Summary
The prognosis of kidney transplant recipients (KTR) with vascular calcification (VC) in the aorto‐iliac arteries is unclear. We performed a systematic review and meta‐analysis to investigate their survival outcomes. Studies from January 1st, 2000 until March 5th, 2019 were included. Outcomes for meta‐analysis were patient survival, (death‐censored) graft survival and delayed graft function (DGF). Twenty‐one studies were identified, eight provided data for meta‐analysis. KTR with VC had a significantly increased mortality risk [1‐year: risk ratio (RR) 2.19 (1.39–3.44), 5‐year: RR 2.28 (1.86–2.79)]. The risk of 1‐year graft loss was three times higher in recipients with VC [RR 3.15 (1.30–7.64)]. The risk of graft loss censored for death [1‐year: RR 2.26 (0.58–2.73), 3‐year: RR 2.19 (0.49–9.82)] and the risk of DGF (RR 1.24, 95% CI 0.98–1.58) were not statistically different. The quality of the evidence was rated as very low. To conclude, the presence of VC was associated with an increased mortality risk and risk of graft loss. In this small sample size, no statistical significant association between VC and DGF or risk of death‐censored graft loss could be demonstrated. For interpretation of the outcomes, the quality and sample size of the evidence should be taken into consideration.
Keywords: atherosclerosis, graft survival, kidney transplantation, meta‐analysis, systematic review
Introduction
As kidney transplant recipients (KTR) are becoming older and vascular disease is more prevalent, the challenge of transplanting a kidney onto atherosclerotic aorto‐iliac arteries is likely to become more common. Nearly 25% of all kidney transplant candidates have vascular calcification (VC) in the aorto‐iliac arteries on lumbar X‐ray 1. Risk factors for VC are common risk factors for vascular disease such as diabetes, smoking, hypertension and dyslipidemia 2, 3. Patients suffering from end‐stage renal disease (ESRD) are at higher risk to develop vascular disease due to added risk factors like chronic uremia, use of calcium‐based phosphate binders and, most importantly, dialysis treatment 4. Severe VC in the aorto‐iliac arteries has been considered as a relative contra‐indication for kidney transplantation (KTx). It has been found that 43% of all transplant candidates who are considered ineligible is due to VC 5.
There are several reasons why KTx in patients with VC in the aorto‐iliac arteries can be problematic. First, the vascular anastomosis itself may be technically challenging. Over the years, transplant surgeons found various ways to overcome this issue. A Fogarty catheter can be used in case of an unclampable iliac artery 6. In case of compromised blood flow in the external iliac artery (EIA) caused by VC, a percutaneous transluminal angioplasty (PTA), endarterectomy or vascular bypass can be performed in a staged or simultaneous procedure 7. Second, vascular complications like steal syndrome or trash foot may be a threat to the vascular challenging transplant candidate 8, 9, 10. As a third reason, patients with VC are considered to have a limited life expectancy due to cardiovascular comorbidities, leading to a high perioperative mortality risk and limiting 5‐year patient survival to 35% 11, 12. Due to organ shortage, it might not be ethical to perform a deceased donor KTx in patients with such a limited life expectancy. On the other hand, the quality of life is significantly improved if a transplant can be performed successfully 13. Therefore, proper recipient evaluation is of paramount importance.
To date, there is scant information about the prognosis of KTR with VC in the aorto‐iliac arteries. Most published studies are too small to provide definite conclusions about survival outcomes. We performed a systematic review and meta‐analysis concerning clinical outcomes after KTx in patients with VC. The primary objective of this systematic review and meta‐analysis was to evaluate the risk of mortality and graft loss in KTR with VC in comparison to KTR without VC. As secondary outcomes, we investigated the risk of DGF and 1‐year kidney function.
Methods
This systematic review and meta‐analysis was performed according to the guidelines for observational studies as described in the Preferred Reporting Items for Systematic review and Meta‐Analysis Protocols (PRISMA) guidelines 14.
Search strategy
Together with the help from a clinical librarian, we searched Embase, Medline, Web of Science, Cochrane and Google Scholar database. A search for the Embase database was created and the search terms for other databases were derived from this one. The search included the following terms: kidney/renal transplantation, atherosclerosis, iliac artery. The first search was performed on August 2nd 2017 and the last search on March 5th, 2019. Detailed search strategies are included in Table S1.
Study selection
The studies were firstly screened on title and abstract by two independent reviewers (ER and JLD). Eligible study designs were cross‐sectional studies, cohort studies and case‐control studies. Studies were included if they reported either patient survival, uncensored graft survival, death‐censored graft survival, DGF or kidney function. Studies were included in meta‐analysis if they compared clinical outcomes between KTR with any degree of VC in the aorto‐iliac arteries and KTR without VC. Also, studies describing KTx outcomes after treatment of VC [through PTA, endarterectomy (EAT) or vascular bypass] were eligible for systematic review. For both the meta‐analysis part as well as the systematic review part, the following exclusion criteria were used: conference abstracts, systematic or narrative reviews, studies published not in the English language, studies including multi‐organ transplantation and studies published before January 1st, 2000. Disagreements were discussed between both reviewers and, if necessary, consulted with a third party (RCM). References were manually checked for relevant studies.
Quality assessment
The quality of the evidence was assessed using the GRADE tool for prognosis studies 15, 16. For the GRADE tool, risk of bias, heterogeneity, directness of the evidence, precision of effect estimates and risk of publication bias were assessed for every outcome. The Newcastle‐Ottawa scale for cohort studies was adopted to assess the quality of each individual study 17. Studies were graded according to selection of study groups, comparability and ascertainment of exposure and outcomes. ER and JLD assessed the studies independently.
Data collection and extraction
Data extraction was completed by two independent authors. The following items were extracted from included studies that did not provide data for meta‐analysis: study design, sample size per group, donor type, age, dialysis treatment, and treatment for aorto‐iliac calcification. For studies included in meta‐analysis, the following data was extracted: study design, sample size per group, and possible confounding factors such as recipient age, sex, smoking history, hypertension, diabetes mellitus, hypercholesterolemia, hemodialysis treatment, donor type, history with myocardial infarction or cerebrovascular accident (CVA)/transient ischemic attack (TIA). The following outcomes were considered for meta‐analysis when compared between patients with and without VC: patient survival, uncensored graft survival, death‐censored graft survival, DGF, and kidney function. Events for survival outcomes were deduced from Kaplan‐Meier survival curves using DataThief software and from numbers and percentages described in the results section 18.
Statistical analysis
For meta‐analysis, pooled risk ratios (RR) with 95% confidence intervals (CI) were calculated at fixed time spans based on the number of events per group as described in the individual studies. Because of the expected observational designs of included studies resulting in high between‐study variance, a random‐effect model was used as described by DerSimonian and Laird 19. The Mantel‐Haenszel analysis method was used with calculation of the overall effect using the Z‐test. To investigate potential confounders, baseline characteristics were collected of included patients without VC and with any VC. For continuous variables, the group mean weighed for number of included patients was reported with pooled standard error, if the included study reported the mean. Normality of the means of the included studies was assumed because of the sample size, according to the central limit theorem. Therefore, baseline characteristics were compared with the unpaired T‐test in case of continuous variables and with chi‐square test for categorical variables using medcalc software (version 16.2). Statistical heterogeneity was visually assessed by judging overlap in the 95% confidence intervals and with I 2. Publication bias was assessed using funnel plots of the logarithm of RR versus their standard errors, which are included in the Supplemental Digital Content 20. A P‐value below 0.05 was considered statistically significant. The program used for meta‐analysis was review manager 5.3 21.
Results
Study selection and characteristics
A total number of 1523 potentially relevant, observational studies were identified. None of the studies describing iliac calcifications distinguished between calcifications of the common iliac arteries or external iliac arteries. Figure 1 presents the PRISMA flow diagram. Twenty‐one studies met the inclusion criteria from which one was added after manual reference check. Eight studies provided data for meta‐analysis. Characteristics of studies included in meta‐analysis are presented in Table 1 and from the studies that did not provide data for meta‐analysis in Table 2. The baseline characteristics of the patients included in meta‐analysis are shown in Table 3. Patients with any degree of VC were older [no vascular calcification (nVC: 42.0 ± 12.4, any VC: 54.3 ± 10.8, P < 0.001)], were more frequently suffering from hypertension (nVC 75.8%, any VC 86.0%, P < 0.001), diabetes mellitus (nVC 10.5%, any VC 32.4%, P < 0.001) and hypercholesterolemia (nVC 27.9%, any VC 44.3%, P < 0.001). Patients with any VC had more often a history with a myocardial infarction (nVC 6.7%, any VC 14.2%, P < 0.001) and received more frequently a living donor kidney transplant (nVC 2.1%, any VC 5.3%, P < 0.001). Patients with any VC were more often preemptively transplanted (nVC 7.3%, any VC 16.3%, P < 0.001).
Figure 1.
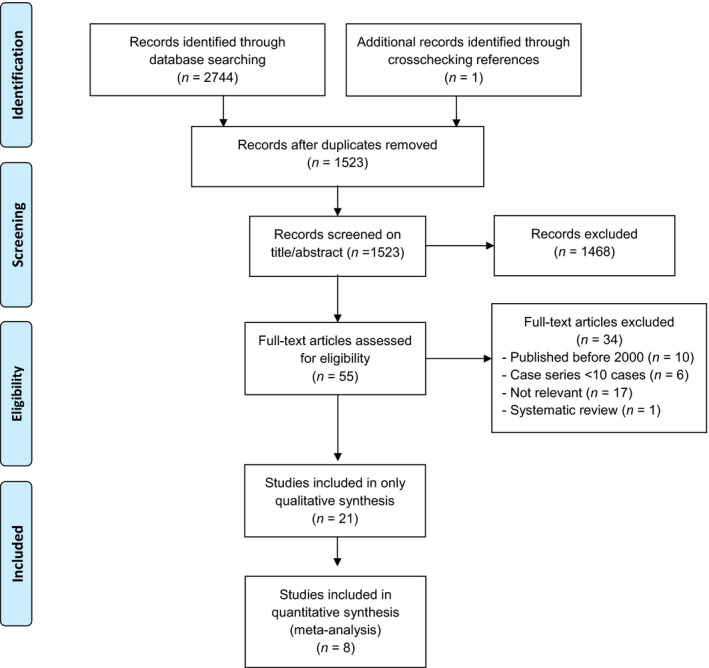
Preferred reporting items for systematic reviews and meta‐analyses flow diagram of the systematic literature search.
Table 1.
Studies providing data for meta‐analysis (n = 8).
| Study | Year | Design | nVC | VC | Diagnosis of VC | Severity VC | Location | Outcome | Newcastle‐Ottawa scale | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Quality | |||||||||
|
Hernandez Spain |
2005 | R OBS | 844 | 273 | Pelvic X‐ray |
No VC Any VC |
IA DA |
1,5 | 4 | 2 |
2 Median FU 49 months, lost to FU unknown |
Good |
|
Droupy France |
2006 | P OBS | 1001 | 69 | Palpable during transplantation |
No VC Any VC |
IA | 1,3,4 | 4 |
0 Not controlled for confounders |
0 FU unknown, lost to FU unknown |
Poor |
|
Aalten Netherlands |
2011 | P OBS | 74 | 35 | Palpable during transplantation |
No VC Any VC |
IA | 1,2,4,5 | 4 |
0 Not controlled for confounders |
0 FU 1 year, lost to FU unknown |
Poor |
|
Aitken Scotland |
2012 | P OBS | 61 | 32 | Pelvic X‐ray |
Minimal VC Moderate/severe VC |
IA | 2,4,5 | 4 |
0 Not controlled for confounders |
3 Complete follow‐up of 5 years for all patients |
Poor |
|
Munguia Spain |
2015 | R OBS | 69 | 50 | Pelvic X‐ray, usage of Kauppila index, L4‐S1 |
No VC (KI 0–2) Any VC (KI 3–24) |
DA | 1,2,3,4,5 | 4 |
0 Not controlled for confounders |
0 FU unknown, lost to FU unknown |
Poor |
|
Davis United states |
2016 | R OBS | 41 | 90 | CT‐scan with calcium score |
No VC Any VC |
IA | 1 | 4 |
0 Not controlled for confounders |
0 Mean FU 34.1 months, lost to FU unknown |
Poor |
|
Benjamens Netherlands |
2018 | R OBS | 434 | 267 | DXA L1‐L4 with score using Schousboe method |
No VC Any VC |
DA | 1 | 4 | 2 |
3 Median FU 5.4 years, lost to FU unknown |
Good |
|
Disthabanchong Thailand |
2018 | P OBS | 108 | 26 | Pelvic X‐ray, usage of Kauppila index |
VC ≤ 1 VC> 1 |
IA | 1 | 4 | 2 |
2 Median FU 62.5 months, lost to FU unknown |
Good |
| Total | 2632 | 842 | ||||||||||
AAC, abdominal aortic calcification; CT, computed tomography; DA, distal aorta; DXA, dual‐energy X‐ray absorptiometry; FU, follow‐up; IA, iliac arteries; KI, Kauppila index; MRA, magnetic resonance angiography; nVC, no vascular calcification; P OBS, prospective observational; R OBS, retrospective observational; VC, vascular calcification.
Outcomes: 1, patient survival; 2, uncensored graft survival; 3, death‐censored graft survival; 4, delayed graft function; 5, kidney function.
Table 2.
Included studies not suitable for meta‐analysis (n = 13).
| Study | Year | Design | Study size | Study population | Treatment | Outcome |
|---|---|---|---|---|---|---|
|
Galazka Poland |
2002 | R OBS |
N = 128 (54 pairs): Group A: N = 54 with VC Group B: N = 54 without VC |
Pairs of recipients who received a kidney from the same donor (1 recipient with VC, 1 without VC) Donor: 0% LD, age: 48 |
Group A: N = 36 EAT, N = 16 anastomosis to EIA, N = 2 anastomosis to CIA Group B: Anastomosis on AII |
1,2 |
|
Tozzi Italy |
2013 | P OBS | N = 21 |
Recipients with TASC II C/D lesions Donor: 0% LD, age: 54 ± 9 |
N = 15 simultaneous EAT N = 4 aorto‐bi‐iliac bypass |
1,2,4,6 |
|
Ozcelik, Germany |
2007 | R OBS | N = 11 |
Recipients who received a kidney transplant on a vascular bypass Donor: 91% LD, age: 57.7 ± 13.9 |
N = 9: reconstruction with ileo‐femoral bypass, N = 1 femoroiliac crossover bypass, N = 1 aortofemoral bypass | 1,2,4,6 |
|
Tsivian Italy |
2009 | R OBS | N = 30 (N = 19 stenotic lesions, N = 11 aneurysms) |
Recipients who received aortoiliac surgery simultaneously with KTx Age: 55 (43–65) |
N = 15 EAT, N = 2 aorto‐iliac bypass, N = 5 aorto‐bi‐iliac bypass, N = 1 aorto‐bifemoral bypass, N = 4 arterioplasty, N = 3 iliac‐iliac bypass | 1,2,5 |
|
Patrono, Belgium |
2013 | R OBS |
N = 27 N = 32 from literature |
Recipients who received a kidney transplant on a prosthetic graft Donor: 7.4% LD, age: 56 (35–75) |
N = 24 implantations on prosthetic graft (N = 22 graft before KTx, N = 2 simultaneously, N = 3 after KTx) | 1,2,5,6 |
|
Han Korea |
2014 | R OBS | N = 748 |
Recipients with asymptomatic TASC II A/B requiring angioplasty Donor: 100% LD, age: 51.4 ± 9.1 |
N = 27 with angioplasty (N = 2 PTFE reconstruction, N = 25 EAT) | 1,2,3,4,6 |
|
Coleman USA |
2014 | R OBS | N = 10 |
Recipients transplanted with usage of a vascular conduit because of severe VC Donor: 20% LD, age: 61 ± 9 |
N = 8 donor iliac artery graft N = 2 saphenous vein graft |
1,2,4,5,6 |
|
Hwang Korea |
2015 | P OBS | N = 90 (N = 48 positive intimal microcalcification, N = 42 negative intimal microcalcification) |
Recipients who gave consent to provide an iliac artery specimen during KTx Donor: 78% LD, age: 42.5 ± 10.3 |
NA | 3,4 |
|
Sagban Germany |
2016 | R OBS |
N = 208 N = 121 from literature |
Recipients who received a kidney transplant on a prosthetic graft Donor: 0% LD, age: 56 |
N = 4 anastomosis on a prosthetic graft N = 121 anastomosis on a prosthetic graft from the literature |
1,2,5,6 |
|
Nanmoku Japan |
2017 | R OBS | N = 13 |
Recipients predicted to have complications with the arterial anastomosis because of VC Donor: 92.3% LD, age: 60.2 ± 10.4, 0% pre‐emptive |
N = 13 EAT before anastomosis (N = 10 anastomosis on EIA, N = 1 on CIA, N = 1 on IIA, N = 1 on prosthetic graft) |
1,2,3,6 |
|
Chavent France |
2017 | R OBS | N = 100 (divided in quartiles, based on calcium score) | Recipients with non‐contrast enhanced abdominal CT‐scan. Donor: 5% LD, age: 60.3 ± 12.8, 5% pre‐emptive | NA | 1,3,5 |
|
Franquet France |
2018 | R OBS | N = 11 |
Recipients who received a kidney transplant on a vascular bypass Age: 61 (57–62.5) |
N = 9 aorto‐femoral bypass N = 1 aorto‐bifemoral bypass N = 1 aorto‐iliac bypass |
1,2,6 |
|
Rijkse Netherlands |
2019 | R OBS | N = 374 (n = 88 with TASC II lesions, n = 286 without TASC II lesions) |
Recipients with aorto‐iliac stenosis compared to recipients without stenosis Donor: 61% LD, age: 59.6 ± 12.7, 17% pre‐emptive |
N = 3 aortic bifurcation prosthesis, N = 1 iliac femoral bypass, N = 2 iliac endarterectomy with patch angioplasty, N = 2 Gore‐Tex angioplasty | 1,2,3,6 |
CIA, common iliac artery; CT, computed tomography; EAT, endarterectomy; EIA, external iliac artery; IIA, internal iliac artery; KTx, kidney transplantation; LD, living donor; NA, not applicable; P OBS, prospective observational; PTFE, polytetrafuoroethylene; R OBS, retrospective observational; TASC, Trans‐Atlantic inter‐Society Consensus.
Outcomes: 1, patient survival; 2, uncensored graft survival; 3, death‐censored graft survival; 4, delayed graft function; 5, kidney function; 6, postoperative complications.
Table 3.
Baseline characteristics of patients included in meta‐analysis.
| Characteristics | Studies | nVC | Total patients | Any VC | Total patients | P‐value |
|---|---|---|---|---|---|---|
| Recipient age, mean (SD) | 61,22,23,25,26,27 | 42.0 (12.4) | 2529 | 54.3 (10.8) | 721 | <0.001* |
| Male sex, n (%) | 51,22,23,26,27 | 977 (63.9) | 1528 | 421 (64.6) | 652 | 0.779 |
| Smoking, n (%) | 222,23 | 99 (19.5) | 508 | 59 (19.5) | 302 | <0.986 |
| Hypertension, n (%) | 41,22,23,27 | 1106 (75.8) | 1459 | 518 (86.0) | 602 | <0.001* |
| DM, n (%) | 51,22,23,26,27 | 160 (10.5) | 1528 | 211 (32.4) | 652 | <0.001* |
| Hypercholesterolemia, n (%) | 31,23,27 | 387 (27.9) | 1385 | 251 (44.3) | 567 | <0.001* |
| Pre‐emptive KTx, n (%) | 51,22,23,25,26 | 176 (7.3) | 2422 | 113 (16.3) | 694 | <0.001* |
| Living donor, n (%) | 31,22,25 | 41 (2.1) | 1919 | 20 (5.3) | 377 | <0.001* |
| History MI, n (%) | 222,23 | 34 (6.7) | 508 | 43 (14.2) | 302 | <0.001* |
| History CVA/TIA, n (%) | 222,23 | 28 (5.5) | 508 | 27 (8.9) | 302 | 0.061 |
CVA, cerebrovascular accident; DM, diabetes mellitus; KTx, kidney transplantation; MI, myocardial infarction; nVC, no vascular calcification; SD, standard deviation; TIA, transient ischemic attack; VC, vascular calcification.
Patient survival
Seven studies provided data for meta‐analysis. The forest plots of the comparisons made for patient survival are shown in Fig. 2. All seven studies investigated 1‐year patient survival and showed an increased mortality risk in patients with any VC with a pooled risk ratio (RR) of 2.19 (n = 3381; 95% CI 1.39–3.44; P < 001) 1, 22, 23, 24, 25, 26, 27. Six studies also noted the risk of 3‐year mortality which was also increased in recipients with any VC (n = 3272; RR: 2.17; 95% CI 1.66–2.83; P < 0.001) 1, 23, 24, 25, 26, 27. Five studies mentioned 5‐year mortality risk. Similar to the pooled results of 1‐year and 3‐year survival, a significantly increased 5‐year mortality risk was shown in patients with any degree of VC (n = 3153; RR: 2.28; 95% CI 1.86–2.79; P < 0.001) 1, 23, 24, 25, 27. Two eligible studies were not suitable for meta‐analysis. Chavent et al. divided patients into four quartiles, based on a CT‐scan based calcification score. No difference was found for patient survival between quartiles after a mean follow‐up of 4.18 ± 1.64 years 28. Rijkse et al. 29 investigated the impact of aorto‐iliac stenosis classified with the TASC II classification within a retrospective cohort study. They found a significantly decreased patient survival in patients with TASC II C/D lesions with a 5‐year survival of 66% in the TASC II A/B group, 26% in the TASC II C/D group and 72% in the control group without stenosis (log‐rank test TASC II A/B: P = 0.078, TASC II C/D: P < 0.001). After adjustment for various confounders, having a TASC II C/D lesion was a strong, independent risk factor for mortality (HR 3.25; 95% CI 1.87–5.67; P < 0.001) 29. Four studies also investigated causes of death between patients with any degree of VC and no VC. Droupy et al. found that death from cardiovascular cause was more frequent in patients with any VC (nVC: 2.7%, any VC: 27%, P < 0.001) 25. Also, Hernandez et al found that death from a cardiovascular cause was more frequent among recipients with any VC (nVC: 3.1%, any VC: 9.5%) 1. Munguia et al. investigated the combined outcome of a Major Cardiovascular Event (MACE) and cardiovascular death. They found a statistical significant difference with an incidence of 6.5% in KTR without VC and 21.7% in KTR with any VC (P = 0.035) 26. Rijkse et al. found that, among deceased patients, death from a cardiovascular cause was more frequent in patients with any TASC II lesion (any TASC II lesion: 35.4%, no TASC II lesion 19.1%, P = 0.035) 29.
Figure 2.
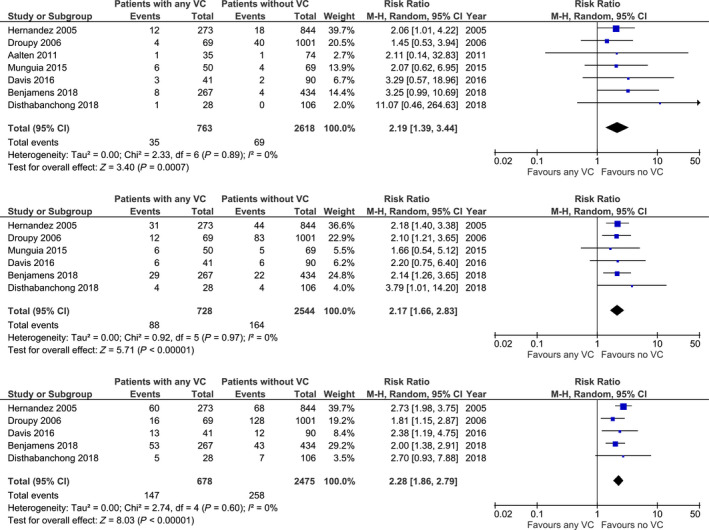
Risk of 1‐, 3‐ and 5‐year mortality in recipients with any degree of vascular calcification (VC) and without VC.
Uncensored graft survival
Four studies, from which three provided data for meta‐analysis, reported uncensored graft survival. Figure 3 shows the pooled results of those studies. The risk of one‐year graft loss was three times higher in KTR with any VC (n = 321; RR: 3.15; 95% CI 1.30–7.64; P = 0.01) 22, 26, 30. The risk of 3‐year graft loss was investigated in two studies. The pooled RR showed no significant higher risk of graft failure in KTR with VC (n = 212; RR: 3.41; 95% CI 0.97–11.96; P = 0.05) 26, 30. Rijkse et al. 29 also found a significant graft survival difference between KTR with a TASC II C/D lesion in comparison to KTR without any TASC II lesions (5‐year graft survival: no TASC II lesions: 60%, TASC II C/D lesion: 22%, log‐rank test: P < 0.001).
Figure 3.
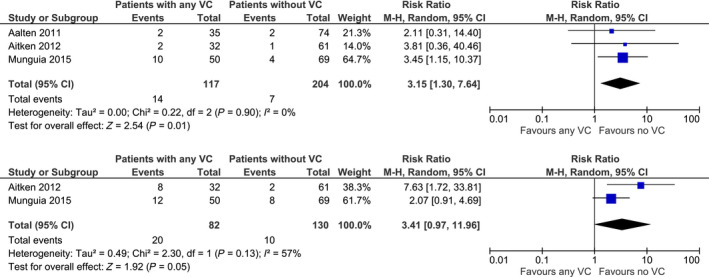
Risk of 1‐ and 3‐year graft loss uncensored for death in recipients with any degree of vascular calcification (VC) and without VC.
Death‐censored graft survival
Five studies reported death‐censored graft survival in recipients with any VC without an additional vascular procedure. Only two provided data for meta‐analysis. Figure 4 shows the forest plots with pooled RR. The risk of death‐censored graft loss was not significantly different between KTR with and without VC with a RR of 2.26 (n = 1189; 95% CI 0.58–8.82; P = 0.24) 25, 26. Also, the risk of 3‐year death‐censored graft loss was statistically similar (n = 1189; RR: 2.19; 95% CI 0.49–9.82; P = 0.31) 25, 26. Three eligible studies were not suitable for meta‐analysis. Chavent et al. reports no difference in overall death‐censored graft survival after a mean follow‐up of 4.18 ± 1.64 years (P = 0.7) 28. Hwang et al. 31 found a significant difference in overall death‐censored graft survival between patients with positive intimal calcification in comparison with patients with negative intimal micro‐calcification (log‐rank test P = 0.017). Rijkse et al. 29 found no significant association between the presence of aorto‐iliac stenosis as classified with the TASC II classification and death‐censored graft loss [TASC II A/B: HR 0.78 (0.41–1.50), TASC II C/D: HR 1.85 (0.74–4.65)].
Figure 4.
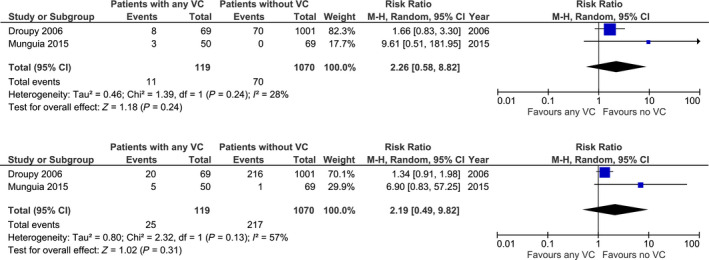
Risk of 1‐ and 3‐year death‐censored graft loss in recipients with any degree of vascular calcification (VC) and without VC.
Delayed graft function
Out of five studies reporting the incidence of DGF, four provided data for meta‐analysis. The pooled RR, as shown in the forest plot depicted in Fig. 5, showed no statistical significant difference for risk of DGF (n = 1391; RR: 1.24; 95% CI 0.98–1.58; P = 0.08) 22, 25, 26, 30. Hwang et al. 31 found no significant difference in the incidence of DGF between patients with and without intimal microcalcification (22.9% vs. 21.4%, P = 0.204).
Figure 5.
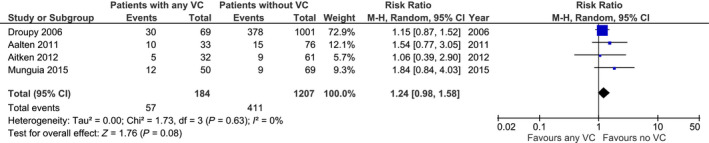
Risk of delayed graft function in recipients with any degree of vascular calcification (VC) and without VC.
Kidney function
Five studies investigated the impact of VC on 1‐year creatinine. Due to the large heterogeneity between studies (I 2 of 81%), it was decided not to pool the results. Aalten et al. 22 found a mean serum creatinine of 130 ± 38 µmol/l in KTR with VC and 131 ± 41 µmol/l in KTR without VC, which was not statistically different. The study of Aitken et al. also found no statistical significant difference (VC: 148.5 ± 9.6, nVC: 140.3 ± 8.9) 30. Munguia et al. investigated serum creatinine after 1 month (nVC: 1.90 ± 0.13 mg/dl, VC: 1.79 ± 0.10 mg/dl), 3 months (nVC: 1.63 ± 0.06 mg/dl, VC: 1.69 ± 0.10 mg/dl), and 1 year (nVC: 1.57 ± 0.07 mg/dl, VC: 1.55 ± 0.11 mg/dl). No significant difference was found 26. Hernandez et al. investigated the percentage of patients with a serum creatinine >2 mg/dl at discharge. He found that the proportions were not significantly different (VC 23.4%, nVC 27.3%, P‐value 0.248) 1. Chavent et al. found that creatinine levels were significantly higher at last‐follow‐up (mean 4.18 ± 1.64) in the fourth quartile with the most severe calcification (P = 0.046), but this result was not significant when the glomerular filtration rate was calculated with the MDRD formula (P = 0.1) 28.
KTx on a prosthetic graft
Nine studies were published in which kidney transplants were connected to a prosthetic graft. In three studies, numbers were too small for further analysis 32, 33, 34. One study did not describe the results of those patients separately 29. Table 4 presents the eligible studies. A total number of 57 cases were described in which the kidney transplant was connected to a prosthetic graft 35, 36, 37, 38, 39. The incidence of DGF was described in 53 patients from which 4 (7.5%) had a DGF 35, 36, 37, 39. The incidence of postoperative complications was 19.3% and complications described were renal vein thrombosis (n = 1), bleeding (n = 5), rejection (n = 1), lower limb amputation (n = 1), thrombosis below the graft (n = 1), infection (n = 2), and surgical wound dehiscence (n = 1) 35, 36, 37, 38, 39. A re‐operation was needed in 7.0% of the recipients 35, 36, 37, 38, 39. Thirty‐day patient, death‐censored and uncensored graft survival was 100%, 90.0% and 90% respectively 35, 36, 38, 39. One‐year patient survival, uncensored and death‐censored graft survival was 93.5%, 93.5% and 89.1% respectively 35, 37, 38, 39. Five‐year survival outcomes were only mentioned in one study. In this study, 5‐year patient survival, death‐censored and uncensored graft survival was 85.2%, 90.3% and 74.1% respectively 37. Coleman et al. described the usage of a vascular conduit in 10 patients (n = 8 donor iliac artery graft, n = 2 saphenous vein graft) to facilitate KTx in case of limited anastomotic options due to iliac calcification 40. No postoperative mortality or graft loss was observed. Two patients had a fascial dehiscence as a complication, and two patients had DGF 40.
Table 4.
Outcomes of KTx on a vascular bypass.
| Study | N | DGF (%) | EC (%) | Re‐operation (%) | Patient survival (%) | Graft survival DC (%) | Uncensored graft survival (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 days | 1 year | 3 years | 5 years | 30 days | 1 year | 3 years | 5 years | 30 days | 1 year | 3 years | 5 years | ||||||
|
Ozcelik Germany |
2007 | 11 | 2/11 (18.2) | 3/11 (27.3) | 1/11 (9.1) | NA | NA | NA | NA | 8/11 (72.7) | NA | NA | NA | 8/11 (72.7) | NA | NA | NA |
|
Patrono Belgium |
2013 | 27 | 2/27 (7.4) | 5/27 (7.4) | 1/27 (3.7) | NA | 24/27 (88.9) | 23/27 (85.2) | 23/27 (85.2) | NA | 26/27 (96.3) | 26/27 (96.3) | 24/27 (88.9) | NA | 24/27 (88.9) | 22/27 (81.5) | 20/27 (74.1) |
|
Tozzi, Italy |
2013 | 4 | 0/4 (0) | 0/4 (0) | 0/4 (0) | 4/4 (100) | 4/4 (100) | NA | NA | 4/4 (100) | 4/4 (100) | NA | NA | 4/4 (100) | 4/4 (100) | NA | NA |
|
Sagban German |
2016 | 4 | NA | 1/4 (25) | 1/4 (25) | 4/4 (100) | 4/4 (100) | NA | NA | 4/4 (100) | 4/4 (100) | 4/4 (100) | 4/4 (100) | 4/4 (100) | 4/4 (100) | NA | NA |
|
Franquet France |
2018 | 11 | 0/11 (0) | 2/11 (27.3) | 1/11 (9.1) | 11/11 (100) | 11/11 (100) | 9/11 (81.8) | NA | 11/11 (100) | 9/11 (81.8) | NA | NA | 11/11 (100) | 9/11 (81.8) | NA | NA |
| Total | 57 | 4/53 (7.5) | 11/57 (19.3) | 4/57 (7.0) | 19/19 (100) | 43/46 (93.5) | 32/38 (84.2) | 23/27 (85.2) | 27/30 (90.0) | 43/46 (93.5) | 30/31 (96.8) | 28/31 (90.3) | 27/30 (90.0) | 41/46 (89.1) | 22/27 (81.5) | 20/27 (74.1) | |
DC, death‐censored; DGF, delayed graft function; EC, early complications; NA, not applicable.
KTx after endarterectomy
Six studies discussed clinical outcomes after KTx in patients who underwent simultaneous iliac artery endarterectomy. All of them mentioned 1‐year patient survival, as shown in Table 5. One‐year patient survival varied from 86.6% till 100% in patients who underwent endarterectomy (EAT) 25, 32, 33, 34, 39, 41. Three studies used a control group of patients without VC and they all showed no statistical significant difference for 1‐year patient survival 25, 32, 41. Three studies also investigated 5‐year patient survival, from which one showed a significant difference in favor of patients without VC (nVC 87 ± 1, VC + EAT 69 ± 8, P < 0.001) 25. Five studies noted 1‐year uncensored graft survival, which varied from 80% to 100% 32, 33, 34, 39, 41. Two studies compared the results with KTR without VC, which was not statistically different 32, 41. One‐year death‐censored graft survival was investigated in three studies and varied from 87–100%, from which two studies used a control group of recipients without VC 33, 34, 39. Droupy et al. found no significant difference for 1‐year death‐censored survival, but 5‐year death‐censored graft survival was inferior in recipients who underwent EAT (70 ± 2, 46 ± 7, P < 0.001) 25. In the study from Han et al., 1‐ and 5‐year death‐censored graft survival was equal between both groups 32. The incidence of DGF was investigated in two studies. Droupy et al. found no difference in the incidence of DGF between KTR without VC and KTR with VC + EAT (37%, 42%, NS) 25. Han et al. 32 confirms these results with an incidence of 2.0% in the KTR without VC group and 7.4% in the KTR with VC + EAT group (P = 0.099).
Table 5.
Patient and graft survival in kidney transplant recipients who underwent endarterectomy.
| Study | Year | Patient survival (%) | Uncensored graft survival (%) | Death‐censored graft survival (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1‐year | 5‐year | 1‐year | 5‐year | 1‐year | 5‐year | ||||||||||||||
| nVC | EAT | P‐value | nVC | EAT | P‐value | nVC | EAT | P‐value | nVC | EAT | P‐value | nVC | EAT | P‐value | nVC | EAT | P ‐value | ||
|
Galazka Poland |
2002 | 94 | 98 | >0.05 | 92 | 96 | >0.05 | 87 | 89 | >0.05 | 66 | 68 | >0.05 | NA | NA | NA | NA | NA | NA |
|
Droupy France |
2006 | 96 | 97.4 | >0.05 | 87 ± 1 | 69 ± 8 | <0.001 | NA | NA | NA | NA | NA | NA | 93 | 87 | >0.05 | 70 ± 2 | 46 ± 7 | <0.001 |
|
Tsivian Italy |
2009 | NA | 86.6 | NA | NA | NA | NA | NA | 80 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
|
Tozzi Italy |
2013 | NA | 100 | NA | NA | NA | NA | NA | 100 | NA | NA | NA | NA | NA | 100 | NA | NA | NA | NA |
|
Han Korea |
2014 | 97.7 | 96.3 | >0.05 | 96.3 | 96.3 | >0.05 | 96.9 | 96.3 | 0.463 | 94.5 | 86.7 | 0.463 | 99.2 | 100 | 0.424 | 97.7 | 90 | 0.424 |
|
Nanmoku Japan |
2017 | NA | 100 | NA | NA | NA | NA | NA | 100 | NA | NA | NA | NA | NA | 100 | NA | NA | NA | NA |
EAT, endarterectomy; NA, not applicable; nVC, without vascular calcification; VC, vascular calcification.
Quality of evidence, publication bias and statistical heterogeneity
Because all included studies were observational, the baseline quality of the evidence was graded as low. The GRADE assessment can be found in Table S2. The final quality assessment for every outcome was downgraded to “very low” because of the accompanying high risk of bias associated with the usage of unadjusted RR. Because of the high risk of bias, the quality of the evidence was not upgraded if the association was strong (RR >2). For the mortality outcome, between study heterogeneity was low with an I 2 of 0% for both 1‐year, 3‐year and 5‐year mortality. For 1‐year graft loss uncensored for death, between study heterogeneity was also low (I 2 0%). For 3‐year graft loss uncensored for death, we found substantial heterogeneity with an I 2 of 57%. For the risk of 1‐year and 3‐year death‐censored graft loss, heterogeneity was either low (1‐year: I 2 28%) or moderate (I 2 57%). For the outcome DGF, heterogeneity was also low (I 2 0%). According to the Newcastle‐Ottawa scale, the quality of three studies was considered good, and for five studies poor. Reasons to consider a study of poor quality were often a combination of no adjustment for confounding, a short follow‐up time or no description of the percentage lost to follow‐up. Funnel plots to assess publication bias are added in the Figs S1–S4. No important publication bias could be found for the studies that provided data for meta‐analysis.
Discussion
Our meta‐analysis demonstrated that the presence of any VC is associated with an increased mortality risk and risk of 1‐year graft loss. This is in line with our expectations, because there is a strong association between large‐vessel peripheral arterial disease and cardiovascular mortality 42. Indeed, the incidence of cardiovascular death was more frequent in deceased KTR with any degree of VC. As expected, risk factors for vascular disease were more prevalent in patients with any degree of VC. Our meta‐analysis did not demonstrate a statistical significant difference for risk of death‐censored graft loss or DGF. However, the pooled RR’s for these outcomes and the wide confidence intervals suggest that this may be attributable to the small sample size. Studies describing results from KTx on a prosthetic graft or after endarterectomy were scarce and results varied largely. Patients who died after bypass surgery and did therefore not receive a kidney transplant are not taken into account, creating guarantee‐time bias. As a result, survival outcomes of kidney transplantation on a prosthetic graft might be too optimistic.
Our meta‐analysis has some important limitations. First, all studies were observational, which means that there is much confounding we could not correct for. We compared baseline characteristics to give an indication of the existing confounders. Confounders known to increase the mortality risk were more prevalent in KTR with any degree of VC. Factors associated with better graft survival, such as a living donor transplant and preemptive transplantation, were also more frequent in KTR with any degree of VC. This may be due to possible selection bias of the transplant surgeon to improve outcomes and to guarantee daytime surgery. Another limitation is instrument variability because of the different methods used to diagnose VC. In two studies, VC was diagnosed during surgery, which may lead to underdetection. Other studies used an X‐ray or CT‐scan to diagnose VC. According to Aitken et al. 30, sensitivity and specificity for pelvic X‐ray to diagnose VC in comparison to CTA were 98.5% and 92.6% respectively. Both pelvic X‐ray (sensitivity 95.5%, specificity 83.1%) and CTA (sensitivity 100%, specificity 92%) correlated well with intraoperative assessment of the vessels 30. Therefore, we think that the usage of different imaging modalities did not largely affect our results. Because of the different scoring systems used and the different methods to diagnose VC, we could not investigate a dose‐response relationship. Therefore, we decided to use the dichotomous outcome of no VC/any degree of VC to decrease misclassification. The studies in our meta‐analysis did not all describe if patients with any VC who received pre‐transplant PTA were excluded. If patients after PTA were included in the study, graft survival estimates could have been diluted towards the null when compared to untreated VC. Also, no distinction could be made between VC and hemodynamically significant stenosis. It may be possible that VC only impacts death‐censored graft survival outcomes in case of a hemodynamically significant stenosis. Rijkse et al. investigated this and did not find a statistical significant difference [TASC II A/B: HR 0.78 (0.41–1.50), TASC II C/D: HR 1.85 (0.74–4.65)] 29. However, in this study, the sample size of KTR with significant stenosis was small. Larger studies are needed to provide definite answers to this question.
This meta‐analysis is the first one to describe the overall prognosis of KTR with any degree of VC. Besides the observational study designs, low between study heterogeneity was observed. We were able to show important factors associated with the less optimistic prognosis of KTR with any degree of VC. For this meta‐analysis, we were only able to look at the dichotomous outcome of any VC/ no VC. Future studies should focus on finding a dose‐response effect of the amount of VC. Also, a standardized classification should be used to reduce heterogeneity between studies and to allow risk stratification. Even though the study designs were not optimal for meta‐analysis, we carefully selected studies to include in our meta‐analysis and therefore, we believe this is the best available evidence on this subject. Because of all confounding factors, we could not investigate whether VC is an independent risk factor for mortality and graft loss. The value of VC as an independent risk factor should be investigated for usage in post‐transplant risk adjustment models, such as the Scientific Registry of Transplant Recipients (SRTR) post‐transplant risk adjustment models 43.
Conclusion
The presence of VC in KTR is associated with an increased mortality risk and increased risk of graft loss. No statistical significant association between VC and DGF or risk of death‐censored graft loss could be demonstrated. However, for the interpretation of the outcomes, the quality, risk of bias and sample size of the available evidence should be taken into consideration.
Authorship
ER, RCM and HJANK: participated in the research design. ER and JLD: screened all studies, selected the studies to be included in this systematic review and analyzed the data. ER, JLD and RCM: wrote the article. JLD, JNMIJ, RCM, JIR and HJANK: revised the manuscript critically.
Funding
The authors received no funding for this work.
Conflict of interest
The authors declare that they do not have any conflicts of interest to disclose.
Supporting information
Table S1. Search terms used for this systematic review and meta‐analysis.
Table S2. Summary of findings table for studies included in meta‐analysis.
Figure S1. Funnel plot for outcome: 1‐year mortality.
Figure S2. Funnel plot for outcome: 1‐year uncensored graft loss.
Figure S3. Funnel plot for outcome: 1‐year death‐censored graft loss.
Figure S4. Funnel plot for outcome: delayed graft function.
Acknowledgement
The authors would like to thank Wichor Bramer for his assistance with the literature search.
Footnotes
* indicates statistical significance.
References
- 1. Hernandez D, Rufino M, Bartolomei S, et al. Clinical impact of preexisting vascular calcifications on mortality after renal transplantation. Kidney Int 2005; 67: 2015. [DOI] [PubMed] [Google Scholar]
- 2. Belch JJ, Topol EJ, Agnelli G, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med 2003; 163: 884. [DOI] [PubMed] [Google Scholar]
- 3. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329. [DOI] [PubMed] [Google Scholar]
- 4. Floege J, Ketteler M. Vascular calcification in patients with end‐stage renal disease. Nephrol Dial Transplant 2004; 19(Suppl 5): V59. [DOI] [PubMed] [Google Scholar]
- 5. Kianda MN, Wissing KM, Broeders NE, et al. Ineligibility for renal transplantation: prevalence, causes and survival in a consecutive cohort of 445 patients. Clin Transplant 2011; 25: 576. [DOI] [PubMed] [Google Scholar]
- 6. Fridell JA, Gage E, Goggins WC, Powelson JA. Complex arterial reconstruction for pancreas transplantation in recipients with advanced arteriosclerosis. Transplantation 2007; 83: 1385. [DOI] [PubMed] [Google Scholar]
- 7. Brekke IB, Lien B, Sodal G, et al. Aortoiliac reconstruction in preparation for renal transplantation. Transpl Int 1993; 6: 161. [DOI] [PubMed] [Google Scholar]
- 8. Baumann DS, McGraw D, Rubin BG, Allen BT, Anderson CB, Sicard GA. An institutional experience with arterial atheroembolism. Ann Vasc Surg 1994; 8: 258. [DOI] [PubMed] [Google Scholar]
- 9. Goldsmith PJ, Fraser SM, Fitzpatrick M, Scott DJ, Ahmad N. Acute lower limb ischemia following pediatric renal transplantation. Pediatr Transplant 2010; 14: E93. [DOI] [PubMed] [Google Scholar]
- 10. Northcutt A, Zibari G, Tan TW, Coulter AH, Zhang WW. Does kidney transplantation to iliac artery deteriorate ischemia in the ipsilateral lower extremity with peripheral arterial disease? Vascular 2015; 23: 490. [DOI] [PubMed] [Google Scholar]
- 11. Neves SE. Anesthesia for patients with peripheral vascular disease and cardiac dysfunction. Anesthesiol Clin 2016; 34: 775. [DOI] [PubMed] [Google Scholar]
- 12. Yang Y, Ning Y, Shang W, et al. Association of peripheral arterial disease with all‐cause and cardiovascular mortality in hemodialysis patients: a meta‐analysis. BMC Nephrol 2016; 17: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011; 11: 2093. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schunemann HJ, Oxman AD, Brozek J, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. Evid Based Med 2008; 13: 162. [DOI] [PubMed] [Google Scholar]
- 16. Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015; 350: h870. [DOI] [PubMed] [Google Scholar]
- 17. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta‐analyses.
- 18. Tummers B. DataThief III, 2006. https://datathief.org/.
- 19. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177. [DOI] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Copenhagen: The Nordic Cochrane Centre TCC. Review Manager (RevMan). 5.3 ed2014.
- 22. Aalten J, Dekker HM, van der Vliet JA, Hoitsma AJ. Does a plain X‐ray of the pelvis predict arterial complications in renal transplantation? A prospective study. Nephrol Dial Transplant 2011; 26: 2007. [DOI] [PubMed] [Google Scholar]
- 23. Benjamens S, Pol RA, Glaudemans A, et al. A high abdominal aortic calcification score by dual X‐ray absorptiometry is associated with cardiovascular events after kidney. Transplantation 2018; 33: 2253. [DOI] [PubMed] [Google Scholar]
- 24. Davis B, Marin D, Hurwitz LM, et al. Application of a novel CT‐based iliac artery calcification scoring system for predicting renal transplant outcomes. AJR Am J Roentgenol. 2016; 206: 436. [DOI] [PubMed] [Google Scholar]
- 25. Droupy S, Eschwege P, Hammoudi Y, Durrbach A, Charpentier B, Benoit G. Consequences of iliac arterial atheroma on renal transplantation. J Urol 2006; 175(3 Pt 1): 1036. [DOI] [PubMed] [Google Scholar]
- 26. Munguia P, Caramelo R, Rubio MV, et al. Pre‐transplant assessment of vascular calcification as a risk factor of mortality, graft loss, and cardiovascular events in renal transplant recipients. Transplant Proc 2015; 47: 2368. [DOI] [PubMed] [Google Scholar]
- 27. Disthabanchong S, Vipattawat K, Phakdeekitcharoen B, Kitiyakara C, Sumethkul V. Abdominal aorta and pelvic artery calcifications on plain radiographs may predict mortality in chronic kidney disease, hemodialysis and renal transplantation. Int Urol Nephrol 2018; 50: 355. [DOI] [PubMed] [Google Scholar]
- 28. Chavent B, Maillard N, Boutet C, Albertini JN, Duprey A, Favre JP. Prognostic value of aortoiliac calcification score in kidney transplantation recipients. Ann Vasc Surg 2017; 44: 245. [DOI] [PubMed] [Google Scholar]
- 29. Rijkse E, Kimenai H, Roodnat JI, et al. Impact of aortoiliac stenosis on graft and patient survival in kidney transplant recipients using the TASC II classification. Transplantation 2019; 103: 2164. [DOI] [PubMed] [Google Scholar]
- 30. Aitken E, Ramjug S, Buist L, Kingsmore D. The prognostic significance of iliac vessel calcification in renal transplantation. Transplant Proc 2012; 44: 2925. [DOI] [PubMed] [Google Scholar]
- 31. Hwang HS, Lim SW, Sun IO, et al. Clinical significance of preexisting microcalcification in the iliac artery in renal transplant recipients. Transplantation 2015; 99: 811. [DOI] [PubMed] [Google Scholar]
- 32. Han DJ, Kim YH, Chung YS, et al. Effects of simultaneous iliac artery angioplasty on graft and patient survival after living‐donor kidney transplantation. Transplantation 2014; 97: 826. [DOI] [PubMed] [Google Scholar]
- 33. Nanmoku K, Watarai Y, Narumi S, et al. Surgical techniques and procedures for kidney transplant recipients with severe atherosclerosis. Exp Clin Transplant 2017; 15: 594. [DOI] [PubMed] [Google Scholar]
- 34. Tsivian M, Neri F, Nardo B, et al. Aortoiliac surgery concomitant with kidney transplantation: a single center experience. Clin Transplant 2009; 23: 164. [DOI] [PubMed] [Google Scholar]
- 35. Franquet Q, Terrier N, Pirvu A, et al. Aortic bypass surgery for asymptomatic patients awaiting a kidney transplant: a word of caution. Clinical Transplantation 2018; 32: e13218. [DOI] [PubMed] [Google Scholar]
- 36. Ozcelik A, Treckmann J, Paul A, et al. Results of kidney transplantation with simultaneous implantation of vascular graft. Transplant Proc 2007; 39: 509. [DOI] [PubMed] [Google Scholar]
- 37. Patrono D, Verhelst R, Buemi A, et al. Renal allograft implantation on prosthetic vascular grafts: short‐ and long‐term results. World J Surg 2013; 37: 1727. [DOI] [PubMed] [Google Scholar]
- 38. Sagban TA, Regus S, Heller K, et al. Results of renal transplantation on alloplastic arterial grafts. Urol Int 2016; 96: 157. [DOI] [PubMed] [Google Scholar]
- 39. Tozzi M, Franchin M, Soldini G, et al. Treatment of aortoiliac occlusive or dilatative disease concomitant with kidney transplantation: how and when? Int J Surg 2013; 11(Suppl 1): S115. [DOI] [PubMed] [Google Scholar]
- 40. Coleman S, Kerr H, Goldfarb D, Krishnamurthi V, Rabets JC. Utilization of vascular conduits to facilitate renal transplantation in patients with significant aortoiliac calcification. Urology 2014; 84: 967. [DOI] [PubMed] [Google Scholar]
- 41. Galazka Z, Szmidt J, Nazarewski S, et al. Long‐term results of kidney transplantation in recipients with atherosclerotic iliac arteries. Transplant Proc 2002; 34: 604. [DOI] [PubMed] [Google Scholar]
- 42. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992; 326: 381. [DOI] [PubMed] [Google Scholar]
- 43. Snyder JJ, Salkowski N, Kim SJ, et al. Developing statistical models to assess transplant outcomes using national registries: the process in the United States. Transplantation 2016; 100: 288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search terms used for this systematic review and meta‐analysis.
Table S2. Summary of findings table for studies included in meta‐analysis.
Figure S1. Funnel plot for outcome: 1‐year mortality.
Figure S2. Funnel plot for outcome: 1‐year uncensored graft loss.
Figure S3. Funnel plot for outcome: 1‐year death‐censored graft loss.
Figure S4. Funnel plot for outcome: delayed graft function.


