ABSTRACT
Denosumab is associated with continued gains in hip and spine BMD with up to 10 years of treatment in postmenopausal women with osteoporosis. Despite potent inhibition of bone remodeling, findings in nonhuman primates suggest modeling‐based bone formation (MBBF) may persist during denosumab treatment. This study assessed whether MBBF in the femoral neck (FN) is preserved in the context of inhibited remodeling in subjects receiving denosumab. This open‐label study enrolled postmenopausal women with osteoporosis who had received two or more doses of denosumab (60 mg subcutaneously every 6 months [Q6M]) per standard of care and were planning elective total hip replacement (THR) owing to osteoarthritis of the hip. Transverse sections of the FN were obtained after THR and analyzed histomorphometrically. MBBF, based on fluorochrome labeling and presence of smooth cement lines, was evaluated in cancellous, endocortical, and periosteal envelopes of the FN. Histomorphometric parameters were used to assess MBBF and remodeling‐based bone formation (RBBF) in denosumab‐treated subjects (n = 4; mean age = 73.5 years; range, 70 to 78 years) and historical female controls (n = 11; mean age = 67.8 years; range, 62 to 80 years) obtained from the placebo group of a prior study and not treated with denosumab. All analyses were descriptive. All subjects in both groups exhibited MBBF in the periosteal envelope; in cancellous and endocortical envelopes, all denosumab‐treated subjects and 81.8% of controls showed evidence of MBBF. Compared with controls, denosumab‐treated subjects showed 9.4‐fold and 2.0‐fold higher mean values of MBBF in cancellous and endocortical envelopes, respectively, whereas RBBF mean values were 5.0‐fold and 5.3‐fold lower. In the periosteal envelope, MBBF and RBBF rates were similar between subjects and controls. These results demonstrate the occurrence of MBBF in the human FN and suggest that denosumab preserves MBBF while inhibiting remodeling, which may contribute to the observed continued gains in BMD over time after remodeling is maximally inhibited. © 2020 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research
Keywords: ANTIRESORPTIVES, BONE HISTOMORPHOMETRY, BONE MODELING AND REMODELING, OSTEOPOROSIS
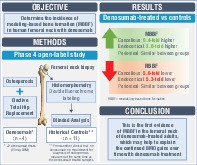
Femoral neck histomorphometry was assessed in adult subjects (n = 4) with osteoporosis who received at least two doses of denosumab and were scheduled for total hip replacement. Compared with historical controls (n = 11), denosumab‐treated subjects showed 9.4‐fold and 2.0‐fold higher values of MBBF in cancellous and endocortical envelopes, respectively. RBBF values were 5.0‐fold and 5.3‐fold lower. Thus, denosumab may preserve MBBF while inhibiting RBBF, resulting in the continued increases in BMD observed with long‐term treatment. MBBF = modeling‐based bone formation; RBBF = remodeling‐based bone formation.
Introduction
Osteoporosis is characterized by an imbalance in bone remodeling, wherein increased bone resorption and decreased bone formation cause bone loss and microarchitectural decay. These changes can lead to fractures associated with morbidity and increased mortality.1, 2, 3 Antiresorptive therapies, including denosumab and bisphosphonates, reduce fracture risk and increase bone mineral density (BMD).4, 5, 6, 7 As an antibody against RANK ligand (RANKL), denosumab is a potent antiresorptive agent that inhibits the activity of osteoclasts and thus bone remodeling. In the pivotal phase 3, randomized Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial and its open‐label extension, long‐term treatment with denosumab for up to 10 years in postmenopausal women with osteoporosis was associated with continued BMD gains at both the spine and hip and a low incidence of fracture.8 In FREEDOM substudies using quantitative computed tomography (QCT), denosumab treatment over 36 months was associated with progressive improvements in bone density at the hip.9, 10 The continued gain in BMD seen with denosumab is distinct from that with other antiresorptive treatments, such as bisphosphonates, for which there is generally little or no further improvement in BMD after 3 to 4 years of treatment.11, 12, 13
The initial, rapid gains in BMD observed with denosumab treatment are likely due to the potent inhibition of remodeling and the refilling of preexisting remodeling units with new bone matrix that mineralizes over time.14 Prolonged secondary mineralization extends the remodeling period through approximately 5 years of denosumab treatment.15 Subsequently, with continued inhibition of bone turnover associated with prolonged denosumab treatment, longer‐term gains in BMD (between 5 and 10 years) may be due to a remodeling‐independent mechanism, namely modeling‐based bone formation (MBBF).
Bone modeling governs skeletal development and growth and has been demonstrated to occur in the adult skeleton with aging and in response to increased mechanical strain.2, 16, 17 In ovariectomized cynomolgus monkeys given high doses of denosumab for 16 months, MBBF was observed concomitantly with continuous increases in BMD and femoral neck strength.18 Although it is currently unknown whether this occurs in humans as well, these results suggest that, in the presence of remodeling‐based bone loss, MBBF may contribute to the clinical observation of sustained BMD gain with long‐term denosumab treatment.
In the current study, we performed bone histomorphometry to assess bone modeling and remodeling at the proximal femur in denosumab‐treated subjects undergoing total hip replacement (THR). The objective of this study was to determine the occurrence of MBBF in the human femoral neck in the context of inhibited remodeling with denosumab treatment.
Subjects and Methods
Subjects
This open‐label, phase 4 study (NCT02576652) enrolled ambulatory postmenopausal women with osteoporosis who had received two or more doses of denosumab (60 mg subcutaneously every 6 months [Q6M]) per standard of care and were planning to undergo elective THR owing to osteoarthritis of the hip. The study was conducted from December 2015 to December 2017. For all subjects, denosumab had been prescribed by the treating physician for the treatment of osteoporosis, and the last dose of denosumab was within 6 months of scheduled THR. Subjects were excluded if they had received osteoporosis treatment with agents other than denosumab during the 1 year prior to THR or had known sensitivity to tetracycline or demeclocycline. Samples from female subjects enrolled in the placebo group of a prior clinical study and not treated with denosumab (NCT01309399) were used as historical controls.19 Control subjects were excluded if they had used glucocorticoids or osteoporosis medication within 3 months, or bisphosphonates within 1 year, before THR. Control femoral neck biopsies were analyzed using the same methodology as for study subjects. Denosumab‐treated and control biopsy specimens were prepared at the same time as described previously20 and were deidentified so that the histomorphometrist would be blinded to treatment.
The study received ethical review board approval from Helen Hayes Hospital and the Hospital for Special Surgery, and subjects provided written informed consent. The study was conducted in compliance with the World Medical Association Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects.
Study design and treatment
After enrollment, subjects self‐administered tetracycline and demeclocycline orally. During cycle 1, tetracycline was administered at either 250 mg four times daily or 500 mg twice daily for 3 days. After a 10‐day break, demeclocycline was administered during cycle 2 at either 150 mg four times daily or 300 mg twice daily for 3 days. THR was performed approximately 5 days after the last demeclocycline dose was administered (Fig. 1).
Figure 1.
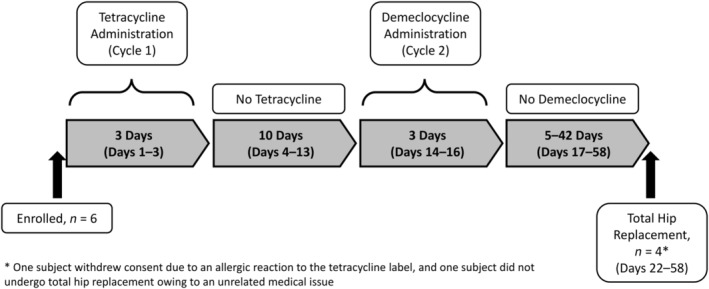
Study design for histomorphometric analysis of bone biopsies.
Fluorochrome labeling and biopsy
During THR, a sample of the mid‐femoral neck was obtained, and the superior and posterior aspects were labeled with ink. The sample consisted of a ring of the femoral neck, ranging in thickness from 1.0 to 1.5 cm, centered on the midpoint of the femoral neck (Supplemental Fig. 1). The specimen was fixed in 10% formalin and embedded without decalcification, as previously described.19 The femoral neck was subsequently sectioned transversely, and three adjacent sections were cut from two levels 100 μm apart. Within each level, one 20‐μm‐thick section was mounted unstained, and two 7‐μm‐thick sections were stained with Goldner trichrome and toluidine blue, respectively. The endocortical, periosteal, and cancellous envelopes were evaluated. The primary objective of this study was to determine the number (%) of subjects in the denosumab‐treated and control groups exhibiting fluorochrome labeling associated with smooth cement lines (indicative of MBBF) in cancellous, endocortical, and periosteal envelopes of the femoral neck. Secondary analyses quantified MBBF using modeling‐based formation units per millimeter of bone surface and remodeling‐based bone formation (RBBF) using remodeling‐based formation units (including overfilled units) per mm of bone surface. Figure 2 provides a schematic illustration of how bone formation was assessed using the double labeling procedure.
Figure 2.
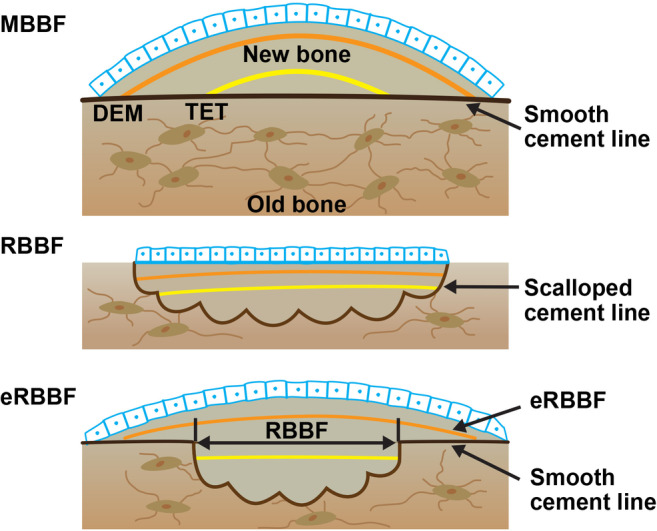
Illustration showing the types of bone formation assessed with quadruple labeling. MBBF = modeling‐based bone formation; RBBF = remodeling‐based bone formation; eRBBF = extended remodeling‐based bone formation; TET = tetracycline (the first set of labels); DEM = demeclocycline (the second set of labels).
The following specialized histomorphometric variables were measured: modeling‐based single‐label surface, modeling‐based double‐label surface, modeling‐based mineralizing surface (MBBF MS), extended remodeling‐based single‐label surface, extended remodeling‐based double‐label surface, extended remodeling‐based mineralizing surface (eRBBF MS), remodeling‐based single‐label surface, remodeling‐based double‐label surface, and remodeling‐based mineralizing surface (RBBF MS). The following conventional histomorphometric variables were also measured: mineralizing surface/bone surface (MS/BS), bone formation rate/bone surface (BFR/BS), and eroded surface/bone surface (ES/BS). All histomorphometric parameters were defined, calculated, and expressed according to the most recent recommendations of the American Society for Bone and Mineral Research.21
Statistical methods
Primary analyses were performed on all enrolled subjects with an evaluable biopsy for fluorochrome labeling. The safety analysis subset included all enrolled subjects who received at least one dose of tetracycline or demeclocycline. No formal hypothesis was tested, and all analyses were descriptive in nature. All histomorphometric parameters were summarized using descriptive statistics for each analyzed surface of the overall femoral neck.
Data Availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/
Results
Subject characteristics
As shown in Fig. 1, six subjects were enrolled in the study. Of these, four subjects underwent THR, had an evaluable biopsy for fluorochrome labeling, and were included in the analysis. The first subject was enrolled in December 2015, and the last subject completed the study in December 2017. Two subjects were not included in the analysis: one subject withdrew consent because of an allergic reaction to the tetracycline label, and one subject did not undergo THR owing to an unrelated medical issue. Female historical controls (n = 11) from the placebo group of a previous clinical study19 were included in the analysis. The baseline demographic characteristics of the study subjects and historical controls are shown in Table 1. The study subjects had a mean age of 73.5 years and mean duration of denosumab use of 1.8 years. The controls had a mean age of 67.8 years, and 72.7% had received osteoporosis treatment (prior to enrollment in the study) that did not include denosumab (but did include bisphosphonates [used more than 1 year before THR] and hormone replacement therapy or selective estrogen receptor modulators [SERMs; used more than 3 months before THR]).
Table 1.
Baseline Characteristics for Study Subjects and Controls
| Historical controls (N = 11) | Denosumab subjects (N = 4) | |
|---|---|---|
| Age (years), mean ± SDa | 67.8 ± 5.0 | 73.5 ± 3.7 |
| Years since menopause, mean ± SD | 17.7 ± 6.0 | 26.6 ± 7.3 |
| Race, white, n (%) | 10 (90.9) | 4 (100.0) |
| Body mass index (kg/m2), mean ± SD | 29.7 ± 6.0 | 25.2 ± 2.6 |
| Prior fracture, n (%) | 8 (72.7) | 3 (75.0) |
| Hip | 1 (9.1) | 1 (25.0) |
| Spine | 0 (0.0) | 0 (0.0) |
| Wrist | 0 (0.0) | 1 (25.0) |
| Other | 7 (63.6) | 2 (50.0) |
| Prior osteoporosis treatment, n (%) | 8 (72.7) | 4 (100.0) |
| Denosumab | 0 (0.0) | 4 (100.0) |
| Number of doses received, mean ± SD | 0 ± 0.0 | 4 ± 3.4 |
| Bisphosphonatesb | 4 (36.4) | 0 (0.0) |
| Hormone replacement therapy/SERMc | 5 (45.5) | 0 (0.0) |
N = number of subjects with an evaluable biopsy for fluorochrome labeling; n = number of subjects with observed data; SERM = selective estrogen receptor modulators.
Age at first administration of fluorochrome treatment.
At least 1 year prior to THR.
At least 3 months prior to THR.
Number and percentage of denosumab‐treated subjects and controls exhibiting MBBF
The number and percentage of subjects exhibiting MBBF are shown in Table 2. All four study subjects exhibited fluorochrome labeling indicative of MBBF in the cancellous, periosteal, and endocortical envelopes. All control subjects exhibited MBBF in the periosteal bone envelope, and the amount of MBBF was similar in the periosteal envelope between subjects and controls. In the cancellous and endocortical envelopes, 81.8% of control subjects showed evidence of MBBF. Representative images of tetracycline‐labeled femoral neck bone from individual subjects in both groups are shown in Supplemental Fig. 2.
Table 2.
Subjects and Controls Exhibiting Modeling‐Based Bone Formation
| Historical controls (N = 11) | Denosumab subjects (N = 4) | |
|---|---|---|
| Modeling‐based fluorochrome labeling at the femoral neck, n (%) | ||
| Cancellous | 9 (81.8) | 4 (100.0) |
| Endocortical | 9 (81.8) | 4 (100.0) |
| Periosteal | 11 (100.0) | 4 (100.0) |
N = number of subjects with an evaluable biopsy for fluorochrome labeling; n = number of subjects with observed data.
Histomorphometric analysis of bone formation parameters
Rates of MBBF and RBBF in subjects and controls are shown in Fig. 3 A. Compared with historical controls, denosumab‐treated subjects showed 9.4‐fold and 2.0‐fold higher mean values of MBBF in the cancellous and endocortical envelopes, respectively, whereas RBBF mean values were 5.0‐fold and 5.3‐fold lower, respectively. Consistent with the bone formation rates in these envelopes, the extent of MBBF MS (mean ± SD) was greater in denosumab‐treated subjects than in controls (5.59 ± 9.70 versus 0.55 ± 0.72 in cancellous; 10.06 ± 14.05 versus 4.10 ± 4.09 in endocortical), whereas that of RBBF MS was less in denosumab‐treated subjects than in controls (0.90 ± 0.97 versus 4.48 ± 3.15 for cancellous; 0.73 ± 0.79 versus 7.44 ± 5.11 for endocortical) (Fig. 3 B). In the periosteal envelope, the rates of MBBF and RBBF and percentage of RBBF MS were similar between subjects and controls. Supplemental Table 1 lists additional specialized histomorphometry variables examined for the three envelopes of the femoral neck in study subjects and controls.
Figure 3.
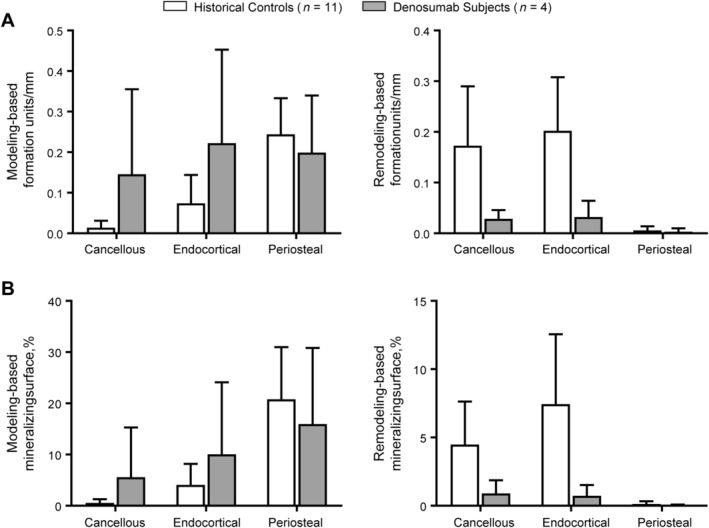
Specialized histomorphometric modeling‐based and remodeling‐based bone formation parameters in study subjects and historical controls. Graphs show the mean ± SD. (A) Modeling‐based and remodeling‐based formation units per mm in each of the three bone envelopes. (B) Percentage of modeling‐based and remodeling‐based mineralizing surface in each of the three bone envelopes.
Conventional histomorphometric parameters are described in Table 3. Denosumab‐treated subjects showed higher ES/BS but lower MS/BS and BFR/BS compared with controls in the cancellous and endocortical envelopes. In contrast, the periosteal envelope showed lower MS/BS and BFR/BS in treated subjects compared with controls.
Table 3.
Conventional Histomorphometric Parameters in Study Subjects and Controls
| Parameter | Historical controls (N = 11) | Denosumab subjects (N = 4) |
|---|---|---|
| Cancellous bone | ||
| Eroded surface/bone surface (%) | 5.59 ± 1.88 | 1.99 ± 1.08 |
| Mineralizing surface/bone surface (%) | 5.36 ± 3.89 | 6.73 ± 10.48 |
| Bone formation rate/bone surface (μm3/μm2/year) | 10.60 ± 8.41 | 20.76 ± 27.48 |
| Endocortical bone | ||
| Eroded surface/bone surface (%) | 5.82 ± 2.10 | 2.03 ± 1.14 |
| Mineralizing surface/bone surface (%) | 11.99 ± 9.28 | 11.10 ± 13.41 |
| Bone formation rate/bone surface (μm3/μm2/year) | 28.89 ± 24.75 | 28.52 ± 37.61 |
| Periosteal bone | ||
| Mineralizing surface/bone surface (%) | 20.91 ± 10.25 | 15.97 ± 14.84 |
| Bone formation rate/bone surface (μm3/μm2/year) | 73.42 ± 33.45 | 66.37 ± 54.72 |
All values shown are mean ± SD.
N = number of subjects with an evaluable biopsy for fluorochrome labeling.
Discussion
This study is the first to show evidence of MBBF in the femoral neck of patients with osteoporosis treated with denosumab per standard of care. Modeling‐based fluorochrome labels, with no evidence of prior resorption, were observed in all study subjects, and rates of MBBF were numerically higher in denosumab‐treated subjects compared with controls in endocortical and cancellous bone envelopes. In this study, the percentage of MBBF MS was also higher, with no reduction in MS/BS, in treated subjects compared with controls, suggesting that bone modeling was preserved or potentially enhanced at the femur with denosumab. Maintained or higher MBBF in association with potent inhibition of remodeling likely reflects a net increase in bone volume and helps to provide a mechanism to explain the continued increases in BMD reported in subjects receiving denosumab for up to 10 years.
Hip fracture is the most serious consequence of osteoporosis because of the associated mortality, morbidity, and healthcare costs.22, 23 If osteoporosis is diagnosed and treated, hip fracture risk can be significantly reduced.24 In FREEDOM, the improvement in total hip BMD with denosumab treatment accounted for roughly 80% of the reduction in nonvertebral fracture risk.25 Furthermore, during the FREEDOM extension study, with up to 10 years of denosumab treatment, the total hip T‐score attained at any time on therapy was a predictor of subsequent fracture risk.26 However, it is challenging to obtain biopsies and assess bone histomorphometry at the femoral neck, the site of approximately 50% of hip fractures,27 because biopsies of the femoral neck would be associated with weakening of the bone. Therefore, only in the context of a THR, where the femoral neck is routinely removed, can this site be examined histomorphometrically.
Typically, the effects of osteoporosis treatments on bone histomorphometry are evaluated using iliac crest biopsies. In the past, iliac crest biopsies were primarily performed to assess normalcy of bone quality and presence and magnitude of bone remodeling inhibition with antiresorptive therapy. Indeed, with denosumab treatment, the primary effect seen in iliac biopsies was potent inhibition of remodeling.28 Bone formation at this site has also been studied, especially in the context of teriparatide treatment.20, 29, 30 These studies demonstrated increased MBBF and RBBF in all three bone envelopes with teriparatide treatment after a range of treatment durations (from 1 to 24 months), corresponding to its anabolic mechanism of action. The current study is the first to report bone formation at the human femoral neck following denosumab treatment.
In nonhuman primates, MBBF was observed in the endocortical surface of the femur following administration of romosozumab31 and the cathepsin K inhibitor odanacatib.32 Ominsky et al.18 provided evidence of active MBBF in the adult primate skeleton following high‐dose treatment with denosumab for 16 months; MBBF was predominantly observed at the superior endocortex and the inferior periosteal surface of the femoral neck. In the current study, the higher amount of MBBF in denosumab‐treated subjects compared with controls was most pronounced in the cancellous and endocortical envelopes. This finding is consistent with the results of Cosman et al. showing that teriparatide rapidly stimulated bone formation at the cancellous and endocortical surfaces of the human femoral neck.19 In both studies, controls and treated subjects both showed extensive bone formation in the periosteum, which is likely due to the fact that periosteal modeling in the femoral neck might be increased in the setting of severe hip osteoarthritis and the effect of treatment was not sufficient to create a measurable difference between groups.33 In contrast, rates of RBBF in the periosteum were very low for both groups in the current analysis.
Bone histomorphometry results from iliac crest biopsies collected at years 2 and 3 of FREEDOM showed marked decreases in bone remodeling parameters with denosumab, namely MS/BS and BFR/BS,28 which is consistent with denosumab‐induced inhibition of osteoclast activity and reduced remodeling activation. The current findings in femoral neck biopsies showed no reduction in MS/BS and BFR/BS for the cancellous and endocortical bone envelopes. However, the mean values for ES/BS were decreased in denosumab‐treated subjects. This observation, together with the decreases in RBBF MS and increases in MBBF MS in these subjects, seems to indicate a greater degree of modeling in the femur with denosumab treatment in this study compared with the iliac crest in FREEDOM. From biopsies in subjects undergoing total hip arthroplasty or femoral head replacement, Sano et al. revealed strong histomorphometric evidence of MBBF on trabeculae in loaded femoral heads, even in elderly subjects.34 Thus, bone in the femoral neck may hold greater potential for modeling because it is weight bearing. Additional studies are needed to address bone histomorphometry and levels of MBBF and RBBF in the femoral neck in the untreated population and those who have been treated with denosumab.
The amount of MBBF was higher in denosumab‐treated subjects compared with controls, although it is unclear whether denosumab had a direct or, more likely, an indirect or simply a permissive effect on bone modeling. The mechanism is not known. This situation may resemble that in patients with adynamic bone disease, a condition characterized by low rates of remodeling due to low serum levels of parathyroid hormone. Such patients have higher levels of MBBF, which may serve as an adaptive response to preserve bone volume.35 Increased levels of PTH, above the upper limit of normal in many patients, have been reported shortly (1 to 3 months) after denosumab injection,36, 37, 38 and this compensatory response to maintain serum calcium levels has been proposed to indirectly stimulate bone formation. We might speculate that, in the face of potent resorption inhibition with denosumab, the stimulus to PTH‐mediated formation may persist and result in an increase in MBBF. In contrast, bone‐forming agents likely have a direct effect on MBBF. With romosozumab treatment in the FRAME trial, the rapid stimulation of bone formation in the first 2 months of treatment was found to be the result of increased MBBF in the cancellous and endocortical envelopes.39
Some limitations of this study should be noted. First, the sample size of denosumab‐treated (n = 4) and control (n = 11) subjects was small, which makes the current analysis entirely exploratory. The small number of enrolled subjects also precluded statistical comparisons between denosumab‐treated and control subjects. Thus, all analyses were descriptive in nature, and interpretations should be made considering this limitation. Second, all biopsies were obtained from subjects with hip osteoarthritis, which is known to induce a remodeling imbalance favoring bone formation at the femoral neck.33 Third, there was no requirement for a diagnosis of osteoporosis among control subjects, and 36% of control subjects had received bisphosphonate treatment more than 1 year before THR. Given the persistence of bisphosphonates in bone, it is possible that such treatment could have been permissive of modeling in the presence of some remodeling inhibition. However, this effect would likely have underestimated the current results showing higher rates of MBBF with lower rates of RBBF in denosumab‐treated subjects compared with controls. Finally, the duration of denosumab treatment prior to THR varied (from two to nine doses) among study subjects, and further studies are needed to assess MBBF in the femoral neck in the context of long‐term denosumab treatment.
This study is the first to provide evidence of MBBF with denosumab treatment in the human femoral neck, a common site of hip fracture associated with osteoporosis. These results support previous nonclinical findings18 in suggesting that denosumab helps to preserve MBBF while inhibiting remodeling at the hip, which may contribute to the observed continued gains in BMD over time after remodeling is maximally inhibited.
Disclosures
DWD has grant/research support, consulting fees, and/or speaker fees/honoraria from Amgen Inc., Eli Lilly, Radius Health, and the National Institutes of Health. AC and LC are employees and shareholders of Amgen Inc. NP and RBW were previously employed by Amgen Inc. MPB has grant/research support from the National Institutes of Health, American Austrian Foundation, Ines Mandl Research Foundation, and Smith & Nephew and consulting fees/royalties from Smith & Nephew. JWN has grant/research support from Eli Lilly, Radius Health, and the National Institutes of Health. HZ has no disclosures to report. FC has grant/research support from Amgen Inc. and Eli Lilly; consulting fees from Amgen Inc., Eli Lilly, Radius Health, and RPharm; and speaker/honoraria from Amgen Inc., Eli Lilly, and Radius.
Supporting information
Supplemental Fig. 1 Representative image of femoral neck specimen for staining. (A) Cross‐section of the femoral neck, toluidine blue stain. (B) Magnified view of the femoral neck indicating the three envelopes that were analyzed. Cn = cancellous; Ec = endocortical; Ps = periosteal. The histomorphometric analysis was performed within each envelope for the entire cross section.
Supplemental Fig. 2 Representative images of tetracycline labels showing RBBF and MBBF in hip biopsies from study subjects and controls. All images are from the cancellous envelope. Fluorescence microscopy images showing fluorochrome labels are in the left‐hand panels with corresponding bright‐field images in the right‐hand panels. (A) RBBF in a control subject. The reversal line underlying the label is scalloped. (B) MBBF in a control subject. The reversal line underlying the label is smooth. (C) RBBF in a denosumab‐treated subject. The reversal line underlying the label is scalloped. (D) MBBF in a denosumab‐treated subject. The reversal line underlying the label is smooth. MBBF = modeling‐based bone formation; RBBF = remodeling‐based bone formation.
Supplemental Table 1 Specialized histomorphometric modeling‐ and remodeling‐based surface parameters in study subjects and controls.
Acknowledgments
This study was funded by Amgen Inc. Kate Smigiel, PhD (Amgen Inc.) provided medical writing support. The authors thank Shuang Huang, PhD (Amgen Inc.) for statistical support.
Authors’ roles: Study design: DWD, FC, JWN, and RBW. Study conduct: FC, DWD, JWN, and MPB. Data collection: HZ and JWN. Data analysis: HZ and JWN. Data interpretation: All authors. Drafting manuscript: DWD, FC, and JWN. Revising manuscript content: All authors. Approving final version of manuscript: All authors. All authors take responsibility for the integrity of the data analysis.
Public clinical trial registration: http://clinicaltrials.gov/show/NCT02576652. Bone Histomorphometry of the Proximal Femur in Denosumab‐treated Subjects Undergoing Total Hip Replacement.
References
- 1. Adachi JD, Ioannidis G, Olszynski WP, et al. The impact of incident vertebral and non‐vertebral fractures on health related quality of life in postmenopausal women. BMC Musculoskelet Disord. 2002;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2016;8(6):225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weycker D, Li X, Barron R, Bornheimer R, Chandler D. Hospitalizations for osteoporosis‐related fractures: economic costs and clinical outcomes. Bone Rep. 2016;5:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41. [DOI] [PubMed] [Google Scholar]
- 5. Black DM, Delmas PD, Eastell R, et al. Once‐yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. [DOI] [PubMed] [Google Scholar]
- 6. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65. [DOI] [PubMed] [Google Scholar]
- 7. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) study group. JAMA. 1999;282(14):1344–52. [DOI] [PubMed] [Google Scholar]
- 8. Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open‐label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–23. [DOI] [PubMed] [Google Scholar]
- 9. Genant HK, Libanati C, Engelke K, et al. Improvements in hip trabecular, subcortical, and cortical density and mass in postmenopausal women with osteoporosis treated with denosumab. Bone. 2013;56(2):482–8. [DOI] [PubMed] [Google Scholar]
- 10. McClung MR, Zanchetta JR, Hoiseth A, et al. Denosumab densitometric changes assessed by quantitative computed tomography at the spine and hip in postmenopausal women with osteoporosis. J Clin Densitom. 2013;16(2):250–6. [DOI] [PubMed] [Google Scholar]
- 11. Bone HG, Hosking D, Devogelaer JP, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–99. [DOI] [PubMed] [Google Scholar]
- 12. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON‐Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27(2):243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mellstrom DD, Sorensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75(6):462–8. [DOI] [PubMed] [Google Scholar]
- 14. Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20(2):177–84. [DOI] [PubMed] [Google Scholar]
- 15. Dempster DW, Brown JP, Fahrleitner‐Pammer A, et al. Effects of long‐term denosumab on bone histomorphometry and mineralization in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103(7):2498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taddei F, Balestri M, Rimondi E, Viceconti M, Manfrini M. Tibia adaptation after fibula harvesting: an in vivo quantitative study. Clin Orthop Relat Res. 2009;467(8):2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruff CB, Hayes WC. Subperiosteal expansion and cortical remodeling of the human femur and tibia with aging. Science. 1982;217(4563):945–8. [DOI] [PubMed] [Google Scholar]
- 18. Ominsky MS, Libanati C, Niu QT, et al. Sustained modeling‐based bone formation during adulthood in cynomolgus monkeys may contribute to continuous BMD gains with denosumab. J Bone Miner Res. 2015;30(7):1280–9. [DOI] [PubMed] [Google Scholar]
- 19. Cosman F, Dempster DW, Nieves JW, et al. Effect of teriparatide on bone formation in the human femoral neck. J Clin Endocrinol Metab. 2016;101(4):1498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short‐term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21(3):366–73. [DOI] [PubMed] [Google Scholar]
- 21. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burge R, Dawson‐Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis‐related fractures in the United States, 2005‐2025. J Bone Miner Res. 2007;22(3):465–75. [DOI] [PubMed] [Google Scholar]
- 23. Haentjens P, Magaziner J, Colon‐Emeric CS, et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewiecki EM, Wright NC, Curtis JR, et al. Correction to: hip fracture trends in the United States, 2002 to 2015. Osteoporos Int. 2018;29(11):2583. [DOI] [PubMed] [Google Scholar]
- 25. Austin M, Yang YC, Vittinghoff E, et al. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res. 2012;27(3):687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T‐score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34(6):1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karagas MR, Lu‐Yao GL, Barrett JA, Beach ML, Baron JA. Heterogeneity of hip fracture: age, race, sex, and geographic patterns of femoral neck and trochanteric fractures among the US elderly. Am J Epidemiol. 1996;143(7):677–82. [DOI] [PubMed] [Google Scholar]
- 28. Reid IR, Miller PD, Brown JP, et al. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2010;25(10):2256–65. [DOI] [PubMed] [Google Scholar]
- 29. Dempster DW, Zhou H, Recker RR, et al. Remodeling‐ and modeling‐based bone formation with teriparatide versus denosumab: a longitudinal analysis from baseline to 3 months in the AVA study. J Bone Miner Res. 2018;33(2):298–306. [DOI] [PubMed] [Google Scholar]
- 30. Ma YL, Zeng Q, Donley DW, et al. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF‐II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21(6):855–64. [DOI] [PubMed] [Google Scholar]
- 31. Ominsky MS, Niu QT, Li C, Li X, Ke HZ. Tissue‐level mechanisms responsible for the increase in bone formation and bone volume by sclerostin antibody. J Bone Miner Res. 2014;29(6):1424–30. [DOI] [PubMed] [Google Scholar]
- 32. Pennypacker BL, Chen CM, Zheng H, et al. Inhibition of cathepsin K increases modeling‐based bone formation, and improves cortical dimension and strength in adult ovariectomized monkeys. J Bone Miner Res. 2014;29(8):1847–58. [DOI] [PubMed] [Google Scholar]
- 33. Jordan GR, Loveridge N, Power J, Clarke MT, Reeve J. Increased cancellous bone in the femoral neck of patients with coxarthrosis (hip osteoarthritis): a positive remodeling imbalance favoring bone formation. Osteoporos Int. 2003;14(2):160–5. [DOI] [PubMed] [Google Scholar]
- 34. Sano H, Kondo N, Shimakura T, et al. Evidence for ongoing modeling‐based bone formation in human femoral head trabeculae via forming minimodeling structures: a study in patients with fractures and arthritis. Front Endocrinol. 2018;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ubara Y, Tagami T, Nakanishi S, et al. Significance of minimodeling in dialysis patients with adynamic bone disease. Kidney Int. 2005;68(2):833–9. [DOI] [PubMed] [Google Scholar]
- 36. Dempster DW, Zhou H, Recker RR, et al. Differential effects of teriparatide and denosumab on intact PTH and bone formation indices: AVA Osteoporosis Study. J Clin Endocrinol Metab. 2016;101(4):1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seeman ELC, Austin M, et al. Association between transitory increase in PTH following denosumab administration and reduced intracortical porosity is a distinctive attribute of denosumab therapy. Bone. 2012;50:S162. [Google Scholar]
- 38. McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821–31. [DOI] [PubMed] [Google Scholar]
- 39. Eriksen EF, Chapurlat R, Boyce R, et al. Extensive modeling‐based bone formation after 2 months of romosozumab treatment: results from the FRAME Clinical Trial. J Bone Miner Res. 2019;32(Suppl 1) [Presented orally at: Annual Meeting American Society for Bone and Mineral Research (ASBMR); 2019 Sep 20‐23; Orlando, FL, USA; Presentation Number: 1049]. Available from: https://www.asbmr.org/education/AbstractDetail?aid=d112527c-6efe-46cd-96af-04ce66040dcd. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1 Representative image of femoral neck specimen for staining. (A) Cross‐section of the femoral neck, toluidine blue stain. (B) Magnified view of the femoral neck indicating the three envelopes that were analyzed. Cn = cancellous; Ec = endocortical; Ps = periosteal. The histomorphometric analysis was performed within each envelope for the entire cross section.
Supplemental Fig. 2 Representative images of tetracycline labels showing RBBF and MBBF in hip biopsies from study subjects and controls. All images are from the cancellous envelope. Fluorescence microscopy images showing fluorochrome labels are in the left‐hand panels with corresponding bright‐field images in the right‐hand panels. (A) RBBF in a control subject. The reversal line underlying the label is scalloped. (B) MBBF in a control subject. The reversal line underlying the label is smooth. (C) RBBF in a denosumab‐treated subject. The reversal line underlying the label is scalloped. (D) MBBF in a denosumab‐treated subject. The reversal line underlying the label is smooth. MBBF = modeling‐based bone formation; RBBF = remodeling‐based bone formation.
Supplemental Table 1 Specialized histomorphometric modeling‐ and remodeling‐based surface parameters in study subjects and controls.
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/


