Abstract
Premise
Anatomically preserved evidence for a novel clade of gymnosperms emphasizes diversity of seed plants immediately prior to the appearance of angiosperm fossils in the paleontological record.
Methods
Cupulate seeds from the Early Cretaceous Apple Bay locality (Vancouver Island) are described from serial cellulose acetate peels and three‐dimensional reconstruction. Phylogenetic context is assessed through the comparative analysis of gymnosperm seed producing fructifications and maximum parsimony analysis of a revised morphological data set for seed plant phylogeny.
Results
Xadzigacalix quatsinoensis gen. et sp. nov. is characterized by an orthotropous ovule with an elongated micropyle and complex integument, enclosed within a radial cupule. The micropylar canal is elongated; and the nucellus extends into the micropyle to seal the post pollination ovule. Except at the apex of the micropyle, the seed is completely enclosed by a parenchymatous cupule with ca. 20 axially elongated secretory ducts. The cupulate seed is produced upon a triangular woody stele, consisting of a parenchymatous pith surrounded by radially aligned tracheids. The stele produces three short terete traces that terminate within the base of the cupule as transfusion tissue at the seed chalaza.
Conclusions
Organography, vascularization, nature of the integument and nucellus, and configuration of the micropylar canal distinguish Xadzigacalix quatsinoensis from all other gymnosperm clades. Cladistic analyses suggest the new plant may have affinities with gnetophytes or angiosperms. These results are complemented with a critical re‐evaluation of ovulate structures for Mesozoic gymnosperms, providing new insight into plant diversity immediately antecedent to the explosive diversification of flowering plants.
Keywords: anthophyte, Apple Bay, Cretaceous, cupule, Doyleales, Gnetales, gnetophytes, pteridosperm, seed plant phylogeny
The late Mesozoic was a time of profound floristic change with the origin, rapid diversification and rise to dominance of angiosperms and the appearance of many seed plant clades (e.g., Crane, 1987; Wing et al., 1993, 2009; Doyle, 2014; Herendeen et al., 2017; Coiro et al., 2019). Although flowering plants became significant components of terrestrial communities throughout the Cretaceous (Crane, 1987; Wolf and Upchurch, 1987; Wing et al., 2009; Leslie et al., 2018; Condamine et al., 2018), the picture of biotic change in the late Mesozoic is not simply one of exclusion or replacement: Pinaceous and cupressaceous conifers reached the zenith of their diversity in the northern hemisphere (Miller, 1976; Klymiuk and Stockey, 2012; Ryberg et al., 2012; Herrera et al., 2016; Falcon‐Lang et al., 2016; Smith et al., 2017; Leslie et al., 2018; Rothwell et al., 2020); gnetophytes flourished and then declined (Crane and Lidgard, 1989; Friis et al., 2007, 2009, 2013, 2019a; Mendes et al., 2008, 2010, 2012, 2020; Rydin and Friis, 2010); and even leptosporangiate ferns enjoyed explosive evolutionary radiations (Lovis, 1977; Rothwell, 1987). While some gymnospermous clades such as the Bennettitales, which had been major components of Mesozoic landscapes, did decline and suffer extinction during the Cretaceous, others such as the Doyleales (Stockey and Rothwell, 2009, 2016; Shi et al., 2016, 2021) first appear in this interval.
Our capacity to recognize novel clades, such as the doylealean plants, owes significantly to the availability of anatomically preserved specimens. Although only a minority of plant fossils are preserved in three dimensions at a cellular level of detail, such assemblages yield a disproportionately large percentage of information about the diversity, growth, development, and reproductive biology of extinct plants. This assertion is particularly true for the Lower Cretaceous (Valanginian) Apple Bay assemblage of northern Vancouver Island. This paleobotanical konservat lagerstätte offers a unique window into vascular plant diversity immediately antecedent to the appearance of angiosperms (sensu Bateman, 2020) in the fossil record. Two decades of collecting efforts at Apple Bay have yielded fossil fungi (Smith et al., 2004; Bronson et al., 2013), lichens (Matsunaga et al., 2013), liverworts and mosses (Steenbock et al., 2011; Shelton et al., 2015, 2016; Tomescu, 2016; Bippus et al., 2017; Savoretti et al., 2018), lycopodialean and selaginellalean lycophytes (Stockey and Rothwell, 2006), Equisetum (Stanich et al., 2009), several families of leptosporangiate ferns (Smith et al., 2003; Hernandez‐Castillo et al., 2006; Little et al., 2006; Rothwell and Stockey, 2006; Stockey et al., 2006; Vavrek et al., 2006; Jud et al., 2008), conifers (Stockey and Wiebe, 2008; Klymiuk and Stockey, 2012; Atkinson et al., 2014a, 2014b; Stockey et al., 2018), gnetophytes (Rothwell and Stockey, 2013), and other enigmatic gymnosperms (Rothwell et al., 2009; Rothwell and Stockey, 2010, 2016; Stockey and Rothwell, 2009; Ray et al., 2014). The Apple Bay assemblage thus offers an unparalleled census of plant diversity during this pivotal interval of Earth history.
In this study, we describe a novel uni‐ovulate cupulate gymnosperm fructification from the Apple Bay assemblage. These fossils, described herein as Xadzigacalix quatsinoensis gen. et sp. nov., do not conform to any currently recognized clade of seed plants and thus illustrate yet another unexpected facet of Cretaceous gymnosperm diversity. This discovery emphasizes that anatomically preserved lagerstätte assemblages continue to significantly expand our appreciation for the complexity of gymnosperm‐dominated assemblages immediately before the radiation of flowering plants.
MATERIALS AND METHODS
Geologic and depositional setting
Specimens presented here are three‐dimensionally preserved at a cellular level of detail within semi‐sideritic, marine carbonate concretions (Gierlowski‐Kordesch et al., 2021) collected from the Apple Bay locality of northern Vancouver Island, British Columbia (50°36′21″N, 127°39′25″W; UTM 9U WG 951068). The locality comprises a 6.2‐m‐thick section of 20 sedimentary beds, 13 of which are fossiliferous. The beds crop out as a rocky beach on the north shore of Holberg Inlet and are composed predominantly of clast‐supported cryptically bioturbated (sensu Pemberton et al., 2008) sandstone cemented with CaCO3. Sandy, stacked amalgamated beds like those at Apple Bay form on delta fronts under hyperpycnal conditions; when associated with seasonal storms, they often contain significant plant debris (MacEachern et al., 2005; Gierlowski‐Kordesch et al., 2021). Taxa preserved at Apple Bay represent a diverse assemblage of plants, including moss and liverwort gametophytes (Tomescu, 2016), fungi (Smith et al., 2004; Bronson et al., 2013), and dipteridaceous ferns (Stockey et al., 2006) consistent with a forest‐floor flora marginal to a river or stream, constituents of which were periodically swept into the marine basin during storm events (Klymiuk and Stockey, 2012). The Apple Bay locality represents one of several sedimentary basins associated with the Wrangellia Terrane (Muller et al., 1974; Hammack et al., 1994) and has been interpreted as a Lower Cretaceous Longarm Formation equivalent (Jeletzky, 1976; Haggart, 1994; Nixon et al., 2011) of possible Barremian age (Sweet, 2000). Oxygen isotope analyses indicate the locality is ~136 Ma, corresponding to the mid‐late Valanginian (D. R. Gröcke, Durham University, personal communication, 2010), and therefore probably represents the zenith of pre‐angiosperm floral diversity (Herendeen, 2017; Coiro et al., 2019).
Specimen preparation
Concretions containing the specimens were sectioned into ~0.75‐cm‐thick wafers, exposing two specimens in oblique transverse section (UAPC‐ALTA P15375 Cbot, D, and P16474 Btop), and one specimen in oblique longitudinal section (P15280 Btop). Using the cellulose acetate peel technique (Joy, 1956), we prepared serial transverse and longitudinal sections of all specimens in their entirety. Specimen peels were permanently mounted on glass slides, using xylene‐soluble Eukitt (O. Kindler GmbH, Freiberg, Germany) mounting medium. Photomicrographs were captured with a Powerphase (Phase One, Copenhagen, Denmark) digital scanning camera mounted on a Leitz Aristophot bellows camera and Zeiss WL compound microscope. Images were processed with Photoshop CS5 (Adobe, San Jose, CA, USA); image processing involved minor color and contrast adjustments applied globally. Three‐dimensional reconstructions were constructed in Avizo v5.1 (FEI Visualization Sciences Group, Hillsboro, OR, USA). Specimens are deposited in the University of Alberta Paleobotanical Collection (UAPC‐ALTA), in Edmonton, Alberta, Canada.
Phylogenetic analyses
Phylogenetic relationships of the new taxon were assessed with a morphological matrix (Appendix S1) of 107 characters and 42 taxa. This matrix is a revision and expansion (Appendix S2) of that previously published by Rothwell and Stockey (2016), and includes character concepts originally developed by Rothwell and Serbet (1994), Hilton and Bateman (2006), and Doyle (2006, 2008). Tree searches were performed under the parsimony ratchet perturbation algorithm (Nixon, 1999), used in the program TNT (Goloboff et al., 2008) spawned through Winclada (implemented as Asado, v1.1 beta, by K. Nixon, Cornell University). If support was ambiguous, branches were collapsed. All characters were treated as nonadditive, with multistate characters unordered. In each of 10 sequential ratchet searches, ratchet perturbation sampled 10% of the parsimony‐informative characters through 10,000 iterations per replication, with 10 trees held per iteration, and a random constraint level of 12 (Goloboff et al., 2018). Only the semi‐strict consensus phylogeny is reported; bootstrap (Felsenstein, 1985) values were generated from 1000 replicates, with 10 trees held for each of 100 multiple tree‐bisection‐reconnection (TBR) search replications. The morphological matrix was also explored using PAUP* v4.0a168 (Swofford, 2003), where we employed maximum parsimony heuristic searching with characters unordered and equally weighted; gaps were treated as missing, and multistate taxa were interpreted as polymorphisms. Starting trees were obtained by random stepwise addition for 10,000 replicates with one tree held at each step. Branch swapping was performed by TBR with a reconnection limit of 8. Trees were unrooted, and branches were collapsed to polytomies if the branch length was zero. Bootstrap values were generated from 100 bootstrap replicates with one tree held for each of 100 TBR heuristic search replications with reconnection limit of 8.
RESULTS
Systematics
Class Spermatopsida
Order Indet.
Family Indet.
Generic diagnosis: Xadzigacalix Klymiuk, Rothwell et Stockey gen. nov.
Gymnospermous plants bearing radial uni‐ovulate cupulate seeds terminally on woody stems. Cylindrical stele dividing into three terete vascular bundles within base of cupule, terminating as transfusion tissue at chalaza of ovule. Ovule orthotropous, radially symmetrical; integument complex, unvascularized, forming elongated micropylar canal. Nucellus with thickened cuticle; apex solid, cellular; attached to integument only at chalaza. Ovule surrounded by radial cupule except at protruding micropylar canal, attached only at chalaza.
Etymology: Xadzigacalix is compounded of Latin (calix = chalice), and transliterated Kwak̓wala (xa̱dziga = plant resin), the language of the Kwakwaka'wakw nations of the northwestern coastline of North America. The Apple Bay locality, from which the new genus is described, occurs within traditional and unceded territory of these Indigenous peoples. The name Xadzigacalix denotes the morphology and histology of the cupule. (pronunciation, styled after the Oxford English Dictionary:/sʧædz:ˌi:'gʌ:keɪ:'lɪks/; styled phonetically: chutdz‐i‐guh kay‐liks’\).
Type species: Xadzigacalix quatsinoensis Klymiuk, Rothwell et Stockey sp. nov.
Specific diagnosis: Cupule ellipsoidal, ca. 5 mm long by 3 mm diameter, glabrous, composed of two zones of parenchyma. Outer parenchyma zone of isodiametric to flattened cells 3–20 µm diameter, containing longitudinal secretory ducts up to 100 × 200 µm diameter. Ducts lined with uniseriate epithelium of secretory cells. Inner parenchyma zone of radially elongated cells 25 µm diameter grading to isodiametric cells 45–60 µm diameter, forming weakly organized palisade layer. Epidermis of abaxial/external surface with zones of radially aligned parenchyma. Ovule tetrahedral, 1.5–1.6 mm wide at midsection. Integument of three layers; outermost 45–75 µm thick composed of thick‐walled parenchyma; middle 95–100 µm thick composed of palisade sclerenchyma; innermost 50 µm thick composed of isodiametric sclerenchyma. Nucellus without thickened cuticle, expanding into micropyle at maturity, containing megagametophyte with megaspore membrane up to 250 µm thick. Micropylar canal nearly sealed at maturity with triradiate opening reflecting three angles of ovule. Cupule at apex of triangular stele 600–700 µm wide with parenchymatous pith and radial rows of helical to scalariform tracheids 6–10 µm diameter. Stele terminating below nucellus as transfusion tracheids 10–18 µm diameter and as terete cupular strands 125–145 µm diameter below each angle of ovule.
Etymology: The specific epithet quatsinoensis refers to Quatsino Sound, Vancouver Island, British Columbia. (pronunciation:/'cwɑt:ˌsi:noʊ:'ɛn:sɪs/)
Holotype hic designatus: Uni‐ovulate cupule in transverse section (P15375 Cbot and D). Paratypes comprise less mature uni‐ovulate cupules in longitudinal (P15280 Btop) and transverse (P16474 Btop) sections. All specimens are prepared as cellulose acetate peels mounted on glass microscope slides; specimens are deposited in the University of Alberta Paleobotanical Collection (UAPC‐ALTA), Edmonton, Alberta, Canada.
Type locality: Apple Bay, Holberg Inlet, Vancouver Island, British Columbia.
Stratigraphy: probable Longarm Formation equivalent.
Age: mid‐Valanginian, ~136 Ma.
Description of Xadzigacalix quatsinoensis
General features: The gymnospermous Xadzigacalix quatsinoensis plant bears uni‐ovulate, radial cupules terminally upon an apparently eustelic woody stem tip. Whether or not individual cupulate seeds form part of a larger fructification is unknown. Seeds are characterized by a complex integument and extended micropylar canal, and the cupule has distinctive longitudinal secretory ducts. Three specimens of this species are presently available for study (Figures 1–7, 21): a histologically mature ovule in transverse section (Figure 1–7, 21), in which the micropyle and subchalazal vascular architecture are preserved; and two histologically immature specimens, in oblique longitudinal (Figure 1–7, 21) and transverse section (Figure 1–7, 21). Together, these specimens reveal features of cupule attachment, seed and integument structure and development, and permit characterization of the nucellus. All three ovules are enclosed in ellipsoidal cupules that are circular in cross section and approximately 5 mm long and 3 mm in diameter at maturity. Cupules appear to achieve mature dimensions and histological differentiation before ovule maturation, as evidenced by the presence of an ovule at an early stage of development within a much larger cupule (Figure 1–7, 21); at maturity, the ovule entirely fills the available space within the cupule (Figure 1–7, 21). Cupules have two histological zones throughout and show small areas of secondary parenchyma formation (Figure 1–7, 21).
Figures 1–7.
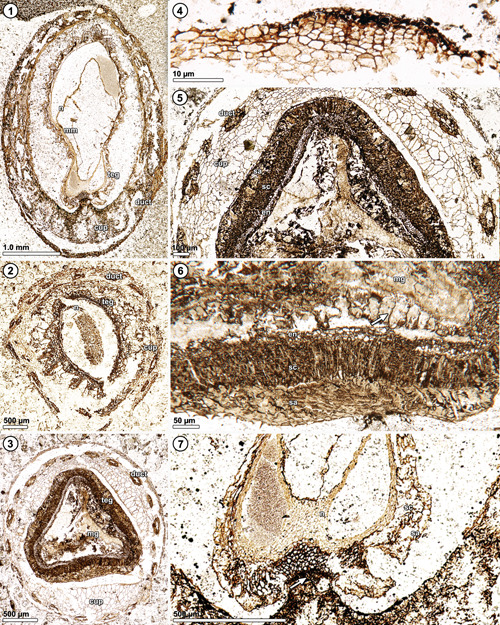
Xadzigacalix quatsinoensis gen. et sp. nov., development and histology of ovule and enclosing cupulate structure. 1. Oblique longitudinal section of immature ovule basally attached to cupulate (cup) structure. Note developing integument (teg) of ovule, nucellus (n) with chalazal attachment, and megaspore membrane (mm), and secretory ducts (duct) throughout cupule. P15280 Btop #1. 2. Immature ovule in transverse section, with developing integument (teg) and nucellus (n), enclosed by cupulate structure (cup) P16474 Btop #25. 3. Mature seed in transverse section, with complex integument (teg) and megagametophyte (mg) tissue. Note histology of enclosing cupulate structure (cup), with outermost zone of thin‐walled isodiametric parenchyma containing secretory ducts (duct) with distinctive epithelial lining. P15375 Cbot #51. 4. Serially arranged files of parenchymatous cells along exterior surface of cupulate structure P15375 Cbot #175. 5. Histology of seed integument showing sarcotesta (sa), sclerotesta (sc), multiseriate endotesta (en), and histology of cupulate structure (cup), showing secretory ducts (duct) lined with darkly pigmented epithelial cells. Note histological zonation of cupule, with large, elongate parenchyma internal to zone of smaller, isodiametric parenchyma containing ducts. P15375 Cbot #67. 6. Histology of seed integument (sarcotesta, sa; sclerotesta, sc; endotesta, en), prominent megaspore membrane (arrow) enclosing megagametophyte (mg). P15375 Cbot #4. 7. Chalaza of immature ovule showing developing integument (Sarcotesta (sa) and sclerotesta (sc) are differentiating), basal attachment of nucellus (n), and transfusion tissue (arrow) at point of attachment with cupulate structure. P15280 Btop #1.
Figure 21.
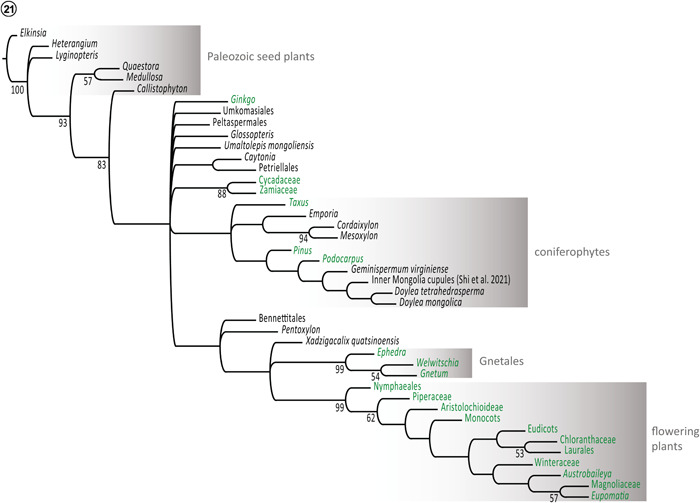
Semi‐strict consensus cladogram of 60 most parsimonious trees. Bootstrap support values >50 are reported.
Ovule: The ovule is ca. 4.5–5.5 mm long, 3.0–3.5 mm in diameter at maturity, tetrahedral and triangular in cross section, and with each side up to 1.1–1.2 mm wide in the midregion (i.e., Figure 1–7, 21). Histologically, the ovule is characterized by a complex integument differentiated into outer sarcotesta with uniseriate epidermis, sclerotesta, and endotesta. The sarcotesta is composed of thick‐walled irregularly shaped parenchyma forming a layer 45–50 µm thick at the corners and thickening to ca. 75 µm along the sides of the ovule. The sclerotesta is 95–100 µm thick and composed of ~five to seven layers of thick‐walled sclereids in a palisade arrangement. Endotesta consists of four to six layers of smaller isodiametric sclerotic cells ca. 50 µm in diameter (Figures 1–7, 21).
Apically, the ovule forms an elongate cylindrical micropyle (Figure 8–10, 30) 310–325 µm in diameter at the base (Figure 8–10, 30), that protrudes at least 100 µm beyond the cupule rim and that narrows (Figures 1–7, 8–10) to ca. 75 µm at the apex (Figure 8–10, 30). The micropylar canal also narrows distally, becoming nearly closed at its apex save for a tri‐radiate opening, ca. 15 µm wide (Figure 8–10, 30, at arrow; Appendix S3).
Figures 8–10.
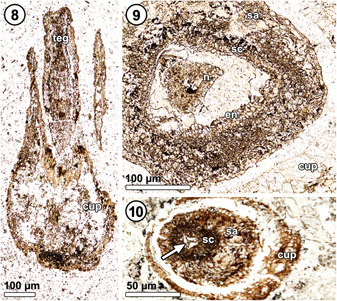
Xadzigacalix quatsinoensis gen. et sp. nov., cupule apex and micropyle of seed. 8. Oblique section through micropylar canal at apex of ovule; ovule integument (teg) extends beyond cupule tissue (cup). P15280 A #1. 9. Transverse section near ovule apex; note solid cellular nucellus (n) and integumentary layers comprising sarcotesta (sa), sclerotesta (sc), and endotesta (en). P15375 D #3. 10. Micropylar apex showing sarcotesta (sa) and sclerotesta (sc) integumentary layers; note nucellar apex (arrow). P15375 D #60.
Figure 30.
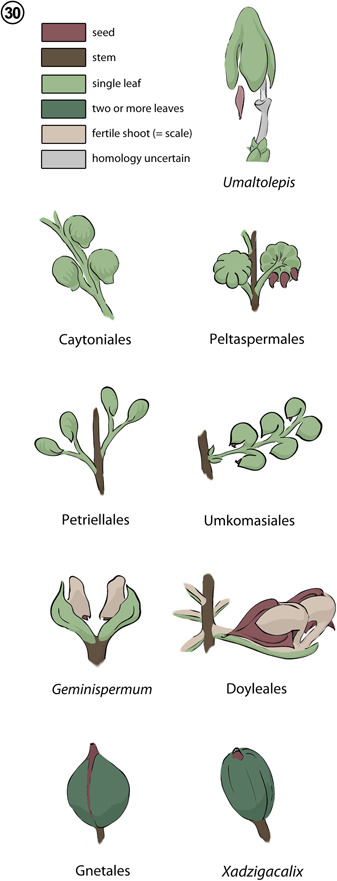
“Cupulate” Mesozoic gymnosperms. Structures are colored to indicate organographic homologues (key at upper left).
Nucellus and megagametophyte: The nucellus is attached to the integument at the chalaza and free at more distal levels (Figure 8–10, 30). It lacks the prominent outer cuticle that characterizes many other gymnospermous ovules (Figures 1–7, 21). Taphonomically altered tissue within the nucellus of the longitudinally sectioned specimen may represent cellular megagametophyte (Figure 1–7, 21). In the most mature specimen, a distinctive megaspore membrane is clearly visible (Figures 1–7, 21) and surrounded by isodiametric to rectangular cells of the nucellus that are up to 25 µm wide and 75 µm high (Figure 1–7, 21, at arrow). The apex of the nucellus shows no evidence of a pollen chamber, but instead remains solid as it projects up into the micropylar canal (Figures 8–10, 30, 8–10, 30; Appendix S3), and assumes a tri‐radiate configuration in cross section (Figure 8–10, 30). There are remnants of megagametophyte or embryonic tissue in one specimen (Figure 1–7, 21), but cellular details are incompletely preserved.
Cupule: The cupule almost completely surrounds and closely adheres to the seed (Figures 1–7, 21), except at the apex where the elongated micropylar tube protrudes beyond the cupule rim (Figures 8–10, 30). It is composed of two zones of thin‐walled parenchyma. The outer zone of parenchyma consists of smaller (13–20 µm diameter) cells (Figures 1–7, 21) and contains distinctive secretory ducts that extend the length of the cupule (Figure 1–7, 21). The secretory ducts are histologically distinctive (Figures 1–7, 21), appearing in cross section either as oval areas of darkened secretory cells (Figure 1–7, 21), or as elliptical rings of such cells surrounding a hollow center (Figures 1–7, 21). Ducts typically measure 100 × 200 µm at their widest (Figure 1–7, 21). Most secretory ducts originate below the level of ovule attachment to the cupule, and several extend most of the length of the cupule (Figure 14). Basal portions of the cupule have discrete areas of radially aligned secondary parenchyma cells near the periphery that are consistent with periderm initiation or wound response parenchyma (Figure 1–7, 21). Cells of the inner parenchyma zone are radially elongate (ca. 25 µm diameter), grading into larger‐diameter (45–60 µm) isodiametric cells, which together form a weakly organized palisade (Figures 1–7, 21). The interior surface of the cupule is lined by a layer of epidermal cells that are 7–10 µm wide.
Figures 14–17.
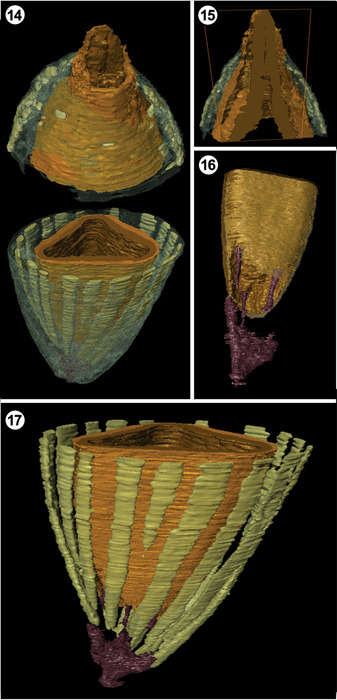
Xadzigacalix quatsinoensis gen. et sp. nov., three‐dimensional reconstruction. 14. Cupulate seed reconstructed from oblique transverse sections of base, P15375 Cbot, and apex, P15375 D. Neither nucellus nor endotesta have been reconstructed; ovule tissues in reconstruction comprise sarcotesta (exterior, golden), sclerotesta (interior, dark brown); secretory ducts (light green) within cupule (translucent green) are absent from one side of specimen owing to abrasion before fossilization. 15. Apex of ovule enclosed by cupulate structure, with digital longitudinal section illustrating extension of micropylar canal beyond cupule. P15375 D. 16. Ovule (sarcotesta = golden, sclerotesta = brown) vascularized by three bundles produced from woody stem (puce). 17. Arrangement of secretory ducts with respect to chalaza of ovule and vascular tissue. P15375 D.
Vascular tissue and architecture: At the basalmost level of the fossils, there is a triangular stele (Figure 11–13, 31) that consists of a woody cylinder with a parenchymatous pith (Figures 11–13, 31, 20). There are at least six rows of radially aligned tracheids 10–14 µm in diameter. Progressing distally, the stele divides to produce three terete vascular strands, 125–145 µm in diameter, that terminate within the base of the cupule on radii that correspond to the angles of the ovule (Figures 11–13, 31, 16, 17). Tracheids of the vascular strands are narrow, 6–10 µm, and have scalariform secondary wall thickenings (Figure 19). The central region of the stele extends into the chalaza of the seed and terminates as a mixture of parenchyma cells and transfusion tracheids at the base of the nucellus (Figures 1–7, 21, 11–13, 31). Transfusion tracheids measure 10–18 µm in diameter and have helical to scalariform secondary wall thickenings (Figure 18).
Figures 11–13.
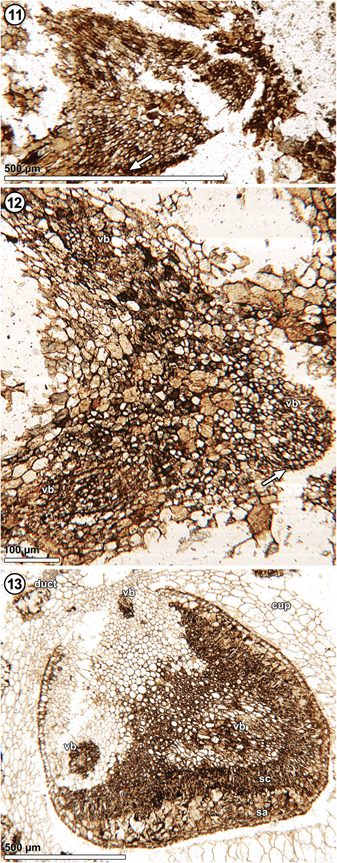
Xadzigacalix quatsinoensis gen. et sp. nov., chalaza and vascular architecture subtending ovule. 11. Incomplete triangular stele at base of cupule; note radially aligned tracheids (arrow) and parenchymatous pith. P15375 Cbot #253. 12. Oblique section distal to triangular stele, showing three vascular bundles (vb); note radially aligned tracheids (arrow). P15375 Cbot #227. 13. Chalaza of mature seed with differentiated integumentary layers (sarcostesta, sa; sclerotesta, sc), and terete vascular bundles (vb) terminating within integument at chalaza (arrow). P15375 Cbot #143.
Figure 31.
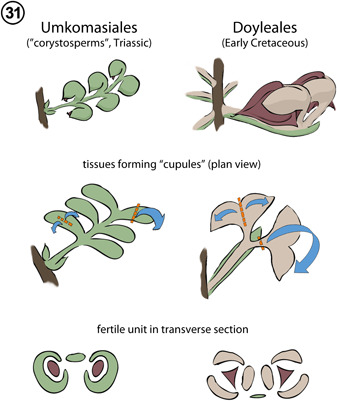
Derivation of cupules in umkomasialean and doylealean plants. Dashed lines and arrows (middle row) indicate planes and direction of folding/enrolling to accomplish enclosure of ovule (in cross section, lower row). Structures colored to indicate organographic homologues, as per Figure 8–10, 30.
Figures 18–20.
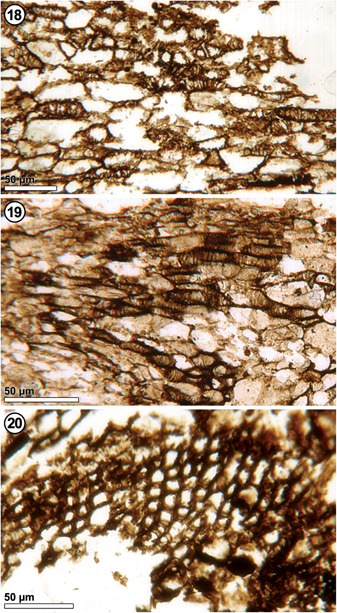
Xadzigacalix quatsinoensis gen. et sp. nov., vascular tissues within cupulate structure. 18. Transfusion tracheids beneath chalaza of ovule. P15280 Btop #240. 19. Tracheids with helical to scalariform thickenings. P15375 Cbot #248. 20. Radially aligned tracheids forming woody cylinder at base of stele. P15280 Btop #206.
Phylogenetic context of Xadzigacalix quatsinoensis
We performed two parallel maximum parsimony analyses of a 42‐terminal, 107‐character morphological matrix (Appendix S1) to hypothesize on the phylogenetic relationships of this novel cupulate gymnosperm. Parsimony analyses employing ratchet perturbation (Nixon, 1999) generated 60 most parsimonious tree topologies (tree length = 363, CI = 0.45, RI = 0.76); 20 most parsimonious trees were recovered with TBR heuristic searching in PAUP* (length = 404, CI = 0.51, RI = 0.77). Consensus topologies of trees generated under both search strategies are identical; therefore, we present only the semi‐strict consensus topology of trees generated with parsimony ratchet perturbation (Figure 1–7, 21).
In the consensus topology (Figure 1–7, 21), extinct Paleozoic seed plants constitute a basal‐grade sister to all Mesozoic and extant seed plants. Living cycadophytes (bootstrap support, BS = 88), angiosperms (BS = 99), and Gnetales (BS = 99) were recovered as monophyletic lineages with high levels of bootstrap support. Operational taxonomic units (OTUs) representing extant Taxaceae, Pinaceae, and Podocarpaceae assort within a coniferophyte clade that lacks basal resolution, but recovers the fossil taxa Cordaixylon Grand'Eury and Mesoxylon D.H. Scott et Maslen as sister taxa (BS = 94). Doylealean cupulate plants and Geminispermum virginense E.M. Friis, P.R. Crane et K.R. Pedersen (2019b) also assort with coniferophytes, where they form a sister clade to podocarpaceous conifers.
The second major clade of Mesozoic through modern seed plants includes the angiosperms, which are resolved as sister to a clade consisting of the gnetophytes and the new plant Xadzigacalix quatsinoensis (BS < 50). Bennettitalean plants + Pentoxylon B.P. Srivast. form a clade that is attached to the stem of the tree immediately basal to the node (BS < 50) where the angiosperms + [gnetophytes + Xadzigacalix] clade is attached (Figure 1–7, 21).
DISCUSSION
Gymnospermous seed plants of the Apple Bay lagerstätte
The Valanginian Apple Bay flora of Vancouver Island, British Columbia provides an exceptional window into the prevalence and diversity of complex seed plants in the Early Cretaceous of western North America. Conifers at Apple Bay are represented by dispersed leaves and leafy shoots (Stockey and Wiebe, 2006, 2008; Atkinson et al., 2014b), pollen cones (Sanders et al., 2004; Stockey et al., 2018), seed cones, and dispersed seeds of Cupressaceae and Pinaceae (Klymiuk and Stockey, 2012; Atkinson et al., 2014a, 2014b). Other gymnospermous plants that populate the assemblage include species of Bennettitales (i.e., Nilssoniopteris corrugata Ray, Rothwell et R.A. Stockey, Williamsonia Carruthers sp., and Foxeoidea connatum Rothwell et R.A. Stockey (Rothwell and Stockey, 2002, 2010; Stockey and Rothwell, 2003; Rothwell et al., 2009; Ray et al., 2014), Gnetales (i.e., Protoephedrites eamesii Rothwell et R.A. Stockey, 2013), and Doyleales (i.e., Doylea tetrahedrasperma R.A. Stockey et Rothwell, 2009; Rothwell and Stockey, 2016). The discovery of Xadzigacalix quatsinoensis further increases the diversity of gymnosperms within the Apple Bay assemblage, emphasizing that North American biomes were systematically diverse immediately preceding the appearance of flowering plants in the fossil record.
All of the Apple Bay gymnosperms display some degree of seed enclosure that presumably functioned in pollination biology, seed protection, and/or seed dispersal (e.g., Leslie et al., 2018). Most conifer seeds, including all currently described from the Apple Bay flora (Klymiuk and Stockey, 2012; Atkinson et al., 2014a, 2014b), are more or less surrounded by parts of the bract/scale complex, while seeds of Bennettitaleans either are immersed among closely packed interseminal scales, or are more or less fused to each other (Rothwell et al., 2009; Rothwell and Stockey, 2010). Among the gnetophytes (e.g., Protoephedrites eamesii; Rothwell and Stockey, 2009), seeds are subtended by a pair of leaf homologues termed bracteoles, which form an outer “integument” or “envelope” in all living species of the clade (e.g., Chamberlain, 1935; Bierhorst, 1971; Friis et al., 2011). The seeds of Doylea spp. are enclosed within compound cones and more or less surrounded by a “cupule” (Rothwell and Stockey, 2016).
The new seed plant Xadzigacalix exhibits a cupulate syndrome that differs substantially from other enclosing syndromes of Apple Bay gymnosperms. Diagnostic characters for inferring organographic homology for “cupulate” gymnosperms include radial or bilateral symmetry of organ morphology; radial or bilateral anatomy of the cupulate organ; presence or absence of a bract subtending the cupulate organ; and presence or absence of the diagnostic seed plant leaf/branch trace vascular anatomy (Rothwell, 1976) of the cupulate organ. In contrast to all other gymnosperms at Apple Bay, the seeds of Xadzigacalix are orthotropous and radial, nonvascularized distal to the chalaza, and enclosed by a uni‐ovulate radial cupule of equivocal (but probably foliar) homologies. The uni‐ovulate cupule occurs at the apex of an organ with morphological and vascular characters like those of a stem (i.e., radial organ with a radial stele; Figures 11–13, 31, 8–10, 30). This novel combination of characters distinguishes Xadzigacalix not only from other Apple Bay seed plants, but all other gymnosperms.
Insights from, and limits of, phylogenetic analyses
While both the sampling and taxonomic breadth of genome‐scale assessment of relationships among living plants is increasing rapidly (Ran et al., 2018b; Wan et al., 2018; Leebens‐Mack et al., 2019), models postulating or testing divergence hypotheses clearly indicate that major clades of living seed plants are evolutionarily separated from one another by hundreds of millions of years (Clark and Donoghue, 2017; Ran et al., 2018a). In most cases, “sister taxa” of living clades of non‐angiospermous seed plants are extinct and may as yet be entirely unknown from the fossil record (Rothwell et al., 2018). Phylogenetic hypotheses for seed plants thus remain equivocal owing to the reality that (1) systematic patterns resolved using only living plants frequently are altered when extinct terminals are added (e.g., Gauthier et al., 1988; Rothwell and Nixon, 2006; see Donoghue et al., 1989 for an analysis of this issue); (2) character sets are based on morphology, requiring nuance in both interpretation and methods of analysis (Coiro et al., 2018; Bateman 2020); (3) the “data set” of all available extinct taxa is incomplete; and (4) we currently have no reasonable method to estimate the severity and scale of these problems.
To develop phylogenetic hypotheses for the affinities of Xadzigacalix, we performed maximum parsimony cladistic analyses of a revised morphological matrix (Appendices S1, S2) for seed plants. The semi‐strict consensus of 60 most parsimonious trees tentatively suggests (BS < 50) that Xadzigacalix represents a lineage more closely allied with gnetophytes than other recognized gymnospermous plants (Figure 1–7, 21). Although the uni‐ovulate cupules of Xadzigacalix are superficially similar to those of some gnetophytes in terms of gross morphology, there are numerous organographic, vascular, and histologic differences that clearly distinguish the new plants from living and fossil Gnetales.
The seed‐bearing structures of gnetophytes have been a source of perplexity and debate for a considerable time (Thompson, 1916; Eames, 1952; Rodin and Kapil, 1969; Rothwell and Stockey, 2013). In most living and fossil gnetophytes, ovules are commonly surrounded by at least two layers of tissue (three layers, i.e., with the integument, for some species of Gnetum; Figures 22–26), which have been dubbed the “inner” and “outer envelopes”, or sometimes even referred to as “integuments”. Takaso and Bouman's study (1986) of the development of these structures in Gnetum L. have helped to clarify their organography. The “outer envelope” originates as a pair of opposite protuberances on either side of the ovule primordium (fig. 1I, 1J of Takaso and Bouman, 1986), fusing to enclose the developing ovule. The integument of the ovule elongates apically to form a micropyle that extends up to (and eventually out of) these outer bracteoles (fig. 3K, 3L of Takaso and Bouman, 1986). Meanwhile, a second pair of bracteoles initiates between the integument and outer whorl (fig. 3I, 3J of Takaso and Bouman, 1986), becoming the so‐called “inner envelope”. The complex, fleshy, enclosing structure surrounding most gnetalean seeds is therefore composed of opposite‐decussate (or whorled) bracteoles that may completely fuse as in Gnetum, or appear slightly separated as in Ephedra L. The micropyle‐forming tissue is of course the integument, which shows little to no histological differentiation into sarcotesta, sclerotesta, or endotesta (Rodin and Kapil, 1969).
Figures 22–29.
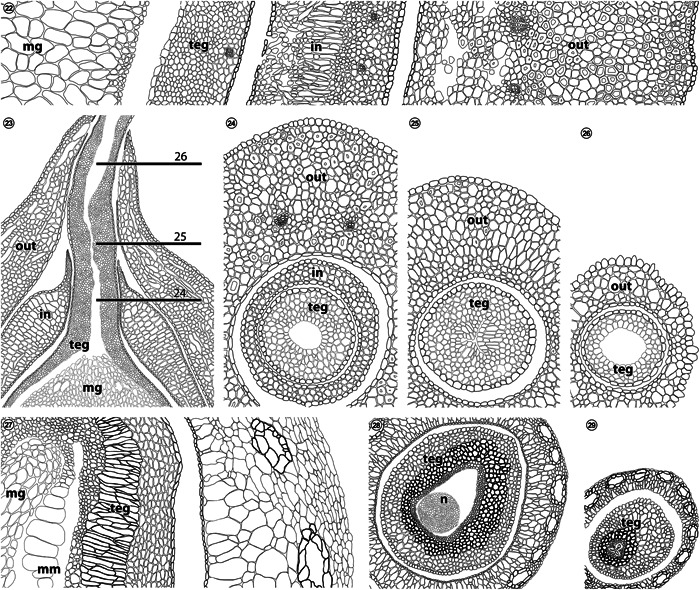
Comparison of integuments and seed‐enclosing structures of Gnetum gnemon with Xadzigacalix quatsinoensis gen. et sp. nov. 22. Histology of G. gnemon in transverse section showing megagametophyte (mg), integument (teg), inner bracteole/“envelope” (in), and outer bracteole/”envelope” (out), illustrated from fig. 3 of Rodin and Kapil (1969). 23. Longitudinal sections of G. gnemon developing ovule with enclosing inner (in) and outer (out) bracteoles with respect to integument (teg) and megagametophyte (mg), illustrated after figs. 6G, H of Takaso and Bouman (1986). Lines 24, 25, and 26 correspond to Figures 24–26, respectively. 24–26. Transverse sections of the micropylar region of G. gnemon ovulate structures, showing the conformation of inner (in) and outer (out) bracteoles with respect to integumentary (teg) tissue of the micropyle. 27. Histology of X. quatsinoensis in transverse section showing probable megagametophyte (mg), megaspore membrane (mm), integument (teg) comprised of endotesta, sclerotesta, and sarcotesta c.f. Figs. 5, 6, and surrounding cupule c.f. Figs. 3, 5. 28, 29. Transverse sections of the micropylar region of X. quatsinoensis, comparable to Figures 24 and 25 (the distalmost portion of the micropyle is abraded in the fossil and thus unavailable for comparison); idealized illustrations after Figures 9, 10, and Appendix S3. Note presence of nucellus (n) within micropyle.
At maturity, the integument of Xadzigacalix seeds is differentiated into sarcotesta, sclerotesta, and endotesta. These integumentary layers are superficially similar to the histology of the inner bracteole whorl of Gnetum gnemon L., as figured by Rodin and Kapil (1969). Some of these similarities may owe to specimen preparation and photography of Gnetum spp. (Rodin and Kapil, 1969). For instance, in Rodin and Kapil's (1969) micrographs, the slightly disorganized mesophyll‐like tissue of the “inner envelope” of G. gnemon appears darkened, heightening the resemblance to sclerotesta of Xadzigacalix, whereas sclereids in G. gnemon are represented as seemingly open voids and cells frequently indistinguishable from parenchyma. Nevertheless, the integument Xadzigacalix can be readily distinguished from the “inner envelope” of gnetaleans on the basis of distal regions of the micropyle, the tissue of which is consistent with the histological complexity of the seed (Figures 8–10, 22–29; Appendix S3).
It is also useful to examine the micropylar regions of these plants to illustrate that no “inner envelope” exists in Xadzigacalix. In Gnetum, the inner bracteoles tightly clasp the base of the micropyle; transverse sections taken below the outer envelope (Figure 24; fig. 6D of Takaso and Bouman, 1986) show that the micropyle is surrounded by a ring of inner bracteole tissue (note epidermal cells on inner/abaxial and outer/adaxial surfaces, clearly demarcating this ring of tissue as separate from the micropyle and the tissue of the fused outer bracteoles). In comparable sections of Xadzigacalix, the micropyle is surrounded only by cupule tissue (Figures 8–10, 22–29; Appendix S3). Similarly, when viewed in longitudinal section (Figures 1, 7), it is clear that the cupule of Xadzigacalix contains only the ovule. Apically, the tissues of gnetalean inner bracteoles are inflated into a void‐filling “flange” (Figure 23; fig. 6G–I of Takaso and Bouman, 1986). No such structures are evident in Xadzigacalix (Figure 1); the absence of similar tissues in Xadzigacalix is unlikely to be an artefact of preservation as even charcoalified gnetalean fossils show evidence of this tissue (fig. 2C of Friis et al., 2007). While the cupule of Xadzigacalix may conceivably have formed from fusion of bracteole‐like initials, there is no evidence for an inner whorl of tissue comparable to that seen in Gnetum spp. (Figures 1–7, 22–29; figs. 3–5 of Rodin and Kapil, 1969).
The cupule of Xadzigacalix is also distinct in that it contains large secretory ducts or canals, arranged in a ring around the entire periphery (Figures 1–7, 21, 1–7, 21). Although secretory structures in the form of laticifers do occur in extant gnetophytes, they are not present in the seed integument or in tissues surrounding the seed (Takaso and Bouman, 1986). Such laticifers are evident in mesophyll of juvenile Gnetum leaves and in stem tissues, becoming inconspicuous at maturity (Carlquist, 1996; Tomlinson and Fisher, 2005). Fossilized gnetalean leaves also have chemical signatures consistent with the presence of laticifers (Roberts et al., 2020). Unlike the conspicuous resin‐ or mucilage‐producing canals in the Xadzigacalix cupule (Figures 1–7, 21, 1–7, 21), however, gnetalean laticifers show little organization within leaf tissue, and are rarely larger than surrounding parenchyma cells (c.f. fig. 8 of Tomlinson and Fisher, 2005). Gnetalean laticifers branch or ramify (Carlquist, 1996), which is not a feature of the secretory structures of Xadzigacalix (Figures 14, 17). Moreover, the bracteoles surrounding gnetalean ovules do not contain obvious laticifers comparable to the secretory structures of the Xadzigacalix cupule; large void‐like structures in G. montanum Markgr. as figured by Rodin and Kapil (1969) are identified by these authors as sclereids.
The abundance and distribution of vascular tissues differs substantially between gnetophytes and Xadzigacalix. Vascular traces can be found in every tissue of the ovulate structure of Gnetum, including the seed integuments (Figure 22; Rodin and Kapil, 1969). In Xadzigacalix, by contrast, the cupule is unvascularized, as is the ovule (except at the chalaza). In this extinct species, three terete vascular strands diverge from the radial stele at the base of the cupule, and pass up to each vertex of the chalaza of the seed. The stele terminates as transfusion tissue below the nucellus (Figures 11–13, 31). By contrast, vascular tissues in the ovulate structures of Gnetum form two distinct rings of numerous vascular bundles at the level of the chalaza (see fig. 13 of Rodin and Kapil, 1969).
Another way in which Xadzigacalix fundamentally differs from gnetophytes is with respect to the nucellus: Gnetophytes have their nucellus fused to the seed integument for more than half the length of the seed and exhibit a pollen chamber (Chamberlain, 1935; Rothwell et al., 2009). By contrast, the nucellus of Xadzigacalix is free from the integument except at the chalaza (Figures 1–7, 21) and shows no evidence of a pollen chamber (c.f. fig. 27 of Rothwell et al., 2009); these nucellar characters are shared by Xadzigacalix and bennettitaleans (Rothwell et al., 2009). It is also worth noting that the uni‐ovulate cupule of the Xadzigacalix plant is borne terminally upon the homologue of a woody stem (Figure 20), whereas ovulate reproductive units of gnetophytes are borne in highly condensed cones or bisporangiate strobili (Ickert‐Bond and Renner, 2016). Finally, the vast majority of gnetophytes currently known from the Cretaceous fossil record have seeds that are four‐lobed or quadrangular in section (e.g., Friis et al., 2007, 2009, 2013, 2019a; Mendes et al., 2012, 2020); Xadzigacalix, by contrast, has a tetrahedral seed that is triangular in transverse section.
Although Xadzigacalix is possibly more closely related to gnetophytes than to any other gymnosperm lineage, it probably does not represent a true sister taxon. It is more likely that gymnosperm diversity remains underrepresented and that seed plant systematics are less fully resolved than commonly thought, owing to a paucity of anatomically preserved taxa throughout the Mesozoic. Phylogenetic placement of fossils using maximum parsimony cladistic analyses is also strongly hampered by probable homoplasy in morphological data sets. As an example, the analysis in this study resolved doylealean taxa within the coniferophytes, as opposed to sharing a sister taxon relationship with gnetophytes and Petriellales + flowering plants (Rothwell and Stockey, 2016). Placement of doylealean taxa within coniferophytes also distinguishes our results from those of Shi et al. (2021), whose analyses of a separate morphological matrix suggest that doylealean taxa share an affinity with Petriellales and Umkomasiales. We suspect that many differences between these analyses derive from character scoring interpretations of cupules either as wholly homologous organs (Shi et al., 2021) or as products of convergent evolution (this paper). Accurately ascertaining the affinities of various cupulate gymnosperms will require untangling the extent to which any of these structures are genuinely homologous.
Morphology, anatomy, and homologies among seed‐bearing structures of “cupulate” Mesozoic gymnosperms
Mesozoic gymnospermous plants bearing seeds within a “cupule” are commonly referred to as “pteridosperms” or “Mesozoic seed ferns”, terms that reflect the traditional recognition that leaf and seed‐bearing structures of these plants are at least superficially similar to those of Paleozoic pteridosperms (Taylor et al., 2006, 2009a). While there is some emerging evidence for phylogenetic continuity for some of these plants across the Permian‐Triassic boundary (Kerp et al, 2001; Hamad et al., 2017; Blomenkemper et al., 2018, 2020; Anderson et al., 2019), most groups are unlikely to share close affinities with Paleozoic seed plants (Figure 1–7, 21). Instead, as is evident from lagerstätte like Apple Bay, many lineages of Mesozoic gymnosperms “experimented” with seed‐enclosing structures. As documented in our current investigation and in previous studies and reviews (e.g., Rothwell and Serbet, 1994; Taylor et al., 1994, 2006, 2009a), the cupules of most Mesozoic gymnosperm clades are not homologous organs (Figure 8–10, 30).
To clarify structural homologies among the heterogeneous assemblage of seed‐enclosing organs that characterize Mesozoic “cupulate” gymnosperms, conifers, and gnetophytes (including dispersed uni‐ovulate structures recognized by some authors as Erdtmanithecales; see Friis et al., 2009, 2013, 2019a; Mendes et al., 2020), we introduce and evaluate major groups of “cupulate” Mesozoic seed plants in terms of homologies to stem/leaf organography (Harris, 1976) and vascularization (Rothwell, 1976; Beck et al., 1982) intrinsic to each. This approach was originally advocated by Florin (1950) for conifers and later elaborated by Harris (1976) for gymnosperms in general and has previously been of great utility in revealing that the seed cones of conifers and taxads are derived from a more or less compound shoot system (e.g., Florin, 1951; Rothwell et al., 2011; Leslie et al., 2018). A comprehensive treatment of structural homologies for Mesozoic gymnosperms will be presented in a separate review. Here, we summarize syndromes for seed enclosure of “cupulate” Mesozoic gymnosperms for which anatomical data are available (Table 1, Figure 8–10, 30), and evaluate the organography of cupulate organs below.
Table 1.
Features and developmental homologies of cupulate Mesozoic gymnosperms.
| Fossil cupulate gymnosperms | Cupule construction | Cupule stalk in axil of leaf/bract | Ovules per cupule | Ovule shape | Apex of micropylar canal | Nucellar attachment | Sclerotic integument | Secretory structures | Age | Key references | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Caytoniales |

|
Abaxially inrolled lamina | – | multiple | ellipsoidal | simple | ? | – | Jurassic | Harris, 1940, 1951, 1964; Reymanowna, 1973 | |
| Peltaspermales |

|
peltate stalk | – | multiple | ellipsoidal | simple | ? | – | Triassic | Kerp, 1988; Poort and Kerp, 1990; Bomfleur et al., 2011 | |
| Petriellales |

|
Ad?axially inrolled lamina | – | 5‐6 | ellipsoidal, triangular in xs | simple | at chalaza only | – | – | Triassic | Taylor et al., 1994 |
| Umkomasiales |

|
Abaxially inrolled lamina | – | 1 (2) | ellipsoidal | bifid | at chalaza only | –? | + | Triassic | Klavins et al., 2002; Anderson et al., 2019 |
| Doyleales |

|
inverted stalk + 2 lateral lobes | yes | 1 | tetrahedral, triangular in xs | pollen horns | at chalaza only | + | – |
Early Cretaceous |
Stockey and Rothwell, 2009; Rothwell and Stockey, 2016; |
| Geminispermum |

|
Inverted hood + stalk | yes | 1 | ellipsoidal, triangular in xs | ?bifid | at chalaza only | –? | +? |
Early Cretaceous |
Friis et al., 2019a |
| Gnetales |

|
opposite‐decussate bracteoles | n/a | 1 | quadrangular | ?bifid | chalaza to midway along seed body | + | + | Cretaceous |
Rothwell and Stockey, 2013 |
| Umaltolepis |

|
apically borne inverted lobes | ? | 4? | elongate, winged | ? | ? | –? | –? |
Early Cretaceous |
Herrera et al., 2017; Dong et al., 2019 |
| Xadzigacalix |

|
aril‐like? | n/a | 1 | tetrahedral, triangular in xs | triradiate opening | at chalaza only | + | + |
Early Cretaceous |
Note: xs, cross section
Broadly, cupulate gymnosperms of the Triassic and Jurassic bore seeds upon foliar organs (=leaf homologues) with enrolled and/or recurved laminar tips forming the “cupules” (Figures 8–10, 30, 11–13, 31); these include Caytoniales, Peltaspermales, Umkomasiales, and Petriellales. Where such leaf homologues are known to be borne on a stem homologue, they form a simple fertile shoot system (sensu Anderson and Anderson, 2003; Anderson et al., 2019). By contrast, “cupulate” gymnosperms that appear first in the Cretaceous bear seeds and their enclosing “cupule” either apically or laterally upon a stem‐like structure (Umaltolepis mongoliensis Herrera et al., 2017; Dong et al., 2019; Nosova, 2020) and/or they bear seeds on compound shoot systems (e.g., Gnetales; Ickert‐Bond and Renner, 2016). For some taxa (i.e., Doyleales, Geminispermum virginiense), these compound shoot systems are broadly comparable to those of extant conifer seed cones, in that they are composed of two orders of cauline and foliar organs (Figure 11–13, 31).
Caytoniales : Seed‐bearing cupules of Caytoniales are multiovulate and occur apically on unbranched lateral units that appear to be pinnately arranged (e.g., Thomas, 1925). The cupule is formed of a bilaterally expanded distal region that is abaxially inverted and laterally enrolled to form a cupule wall with a basally directed lip through which pollination occurs (Harris, 1951, 1964; Reymanowna, 1973; Dilcher, 1979). These cupulate units are parts of a pinnate organ, in which their bilateral morphology indicates that they represent foliar homologues. This interpretation is consistent with the absence of a bract subtending each unit, but remains to be confirmed by the discovery of permineralized specimens with preserved vascular anatomy.
Peltaspermales : “Cupules” of peltasperms are either laminar (i.e., Autunia conferta J.H.F. Kerp, 1988) or peltate, have several abaxially borne seeds, and are helically arranged on an axis (Kerp, 1988; Poort and Kerp, 1990; Kerp et al., 2001). This morphology and the absence of a subtending bract imply that individual cupulate units are foliar and part of a simple shoot. As with Caytoniales, the morphological interpretation of homology for peltasperm cupulate systems remains to be confirmed by the discovery of vascular anatomy.
Umkomasiales : The organography of “cupulate” fructifications of umkomasialean plants has been subject to significant debate (Shi et al., 2016, 2019; Rothwell and Stockey, 2016; Anderson et al., 2019). Here, we clarify that umkomasialean plants produce cupulate seeds upon structures that are homologous to leaves. Umkomasialean plants produce lateral cupule‐bearing units of varying complexities, borne in a helical arrangement upon a larger axis (e.g., Anderson et al., 2019). Each lateral unit (Figure 11–13, 31) typically bears paired uni‐ovulate (rarely bi‐ovulate, see Klavins et al., 2002) cupules (Thomas, 1933; Holmes and Ash, 1979; Holmes, 1987; Klavins et al., 2002; Anderson and Anderson, 2003; Pattemore, 2016; Anderson et al., 2019). Species with simple lateral units may bear only a single cupule (e.g., Umkomasia resinosa S. Klavins, T.N. Taylor & E.L. Taylor, 2002), while other species have two or more terminal cupules (ranging up to ~14 in U. dissecta J.M. Anderson and H.M. Anderson, 2003; Anderson et al., 2019), that are more or less arranged in opposite pairs. Where lateral units contain multiple pairs of cupules, they are occasionally subtended by additional spine‐like appendages termed “bracteoles” by Thomas (1933) and Anderson et al. (2019). Where one scale‐like appendage is present at the base of a cupulate unit, that structure has been interpreted by Thomas (1933) and several more recent authors (e.g., Anderson and Anderson, 2003) as a subtending bract. However, there are numerous specimens that show no evidence of a subtending bract (e.g., plate 85, fig. 1 and Plate 87, figs. 8 and 9 of Anderson and Anderson, 2003; figs. 1, 2, and 4 of Shuqin et al., 2008). It is the presence or absence of a subtending bract that is at the heart of opposing interpretations for the homologies of the lateral cupulate units of Umkomasia spp. (Rothwell and Stockey, 2016; Shi et al., 2016, 2019). If each unit is subtended by a bract (=leaf homologue), then the cupulate unit must represent an axillary shoot (Harris, 1976), and the base of the cupulate units will have radial vascular architecture, as is characteristic of stems. If, on the other hand, the cupule‐bearing lateral units of Umkomasia spp. are not subtended by a bract, are not radial, and have bilateral vascular anatomy, then they represent leaf homologues as interpreted by Rothwell and Stockey (2016) and Anderson et al. (2019).
Anatomically preserved cupulate systems of Umkomasia resinosa provide data needed to resolve these competing hypotheses (Klavins et al., 2002). Ovulate fructifications of U. resinosa consist of a radial axis with a radial stele (diagnostic of a stem homologue) that produces bilateral cupulate laterals with bilateral vascular anatomy (diagnostic of a leaf homologue). Moreover, there clearly is no bract or bract trace subtending each of the cupulate units, thus strongly supporting the hypothesis that cupulate units of umkomasialean plants are leaf homologues borne on a stem homologue (Figure 11–13, 31). The cupule wall in Umkomasia spp. therefore consists of a broadened laminar (i.e., leaf or leaflet homologue) tip that is inverted and inrolled toward the abaxial surface, producing a cupule that consists entirely of leaf tissue (Klavins et al., 2002; Anderson et al., 2019; Figure 11–13, 31).
Petriellales : ‘Cupules’ of Petriellales are formed from a bilaterally expanded distal region of a leaf or leaflet homologue that is inverted and laterally enrolled to enclose several seeds (Taylor et al., 1994). As originally interpreted, cupulate axes of Petriellaea triangulata T.N Taylor, G.M. del Fueyo & E.L. Taylor occur in pairs at the apex of a forking structure that may be borne on a subtending axis in a lax simple strobilus (Bomfleur et al., 2014). However, this interpretation has yet to be verified by the discovery of articulated cupulate systems (B. Bomfleur, Westfälische Wilhelms‐Universität Münster, personal communication, 2021). Nevertheless, both the bilateral morphology and vascular anatomy of the P. triangulata cupulate organ are consistent with the interpretation that such cupules represent foliar homologues (Klavins et al., 2002). As in Caytonia Harris and Umkomasia Thomas, the wall of Petriellaea cupules is formed from a laterally expanded, laminar pinnule or pinna tip. In contrast to all other known Mesozoic gymnosperm cupules, those of Petriellaea have been described as being inrolled with the adaxial surface of the cupule wall positioned to the inside (rather than to the outside) and have adaxially borne seeds (Taylor et al., 1994). The adaxial seed attachment for Petriellaea has been questioned recently (Shi et al., 2021), and more evidence is necessary to resolve the question. The nature of seed attachment is a most important character because Petriellaea is the only seed plant other than angiosperms reported to bear seeds on the adaxial surface of the enclosing structure.
Gnetales : Among living species of the Gnetales, seeds occur at the tip of the axis (i.e., a stem homologue; Harris, 1976; Figure 8–10, 30) within a compound cone (Chamberlain, 1935; Sporne, 1965; Bierhorst, 1971). Based on the transformational series of morphologies proposed by Eames (1952) and fossil evidence for an extinct intermediate in that series (Rothwell and Stockey, 2013), the cauline attachment of gnetophyte seeds appears to be the result of reduction and condensing from an ancestral condition where seeds are borne on fertile shoots in the axils of leaves (see fig. 8 of Rothwell and Stockey, 2013). This condition is comparable to that in the conifer Taxus L., in which seeds are terminal (i.e., on a stem homologue) in the axil of a leaf on an otherwise vegetative leafy shoot system, but may actually represent a highly condensed and reduced compound cone (e.g., Leslie et al., 2018).
Doyleales : The ovulate reproductive structures of a number of “cupulate” Mesozoic gymnosperms can be equated to the compound seed cones of conifers, wherein the seed‐bearing and seed‐enclosing structure is attached to a cone axis and subtended by a bract (Figure 11–13, 31). Such homologies are most obvious for species of the Doyleales (i.e., Doylea tetrahedrasperma, D. mongolica, an unnamed new doylealean species, Shi et al., 2021; Figures 8–10, 30, 11–13, 31), and for dispersed bract/scale complexes of two additional species originally described as Umkomasia (i.e., U. corniculata and U. trilobata G. Shi, P.R. Crane, Herend., Ichinorov, Mas.Takah, & F. Herrerra, 2019), but clearly assignable to Doylea on the basis of compound construction. The bract/scale complexes of all of these species divide at the base to produce a bract abaxially and the cupulate unit adaxially. The cupulate unit then divides laterally to produce one (unnamed new species; Shi et al., 2021), two (D. tetrahedrasperma; Stockey and Rothwell, 2009), or three (D. trilobata; Shi et al., 2019) ovuliferous scale homologues, each of which terminate in a uni‐ovulate cupule bearing a tetrahedral seed (Figures 8–10, 30, 11–13, 31; c.f. Rothwell and Stockey, 2016 to Shi et al., 2019). The recently described, unnamed doylealean from Upper Mongolia (Shi et al., 2021) illustrates a condition in which the cupulate unit does not undergo a lateral division to form two fertile stalks (forked ovuliferous scale homologue). Instead, the two vascular strands persist within a single stalk, bifurcating to vascularize two seeds within a bi‐ovulate cupule (see extended data fig. 3 of Shi et al., 2021). Doylealean cupules are formed by lateral inflation of the distal (inverted or recurved) portions of fertile stalk/ovuliferous scale (Figure 11–13, 31). Thus, when viewed in cross section, doylealean cupules consist of fertile stalk tissue on one side of the seed and laminar lobes along the other sides of each seed (c.f. figs. 24–26 of Rothwell and Stockey, 2016, fig. 4 of Shi et al., 2016, fig. 6 of Shi et al., 2019).
Geminispermum virginiense : Less‐obvious compound seed cone structure and homologies are displayed by G. virginiense (Friis et al., 2019b), which constitutes an axis that branches to form two units, each consisting of an abaxial bract and an adaxial uni‐ovulate cupule (Figure 8–10, 30; Friis et al., 2019b). Because the base of this fossil is radial and has a radial vascular system (fig. 2E of Friis et al., 2019b), it is comparable to a stem in both morphology and vascular anatomy. Distal to the branching of the primary axis or main stem, each cupulate unit has a C‐shaped vascular strand (fig. 2D of Friis et al., 2019b), which branches into three traces; the central trace enters the bract, and the two lateral traces enter the base of the cupulate structure (fig. 3 of Friis et al., 2019b). This vascular syndrome is strictly comparable to the vascularization of a leaf and accompanying axillary branch on the vegetative system of typical gymnosperms (Rothwell, 1976). Thus, we interpret G. virginiense to be a determinate compound seed cone composed of a single pair of oppositely arranged bract/scale complexes. This morphology is slightly simpler in overall cone structure, but otherwise comparable to the oppositely arranged bract/scale seed cone complexes of some Cupressaceae [i.e., one opposite pair, rather than two decussate pairs; e.g., Tetraclinis articulata (Vahl) Mast., fig. 4a of Farjon, 2005] or the bract‐scale complexes of some living podocarps (e.g., Chamberlain, 1935; Sporne, 1965).
Xadzigacalix quatsinoensis : The uni‐ovulate cupule of Xadzigacalix is borne apically upon a radial stem homologue exhibiting a radial stele with secondary growth, i.e., wood. The stele ramifies within the base of the cupule to produce three terete strands that terminate at the base of the seed. There is no histological evidence that the cupule of Xadzigacalix is constructed of fused leaf homologues like the bracteoles of gnetophytes because the three vascular strands do not pass into the enclosing tissue of the cupule. Rather, it appears to be more like the aril of some taxaceous conifers (see Farjon, 2005). Additional specimens of this plant are needed to resolve whether the cupulate units are solitary upon the stem or borne in an aggregate (cone‐like) fructification.
SUMMARY AND CONCLUSIONS
Relationships among disparate clades of Paleozoic and Mesozoic non‐coniferophyte gymnosperms remain incompletely resolved (Nixon et al., 1994; Hilton and Bateman, 2006; Taylor et al., 2006; Taylor and Taylor, 2009b; Rothwell and Stockey, 2016). This state of affairs owes largely to a combination of incomplete and differential preservation of the fossils, inconsistent terminology used for the structures of different taxa, and a lack of understanding for the underlying homologies of the various structures that make up the cupulate system. As detailed here, cupules and cupule‐bearing structures show a wide range of structural heterogeneity among the various groups of Mesozoic gymnosperms and almost certainly do not represent homologous organ systems across the known range of taxonomic groups (Figure 8–10, 30, Table 1). The hypotheses we have introduced here, following Harris (1976), provide a working framework to interpret new additions to the gymnosperm fossil record.
Several clades of cupulate Mesozoic gymnosperms have sparked interest as possible antecedent or sister clades to flowering plants (Harris, 1964; Doyle, 2006, 2014; Stockey and Rothwell, 2009; Taylor and Taylor, 2009a; Doyle and Endress, 2011). The apparent abrupt appearance of angiosperms in the fossil record (Herendeen et al., 2017; Bateman, 2020) and the persistent difficulty in ascertaining their phylogenetic context with respect to other spermatophytes has been called an “abominable mystery” (attributed to Darwin; e.g., Davies et al., 2004; but see Friedman, 2009; Buggs et al., 2021). It is becoming obvious that some of this mystery stems from a woefully incomplete sampling of outgroup taxa. Most extant gymnosperm species are relicts of clades that were more highly diverse throughout the Mesozoic. Until recently (i.e., Rothwell and Stockey, 2016; Herendeen et al., 2017), it was assumed that most major lineages of gymnospermous plants had been recognized in the fossil record. Their evolutionary relationships to one another might remain inscrutable, but few botanists seriously entertained the opinion that we might still be missing wide swathes of vascular plant diversity. The unprecedented organography exhibited by the Xadzigacalix quatsinoensis plant—which has a tetrahedral seed with complex integument within a resinous cupule, borne apically upon a woody stem—illustrates that we still stand to discover new clades of Mesozoic gymnosperms. Xadzigacalix likely constitutes a new order of seed plants, perhaps with affinities to gnetophytes or angiosperms. Given that we are still discovering novel gymnosperm clades from the geologic interval immediately antecedent to the rapid diversification of angiosperms, Xadzigacalix fossils also underscore that we continue to lack crucial fossil data necessary for understanding the evolutionary context of flowering plants.
AUTHOR CONTRIBUTIONS
This study was conceived by A.A.K., G.W.R., and R.A.S. Specimens were discovered and prepared by A.A.K. and photographed by G.W.R. and A.A.K.; cladistic analyses and character scoring were performed by G.W.R., A.A.K., and R.A.S.; 3D reconstructions, photographic plates, illustrations, and cladograms were created by A.A.K. The manuscript was written and revised by A.A.K., G.W.R., and R.A.S.
Supporting information
Appendix S1. Morphological matrix for cladistic analysis (NEXUS file format), including a tree block of 60 most‐parsimonious trees found under parsimony ratchet perturbation. Archived as Morphobank Project P4231 and also available as a Winclada‐executable file by request.
Appendix S2. Table of concordance explaining character revisions and character state changes made to the previous version of this matrix published by Rothwell and Stockey ( 2016; Morphobank Project 23745).
Appendix S3. Serial sections of apical/micropylar region and seed chalaza/cupule base of Xadzigacalix quatsinoensis in PowerPoint (.ppt) format.
ACKNOWLEDGMENTS
This research was supported in part by: Natural Sciences and Engineering Research Council of Canada (NSERC) grant A‐6908 to R.S.; National Science Foundation grant EF‐0629819 to G.R., Gene Mapes, and R.S.; and NSERC PGS‐D2‐438186‐2013 to A.K. We thank Thomas N. Taylor (deceased and Edith L. Taylor (University of Kansas) for access to 3D reconstruction software in 2015. We also gratefully acknowledge Patrick Herendeen, Fabiany Herrera, and Peter Crane (Chicago Botanic Garden, Field Museum) for enlightening discussions and access to data sets. As well, we thank Anne‐Laure Decombeix (CNRS ‐ UMR AMAP Montpellier) and Kathleen Pigg (Arizona State University) for entertaining our questions on characters and providing timely data. Comments from an anonymous reviewer enhanced the manuscript.
Klymiuk, A. A. , Rothwell G. W., and Stockey R. A.. 2022. A novel cupulate seed plant, Xadzigacalix quatsinoensis gen. et sp. nov., provides new insight into the Mesozoic radiation of gymnosperms. American Journal of Botany 109(6): 966–985. 10.1002/ajb2.1853
DATA AVAILABILITY STATEMENT
Our morphological matrix for seed plant phylogeny is available as Appendix S1 and archived as Morphobank Project P4231. Serial section “labelfield” image files for 3D reconstruction in Avizo/Amira are available via Figshare (https://doi.org/10.6084/m9.figshare.19597300, https://doi.org/10.6084/m9.figshare.19597453) or by request.
REFERENCES
- Anderson, J. M. , and Anderson H. M.. 2003. Heyday of the gymnosperms: systematics and biodiversity of the Late Triassic Molteno fructifications. Strelitzia 15: 1–398. [Google Scholar]
- Anderson, H. M. , Barbacka M. K., Bamford M. K., Holmes W. K., and Anderson J. M.. 2019. Umkomasia (megasporophyll): part 1 of a reassessment of Gondwana Triassic plant genera and a reclassification of some previously attributed. Alcheringa: An Australasian Journal of Palaeontology 43: 43–70. [Google Scholar]
- Atkinson, B. A. , Rothwell G. W., and Stockey R. A.. 2014a. Hughmillerites vancouverensis sp. nov. and the Cretaceous diversification of Cupressaceae. American Journal of Botany 101: 2136–2147. [DOI] [PubMed] [Google Scholar]
- Atkinson, B. A. , Rothwell G. W., and Stockey R. A.. 2014b. Hubbardiastrobus cunninghamioides gen. et sp. nov., evidence for a Lower Cretaceous diversification of cunninghamioid Cupressaceae. International Journal of Plant Sciences 175: 256–269. [Google Scholar]
- Bateman, R. M. 2020. Hunting the snark: the flawed search for mythical Jurassic angiosperms. Journal of Experimental Botany 71: 22–35. [DOI] [PubMed] [Google Scholar]
- Bierhorst, D. W. 1971. Morphology of vascular plants. Macmillan, NY, NY, USA. [Google Scholar]
- Bippus, A. C. , Stockey R. A., Rothwell G. W., and Tomescu A. M.. 2017. Extending the fossil record of Polytrichaceae: Early Cretaceous Meantoinea alophosioides gen. et sp. nov., permineralized gametophytes with gemma cups from Vancouver Island. American Journal of Botany 104: 584–597. [DOI] [PubMed] [Google Scholar]
- Blomenkemper, P. , Kerp H., Hamad A. A., and Bomfleur B.. 2020. Contributions towards whole‐plant reconstructions of Dicroidium plants (Umkomasiaceae) from the Permian of Jordan. Review of Palaeobotany and Palynology 13: 104210. [Google Scholar]
- Blomenkemper, P. , Kerp H., Hamad A. A., DiMichele W. A., and Bomfleur B.. 2018. A hidden cradle of plant evolution in Permian tropical lowlands. Science 362: 1414–1416. [DOI] [PubMed] [Google Scholar]
- Bomfleur, B. , Decombeix A. L., Schwendemann A. B., Escapa I. H., Taylor E. L., Taylor T. N., and McLoughlin S.. 2014. Habit and ecology of the Petriellales, an unusual group of seed plants from the Triassic of Gondwana. International Journal of Plant Sciences 175: 1062–1075. [Google Scholar]
- Bomfleur, B ., Taylor E. L., Taylor T. N., Serbet R., Krings M., and Kerp H. 2011. Systematics and paleoecology of a new peltaspermalean seed fern from the Triassic polar vegetation of Gondwana. International Journal of Plant Sciences 172: 807–835. [Google Scholar]
- Bronson, A. W. , Klymiuk A. A., Stockey R. A., and Tomescu A. M.. 2013. A perithecial sordariomycete (Ascomycota, Diaporthales) from the lower cretaceous of Vancouver Island, British Columbia, Canada. International Journal of Plant Sciences 174: 278–292. [Google Scholar]
- Buggs, R. J. 2021. The origin of Darwin's “abominable mystery”. American Journal of Botany 108: 22–36. [DOI] [PubMed] [Google Scholar]
- Carlquist, S. 1996. Wood, bark, and stem anatomy of Gnetales: a summary. International Journal of Plant Sciences 157: S58–76. [Google Scholar]
- Chamberlain, C. J. 1935. Gymnosperms. Structure and evolution. University of Chicago Press, Chicago, IL, USA. [Google Scholar]
- Clark, J. W. , and Donoghue P. C.. 2017. Constraining the timing of whole genome duplication in plant evolutionary history. Proceedings of the Royal Society, B, Biological Sciences 284: 20170912. [DOI] [PMC free article] [PubMed]
- Coiro, M. , Chomicki G., and Doyle J. A.. 2018. Experimental signal dissection and method sensitivity analyses reaffirm the potential of fossils and morphology in the resolution of the relationship of angiosperms and Gnetales. Paleobiology 44: 1–21. [Google Scholar]
- Coiro, M. , Doyle J. A., and Hilton J.. 2019. How deep is the conflict between molecular and fossil evidence on the origin of angiosperms. New Phytologist 223: 83–99. [DOI] [PubMed] [Google Scholar]
- Condamine, F. L. , Silvestro D., Koppelhus E. B., and Antonelli A.. 2018. The rise of angiosperms pushed conifers to decline during global cooling. Proceedings of the National Academy of Sciences, USA 117: 28867–28875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane, P. R. 1987. Vegetational consequences of angiosperm diversification. In Friis E. M., Chaloner W. G., and Crane P. R. [eds.], The origins of angiosperms and their biological consequences, 107–144, Cambridge University Press, Cambridge, UK. [Google Scholar]
- Crane, P. R. , and Lidgard S.. 1989. Angiosperm diversification and paleolatitudinal gradients in Cretaceous floristic diversity. Science 246: 675–678. [DOI] [PubMed] [Google Scholar]
- Davies, T. J. , Barraclough T. G., Chase M. W., Soltis P. S., Soltis D. E., and Savolainen V.. 2004. Darwin's abominable mystery: insights from a supertree of the angiosperms. Proceedings of the National Academy of Sciences, USA 101: 1904–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilcher, D. L. 1979. Early angiosperm reproduction: an introductory report. Review of Palaeobotany and Palynology 27: 291–328. [Google Scholar]
- Dong, C. , Zhou Z., Zhang B., Wang Y., and Shi G.. 2019. Umaltolepis and associated Pseudotorellia leaves from the Middle Jurassic of Yima in Henan Province, Central China. Review of Palaeobotany and Palynology 271: 104111. [Google Scholar]
- Donoghue, J. D. , Doyle J. A., Gautier J., Kluge A. G., and Rowe T.. 1989. The importance of fossils in phylogeny reconstruction. Annual Review of Ecology and Systematics 20: 431–460. [Google Scholar]
- Doyle, J. A. 2006. Seed ferns and the origin of angiosperms. Journal of the Torrey Botanical Society 133: 169–209. [Google Scholar]
- Doyle, J. A. 2008. Integrating molecular phylogenetic and paleobotanical evidence on origin of the flower. International Journal of Plant Sciences 169: 816–843. [Google Scholar]
- Doyle, J. A. 2014. Recognising angiosperm clades in the Early Cretaceous fossil record. Historical Biology 27: 414–429. [Google Scholar]
- Doyle, J. A. , and Endress P. K.. 2011. Tracing the early evolutionary diversification of the angiosperm flower. In Wanntorp L. and Ronse de Craene L. P. [eds.], Flowers on the tree of life, 88–119, Cambridge University Press, Cambridge, UK. [Google Scholar]
- Eames, A. J. 1952. Relationships of the Ephedrales. Phytomorphology 2: 79–100. [Google Scholar]
- Falcon‐Lang, H. J. , Mages V., and Collinson M.. 2016. The oldest Pinus and its preservation by fire. Geology 44: 303–306. [Google Scholar]
- Farjon, A. 2005. Monograph of Cupressaceae and Sciadopitys. Royal Botanic Gardens, Kew, UK. [Google Scholar]
- Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Florin, R. 1950. Upper Carboniferous and lower Permian conifers. Botanical Review 16: 258–282. [Google Scholar]
- Florin, R. 1951. Evolution in cordaites and conifers. Acta Horti Bergiani 15: 286–388. [Google Scholar]
- Friedman, W. E. 2009. The meaning of Darwin's “abominable mystery”. American Journal of Botany 96: 5–21. [DOI] [PubMed] [Google Scholar]
- Friis, E. M. , Crane P. R., and Pedersen K. R.. 2011. Early flowers and angiosperm evolution. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Friis, E. M. , Crane P. R., and Pedersen K. R.. 2019a. Chlamydospermous seeds document the diversity and abundance of extinct gnetalean relatives in Early Cretaceous vegetation. International Journal of Plant Sciences 180: 643–666. [Google Scholar]
- Friis, E. M. , Crane P. R., and Pedersen K. R.. 2019b. Geminispermum, an Early Cretaceous (early–middle Albian) cupulate unit from the angiosperm‐dominated Puddledock flora of eastern North America. Acta Palaeobotanica 59: 229–239. [Google Scholar]
- Friis, E. M. , Crane P. R., Pedersen K. R., Bengtson S., Donoghue P. C., Grimm G. W., and Stampanoni M.. 2007. Phase‐contrast X‐ray microtomography links Cretaceous seeds with Gnetales and Bennettitales. Nature 450: 549–552. [DOI] [PubMed] [Google Scholar]
- Friis, E. M. , Pedersen K. R., and Crane P. R.. 2009. Early Cretaceous mesofossils from Portugal and eastern North America related to the Bennettitales‐Erdtmanithecales‐Gnetales group. American Journal of Botany 96: 252–283. [DOI] [PubMed] [Google Scholar]
- Friis, E. M. , Pedersen K. R., and Crane P. R.. 2013. New diversity among chlamydospermous seeds from the Early Cretaceous of Portugal and North America. International Journal of Plant Sciences 174: 530–558. [Google Scholar]
- Gauthier, J. , Kluge A. G., and Rowe T.. 1988. Amniote phylogeny and the importance of fossils. Cladistics 4: 105–209. [DOI] [PubMed] [Google Scholar]
- Gierlowski‐Kordesch, E. H. , Rothwell G. W., Stockey R. A., and Finkelstein D. B.. 2021. Submarine groundwater discharge as a catalyst for eodiagenetic carbonate cements within marine sedimentary basins. In Rosen M. R., Park‐Boush L., Finkelstein D. B., and Pueyo S.P. [eds.], Limnogeology: progress, challenges and opportunities: a tribute to Elizabeth Gierlowski‐Kordesch, 445–470, Springer Nature, Cham, Switzerland. [Google Scholar]
- Goloboff, P. A. , Farris J. S., and Nixon K. C.. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24: 774–786. [Google Scholar]
- Goloboff, P. A ., Torres A., and Arias J. S.. 2018. Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics 34: 407–437. [DOI] [PubMed] [Google Scholar]
- Haggart, J. W. 1994. New results in Jura‐Cretaceous stratigraphy, northern Vancouver Island, British Columbia. Geological Survey of Canada Current Research 994: 59–66. [Google Scholar]
- Hamad A. A., Blomenkemper P., Kerp H., and Bomfleur B.. 2017. Dicroidium bandeliisp. nov.(corystospermalean foliage) from the Permian of Jordan. PalZ 91: 641–648. [Google Scholar]
- Hammack, J. L. , Nixon G. T., Koyan V., Payie G. J., Panteleyev A., Massey N. W., Hamilton J. V., and Haggard J. W.. 1994. Preliminary geology of the Quatsino‐Port McNeill Area, northern Vancouver Island. BC Ministry of Energy, Mines, and Petroleum Resources, Open File 1994: 15. [Google Scholar]
- Harris, T. M . 1940. Caytonia. Annals of Botany 4: 713–734. [Google Scholar]
- Harris, T. M. 1951. The relationships of the Caytoniales. Phytomorphology 1: 29–39. [Google Scholar]
- Harris, T. M. , 1964. The Yorkshire Jurassic flora, II. Caytoniales, Cycadales and pteridosperms. British Museum (Natural History), London, UK. [Google Scholar]
- Harris, T. M. 1976. The mesozoic gymnosperms. Review of Palaeobotany and Palynology 21: 119–134. [Google Scholar]
- Herendeen, P. S. , Friis E. M., Pedersen K. R., and Crane P. R., 2017. Paleobotanical redux: revisiting the age of the angiosperms. Nature Plants 3: 1705. [DOI] [PubMed] [Google Scholar]
- Hernandez‐Castillo, G. R. , Stockey R. A., and Rothwell G. W.. 2006. Anemia quatsinoensis sp. nov. (Schizaeaceae), a permineralized fern from the Lower Cretaceous of Vancouver Island. International Journal of Plant Sciences 167: 665–674. [Google Scholar]
- Herrera, F. , Leslie A. B., Shi G., Knopf P., Ichinnorov N., Takahashi M., Crane P. R., and Herendeen P. S.. 2016. New fossil Pinaceae from the Early Cretaceous of Mongolia. Botany 94: 885–915. [Google Scholar]
- Herrera, F. , Shi G., Ichinnorov N., Takahashi M., Bugdaeva E. V., Herendeen P. S., and Crane P. R.. 2017. The presumed ginkgophyte Umaltolepis has seed‐bearing structures resembling those of Peltaspermales and Umkomasiales. Proceedings of the National Academy of Sciences, USA 114: E2385–E2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton, J. , and Bateman R.. 2006. Pteridosperms are the backbone of seed‐plant phylogeny. Journal of the Torrey Botanical Society 133: 119–168. [Google Scholar]
- Holmes, W. B. K. 1987. New corystosperm ovulate fructifications from the Middle Triassic of eastern Australia. Alcheringa 11: 165–173. [Google Scholar]
- Holmes, W. B. K. , and Ash S. R.. 1979. An early Triassic megafossil flora from the Lorne Basin, New South Wales. Proceedings of the Linnean Society of New South Wales 103: 47–70. [Google Scholar]
- Ickert‐Bond, S. M. , and Renner S. S.. 2016. The Gnetales: recent insights on their morphology, reproductive biology, chromosome numbers, biogeography, and divergence times. Journal of Systematics and Evolution 54: 1–16. [Google Scholar]
- Joy, K. W. 1956. A rapid cellulose acetate peel method in palaeobotany. Annals of Botany 20: 635–637. [Google Scholar]
- Jeletzky, J. A. 1976. Mesozoic and Tertiary rocks of Quatsino Sound, Vancouver Island, British Columbia. Geological Survey of Canada Bulletin 242: 1–243. [Google Scholar]
- Jud, N. A. , Rothwell G. W., and Stockey R. A.. 2008. Todea from the Lower Cretaceous of western North America: implications for the phylogeny, systematics, and evolution of modern Osmundaceae. American Journal of Botany 95: 330–339. [DOI] [PubMed] [Google Scholar]
- Kerp, H. J. 1988. Aspects of Permian palaeobotany and palynology. X. The west and central European species of the genus Autunia Krasser emend. Kerp (Peltaspermaceae) and the form‐genus Rhachiphyllum Kerp (callipterid foliage). Review of Palaeobotany and Palynology 54: 249–360. [Google Scholar]
- Kerp, H. , Broutin J., Lausberg S., and Aassoumi H.. 2001. Discovery of latest Carboniferous–Early Permian radially symmetrical peltaspermaceous megasporophylls from Europe and North Africa. Comptes Rendus de l'Académie des Sciences‐Series IIA‐Earth and Planetary Science 332: 513–519. [Google Scholar]
- Klavins, S. D. , Taylor T. N., and Taylor E. L.. 2002. Anatomy of Umkomasia (corystospermales) from the Triassic of Antarctica. American Journal of Botany 89: 664–676. [DOI] [PubMed] [Google Scholar]
- Klymiuk, A. A. , and Stockey R. A.. 2012. A Lower Cretaceous (Valanginian) seed cone provides the earliest fossil record for Picea (Pinaceae). American Journal of Botany 99: 1069–1082. [DOI] [PubMed] [Google Scholar]
- Leebens‐Mack, J. H. , Barker M. S., Carpenter E. J., Deyholos M. K., Gitzendanner M. A., Graham S. W., Grosse I., et al. 2019. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie, A. B. , Beaulieu J., Holman G., Campbell C. S., Mei W., Raubeson L. R., and Mathews S.. 2018. An overview of extant conifer evolution from the perspective of the fossil record. American Journal of Botany 105: 1–14. [DOI] [PubMed] [Google Scholar]
- Little, S. A. , Stockey R. A., and Rothwell G. W.. 2006. Solenostelopteris skogiae sp. nov. from the Lower Cretaceous of Vancouver Island. Journal of Plant Research 119: 525–532. [DOI] [PubMed] [Google Scholar]
- Lovis, J. D. 1977. Evolutionary patterns and processes in ferns. In Preston R. D. and Woolhouse H. W. [eds.], Advances in botanical research, 4, 229–440. Academic Press, London, UK. [Google Scholar]
- MacEachern, J. A. , Bann K. L., Bhattacharya J. P., and C. D. Howell, Jr. 2005. Ichnology of deltas: organism responses to the dynamic interplay of rivers, waves, storms, and tides. In Bhattacharya B. P. and Giosan L. [eds.], SEPM Special Publication 83, River deltas: concepts, models, and examples, 1–37. Society for Sedimentary Geology, Tulsa, OK, USA. [Google Scholar]
- Matsunaga, K. K. , Stockey R. A., and Tomescu A. M.. 2013. Honeggeriella complexa gen. et sp. nov., a heteromerous lichen from the Lower Cretaceous of Vancouver Island (British Columbia, Canada). American Journal of Botany 100: 450–459. [DOI] [PubMed] [Google Scholar]
- Mendes, M. M. , Dinis J. L., Balbino A. C., and Pais J.. 2012. Bennettitales, Erdtmanithecales e Gnetales do Cretácico Inferior da Bacia Lusitânica (litoral Centro‐Oeste de Portugal): síntese e enquadramento estratigráfico. Comunicaçõe Geológicas 99: 11–18. [Google Scholar]
- Mendes, M. M. , Friis E. M., and Pais J.. 2008. Erdtmanispermum juncalense sp. nov., a new species of the extinct order Erdtmanithecales from the Early Cretaceous (probably Berriasian) of Portugal. Review of Palaeobotany and Palynology 149: 50–56. [Google Scholar]
- Mendes, M. M. , Pais J., Pedersen K. R., and Friis E. M.. 2010. Erdtmanitheca portucalensis, a new pollen organ from the Early Cretaceous (Aptian–Albian) of Portugal with Eucommiidites‐type pollen. Grana 49: 26–36. [Google Scholar]
- Mendes, M. M. , Pedersen K. R., and Friis E. M.. 2020. Battenispermum hirsutum gen. et sp. nov., a new Early Cretaceous seed from Portugal with chlamydospermous organisation. Cretaceous Research 109: 104376. [Google Scholar]
- Miller, C. N., Jr. 1976. Early evolution in the Pinaceae. Review of Palaeobotany and Palynology 21: 101–117. [Google Scholar]
- Muller, J. E. , Carlisle D., and Northcote K. E.. 1974. Geology and mineral deposits of Alert Bay‐Cape Scott Map‐area (92 L‐102 I), Vancouver Island, British Columbia. Geological Survey of Canada Paper 74, Natural Resources Canada/Ressources Naturelles Canada, Ottawa, ON, Canada.
- Nixon, K. C. 1999. The parsimony ratchet, a new method for rapid parsimony analysis. Cladistics 15: 407–414. [DOI] [PubMed] [Google Scholar]
- Nixon, K. C. , Crepet W. L., Stevenson D., and Friis E. M.. 1994. A reevaluation of seed plant phylogeny. Annals of the Missouri Botanical Garden 81: 484–533. [Google Scholar]
- Nixon, G. T. , Hammack J. L., Koyanagi V. M., Snyder L. D., Payie G. J., Panteleyev A., Massey N. W. D., et al. 2011. Geology, geochronolgy, lithogeochemistry and metamorphism of the Holberg‐Winter Harbour Area, northern Vancouver Island (parts of 92L/5,12,13; 102I/8,9,16). BC Geological Survey Parts of NTS 092L/05, 12, 13; 102I/08, 09 & 16 GEOSCIENCE MAP 2011‐1, BC Geological Survey, Victoria, BC, Canada.
- Nosova, N. 2020. Female reproductive structures of Umaltolepis Krassilov and associated short shoots, buds and leaves of Pseudotorellia Florin from the Middle Jurassic of Angren, Uzbekistan. Review of Palaeobotany and Palynology 20: 104266. [Google Scholar]
- Pattemore, G. A. 2016. The structure of umkomasiacean fructifications from the Triassic of Queensland. Acta Palaeobotanica 56: 17–40. [Google Scholar]
- Pemberton, S. G. , MacEachern J. A., Gingras M. K., and Saunders T. D.. 2008. Biogenic chaos: cryptobioturbation and the work of sedimentologically friendly organisms. Palaeogeography, Palaeoclimatology, Palaeoecology 270: 273–279. [Google Scholar]
- Poort, R. J. , and Kerp H. F.. 1990. Aspects of Permian palaeobotany and palynology. XI. On the recognition of true peltasperms in the Upper Permian of western and central Europe and reclassification of species formerly included. Review of Palaeobotany and Palynology 63: 197–225. [Google Scholar]
- Ran, J. H. , Shen T. T., Wang M. M., and Wang X. Q.. 2018a. Phylogenomics resolves the deep phylogeny of seed plants and indicates partial convergent or homoplastic evolution between Gnetales and angiosperms. Proceedings of the Royal Society, B, Biological Sciences 285: 20181012. [DOI] [PMC free article] [PubMed]
- Ran, J. H. , Shen T. T., Wu H., Gong X., and Wang XQ. X. Q. 2018b. Phylogeny and evolutionary history of Pinaceae updated by transcriptomic analysis. Molecular Phylogenetics and Evolution 129: 106–116. [DOI] [PubMed] [Google Scholar]
- Ray, M. M. , Rothwell G. W., and Stockey R. A.. 2014. Anatomically preserved Early Cretaceous bennettitalean leaves: Nilssoniopteris corrugata n. sp. from Vancouver Island, Canada. Journal of Paleontology 88: 1085–1093. [Google Scholar]
- Reymanowna, M. A. 1973. The Jurassic flora from Grojec near Kraków in Poland. Part II. Caytoniales and anatomy of Caytonia . Acta Palaeobotanica 14: 45–87. [Google Scholar]
- Roberts, E. A. , Seyfullah L. J., Loveridge R. F., Garside P., and Martill D. M.. 2020. Cretaceous gnetalean yields first preserved plant gum. Scientific Reports 10: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin, R. J. , and Kapil R. N.. 1969. Comparative anatomy of the seed coats of Gnetum and their probable evolution. American Journal of Botany 56: 420–431. [Google Scholar]
- Rothwell, G. W. 1976. Primary vasculature and gymnosperm systematics. Review of Palaeobotany and Palynology 22: 193–206. [Google Scholar]
- Rothwell, G. W. 1987. Complex Paleozoic filicaleds in the evolutionary radiation of ferns. American Journal of Botany 74: 458–461. [Google Scholar]
- Rothwell, G. W ., and Nixon K. C.. 2006. How does the inclusion of fossil data change our conclusions about the phylogenetic history of euphyllophytes? International Journal of Plant Sciences 167: 737–749. [Google Scholar]
- Rothwell, G. W. , and Serbet R.. 1994. Lignophyte phylogeny and the evolution of spermatophytes: a numerical cladistic analysis. Systematic Botany 19: 443–482. [Google Scholar]
- Rothwell, G. W. , and Stockey R. A.. 2002. Anatomically preserved Cycadeoidea (Cycadeoidaceae), with a reevaluation of the systematic characters for the seed cones of Bennettitales. American Journal of Botany 89: 1447–1458. [DOI] [PubMed] [Google Scholar]
- Rothwell, G. W. , and Stockey R. A.. 2006. Combining characters of Pteridaceae and tree ferns: Pterisorus radiata gen. et sp. nov., a permineralized Lower Cretaceous filicalean with radial sori. International Journal of Plant Sciences 167: 695–701. [Google Scholar]
- Rothwell, G. W. , and Stockey R. A.. 2016. Phylogenetic diversification of Early Cretaceous seed plants: the compound seed cone of Doylea tetrahedrasperma . American Journal of Botany 103: 923–937. [DOI] [PubMed] [Google Scholar]
- Rothwell, G. W. , and Stockey R. A.. 2010. Independent evolution of seed enclosure in the Bennettitales: Evidence from the anatomically preserved cone Foxeoidea connatum gen. et sp. nov. In Gee C. L. [ed.], Plants in Mesozoic Time: morphological innovations, phylogeny, ecosystems, 51–64. Indiana University Press, Bloomington, IN, USA. [Google Scholar]
- Rothwell, G. W. , and Stockey R. A.. 2013. Evolution and phylogeny of gnetophytes: evidence from the anatomically preserved seed cone Protoephedrites eamesii gen. et sp. nov. and the seeds of several bennettitalean species. International Journal of Plant Sciences 174: 511–529. [Google Scholar]
- Rothwell, G. W. , Crepet W. L., and Stockey R. A.. 2009. Is the anthophyte hypothesis alive and well? New evidence from the reproductive structures of Bennettitales. American Journal of Botany 96: 296–322. [DOI] [PubMed] [Google Scholar]
- Rothwell, G. W. , Escapa I. H., and Tomescu A. M.. 2018. Tree of death: the role of fossils in resolving the overall pattern of plant phylogeny. American Journal of Botany 105: 1239–1242. [DOI] [PubMed] [Google Scholar]
- Rothwell, G. W. , Stockey R. A., Mapes G., and Hilton J.. 2011. Structure and relationships of the Jurassic conifer seed cone Hughmillerites juddii gen. et comb. nov.: implications for the origin and evolution of Cupressaceae. Review of Palaeobotany and Palynology 164: 45–59. [Google Scholar]
- Rothwell, G. W. , Stockey R. A., and Smith S. Y.. 2020. Revisiting the Late Cretaceous Parataxodium wigginsii flora from the North Slope of Alaska, a high‐latitude temperate forest. Cretaceous Research 116: 104592. [Google Scholar]
- Ryberg, P. E. , Rothwell G. W., Stockey R. A., Hilton J., Mapes G., and Riding J. B.. 2012. Reconsidering relationships among stem and crown group Pinaceae: oldest record of the genus Pinus from the Early Cretaceous of Yorkshire, United Kingdom. International Journal of Plant Sciences 173: 917–932. [Google Scholar]
- Rydin, C. , and Friis E. M.. 2010. A new Early Cretaceous relative of Gnetales: Siphonospermum simplex gen. et sp. nov. from the Yixian Formation of Northeast China. BMC Evolutionary Biology 10: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, H. , Rothwell G. W., and Stockey R. A.. 2004. Diversity of Cretaceous conifers: a species with pollen cones that bear adaxial pollen sacs. Botany 2004: Alpine diversity: adapted to the peaks, Snowbird, UT, USA. Abstracts 344: 89.
- Savoretti, A. , Bippus A. C., Stockey R. A., Rothwell G. W., and Tomescu A. M.. 2018. Grimmiaceae in the Early Cretaceous: Tricarinella crassiphylla gen. et sp. nov. and the value of anatomically preserved bryophytes. Annals of Botany 121: 1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton, G. W. , Stockey R. A., Rothwell G. W., and Tomescu A. M.. 2015. Exploring the fossil history of pleurocarpous mosses: Tricostaceae fam. nov. from the Cretaceous of Vancouver Island, Canada. American Journal of Botany 102: 1883–1900. [DOI] [PubMed] [Google Scholar]
- Shelton, G. W. , Stockey R. A., Rothwell G. W., and Tomescu A. M.. 2016. Krassiloviella limbelloides gen. et sp. nov.: additional diversity in the hypnanaean moss family Tricostaceae (Valanginian, Vancouver Island, British Columbia). International Journal of Plant Sciences 177: 792–808. [Google Scholar]
- Shi, G. , Crane P. R., Herendeen P. S., Ichinnorov N., Takahashi M., and Herrera F.. 2019. Diversity and homologies of corystosperm seed‐bearing structures from the Early Cretaceous of Mongolia. Journal of Systematic Palaeontology 17: 997–1029. [Google Scholar]
- Shi, G. , Herrera F., Herendeen P. S., Clark E. G., and Crane P. R.. 2021. Mesozoic cupules and the origin of the angiosperm second integument. Nature 594: 223–226. [DOI] [PubMed] [Google Scholar]
- Shi, G. , Leslie A. B., Herendeen P. S., Herrera F., Ichinnorov N., Takahashi M., Knopf P., and Crane P. R.. 2016. Early Cretaceous Umkomasia from Mongolia: implications for homology of corystosperm cupules. New Phytologist 210: 1418–1429. [DOI] [PubMed] [Google Scholar]
- Shuqin, Z. , Axsmith B. J., Fraser N. C., Fengxiang L., and Dehe X.. 2008. New evidence for Laurasian corystosperms: Umkomasia from the Upper Triassic of Northern China. Review of Palaeobotany and Palynology 149: 202–207. [Google Scholar]
- Smith, S. Y. , Currah R. S., and Stockey R. A.. 2004. Cretaceous and Eocene poroid hymenophores from Vancouver Island, British Columbia. Mycologia 96: 180–186. [PubMed] [Google Scholar]
- Smith, S. Y. , Rothwell G. W., and Stockey R. A.. 2003. Cyathea cranhamii sp. nov. (Cyatheaceae), anatomically preserved tree fern sori from the Lower Cretaceous of Vancouver Island, British Columbia. American Journal of Botany 90: 755–760. [DOI] [PubMed] [Google Scholar]
- Smith, S. Y. , Stockey R. A., Rothwell G. W., and Little S. A.. 2017. A new species of Pityostrobus (Pinaceae) from the Cretaceous of California: moving towards understanding the Cretaceous radiation of Pinaceae. Journal of Systematic Palaeontology 15: 69–81. [Google Scholar]
- Sporne, K. R. 1965. The morphology of gymnosperms. Hutchinson University Library, London, UK. [Google Scholar]
- Stanich, N. A. , Rothwell G. W., and Stockey R. A.. 2009. Phylogenetic diversification of Equisetum (Equisetales) as inferred from lower cretaceous species of British Columbia, Canada. American Journal of Botany 96: 1289–1299. [DOI] [PubMed] [Google Scholar]
- Steenbock, C. M. , Stockey R. A., Beard G., and Tomescu A. M.. 2011. A new family of leafy liverworts from the middle Eocene of Vancouver Island, British Columbia, Canada. American Journal of Botany 98: 998–1006. [DOI] [PubMed] [Google Scholar]
- Stockey, R. A. , and Wiebe N. J. P.. 2006. Mosaic evolution of Early Cretaceous Pinaceae and the origins of extant genera. Botany 2006: 569. [Google Scholar]
- Stockey, R. A. , and Wiebe N. J.. 2008. Lower Cretaceous conifers from Apple Bay, Vancouver Island: Picea‐like leaves, Midoriphyllum piceoides gen. et sp. nov. (Pinaceae). Botany 86: 649–657. [Google Scholar]
- Stockey, R. A. , and Rothwell G. W.. 2003. Anatomically preserved Williamsonia (Williamsoniaceae): evidence for bennettitalean reproduction in the Late Cretaceous of western North America. International Journal of Plant Sciences 164: 251–262. [Google Scholar]
- Stockey, R. A. , and Rothwell G. W.. 2006. The last of the pre‐angiospermous vegetation: a Lower Cretaceous flora from Apple Bay, Vancouver Island. In Proceedings of Advances in Paleobotany Conference: recognizing the contributions of David L. Dilcher and Jack A. Wolfe on the occasion of their 70th birthday, contribution no. 45, 2006, Gainesville, FL, USA.
- Stockey, R. A. , and Rothwell G. W.. 2009. Distinguishing angiophytes from the earliest angiosperms: a Lower Cretaceous (Valanginian‐Hauterivian) fruit‐like reproductive structure. American Journal of Botany 96: 323–335. [DOI] [PubMed] [Google Scholar]
- Stockey, R. A. , Rothwell G. W., and Little S. A.. 2006. Relationships among fossil and living Dipteridaceae: anatomically preserved Hausmannia from the Lower Cretaceous of Vancouver Island. International Journal of Plant Sciences 167: 649–663. [Google Scholar]
- Stockey, R. A. , Wiebe N. J., Atkinson B. A., and Rothwell G. W.. 2018. Cupressaceous pollen cones from the Early Cretaceous of Vancouver Island, British Columbia: Morinostrobus holbergensis gen. et sp. nov. International Journal of Plant Sciences 179: 402–414. [Google Scholar]
- Sweet, A. R. 2000. Applied research report on two samples of Cretaceous age from Vancouver Island, British Columbia as requested by J. Haggart (GSC Pacific, Vancouver). Geological Survey of Canada Paleontological Report ARS‐2000‐02. Geological Survey of Canada, Calgary, AB, Canada.
- Swofford, D. L . 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.0a169. Sinauer Associates, Sunderland, Massachusetts.
- Takaso T., and Bouman F.. 1986. Ovule and seed ontogeny in Gnetum gnemon L. Botanical Magazine (Shokubutsu‐gaku‐zasshi) 99: 241–266. [Google Scholar]
- Taylor, E. L. , and Taylor T. N.. 2009a. Seed ferns from the late Paleozoic and Mesozoic: Any angiosperm ancestors lurking there? American Journal of Botany 96: 237–251. [DOI] [PubMed] [Google Scholar]
- Taylor, E. L. , Taylor T. N., Kerp H., and Hermsen E. J.. 2006. Mesozoic seed ferns: old paradigms, new discoveries. Journal of the Torrey Botanical Society 133: 62–82. [Google Scholar]
- Taylor, T. N. , Fueyo G. M., and Taylor E. L.. 1994. Permineralized seed fern cupules from the Triassic of Antarctica: implications for cupule and carpel evolution. American Journal of Botany 81: 666–677. [Google Scholar]
- Taylor, T. N. , Taylor E. L., and Krings M.. 2009b. Paleobotany: the biology and evolution of fossil plants. Academic Press, Amsterdam, Netherlands. [Google Scholar]
- Thomas, H. H. 1925. VI. The Caytoniales, a new group of angiospermous plants from the Jurassic Rocks of Yorkshire. Philosophical Transactions of the Royal Society of London, B, Containing Papers of a Biological Character 213: 299–363. [Google Scholar]
- Thomas, H. H. 1933. VII. On some pteridospermous plants from the Mesozoic rocks of South Africa. Philosophical Transactions of the Royal Society of London, B, Containing Papers of a Biological Character 222: 193–265. [Google Scholar]
- Thompson, W. P. 1916. The morphology and affinities of Gnetum. American Journal of Botany 1: 135–184. [Google Scholar]
- Tomescu, A. M. 2016. The Early Cretaceous Apple Bay flora of Vancouver Island: a hotspot of fossil bryophyte diversity. Botany 94: 683–695. [Google Scholar]
- Tomlinson, P. B. , and Fisher J. B.. 2005. Development of nonlignified fibers in leaves of Gnetum gnemon (Gnetales). American Journal of Botany. 92: 383–389. [DOI] [PubMed] [Google Scholar]
- Vavrek, M. J. , Stockey R. A., and Rothwell G. W.. 2006. Osmunda vancouverensis sp. nov. (Osmundaceae), permineralized fertile frond segments from the Lower Cretaceous of British Columbia, Canada. International Journal of Plant Sciences 167: 631–637. [Google Scholar]
- Wan, T. , Liu Z. M., Li L. F., Leitch A. R., Leitch I. J., Lohaus R., Liu Z. J., et al. 2018. A genome for gnetophytes and early evolution of seed plants. Nature Plants 4: 82–89. [DOI] [PubMed] [Google Scholar]
- Wing, S. L. , Herrera F., Jaramillo C. A., Gómez‐Navarro C., Wilf P., and Labandeira C. C.. 2009. Late Paleocene fossils from the Cerrejón Formation, Colombia, are the earliest record of Neotropical rainforest. Proceedings of the National Academy of Sciences, USA 106: 18627–18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing, S. L. , Hickey L. J., and Swisher C. C.. 1993. Implications of an exceptional fossil flora for Late Cretaceous vegetation. Nature 363: 342–344. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Morphological matrix for cladistic analysis (NEXUS file format), including a tree block of 60 most‐parsimonious trees found under parsimony ratchet perturbation. Archived as Morphobank Project P4231 and also available as a Winclada‐executable file by request.
Appendix S2. Table of concordance explaining character revisions and character state changes made to the previous version of this matrix published by Rothwell and Stockey ( 2016; Morphobank Project 23745).
Appendix S3. Serial sections of apical/micropylar region and seed chalaza/cupule base of Xadzigacalix quatsinoensis in PowerPoint (.ppt) format.
Data Availability Statement
Our morphological matrix for seed plant phylogeny is available as Appendix S1 and archived as Morphobank Project P4231. Serial section “labelfield” image files for 3D reconstruction in Avizo/Amira are available via Figshare (https://doi.org/10.6084/m9.figshare.19597300, https://doi.org/10.6084/m9.figshare.19597453) or by request.


