Abstract
Background
Admission to a pediatric intensive care unit (PICU) has been associated with respiratory consequences in children with asthma and carries major implications for management control. Whereas respiratory syncytial virus (RSV) bronchiolitis has been associated with increasing intensity of wheezing, the relationship between RSV‐bronchiolitis PICU admission and future asthma is unclear. This retrospective case‐control study evaluated whether hospitalization in the PICU due to RSV bronchiolitis is more likely to be associated with future asthma in early life compared with hospitalization in a general pediatric ward.
Methods
Children hospitalized due to RSV bronchiolitis between 2007 and 2019 in the PICU (study group) were compared to those hospitalized in a general pediatric ward (controls). Asthma prevalence was assessed by a follow‐up questionnaire based on The International Study of Asthma and Allergies in Childhood questionnaire.
Results
Sixty‐three PICU patients and 66 controls were included. The PICU patients presented with more severe disease during RSV hospitalization. At follow‐up, significantly more PICU patients aged 3–6 years had physician‐diagnosed asthma, respiratory symptoms during the previous 12 months, and underwent respiratory treatment since hospital discharge compared to controls (14 [60.9%] vs. 4 [18.2%] patients; 15 [65.2%] vs. 6 [27.3%]; and 16 [69.6%] vs. 8 [36.4%]; respectively). These differences were no longer observed after 6 years of age.
Conclusions
Children admitted to the PICU for RSV bronchiolitis are at higher risk for asthma in subsequent pre‐school years and will require close respiratory follow‐up than those admitted to general pediatric wards. Admission venue should be queried when asthma is suspected.
Keywords: asthma, intensive care unit, respiratory syncytial virus
1. INTRODUCTION
Asthma is the most common chronic disease of childhood, and correct diagnosis and treatment have a significant effect on asthma‐related morbidity and mortality. Assessment of asthma control is based upon careful history taking and evaluation of risk factors, one of which is previous admission to a pediatric intensive care unit (PICU) due to severe asthma exacerbation. 1 The role of a previous non‐asthma respiratory‐related PICU admission, such as bronchiolitis, has not been fully explored in children with an established or suspected diagnosis of asthma.
Bronchiolitis is an acute lower respiratory tract infection caused mostly by viruses, mainly the respiratory syncytial virus (RSV), which is the pathogen responsible for 50‐80% of cases. 2 Between 2% and 3% of all children younger than 12 months of age are hospitalized with a diagnosis of bronchiolitis, 3 , 4 , 5 and ~10% of them are admitted to the PICU. 6 A number of studies have shown that RSV bronchiolitis early in life is frequently followed by lingering abnormalities in airway function, including recurrent wheezing, reduced oxygenation, and deficits in pulmonary function tests suggestive of peripheral airway obstruction. 4 , 7 , 8 The association of viral lower respiratory tract infection during infancy and subsequent childhood wheezing is well established. 4 , 7 , 8 Previous studies have also shown a threefold increased risk of current wheezing and of current asthma in hospitalized RSV bronchiolitis pediatric patients compared to nonhospitalized children. 9 , 10 The association of severity of the bronchiolitis with future childhood asthma risk has also been suggested, 11 but this suggestion was based upon disease severity as defined according to whether treatment was provided in an outpatient setting, an emergency department (ED), or during hospitalization. The predictive value of PICU admission specifically for RSV bronchiolitis in future asthma development has not been fully explored.
The purpose of the present study was to evaluate if hospitalization in the PICU, as opposed to a general pediatric ward, can contribute to the identification of children with RSV bronchiolitis who will be likely to develop childhood asthma.
2. METHODS
This was a retrospective case‐control study. Demographic and clinical data of participants were obtained from their medical records. The participants' caregivers were interviewed about the presence of asthma in their children 3–10 years after PICU or pediatric ward hospitalization for RSV bronchiolitis. The study was approved by the Tel‐Aviv Sourasky Medical Center ethics committee (approval no. TLV‐0036‐18).
2.1. Participants
The study population included children who were admitted to the PICU at Dana–Dwek Children's Hospital with the diagnosis of RSV bronchiolitis between 2007 and 2019. The control group included matched children who were admitted to the general pediatric ward due to RSV bronchiolitis during the same period. The study inclusion criteria were a positive RSV nasal swab by either polymerase chain reaction (PCR) or by the immunofluorescence method, PICU hospitalization for the study group and general pediatric ward hospitalization for the control group and available hospitalization data from the hospital's medical database. The exclusion criteria were severe background illness (e.g., cyanotic cardiac malformation, chronic pulmonary disease, severe neurologic developmental delay, neoplasm, etc.) or if RSV bronchiolitis was incidentally diagnosed during hospitalization for another reason.
2.2. Study protocol
The study participants were first identified by their having positive RSV nasal swab test results as recorded in the hospital's virology database. The study group consisted of patients who were hospitalized in the PICU, while the control group patients were those who were hospitalized in the pediatric ward during the same period. Each patient who was hospitalized in the PICU was matched to the consecutive patient who was hospitalized in the ward. The study and control groups were matched according to sex, gestational week of birth (preterm/term), age group at hospitalization (0–2, 2–6, 6–12, and >12 months), and age at follow‐up (3–6/>6 years). The data collected from the medical records included; demographics (sex, gestational week of birth, age at hospitalization, birth weight, perinatal complications, and vaccinations), prehospitalization information (days since first symptom, clinical symptoms, use of primary medical care, and treatments), ED presentation (vital signs, physical findings, chest X‐ray, and laboratory findings), hospitalization information (length of stay, treatments, type, and duration of respiratory support) and follow‐up information collected by a structured telephone asthma questionnaire (Appendix A) designed for caregivers. The questionnaire was based on The International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire. 12
2.3. Outcomes
The main outcome was the presence of childhood asthma, according to the definition of the ISAAC questionnaire as follows 12 : physician‐diagnosed asthma, or respiratory symptoms (wheezing, whistling in the chest, shortness of breath) during the past 12 months, or respiratory treatment (e.g., inhalers, inhalations or systemic steroids) given at least once since hospital discharge.
2.4. Statistical analyses
The statistical analyses were performed with IBM SPSS statistics 25. The patients' characteristics were reported by descriptive statistics. Pearson's chi‐squared test assessed associations between the cases or controls and the following categorical variables: sex, age at hospitalization (0–2, 2–6, 6–12, and 12+ months), gestational week of birth (preterm and term), community clinic attendance and treatment, presenting symptoms, drugs administered during hospitalization, oxygen support, complications, and asthma diagnosis at follow‐up. The risk of these variables was assessed using odds ratio (OR). The independent samples t‐test assessed differences between the study patients and the controls with respect to continuous variables (birth weight, weight at hospitalization, laboratory values, body temperature, and length of hospitalization). A multivariable analysis was performed using logistic regression to predict asthma at follow‐up.
3. RESULTS
Two thousand two hundred thirty‐five RSV bronchiolitis patients were hospitalized during the study period, of whom 129 RSV bronchiolitis patients were included in the final analysis: 63 were the PICU patients and 66 were the pediatric ward patients. The PICU patients included 56 children who were admitted directly from the ED to the PICU and 7 children who were admitted first to the pediatric ward and then transferred to the PICU due to respiratory deterioration (Figure 1). The demographics were similar between the study and control groups with regard to sex, gestational week of birth, birth weight, age group at hospitalization, and age group at follow‐up (Table 1). The gestational age was similar between the two groups with no significant difference (study group 36.76 ± 2.92 weeks, control group 37.54 ± 2.47 weeks; p = 0.136).
Figure 1.
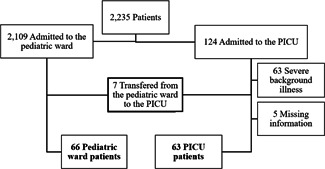
Flowchart of study entry selection
Table 1.
Demographic characteristics and prehospitalization data
| Variable | PICU | Pediatric ward | p Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age group (months) | 0.691 | ||
| 0–2 | 36 (57%) | 36 (55%) | |
| 2–6 | 12 (19%) | 15 (22%) | |
| 6–12 | 6 (10%) | 9 (13%) | |
| >12 | 9 (14%) | 6 (9%) | |
| Age group at follow‐up | 0.705 | ||
| 3–6 years | 23 (36%) | 22 (33%) | |
| >6 years | 40 (63%) | 44 (66%) | |
| Sex – male | 32 (51%) | 33 (50%) | 0.928 |
| Gestational age of birth, weeks (mean ± SD) | 36.76 ± 2.92 | 37.54 ± 2.47 | 0.136 |
| Gestational week of birth – preterm | 41 (65%) | 43 (65%) | 0.898 |
| Birth weight (kg) (mean ± SD) | 2.773 ± 0.75 | 3.047 ± 1.02 | 0.106 |
| Prehospitalization data | |||
| Number of days since 1st symptom (mean ± SD) | 2.9 ± 1.4 | 3.8 ± 3.9 | 0.090 |
| Symptoms before hospitalization, number of patients | |||
| Cough and/or nasal congestion | 53 (84.1%) | 60 (90.9%) | 0.243 |
| Shortness of breath | 29 (46%) | 26 (39.4%) | 0.446 |
| Fever | 19 (30.2%) | 31 (47%) | 0.050 |
| Caregiver‐reported apnea episodes | 16 (25.4%) | 4 (6.1%) | 0.002 |
Note: Bold indicates significant.
Abbreviations: PICU, pediatric intensive unit; SD, standard deviation.
Before hospitalization, more PICU patients sustained apnea episodes 16 (25.4%) versus 4 (6.1%) patients (p = 0.002) as reported by the caregivers, but other prehospitalization characteristics were similar between the groups (Table 1).
During hospitalization, the PICU group had a longer length of stay than the control group (9.83 ± 5.6 vs. 4.23 ± 2.5 mean days, p < 0.001). In addition, more PICU patients were treated with antibiotics and hemodynamic stabilization interventions compared to the controls (40 [63.5%] vs. 30 [45.5%] patients; p = 0.04 and 19 [30.3%] vs. 0 [0%] patients; p < 0.001, respectively). Finally, more PICU patients needed advanced respiratory support (e.g., high‐flow nasal cannula or mechanical ventilation) compared to the controls (Table 2). A comparison of the chest X‐rays performed at admission revealed that more PICU patients had lung infiltrates and atelectasis compared to the controls (39 [69.6%] vs. 28 [50.9%]; p = 0.044).
Table 2.
Hospitalization data of PICU and pediatric ward patients
| Variable | PICU | Pediatric ward | p Value |
|---|---|---|---|
| Length of stay (mean days ± SD) | 9.83 ± 5.6 | 4.23 ± 2.5 | <0.001 |
| Treatments, number of patients | |||
| Nebulized hypertonic saline 3% | 29 (46%) | 42 (63.6%) | 0.045 |
| Bronchodilator inhalations | 33 (52.4%) | 33 (50%) | 0.787 |
| Inhaled or systemic steroids | 11 (17.5%) | 11 (16.7%) | 0.905 |
| Antibiotic treatment | 40 (63.5%) | 30 (45.5%) | 0.040 |
| Hemodynamic stabilization treatment | 19 (30.3%) | 0 (0%) | <0.001 |
| Respiratory support | 60 (95%) | 40 (60%) | <0.001 |
| Mean duration of support (days ± SD) | 4.19 ± 2.8 | 2.86 ± 2.8 | 0.128 |
| High‐flow nasal cannula, number of patients | 22 (36.6%) | 0 (0%) | <0.001 |
| Mechanical ventilation, number of patients | 19 (31.6%) | 0 (0%) | <0.001 |
| Chest x‐ray evidence of infiltrates and atelectasis ‐ no. of patients | 39 (69.6%) | 28 (50.9%) | 0.044 |
Note: Bold indicates significant.
Abbreviations: PICU, pediatric intensive unit; SD, standard deviation.
All 129 participants responded to the follow‐up ISAAC questionnaire. At follow‐up, the study and the control groups were divided into two age groups, one of children 3–6 years of age and the other of children >6 years of age, with similar numbers of patients in each group (3–6 years: 23 [36%] in the study group and 22 [33%] in the control group, 6+ years: 40 [63%] in the study group, 44 [66%] in the control group, p = 0.705).
There was a significant difference in the number of children with respiratory symptoms during the previous 12 months between study and control groups (24 [38.1%] vs. 12 [18.2%] patients, respectively; p < 0.001; Table 3). There were significant group differences for all three assessed parameters for children between 3 and 6 years of age. Moreover, PICU hospitalization for RSV bronchiolitis increased the risk of physician‐diagnosed asthma (OR 7.00 [1.78–27.528]), respiratory symptoms in the previous 12 months (OR 5.00 [1.402–17.83]), and respiratory treatment given since hospital discharge (OR 4.00 [1.155–13.855]) among this young age group. These differences, however, were not found for children older than 6 years of age. Finally, a multivariable analysis was performed using logistic regression to predict asthma at follow‐up, and the results were not significant.
Table 3.
Asthma at follow‐up among PICU and pediatric ward patients
| PICU | Pediatric ward | p Value | Odds ratio (95% CI) | |
|---|---|---|---|---|
| Entire cohort | 63 (100%) | 66 (100%) | ||
| Physician‐diagnosed asthma | 23 (36.5%) | 16 (24.2%) | 0.129 | 1.797 (0.839–3.848) |
| Respiratory symptoms in the previous 12 months | 24 (38.1%) | 12 (18.2%) | 0.012 | 2.769 (1.237–6.201) |
| Respiratory treatment given since hospital discharge | 34 (54%) | 30 (45.5%) | 0.379 | 1.407 (0.704–2.813) |
| Ages 3–6 years | 23 (100%) | 22 (100%) | ||
| Doctor‐diagnosed asthma | 14 (60.9%) | 4 (18.2%) | 0.003 | 7.00 (1.78–27.528) |
| Respiratory symptoms in the previous 12 months | 15 (65.2%) | 6 (27.3%) | 0.011 | 5.00 (1.402–17.83) |
| Respiratory treatment given since hospital discharge | 16 (69.6%) | 8 (36.4%) | 0.026 | 4.00 (1.155–13.855) |
| Ages 6+ years | 40 (100%) | 44 (100%) | ||
| Physician‐diagnosed asthma | 9 (22.5%) | 12 (27.3%) | 0.614 | 0.744 (0.286–2.095) |
| Respiratory symptoms in the previous 12 months | 9 (22.5%) | 6 (13.6%) | 0.289 | 1.839 (0.59–5.73) |
| Respiratory treatment given since hospital discharge | 18 (45%) | 22 (50%) | 0.559 | 0.818 (0.347–1.931) |
Note: Bold indicates significant.
Abbreviations: CI, confidence interval; PICU, pediatric intensive unit
.
4. DISCUSSION
The identification of childhood conditions that predispose a child to asthma later in life is highly important since it alerts to the potential need for future follow‐up and treatment. The findings of this study show that hospitalization in the PICU due to RSV bronchiolitis may be a greater risk factor for future childhood asthma than hospitalization for RSV bronchiolitis in a general pediatric ward. Comparison of the follow‐up data of the cohort revealed that PICU patients had significantly higher rates of childhood asthma between the ages of 3–6 years compared to the children who were hospitalized in a general pediatric ward. Indeed, PICU hospitalization for RSV bronchiolitis increased the risk of physician‐diagnosed asthma, respiratory symptoms during the previous 12 months, and prescribed respiratory treatment since hospital discharge with an OR ranging between 4 and 7. At ages older than 6 years, there was no significant difference between these two groups. This is in line with most of the previous publications which showed that childhood asthma or childhood wheezing as a sequela of RSV bronchiolitis is more predominant in younger ages, especially those under 5 years. 13 , 14
It was recently reported that the mere hospitalization in a PICU and/or the implementation of invasive ventilation due to an asthma attack is a significant prognostic parameter when assessing asthma control including the potential for life‐threatening asthma attacks in patients diagnosed as having asthma. 1 Moreover, a relationship between the severity of bronchiolitis and the increasing odds for both early childhood asthma and asthma‐specific morbidity has also been suggested., 11 Although RSV bronchiolitis has been frequently associated with an increased risk of developing recurrent wheezing and asthma later in life, 7 , 8 , 9 , 11 , 13 no studies had explored the long‐term impact of mere PICU hospitalization for RSV bronchiolitis in young children on the subsequent development of asthma.
Our current findings suggest that hospitalization in the PICU for RSV bronchiolitis should itself be considered a significant risk factor for asthma development in younger ages. Not surprisingly, the patients admitted to the PICU had indeed presented with a more severe degree of RSV bronchiolitis than those admitted to the general pediatric ward, reflected by a longer length of stay and a greater need for hemodynamic stabilization, antibiotic treatment, and respiratory support than the controls. As expected, all of these findings are in line with previous studies that reported more severe bronchiolitis in patients admitted to a PICU. 5 , 15 However, regardless of disease severity, we now propose that any history of hospitalization in the PICU due to bronchiolitis should be part of the assessment of a patient with suspected asthma.
There are a few limitations that bear mention. First, we acknowledge that the increased risk for future asthma may be due to the more severe disease rather than the PICU hospitalization itself. Nevertheless, given that it may not be possible to differentiate between these two factors, we felt that it would be still important to review asthma prevalence specifically after PICU admission. In this context, we cannot rule out that the genetic factor and not the impact of PICU hospitalization Perce, dictate both the severity of RSV bronchiolitis disease and the likelihood for PICU hospitalization and also predisposing for future asthma in the 3–6 preschool children. 16 , 17 Second, the hospitalization data were collected retrospectively by a telephonic interview, and some data may be missing or inaccurate. The relatively small number of patients admitted to the PICU due to RSV bronchiolitis may also be a limitation, however, it reflects disease's burden in a tertiary medical center for over a decade.
In conclusion, our study results demonstrated that children hospitalized in the PICU due to RSV bronchiolitis have a higher risk of developing childhood asthma or wheezing at early ages than children hospitalized in a general pediatric ward. A history of hospitalization in a PICU for RSV bronchiolitis should alert pediatricians and caregivers to possible future asthma. Moreover, past PICU admission for RSV bronchiolitis should be part of the history taking when suspecting the diagnosis of asthma.
AUTHOR CONTRIBUTIONS
Moria Be'er: Data curation (equal); investigation (lead); methodology (equal); visualization (lead); writing–original draft (equal); writing–review and editing (lead). Shai Bushmitz: Data curation (equal); investigation (equal); methodology (equal); writing–original draft (lead); writing–review and editing (equal). Michal Cahal: Data curation (equal); investigation (supporting); methodology (supporting); validation (supporting); writing–review and editing (supporting). Efraim Sadot: Data curation (equal); validation (supporting); writing–review and editing (supporting). Sivan Yochpaz: Data curation (lead); project administration (supporting); validation (supporting); writing–review and editing (supporting). Omri Besor: Data curation (equal); formal analysis (lead); methodology (supporting); writing–review and editing (supporting). Israel Amirav: Conceptualization (lead); methodology (equal); supervision (lead); writing–review and editing (equal). Moran Lavie: Conceptualization (lead); investigation (equal); methodology (equal); supervision (lead); writing–original draft (equal); writing–review and editing (equal).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
The authors would like to acknowledge Mrs. Ora Halutz for her assistance with data curation.
APPENDIX A. STRUCTURED QUESTIONNAIRE DESIGNED FOR PARENTS OF CASES AND CONTROLS 12
1. Has your child ever had wheezing or whistling in the chest at any time in the past?
No
Yes
IF YOU HAVE ANSWERED “NO” PLEASE SKIP TO QUESTION 6
2. Has your child had wheezing or whistling in the chest in the past 12 months?
No
Yes
IF YOU HAVE ANSWERED “NO” PLEASE SKIP TO QUESTION 6
3. How many attacks of wheezing has your child had in the past 12 months?
None
1–3
4–12
More than 12
4. In the past 12 months, how often, on average, has your child's sleep been disturbed due to wheezing?
Never woken with wheezing
Less than one night per week
One of more nights per week
5. In the past 12 months, has wheezing ever been severe enough to limit your child's speech to only one or two words at a time between breaths?
No
Yes
6. Has your child ever had asthma?
No
Yes
7. In the past 12 months, has your child's chest sounded wheezy during or after exercise?
No
Yes
8. In the past 12 months, has your child had a dry cough at night, apart from a cough associated with a cold or chest infection?
No
Yes
9. In the past 12 months, has your child visited the emergency department due to wheezing, shortness of breath, or asthma?
No
Yes, how many times? ____
10. In the past 12 months, has your child been hospitalized due to wheezing, shortness of breath, or asthma?
No
Yes, how many times? ____
11. Does your child visit a pulmonology or allergy clinic on a regular basis due to asthma?
No
Yes
12. Has your child ever received any respiratory treatment such as inhalers or steroids?
No
Yes
Be'er M, Bushmitz S, Cahal M, et al. Asthma risk after a pediatric intensive care unit admission for respiratory syncytial virus bronchiolitis. Pediatric Pulmonology. 2022;57:1677‐1683. 10.1002/ppul.25953
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Global Initiative for Asthma . Global strategy for asthma management and prevention (Updated 2020). Rev Frd'Allergologie d'Immunologie Clin [Internet]. 2020;36(6):685‐704. https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdfbox2.2page35 [Google Scholar]
- 2. Lin JA, Madikians A. From bronchiolitis guideline to practice: a critical care perspective. World J Crit Care Med. 2015;4(3):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Article R. More on viral bronchiolitis in children. N Engl J Med. 2016;375(12):1199‐1200. [DOI] [PubMed] [Google Scholar]
- 4. Øymar K, Skjerven HO, Mikalsen IB. Acute bronchiolitis in infants, a review. Scand J Trauma Resusc Emerg Med. 2014;22(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caballero MT, Polack FP, Stein RT. Bronquiolite viral em neonatos jovens: novas perspectivas para manejo e tratamento. J Pediatr (Rio J) [Internet]. 2017;93:75‐83. 10.1016/j.jped.2017.07.003 [DOI] [Google Scholar]
- 6. Oakley E, Chong V, Borland M, et al. Intensive care unit admissions and ventilation support in infants with bronchiolitis. Emerg Med Australas. 2017;29(4):421‐428. [DOI] [PubMed] [Google Scholar]
- 7. Gern JE, Rosenthal LA, Sorkness RL, Lemanske RF Jr. Effects of viral respiratory infections on lung development and childhood asthma. J Allergy Clin Immunol. 2005;115(4):668‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramilo O, Mejias A. Respiratory syncytial virus‐induced acute disease severity and long‐term wheezing: uncovering the unexpected. Am J Respir Crit Care Med. 2018;198(8):984‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zomer‐Kooijker K, Van Der Ent CK, Ermers MJJ, Uiterwaal CSPM, Rovers MM, Bont LJ. Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS One. 2014;9(1):3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silver AH, Nazif JM. Bronchiolitis. Pediatr Rev. 2019;40(11):568‐557. [DOI] [PubMed] [Google Scholar]
- 11. Carroll KN, Wu P, Gebretsadik T, et al. The severity‐dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123(5):1055‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483‐491. [DOI] [PubMed] [Google Scholar]
- 13. Régnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta‐analysis. Pediatr Infect Dis J. 2013;32(8):820‐826. [DOI] [PubMed] [Google Scholar]
- 14. Szabo SM, Levy AR, Gooch KL, Bradt P, Wijaya H, Mitchell I. Elevated risk of asthma after hospitalization for respiratory syncytial virus infection in infancy. Paediatr Respir Rev. 2013;13(Suppl 2):S9‐S15. [DOI] [PubMed] [Google Scholar]
- 15. Damore D, Mansbach JM, Clark S, Ramundo M, Camargo CA Jr. Prospective multicenter bronchiolitis study: predicting intensive care unit admissions. Acad Emerg Med. 2008;15(10):887‐894. [DOI] [PubMed] [Google Scholar]
- 16. Tal G, Mandelberg A, Dalal I, et al. Association between common Toll‐like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis. 2004;189(11):2057‐2063. [DOI] [PubMed] [Google Scholar]
- 17. Mandelberg A, Tal G, Naugolny L, et al. Lipopolysaccharide hyporesponsiveness as a risk factor for intensive care unit hospitalization in infants with respiratory syncitial virus bronchiolitis. Clin Exp Immunol. 2006;144(1):48‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


