants in patients with atrial fibrillation and their associations with stroke risk. Eur Heart J Cardiovasc Pharmacother. 2020. 10.1093/ehjcvp/pvaa017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43‐46. [PubMed] [Google Scholar]
- 39. Ruigomez A, Brobert G, Suzart‐Woischnik K, Garcia‐Rodriguez LA. Ascertainment and validation of major bleeding events in a primary care database. Pharmacoepidemiol Drug Saf. 2019;28(2):148‐155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sharing of data is not allowed due to regulations imposed by the data suppliers.


