ABSTRACT
Objectives
To evaluate whether the Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis need to be better defined and, if deemed necessary, to reach consensus on the updated definitions.
Methods
A modified Delphi procedure was performed among European gynecologists with expertise in ultrasound diagnosis of adenomyosis. To identify MUSA features that might need revision, 15 two‐dimensional (2D) video recordings (four recordings also included three‐dimensional (3D) still images) of transvaginal ultrasound (TVS) examinations of the uterus were presented in the first Delphi round (online questionnaire). Experts were asked to confirm or refute the presence of each of the nine MUSA features of adenomyosis (described in the original MUSA consensus statement) in each of the 15 videoclips and to provide comments. In the second Delphi round (online questionnaire), the results of the first round and suggestions for revision of MUSA features were shared with the experts before they were asked to assess a new set of 2D and 3D still images of TVS examinations and to provide feedback on the proposed revisions. A third Delphi round (virtual group meeting) was conducted to discuss and reach final consensus on revised definitions of MUSA features. Consensus was predefined as at least 66.7% agreement between experts.
Results
Of 18 invited experts, 16 agreed to participate in the Delphi procedure. Eleven experts completed and four experts partly finished the first round. The experts identified a need for more detailed definitions of some MUSA features. They recommended use of 3D ultrasound to optimize visualization of the junctional zone. Fifteen experts participated in the second round and reached consensus on the presence or absence of ultrasound features of adenomyosis in most of the still images. Consensus was reached for all revised definitions except those for subendometrial lines and buds and interrupted junctional zone. Thirteen experts joined the online meeting, in which they discussed and agreed on final revisions of the MUSA definitions. There was consensus on the need to distinguish between direct features of adenomyosis, i.e. features indicating presence of ectopic endometrial tissue in the myometrium, and indirect features, i.e. features reflecting changes in the myometrium secondary to presence of endometrial tissue in the myometrium. Myometrial cysts, hyperechogenic islands and echogenic subendometrial lines and buds were classified unanimously as direct features of adenomyosis. Globular uterus, asymmetrical myometrial thickening, fan‐shaped shadowing, translesional vascularity, irregular junctional zone and interrupted junctional zone were classified as indirect features of adenomyosis.
Conclusion
Consensus between gynecologists with expertise in ultrasound diagnosis of adenomyosis was achieved regarding revised definitions of the MUSA features of adenomyosis and on the classification of MUSA features as direct or indirect signs of adenomyosis. © 2021 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: adenomyosis, consensus, Delphi technique, ultrasonography
Short abstract
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
RESUMEN
Consenso sobre las definiciones revisadas de las características de la Evaluación Ecográfica de la Morfología del Útero (MUSA, por sus siglas en inglés) de la adenomiosis: resultados de un procedimiento Delphi modificado
Objetivos
Evaluar si es necesario definir mejor las características de la Evaluación Ecográfica de la Morfología del Útero (MUSA) de la adenomiosis y, si fuera necesario, llegar a un consenso sobre las definiciones actualizadas.
Métodos
Se aplicó un procedimiento Delphi modificado entre especialistas en ginecología de Europa con experiencia en el diagnóstico ecográfico de la adenomiosis. Para identificar las características de la MUSA que podrían necesitar revisión, en la primera ronda Delphi (cuestionario en línea) se presentaron 15 grabaciones de vídeo bidimensionales (2D), cuatro de las cuales incluían también fotografías tridimensionales (3D), de exámenes mediante ecografía transvaginal (ETV) del útero. Se pidió a los especialistas que confirmaran o refutaran la presencia de cada una de las nueve características de la MUSA de la adenomiosis (descritas en la declaración original de consenso de la MUSA) en cada uno de los 15 vídeos y que aportaran comentarios. En la segunda ronda Delphi (cuestionario en línea), se compartieron con los especialistas los resultados de la primera ronda y las sugerencias sobre la revisión de las características de la MUSA, antes de pedirles que evaluaran un nuevo grupo de imágenes fotográficas en 2D y 3D de los exámenes de ETV y que aportaran sus comentarios sobre las revisiones propuestas. Se llevó a cabo una tercera ronda Delphi (reunión virtual en grupo) para debatir y alcanzar un consenso final sobre las definiciones revisadas de las características de la MUSA. El consenso se había definido previamente como un acuerdo de al menos el 66,7% entre los especialistas.
Resultados
De los 18 especialistas invitados, 16 aceptaron participar en el procedimiento Delphi. Once especialistas completaron la primera ronda y cuatro parcialmente. Los especialistas identificaron la necesidad de definiciones más detalladas de algunas características de la MUSA.
Se recomendó el uso de la ecografía 3D para optimizar la visualización de la zona de unión. En la segunda ronda participaron quince especialistas y llegaron a un consenso sobre la presencia o ausencia de características ecográficas de la adenomiosis en la mayoría de las imágenes fotográficas. Se llegó a un consenso para todas las definiciones revisadas, excepto para las líneas y brotes subendometriales y la zona de unión interrumpida. Trece especialistas participaron en la reunión en línea, en la que debatieron y acordaron las revisiones finales de las definiciones de la MUSA. Se llegó a un consenso sobre la necesidad de distinguir entre las características directas de la adenomiosis, es decir, las que señalan la presencia de tejido endometrial ectópico en el miometrio, y las características indirectas, es decir, las que reflejan cambios en el miometrio secundarios a la presencia de tejido endometrial en el miometrio. Los quistes miometriales, las islas hiperecogénicas y las líneas y brotes subendometriales ecogénicos se clasificaron unánimemente como características directas de la adenomiosis. El útero globular, el engrosamiento asimétrico del miometrio, el oscurecimiento en forma de abanico, la vascularidad translesional, la zona de unión irregular y la zona de unión interrumpida se clasificaron como características indirectas de la adenomiosis.
Conclusión
Se llegó a un consenso entre ginecólogos con experiencia en el diagnóstico ecográfico de la adenomiosis con respecto a las definiciones revisadas de las características de la MUSA de la adenomiosis y a la clasificación de las características de la MUSA como indicios directos o indirectos de la adenomiosis.
摘要
基于子宫腺肌症的形态学子宫超声评估(MUSA)特征达成修订定义共识:改良德尔菲法结果
目的
评估子宫腺肌症的形态学子宫超声评估(MUSA)特征,确认是否需要修订其定义,若认为有必要修订,则就更新最新定义达成共识。
方法
由专业的欧洲子宫腺肌症超声诊断妇科医生应用改良德尔菲法。为了确定可能需要修订的MUSA特征,在第一轮德尔菲法程序(在线问卷)中呈现15份经阴道超声(TVS)检查的二维(2D)视频记录(其中4份记录还包括三维(3D)静态影像)。我们要求专家组逐一确认或否认15份视频短片中是否存在子宫腺肌症的九个MUSA特征(根据原始MUSA共识声明中的描述)并发表意见。在第二轮德尔菲法程序(在线问卷)中向专家组通报第一轮的结果以及对MUSA特征的修订建议,然后要求他们评估一组新的TVS检查二维和三维静态图像,并且对拟采取的修订提供反馈。第三轮德尔菲法程序(虚拟小组会议)讨论MUSA特征的修订定义并达成最终共识。共识被预先定义为至少66.7%的专家取得一致意见。
结果
我们邀请18位专家,其中16位同意参加德尔菲法程序。 11位专家完成第一轮,而4位专家部分完成第一轮。专家组认为需要对某些MUSA特征给出
更为详细的定义。他们建议通过三维超声优化交界区的可视化。15位专家参加第二轮并就多数静态影像中是否存在子宫腺肌症的超声特征达成共识。除了子宫内膜下线纹以及中断交界区的定义外,专家组就所有其他修订定义达成共识。13位专家参加在线会议,讨论并同意对MUSA定义的最终修订。专家组一致认为需要区分子宫腺肌症的直接特征(即表明子宫肌层存在异位子宫内膜组织的特征)和间接特征(即反映子宫肌层继发于子宫内膜组织的变化特征)。子宫肌层囊肿、高回声团以及子宫内膜下回声线纹被一致归为子宫腺肌症的直接特征。子宫呈球形,不对称的子宫肌层增厚,扇形阴影,跨病变血管分布,不规则交界区和中断交界区则被列为是子宫腺肌症的间接特征。
结论
专业的子宫腺肌症超声诊断妇科医生在修订子宫腺肌症MUSA特征的定义以及MUSA特征作为子宫腺肌症的直接或间接标志的分类方面达成共识。
CONTRIBUTION —
What are the novel findings of this work?
In this modified Delphi study, 13 experts on ultrasound diagnosis of adenomyosis reached consensus on revised definitions of the Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis and classified these into direct and indirect sonographic signs of adenomyosis.
What are the clinical implications of this work?
The revised definitions of MUSA features of adenomyosis and the distinction between direct and indirect signs should facilitate recognition and diagnosis of adenomyosis in clinical practice. The updated definitions are important for future studies on the relationship between the MUSA features of adenomyosis and clinical symptoms and reproductive outcome.
INTRODUCTION
Adenomyosis is a benign uterine pathology defined as the presence of endometrial glands and stroma in the myometrium 1 . It can appear as a focal or diffuse lesion in the inner or outer myometrium, with or without surrounding hypertrophied myometrium. If adenomyosis is limited to the outer myometrium, it may be a continuation of endometriosis growing into the uterus from outside 2 . In women attending a gynecologic clinic for contraception counseling or for other reasons, an ultrasound diagnosis of adenomyosis was made in 34.5% and 20.9%, respectively 3 , 4 . Nowadays, transvaginal ultrasonography (TVS) is the first‐line imaging method to diagnose adenomyosis. It has been shown to be sufficiently accurate when using histopathology of hysterectomy specimens as the reference standard, as seen in a recent meta‐analysis 5 , which reported both sensitivity and specificity to be 78%. Three‐dimensional (3D) TVS has been reported to improve the diagnostic accuracy for adenomyosis, because it enables visualization of changes in the junctional zone in greater detail than two‐dimensional (2D) ultrasound 5 .
Thus far, a uniformly accepted or validated system to diagnose or classify the severity of adenomyosis based on imaging findings is lacking 2 . In 2015, the international Morphological Uterus Sonographic Assessment (MUSA) group published a consensus on which terminology to use when describing ultrasound images of adenomyosis 6 . In 2019, the MUSA group suggested a uniform classification and reporting system to be used when describing morphological variations of adenomyosis and its extent on ultrasound 7 . In a pilot study, the inter‐rater agreement when using the MUSA features to describe ultrasound images of adenomyosis was poor both among highly experienced and moderately experienced raters 8 . Inter‐rater agreement in diagnosing adenomyosis between an expert and a non‐expert rater trained in pattern recognition showed good inter‐rater agreement for 2D‐TVS images but poor agreement for 3D‐TVS images 9 . Poor inter‐rater agreement might be explained by unclear definitions of the MUSA features. Therefore, it is important to investigate if the definitions of the MUSA features are sufficiently clear, and if all or some of them need to be revised.
The aim of this study was to explore which, if any, MUSA features need to be better defined and to reach consensus on updated definitions by performing a modified Delphi study among gynecologists with expertise in ultrasound diagnosis of adenomyosis.
METHODS
A three‐round modified Delphi procedure was designed to achieve consensus among gynecologists with expertise in ultrasound diagnosis of adenomyosis. A Delphi procedure is a qualitative study method to identify the collective opinion of experts on a particular topic. Two rounds of questionnaires were held. The questionnaires included ultrasound images and videoclips of uteri of women suspected to have adenomyosis. The aims of showing images and videoclips were: (1) to explore agreement between the experts on the presence of MUSA features so as to identify MUSA features that might need a revised definition because of poor agreement; (2) to collect suggestions for revised definitions; and (3) reach consensus on suggested revised definitions. It is important to emphasize that, even though agreement between experts was assessed, this study is not an interobserver agreement study. A third Delphi round, which involved an online meeting, was organized to discuss and reach final consensus on revised definitions of MUSA features. The Delphi procedure was conducted online. Questionnaires were sent out using Research Survey® (IDEACT, Zwaag, The Netherlands) to facilitate participation by experts from different areas and reduce cost and time 10 . The approach of a modified Delphi procedure was chosen to use optimally the comments from the experts by identifying points of agreement and resolving points of disagreement.
The TVS images and recordings were selected from those stored for educational and research purposes in the University Hospital of Leuven, Leuven, Belgium, and Amsterdam UMC, location VUMC and AMC, Amsterdam, The Netherlands. All images were from patients who were suspected to have adenomyosis based on ultrasound findings. The patients had been examined with ultrasound by different experienced gynecologists during a specialist consultation in a tertiary referral center. The high‐end Voluson E8 Expert machine (GE Healthcare, Zipf, Austria) equipped with a multifrequency (4–9 MHz) endovaginal probe and the Samsung WS80A ultrasound system (Samsung Medison, Seoul, Korea) equipped with a multifrequency (5–9‐MHz) endovaginal probe were used. All images with sufficient ultrasound resolution and videoclips in which both the sagittal and transverse planes of the uterus were shown were eligible. Images or recordings showing a dominant pathology other than adenomyosis, such as a uterine fibroid, uterine niche, deep endometriosis or malignancy, were ineligible for inclusion. The images and videoclips to be used in the Delphi procedure were selected by the first author (M.J.H), who has been trained in the assessment of the MUSA features of adenomyosis through close supervision from the senior authors R.A.d.L., F.G., W.J.K.H. and J.A.F.H. in her ultrasound performance during outpatient consultations in a tertiary clinic for 2 years. M.J.H. did not participate in the Delphi procedure, but some of the gynecologists that had stored the images for education and research purposes did. The images and video recordings contained no identifiable patient data. The authors J.A.F.H., R.A.d.L. and M.J.H. decided which ultrasound features of adenomyosis should be assessed in the Delphi procedure (n = 9, see below). They based their choices on two MUSA publications 6 , 7 . The participating experts were asked to confirm or negate the presence of the following MUSA features: (1) globular uterus, (2) asymmetrical thickening of myometrial walls, (3) cysts within the myometrium, (4) fan‐shaped shadowing, (5) translesional vascularity, (6) hyperechogenic islands within the myometrium, (7) echogenic subendometrial lines and buds, (8) irregular junctional zone and (9) interrupted junctional zone. Ultrasound images of the features are presented in Figure 1.
Figure 1.
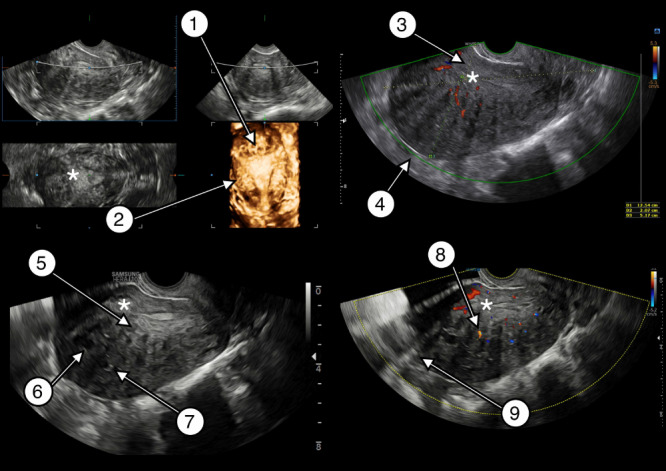
Transvaginal two‐dimensional and three‐dimensional ultrasound images of a uterus affected by adenomyosis depicting all Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis: 1, interrupted junctional zone; 2, irregular junctional zone; 3, asymmetrical myometrial thickening; 4, globular uterus; 5, echogenic subendometrial lines and buds; 6, myometrial cysts; 7, hyperechogenic islands; 8, translesional vascularity; 9, fan‐shaped shadowing.  , endometrium.
, endometrium.
The percentage of agreement in all videoclips and images after each round was calculated. All comments were analyzed and summarized by authors M.J.H. and R.A.d.L. and reported back to the participating experts in the next round. The experts were blinded to each other's opinions. The data were collected between November 2018 and May 2020. This study was approved by the ethics committee of the co‐ordinating hospital (Amsterdam UMC, location Vrije Universiteit; nWMO 2018.669).
Expert panel recruitment
Gynecologists with expertise in the ultrasound diagnosis of adenomyosis were invited to participate. Expertise was defined as demonstrable experience in gynecological ultrasound in clinical practice, involvement in research projects on adenomyosis and publications on this topic in international scientific peer‐reviewed journals. All experts were free to suggest another expert fulfilling the criteria of expertise to participate. There was no restriction on the invitation of experts from the same institution or country. An invitation email was sent to 18 potential participants. Those who agreed to participate received an electronic link to the questionnaire.
Questionnaires to assess MUSA features of adenomyosis
In the first Delphi round, the experts were presented with 15 videoclips of ultrasound examinations of the uterus (four videoclips included 3D still images at the end of the recording). They were asked to assess every video recording for the presence or absence of each of the nine MUSA features listed above using a four‐point Likert scale (‘very sure’, ‘probably’, ‘probably not’, ‘certainly not’). In this round, experts could also select ‘don't know’ as an answer. This option was added to identify features that were particularly difficult to assess. The experts could provide comments on all questions. If their answer was ‘don't know’, they were asked to explain why they were in doubt. An example of a question from round one is presented in Figure 2. Videoclip S1 is an example of a video recording presented to the experts. In this round we also collected information on the experts themselves, including country of residence, year of birth, years of experience as an ultrasound examiner, number of patients examined with TVS and found to have adenomyosis per month and number of publications on adenomyosis (either an article on adenomyosis in a peer‐reviewed journal or a presentation on ultrasound and adenomyosis at a national or international scientific meeting).
Figure 2.
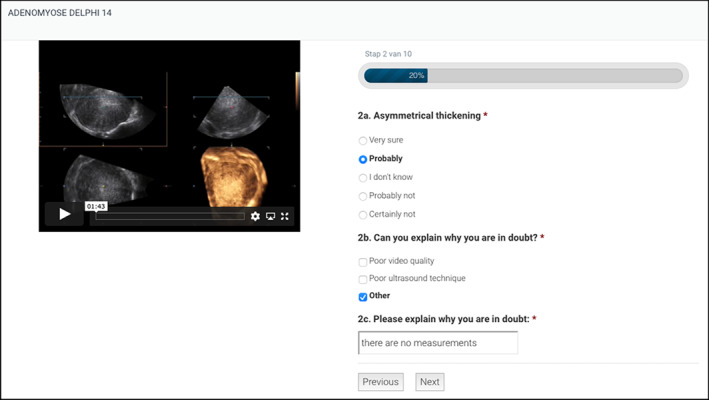
Example of a question in Round 1 (online survey) of the modified Delphi procedure on definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis, depicting a videoclip of a three‐dimensional transvaginal ultrasound examination and the question to the participant.
In the second Delphi round, a new set of 60 2D‐TVS still images of the sagittal plane of the uterus and a separate set of 15 3D‐TVS images (not volumes), were presented to the experts. The 3D images showed the 2D sagittal, 2D transverse and rendered coronal planes in a multiplanar image. We asked the experts to rate five to seven images per MUSA feature that had a high rate of agreement in the first round, and 11 to 13 images per MUSA feature that had a low rate of agreement in the first round. We used still images for this round to ensure that all experts assessed the same image. The experts were asked to confirm (‘yes’) or deny (‘no’) the presence of each feature and to add comments. An example of a question in round two is presented in Figure 3. J.A.F.H., R.A.d.L. and M.J.H. analyzed together the comments made in the first round. Based on these, they suggested revised definitions of each MUSA feature. The suggested revisions were proposed to the experts in the second Delphi round. The experts were asked to indicate whether they agreed with the suggested revision of the definition (‘yes’), partly agreed (‘yes, with comment’) or disagreed (‘no, but instead: …’). They were also encouraged to present their own suggestions for revision. An example of a question considering revision of a definition is shown in Figure 4. The comments from the experts on the suggested revisions were collected by authors M.J.H. and R.A.d.L., who prepared them for sharing with the experts in the third Delphi round.
Figure 3.
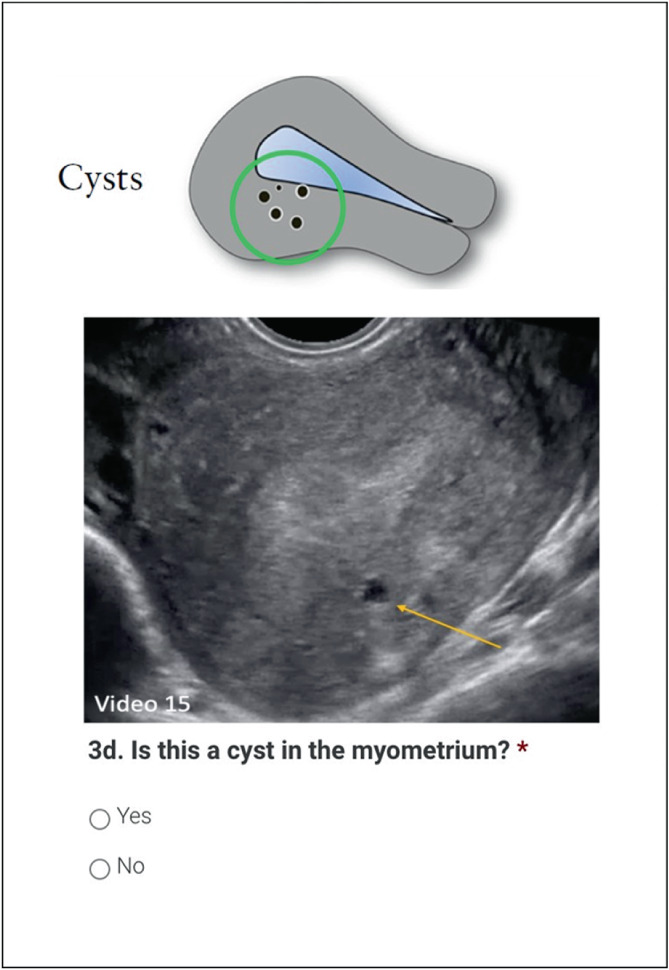
Example of a question in Round 2 (online survey) of the modified Delphi procedure on definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis, depicting a two‐dimensional still image of a transvaginal ultrasound examination.
Figure 4.
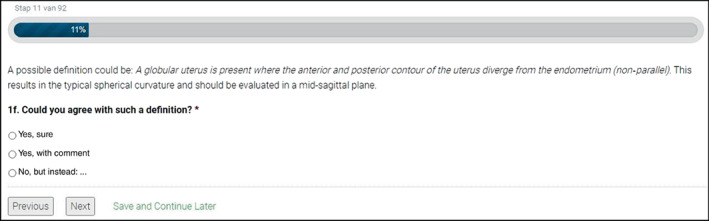
Example of a question in Round 2 (online survey) of the modified Delphi procedure on definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis, showing proposed definition of globular uterus.
The third Delphi round was an online consensus meeting with all participating experts, held on 8 May 2020. It was hosted by senior author R.A.d.L. and junior author M.J.H., and recorded and reviewed by M.J.H.. The results of the first and second rounds of the Delphi study regarding the agreement on the presence of the MUSA features in videoclips and still images, and the agreement on the proposed revised definitions, were presented by the meeting hosts (M.J.H. and R.A.d.L.). The comments on the suggested revised definitions that had been made in Round 2 were discussed. New suggestions for revisions made by the participating experts during the third round were summarized by the meeting host (R.A.d.L.). Consensus on a revised definition was established when no participating expert had any additional comments and agreed. If no agreement could be reached after thorough discussion, the conclusion was that there was no consensus concerning the feature.
Data analysis
The experts' responses to the questionnaires were described using descriptive statistics (n (%) or n/N (%)). Consensus was defined a priori as at least 66.7% of the participating experts having the same opinion on the presence or absence of a feature (agreeing/strongly agreeing or disagreeing/strongly disagreeing) or on the proposed revision of a definition (‘yes’ plus ‘yes, with comment’) or disagreeing (‘no, with comment’). In the first Delphi round, the ‘don't know’ responses were excluded from the calculation because this reply indicates that the expert did not have an opinion. There is debate on how to best define consensus, with previous studies 10 using agreement between 51% and 80%. The agreement rate of ≥ 66.7% has been used in previous studies applying the Delphi method 11 , 12 , 13 . Statistical analysis was performed using IBM SPSS statistical software (version 20.0; IBM Corp., Armonk, NY, USA).
RESULTS
Of 18 invited experts, 16 agreed to participate and two did not respond to the invitation. The participating experts were from seven different countries. Their median age was 43 (interquartile range (IQR), 40–58) years, and they had a median of 15 (IQR, 10–30) years of experience as a gynecologist. Most of the experts (13/16) examined by TVS more than 10 women who were found to have adenomyosis per month (irrespective of the time spent at the outpatient clinic) (Table S1).
Eleven participants completed the first Delphi round. Four experts started the questionnaire but did not finish (one participant assessed two videos, another nine videos and two others 10 videos). One further expert did not respond to the invitation for this round. In the first round, consensus was reached for ≥ 80% of the videoclips for the presence of all MUSA features (Table 1). The median rate of ‘don't know’ answers per feature was highest for irregular and interrupted junctional zone (Table 1). A frequent comment given by the experts in the first round was that features were difficult to assess because there was no clear definition of the feature. Therefore, it was decided to propose a revised definition of each MUSA feature of adenomyosis by using the comments made by the experts in the first Delphi round and to investigate agreement on these proposed revisions in the second round.
Table 1.
Summary of results of first (n = 11 experts) and second (n = 15 experts) rounds of modified Delphi procedure on definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis
| MUSA feature | Round 1* | Round 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Videoclips with agreement ≥ 66.7% | ‘Don't know’ responses† | Still images with agreement ≥ 66.7%‡ | Suggested revision of MUSA definition based on results of Round 1 | Agreement with proposed revision | ||||
| ‘Yes’ | ‘Yes, with comment’§ | ‘No, with comment’§ | Total agreement¶ | |||||
| Globular uterus | 13/15 (86.7) | 1 (1–1) | 7/8 (85.7) | Globular uterus is present when the myometrial serosa of the anterior and/or posterior wall diverges from the cervix instead of following a trajectory parallel to the endometrium; this results in the typical spherical shape of a globular uterus | 8 (53.3) | 5 (33.3) | 2 (13.3) | 13 (86.7) |
| Specify globally enlarged vs globally shaped | 6 (40.0) | 2 (13.3) | 7 (46.7) | 8 (53.3) | ||||
| Both myometrial walls should diverge | 9 (60.0) | 2 (13.3) | 4 (26.7) | 11 (73.3) | ||||
| Asymmetrical myometrial thickening | 12/15 (80) | 0 (0–0) | 5/5 (100) | Difference in myometrial wall thickness > 5 mm | 6 (40.0) | 7 (46.7) | 2 (13.3) | 13 (86.7) |
| Myometrial cysts | 14/15 (93.3) | 0 (0–0.5) | 6/7 (85.7) | Either surrounded by hyperechogenic rim or have minimum size of 3 mm | 7 (46.7) | 5 (33.3) | 3 (20.0) | 12 (80.0) |
| Fan‐shaped shadowing | 12/15 (80) | 0 (0–0) | 9/9 (100) | Present behind the myometrial lesion | 12 (80.0) | 3 (20.0) | 0 (0) | 15 (100) |
| Translesional vascularity | 13/15 (86.7) | 0 (0–1) | 4/5 (80) | Diffuse adenomyosis: translesional vascularity | 10 (66.7) | 2 (13.3) | 3 (20.0) | 12 (80.0) |
| Adenomyoma: circumferential vascularity | 9 (60.0) | 2 (13.3) | 4 (26.7) | 11 (73.3) | ||||
| Hyperechogenic islands | 13/15 (86.7) | 1 (0–1) | 9/13 (69.2) | No minimum diameter | 12 (80.0) | 2 (13.3) | 1 (6.7) | 14 (93.3) |
| Minimum distance from endometrium of 3 mm | 9 (60.0) | 2 (13.3) | 4 (26.7) | 11 (73.3) | ||||
| Minimum of three hyperechogenic islands | 3 (20.0) | 0 (0) | 12 (80.0) | 12 (80.0) | ||||
| Echogenic subendometrial lines and buds | 13/15 (86.7) | 1 (0.5–2) | 4/6 (66.7) | 3D‐US and a high‐quality still image of area of interest is needed | 5 (33.3) | 4 (26.7) | 6 (40.0) | 9 (60.0) |
| Irregular JZ | 14/15 (93.3) | 2 (2–3) | 7/11 (63.6) | High‐quality still image of area of interest is needed | 8 (53.3) | 2 (13.3) | 5 (33.3) | 10 (66.7) |
| JZ of > 12 mm is irregular per definition | 2 (13.3) | 2 (13.3) | 11 (73.3) | 11 (73.3) | ||||
| Interrupted JZ | 14/15 (93.3) | 3 (2–4.5) | 7/12 (58.3) | High‐quality still image of area of interest is needed | 8 (53.3) | 1 (6.7) | 6 (40.0) | 9 (60.0) |
| JZ is interrupted if not visible in sagittal plane | 4 (26.7) | 2 (13.3) | 9 (60.0) | 9 (60.0) | ||||
| Only assess JZ in late follicular phase | 3 (20.0) | 5 (33.3) | 7 (46.7) | 8 (53.3) | ||||
Data are given as n/N (%), median (interquartile range) or n (%).
Consensus was defined as agreement ≥ 66.7%.
In Round 1, experts assessed 15 videoclips for presence or absence of each MUSA feature; percentage of videoclips per MUSA feature for which agreement was achieved is shown.
‘Don't know’ responses were excluded when calculating percentage of agreement.
In Round 2, experts confirmed or denied presence of a feature in a set of still images for each MUSA feature; percentage of still images per MUSA feature for which agreement was achieved is shown.
Experts could comment on proposed revision of the definition of each MUSA feature.
Total agreement was calculated as percentage of experts agreeing (‘Yes’ plus ‘Yes, with comment’) or disagreeing (‘No, with comment’).
3D, three‐dimensional; JZ, junctional zone; US, ultrasound.
Fifteen experts participated in the second round, as one expert (the same as in Round 1) did not respond to the invitation. All participating experts completed this round. The results of the second Delphi round are summarized in Table 1. There was consensus (≥ 66.7%) on the presence or absence of the MUSA features for adenomyosis in most images. For the feature irregular junctional zone, consensus was reached in 3/6 (50%) 3D images and 4/5 (80%) 2D images, and for the feature interrupted junctional zone in 3/8 (37.5%) 3D images and in 4/4 (100%) 2D images. Even though consensus was reached for most individual features, there was consensus among the experts that the MUSA features needed to be better defined. In the second Delphi round, there was consensus on the proposed revised definitions of the following MUSA features: globular uterus, asymmetrical myometrial thickening, myometrial cysts, fan‐shaped shadowing, translesional vascularity, hyperechogenic islands and irregular junctional zone. There was no consensus on the suggested revisions of the definitions of echogenic subendometrial lines and buds and interrupted junctional zone. In the second round, many experts commented that the MUSA features needed to be classified as direct or indirect signs of adenomyosis.
Thirteen experts joined the third Delphi round in a virtual meeting on 8 May 2020. The experts discussed, first, which features are direct and which are indirect signs of adenomyosis, second, the assessment of the junctional zone, and third, how to revise the definition of each feature. The results of this discussion and the outcome for each feature are summarized in Tables 2 and 3 and described in detail below.
Table 2.
Summary of general consensus statements agreed in Round 3 of modified Delphi procedure on definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis
| Distinction between direct and indirect features |
| Ultrasound features that are typical of adenomyosis are direct features, while ultrasound features that are a consequence of ectopic endometrium in the myometrium are indirect features. |
| In the absence of intramyometrial abnormalities (myometrial cysts, hyperechogenic islands or subendometrial lines or buds), indirect features are not conclusive for the presence of adenomyosis. |
| Currently, the importance of each individual ultrasound feature of adenomyosis is unknown. Prospective studies are needed to elucidate the clinical relevance of each individual feature. |
| Direct features: cysts in the myometrium; hyperechogenic islands; echogenic subendometrial lines or buds. |
| Indirect features: globular uterus; asymmetrical myometrial thickening; fan‐shaped shadowing; translesional vascularity; irregular JZ; interrupted JZ. |
| Clinical relevance of endometrial–myometrial JZ |
| Although multiplanar assessment of the JZ in a 3D ultrasound volume is difficult technically, an abnormal JZ in 3D ultrasound images indicates possible adenomyosis. Referral to a specialized gynecological practice for 3D ultrasound might be useful if there is uncertainty about the diagnosis. |
| A regular, uninterrupted JZ is an indicator of absence of adenomyosis. |
3D, three‐dimensional; JZ, junctional zone.
Table 3.
Consensus on definitions of Morphological Uterus Sonographic Assessment (MUSA) features for diagnosing adenomyosis reached in Round 3 of modified Delphi procedure
| Direct features of adenomyosis | |
| Myometrial cysts | |
| Definition in MUSA consensus 6 | Rounded lesions within the myometrium. Cystic contents may be anechoic, of low‐level echogenicity, of ground‐glass appearance or of mixed echogenicity. May be surrounded by a hyperechogenic rim. |
| Consensus statement | Any size of myometrial cyst is relevant (no minimum or maximum size). |
| Hyperechogenic rim is not obligatory. | |
| As a rule of thumb, color Doppler should be used to differentiate between blood vessels and myometrial cysts. | |
| Suggested revised definition | Rounded or oval cystic spaces of any size within the myometrium. |
| Hyperechogenic islands | |
| Definition in MUSA consensus 6 | Hyperechogenic areas within the myometrium that may be regular, irregular or ill‐defined. |
| Consensus statement | No minimum diameter and no minimum number of hyperechogenic islands are defined. |
| Hyperechogenic islands should have no connection with the endometrium. | |
| There is no minimum distance from the endometrium. | |
| Suggested revised definition | Hyperechogenic areas within the myometrium that have no connection with the endometrium (no minimum distance, no minimum number). They may be regular, irregular or ill‐defined. |
| Echogenic subendometrial lines and buds | |
| Definition in MUSA consensus 6 | Hyperechogenic subendometrial lines or buds may be observed disrupting the JZ. Hyperechogenic subendometrial lines are (almost) perpendicular to the endometrial cavity and are in continuum with the endometrium. |
| Consensus statement | As a rule of thumb, any form of invasion of endometrial tissue into the myometrium is a feature of adenomyosis, even if its appearance is not that of lines or buds. |
| Suggested revised definition | None. |
| Indirect features of adenomyosis | |
| Globular uterus | |
| Definition in MUSA consensus 6 | None. |
| Consensus statement |
This feature can be false positive in the presence of fibroids or intracavitary abnormality. No need to specify enlargement in terms of measurements because globular describes uterine shape, not size. |
| Suggested definition | Globular uterus is present when the myometrial serosa diverges from the cervix in at least two directions (anterior/posterior/lateral), instead of following a trajectory parallel to the endometrium, and measured diameters (length/width/depth) of the uterine corpus are approximately equal. This results in the typical spherical shape of a globular uterus. |
| Asymmetrical myometrial thickening | |
| Definition in MUSA consensus 6 | The anterior and posterior myometrial walls are measured from the external uterine serosa to the external endometrial contour and should include the JZ but not the endometrium. The myometrial walls are measured in the sagittal plane, perpendicular to the endometrium. Both measurements are performed in the same plane, and the measurements should be obtained from the thickest point of the myometrial wall. The ratio between the anterior and posterior wall thickness is calculated. A ratio of around 1 indicates that the myometrial walls are symmetrical and a ratio well above or below 1 indicates asymmetry, although this may also be estimated subjectively. |
| Consensus statement | There is no evidence‐based cut‐off to define asymmetry. Cut‐off of ≥ 5 mm difference in myometrial wall thickness, or ratio between the anterior and posterior wall thickness well above 1 or well below 1, should be used only as a rule of thumb. |
| Caution: different planes/rotated uteri/myometrial contraction can imitate asymmetrical myometrial thickening. | |
| Suggested revised definition | As a rule of thumb only, asymmetrical thickening is present when the difference in thickness between the anterior and the posterior myometrial wall exceeds 5 mm, or when the ratio between the anterior and posterior wall thickness is well above 1 or well below 1. |
| Fan‐shaped shadowing | |
| Definition in MUSA consensus 6 | Presence of hypoechogenic linear stripes, sometimes alternating with linear hyperechogenic stripes. (The degree of shadowing is recorded subjectively as slight, moderate or strong.) |
| Consensus statement | Fan‐shaped shadowing should be present behind the myometrial lesion. |
| As a rule of thumb, finding edge shadows lateral to the lesion might indicate the presence of a fibroid or fibrosis due to a CS scar rather than adenomyosis. | |
| This feature is best assessed in grayscale images without the use of color Doppler. | |
| Suggested revised definition | Presence of hypoechogenic stripes behind the myometrial lesion, sometimes alternating with linear hyperechogenic stripes (slight/moderate/strong). This feature is best assessed in grayscale images without the use of color Doppler. |
| Translesional vascularity | |
| Definition in MUSA consensus 6 | Translesional vascularity is characterized by the presence of vessels perpendicular to the uterine cavity/serosa crossing the lesion (hyperechogenic islands in the myometrium). |
| Consensus statement |
Assessing vascularity is helpful in discriminating between fibroids and adenomyosis, and between subendometrial cysts and blood vessels. As a rule of thumb, translesional vascularity is more likely to be present in diffuse adenomyosis, while circumferential vascularity may be present when there is an adenomyoma. |
| Suggested revised definition | None. |
| Irregular JZ | |
| Definition in MUSA consensus 6 | The JZ can be irregular because of cystic areas, hyperechogenic dots, and hyperechogenic buds and lines. Magnitude of JZ irregularity: JZdif = JZmax − JZmin. Extent of JZ irregularity: percentage of JZ that is irregular (< 50% or ≥ 50%, assessed subjectively). |
| Consensus statement |
Obtaining a high‐quality 2D or 3D still image of the area of interest, and assessment in the sagittal, transverse and coronal planes using 3D ultrasound, could aid in the assessment of JZ. Ultrasound measurement of JZ thickness has currently no role in clinical practice. |
| Suggested revised definition | The JZ can be irregular because of cystic areas, hyperechogenic dots, and hyperechogenic buds and lines. Ultrasound measurement of JZ thickness has currently no role in clinical practice. |
| Interrupted JZ | |
| Definition in MUSA consensus 6 | There is interruption of the JZ when a proportion of the JZ cannot be visualized (< 50% or ≥ 50%, assessed subjectively). |
| Consensus statement | It is impossible to specify what proportion (< 50% or ≥ 50%) of the JZ is interrupted. |
| Suggested revised definition | There is interruption of the JZ when a proportion of the JZ cannot be visualized on either 2D or 3D transvaginal ultrasound in any plane. An uninterrupted JZ means that the JZ is clearly seen in all planes on 2D ultrasound or in all planes on 3D ultrasound. |
2D, two dimensional; 3D, three dimensional; CS, Cesarean section; dif, difference; JZ, junctional zone; JZmax, maximum JZ thickness; JZmin, minimum JZ thickness.
General consensus points
Direct and indirect features of adenomyosis
There was consensus that the MUSA features should be divided into direct and indirect ultrasound signs of adenomyosis (Figure 5) as suggested by others 14 , 15 , 16 . Direct features indicate the presence of ectopic endometrial tissue in the myometrium 1 , 7 . Indirect features are those that are secondary to the presence of endometrial tissue in the myometrium, such as muscular hypertrophy (globular uterus) or artifacts (e.g. shadowing). After discussion, the experts agreed which features should be classified into which group (Table 2, Figure 5).
Figure 5.
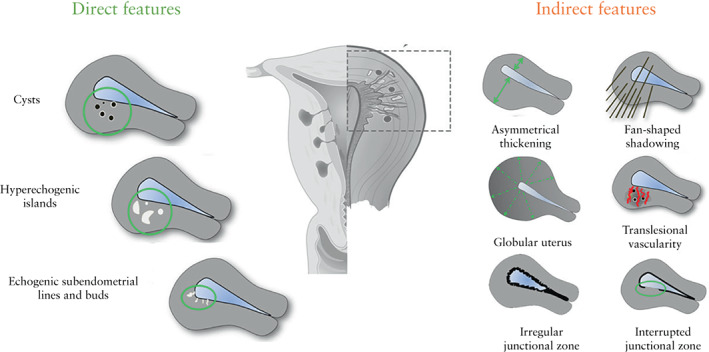
Schematic representation of direct and indirect Morphological Uterus Sonographic Assessment (MUSA) features of uterine adenomyosis (not endometriosis), according to modified Delphi procedure. Adapted from Van den Bosch et al. 6 .
Assessment of junctional zone
The experts agreed that assessment of the junctional zone is useful in case of uncertainty about the diagnosis. A regular, uninterrupted junctional zone indicates absence of adenomyosis. The experts suggested to assess the regularity of the junctional zone in multiple planes using 3D ultrasound (any plane slicing). The suggestion, formulated based on the answers given in the first round (Table 1), to measure the maximal thickness of the junctional zone, was dismissed by the experts, because of lack of evidence of the clinical relevance of this measurement 5 . There was consensus that assessment of the junctional zone on 3D ultrasound in multiple planes requires technical expertise, that this technique might be unavailable in general gynecological practice, and that therefore referral to a specialized gynecological practice might be needed (Table 2).
Revision of definitions of MUSA features
The experts agreed on adjustments in, or additions to, the definition of each MUSA feature (Table 3).
Direct features of adenomyosis
Myometrial cysts
The experts agreed (agreement ≥ 66.7%) on the presence of myometrial cysts in 14/15 videoclips in the first round and in 6/7 images in the second round. Common remarks in Rounds 1 and 2 were that the cysts were minimal or small and that a definition based on size is lacking. In Round 2, there was consensus (agreement 80%) on a revised definition of myometrial cysts. The published MUSA statement 6 defines cysts as: ‘Rounded lesions within the myometrium. Contents may be anechoic, of low‐level echogenicity, of ground‐glass appearance or of mixed echogenicity. Cysts may be surrounded by a hyperechogenic rim’. In Round 3, the consensus was that any size of a myometrial cyst is relevant (no minimum or maximum size) and that a hyperechogenic rim is not obligatory. The experts recommended using color Doppler to distinguish blood vessels from myometrial cysts (Table 3). In the third Delphi round, all experts agreed that myometrial cysts are a direct feature of adenomyosis (Table 2, Figure 5).
Hyperechogenic islands
There was agreement on the presence of hyperechogenic islands in 13/15 videoclips in Round 1 and in 9/13 images in Round 2 (Table 1). The only comment in Round 2 was the concern that echo enhancement might mimic hyperechogenic islands. In the first MUSA consensus statement 6 , hyperechogenic islands were defined as ‘hyperechogenic areas within the myometrium and they may be regular, irregular or ill‐defined’. In Round 3, the experts agreed to revise the definition by adding the criterion that hyperechogenic islands should have no connection with the endometrium. A minimum distance from the endometrium was not defined as it would be arbitrary. The same applies to a minimum diameter or a certain number of hyperechogenic islands (Table 3). In the third Delphi round, the experts agreed unanimously that hyperechogenic islands are a direct feature of adenomyosis, as they represent ectopic endometrium within the myometrium (Table 2, Figure 5).
Echogenic subendometrial lines and buds
There was consensus regarding the presence of echogenic subendometrial lines and buds in 13/15 videoclips in Round 1 and in 4/6 still images in Round 2 (Table 1). In the first and second rounds, the experts commented that the assessment of this feature was made difficult by: lack of 3D ultrasound images, difficulty with discerning the endometrial–myometrial border and invisible junctional zone. The definition of this feature in the MUSA consensus statement 6 was: ‘Hyperechogenic subendometrial lines or buds may be observed disrupting the junctional zone. Hyperechogenic subendometrial lines are (almost) perpendicular to the endometrial cavity and are in continuum with the endometrium’. In the third Delphi round, the experts agreed that the original definition needed no adjustment, and that echogenic subendometrial lines and buds are a direct feature of adenomyosis. However, they agreed to add that any form of invasion of endometrial tissue into the myometrium may be a sign of adenomyosis, even if it does not have the appearance of lines or buds (Tables 2 and 3, Figure 5).
Indirect features of adenomyosis
Globular uterus
The experts agreed on the presence of a globular uterus in 13/15 videoclips in Round 1 and in 7/8 still images in Round 2 (Table 1). The following definition was agreed on in the second round (agreement 86.7%) and accepted in Round 3: ‘A globular uterus is present when the myometrial serosa diverges from the cervix in at least two directions (anterior/posterior/lateral), instead of following a trajectory parallel to the endometrium, and measured diameters (length/width/depth) of the uterine corpus are approximately equal. This results in the typical spherical shape of a globular uterus’ (Tables 1 and 3, Figure 6). There was consensus that this sign can be false positive if there is a fibroid or an intracavitary abnormality, and that globular describes the shape, not the size, of the uterus. In the third round, all experts agreed that globular uterus is an indirect feature of adenomyosis (Table 2, Figure 5).
Figure 6.
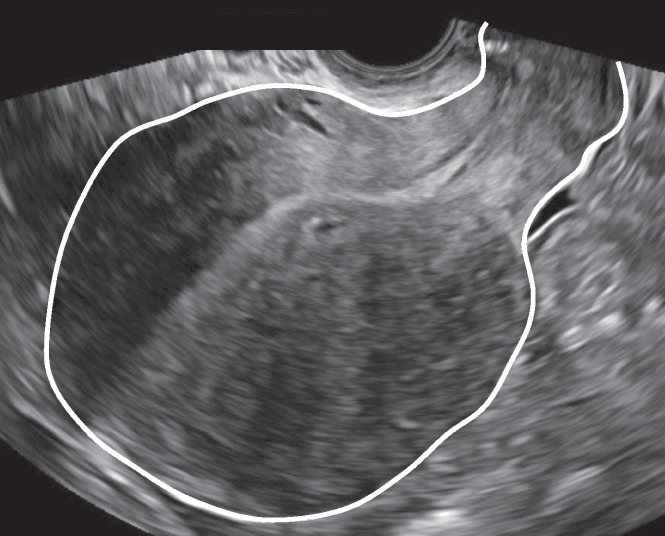
Transvaginal ultrasound image showing typical spherical shape of a globular uterus.
Asymmetrical myometrial thickening
There was agreement on the presence of asymmetrical myometrial thickening in 12/15 videoclips in Round 1 and in 5/5 still images in Round 2 (Table 1). In the first and second Delphi rounds, experts mentioned that the lack of a metric definition of asymmetry complicated the assessment of asymmetrical thickening of the myometrium. With respect to measurement of the myometrial walls, the MUSA consensus statement 6 specified that: ‘The ratio between the anterior and posterior wall thickness is calculated. A ratio of around 1 indicates that the myometrial walls are symmetrical and a ratio well above or well below 1 indicates asymmetry, although this may also be estimated subjectively’. Despite the consensus in the second Delphi round that a cut‐off for asymmetrical myometrial thickening is needed (agreement 86.7%), it was agreed in the third round that there is no evidence supporting the use of a cut‐off value, and that therefore no specific recommendations can be made. The cut‐off value of 5 mm difference in myometrial wall thickness proposed in the second Delphi round, or a ratio between the thickness of the anterior and posterior uterine wall of well above or well below 1 as mentioned in the MUSA consensus statement 6 , can be used only as a rule of thumb. Potential diagnostic pitfalls regarding the assessment of asymmetry are transient uterine contractions, or the presence of uterine fibroids (Table 3). All experts agreed in Round 3 that asymmetrical thickening is an indirect feature of adenomyosis (Table 2, Figure 5).
Fan‐shaped shadowing
In 12/15 videoclips in the first Delphi round and in 9/9 still images in the second round, the agreement on the presence or absence of fan‐shaped shadowing was ≥ 66.7%. According to the original MUSA definition 6 , fan‐shaped shadowing ‘is defined by the presence of hypoechogenic linear stripes, sometimes alternating with linear hyperechogenic stripes’. In Delphi Round 2, it was agreed that shadowing must be present behind the myometrial lesion (agreement 100%). This was accepted in Round 3. In the third round, the experts agreed that fan‐shaped shadowing is best assessed in grayscale mode and added that diagnostic problems may be caused by other lesions that generate shadowing, such as fibroids or fibrosis in a Cesarean section scar (Table 3). The experts agreed that fan‐shaped shadowing is an indirect feature of adenomyosis (Table 2, Figure 5).
Translesional vascularity
Consensus on the presence of translesional vascularity was reached in 13/15 videoclips in the first Delphi round and in 4/5 still images in the second two (Table 1). A common comment in the first and second rounds was whether vascularity could be called translesional if no apparent lesion was present. According to the MUSA consensus statement 6 , ‘Translesional vascularity is characterized by the presence of blood vessels perpendicular to the uterine cavity/serosa crossing the lesion’. In Round 2, the experts agreed (agreement 80%) that translesional vascularity is likely to be present in diffuse adenomyosis, but that circumferential vascularity, which is typically seen around fibroids, can also be present when there is an adenomyoma (agreement 73.3%) (Table 1). The experts agreed in Round 3 that although intralesional vessels may be present in fibroids, translesional vascularity, meaning vessels crossing the lesion, are not seen in fibroids. For that reason, they agreed that this feature is suited to distinguish adenomyosis from fibroids. The definition was not adjusted, but a rule of thumb was added that translesional vascularity is more likely to be present in diffuse adenomyosis and circumferential vascularity when there is an adenomyoma (Table 3). The experts agreed that translesional vascularity is an indirect sign of adenomyosis (Table 2, Figure 5).
Irregular junctional zone
There was consensus on the presence of irregular junctional zone in 14/15 videoclips in Round 1, and in 3/6 of the 3D images and 4/5 of the 2D images in Round 2 (Table 1). However, in 3/15 videoclips in the first round, ≥ 30% of the raters answered ‘don't know’. The most common remark (> 50% of the comments in the 11 videoclips without 3D images in Round 1) was that it was difficult to assess the junctional zone because there were no 3D ultrasound images. According to the original MUSA statement 6 , the junctional zone can be irregular because of cystic areas, hyperechogenic dots, and hyperechogenic buds and lines. The magnitude of a junctional zone irregularity is expressed as the difference between the maximum and minimum junctional zone thickness. The extent of the irregularity is reported as the subjective estimation of the percentage of the junctional zone that is irregular (< 50% or ≥ 50%) 6 . In the second Delphi round, there was consensus that obtaining a high‐quality still image of the area of interest could aid in the assessment of the junctional zone (agreement 66.7%) and that a cut‐off for junctional zone thickness should not be used as a diagnostic feature for adenomyosis (agreement 73.3%). In Round 3, the experts agreed that the junctional zone should be evaluated using 3D‐TVS in the sagittal, transverse and coronal planes, but that there is no scientific evidence to recommend a cut‐off for junctional zone thickness to diagnose adenomyosis (Table 3). In the third Delphi round, the experts agreed that an irregular junctional zone is an indirect feature of adenomyosis (Table 2, Figure 5).
Interrupted junctional zone
There was consensus regarding the presence of an interrupted junctional zone in 14/15 videoclips in the first Delphi round and in 3/8 of the 3D images and 4/4 of the 2D images in the second round. However, in 6/15 videoclips in Round 1, ≥ 30% of the experts answered ‘don't know’. In the first and second rounds, the experts commented that assessment of the junctional zone was difficult if no 3D ultrasound images were available. In Round 2, there was no consensus on the suggested revisions of the definition. According to the MUSA consensus statement 6 , ‘there is interruption of the junctional zone when a proportion of the junctional zone cannot be visualized (< 50% or ≥ 50%)’. In the third round, the experts agreed that it is impossible to specify what proportion (< 50% or ≥ 50%) of the junctional zone is interrupted, and that the revised definition of interrupted junctional zone should read, ‘There is interruption of the junctional zone when a proportion of the junctional zone cannot be visualized in either 2D or 3D transvaginal ultrasound in any plane. An uninterrupted junctional zone means that the junctional zone is clearly seen in all planes on 2D ultrasound, or in all planes on 3D ultrasound.’ (Table 3). The experts agreed unanimously that interruption of the junctional zone is an indirect feature of adenomyosis (Table 2, Figure 5).
DISCUSSION
Main findings
The most important points of consensus were the revised definitions of the MUSA features and the classification of features as direct or indirect. This distinction is relevant because it reflects the pathophysiology of adenomyosis and the diagnostic potential of the features. The direct features represent ectopic endometrial glands and stroma beyond the subendometrial layer. The indirect features can be present in the absence of direct features, in which case the diagnosis is uncertain. By consistently using the MUSA features for adenomyosis to describe ultrasound images of the uterus, and by classifying the features as either direct or indirect signs of adenomyosis, the goal of consistent detection, description and classification of adenomyosis can be reached. The experts agreed that the assessment of the junctional zone may be important in case of uncertainty about the diagnosis of adenomyosis and that it is important for research purposes, but that its assessment requires expertise in 3D ultrasonography.
Comparison with other studies
Other imaging experts have classified myometrial cysts as a direct sign and features reflecting hypertrophic myometrium as indirect signs of adenomyosis 14 , 15 , 16 . Direct features are often subtle and may not be easily detectable, and all three direct features may not be present in the same uterus. However, because they represent endometrial tissue in the myometrium, they are expected to be reliable indicators of adenomyosis. This is reflected by the high specificity of myometrial cysts, echogenic subendometrial lines and buds, and hyperechogenic islands (86–98%, 83–95.5% and 78%, respectively), but their low sensitivity (47–55%, 12–54% and 51%, respectively) for diagnosing adenomyosis 15 , 17 , 18 , 19 , 20 , 21 . Indirect features may be easier to detect than direct ones. The more advanced the disease and the more the muscular hypertrophy has progressed, the easier it should be to recognize the changes. Thus, the extent to which ultrasound signs of adenomyosis are present depends on the population examined. The ability to recognize ultrasound features of adenomyosis, in particular those involving the junctional zone, is likely to depend on the examiner's experience, equipment quality, availability of 3D‐TVS and ability to acquire, manipulate and interpret 3D‐TVS volumes 8 , 9 . Greater expertise improves the recognition of features of adenomyosis 9 , 22 . The heterogeneity in the reported sensitivity of asymmetric myometrial walls (23–62%) 17 , 19 , 20 , 21 , 23 , 24 and globular uterus (51–74%) 19 , 20 , 23 , 24 in studies performed in women that underwent hysterectomy may be explained both by differences in disease severity and ultrasound expertise. Fibroids may also explain uterine asymmetry or a globular shape of the uterus. This study did not investigate the ability of uterine asymmetry or globular shape to discriminate between adenomyosis and other conditions, but it would be interesting to investigate their discriminative ability in future studies using the updated MUSA terminology.
Remarks on junctional zone, cysts in the myometrium, and hyperechogenic lines and buds
The agreement between ultrasound experts, based on our Delphi procedure, on the presence of an irregular or interrupted junctional zone was poorer than for any other ultrasound feature of adenomyosis. In a recent meta‐analysis, the pooled area under the receiver‐operating‐characteristics curve for diagnosing adenomyosis based on finding an irregular junctional zone on 3D TVS was 0.81, reflecting good discriminative ability of this feature 5 . This is likely to be explained by the ultrasound examiners being highly experienced in those studies that found a good diagnostic performance of junctional zone changes when using 3D ultrasound 17 , 21 . Rasmussen et al. 8 reported that inter‐rater agreement for assessment of the variables interrupted and irregular junctional zone was better among highly experienced raters than among raters with medium experience. With improving expertise in 3D ultrasonography among gynecological ultrasound examiners, 3D ultrasound assessment of the uterus is expected to become more reliable. It is interesting to note that the experts taking part in this Delphi study agreed that assessment of the junctional zone in multiple planes using 3D TVS is essential to visualize the junctional zone properly, and therefore relevant in the diagnosis of adenomyosis. However, the poor intra‐ and interobserver agreement for irregular or interrupted junctional zone among ultrasound examiners with moderate experience in gynecological ultrasound 8 limits the usefulness of abnormal junctional zone as a feature to diagnose adenomyosis in general gynecological practice. An irregular, interrupted or not visible junctional zone indicates that adenomyosis may be present (indirect feature) and should prompt the ultrasound examiner to scrutinize the myometrium for direct features. However, whether the assessment of the junctional zone could aid in patient management when there is uncertainty about a diagnosis of adenomyosis is a relevant question for future research. Referral to a specialized gynecological practice might be needed in case of uncertainty about the diagnosis.
Invasion of endometrial tissue into the myometrium should be interpreted with caution in older and postmenopausal women as this might be a sign of malignancy and not of adenomyosis (subendometrial buds and lines). The spatial resolution of the ultrasound device determines the minimum size of a cyst in the myometrium that can be detected. A large cyst may be a cystic adenomyoma. Despite this, there was consensus among the experts in this Delphi procedure that cysts in the myometrium of any size should be taken into account when looking for the MUSA feature of endometrial cysts.
Strengths and limitations
The strength of this study is the application of the modified Delphi methodology, which allows use of the comments from the experts to identify disagreement and formulate suggestions to revise the definitions of the MUSA features. Having experts from different clinical and research environments increases the likelihood of diverse opinions. Group dynamics are important in a Delphi study, and a minimum of 12 participating experts is a generally accepted number when trying to achieve consensus 25 , 26 .
The selection of participating experts might be considered a limitation. No radiologists were included, and the participating experts do not represent all international experts with skill and knowledge in the ultrasound diagnosis of adenomyosis. The participants represented nine institutions from seven European countries, and there were four experts from the same institution (R.A.d.L., F.G., W.J.K.H. and J.A.F.H.). These four experts represented one institution but only two of them (R.A.d.L. and J.A.F.H.) managed the project and participated in the final consensus meeting. The definition of an expert may be considered arbitrary. Another limitation is that not all experts participated in all rounds of the Delphi study, and that some experts did not assess all videoclips in the first round. Some videos in Round 1 were assessed by fewer experts than other videos, and the rate of ‘don't know’ answers was higher for some videos than for others. This means that the number of experts used to calculate agreement differed between videos. It is important to emphasize, however, that this study is not an interobserver agreement or diagnostic accuracy study 27 . Agreement was assessed only to guide the discussion on a possible need to update the definitions of the MUSA features. A further limitation is the loss of image resolution when presenting ultrasound images in an online survey. Moreover, 3D TVS images should be assessed in a volume on a high‐end ultrasound device where they can be manipulated by the operator. We speculate that the loss of image quality and lack of manipulation of the 3D volumes probably contributed to the low rates of agreement for 3D images of the junctional zone. Even though video recordings are optimal for assessment of ultrasound images, we used still images in the second Delphi round. This was to ensure that all the participating experts used the same image to assess a particular feature.
Future perspectives
Clearly defined ultrasound features of adenomyosis are prerequisites for a correct diagnosis that can serve both clinicians and researchers. To estimate the usefulness of the direct and indirect MUSA features, in particular those concerning the junctional zone, more intra‐ and inter‐rater reliability studies are needed. These should include raters with different levels of expertise who have all had sufficient training in the recognition of the updated MUSA features. It would be interesting to see the results of a Delphi procedure regarding the updated MUSA definitions performed among a panel of experts including radiologists. A few studies described the relationship between ultrasound features of adenomyosis and clinical outcomes 4 , 28 , 29 , but the MUSA definitions of the ultrasound features were only referred to in one of them 29 . The association between the presence of one or more direct or indirect MUSA features and clinical symptoms needs to be investigated further, as well as the association between the number and size of features and their location (relation to the junctional zone, uterine layer involvement) and symptoms 30 . The accuracy of the presence of one or more direct and/or indirect features to diagnose adenomyosis needs to be investigated further. However, clinically useful diagnostic accuracy studies are difficult to perform since the reference standard is hysterectomy, and not all women with suspected adenomyosis undergo hysterectomy. Moreover, there is no international agreement between pathologists on how to diagnose adenomyosis 7 .
Conclusions
We present an update of the terms and definitions to describe adenomyosis with ultrasound, which were first presented by the MUSA group in 2015 6 . The present consensus was reached through a Delphi study amongst 13 gynecologists with expertise in ultrasound diagnosis of adenomyosis. The updated definitions distinguish between direct and indirect ultrasound features of adenomyosis. The relationship between direct and indirect MUSA features of adenomyosis and the correlation of disease location, extent and size with clinical symptoms and therapeutic outcomes needs to be investigated in prospective studies. Use of the MUSA terminology will aid comparison of results of future studies.
Disclosures
T.T. and C.E. receive fees from GE Healthcare as invited speakers. J.H. received research grants and compensation for costs as invited speaker from Samsung; the topics were not related to the current publication.
Supporting information
Videoclip S1 Example of a videoclip used in Round 1 of Delphi procedure (online survey).
Table S1 Characteristics of experts who participated in Delphi procedure
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update 1998; 4: 312–322. [DOI] [PubMed] [Google Scholar]
- 2. Munro MG. Classification and Reporting Systems for Adenomyosis. J Minim Invasive Gynecol 2020; 27: 296–308. [DOI] [PubMed] [Google Scholar]
- 3. Naftalin J, Hoo W, Pateman K, Mavrelos D, Holland T, Jurkovic D. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod 2012; 27: 3432–3439. [DOI] [PubMed] [Google Scholar]
- 4. Pinzauti S, Lazzeri L, Tosti C, Centini G, Orlandini C, Luisi S, Zupi E, Exacoustos C, Petraglia F. Transvaginal sonographic features of diffuse adenomyosis in 18–30‐year‐old nulligravid women without endometriosis: association with symptoms. Ultrasound Obstet Gynecol 2015; 46: 730–736. [DOI] [PubMed] [Google Scholar]
- 5. Tellum T, Nygaard S, Lieng M. Noninvasive Diagnosis of Adenomyosis: A Structured Review and Meta‐analysis of Diagnostic Accuracy in Imaging. J Minim Invasive Gynecol 2020; 27: 408–418e3. [DOI] [PubMed] [Google Scholar]
- 6. Van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A, Van Schoubroeck D, Landolfo C, Installe AJ, Guerriero S, Exacoustos C, Gordts S, Benacerraf B, D'Hooghe T, De Moor B, Brolmann H, Goldstein S, Epstein E, Bourne T, Timmerman D. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 2015; 46: 284–298. [DOI] [PubMed] [Google Scholar]
- 7. Van den Bosch T, de Bruijn AM, de Leeuw RA, Dueholm M, Exacoustos C, Valentin L, Bourne T, Timmerman D, Huirne JAF. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol 2019; 53: 576–582. [DOI] [PubMed] [Google Scholar]
- 8. Rasmussen CK, Van den Bosch T, Exacoustos C, Manegold‐Brauer G, Benacerraf BR, Froyman W, Landolfo C, Condorelli M, Egekvist AG, Josefsson H, Leone FPG, Jokubkiene L, Zannoni L, Epstein E, Installe A, Dueholm M. Intra‐ and Inter‐Rater Agreement Describing Myometrial Lesions Using Morphologic Uterus Sonographic Assessment: A Pilot Study. J Ultrasound Med 2019; 38: 2673–2683. [DOI] [PubMed] [Google Scholar]
- 9. Rasmussen CK, Hansen ES, Dueholm M. Inter‐rater agreement in the diagnosis of adenomyosis by 2‐ and 3‐dimensional transvaginal ultrasonography. J Ultrasound Med 2019; 38: 657–666. [DOI] [PubMed] [Google Scholar]
- 10. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–1015. [PubMed] [Google Scholar]
- 11. Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014; 67: 401–409. [DOI] [PubMed] [Google Scholar]
- 12. de Villiers MR, de Villiers PJ, Kent AP. The Delphi technique in health sciences education research. Med Teach 2005; 27: 639–643. [DOI] [PubMed] [Google Scholar]
- 13. Vogel C, Zwolinsky S, Griffiths C, Hobbs M, Henderson E, Wilkins E. A Delphi study to build consensus on the definition and use of big data in obesity research. Int J Obes (Lond) 2019; 43: 2573–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Novellas S, Chassang M, Delotte J, Toullalan O, Chevallier A, Bouaziz J, Chevallier P. MRI characteristics of the uterine junctional zone: from normal to the diagnosis of adenomyosis. AJR Am J Roentgenol 2011; 196: 1206–1213. [DOI] [PubMed] [Google Scholar]
- 15. Bazot M, Darai E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril 2018; 109: 389–397. [DOI] [PubMed] [Google Scholar]
- 16. Bluhm M, Dueholm M. Imaging for Adenomyosis: Making the Diagnosis by Sonography. J Minim Invasive Gynecol 2020; 27: 267. [DOI] [PubMed] [Google Scholar]
- 17. Exacoustos C, Brienza L, Di Giovanni A, Szabolcs B, Romanini ME, Zupi E, Arduini D. Adenomyosis: three‐dimensional sonographic findings of the junctional zone and correlation with histology. Ultrasound Obstet Gynecol 2011; 37: 471–479. [DOI] [PubMed] [Google Scholar]
- 18. Rasmussen CK, Hansen ES, Ernst E, Dueholm M. Two‐ and three‐dimensional transvaginal ultrasonography for diagnosis of adenomyosis of the inner myometrium. Reprod Biomed Online 2019; 38: 750–760. [DOI] [PubMed] [Google Scholar]
- 19. Tellum T, Nygaard S, Skovholt EK, Qvigstad E, Lieng M. Development of a clinical prediction model for diagnosing adenomyosis. Fertil Steril 2018; 110: 957–964e3. [DOI] [PubMed] [Google Scholar]
- 20. Kepkep K, Tuncay YA, Goynumer G, Tutal E. Transvaginal sonography in the diagnosis of adenomyosis: which findings are most accurate? Ultrasound Obstet Gynecol 2007; 30: 341–345. [DOI] [PubMed] [Google Scholar]
- 21. Luciano DE, Exacoustos C, Albrecht L, LaMonica R, Proffer A, Zupi E, Luciano AA. Three‐dimensional ultrasound in diagnosis of adenomyosis: histologic correlation with ultrasound targeted biopsies of the uterus. J Minim Invasive Gynecol 2013; 20: 803–810. [DOI] [PubMed] [Google Scholar]
- 22. Lazzeri L, Morosetti G, Centini G, Monti G, Zupi E, Piccione E, Exacoustos C. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril 2018; 110: 1154–1161e3. [DOI] [PubMed] [Google Scholar]
- 23. Bazot M, Darai E, Rouger J, Detchev R, Cortez A, Uzan S. Limitations of transvaginal sonography for the diagnosis of adenomyosis, with histopathological correlation. Ultrasound Obstet Gynecol 2002; 20: 605–611. [DOI] [PubMed] [Google Scholar]
- 24. Sun YL, Wang CB, Lee CY, Wun TH, Lin P, Lin YH, Tseng CC, Chen CH, Tseng CJ. Transvaginal sonographic criteria for the diagnosis of adenomyosis based on histopathologic correlation. Taiwan J Obstet Gynecol 2010; 49: 40–44. [DOI] [PubMed] [Google Scholar]
- 25. Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, Marteau T. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998; 2: i–iv, 1–88. [PubMed] [Google Scholar]
- 26. Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf Manag 2004; 42: 15–29. [Google Scholar]
- 27. Kottner J, Audige L, Brorson S, Donner A, Gajewski BJ, Hrobjartsson A, Roberts C, Shoukri M, Streiner DL. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol 2011; 64: 96–106. [DOI] [PubMed] [Google Scholar]
- 28. Naftalin J, Hoo W, Pateman K, Mavrelos D, Foo X, Jurkovic D. Is adenomyosis associated with menorrhagia? Hum Reprod 2014; 29: 473–479. [DOI] [PubMed] [Google Scholar]
- 29. Naftalin J, Hoo W, Nunes N, Holland T, Mavrelos D, Jurkovic D. Association between ultrasound features of adenomyosis and severity of menstrual pain. Ultrasound Obstet Gynecol 2016; 47: 779–783. [DOI] [PubMed] [Google Scholar]
- 30. Chapron C, Vannuccini S, Santulli P, Abrao MS, Carmona F, Fraser IS, Gordts S, Guo SW, Just PA, Noel JC, Pistofidis G, Van den Bosch T, Petraglia F. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update 2020; 26: 392–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videoclip S1 Example of a videoclip used in Round 1 of Delphi procedure (online survey).
Table S1 Characteristics of experts who participated in Delphi procedure
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


