Abstract
Aims
Prescribing medication is a complex process that, when done inappropriately, can lead to adverse drug events, resulting in patient harm and hospital admissions. Worldwide cost is estimated at 42 billion USD each year. Despite several efforts in the past years, medication‐related harm has not declined. The aim was to determine whether a prescriber‐focussed participatory action intervention, initiated by a multidisciplinary pharmacotherapy team, is able to reduce the number of in‐hospital prescriptions containing ≥1 prescribing error (PE), by identifying and reducing challenges in appropriate prescribing.
Methods
A prospective single‐centre before‐ and after study was conducted in an academic hospital in the Netherlands. Twelve clinical wards (medical, surgical, mixed and paediatric) were recruited.
Results
Overall, 321 patients with a total of 2978 prescriptions at baseline were compared with 201 patients with 2438 prescriptions postintervention. Of these, m456 prescriptions contained ≥1 PE (15.3%) at baseline and 357 prescriptions contained ≥1 PEs (14.6%) postintervention. PEs were determined in multidisciplinary consensus. On some study wards, a trend toward a decreasing number of PEs was observed. The intervention was associated with a nonsignificant difference in PEs (incidence rate ratio 0.96, 95% confidence interval 0.83–1.10), which was unaltered after correction. The most important identified challenges were insufficient knowledge beyond own expertise, unawareness of guidelines and a heavy workload.
Conclusion
The tailored interventions developed with and implemented by stakeholders led to a statistically nonsignificant reduction in inappropriate in‐hospital prescribing after a 6‐month intervention period. Our prescriber‐focussed participatory action intervention identified challenges in appropriate in‐hospital prescribing on prescriber‐ and organizational level.
Keywords: clinical pharmacology, medical education, medication errors, medication safety, prescribing
What is already known about this subject
Despite several interventions over recent years, medication‐related harm due to inappropriate prescribing still occurs.
Intervention studies conducted thus far have focused on specific patient populations, have been mainly pharmacist‐led and often solely focus on 1 factor influencing prescribing.
What this study adds
To succeed, participatory action research requires substantial commitment of stakeholders.
Reducing inappropriate prescribing is not a quick fix but instead requires commitment to improve and situational awareness on medication safety.
Future studies should therefore involve in‐depth research to identify factors contributing to inappropriate prescribing (technical and nontechnical skills) and mitigation strategies to reduce this complex challenge.
1. INTRODUCTION
Prescribing is a complex process influenced by various factors, such as pharmacotherapeutical knowledge, 1 , 2 prescribing skills, 1 , 3 access to and use of local and national guidelines, 2 workload, 4 , 5 and patient‐related factors (e.g. impaired renal function, age, polypharmacy). 4 Appropriate prescribing can be defined as the 5 rights: the right drug, right dose, right route, right timing (frequency and duration), and right patient. 6 Inappropriate prescribing can lead to adverse drug events (ADEs), resulting in patient harm, hospital admissions, and healthcare costs. 7 Recent reports estimate that 5–7% of all hospital admissions in economically developed countries are medication related. Approximately 66% are due to inappropriate prescribing. Associated worldwide costs are estimated at 42 billion USD each year. 7 , 8
Over the past decade, steps have been taken to solve this problem including programmes with a focus on specific medications and their harmful ADEs (e.g. nonsteroidal anti‐inflammatory drugs and antiplatelet drugs causing gastrointestinal tract bleeding 9 , 10 ), medication reconciliation 11 and the implementation of computerized physician order entry (CPOE) systems, often in combination with clinical decision support systems. Nonetheless, ADEs still occur. A recent report, commissioned by the Dutch government, 12 revealed a rise of 26% in (the absolute number of) medication‐related hospital admissions between 2008 and 2013. This is in line with international publications. 13
Intervention studies conducted thus far have focused on specific populations, such as intensive care unit patients, surgical patients or elderly, have been mainly pharmacist led and often solely focussing on 1 factor influencing prescribing. 14 , 15 , 16 However, keeping in mind that prescribing is multifactorial, not every prescriber or clinical facility faces the same challenges in appropriate prescribing, rejecting a one‐size‐fits all intervention. Therefore, we hypothesized that a participatory action research approach might be effective and sustainable, by tailoring interventions to prescribers in various clinical settings.
Participatory action research is characterized by the involvement of relevant stakeholders in the analysis of complex problems and in leading the change process. 17 Stakeholders are empowered to identify the problem's root causes as well as opportunities to improve, develop and implement a tailored plan of improvement. This process should result in sustainable improvement as stakeholders place value on an intervention they partially created (the IKEA effect 18 ). In primary care, this approach improved appropriate benzodiazepine prescribing for insomnia. 19 , 20 In in‐hospital care, a participatory action intervention was be effective in improving appropriate antimicrobial prescribing. 21
However, to our knowledge, no previous studies have investigated the effect of a participatory action intervention strategy in general in‐hospital prescribing. Our aim was to investigate whether this prescriber‐centred strategy, implemented and supervised by a multidisciplinary team consisting of doctors and pharmacists, can be used to (i) improve appropriate in‐hospital prescribing by (ii) identifying which factors influence prescribing.
2. METHODS
2.1. Study design and setting
This prospective single‐centre study was performed between June 2015 and April 2018 in Amsterdam UMC—location VU Medical Center (VUmc), a 733‐bed tertiary centre in the Netherlands. From all clinical wards, 12 clinical (nonintensive care unit) wards with the highest average number of admissions and prescriptions per patient were selected (hereafter referred to as study wards). All study wards (5 medical, 5 surgical and 2 mixed [1 acute admission and 1 paediatric]) voluntarily agreed to participate in this study. Each ward underwent a 10‐month intervention period including a baseline‐ and postintervention measurement (Figure 1). In line with participatory action research, each study ward themselves decided when the 10‐month intervention period started. During 3 weeks, the baseline and postintervention measurements were performed. The medical ethics review board of the Amsterdam UMC—location VUmc approved all study procedures (no. 2015.336).
FIGURE 1.
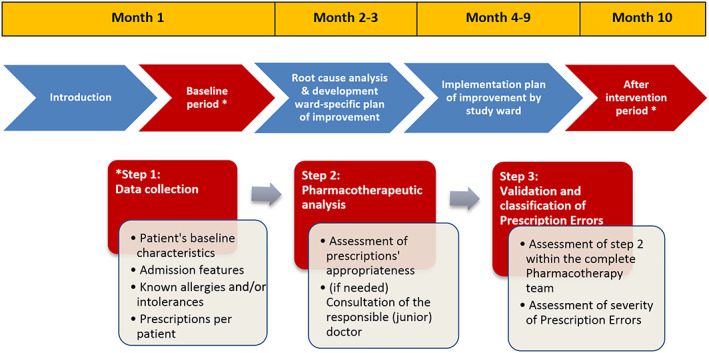
Flowchart of the 10‐month trajectory per study ward
The study was carried out by a multidisciplinary in‐hospital team (Pharmacotherapy team) consisting of an internist, a hospital pharmacist and 2 doctors, all clinical pharmacologists in training. These team members were selected since they represented both experts as well as key players in the prescribing process. The Pharmacotherapy team was supported by a quality improvement expert with extensive experience in root cause analysis and by medical students.
2.2. Outcome measures
The primary outcome was improvement in appropriate prescribing, defined as a 40% reduction in the number of prescriptions with ≥1 prescription errors (PEs) compared between baseline and postintervention period. A PE was defined in consensus within the team based on the definition by Dean et al.: an error in prescribing decision(s) and/or the (electronic) prescription writing process that could result in clinically relevant and significant harm to the patient or to a diminished effect of treatment. 22 Secondary outcomes were identification of challenges in appropriate prescribing by root cause analysis, the prevalence of patients with a PE and the prevalence of drugs that were associated with a PE categorized by their Anatomical Therapeutic Chemical (ATC) classification code.
2.3. Data collection
Patients were considered eligible if they were admitted for ≥24 hours to the study ward, had ≥5 drug prescriptions, and were not discharged from the study ward on the day of data collection. Two doctors/clinical pharmacologists in training of the Pharmacotherapy team, assisted by medical students, collected data from eligible patients, including patient characteristics (e.g. age, sex, medical history), admission features (e.g. date and reason of admission, if medication reconciliation had taken place), characteristics of registered medications (e.g. dosage, route of administration), relevant laboratory data (e.g. kidney function, international normalized ratio, electrolytes), registered drug allergies, intolerances and contraindications.
The appropriateness of each prescription was determined by assessing the indication, dosing frequency, dosage, route of administration, duration of therapy, clinically relevant interactions (e.g. drug–drug), and, when known, drug allergies and contraindications. Additionally, duplications and omissions were assessed based on pre‐hospital medication use). Appropriateness was defined as a prescription consistent with the recommendations of in‐hospital, national (e.g. The Royal Dutch Pharmacists Association database [KNMP Kennisbank]) or international evidence‐based guidelines. When deviated from these evidence‐based guidelines but with pathophysiological and/or evidence‐based arguments recorded in the patient's medical record, prescriptions were also considered appropriate. In case of queries and/or insufficient data from the patient record to assess appropriateness, the prescribing doctor or doctor responsible for the included patient was consulted by telephone or in person. Subsequently, all collected data and each inappropriate prescription according to these criteria including communication outcomes with involved doctors, were documented in a password‐protected Microsoft Access 2010 database (Microsoft Corporation, Redmond WA, USA).
Collected data were then assessed during consensus meetings, attended by the members of the Pharmacotherapy team, to identify PEs. The severity of identified PEs at time of detection was classified using the index of the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP; Table S1). 23 , 24 PEs that were not identified and corrected before administration to the patient were considered clinically relevant (NCC MERP category C–I). PEs that were detected and corrected before administration or did not reach the patient were considered clinically nonrelevant (NCC MERP category B). NCC MERP‐category A does not categorize a PE but rather describes circumstances or events that could result in a PE; this category was not included in our results. Data were collected prospectively but inappropriateness assessments were made retrospectively to enable multidisciplinary medication assessment.
2.4. Root cause analysis
At the start of baseline measurements, the Pharmacotherapy team and study objectives were introduced to each study ward separately, through a face‐to‐face introduction meeting. Relevant stakeholders (junior doctors, clerks, medical specialists and nurses) were identified and invited to participate in this and coming meetings in the 10‐month trajectory.
After the baseline period, data and observations were presented to each individual study ward. Subsequently, during face‐to‐face meetings, stakeholders were asked to identify factors (root causes) that, in their opinion, challenged appropriate prescribing. There were 3 such meetings, maximally 3 weeks apart, supervised by both the quality improvement expert and the Pharmacotherapy team. The results of the root cause analysis were visualized using the BowTie model. 25 Based on the identified challenges, stakeholders were stimulated to develop a ward‐specific intervention plan of improvement. In the subsequent 6 months, stakeholders implemented their tailored plan themselves on their ward. Progress was monitored and, when necessary, coached by the Pharmacotherapy team in ≥2 face‐to‐face meetings during these 6 months. After the implementation phase, data were again collected and analysed as described for the baseline period.
2.5. Data analysis and statistics
A standard sample size calculation was performed to detect a statistically significant reduction in the number of prescriptions containing a PE assuming 2 independent samples of medication orders (before and after the intervention), based on a power of 80%, a type 1 error (α) of 5%, an estimated PE baseline prevalence of 8%. 26 To demonstrate a 40% reduction after the intervention, a sample of 1834 prescriptions was required, i.e. 917 prescriptions before and 917 prescriptions after the intervention. Given that the included wards were heterogeneous clinical settings, we decided to also perform stratified analyses for type of ward (i.e. medical vs surgical wards). To have enough power for the stratified analyses, and anticipating 10% missing data among prescriptions, we aimed for a sample size of 1009 prescriptions before and after the intervention at each type of ward (medical and surgical); yielding a total of 2018 prescriptions at baseline and postintervention with a total yield of ≥4036 prescriptions.
All analyses were performed using SPSS 22 (IBM Corp., Armonk, NY, USA). Differences between the baseline and postintervention period in number of prescriptions per patient and patient age were assessed using Mann–Whitney U tests. Differences in the number of patients and patient sex per ward type (medical, surgical and mixed) were assessed using Fisher‐exact tests. The number of PEs categorized as clinically relevant and clinically nonrelevant were compared by calculating the incidence rate ratio (IRR) between the baseline and postintervention periods, using generalized linear mixed models with a Poisson log‐linear link function and the number of prescriptions as offset for each patient. Omitted prescriptions (necessary drugs but not prescribed, for example prescribed before hospitalization but not continued after hospital admission), dichotomized within a patient as 0 vs ≥1, and the number of patients with ≥1 PE were compared by calculating the odds ratio between the baseline and postintervention periods, using a generalized linear mixed model with logit‐link function. The models were subsequently adjusted for ward, ward type and ward nested within ward type by adding the ward (type) as (nested) level to the generalized linear mixed models.
3. RESULTS
Overall, 321 patients with a total of 2978 prescriptions with a median of 9 (IQR 6–12) per patient at baseline were compared with 201 patients with a total of 2438 prescriptions with a median of 11 (interquartile range 9–15) per patient postintervention. The number of prescriptions per patient between baseline and postintervention was statistically significant different (P < .001). No difference in age or sex between the 2 groups was observed. Demographics of patients admitted per study ward during the baseline and postintervention periods are presented in Table 1.
TABLE 1.
Patient characteristics
| Baseline (n = 321) | Post intervention (n = 201) | P‐value | ||
|---|---|---|---|---|
| Number of patients included | .19 e | |||
| Medical a | n (%) | 153 (47.7) | 81 (40.3) | |
| Surgery b | n (%) | 103 (32.1) | 79 (39.3) | |
| Mixed ward c | n (%) | 65 (20.2) | 41 (20.4) | |
| Age in years | ||||
| Adults | Median (IQR d ) | 67.0 (55.5–76.0) | 69.0 (60.0–77.0) | .08 f |
| Paediatric | Median (IQR d ) | 1.96 (0.33–13.0) | 1.33 (0.75–11.0) | .62 f |
| Sex, female | n (%) | 138 (43.0) | 80 (39.8) | .47 e |
| Number of prescriptions per patient | Median (IQR d ) | 9 (6–12) | 11 (9–15) | <.001 f |
Internal medicine, neurology, pulmonary medicine, cardiology, gastroenterology disorders.
Vascular surgery, urology, gastrointestinal surgery, otolaryngology, trauma surgery.
Acute admission, paediatrics.
Interquartile range with lower and upper quartile
Fisher exact test.
Mann–Whitney U test.
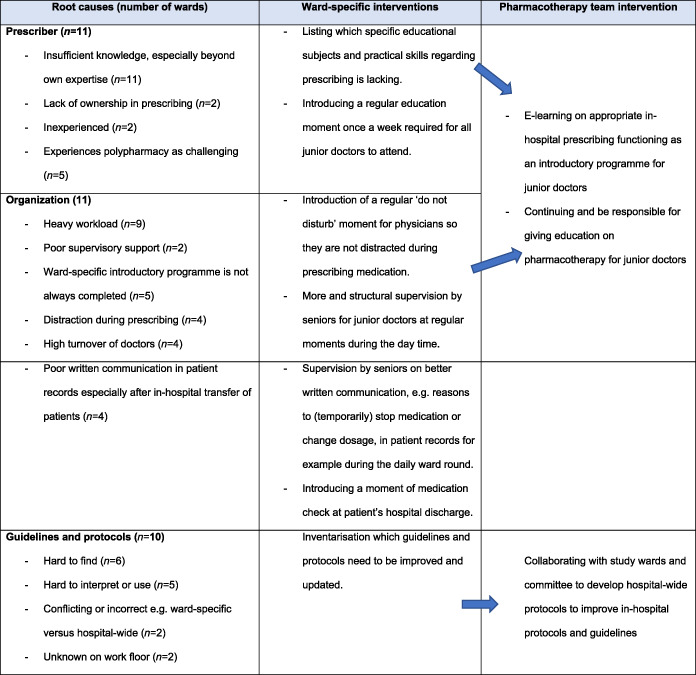
3.1. Root cause analysis and tailored interventions
The root cause analyses identified several challenges to appropriate prescribing. Stakeholders on all 12 study wards reported several miscommunications regarding medication reconciliation at hospital admission. The stakeholders' intervention addressing this root cause was incorporation of a daily check on ward‐admitted patient's current medication and on written communication in the discharge letters, e.g. reasons to (temporarily) stop medication or change dosage, during the ward rounds by the ward physicians.
Eleven out of 12 wards addressed organizational and prescriber‐related challenges such as a high workload, distractions during prescribing, poor supervision and insufficient (drug) knowledge, especially beyond own expertise. Examples of stakeholders' interventions to distractions during prescribing were the introduction of a regular do not disturb moment for physicians; more and structural supervision on written communication in patient records and rationale behind medication policy by seniors at set moments during the day. Stakeholders believed these interventions would eventually reduce the workload. The interventions addressing insufficient (drug) knowledge was listing which specific educational subjects was lacking and thus should be taught during regular education moments.
Ten out of 12 study wards encountered difficulties with guidelines and protocols at different levels (e.g. unawareness on the work floor, hard to find, hard to interpret and thus to apply). Stakeholders' interventions included listing which guidelines and protocols needed to be improved or updated. The Pharmacotherapy team then enabled collaboration between study wards and the in‐hospital guideline committee.
Finally, 8/12 study wards addressed difficulties with the newly implemented CPOE. Stakeholder intervention consisted of providing user feedback on the newly implemented CPOE. The Pharmacotherapy team would subsequently collect user feedback on a regular base for the IT department in order to improve its user friendliness (Table 2).
TABLE 2.
Result of root cause analysis performed on 12 study wards
CPOE = computerized physician order entry
During the face‐to‐face meetings throughout the 10‐month trajectory, the Pharmacotherapy team observed that stakeholders on some study wards were more motivated to address the challenges on their ward. These study wards initiated more ward‐specific interventions, as listed in Table 2.
Due to overarching similar challenges identified by study wards, the Pharmacotherapy team proposed and developed 4 hospital‐wide improvements: (i) the development of an E‐learning programme for appropriate in‐hospital prescribing, to be offered to all junior doctors; (ii) continuing education on various pharmacotherapy topics for junior doctors and prescribing nurses; (iii) improvement of specific in‐hospital protocols and guidelines; and (iv) collecting user feedback about the newly implemented CPOE and passing this information on to the IT Department in order to improve the user friendliness of the system.
3.2. Inappropriate prescribing
Overall, a total of 456 prescriptions were in multidisciplinary consensus identified containing ≥1 PE (15.3%) at baseline and 357 prescriptions (14.6%) postintervention. The intervention was associated with a nonsignificant difference in PEs (IRR 0.96, 95% confidence interval [CI] 0.83–1.10), even after correction respectively for study ward, ward type (medical, surgical or mixed) and study ward nested within ward type (Table 3).
TABLE 3.
Outcomes
| Baseline (%) | Postintervention (%) | Uncorrected | Corrected for study ward a | Corrected for ward type b | Corrected for study ward a nested within ward type b | ||
|---|---|---|---|---|---|---|---|
| All wards (n = 12) | |||||||
| Total number of prescriptions | 2978 | 2438 | ‐ | ‐ | ‐ | ‐ | |
| Total number of patients | 321 | 201 | ‐ | ‐ | ‐ | ‐ | |
| Number of prescriptions with ≥1 prescribing errors | IRR (95% CI) | 456 (15.3) | 357 (14.6) | 0.96 (0.83–1.10) | 0.95 (0.68–1.31) | 0.94 (0.82–1.08) | 0.95 (0.68–1.31) |
| Number of prescriptions with ≥1 clinically relevant prescription error c | IRR (95% CI) | 144 (4.8) | 139 (5.7) | 1.18 (0.93–1.49) | 1.18 (0.89–1.56) | 1.15 (0.78–1.68) | 1.18 (0.89–1.56) |
| Number of prescriptions with ≥1 clinically nonrelevant prescription error d | IRR (95% CI) | 312 (10.5) | 218 (8.9) | 0.85 (0.72–1.02) | 0.84 (0.54–1.30) | 0.83 (0.78–0.89) | 0.84 (0.54–1.30) |
| Total number of omitted prescriptions | 145 | 99 | |||||
| Number of patients with ≥1 prescription error | OR (95% CI) | 176 (54.8) | 138 (68.7) | 1.81 (1.25–2.61) | 1.68 (0.94–2.99) | 1.80 (1.10–2.94) | 1.68 (0.94–2.99) |
| Number of patients with ≥1 drug omission | OR (95% CI) | 105 (32.7) | 55 (27.4) | 0.78 (0.53–1.14) | 0.76 (0.39–1.50) | 0.76 (0.54–1.06) | 0.76 (0.39–1.50) |
| Medical wards (n = 5) | |||||||
| Total number of prescriptions | 1,368 | 974 | ‐ | ‐ | ‐ | ‐ | |
| Total number of patients | 153 | 81 | ‐ | ‐ | ‐ | ‐ | |
| Number of prescriptions with at ≥1 prescribing errors | IRR (95% CI) | 172 (12.6) | 115 (11.8) | 0.94 (0.74–1.19) | 0.94 (0.63–1.40) | ‐ | ‐ |
| Number of prescriptions with ≥1 clinically relevant prescription error c | IRR (95% CI) | 65 (4.8) | 58 (6.0) | 1.25 (0.88–1.79) | 1.30 (1.04–1.61) | ‐ | ‐ |
| Number of prescriptions with ≥1 clinically nonrelevant prescription error d | IRR (95% CI) | 107 (7.8) | 57 (5.9) | 0.75 (0.54–1.03) | 0.73 (0.35–1.50) | ‐ | ‐ |
| Number of omitted prescriptions | 74 | 43 | 1.08 (0.75–1.58) | 1.06 (0.73–1.54) | ‐ | ‐ | |
| Number of patients with a prescription error | OR (95% CI) | 85 (55.6) | 55 (67.9) | 1.69 (0.96–2.98) | 1.36 (0.91–2.01) | ‐ | ‐ |
| Number of patients with a drug omission | OR (95% CI) | 50 (32.7) | 22 (27.2) | 0.77 (0.42–1.39) | 0.70 (0.45–1.08) | ‐ | ‐ |
| Surgical wards (n = 5) | |||||||
| Total number of prescriptions | 1,110 | 1,052 | |||||
| Total number of patients | 103 | 79 | |||||
| Number of prescriptions with at ≥1 prescribing errors | IRR (95% CI) | 195 (17.6) | 152 (14.4) | 0.82(0.67–1.02) | 0.79 (0.48–1.31) | ‐ | ‐ |
| Number of prescriptions with ≥1 clinically relevant prescription error c | IRR (95% CI) | 63 (5.7) | 48 (4.6) | 0.80 (0.55–1.17) | 0.79 (0.54–1.17) | ‐ | ‐ |
| Number of prescriptions with ≥1 clinically nonrelevant prescription error d | IRR (95% CI) | 132 (11.9) | 104 (9.9) | 0.83 (0.64–1.08) | 0.80 (0.44–1.45) | ‐ | ‐ |
| Number of omitted prescriptions | 50 | 27 | 0.71 (0.45–1.14) | 0.79 (0.48–1.31) | ‐ | ‐ | |
| Number of patients with ≥1 prescription error | OR (95% CI) | 67 | 53 | 1.10 (0.59–2.04) | 1.89 (1.11–3.23) | ‐ | ‐ |
| Number of patients with ≥1 drug omission | OR (95% CI) | 35 (34.0) | 18 (22.8) | 0.57 (0.29–1.15) | 0.59 (0.25–1.39) | ‐ | ‐ |
| Mixed wards (n = 2) | |||||||
| Total number of prescriptions | 500 | 412 | |||||
| Total number of patients | 65 | 41 | |||||
| Number of prescriptions with at ≥1 prescribing errors | IRR (95% CI) | 89 (17.8) | 90 (21.8) | 1.23 (0.92–1.65) | 1.22 (0.54–2.75) | ‐ | ‐ |
| Number of prescriptions with ≥1 clinically relevant prescription error c | IRR (95% CI) | 16 (3.2) | 33 (8.0) | 2.50 (1.38–4.55) | 2.26 (1.28–3.99) | ‐ | ‐ |
| Number of prescriptions with ≥1 clinically nonrelevant prescription error d | IRR (95% CI) | 73 (14.6) | 57 (13.8) | 0.95 (0.67–1.34) | 0.98 (0.37–2.62) | ‐ | ‐ |
| Number of omitted prescriptions | 21 | 29 | |||||
| Number of patients with ≥1 prescription error | OR (95% CI) | 24 (36.9) | 30 (73.2) | 4.66 (1.98–11.0) | 0.64 (0.30–1.38) | ‐ | ‐ |
| Number of patients with ≥1 drug omission | OR (95% CI) | 20 (30.8) | 15 (36.6) | 1.30 (0.57–2.96) | 1.35 (0.10–17.4) | ‐ | ‐ |
Internal medicine, neurology, pulmonary medicine, cardiology, gastroenterology disorders, vascular surgery, urology, gastrointestinal surgery, otolaryngology, trauma surgery, acute admission and paediatrics.
Medical, surgical or mixed.
Clinically relevant errors (NCC MERP category C, D and E).
Clinically nonrelevant errors (NCC MERP category B).
CI, confidence interval; IRR, incidence rate ratio; OR, odds ratio.
Of all identified prescriptions containing ≥1 PE, 144 (4.8%) were identified as clinically relevant (containing a PE classified as NCC MERP category C–E) at baseline vs 139 (5.7%) postintervention. This difference was associated with a nonsignificant difference (IRR 1.18, 95% CI 0.93–1.49), even after correction. There were no prescriptions identified in our study containing PEs classified as NCC MERP category F–I PEs. Examples of PEs indexed according to NCC MERP classification are presented in Table S2.
Overall, the prescriptions of 176 patients (54.8%) contained a PE (NCC MERP classification B–E) at baseline compared to 138 patients (68.7%) in the postintervention period. This was associated with a significant increase (IRR 1.81, 95% CI 1.25–2.61), which was unaltered after correction for ward type (IRR 1.80, 95% CI 1.10–2.94). However, after correcting for study ward and study ward nested within ward type this was associated with a nonsignificant difference.
In total, 105 patients (32.7%) had a drug omission at baseline and 55 patients (27.4%) postintervention. This difference was not significant (odds ratio 0.78, 95% CI 0.53–1.14), even after correction. Hypothesis‐generating analyses of data for individual wards showed that some wards did achieve a reduction in PEs, but the majority did not (for 2 examples of results of individual study wards see Figures S1 and S2).
When zooming in on the different ward types, a significant increase in the number of prescriptions with ≥1 clinically relevant PE on the medical wards is observed postintervention after correcting for study ward (IRR 1.30, 95% CI 1.04–1.61). In the surgical wards, a significant increase in the number of patients with ≥1 PE is observed postintervention after correction for study ward (IRR 1.89, 95% CI 1.11–3.23).
The most frequent type of PE was incorrect instructions for medication use or administration, followed by the categories no, unknown or incorrect indication and incorrect dosing (Table 4).
TABLE 4.
Types of identified prescribing errors
| Type of PE | Preintervention (464) (%) | Postintervention (366) (%) | Case(s) |
|---|---|---|---|
| No, unknown or incorrect indication | 72 (15.5) | 73 (19.9) | ‐ use of a proton‐pump inhibitor in prophylactic dose without indication. |
| ‐ nitrazepam use in a patient with a high risk of falling and fractures. | |||
| Nonadherence to guidelines | 18 (3.9) | 4 (1.1) | Initiation of a prophylactic proton‐pump inhibitor as monotherapy when not indicated. |
| Incorrect dosing | 99 (21.3) | 76 (20.8) | |
| Under‐ or overdosing | 54 (11.6) | 57 (15.6) | |
| No maximum dose if prescribed as needed | 37 (8.0) | 0 (0) | |
| Incorrect dosing frequency | 8 (0.9) | 19 (1.9) | |
| Incorrect or no dosing unit registered | 3 (0.6) | 0 (0) | ‐ sodium phosphate enema dosed as 1 mL instead of 1 piece. |
| ‐ paracetamol 4 times daily 1 instead of 4 times daily 1 g. | |||
| Incorrect duration of medication therapy | 3 (0.6) | 11 (3.0) | |
| Inadequate instructions for medication use or administration | 221 (47.6) | 168 (45.9) | |
| Incorrect route of administration | 4 (0.9) | 19 (5.2) | Paracetamol administered intravenously while patient was taking other medication orally. |
| Incorrect registration of administration route | 165 (35.6) | 113 (36.3) | Registered as per os while medication administered over nasogastric tube. |
| No or incorrect instructions for use | 44 (9.5) | 34 (9.3) | |
| Incorrectly registered administration time | 8 (1.7) | 2 (0.5) | |
| Incorrect medication form | 4 (0.9) | 4 (1.1) | Metoprolol succinate instead of tartrate. |
| (pseudo) medication duplication | 23 (5.0) | 14 (3.8) | ‐ simultaneous use of diazepam and temazepam for insomnia as lower back pain. |
| ‐ psyllium fibres and simultaneous use of macrogol with electrolytes (movicolon) for obstipation, while the guidelines state that the psyllium fibres need to be stopped as obstipation occurs during use and another laxative is indicated. | |||
| Surveillance | 45 (9.7) | 24 (6.6) | |
| Inadequate nonadherence to guidelines without registered explanation in electronic patient record | 18 (3.9) | 4 (1.1) | |
| Inadequate or no action based on clinical decision support system or computerized physician order entry alert | 4 (0.9) | 6 (1.6) | |
| No follow‐up on medication therapy | 15 (3.2) | 5 (1.4) | |
| Inadequate action for relevant contraindication | 5 (1.1) | 4 (1.1) |
4. DISCUSSION
To our knowledge, this is the first in‐hospital participatory action intervention study carried out to reduce inappropriate general in‐hospital prescribing. As part of the baseline measurement, 15% of prescriptions were found to contain an error. Root cause analysis revealed challenges in appropriate prescribing on prescriber‐ and organizational level. Stakeholders on the 12 clinical wards involved in this study developed, in close collaboration with the Pharmacotherapy team, tailored interventions to address these challenges. Despite these efforts, no reduction in inappropriate prescriptions after a 6‐month intervention period was achieved.
One of the explanations for the negative results may be that some of the identified root causes, such as challenges on organizational level, i.e. a high workload, cannot be easily changed on ward level. The high turnover of doctors and fellows on the study wards is another factor that could not be addressed by either the Pharmacotherapy team or on‐ward stakeholders. Addressing these barriers on organizational level requires change at hospital management level.
A second potential explanation is the observed lack of stakeholder commitment on some study wards to implement and adhere to their proposed interventions, compared to others. This observation was supported by the finding that on some wards a marked reduction of PEs was observed (Figure S1 and S2). To succeed, participatory action research requires substantial commitment on the part of stakeholders. 17 , 21 , 27 The aforementioned workload and high turnover of doctors is also likely to have impaired long‐term commitment of prescribers on the study wards.
Despite the absence of a reduction in PEs in our study, the participatory action method was very valuable to identify barriers to appropriate prescribing. This study design enabled relevant stakeholders involved in prescribing (such as medical specialists, junior doctors, clerks, nurses, clinical pharmacologists, and pharmacists) to elaborate on challenges faced as healthcare professionals in their daily practice. In general, this method resulted in open communication. During the face‐to‐face meetings, we observed that awareness about medication safety was increased among stakeholders. By increasing and sustaining awareness, we hope to tackle the lack of commitment and motivation in prescribers to reduce inappropriate prescribing.
This study provided us with important information on the frequency, type and severity of PEs in our hospital, which can be used for future programmes to monitor medication safety. The most inappropriately prescribed drugs were those for acid‐related disorders (ATC code A02), specific proton‐pump inhibitors (ATC code A02BC) due to wrong equivalent conversion dosage and lack of indication (Table 5). Training prescribers not only in appropriate prescribing but also in deprescribing 28 is low‐hanging fruit to improve outcomes.
TABLE 5.
Number of prescribing errors per Anatomical Therapeutic Chemical (ATC) category group during baseline and postintervention period of all study wards
| Baseline | Postintervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ATC code | Drug categories based on ATC code | Number of prescriptions | Number of prescribing errors (%) | ATC code | Drug categories based on ATC code | Number of prescriptions | Number of prescription errors (%) | ||
| 1. | N02 | Analgesics | 454 | 52 (11.5) | 1. | A02 | Drugs for acid related disorders | 147 | 54 (36.7) |
| A02 | Drugs for acid related disorders | 195 | 52 (26.7) | 2. | N02 | Analgesics | 410 | 47 (11.5) | |
| 2. | N05 | Psycholeptica | 172 | 38 (22.1) | 3. | N05 | Psycholeptics | 133 | 28 (21.1) |
| 3. | A06 | Drugs for constipation | 154 | 34 (22.7) | 4. | A03 | Drugs for functional gastrointestinal disorders | 117 | 24 (20.5) |
| 4. | B01 | Antithrombotic agents | 305 | 29 (9.5) | J01 | Antibiotics | 92 | 24 (26.1) | |
| 5. | A04 | Antiemetics and antinauseants | 65 | 22 (33.8) | 5. | A11 | Vitamins | 85 | 22 (25.9) |
| 6. | A03 | Drugs for functional gastrointestinal disorders | 57 | 21 (36.8) | 6. | B01 | Antithrombotic agents | 193 | 21 (10.9) |
| 7. | A11 | Vitamins | 131 | 17 (13.0) | 7. | A04 | Antiemetics and antinauseants | 69 | 19 (27.5) |
| 8. | A10 | Antidiabetic drugs | 114 | 16 (14.0) | 8. | A06 | Drugs for constipation | 132 | 13 (9.8) |
| 9. | C10 | Lipid modifying agents | 86 | 15 (17.4) | 9. | C07 | Beta‐blocking agents | 58 | 9 (15.5) |
| 10. | N03 | Antiepileptics | 59 | 14 (23.7) | 10. | C03 | Diuretics | 60 | 8 (13.3) |
We did not identify PEs that could be categorized as NCC MERP category F–I. These findings are not in line with similar studies, 15 , 29 , 30 which identified more severe PEs according to the NCC MERP index. Several possible explanations for this discrepancy. First, assessment and subsequent classification of PEs using any index tool is dependent on the assessor's interpretation. In our study, we therefore composed a team with members of different expertise to ascertain consensus regarding the classification of PEs. For maximum transparency, we described examples of identified PEs including their classification (Table S2). Second, we assessed PEs in patients who had been admitted for more than 24 hours. The current Dutch in‐hospital standard of care requires hospital pharmacists to assess prescriptions within 24 hours of their being written out, meaning that PEs that could potentially cause serious or acute harm (NCC MERP category F–I) should have been intercepted before the assessment of our team. Implementation of a CPOE including a clinical decision support system could also facilitate early detection and correction of errors by prescribers. The low incidence of NCC MERP category E and the nonoccurrence of category F PEs is a positive finding. Most PEs in our study were NCC MERP category C and D. These PEs were clinically relevant and required an intervention to prevent patient harm. 24 This finding implies that the current Dutch and our in‐hospital standard of care is not able to identify nonacute PEs. In the long‐term, these PEs could still very well cause severe patient harm if not identified and corrected.
4.1. Strengths
We believe that our study had several major strengths. First, the multidisciplinary character of our intervention team combined the clinical skills of doctors and the pharmacotherapeutic knowledge of pharmacists. 15 , 29 , 30 , 31 , 32 , 33 The different perspectives of the team members on (in)appropriate prescribing resulted in a weighed interpretation and assessment of the identified PEs and their severity. Within the team, this enabled both active and passive interdisciplinary knowledge transfer between members, which resulted in a better understanding of the challenges faced by various health professionals in daily practice. Also stakeholders felt at ease and well understood due to the multidisciplinary character.
Second, the performed root cause analyses provided valuable information and insight on factors and circumstances influencing prescribing. This is used to improve both current and future daily practice of healthcare professionals as well as patient safety in this hospital.
4.2. Limitations
One of the limitations of our study was that the electronic patient record (EPD), including a new CPOE system, was introduced 6–7 months after our study started. This means that the EPD had been introduced to all wards by the time of the postintervention assessment. We observed that more prescriptions were written out after EPD introduction, i.e. the number of prescriptions per patient was (significantly) higher. This may have been due to the fact that the EPD improved registration of all prescribed medications. It is possible that more PEs were detected by our study team because of this improved registration as the EPD might make it easier to detect PEs. This study design made it impossible to correct for events possibly influencing study outcomes, such as the introduction of the new CPOE and that the stakeholders on the wards were told about the study in advance. Potential awareness on study wards may have led to a change in prescribing behaviour in 2 ways—it may have increased awareness of appropriate prescribing or it could have made prescribers sloppy in their prescribing because they knew that PEs would be detected and corrected (the Hawthorn effect 34 ). To reduce the potential influence of these factors, multiple measurements over a certain time are needed, like in a stepped‐wedge study design; however, for practical reasons this was not feasible in our study.
Another limitation of this study is the seemingly high financial start‐up costs and the amounts of effort of the presented improvement strategy. Improvement of in‐hospital medication safety should involve all key players in the process, pleading for a multidisciplinary approach. Initiatives should also include a hospital‐wide approach and should not focus solely on a local (ward) level. These criteria imply cost and effort. Despite the nonsignificant improvement obtained, the Pharmacotherapy team meets these mentioned requirements and maintains the implemented interventions like the e‐learning, education and improvement of guidelines and protocols.
4.3. Future research
We conducted a before and after study involving a single period of participatory action interventions. For future programmes to improve medication safety, we would recommend to either perform recurring interventions or provide structural (i.e. weekly) support to prescribers by a dedicated team such as the Pharmacotherapy team. Such a team should also be available to provide for example structured medication reviews for patients with polypharmacy, upon request. Based on our experiences, we believe such an approach is essential in order to maintain awareness and commitment to improve prescribing. Structural presence of a dedicated team also circumvents some of the most important identified challenges to appropriate prescribing, such as the high turnover of (junior) doctors. Furthermore, the majority of errors involved very common medications, such as proton‐pump inhibitors. This underscores the importance of easy access and applicability of guidelines and protocols and easy access to educational resources for prescribers. Pharmacotherapy education should be in different phases, from medical school until after graduation, an essential element to improve and sustain medication safety.
5. CONCLUSION
In‐hospital prescribing is a complex process, influenced by various factors. This participatory action research study, involving 12 clinical wards in a Dutch academic hospital, did not lead to a statistically significant improvement in appropriate in‐hospital prescribing. Nevertheless, it provided insight in prescriber‐identified challenges in appropriate in‐hospital prescribing and into the severity and type of identified PEs. This information can inform future programmes to monitor and improve medication safety. From the experience and results of this study can be concluded that reducing inappropriate prescribing is not a quick fix but instead requires structural commitment to improve and situational awareness on medication safety, which a dedicated, multidisciplinary team is able to provide. Future studies should therefore involve in‐depth research to identify factors contributing to inappropriate prescribing (technical and nontechnical skills) and mitigation strategies to reduce this complex challenge.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
R.M., K.S., J.B., M.D., D.B., M.K., M.v.B., B.L‐W., J.T., M.v.A. wrote the article; M.v.A., M.D., J.B., D.B., M.K. designed the research; R.M., K.S., J.B., M.D., D.B., M.K., M.v.B., M.v.A. performed the research; R.M., B.L‐W. analysed the data.
Supporting information
TABLE S1 National Coordinating Council for Medication Error Reporting and Prevention Index
TABLE S2 Examples of prescribing errors in National Coordinating Council for Medication Error Reporting and Prevention category B–E.
FIGURE S1 Examples of a study ward in which the intervention was effective in reduction
FIGURE S2 Example of a study ward with which the intervention was not effective reduction
ACKNOWLEDGEMENTS
This study was a project initiated within the Commission Medication Safety of the Amsterdam UMC—location VUmc. We are additionally grateful to all members of the Commission Medication Safety of the Amsterdam UMC—location VUmc, in particular to Leo de Haan for his support to this project and our team. We would also like to thank all stakeholders of the participating study wards of this study. This project was funded by the Commission Medication Safety from the Amsterdam UMC—location VUmc.
Mahomedradja RF, Sigaloff KCE, Bekema JK, et al. The pharmacotherapy team: A novel strategy to improve appropriate in‐hospital prescribing using a participatory intervention action method. Br J Clin Pharmacol. 2021;87:565–576. 10.1111/bcp.14418
The authors confirm that the Principal Investigator for this paper is Michiel van Agtmael and that he had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, R.F.M., upon reasonable request.
REFERENCES
- 1. Lesar TS, Briceland L, Stein DS. Factors related to errors in medication prescribing. JAMA. 1997;277(4):312‐317. [PubMed] [Google Scholar]
- 2. Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A Framework for Improvement. JAMA. 1999;282(15):1458‐1465. [DOI] [PubMed] [Google Scholar]
- 3. Aronson JK, Henderson G, Webb DJ, Rawlins MD. A prescription for better prescribing. BMJ. 2006;333:459‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saedder EA, Lisby M, Nielsen LP, Bonnerup DK, Brock B. Number of drugs most frequently found to be independent risk factors for serious adverse reactions: a systematic literature review. Br J Clin Pharmacol. 2015;80(4):808‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hutter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607‐2613. [DOI] [PubMed] [Google Scholar]
- 6. Velo GP, Minuz P. Medication errors: prescribing faults and prescription errors. Br J Clin Pharmacol. 2009;67(6):624‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Assiri GA, Shebl NA, Mahmoud MA, et al. What is the epidemiology of medication errors, error‐related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature. BMJ Open. 2018;8:e019101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Organization, G.W.H . Medication Errors ‐ Technical Series on Safer Primary Care; 2016.
- 9. Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM, Group, H.S . Frequency of and risk factors for preventable medication‐related hospital admissions in the Netherlands. Arch Intern Med. 2008;168(17):1890‐1896. [DOI] [PubMed] [Google Scholar]
- 10. Warle‐van Herwaarden MF, Kramers C, Sturkenboom MC, van den Bemt PM, De Smet PA, Dutch H‐WTF. Targeting outpatient drug safety: recommendations of the Dutch HARM‐wrestling task force. Drug Saf. 2012;35(3):245‐259. [DOI] [PubMed] [Google Scholar]
- 11. Pronovost P, Weast B, Schwarz M, et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201‐205. [DOI] [PubMed] [Google Scholar]
- 12. Sturkenboom M. Eindrapport: vervolgonderzoek medicatieveiligheid; 2017.
- 13. Veeren JC, Weiss M. Trends in emergency hospital admissions in England due to adverse drug reactions: 2008‐2015. J Pharm Health Serv Res. 2017;8:5‐11. [Google Scholar]
- 14. Ahmed F, Ahmed N, Briggs TWR, et al. Can reverse innovation catalyse better value health care? Lancet Glob Health. 2017;5(10):e967‐e968. [DOI] [PubMed] [Google Scholar]
- 15. Klopotowska JE, Kuiper R, van Kan HJ, et al. On‐ward participation of a hospital pharmacist in a Dutch intensive care unit reduces prescribing errors and related patient harm: an intervention study. Crit Care. 2010;14(5):R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Surgery and Pharmacy in Liaison (SUREPILL) Study Group . Effect of a ward‐based pharmacy team on preventable adverse drug events in surgical patients (SUREPILL study). Br J Surg. 2015;102:1204‐1212. [DOI] [PubMed] [Google Scholar]
- 17. Baum F, MacDougall C, Smith D. Participatory action research. J Epidemiol Community Health. 2006;60:854‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Norton MI, Mochon D, Ariely D. The IKEA effect: when labor leads to love. J Consum Psychol. 2012;22:453‐460. [Google Scholar]
- 19. Dollman WB, Leblanc VT, Stevens L, O'Connor PJ, Roughead EE, Gilbert AL. Achieving a sustained reduction in benzodiazepine use through implementation of an area‐wide multi‐strategic approach. J Clin Pharm Ther. 2005;30(5):425‐432. [DOI] [PubMed] [Google Scholar]
- 20. Dowell J, Jones A, Snadden D. Exploring medication use to seek concordance with 'non‐adherent' patients: a qualitative study. Br J Gen Pract. 2002;52(474):24‐32. [PMC free article] [PubMed] [Google Scholar]
- 21. Sikkens JJ, van Agtmael M, Peters EJG, et al. Behavioral approach to appropriate antimicrobial prescribing in hospitals: the Dutch unique method for antimicrobial stewardship (DUMAS) participatory intervention study. JAMA Intern Med. 2017;177(8):1130‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dean B, Barber N, Schachter M. What is a prescribing error? Qual Health Care. 2000;9(4):232‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hartwig SC, Denger SD, Schneider PJ. Severity‐indexed, incident report‐based medication error‐reporting program. Am J Hosp Pharm. 1991;48(12):2611‐2616. [PubMed] [Google Scholar]
- 24. Forrey RA, Pedersen CA, Schneider PJ. Interrater agreement with a standard scheme for classifying medication errors. Am J Health Syst Pharm. 2007;64(2):175‐181. [DOI] [PubMed] [Google Scholar]
- 25. Wierenga PC, Lie AHL, de Rooij SE, Klazinga NS, Guchelaar HJ, Smorenburg SM. Application of the Bow‐tie model in medication safety risk analysis: consecutive experience in two hospitals in the Netherlands. Drug Saf. 2009;32:663‐673. [DOI] [PubMed] [Google Scholar]
- 26. Tim Dornan (Principal Investigator) , Heathfield DAH, Lewis, P , Miles, J , Taylor, D , Tully M, Wass V. An in depth investigation into causes of prescribing errors by foundation trainees in relation to their medical education. EQUIP study ; 2009.
- 27. van Buul LW, Sikkens JJ, van Agtmael MA, Kramer MH, van der Steen JT, Hertogh CM. Participatory action research in antimicrobial stewardship: a novel approach to improving antimicrobial prescribing in hospitals and long‐term care facilities. J Antimicrob Chemother. 2014;69(7):1734‐1741. [DOI] [PubMed] [Google Scholar]
- 28. Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging de fi nition of 'deprescribing' with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015;80(6):1254‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gleason KM, McDaniel M, Feinglass J, et al. Results of the medications at transitions and clinical handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bos JM, van den Bemt PMLA, Kievit W, et al. A multifaceted intervention to reduce drug‐related complications in surgical patients. Br J Clin Pharmacol. 2017;83(3):664‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dolovich L, Pottie K, Kaczorowski J, et al. Integrating family medicine and pharmacy to advance primary care therapeutics. Clin Pharmacol Ther. 2008;83(6):913‐917. [DOI] [PubMed] [Google Scholar]
- 32. Tan EC, Stewart K, Elliott RA, George J. Pharmacist consultations in general practice clinics: the pharmacists in practice study (PIPS). Res Social Adm Pharm. 2014;10(4):623‐632. [DOI] [PubMed] [Google Scholar]
- 33. Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study [NCT00351676]. Med Care. 2009;47:642‐650. [DOI] [PubMed] [Google Scholar]
- 34. Taylor FW. The principles of scientific management. New York: Harper & Brothers; 1911. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 National Coordinating Council for Medication Error Reporting and Prevention Index
TABLE S2 Examples of prescribing errors in National Coordinating Council for Medication Error Reporting and Prevention category B–E.
FIGURE S1 Examples of a study ward in which the intervention was effective in reduction
FIGURE S2 Example of a study ward with which the intervention was not effective reduction
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, R.F.M., upon reasonable request.


