ABSTRACT
Recent studies suggest that the RANK/RANKL system impacts muscle function and/or mass. In the pivotal placebo‐controlled fracture trial of the RANKL inhibitor denosumab in women with postmenopausal osteoporosis, treatment was associated with a lower incidence of non‐fracture‐related falls (p = 0.02). This ad hoc exploratory analysis pooled data from five placebo‐controlled trials of denosumab to determine consistency across trials, if any, of the reduction of fall incidence. The analysis included trials in women with postmenopausal osteoporosis and low bone mass, men with osteoporosis, women receiving adjuvant aromatase inhibitors for breast cancer, and men receiving androgen deprivation therapy for prostate cancer. The analysis was stratified by trial, and only included data from the placebo‐controlled period of each trial. A time‐to‐event analysis of first fall and exposure‐adjusted subject incidence rates of falls were analyzed. Falls were reported and captured as adverse events. The analysis comprised 10,036 individuals; 5030 received denosumab 60 mg subcutaneously once every 6 months for 12 to 36 months and 5006 received placebo. Kaplan–Meier estimates showed an occurrence of falls in 6.5% of subjects in the placebo group compared with 5.2% of subjects in the denosumab group (hazard ratio = 0.79; 95% confidence interval 0.66–0.93; p = 0.0061). Heterogeneity in study designs did not permit overall assessment of association with fracture outcomes. In conclusion, denosumab may reduce the risk of falls in addition to its established fracture risk reduction by reducing bone resorption and increasing bone mass. These observations require further exploration and confirmation in studies with muscle function or falls as the primary outcome. © 2020 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research..
Keywords: AGING, ANTIRESORPTIVES, FRACTURE PREVENTION, OSTEOPOROSIS
Introduction
A common cause of fracture in osteoporosis subjects is a fall from a standing height or less; 90% of hip fractures result from a fall.1 Therefore, falls risk reduction is an important component of primary and secondary fragility fracture prevention strategies, usually delivered via multidisciplinary approaches.2, 3, 4, 5 Such approaches include falls risk assessment, exercises, medication review (that includes an assessment of any association with falls for each medication type being administered, as well as dose reductions or switching to alternatives that may be less likely to result in falls, if applicable), and vitamin D supplementation (with or without calcium).6, 7, 8, 9 This multifaceted approach to falls risk reduction is often provided in parallel with bone‐targeted therapies to further reduce fracture risk, as it has been assumed that osteoporosis drugs, per se, are unlikely to also impact muscle function or falls risk.
Denosumab is a fully human monoclonal antibody to receptor activator of NF‐κB ligand (RANKL).10 The clearance of RANKL by denosumab prevents binding to its receptor RANK, inhibiting the development, function, and survival of osteoclasts.11 The RANK/RANKL pathway has also recently been implicated in a role in muscle strength in a muscular dystrophy murine model.12, 13 In addition, denosumab treatment has been shown to reverse an impairment of muscle function and reduction in muscle mass observed in mice overexpressing RANKL.14 Of note, a single case of a woman with facioscapulohumeral muscular dystrophy with osteoporosis who was administered denosumab and within 24 hours showed transient increased grip strength and reduced dystrophic symptoms indicates that there may be a role of RANK/RANKL in the management of this disease.15 Furthermore, a recent review presented evidence indicating that RANKL inhibition with denosumab may benefit patients with Duchenne muscular dystrophy.16 Preliminary data also support a positive impact of denosumab on muscle function in women with osteoporosis.14 These studies prompted us to revisit the observation, largely ignored at the time of study completion, of a significantly lower incidence of falls in the denosumab arm of the pivotal Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial.
In this large, placebo‐controlled trial in postmenopausal women with osteoporosis, 4.5% of the women receiving denosumab during the 3‐year study reported falls as adverse events (AEs) compared with 5.7% of women in the placebo group (log‐rank p = 0.02).10 Notably, this observation excluded falls associated with fracture to try to minimize recall and reporting bias. To further test the hypothesis that denosumab could modify falls risk, we undertook an exploratory pooled analysis of all placebo‐controlled trials of denosumab in the treatment of bone loss.
Materials and Methods
Study design
The analysis comprised data from five placebo‐controlled trials that contributed to the FDA approval of denosumab for use in bone loss indications. The subjects included postmenopausal women with osteoporosis (FREEDOM, trial 1), postmenopausal women with low bone mass (trial 2), men with osteoporosis (trial 3), women with nonmetastatic breast cancer receiving adjuvant aromatase inhibitors (trial 4), and men with nonmetastatic prostate cancer receiving androgen deprivation therapy (trial 5).10, 17, 18, 19, 20 The baseline characteristics of the subjects are shown (Table 1), and the eligibility criteria of each trial are summarized (Table 2).
Table 1.
Description of the Double‐blind Denosumab Versus Placebo‐controlled Studies Included in the Present Analysis
| Trial | Study population | No. of subjects (placebo/denosumab) | Age (years) (placebo/denosumab), mean (SD) | All adverse events (placebo/denosumab), n (%) | Denosumab exposure, months | Reference |
|---|---|---|---|---|---|---|
| Osteoporosis | ||||||
| 1 | Postmenopausal osteoporosis | 3876/3886 | 72.3 (5.2)/72.3 (5.2) | 3607 (93.1)/3605 (92.8) | 36 | 10 |
| 2 | Postmenopausal women with low bone mass | 165/164 | 58.9 (7.5)/59.8 (7.4) | 157 (95.2)/156 (95.1) | 24 | 17 |
| 3 | Men with osteoporosis | 120/120 | 65.1 (9.2)/65.0 (10.2) | 87 (73)/87 (73) | 12 | 18 |
| Cancer treatment‐induced bone loss (CTIBL) | ||||||
| 4 | Women with aromatase inhibitor therapy for breast cancer | 120/129 | 59.7 (9.7)/59.2 (8.9) | 108 (90)/117 (90.7) | 24 | 19 |
| 5 | Men with androgen‐deprivation therapy for prostate cancer | 725/731 | 75.5 (7.1)/75.3 (7.0) | 627 (86.5)/638 (87.3) | 36 | 20 |
Table 2.
Summary of Inclusion and Exclusion Criteria for Each Trial
| Trial | Inclusion | Exclusion | Clinicaltrials.gov identifiers |
|---|---|---|---|
| 110 |
|
|
NCT00089791 |
| 217 |
|
|
NCT00091793 |
| 318 |
|
|
NCT00980174 |
| 419 |
|
|
NCT00089661 |
| 520 |
|
|
NCT00089674 |
BMD = bone mineral density; DXA = dual‐energy x‐ray absorptiometry; PSA = prostate‐specific antigen; PTH = parathyroid hormone.
In each study, subjects were randomly assigned to receive denosumab 60 mg or placebo by subcutaneous injection every 6 months. Calcium and vitamin D supplementation varied between the trials but was applied equally to the denosumab and placebo groups; for example, in trials 1 and 2, subjects received a daily supplement containing at least 1000 mg of calcium, with concomitant vitamin D dosing determined by the baseline 25‐hydroxyvitamin D level.10, 17 None of the studies included specific advice about exercise or falls prevention.
A fall was defined as a sudden, unintentional coming to rest on the ground, floor, or other lower level, regardless of whether an injury had occurred as a result. Subjects were interviewed by study staff at clinic visits where the number and associated features of reported falls were recorded on an AE questionnaire. Preferred terms were coded using MedDRA (MedDRA, McLean, VA, USA) version 9.0 for trials 2 and 4, version 11.0 for trials 1 and 5, and version 14.0 for trial 3.
Statistical analysis
The Cox proportional hazards model was used to estimate the hazard ratio (HR) for incident falls during denosumab treatment relative to placebo as the reference group using both individual study data and pooled data. Interactions between treatment efficacy and baseline age were examined using age as a continuous variable, and as distinct age groups (<75 years and ≥75 years). The stratification at age 75 years was selected to mirror the prespecified subgroup analyses within the FREEDOM trial.10 Interactions of treatment and falls reduction with a history of nonvertebral fracture and prevalent vertebral fracture were also explored in the Cox proportional hazards models. The following baseline covariates, commonly considered to be associated with nonvertebral fracture risk, were added to the Cox models (with and without the interaction term) to explore the robustness of the unadjusted HRs including history of nonvertebral fracture, total hip BMD T‐score, and geographic region. A different set of adjusted Cox models were fitted using the following covariates in the pooled data using treatment group, baseline age (as a continuous variable), history of nonvertebral fracture, total hip BMD T‐score, and geographic region. All Cox models fitted with the pooled data were stratified by study to account for population differences across trials.
In the main analysis, all falls were included in the outcome, regardless of whether a fracture occurred on the same day; all analyses were then repeated for falls excluding those reports that contained a fracture event on the same day. Finally, a sensitivity analysis was carried out using time to the first fall, or nonvertebral fracture, as the composite outcome, given the assumption that nonvertebral fractures usually occur as the result of a fall. All aforementioned analyses were repeated using the time to the first fall/nonvertebral fracture outcome. Significance level was set at alpha = 0.05 for all analyses. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
Results
The analysis population comprised a total of 10,036 subjects, 16.9% of whom were men. The baseline characteristics of the pooled subjects in the denosumab and placebo arms from the five trials are shown (Table 3). The mean age was approximately 72 years, and just under half had sustained a prior fracture. At baseline, 6682 subjects were aged <75 years (5856 women, 826 men), and 3354 subjects were aged ≥75 years (2484 women, 870 men). Overall, the baseline characteristics were similar in both groups, with median vitamin D values being around 21 ng/mL (53 nmol/L) in all subjects.
Table 3.
Pooled Subject Characteristics
| Baseline characteristics | Placebo | Denosumab |
|---|---|---|
| n = 5006 | n = 5030 | |
| Age (years), mean (SD) | 71.9 (6.9) | 71.8 (6.8) |
| Women, n (%) | 4161 (83.1) | 4179 (83.1) |
| Years since menopause, mean (SD) | 23.3 (8.3)* | 23.3 (8.3)* |
| Any historical fracture, n (%) | 2446 (48.9) | 2438 (48.5) |
| Any historical nonvertebral fracture, n (%) | 1765 (35.3) | 1732 (34.4) |
| Prevalent vertebral fracture, n (%) | 1116 (22.3) | 1125 (22.4) |
| Baseline BMD T‐score, mean (SD) | ||
| Lumbar spine | −2.38 (1.29) | −2.35 (1.31) |
| Total hip | −1.70 (0.93) | −1.68 (0.92) |
| Serum CTX (ng/mL), median (Q1, Q3) | 0.54 (0.38, 0.73) | 0.54 (0.38, 0.73) |
| Serum 25‐OH vitamin D (ng/mL), median (Q1, Q3) | 21.1 (16.4, 28.3) | 21.0 (16.4, 28.5) |
These results exclude the two studies in men.
Median (Q1, Q3) duration of follow‐up in the pooled denosumab group was 36.0 (35.2, 36.3) months versus 35.9 (31.7, 36.2) in the placebo group. The number of subjects reporting at least one fall was 5.8% in the pooled placebo group (289 falls in 5006 subjects) compared with 4.6% in the pooled denosumab group (231 falls in 5030 subjects) (Fig. 1). Of the total 520 incident falls, 40 (7.7%) were serious AEs (SAEs). The exposure‐adjusted incidence rate of falls (calculated as the number of subjects who experienced the event divided by the sum of subject exposure time in years) was 2.3 in the pooled placebo group compared with 1.8 in the pooled denosumab group, for a rate ratio (95% confidence interval [CI]) of 0.77 (0.66, 0.90). The exposure‐adjusted incidence rate of falls excluding fracture was 2.0 in the pooled placebo group compared with 1.5 in the pooled denosumab group, for a rate ratio (95% CI) of 0.77 (0.65, 0.91).
Figure 1.
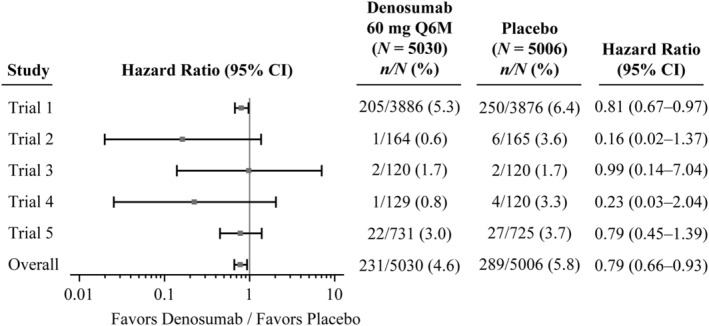
Forest plot of time to first occurrence of fall according to individual studies and the overall. N = number of subjects who received at least one dose of investigational product in trial 1 (placebo‐controlled 36 months), trial 2 (placebo‐controlled only first 24 months), trial 3 (placebo‐controlled only first 12 months), trial 4 (placebo‐controlled only first 24 months), trial 5 (placebo‐controlled only first 36 months). Hazard ratio and 95% CIs are based on Cox proportional hazards model; overall estimates are based on Cox proportional hazards model stratified by study. Q6M = every 6 months.
In each of the studies, the proportion of fallers was either similar or, more commonly, lower in the denosumab arm compared with the placebo arm. Hazard ratios, estimated by Cox regression, ranged from 0.16 (95% CI 0.02–1.37) to 0.99 (95% CI 0.14–7.04) with an overall effect of a 21% reduction in fallers (HR = 0.79, 95% CI 0.66–0.93) (Fig. 1).
The estimated Kaplan–Meier incidence of falls was 6.5% of subjects in the pooled placebo group compared with 5.2% in the pooled denosumab group, with an HR of 0.79 (95% CI 0.66–0.93; p = 0.0061). The time to first fall in subjects randomized to denosumab compared with those receiving placebo is shown in Fig. 2.
Figure 2.
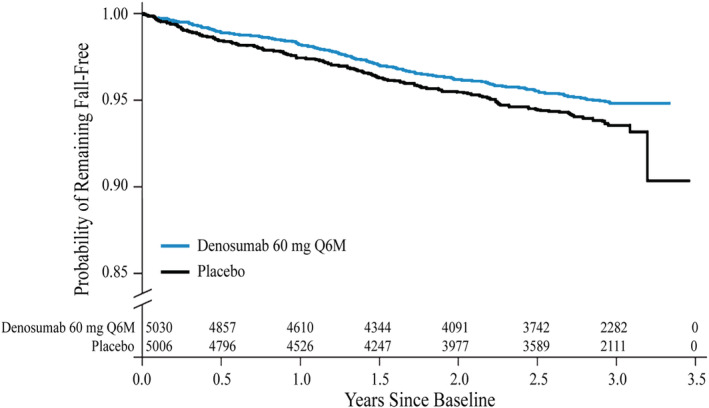
Kaplan–Meier plot of time to first fall by treatment group from pooled data of five placebo‐controlled studies. Numbers at risk for each group for the different time points are shown. Q6M = every 6 months.
Importantly, the pooled HR estimate for falls was similar after adjusting for baseline age (as a continuous variable), total hip BMD T‐score, history of nonvertebral fracture, and geographic region. In addition, there was no significant interaction with a baseline history of nonvertebral fracture or prevalent vertebral fracture. There was, however, a significant interaction between denosumab therapy and age on falls risk when age was considered as a continuous variable (p = 0.045) (Supplemental Fig. S1). A greater reduction in falls was observed with denosumab treatment in subjects aged <75 years versus subjects ≥75 years (interaction p value = 0.012). Thus, denosumab treatment was associated with a 35% reduction in fall risk in subjects <75 years (HR = 0.65, 95% CI 0.52–0.82) but no apparent reduction in subjects ≥75 years (HR = 1.01, 95% CI 0.78–1.31), though a possible early effect on falls risk was suggested (Fig. 3). In contrast to age, no significant interaction was found between the reduction in falls risk and sex (p = 0.96).
Figure 3.

Kaplan–Meier plot of time to first fall by treatment group and age group stratified at the age of 75 years from pooled data of five placebo‐controlled studies. Numbers at risk for each group for the different time points are shown. Q6M = every 6 months.
Discussion
Using pooled data from five placebo‐controlled studies of denosumab, this exploratory analysis suggests that 6‐monthly subcutaneous denosumab can reduce the number of fallers by approximately 20%. Most fractures, including virtually all nonvertebral fractures, occur in the setting of a fall‐related injury, leading to the use of two parallel management strategies, namely to reduce falls risk and improve bone strength. The possibility that treatments targeted to one strategy might impact on the other is not new; for example, it has long been assumed that the effects of vitamin D supplementation might have beneficial effects on both, though the effects are modest.9, 21 However, the observation that therapies developed as specific bone‐targeted agents, such as denosumab, might also impact muscle function and falls risk is a novel development.
The apparent reduction in falls risk observed here is of clinical importance and is similar in magnitude to that achieved by interventions targeted directly at falls risk. For example, in a recent systematic review, multifactorial interventions, which varied substantially across the studies but included exercise, nutritional therapy, knowledge, medication management, urinary incontinence management, environmental modifications, and referral to physical or occupational therapy or other specialties, were found to reduce the incidence of falls in older adults by an identical amount (21%; incidence rate ratio [IRR] = 0.79, 95% CI 0.68–0.91) to that observed with denosumab.3 Interventions based on exercise have also shown a similar reduction (19%; IRR = 0.81, 95% CI 0.73–0.90) in injurious falls, though not an overall reduction in all falls (IRR = 0.87, 95% CI 0.75–1.00).3 Exercise‐based trials have also usually demonstrated high dropout rates, despite relatively short durations and follow‐up periods.22 Compliance with exercise is also not high, with one analysis reporting that approximately 22% of subjects were noncompliant and 41% were partially compliant.23 Vitamin D without calcium supplementation also shows inconclusive results from several meta‐analyses, largely depending on study populations. Vitamin D at doses of 200 to 1000 IU/d is associated with a 14% falls reduction (relative risk = 0.86, 95% CI 0.79–0.93) for older adults aged 60 years or older.8 In another analysis, vitamin D at doses of 700 to 1000 IU/d reduced falls risk by 19% (RR = 0.81, 95% CI 0.71–0.92), whereas a lower dose was ineffective (RR = 1.10, 95% CI 0.89–1.35).24 However, a recent systematic review concluded that vitamin D supplementation (either with or without calcium) showed mixed results in adults without vitamin D deficiency or osteoporosis of increased, decreased, or no difference in falls prevention between control and intervention groups; however, another recent study showed that vitamin D supplementation is beneficial in institutionalized persons and in people with vitamin D deficiency at risk for fractures (ie, with a history of falls or with mobility, gait, or balance problems).3, 25
The question arises as to what potential mechanism might underlie our observations. While much early focus on the impact of inhibition of RANK/RANKL centered on its key role as a final mediator in the regulation of bone remodeling, it is well established that the pathway also exists in other tissues, including skeletal muscle.11, 26 Indeed, there has been interest shown in the potential benefits of RANKL inhibition in muscular dystrophy. For example, a systemic injection of osteoprotegerin (OPG), a soluble decoy receptor of RANKL, has been shown to restore muscle force and improve muscle histology in dystrophic mdx mice, which have the same dystrophin mutation as human patients.27 The same research group found that muscle RANK is a key regulator for calcium ion storage and sarco/endoplasmic reticulum Ca2+ATPase (SERCA) activity and function of fast‐twitch skeletal muscles.28 That pathways in addition to RANKL/RANK may play an important role in muscular dystrophy was recently suggested by the observation that full‐length OPG‐Fc was superior to anti‐RANKL in alleviating muscular dystrophy in the mouse model.12
Outside the setting of muscular dystrophy, RANKL overexpression in a transgenic mouse model has been shown to induce lower maximal speed and force of the limb, with significant reductions in the lower gastrocnemius and soleus muscle mass, as well as severe osteoporosis.29 Treatment with OPG‐Fc was associated with reversal of the functional findings, with an increase in the maximal speed and force of the limb. In a more recent study in the same mouse model, denosumab has also been shown to increase limb force and gastrocnemius muscle mass (+34.7% and +9.8% versus vehicle, respectively, p < 0.05). Importantly, in a small cohort of postmenopausal women receiving denosumab, the same research group showed that treatment was associated with improved lumbar spine BMD, appendicular lean mass, and handgrip over 3 years when compared with controls.14 It should be noted, however, that in a larger cohort of 441 women studied within the placebo‐controlled FREEDOM study, denosumab was not found to have any significant impact on total lean body mass.30 A further possibility is that of cross‐talk between bone and muscle, whereby osteokines released from bone during bone resorption may induce muscle loss and/or weakness.31, 32 If applicable, then other inhibitors of bone resorption might also impact muscle function and falls risk. Several reports using the combination of vitamin D and alendronate show improved muscle function.33, 34, 35 Whether such effects occur in humans in the setting of osteoporosis remain to be determined.
Our analysis also found a significant interaction between denosumab, falls risk, and subject age, in that the protective effect of denosumab on falls risk seemed to be decreased at older ages. There was a higher proportion of men in the older age group (≥75 years) than the younger age group (25.9% versus 12.4%, respectively), raising the possibility of a sex difference in the effect of denosumab; this was countered by the finding of no interaction between sex and efficacy (p = 0.96). A more likely explanation is that, while low muscle mass and impaired muscle function are undoubtedly of importance in falls risk in older people, multimorbidity, frailty, and other deficits are also likely to contribute, eg, polypharmacy, orthostatic hypotension, vestibular disorders, cataracts, and macular degeneration.36, 37, 38
Several limitations of the present analysis need to be noted. Importantly, this is a post hoc study, and although conducted in the setting of randomized controlled clinical trials, none were designed to prospectively or retrospectively collect and validate falls data. Fall events were derived from AE and SAE reports; although the potential impact of treatment on injurious falls would be of more clinical importance, only 40 (7.7%) of the reports were SAEs, suggesting an event that required hospital attendance or admission. This does not imply that the other falls were injury‐free or without impact on quality of life or fear of falling.39 Causes of falls were also not collected in the trials, so we are unable to discriminate falls that might result from muscle dysfunction from those that relate to other causes discussed above. None of the studies collected specific falls‐related risk factors at baseline (eg, prior falls, muscle strength, gait speed, and timed up and go), so no adjustment was possible for such measures, and it was not possible to examine the effect of denosumab specifically in high fall risk subjects. Adjustment for prior fracture was undertaken to at least partially account for prior falls as most fractures would have resulted from a fall‐related injury. Furthermore, the primary analyses were conducted within the individual studies, and the likelihood of significant imbalance in event recall or measures of muscle strength or function is low. Certainly, such measures should be included in future studies. Prospective measurements of vitamin D levels were not undertaken during the studies, but as noted above, the impact of vitamin D on falls risk is uncertain, and the randomized nature of the studies suggests that differences in uptake of vitamin D supplementation between trial arms of sufficient magnitude to mediate the result is highly unlikely.
In addition to the bone turnover and density‐mediated fracture risk reduction in subjects with postmenopausal osteoporosis, low bone mass, male osteoporosis, and cancer treatment‐induced bone loss, denosumab may also reduce the risk of falls in these patients. This observation identifies the need for further high‐quality, appropriately powered and designed studies to explore the effect of denosumab on muscle mass and function.
Disclosures
PC was on the speaker's bureau for Amgen, Pfizer Consumer, MSD, Eli Lilly, Roche, and Novartis and has received travel assistance from Amgen and Teva. EM has received research support from Amgen, ActiveSignal, AstraZeneca, Consilient Healthcare, Fresenius Kabi, GSK, Hologic, I3 Innovus, Internis, IOF, Eli Lilly, Medtronic, Merck, MRC, Novartis, Pfizer, Roche, Sanofi‐Aventis, Servier, Synexus, Tethys, UCB, Unilever, Versus Arthritis, and Warner Chilcott; has received consulting fees from Amgen, ActiveSignal, Consilient Healthcare, GSK, Synexus, and UCB; and has received honoraria from Amgen, AstraZeneca, Consilient Healthcare, GSK, Hologic, Internis, Eli Lilly, Pfizer, Roche, Servier, and UCB. RE has received research funding from Nittobo, IDS, Roche, Amgen, and Alexion and has received consulting fees from IDS, Roche Diagnostics, GSK Nutrition, FNIH, Mereo, Eli Lilly, Sandoz, Nittobo, AbbVie, Samsung, Haoma Medica, CL Bio, Biocon, and Viking. MRM has received consulting fees and honoraria from Amgen. EG has participated in advisory boards for UCB, Amgen, and Takeda and was on the speaker's bureau for Sandoz and Amgen. JG has nothing to disclose. MM, AC, and SH are employees of and own stock in Amgen. SRC has received research support from Amgen; has received consulting fees from Amgen, Radius, UCB, Google Health, Calico, and GE; and has received honoraria from Amgen.
Supporting information
Supplemental Fig. S1. Impact of denosumab on falls risk (defined as one fall or more during follow‐up) compared with the placebo arm, expressed as hazard ratio, across the range of age at baseline.
Acknowledgments
This study was sponsored by Amgen Inc. The authors thank James Ziobro and Martha Mutomba (funded by Amgen Inc.) for providing medical writing assistance in the preparation of this manuscript. We also thank Ms Wachirapan Narktang of the Division of Research, Department of Orthopaedic Surgery, Faculty of Medicine, Siriraj Hospital for her assistance with artwork and illustration of the figures.
Authors' roles: PC and EM had full access to the data and developed the initial and subsequent drafts of the manuscript. PC, EM, RE, MRM, EG, JG, MM, AC, SH, and SRC interpreted the data and reviewed and revised subsequent drafts. SH performed the statistical analyses. All authors approved the final version of the manuscript and approved the decision to submit the manuscript for publication.
References
- 1. Wongtriratanachai P, Luevitoonvechkij S, Songpatanasilp T, et al. Increasing incidence of hip fracture in Chiang Mai, Thailand. J Clin Densitom. 2013;16(3):347–52. [DOI] [PubMed] [Google Scholar]
- 2. Amacher AE, Nast I, Zindel B, Schmid L, Krafft V, Niedermann K. Experiences of general practitioners, home care nurses, physiotherapists and seniors involved in a multidisciplinary home‐based fall prevention programme: a mixed method study. BMC Health Serv Res. 2016;16:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guirguis‐Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(16):1705–16. [DOI] [PubMed] [Google Scholar]
- 4. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7:CD012221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta‐analysis. JAMA. 2017;318(17):1687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooper JW, Burfield AH. Medication interventions for fall prevention in the older adult. J Am Pharm Assoc (2003). 2009;49(3):e70–82 quiz e3–4. [DOI] [PubMed] [Google Scholar]
- 8. Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews DC. Vitamin D treatment for the prevention of falls in older adults: systematic review and meta‐analysis. J Am Geriatr Soc. 2010;58(7):1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu H, Pang Q. The effect of vitamin D and calcium supplementation on falls in older adults: a systematic review and meta‐analysis. Orthopade. 2017;46(9):729–36. [DOI] [PubMed] [Google Scholar]
- 10. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65. [DOI] [PubMed] [Google Scholar]
- 11. Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. [DOI] [PubMed] [Google Scholar]
- 12. Dufresne SS, Boulanger‐Piette A, Bosse S, et al. Genetic deletion of muscle RANK or selective inhibition of RANKL is not as effective as full‐length OPG‐fc in mitigating muscular dystrophy. Acta Neuropathol Commun. 2018;6(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dufresne SS, Boulanger‐Piette A, Bosse S, Frenette J. Physiological role of receptor activator nuclear factor‐kB (RANK) in denervation‐induced muscle atrophy and dysfunction. Receptors Clin Investig. 2016;3(2):e13231–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonnet N, Biver E, Bourgoin L, et al. Denosumab improves muscle function and glucose homeostasis. Calcif Tissue Int. 2018;102:S10–1. [Google Scholar]
- 15. Lefkowitz SS, Lefkowitz DL, Kethley J. Treatment of facioscapulohumeral muscular dystrophy with denosumab. Am J Case Rep. 2012;13:66–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boulanger Piette A, Hamoudi D, Marcadet L, et al. Targeting the muscle‐bone unit: filling two needs with one deed in the treatment of Duchenne muscular dystrophy. Curr Osteoporos Rep. 2018;16(5):541–53. [DOI] [PubMed] [Google Scholar]
- 17. Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2008;93(6):2149–57. [DOI] [PubMed] [Google Scholar]
- 18. Langdahl BL, Teglbjaerg CS, Ho PR, et al. A 24‐month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. J Clin Endocrinol Metab. 2015;100(4):1335–42. [DOI] [PubMed] [Google Scholar]
- 19. Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26(30):4875–82. [DOI] [PubMed] [Google Scholar]
- 20. Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen‐deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta‐analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27(1):367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dishman RK. Exercise compliance: a new view for public health. Phys Sportsmed. 1986;14(5):127–45. [DOI] [PubMed] [Google Scholar]
- 23. Sluijs EM, Kok GJ, van der Zee J. Correlates of exercise compliance in physical therapy. Phys Ther. 1993;73(11):771–82 discussion 83–6. [DOI] [PubMed] [Google Scholar]
- 24. Bischoff‐Ferrari HA, Dawson‐Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta‐analysis of randomised controlled trials. BMJ. 2009;339:b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bischoff‐Ferrari HA, Bhasin S, Manson JE. Preventing fractures and falls: a limited role for calcium and vitamin D supplements? JAMA. 2018;319(15):1552–3. [DOI] [PubMed] [Google Scholar]
- 26. Kartsogiannis V, Zhou H, Horwood NJ, et al. Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25(5):525–34. [DOI] [PubMed] [Google Scholar]
- 27. Dufresne SS, Dumont NA, Bouchard P, Lavergne E, Penninger JM, Frenette J. Osteoprotegerin protects against muscular dystrophy. Am J Pathol. 2015;185(4):920–6. [DOI] [PubMed] [Google Scholar]
- 28. Dufresne SS, Dumont NA, Boulanger‐Piette A, et al. Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast‐twitch skeletal muscles. Am J Physiol Cell Physiol. 2016;310(8):C663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonnet N. Overexpression of human RANKL decreases skeletal muscle function and engenders insulin resistance. Calcif Tissue Int. 2017;100:S12. [Google Scholar]
- 30. Rolland Y, de Souto Baretto P, Cesar iM, et al. Denosumab (DMAb) and total lean body mass: exploratory analyses from the FREEDOM Study. ASBMR 2016 Annual Meeting; Atlanta, GA, USA; 2016.
- 31. Maurel DB, Jahn K, Lara‐Castillo N. Muscle‐bone crosstalk: emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicine. 2017;5(4):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bettis T, Kim BJ, Hamrick MW. Impact of muscle atrophy on bone metabolism and bone strength: implications for muscle‐bone crosstalk with aging and disuse. Osteoporos Int. 2018;29(8):1713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park JH, Park KH, Cho S, et al. Concomitant increase in muscle strength and bone mineral density with decreasing IL‐6 levels after combination therapy with alendronate and calcitriol in postmenopausal women. Menopause. 2013;20(7):747–53. [DOI] [PubMed] [Google Scholar]
- 34. Ringe JD, Schacht E, Dukas L, Mazor Z. Potency of a combined alfacalcidol‐alendronate therapy to reduce the risk of falls and fractures in elderly patients with glucocorticoid‐induced osteoporosis. Arzneimittelforschung. 2011;61(2):104–11. [DOI] [PubMed] [Google Scholar]
- 35. Schacht E, Ringe JD. Risk reduction of falls and fractures, reduction of back pain and safety in elderly high risk patients receiving combined therapy with alfacalcidol and alendronate: a prospective study. Arzneimittelforschung. 2011;61(1):40–54. [DOI] [PubMed] [Google Scholar]
- 36. Kario K, Tobin JN, Wolfson LI, et al. Lower standing systolic blood pressure as a predictor of falls in the elderly: a community‐based prospective study. J Am Coll Cardiol. 2001;38(1):246–52. [DOI] [PubMed] [Google Scholar]
- 37. Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19(6 Pt 1):508–19. [DOI] [PubMed] [Google Scholar]
- 38. Furman JM, Raz Y, Whitney SL. Geriatric vestibulopathy assessment and management. Curr Opin Otolaryngol Head Neck Surg. 2010;18(5):386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terroso M, Rosa N, Marques AT, Simoes R. Physical consequences of falls in the elderly: a literature review from 1995 to 2010. Eur Rev Aging Phys Act. 2014;11:51–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1. Impact of denosumab on falls risk (defined as one fall or more during follow‐up) compared with the placebo arm, expressed as hazard ratio, across the range of age at baseline.


