Abstract
Key points
The right ventricle of the mammal heart is highly sensitive to the afterload imposed by a combination of the pulmonary circulation and the retrograde contribution of the left heart.
Right ventricular afterload can be analysed in terms of pulmonary artery input impedance, which we were able to decompose as the result of the harmonic frequency responses of the pulmonary vessels and the left heart attached in series.
Using spectral methods, we found a natural matching between the pulmonary vasculature and the left chambers of the heart. This coupling implies that the upstream transmission of the left heart frequency‐response has favourable effects on the pulmonary tree.
This physiological mechanism protects the right ventricle against acute changes in preload, and its impairment may be a relevant contribution to right ventricle dysfunction in pulmonary hypertension.
Abstract
The right ventricle (RV) of the mammal heart is highly sensitive to the afterload imposed by the pulmonary circulation, and the left heart (LH) retrogradely contributes significantly to this vascular load. Transmission‐line theory anticipates that the degree of matching between the frequency responses of the pulmonary vasculature and the LH should modulate the global right haemodynamic burden. We measured simultaneous high‐fidelity flow (pulmonary artery) and pressure (pulmonary artery and left atrium) in 18 healthy minipigs under acute haemodynamic interventions. From these data, we decomposed the impedance spectra of the total right‐circulation system into the impedance of the pulmonary vessels and the harmonic response of the LH. For frequencies above the first harmonic, total impedance was below the pulmonary impedance during all phases (P < 0.001; pooled phases), demonstrating a favourable effect of the LH harmonic response on RV pulsatile load: the LH harmonic response was responsible for a 20% reduction of pulse pulmonary artery pressure (P < 0.001 vs. a theoretical purely‐resistive response) and a 15% increase of pulmonary compliance (P = 0.009). This effect on compliance was highest during acute volume overload. In the normal right circulation, the longitudinal impedance of the pulmonary vasculature is matched to the harmonic response of the LH in a way that efficiently reduces the pulmonary pulsatile vascular load. This source of interaction between the right and left circulations of mammals protects the RV against excessive afterload during acute volume transients and its disruption may be an important contributor to pulmonary hypertension.
Keywords: pulmonary circulation, hemodynamics, impedance, vascular function
Key points
The right ventricle of the mammal heart is highly sensitive to the afterload imposed by a combination of the pulmonary circulation and the retrograde contribution of the left heart.
Right ventricular afterload can be analysed in terms of pulmonary artery input impedance, which we were able to decompose as the result of the harmonic frequency responses of the pulmonary vessels and the left heart attached in series.
Using spectral methods, we found a natural matching between the pulmonary vasculature and the left chambers of the heart. This coupling implies that the upstream transmission of the left heart frequency‐response has favourable effects on the pulmonary tree.
This physiological mechanism protects the right ventricle against acute changes in preload, and its impairment may be a relevant contribution to right ventricle dysfunction in pulmonary hypertension.
Introduction
The right and left circulations of the mammal circulation are attached in series. Retrograde transmission of left heart (LH) pressure significantly impacts the right circulation because of the lack of valves in the pulmonary veins and the low pressure of the pulmonary circuit. Importantly, exaggerated pressure transmission is a major source of cardiovascular disease. Associated with important mortality and disability, pulmonary hypertension (PH) has a prevalence of ∼1% in the global population and up to 10% in subjects aged older than 65 years (Hoeper et al. 2016). LH disease is responsible for almost one‐half of the cases of PH in western countries (Hoeper et al. 2016) and PH as a result of LH disease (PH‐LHD) is initiated and sustained by the retrograde transmission of LH pressure (Galie et al. 2016). Understanding the foundations of backwards pressure transmission is therefore of major clinical relevance.
The metric that best describes the vascular load is input impedance. Impedance is defined as the pressure–flow relationship in the frequency domain, comprising both the steady‐flow and pulsatile properties of the vascular tree (Weinberg et al. 2004; Nichols et al. 2011). In the pulmonary circulation, pulsatile properties cannot be ignored because they are responsible for 30–40% of the right ventricle (RV) vascular load (Milnor et al. 1966, 1969; O'Rourke & Milnor, 1971; Tedford et al. 2012). This direct impact on RV afterload explains why pulmonary vascular compliance (PVC) is a major determinant of clinical outcomes of patients with PH‐LHD (Al‐Naamani et al. 2015; Caravita et al. 2018; Tampakakis et al. 2018).
Splitting the right ventricular (RV) vascular load into its pre‐ and postcapillary components is one of the grounds of clinical decision making in PH‐LHD (Galie et al. 2016). Pulmonary capillary wedge pressure and pulmonary vascular compliance (PVC) are inversely related, demonstrating that downstream LA pressure modifies RV vascular load in a more complex way than previously assumed (Tedford et al. 2012). Although hydrodynamic waves are known to be transmitted from the left chambers to the RV by different mechanisms (Smiseth et al. 1999; Hollander et al. 2004), how these waves impact pulmonary impedance remains unknown. Transmission‐line theory states that the global behaviour of a system is determined by the relationship between the harmonic response of each of its elements (Magnusson et al. 2000). In circuit design, matching impedances is key to avoiding reflections and increasing system efficiency. Thus, understanding the full haemodynamic bases of PH entails addressing the full frequency interactions in the system.
On this basis, the present study aimed to investigate the impact of the harmonic responses of the pulmonary vasculature and the LH on the total RV vascular load. We measured high‐fidelity pressure and flow signals in healthy pigs in an open‐chest instrumentation set‐up during acute haemodynamic interventions. We used state‐of‐the‐art methods to estimate frequency domain response functions of three compartments: (i) total pulmonary input impedance, Z T , ; (ii) longitudinal impedance of the pulmonary vessels, Z L; and (iii) harmonic impedance spectra of the LH,. This allowed us to quantify the effects of the LH frequency response on pulmonary artery pressures, pulsatile power and PVC. Finally, we analysed the relationship between LV chamber properties and the impact of the LH frequency response on PVC.
Methods
Ethical approval
The experimental protocol was approved by the local Institute Animal Care Committee (ES280790000087) and all the procedures were in accordance with the European Directive 2010/63/EU and the Spanish law for animal research (53/2013). All investigators worked under the ethical principles of The Journal of Physiology, and their work complied with the ARRIVE guideline and the journal's animal ethics checklist (Grundy, 2015).
Animals were provided by ‘La Chimenea’ farm (Madrid Institute for Research and Rural Development in Food and Agriculture, IMIDRA) in Aranjuez, Madrid. Animals were housed in the Department of Experimental Medicine and Surgery of the Hospital General Universitario Gregorio Marañón at least 48 h before the procedure. All animals were housed under a 12:12 h light/dark cycle in temperature‐regulated rooms with access to food and water available ad libitum.
Study protocol
We studied 18 adult minipigs (13 males; weight 46 ± 7 kg) in an open‐chest instrumentation set‐up. After anaesthetic induction with propofol (1.5 mg kg−1) and atracurium (0.3 mg kg−1), animals were endotracheally intubated and mechanically ventilated without end‐expiratory positive pressure. Complete anaesthesia and relaxation were maintained by propofol (0.2 mg kg−1 min−1), fentanyl (0.1 µg kg−1 min−1 i.v.) and repeated bolus of atracurium (0.3 mg kg−1 h−1). Deep anaesthesia was systematically confirmed before administration of the neuromuscular blocker. In animals undergoing ischaemia, propofol was replaced by thiopental (5 mg kg−1 h−1). Oxygenation and ventilation were checked by monitoring arterial blood gases. Animals were killed at the end of the experiments with i.v. sodium pentobarbital (100 mg kg−1).
We performed a mid‐line sternotomy and pericardiotomy and placed a transit‐time ultrasonic perivascular flow probe (16 mm) (Transonic, Ithaca, NY, USA) around the main pulmonary artery, 2 cm distal to the pulmonary valve. We took special care to avoid a tight fit of the flow‐probe around the pulmonary artery and prevented the loss of probe contact using ultrasound gel. We inserted a 5‐Fr micromanometer‐tipped catheter (Millar Instruments Inc., Houston, TX, USA) anterogradely through the wall of the pulmonary artery and placed it with the pressure sensor at the closest position to the flow probe. We dissected the right inferior pulmonary vein, and advanced either a 2‐Fr micromanometer‐tipped catheter (n = 5) or a 0.014‐inch diameter pressure guidewire (Volcano Corp., San Diego, CA, USA) (n = 13) in the pulmonary vein at the level of the junction with the left atrium (LA). We placed a 7‐Fr pigtail micromanometer‐tipped catheter (Millar Instruments Inc.) in the RV through the right jugular vein. We placed additional 2‐Fr and 5‐Fr micromanometer‐tipped catheters (Millar Instruments Inc.) in the right atrium and in the LV through the right jugular vein and left carotid artery, respectively (Fig. 1 A). We performed epicardial 3‐D B‐mode echocardiography from apical views to calculate simultaneous LV and RV volumes and ejection fractions, as described previously (Perez Del Villar et al. 2015).
Figure 1. Experimental set‐up and procedure.
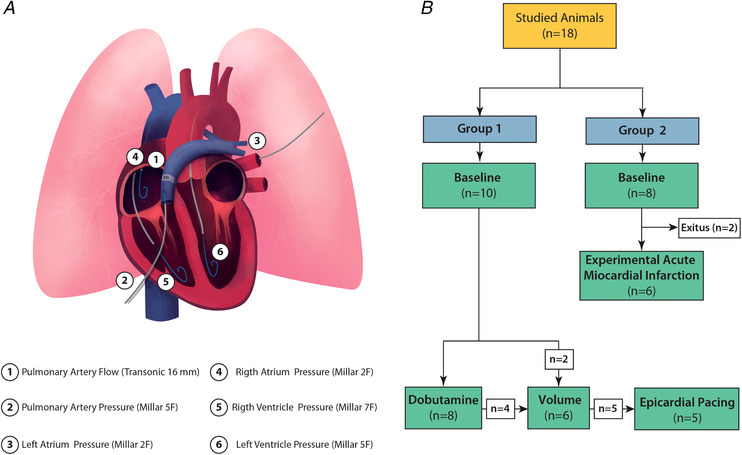
A, sketch of the instrumentation. B, experimental procedure. [Color figure can be viewed at wileyonlinelibrary.com]
We studied two animal groups undergoing different experimental phases (Fig. 1 B). Group 1 (n = 10) underwent inotropic stimulation (dobutamine infusion 2.5 µg kg−1 min−1; n = 8), acute volume loading (1000–1500 mL of isotonic saline solution in 5–10 min; n = 6) and/or epicardial pacing (VVI and DDD modes at 70 to 100 beats min–1 (intervals of 10 beats min–1) after 500 µg kg−1 of i.v. zatebradine to induce sinus bradycardia; n = 5). Group 2 (n = 8) underwent an experimental anterior myocardial infarction (AMI). AMI was achieved by epicardial ligation of left anterior descending coronary artery. Ligation of the artery was performed using a preconditioning protocol consisting of three phases of 10 s intermittent occlusion/release intervals to avoid life‐threatening arrhythmias induced by ischaemia. Data were acquired after 45 s of the final occlusion with the artery still occluded. Two animals died during the AMI procedure. We analysed pacing phases separately as part of a sensitivity analysis.
We stored simultaneous high‐fidelity pressure and flow data thrice at baseline and during each haemodynamic phase in runs of 15–20 s in duration, disconnecting the endotracheal tube. Pressure signals were carefully balanced and checked for drift as reported previously (Yotti et al. 2005). Digital signals were recorded at 1000 Hz on a dedicated computer using custom‐built amplifiers, a 16‐channel analogue‐to‐digital converter board and virtual instrumentation software.
Data analysis
The non‐linear time‐varying properties of the LV preclude its formal characterization in terms of impedance (Nichols et al. 2011). However, obtaining the response function in the frequency domain of this type of elements is a suitable method for understanding their physical behaviour (Zadeh, 1950; Victor et al. 1977), particularly when time‐varying properties are periodical. The term ‘harmonic impedance spectra identification’ has been coined for frequency domain analyses of time‐varying systems (Sanchez et al. 2013). On this basis, we obtained the harmonic impedance spectra of the LH () and analysed its impact on Z T. We hypothesized that Z T (the total vascular load of the pulmonary circulation) can be decomposed in the frequency domain as the result of Z L and attached in series, (Fig. 2). Following the definition of vascular impedance (Nichols et al. 2011), Z T = P PA /Q PA and , where P PA and P LA account for pressure in the main pulmonary artery and LA, respectively, and Q PA accounts for flow in the pulmonary artery (both in the frequency domain). These definitions of Z T and Z L are conceptually equivalent to the one‐input/one‐output and the two‐input/one‐output systems described previously (Fukumitsu et al. 2018).
Figure 2. Theoretical model for frequency domain analyses.
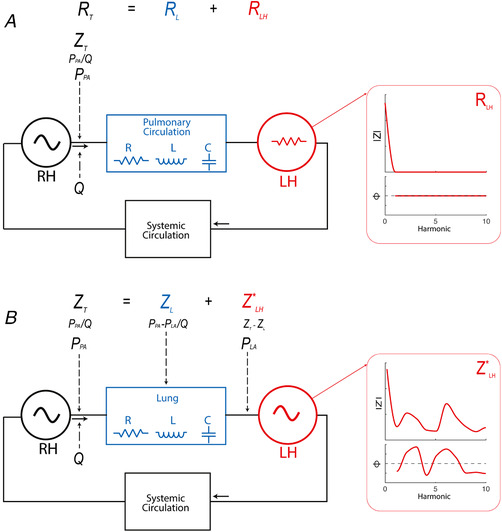
A, classical frameworks in which, although Z T is characterized, only the resistive component of the LH is taken into account. B, frequency domain analysis in which Z T is results of the sum of the longitudinal impedance of the pulmonary vasculature (Z L) plus the harmonic spectra impedance of the periodical time‐varying system of the LH (). Both Z L and are identified in terms of their moduli and phase. [Color figure can be viewed at wileyonlinelibrary.com]
To obtain Z T and Z L, we ensemble averaged 20 to 30 beats in each haemodynamic run before Fourier transforming pressure, P (P PA for Z T; for Z L) and Q signals up to 10 harmonics of the cardiac frequency (Yotti et al. 2015). Moduli of each impedance, , were obtained at each frequency as and their phases were calculated as: . We calculated the harmonic spectra of the LH as , which can be written in their complex form as:
Note that we calculated without the need of measuring flow in the left chambers. Characteristic impedance (Z ch) for each compartment was obtained by averaging above the fourth harmonic, excluding outlier values >3 times the median. All signals underwent digital low‐pass (50 Hz) filtering before processing.
To address the impact of LH pulsatility on RV load, we compared measured haemodynamic indices with those resulting from a hypothetical Z T that would be found if the LH would be purely resistive (obtained by reconstructing the pressure via inverse Fourier transform), in other words if only held a resistance component, neglecting all values of above the zero harmonic.
We calculated total PVC as stroke volume (SV)/pulmonary artery pulse pressure, which is a general method suitable for any pressure waveform morphology that is not based on a specific lumped‐parameter model (Segers et al. 1999). Pulmonary arterial elastance (E a) was calculated as SV/end‐systolic pulmonary artery pressure. Pulsatile power fraction (the fraction of total hydraulic energy dumped by oscillatory phenomena) was obtained as the ratio of pulsatile power to total hydraulic power in the pulmonary artery (Grignola et al. 2007).The time constant of LV relaxation (tau) was obtained assuming a non‐zero asymptote. All data were analysed using custom‐built algorithms (Matlab; MathWorks Inc., Natick, MA, USA).
Sensitivity analysis
We performed a sensitivity analysis aiming to investigate: (i) whether the main effects of the LH harmonic response were sensitive to increased retrograde waves generated by asynchronous VVI pacing and (ii) the method used for impedance estimation. For this purpose, we analysed data from the five animals (of Group 1) undergoing epicardial VVI and DDD pacing experiments using the spectral decomposition method to obtain frequency domain metrics. For non‐periodic signals such as those obtained during pacing, we used the spectral decomposition method (Taylor, 1966); we obtained the power spectra of P and Q, , together with their cospectral and quadrature spectra, . Following the definition of impedance, , and .
Using this formulation, the coherence of the signal, which is an estimate of the power transfer between pressure and flow, can be calculated as . Analogous to a correlation coefficient, coherence varies between 1 and 0. Unity would represent an ideal, linear and time‐invariant system relating pressure and flow at a given harmonic.
Statistical analysis
We used linear mixed‐effects models accounting for repeated measures to: (i) compare frequency domain components; (ii) address the effects of haemodynamic interventions; and (iii) analyse the relationship with LV chamber properties. Values are expressed as least squares mean ± SE of these models. Dunnett contrasts were used to compare haemodynamic interventions against baseline values. Paired t tests and pairwise contrasts in the linear mixed models were used when comparing pooled data of the measured LH harmonic response to the resistive model. All analyses and plots were performed using R, version 3.5 (R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was considered statistically significant.
Results
Haemodynamic interventions
The effects of haemodynamic interventions are shown in Table 1. Acute volume overload increased LV end‐diastolic and mean LA pressures [from 8 ± 1 to 14 ± 1 mmHg (least squares mean ± SE), P < 0.001], which significantly impacted pulmonary pressures, although pulmonary vascular resistance remained unchanged (from 667 ± 115 to 671 ± 138 dyn·s cm−5, P > 0.05). Dobutamine induced significant changes on most haemodynamic indices. First, dobutamine significantly increased the metrics of left and right ventricles (LV and RV) systolic chamber function. Second, dobutamine increased mean, systolic and diastolic pulmonary pressures, whereas LA pressure remained unchanged. Third, dobutamine reduced PVC to one‐half of baseline values (from 2.2 ± 0.2 to 1.1 ± 0.2 ml mmHg−1, P < 0.001). Fourth, dobutamine significantly increased total, steady and pulsatile power, whereas the pulsatile power fraction remained unchanged. Experimental AMI had significant effects on RV and LV volumes and ejection fraction. On pulmonary haemodynamics, AMI significantly lowered PVC down to 1.6 ± 0.2 ml mmHg−1 (P < 0.001 vs. baseline), although pulmonary and LA pressures did not change significantly.
Table 1.
Haemodynamic data
| Baseline | Volume | Dobutamine | AMI | |
|---|---|---|---|---|
| Number of animals (n) | 18 | 6 | 8 | 6 |
| Heart rate (beats min–1) | 72 ± 3 | 63 ± 4* | 112 ± 3* | 74 ± 4 |
| Stroke volume (mL) | 37 ± 2 | 36 ± 3 | 28 ± 3* | 28 ± 3* |
| Cardiac index (L min−1 m−2) | 2.1 ± 0.2 | 1.8 ± 0.2 | 2.5 ± 0.2 | 1.8 ± 0.2 |
| Pulmonary haemodynamics | ||||
| Pulmonary systolic arterial pressure (mmHg) | 34 ± 2 | 39 ± 2 | 52 ± 2* | 36 ± 2 |
| Pulmonary diastolic arterial pressure (mmHg) | 16 ± 1 | 20 ± 1* | 22 ± 1* | 17 ± 1 |
| Pulmonary mean pressure (mmHg) | 25 ± 1 | 29 ± 1* | 34 ± 1* | 27 ± 2 |
| Left atrial mean pressure (mmHg) | 8 ± 1 | 14 ± 1* | 8 ± 1 | 8 ± 1 |
| Pulmonary vascular resistance (dyn s cm−5) | 667 ± 115 | 671 ± 138 | 782 ± 131 | 778 ± 140 |
| Pulmonary arterial elastance (mmHg mL−1) | 0.88 ± 0.14 | 0.99 ± 0.17 | 1.53 ± 0.16* | 1.04 ± 0.17 |
| Pulmonary vascular compliance (mL mmHg−1) | 2.2 ± 0.2 | 2.0 ± 0.2 | 1.1 ± 0.2* | 1.6 ± 0.2* |
| Total hydraulic power (mW) | 207 ± 29 | 195 ± 45 | 553 ± 40* | 182 ± 46 |
| Steady hydraulic power (mW) | 176 ± 24 | 176 ± 37 | 453 ± 34* | 153 ± 38 |
| Pulsatile hydraulic power (mW) | 31 ± 6 | 20 ± 9 | 100 ± 8* | 28 ± 9 |
| Pulmonary pulsatile power fraction | 0.16 ± 0.01 | 0.13 ± 0.01 | 0.18 ± 0.01 | 0.16 ± 0.01 |
| Left ventricle | ||||
| LV systolic pressure (mmHg) | 81 ± 3 | 88 ± 5 | 111 ± 4* | 76 ± 4 |
| LV Tau (ms) | 65 ± 3 | 70 ± 5 | 40 ± 4* | 64 ± 4 |
| LV end diastolic pressure (mmHg) | 7 ± 1 | 13 ± 2* | 7 ± 1 | 7 ± 1 |
| LV End‐diastolic volume (mL) | 48 ± 2 | 55 ± 4 | 39 ± 3 | 52 ± 4 |
| LV End‐systolic volume (mL) | 15 ± 1 | 18 ± 2 | 13 ± 2 | 23 ± 2* |
| LV Ejection fraction (%) | 68 ± 2 | 68 ± 4 | 67 ± 3 | 55 ± 3* |
| LV dP/dt max (mmHg s−1) | 1622 ± 183 | 2007 ± 367 | 4843 ± 249* | 1434 ± 283 |
| Right ventricle | ||||
| RV Tau (ms) | 60 ± 3 | 66 ± 5 | 37 ± 4* | 69 ± 4* |
| RV end diastolic pressure (mmHg) | 5 ± 0 | 11 ± 1* | 4 ± 1* | 6 ± 1 |
| RV End‐diastolic volume (mL) | 43 ± 3 | 71 ± 4* | 35 ± 3* | 51 ± 3* |
| RV End‐systolic volume (mL) | 23 ± 2 | 42 ± 2* | 18 ± 2* | 31 ± 2* |
| RV Ejection fraction (%) | 46 ± 2 | 42 ± 3 | 48 ± 2 | 40 ± 2* |
| RV dP/dt max (mmHg s−1) | 582 ± 48 | 702 ± 115 | 1360 ± 71* | 605 ± 78 |
Values show least squares mean ± SE. dP/dt max, peak of the temporal derivative of pressure, Tau, time‐constant of ventricular relaxation.
* P < 0.05 vs. baseline
Frequency domain analysis
Above the fundamental frequency (over the first harmonic), the input impedance, Z T, of the pulmonary artery, was always below the longitudinal impedance of the pulmonary vessels, Z L, (Fig. 3 and Table 2), demonstrating a favourable effect of the LH response on Z T. Indeed, the moduli of Z T were 43%, 35% and 22% lower than those of Z L at frequencies of the second, third and fourth harmonics, respectively (P < 0.001 for all three; pooled data at all phases). Z T was below Z L because, although the moduli of the harmonic impedance spectra of the LH, was ∼20–35% of |Z T |, the sign of its phase, , was often opposite to that of the pulmonary vessels, ϕL. Also, the characteristic impedance, Z ch, was a 21% lower for Z T than for Z L (164 ± 16 vs. 129 ± 16 dyn·s cm−5; P < 0.001, pooled data from all phases).
Figure 3. Frequency domain analyses.
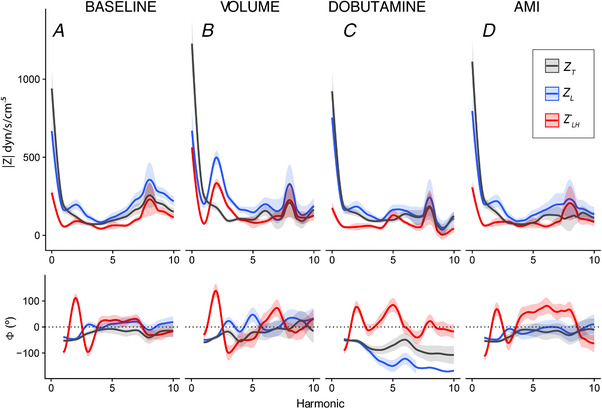
Solid lines show the least squares means and the ribbon shows their SE. Moduli () is shown in the top row whereas the bottom row shows the phase (ϕ). Results are shown for the baseline (A) (n = 18 animals), volume (B) (n = 6), dobutamine (C) (n = 8) and AMI (D) (n = 8) phases. Pulmonary input impedance (Z T) is shown in black and longitudinal impedance of the pulmonary vessels (Z L) is shown in blue, whereas the harmonic spectra impedance of the periodical time‐varying system of the LH () is shown in red. Note that, for most harmonics, Z T is below Z L, demonstrating a favourable effect of the harmonic response of the LH on Z T. [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Results of frequency domain analyses
| Phase | Z 0 | Z 1 | Z 2 | Z 3 | Z 4 | Z ch | |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| Z T | 925 ± 89 | 186 ± 20 | 119 ± 23 | 78 ± 16 | 71 ± 11 | 135 ± 15 | |
| Z L | 653 ± 89* | 162 ± 20* | 197 ± 23* | 115 ± 16* | 85 ± 11 | 171 ± 15* | |
|
|
304 ± 89 | 55 ± 20 | 90 ± 23 | 72 ± 16 | 44 ± 11 | 85 ± 15 | |
| Volume | |||||||
| Z T | 1162 ± 117# | 244 ± 24# | 154 ± 31 | 90 ± 20 | 101 ± 16 | 114 ± 22 | |
| Z L | 572 ± 117* | 195 ± 24* | 500 ± 31* , # | 238 ± 20* , # | 167 ± 16* , # | 187 ± 22* | |
|
|
726 ± 117# | 85 ± 24 | 353 ± 31# | 193 ± 20# | 114 ± 16# | 134 ± 22 | |
| Dobutamine | |||||||
| Z T | 954 ± 100 | 192 ± 22 | 138 ± 26 | 96 ± 18 | 90 ± 13 | 115 ± 18 | |
| Z L | 731 ± 101* | 176 ± 22 | 192 ± 27* | 126 ± 18* | 95 ± 13 | 129 ± 18* | |
|
|
151 ± 101 | 25 ± 22 | 45 ± 27 | 67 ± 18 | 57 ± 13 | 68 ± 18 | |
| AMI | |||||||
| Z T | 1080 ± 111 | 213 ± 24 | 153 ± 29 | 95 ± 19 | 62 ± 15 | 126 ± 20 | |
| Z L | 850 ± 111* | 194 ± 24 | 204 ± 29 | 111 ± 19 | 97 ± 15* | 163 ± 20* | |
|
|
298 ± 111 | 78 ± 24 | 98 ± 29 | 79 ± 19 | 51 ± 15 | 64 ± 20 |
Values show the least squares mean estimate ± SE of spectra moduli. All values expressed in dyn·s cm−5.
* P < 0.05 (Z T vs. ZL);
# P < 0.05 (phase vs. baseline). Z 0, steady‐flow component. Z 1 … 4, values at harmonics 1 to 4. Z ch, characteristic impedance.
The reduction of Z T below Z L was particularly relevant during acute volume overload, at all frequencies beyond the first harmonic. Although Z L at frequencies between the second and fifth harmonics increased during volume overload, the simultaneous increase in opposed‐phase lowered the resultant Z T towards baseline values. During dobutamine infusion and experimental AMI, also consistently reduced Z T below Z L for almost all frequencies above the first harmonic (Fig. 3 and Table 2). By contrast, Z T at the first harmonic was significantly higher than Z L at baseline and during volume overload and showed similar values during dobutamine and AMI phases.
Impact of LH harmonic response on the pulmonary vascular load
The measured pulse pulmonary pressure was 20% lower than it would be in a pure resistive LH, without frequency response (20 ± 8 vs. 25 ± 8 mmHg, P < 0.001, pooled phases) as a result of significant effects on diastolic and systolic pulmonary pressures (Fig. 4). The harmonic response of the LH was responsible for reducing pulmonary pulsatile power fraction by 12% (0.16 ± 0.01 vs. 0.18 ± 0.01, P = 0.02) and increasing PVC by 15% (1.9 ± 0.2 vs. 1.6 ± 0.2 ml mmHg−1, P = 0.009; pooled phases). These favourable effects of harmonic response matching were consistently significant among all phases (Fig. 5). The increase in PVC caused by harmonic response matching was related to the time constant of LV relaxation (P < 0.001) and dP/dt min (P = 0.03). Instead, no significant relationship was observed with either mean left atrial (P = 0.37) or LV end‐diastolic pressures (P = 0.32).
Figure 4. Effects of the harmonic response of the LH on pulmonary artery pressures for pooled data (n = 18).
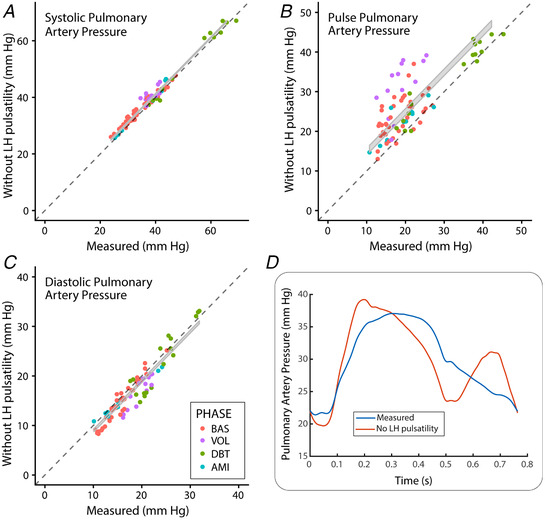
A and C, results for systolic and diastolic pressures, respectively. B, pulse pressure. Measured pressures are shown on the horizontal axes, whereas values obtained if the LH had no harmonic response are shown on the vertical axes. The grey ribbon shows the 95% confidence interval of the paired t test difference. D, waveforms obtained in a representative example. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 5. Impact of the harmonic response of the LH.
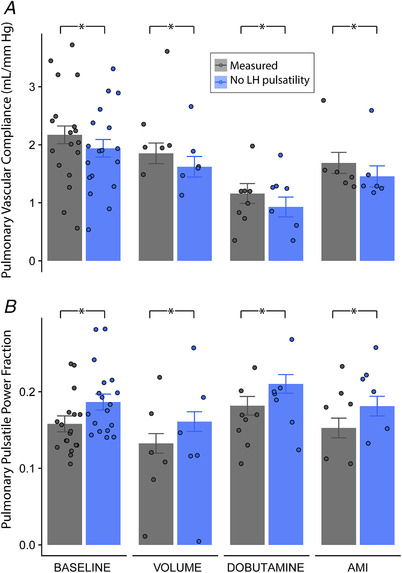
(A) Impact on pulmonary vascular compliance and (B) impact on pulmonary pulsatile power fraction at baseline, volume overload, dobutamine infusion and AMI phases (n = 18 animals). Bars show least squares mean estimates and their SE, whereas scattered dots show individual data. Measured values (grey) are compared with the theoretical values that would be obtained in the presence of a pure resistive LH (blue) by pairwise contrasts in the linear mixed models accounting for repeated measures. [Color figure can be viewed at wileyonlinelibrary.com]
Sensitivity analysis
Identical favourable results on pulsatility were obtained when was computed using a spectral decomposition method instead of fast Fourier transformation of ensemble‐averaged signals. This sensitivity analysis also showed that Z T remained below Z L at frequencies above the first harmonic both during DDD and VVI pacing (Fig. 6 and Table 3). At the first harmonic during VVI pacing, Z T was above Z L as a result of the reduction in Z L. The simultaneous increase in opposed‐phase explained why Z T did not change.
Figure 6. Results of frequency domain analyses using the spectral decomposition method.
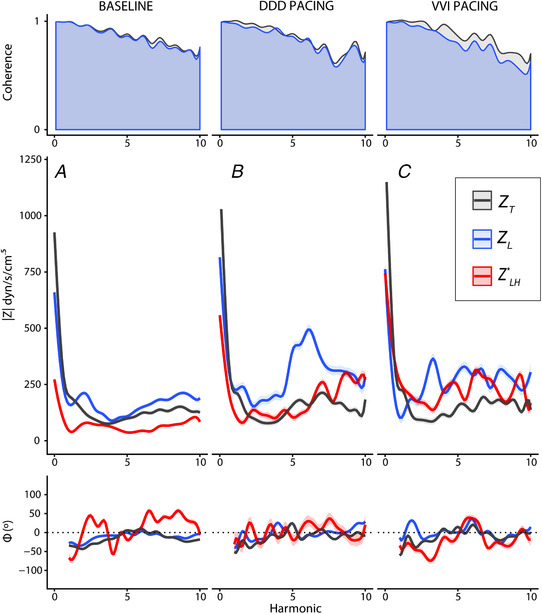
Values are shown at baseline (A) (n = 18 animals), as well as during DDD (B) (n = 5) and VVI pacing (C) (n = 5). The top row shows results of the coherence analysis for Z T (grey) and Z L (blue), overlaid. The mid row shows results for moduli (), whereas the bottom row shows the phase (ϕ) analysis. Solid lines show the least squares means and the ribbon shows their SE. Pulmonary input impedance (Z T) is shown in black and longitudinal impedance of the pulmonary vasculature (Z L) is shown in blue, whereas the harmonic spectra impedance of the periodical time‐varying system of the LH () is shown in red. [Color figure can be viewed at wileyonlinelibrary.com]
Table 3.
Frequency domain analyses of pacing data using the spectral decomposition method
| Phase | Z 0 | Z 1 | Z 2 | Z 3 | Z 4 | Z ch | |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| Z T | 931 ± 100 | 186 ± 19 | 120 ± 18 | 77 ± 15 | 75 ± 13 | 151 ± 17 | |
| Z L | 661 ± 100* | 161 ± 19 | 201 ± 18* | 118 ± 15* | 87 ± 13 | 120 ± 17 | |
|
|
272 ± 100 | 46 ± 19 | 80 ± 18 | 69 ± 15 | 65 ± 13 | 63 ± 17 | |
| DDD pacing | |||||||
| Z T | 1225 ± 122# | 197 ± 25 | 93 ± 26 | 75 ± 21 | 84 ± 20 | 307 ± 27 | |
| Z L | 818 ± 122* | 221 ± 25# | 160 ± 26* | 166 ± 21* , # | 190 ± 20* , # | 167 ± 27 | |
|
|
559 ± 122# | 119 ± 25# | 98 ± 26 | 118 ± 21# | 115 ± 20# | 217 ± 27 | |
| VVI pacing | |||||||
| Z T | 1375 ± 121# | 206 ± 24 | 87 ± 26 | 76 ± 21 | 111 ± 19 | 266 ± 26 | |
| Z L | 762 ± 121* | 108 ± 24# , * | 179 ± 26* | 311 ± 21# , * | 217 ± 19# , * | 157 ± 26 | |
|
|
745 ± 121# | 257 ± 24# | 163 ± 26# | 152 ± 21# | 245 ± 19# | 212 ± 26 |
Values show the least squares mean estimate ± SE of spectra moduli. All values expressed in dyn·s cm−5.
* P < 0.05 (Z T vs. ZL);
# P < 0.05 (phase vs. baseline). Z 0, steady‐flow component. Z 1 … 4, values at harmonics 1 to 4. Z ch, characteristic impedance.
Discussion
We demonstrate for the first time that the frequency response of the LH lowers RV afterload because it is physiologically matched to the longitudinal impedance of the pulmonary vessels. (Herein, we use the concept of ‘physiological matching’ in a general way to designate the relationship between the frequency responses of the pulmonary vessels and the LH that lowers the pulsatile load. In electric circuit systems, the term is reserved for the specific impedance relationship that maximizes power transfer or reduces signal reflections.) Frequency responses of the LH and the pulmonary longitudinal impedance build a source of left–right interaction that favours compliance and lowers energetic waste as a result of arterial pulsatility. This favourable effect protects the RV against afterload mismatching during acute volume transients.
Although the nature of these effects is necessarily speculative, three mechanisms are probably involved. First, the time‐varying systolic and diastolic properties of LH chambers alternate flow pushing and pulling effects that are retrogradely transmitted (Smiseth et al. 1999; Hollander et al. 2004). Note that, during every cardiac cycle, pulmonary vein flow (systolic, diastolic and atrial reversal waves) and pressure (‘a’, ‘c’, ‘x’, ‘v’ and ‘y’ waves) undergo multiple deflections. This multiphasic behaviour may lead to potentially complex pressure/flow spectral responses at frequencies that can reach well beyond the first harmonic. Second, the LH may exert a favourable effect by reducing wave reflections generated by the RV (Nichols et al. 2011; Tedford, 2014); counter‐phase backwards waves rising from the LH probably interact with the forward waves generated by RV ejection and relaxation (Hollander et al. 2004). Third, a direct transmission of left atrial compression (and secondary expansion) waves to the proximal pulmonary tree has been reported in well‐controlled counter pulsation experiments (Hollander et al. 2004). Approximately 20% of ‘V’ atrial waves reach the pulmonary arteries by this direct mechanism that is assumed to take place travelling across the heart.
Potential frequency cross‐talk precludes unequivocally correlating spectral findings with a specific level of the vascular tree. However, full spectral findings suggest that the three mechanisms described above contribute to some degree to the physiological effects of the LH on the pulmonary circulation. The impact of the LH on Z T observed at low frequencies (below the third harmonic) is equivalent to lowering distal wave reflections (Nichols et al. 2011). Although a wave‐intensity analysis was beyond the scope of the present study, an effect on reflections is supported by the lower pulsatile power fraction measured when compared to a pure resistive behaviour. The impact of the LH on Z T observed at high frequencies (Z ch) is equivalent to increasing proximal effective cross‐sectional area of the pulmonary arterial bed and slowing pulse wave velocity (Nichols et al. 2011), hypothetically as a result of direct transmission. Further research should aim to decipher the intrinsic mechanisms of this source of left‐right interaction in the pulmonary circulation.
To our knowledge, only two studies have attempted to infer the impact of the LH on the pulmonary circulation in the frequency domain. In a small rat study, Fukumitsu et al. (2018) did not find a significant effect of LA pressure on Windkessel‐derived parameters of the pulmonary vascular tree. By combining pulmonary vein flow and pulmonary wedge pressure data, Dernellis and Panaretou (2005) described ‘impedance’ properties of the LA as a metric of diastolic dysfunction. The results of their study, as well as our own, show that the magnitudes of the spectra moduli of the LH for frequencies of the first harmonic and beyond are similar to those of the steady component (Fig. 2). These data suggest that the upstream impact of the LH harmonic response may be of higher magnitude than previously proposed (Milnor et al. 1966; Fukumitsu et al. 2018). Interestingly, the study by Dernellis and Panaretou (2005) also described a sign variation pattern of impedance phase between the first and fifth harmonics. Although there were important methodological differences compared to our study, it is remarkable that their ‘W’ phase pattern is almost identical to our findings in the same frequency range.
Clinical implications
In the clinical setting, classifying and treating post‐capillary PH relies on considering the LH only as a pressure load that upstream adds to the pulmonary vessels to build total pulmonary resistance (Galie et al. 2016). It could be expected that this additive effect would also take place on the full frequency spectrum of pulmonary input impedance. By contrast, in the present study, we demonstrate that, in the normal pulmonary circulation, the LH reduces pulmonary artery input impedance moduli beyond the first harmonic. Our results emphasize that PVC should be interpreted to result from the performance of global pulmonary circulation and not exclusively the distensibility of proximal conductance arteries (Lammers et al. 2012).
How the harmonic response matching may change in chronic PH scenarios deserves further investigation. How it is modified by structural remodelling in the arterial, venous, indeterminate or capillary vessels also needs to be addressed in future clinical and animal studies. Although a physiological effect of the LH increasing PVC by 15% may appear to be of limited clinical significance, note that we compared measured values in animals without pulmonary artery disease with those that would be obtained dismissing any LH frequency response. However, under given circumstances, unmatched responses could have an adverse effect on RV afterload; if frequency responses for the LH and the pulmonary vasculature were to take place in‐phase, Z T would have been even much higher than Z L in the full harmonic range.
We observed that the effect of the LH on PVC was related to the rate of relaxation of the LV. This confirms that time‐varying properties of the LA and the LV determine the instantaneous pressure flow relationship transmitted retrogradely (Wu & Kovacs, 2006), with the pulmonary veins (Grimes et al. 1995) and the mitral valve (Flachskampf et al. 1993) behaving as passive inertances. How acute or chronic changes in LH frequency response impacts RV afterload should be addressed because this may be an important contributor to PH‐LHD. Heart failure with preserved ejection fraction, valvular heart disease and left ventricular assist devices are particularly relevant clinical scenarios in which the degree of RV involvement is frequently unexplained by conventional steady‐flow analyses of PH. We consider that the observation of a relationship between LH harmonic response and LV chamber properties paves the road for future exploration on therapeutic modulation.
The results of our sensitivity analyses showed a favourable effect of LH harmonic response even during VVI pacing. Thus, atrial synchronicity does not appear to be a major requirement for this effect, and intrinsic chamber properties and interventricular synchrony may be more relevant. The ‘stiff LA syndrome’ was coined to designate a condition in which the degree of right heart failure is disproportionate to the degree of LH involvement, principally as a result of reduced LA reservoir function (Pilote et al. 1988). This entity may be responsible for persistent PH after successful valve correction (Bermejo et al. 2018) or atrial radiofrequency ablation procedures (Gibson et al. 2011). Chronic atrial fibrillation is also known to be independently associated with RV dysfunction beyond the degree of PH (Gorter et al. 2018). The role of impaired harmonic response matching in these syndromes should be investigated.
The findings of the present study may be also important with respect to understanding types of PH unrelated to LH diseases. RV systolic function is also a major determinant of outcome in pulmonary arterial hypertension, PH as a result of lung diseases and chronic thromboembolic PH (Galie et al. 2016); under these conditions, RV afterload mismatch is frequently disproportionate to the elevation of pulmonary vascular resistance. Note that, even without any changes in the LH compartment, subtle changes in the phase of Z L (the ‘precapillary’ compartment) would also lead to unmatched harmonic responses. The resulting increase in total RV vascular load would not be detected by conventional indices of PH. Interestingly, intima thickening of small pulmonary veins and indeterminant vessels have been described recently in patients with PH as a result of LHD (Fayyaz et al. 2018), comprising structural changes that could eventually modify harmonic matching also by changing Z L. Thus, we consider that frequency domain compartmental analyses should be explored in all types of PH. Future clinical studies should aim to clarify the role of unmatched harmonic responses in chronic PH.
Limitations
A comprehensive correlation of the LH frequency response with LV chamber properties was beyond the scope of the present study. The value and limitations of harmonic impedance spectra of periodical time‐varying systems such as the LH have been discussed above. The effects of acute myocardial infarction on LV chamber properties and atrial pressures were lower than expected. Although pericardial opening may have reduced the direct transmission of atrial waves to the pulmonary artery, direct transmission is also present with an open pericardium (Hollander et al. 2004). To obtain high‐fidelity recordings of pressure and flow, we employed a highly interventional experimental open‐chest protocol using minipigs under mechanical ventilation (Maggiorini et al. 1998). However, combined pressure–flow guidewires can be used to reliably characterize arterial impedance in the clinical setting (Yotti et al. 2015). Although appropriate experimental validation studies are warranted, capillary pressure recordings from balloon‐inflation catheters could be a suitable method for approximating atrial pressure either with (Sharman et al. 2006) or without (Dernellis & Panaretou, 2005) appropriate transfer functions.
Conclusions
In the mammal circulation, RV afterload is reduced by a natural matching between the longitudinal impedance of the pulmonary vasculature and the harmonic response of the LH. This is the consequence of relevant upstream transmission of LH oscillatory behaviour, which has favourable effects on the pulmonary tree and is particularly efficient during acute volume overload. Harmonic response matching is a previously undescribed source of interaction between the right and left mammal circulations that protects the RV against excessive afterload during acute volume changes.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
CPV, PML, RY and JB conceived and designed the research. CPV, TM, CCP, DRP, YB, MMD, AB and JCA performed the experiments. CPV, PML, JB, CCP, YB, MMD, RY and JB analysed the data. CPV, PML, TM, CCP, DRP, EGI, JCdA, JE, RY and JB interpreted the results of the experiments. CPV, PML, CCP, RY and JB prepared the figures. CPV, PML, TY and JB drafted the manuscript. CPV, TM, PML, EGI, JCdA, JE, JCA, FFA, RY and JB edited and revised the final manuscript. All authors have approved the final version of the manuscript submitted for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the Instituto de Salud Carlos III [grants PI12/02878 (to RY) and Juan Rodés Fellowship (JR15/00039) (to CPV)], the Ministerio de Economía y Competitividad of Spain [Juan de la Cierva Incorporación fellowship (IJCI‐2014‐19507) (to PML)], the University of California San Diego CTRI Galvanizing Engineering and Medicine Program and the American Heart Association [Grant 16GRNT27250262 (to JCdA)] and by the EU – European Regional Development Fund. PML is also funded by the CIBERCV.
Acknowledgements
We thank all the personnel of the Department of Experimental Medicine and Surgery of the Hospital General Universitario Gregorio Marañón for their support with the animal experiments. We also thank Ana Fernández‐Baza for her kind assistance with all administrative issues, as well as Diana Bóveda for her assistance and the artwork used in Fig. 1.
Biographies
Candelas Pérez del Villar graduated in Medicine from the University of Salamanca with Mention in the Call for National End‐of‐Career Awards in 2006. She completed her specialized medical training in the Cardiology Department of Hospital Gregorio Marañón (2012). Subsequently, she has acquired a solid training in the field of cardiovascular physiology and imaging. In 2015, she received the degree of Doctor of Medicine with Honors and, in 2017, she received the JACC Cardiovascular Imaging Young Author Achievement Award.

Pablo Martinez‐Legazpi graduated in Mechanical Engineering in 2005 and finished his PhD thesis in 2011. In 2012, he moved to the University of California San Diego, serving as postdoctoral fellow for 2 years. He joined the Cardiology Department of the Hospital General Universitario Gregorio Marañón in 2014, aiming to apply fluid mechanics and engineering modelling to cardiovascular problems. He was awarded with the William W. Parmley Young Achievement Award by the ACC in 2015.
Edited by: Harold Schultz & Satoshi Matsuoka
Contributor Information
Raquel Yotti, Email: raquel.yotti@salud.madrid.org.
Javier Bermejo, Email: javier.bermejo@salud.madrid.org.
References
- Al‐Naamani N, Preston IR, Paulus JK, Hill NS & Roberts KE (2015). Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail 3, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo J, Yotti R, Garcia‐Orta R, Sanchez‐Fernandez PL, Castano M, Segovia‐Cubero J, Escribano‐Subias P, San Roman JA, Borras X, Alonso‐Gomez A, Botas J, Crespo‐Leiro MG, Velasco S, Bayes‐Genis A, Lopez A, Munoz‐Aguilera R, de Teresa E, Gonzalez‐Juanatey JR, Evangelista A, Mombiela T, Gonzalez‐Mansilla A, Elizaga J, Martin‐Moreiras J, Gonzalez‐Santos JM, Moreno‐Escobar E & Fernandez‐Aviles F (2018). Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double‐blind, randomized clinical trial. Eur Heart J 39, 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravita S, Faini A, Carolino D'Araujo S, Dewachter C, Chomette L, Bondue A, Naeije R, Parati G & Vachiery JL (2018). Clinical phenotypes and outcomes of pulmonary hypertension due to left heart disease: role of the pre‐capillary component. PLoS ONE 13, e0199164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernellis J & Panaretou M (2005). Assessment of left atrial input impedance in normal subjects and in hypertensive patients. Eur J Heart Fail 7, 63–68. [DOI] [PubMed] [Google Scholar]
- Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM & Redfield MM (2018). Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 137, 1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachskampf FA, Rodriguez L, Chen C, Guerrero JL, Weyman AE & Thomas JD (1993). Analysis of mitral inertance: a factor critical for early transmitral filling. J Am Soc Echocardiogr 6, 422–432. [DOI] [PubMed] [Google Scholar]
- Fukumitsu M, Kawada T, Shimizu S & Sugimachi M (2018). The pulsatile component of left atrial pressure has little effect on pulmonary artery impedance estimation in normal rats. Physiol Rep 6, e13946–e13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck‐Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H & Luis Zamorano J (2016). 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37, 67–119.26320113 [Google Scholar]
- Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J, Burkhardt JD, Heywood JT, Johnson AD, Rubenson DS, Horton R, Gallinghouse GJ, Beheiry S, Curtis GP, Cohen DN, Lee MY, Smith MR, Gopinath D, Lewis WR & Natale A (2011). Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 8, 1364–1371. [DOI] [PubMed] [Google Scholar]
- Gorter TM, van Melle JP, Rienstra M, Borlaug BA, Hummel YM, van Gelder IC, Hoendermis ES, Voors AA, van Veldhuisen DJ & Lam CSP (2018). Right heart dysfunction in heart failure with preserved ejection fraction: the impact of atrial fibrillation. J Card Fail 24, 177–185. [DOI] [PubMed] [Google Scholar]
- Grignola JC, Gines F, Bia D & Armentano R (2007). Improved right ventricular–vascular coupling during active pulmonary hypertension. Int J Cardiol 115, 171–182. [DOI] [PubMed] [Google Scholar]
- Grimes RY, Levine RA, Walker PG & Yoganathan AP (1995). Dynamics of systolic pulmonary venous flow in mitral regurgitation: mathematical modeling of the pulmonary venous system and atrium. J Am Soc Echocardiogr 8, 631–642. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa‐Hahnle K, Jing ZC & Gibbs JS (2016). A global view of pulmonary hypertension. Lancet Respir Med 4, 306–322. [DOI] [PubMed] [Google Scholar]
- Hollander EH, Dobson GM, Wang JJ, Parker KH & Tyberg JV (2004). Direct and series transmission of left atrial pressure perturbations to the pulmonary artery: a study using wave‐intensity analysis. Am J Physiol Heart Circ Physiol 286, H267–H275. [DOI] [PubMed] [Google Scholar]
- Lammers S, Scott D, Hunter K, Tan W, Shandas R & Stenmark KR (2012). Mechanics and function of the pulmonary vasculature: implications for pulmonary vascular disease and right ventricular function. Compr Physiol 2, 295–319. [DOI] [PubMed] [Google Scholar]
- Maggiorini M, Brimioulle S, De Canniere D, Delcroix M & Naeije R (1998). Effects of pulmonary embolism on pulmonary vascular impedance in dogs and minipigs. J Appl Physiol (1985) 84, 815–821. [DOI] [PubMed] [Google Scholar]
- Magnusson PC, Weisshaar A, Tripathi VK & Alexander GC (2000). Transmission Lines and Wave Propagation. Taylor & Francis, Boca Raton, FL, USA. [Google Scholar]
- Milnor WR, Bergel DH & Bargainer JD (1966). Hydraulic power associated with pulmonary blood flow and its relation to heart rate. Circ Res 19, 467–480. [DOI] [PubMed] [Google Scholar]
- Milnor WR, Conti CR, Lewis KB & O'Rourke MF (1969). Pulmonary arterial pulse wave velocity and impedance in man. Circ Res 25, 637–649. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O'Rourke MF & Vlachopoulos C (2011). Vascular impedance. In McDonald's Blood Flow in Arteries Theoretical, Experimental and Clinical Principles, pp. 273–310. CRC Press, Taylor and Francis Group, Boca Raton, FL, USA. [Google Scholar]
- O'Rourke MF & Milnor WR (1971). Relation between differential pressure and flow in the pulmonary artery of the dog. Cardiovasc Res 5, 558–565. [DOI] [PubMed] [Google Scholar]
- Perez Del Villar C, Bermejo J, Rodriguez‐Perez D, Martinez‐Legazpi P, Benito Y, Antoranz JC, Desco MM, Ortuno JE, Barrio A, Mombiela T, Yotti R, Ledesma‐Carbayo MJ, Del Alamo JC & Fernandez‐Aviles F (2015). The role of elastic restoring forces in right‐ventricular filling. Cardiovasc Res 107, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilote L, Huttner I, Marpole D & Sniderman A (1988). Stiff left atrial syndrome. Can J Cardiol 4, 255–257. [PubMed] [Google Scholar]
- Sanchez B, Louarroudi E, Bragos R & Pintelon R (2013). Harmonic impedance spectra identification from time‐varying bioimpedance: theory and validation. Physiol Meas 34, 1217–1238. [DOI] [PubMed] [Google Scholar]
- Segers P, Brimioulle S, Stergiopulos N, Westerhof N, Naeije R, Maggiorini M & Verdonck P (1999). Pulmonary arterial compliance in dogs and pigs: the three‐element windkessel model revisited. Am J Physiol Heart Circ Physiol 277, H725–H731. [DOI] [PubMed] [Google Scholar]
- Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB & Marwick TH (2006). Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 47, 1203–1208. [DOI] [PubMed] [Google Scholar]
- Smiseth OA, Thompson CR, Lohavanichbutr K, Ling H, Abel JG, Miyagishima RT, Lichtenstein SV & Bowering J (1999). The pulmonary venous systolic flow pulse – its origin and relationship to left atrial pressure. J Am Coll Cardiol 34, 802–809. [DOI] [PubMed] [Google Scholar]
- Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, Kelemen BW, Houston BA, Kolb TM, Damico R, Mathai SC, Kasper EK, Hassoun PM, Kass DA & Tedford RJ (2018). Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail 11, e004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MG (1966). Use of random excitation and spectral analysis in the study of frequency‐dependent parameters of the cardiovascular system. Circ Res 18, 585–595. [DOI] [PubMed] [Google Scholar]
- Tedford RJ (2014). Determinants of right ventricular afterload (2013 Grover Conference series). Pulm Circ 4, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ & Kass DA (2012). Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 125, 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD, Shapley RM & Knight BW (1977). Nonlinear analysis of cat retinal ganglion cells in the frequency domain. Proc Natl Acad Sci U S A 74, 3068–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg CE, Hertzberg JR, Ivy DD, Kirby KS, Chan KC, Valdes‐Cruz L & Shandas R (2004). Extraction of pulmonary vascular compliance, pulmonary vascular resistance, and right ventricular work from single‐pressure and Doppler flow measurements in children with pulmonary hypertension: a new method for evaluating reactivity: in vitro and clinical studies. Circulation 110, 2609–2617. [DOI] [PubMed] [Google Scholar]
- Wu Y & Kovacs SJ (2006). Frequency‐based analysis of the early rapid filling pressure‐flow relation elucidates diastolic efficiency mechanisms. Am J Physiol Heart Circ Physiol 291, H2942–H2949. [DOI] [PubMed] [Google Scholar]
- Yotti R, Bermejo J, Desco MM, Antoranz JC, Rojo‐Alvarez JL, Cortina C, Allue C, Rodriguez‐Abella H, Moreno M & Garcia‐Fernandez MA (2005). Doppler‐derived ejection intraventricular pressure gradients provide a reliable assessment of left ventricular systolic chamber function. Circulation 112, 1771–1779. [DOI] [PubMed] [Google Scholar]
- Yotti R, Bermejo J, Gutierrez‐Ibanes E, Perez del Villar C, Mombiela T, Elizaga J, Benito Y, Gonzalez‐Mansilla A, Barrio A, Rodriguez‐Perez D, Martinez‐Legazpi P & Fernandez‐Aviles F (2015). Systemic vascular load in calcific degenerative aortic valve stenosis: insight from percutaneous valve replacement. J Am Coll Cardiol 65, 423–433. [DOI] [PubMed] [Google Scholar]
- Zadeh LA (1950). Frequency analysis of variable networks. Proc IRE 38, 291–299. [Google Scholar]


