Abstract
Background
The relationship between albuminuria and insulin resistance (IR) has not been clarified in previous studies. This study was conducted to examine whether IR is associated with albuminuria in subjects with diverse blood pressure and glycometabolism statuses.
Methods
This study included 34 136 participants whose data were drawn from a cross‐sectional survey named the 2011 REACTION study. The participants were divided into six groups. The urinary albumin‐creatinine ratio (UACR) and glomerular filtration rate (GFR) were used as markers of chronic kidney disease (CKD). Variance tests and logistic regression models were performed for homeostatic model assessment of insulin resistance (HOMA‐IR) in relation to UACR and eGFR.
Results
First, UACR levels and HOMA‐IR exhibited a positive correlation among participants (P < 0.05), and a negative correlation existed between GFR and HOMA‐IR (P < 0.05). Second, in the hypertension with diabetes group, in individuals whose body mass index (BMI) was 18.5‐24.0 kg/m2, age was 50‐60 years old, low density lipoprotein cholesterol (LDL‐C) was 2.6‐3.4 mmol/L or high density lipoprotein cholesterol (HDL‐C) was 0.9‐1.55 mmol/L, HOMA‐IR was positively associated with UACR (P < 0.05). However, there was a negative correlation between GFR and HOMA‐IR in the hypertension with diabetes group in individuals whose BMI was 18.5‐24.0 kg/m2 or whose age was over 65 years old (P < 0.05).
Conclusions
In the context of different blood pressure and glycometabolism statuses, the positive correlation between UACR levels and HOMA‐IR was affected by BMI, age, LDL‐C, HDL‐C, and GFR. In patients with hypertension and diabetes, the early detection and intervention of IR and related risk factors in patients with normal BMI may reduce the occurrence of microalbuminuria and delay the progression of CKD.
Keywords: Albuminuria; Diabetes; Hypertension; Insulin resistance [Correction added on 2 April 2020, after first online publication: Keywords have been added.]
Highlights
In patients with hypertension and diabetes, early detection and intervention of insulin resistance and related risk factors in patients with normal BMI may reduce the occurrence of urinary microalbumin and delay the progression of CKD.
摘要
目的
既往文献研究中未阐明尿白蛋白与胰岛素抵抗的关系, 因此我们进一步探讨了胰岛素抵抗与尿白蛋白在中国不同血压和糖代谢状态人群中的关系。
方法
本研究共筛选纳入了34136名受试者, 研究数据来源于一项横断面调查, 即2011年“中国糖尿病患者肿瘤发生风险的纵向研究”(Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal study,REACTION)。将总人群根据不同血压及糖代谢状态分为六组。肾小球滤过率(GFR)和尿微量白蛋与肌酐比值(UACR)作为评估慢性肾脏疾病的标志物。计算稳态模型评估胰岛素抵抗指数(HOMA‐IR), 采用方差检验和多因素Logistic回归模型来研究UACR、GFR与HOMA‐IR的关系。
结果
首先, 总人群中UACR水平与HOMA‐IR有显著正相关关系(P<0.05), 而GFR和HOMA‐IR之间呈负相关关系(P <0.05)。其次, 在同时患有糖尿病和高血压的人群中, 体重指数(BMI)处于18.5‐24.0 kg/m2, 年龄为50‐60岁, 低密度脂蛋白胆固醇(LDL‐C)为2.6‐3.4 mmol/L或高密度脂蛋白胆固醇(HDL‐C)为0.9‐1.55 mmol/L的亚组人群里, HOMA‐IR与UACR呈正相关(P <0.05)。然而, 在BMI为18.5‐24.0 kg / m2或年龄超过65岁的高血压合并糖尿病人群中, GFR与HOMA‐IR存在负相关关系(P <0.05)。
结论
在不同的血压和糖代谢状态下, UACR水平与HOMA‐IR的正相关关系受BMI、年龄、LDL‐C、HDL‐C和GFR的影响。在高血压合并糖尿病患者中, 若早期对正常BMI患者的胰岛素抵抗及其相关的危险因素进行检测及干预, 可能会减低尿微量白蛋白的发生和延缓慢性肾脏疾病的进展。
Keywords: 尿微量白蛋白, 胰岛素抵抗, 高血压, 糖尿病
1. INTRODUCTION
Chronic kidney disease (CKD) is a common global public health problem. In recent years, as people's dietary habits and lifestyles have changed, the intake of sugar, fat, protein, and other substances has increased significantly, which has been coupled with a lack of physical exercise; the prevalence of CKD has increased year by year, and the prevalence rate is approximately 8%‐16%.1 Diabetes and hypertension are the most common causes of CKD and can result in the worsening of the disease2 by speeding up the progression of kidney disease, increasing cardiovascular mortality, and leading to acute kidney injury, impaired cognitive function, anemia, imbalanced bone salt metabolism, fracture, and other complications. It is estimated that by 2025, the number of people with diabetes worldwide will increase to 300 million.3 Therefore, the prevention of diabetes‐ or hypertension‐related kidney disease may be the key to reducing the social and economic burden of end‐stage renal disease (ESRD)‐induced diseases.4
Microalbuminuria is currently recognized as an independent risk factor for cardiovascular and cerebrovascular diseases and is also an indicator of the stability of CKD; microalbuminuria has been widely used in the clinic as the most sensitive and reliable early indicator of kidney disease. The prevalence of microalbuminuria in diabetic patients is 2‐3 times higher than that in healthy people, and the prevalence rate is more than 10%.5 The prevalence increases with increasing blood glucose levels; however, reduced insulin sensitivity may augment the risk of albuminuria.6 Studies have shown that microalbuminuria can occur concurrently with diabetes and that microalbuminuria predicts the risk of type 2 diabetes mellitus (T2DM). Microalbuminuria and metabolic syndrome (MetS) as well as its components (including abdominal obesity, blood lipids, blood pressure, blood glucose, etc.), insulin sensitivity, and insulin resistance (IR) are closely related. Currently, this area has become a popular research focus.
IR refers to the reduced in vivo activity of insulin in the promotion of peripheral tissue uptake and utilization of glucose, decreasing the biological effects of hepatic glycogen output inhibition, thus resulting in hyperinsulinemia and/or elevated blood glucose. IR often occurs in patients with MetS and is the central component of the pathogenesis of MetS. IR is mainly caused by abdominal obesity,7 and studies have shown that IR and obesity in individuals with diabetic renal injury are closely related.7, 8, 9, 10 Another study demonstrated that IgA nephropathy exists in most patients with IR as well.11 Therefore, IR is not only a risk factor for new‐onset diabetes, cardiovascular events, and all‐cause mortality but is also an independent risk factor for renal damage and ultimately renal failure. Recently, research on CKD and IR has been carried out throughout the world. However, the relationship between CKD and IR is not consistent among all ethnic groups and countries.12, 13, 14 In this regard, we conducted related research on blood pressure and glucose metabolism status based on data from China's Risk Evaluation of cAncers in Chinese diabeTic Individuals, a lONgitudinal (REACTION) study.
2. METHODS
2.1. Study subjects
A total of 53 639 participants from the REACTION study were investigated; the participants were recruited from 8 regional centers: Gansu, Guangdong, Guangxi, Henan, Hubei, Liaoning, Shanghai, and Sichuan. Subjects with primary kidney diseases, daily use of angiotensin converting enzyme inhibitor/angiotensin receptor blocker medicine, and unreasonable and missing data were excluded, and 34 136 participants were finally selected for baseline inclusion.
2.2. Medical history
The participants' basic information, including their general situation, past medical history, current medication situation, lifestyle, physical exercise, smoking and alcohol consumption habits, family history and other basic information, was collected using a standardized questionnaire. All involved investigators were formally trained.
2.3. Physical examination
The height, weight, waist circumference (WC), and hip circumference (HC) of the subjects were measured and recorded. Participants were required to take off their shoes, hats, and coats before the measurements were taken. While the participants stood with their feet shoulder width apart, their WC was measured in the horizontal plane at the midpoint of the ligature between the anterior superior spine and the inferior margin of the 12th rib. HC was measured as the horizontal circumference of the most prominent part of the hips while the participants stood with their feet together. All data were accurate to one decimal place.
2.4. Urinary albumin‐creatinine ratio (UACR) measurement and data processing
Urine samples were collected in the morning (midstream urine) for UACR measurements. Because the kits for measuring UACR in each center and the range of normal values are different, the value of UACR divided by the median of all values in the center at which the subject was attended (UACR/median) was used to estimate albuminuria. According to the statistics, natural logarithm transformation made albuminuria normally distributed. UACR data were categorized into four groups based on quartiles of logistic regression analysis: less than 25%, 25%‐50%, 50%‐75%, and greater than 75%.
2.5. Blood pressure measurement
Blood pressure was measured three times at 1 minute intervals after the subjects sat and rested for 5 minutes. The average of the three values was used for the analysis.
2.6. Laboratory index measurement
Blood samples were drawn in the morning after subjects fasted for 8 hours the previous night. Participants without a history of diabetes underwent a 75 g oral glucose tolerance test (OGTT), and their venous blood samples were drawn at 0 and 120 minutes. The biochemical indexes, included triglycerides (TGs), cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), serum creatinine (Scr), blood urea nitrogen (BUN), liver function indexes (alanine transaminase [ALT], aspartate aminotransferase [AST], gamma‐glutamyl transferase [GGT]), fasting blood glucose (FBG), postprandial blood glucose (PBG), glycosylated hemoglobin (HbA1c), fasting blood insulin, and postprandial blood insulin were measured by the glucose oxidase‐peroxidase method. Glomerular filtration rate (GFR) was estimated from the simplified equation developed using the Modification of Diet in Renal Disease (MDRD) data: GFR = 175 × (Scr in mg/dL)−1.154 × age−0.203 × (0.742 for women) × (1.212 if African American).15
2.7. Insulin resistance index
IR was assessed using the homeostatic model of insulin resistance index (HOMA‐IR) formula. HOMA‐IR was calculated as fasting plasma glucose (mmol/L) × fasting insulin (mU/L) / 22.5.16 According to the statistical requirements, natural logarithm conversion was performed to achieve a normal distribution. According to logistic regression analysis (HOMA‐IR percentiles) quartiles, HOMA‐IR data were divided into four groups: less than 25%, 25%‐50%, 50%‐75%, and more than 75%.
2.8. Statistical analysis
Statistical analysis was performed using SPSS software, version 20.0. All continuous variables are presented as the mean values and standard deviations (SDs). Measurement data were tested by analysis of variance or t test. Enumeration data were examined by chi‐square test. Pearson correlation analysis examined the relationships between two quantitative variables. The two classification variables were tested by a stepwise logistic regression analysis. P < 0.05 was considered statistically significant.
3. RESULTS
1. Baseline information: A total of 34 136 participants were included in the study. According to different blood pressure and glycometabolism statuses, the participants were divided into six groups: hypertension (HT, systolic blood pressure [SBP] ≥ 140 mmHg or diastolic blood pressure [DBP] ≥ 90 mmHg) with normal glucose tolerance (NGT, according to the OGTT), HT with prediabetes (pre‐DM), HT with diabetes mellitus (DM), normotension (NT) with NGT, NT with pre‐DM. and NT with DM. The clinical characteristics of the study participants are presented in Table 1 and Table 2. As the data showed, there were significant differences between the groups in regard to age, sex, body mass index (BMI), WC‐HC, ALT‐AST, TGs, TC, LDL‐C, GFR, and HOMA‐IR (P < 0.05). In the HT with pre‐DM or DM groups and the NT with pre‐DM or DM groups, UACR was associated with HOMA‐IR (P < 0.05). There was no correlation between UACR and HOMA‐IR in the other two groups (P > 0.05). There were significant differences between smoking/alcohol consumption habits and HOMA‐IR in the HT with NGT, pre‐DM or DM groups and in the NT with NGT or pre‐DM groups (P < 0.05). The other group exhibited the opposite result (P > 0.05).
Table 1.
Characteristics of study population by HOMA‐IR quartiles of HT
| HT with NGT group (n = 5286) | HT with pre‐DM group (n = 3964) | HT with DM group (n = 4459) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | L4nHOMA‐IR(1) | LnHOMA‐IR(2) | LnHOMA‐IR(3) | LnHOMA‐IR(4) | P value | LnHOMA‐IR(1) | LnHOMA‐IR(2) | LnHOMA‐IR(3) | LnHOMA‐IR(4) | P value | LnHOMA‐IR(1) | LnHOMA‐IR(2) | LnHOMA‐IR(3) | LnHOMA‐IR(4) | P value |
| n = 1339 | n = 1538 | n = 1516 | n = 893 | n = 537 | n = 812 | n = 1229 | n = 1386 | n = 318 | n = 538 | n = 1010 | n = 2593 | ||||
| Age | 60.93 ± 9.45 | 60.43 ± 9.21 | 59.20 ± 9.08 | 58.97 ± 9.41 | <0.001 | 63.67 ± 10.28 | 62.16 ± 9.09 | 61.62 ± 9.18 | 60.82 ± 9.36 | <0.001 | 64.21 ± 9.15 | 64.76 ± 9.29 | 63.68 ± 9.06 | 62.69 ± 8.88 | <0.001 |
| BMI,Kg/m2 | 23.31 ± 3.47 | 24.72 ± 3.31 | 25.76 ± 3.05 | 27.38 ± 3.17 | <0.001 | 23.22 ± 3.00 | 24.87 ± 3.08 | 26.01 ± 3.63 | 27.50 ± 3.50 | <0.001 | 23.20 ± 3.10 | 24.46 ± 3.05 | 25.47 ± 3.04 | 27.18 ± 3.70 | <0.001 |
| Wc‐hc | 0.89 ± 0.07 | 0.89 ± 0.07 | 0.90 ± 0.06 | 0.91 ± 0.06 | <0.001 | 0.89 ± 0.07 | 0.90 ± 0.07 | 0.90 ± 0.07 | 0.91 ± 0.07 | <0.001 | 0.89 ± 0.07 | 0.90 ± 0.06 | 0.91 ± 0.07 | 0.92 ± 0.06 | <0.001 |
| AST‐ALT | 1.65 ± 0.68 | 1.50 ± 0.51 | 1.40 ± 0.48 | 1.27 ± 0.50 | <0.001 | 1.67 ± 0.57 | 1.51 ± 0.51 | 1.40 ± 0.48 | 1.25 ± 0.45 | <0.001 | 1.65 ± 0.66 | 1.45 ± 0.53 | 1.37 ± 0.50 | 1.23 ± 0.47 | <0.001 |
| TG,mmol/L | 1.22 ± 0.73 | 1.46 ± 0.91 | 1.70 ± 1.04 | 2.03 ± 1.37 | <0.001 | 1.41 ± 0.97 | 1.64 ± 1.01 | 1.90 ± 1.19 | 2.12 ± 1.37 | <0.001 | 1.40 ± 1.07 | 1.65 ± 0.99 | 1.91 ± 1.18 | 2.28 ± 1.60 | <0.001 |
| CHOL,mmol/L | 4.96 ± 1.11 | 5.11 ± 1.15 | 5.21 ± 1.15 | 5.24 ± 1.13 | <0.001 | 4.89 ± 1.10 | 5.07 ± 1.17 | 5.18 ± 1.20 | 5.15 ± 1.19 | <0.001 | 4.83 ± 1.21 | 5.03 ± 1.23 | 5.13 ± 1.23 | 5.18 ± 1.23 | <0.001 |
| LDL,mmol/L | 2.92 ± 0.88 | 3.06 ± 0.91 | 3.11 ± 0.90 | 3.13 ± 0.90 | <0.001 | 2.82 ± 0.86 | 3.00 ± 0.91 | 3.05 ± 0.93 | 3.05 ± 0.92 | <0.001 | 2.79 ± 0.97 | 3.00 ± 0.93 | 3.01 ± 0.94 | 3.03 ± 0.97 | <0.001 |
| HDL,mmol/L | 1.42 ± 0.37 | 1.34 ± 0.35 | 1.29 ± 0.32 | 1.22 ± 0.29 | <0.001 | 1.37 ± 0.38 | 1.28 ± 0.34 | 1.26 ± 0.33 | 1.20 ± 0.30 | <0.001 | 1.34 ± 0.36 | 1.26 ± 0.34 | 1.24 ± 0.32 | 1.20 ± 0.30 | <0.001 |
| GFR(mL/min*1.73m2) | 94.80 ± 20.61 | 93.23 ± 19.52 | 91.60 ± 19.51 | 90.87 ± 19.43 | <0.001 | 94.94 ± 21.81 | 93.75 ± 21.33 | 92.07 ± 20.76 | 91.23 ± 20.55 | 0.001 | 95.45 ± 19.81 | 91.73 ± 26.17 | 89.86 ± 21.16 | 88.79 ± 20.77 | <0.001 |
| Ln(UACR/Median) | 0.20 ± 1.05 | 0.20 ± 1.13 | 0.23 ± 1.16 | 0.31 ± 1.28 | 0.099 | 0.22 ± 1.01 | 0.21 ± 1.00 | 0.32 ± 1.05 | 0.37 ± 1.24 | 0.004 | 0.30 ± 1.11 | 0.42 ± 1.09 | 0.47 ± 1.22 | 0.70 ± 1.31 | <0.001 |
| Sex(male) | 41.6(557) | 34.9(537) | 30.1(457) | 31.7 (283) | <0.001 | 48.9 (262) | 41.6 (338) | 399(32.5) | 31.5(436) | <0.001 | 58.2(185) | 48.0 (258) | 40.9(413) | 35.5 (920) | <0.001 |
| Smoking habits,% | <0.001 | <0.001 | <0.001 | ||||||||||||
| Never smoker | 79.9(1067) | 86.2(1317) | 88.1(1326) | 87.4(774) | 79.5(427) | 84.2(678) | 88.1(1072) | 88.9(1223) | 79.0(248) | 82.8(442) | 86.6(869) | 87.1(2245) | |||
| Sometimes smoker | 2.9(39) | 2.4(37) | 1.9(28) | 1.8(16) | 3.0(16) | 2.7(22) | 2.6(32) | 2.2(30) | 1.6(5) | 2.8(15) | 2.7(27) | 2.5(65) | |||
| Regular smoker | 17.2(229) | 11.4(174) | 10.0(151) | 10.8(96) | 17.5(94) | 13.0(105) | 9.3(113) | 8.9(122) | 19.4(61) | 14.4(77) | 10.7(107) | 10.4(267) | |||
| Drinking habits,% | <0.001 | <0.001 | <0.001 | ||||||||||||
| Never drinker | 71.1(949) | 75.7(1155) | 76.8(1159) | 74.9(665) | 69.6(374) | 71.1(572) | 77.0(936) | 77.5(1067) | 66.5(210) | 73.9(394) | 76.5(770) | 78.4(2019) | |||
| Sometimes drinker | 17.2(229) | 16.9(258) | 16.8(254) | 18.4(163) | 16.2(87) | 19.4(156) | 14.7(179) | 15.5(213) | 19.6(62) | 18.6(99) | 16.0(161) | 13.6(351) | |||
| Regular drinker | 11.7(156) | 7.4(113) | 6.4(97) | 6.8(60) | 14.2(76) | 9.5(76) | 8.3(101) | 7.0(96) | 13.9(44) | 7.5(40) | 7.5(76) | 8.0(205) | |||
Note: Data were mean ± SD or median (IQR) for skewed variables or numbers (proportions) for categorical variables.
AST‐ALT, aspartate aminotransferase to alanine aminotransferase rate; CHOL, cholesterol; BMI, body mass index; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL‐C, high density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hypertension (HT, SBP ≥140 mmHg or DBP ≥90 mmHg); LDL‐C, low density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglycerides; UACR, urinary albumin‐creatinine ratio; Wc‐hc,waist‐to‐height ratio.
Table 2.
Characteristics of study population by HOMA‐IR quartiles of NT
| NT with NGT group (n=11949) | NT with pre‐DM group (n=6205) | NT with DM group (n=2273) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | LnHOMA‐IR(1) | LnHOMA‐IR(2) | LnHOMA‐IR(3) | LnHOMA‐IR(4) | P value | LnHOMA‐IR(1) | LnHOMA‐IR(2) | LnHOMA‐IR(3) | LnHOMA‐IR(4) | P value | LnHOMA‐IR(1) | LnHOMA‐IR(2) | LnHOMA‐IR(3) | LnHOMA‐IR(4) | P value |
| n=4883 | n=3825 | n=2295 | n=946 | n=1305 | n=1565 | n=1772 | n=1563 | n=208 | n=338 | n=564 | n=1163 | ||||
| Age,years | 54.60±8.17 | 54.33±8.13 | 53.89±7.83 | 53.98±7.96 | 0.002 | 57.96±9.76 | 56.88±9.02 | 56.53±8.59 | 56.07±8.37 | <0.001 | 60.22±8.69 | 59.17±8.97 | 59.22±8.87 | 58.43±8.92 | 0.03 |
| BMI,Kg/m2 | 22.10±3.07 | 23.55±2.96 | 24.69±3.23 | 25.95±3.40 | <0.001 | 22.12±3.12 | 23.77±3.26 | 24.79±3.10 | 26.42±3.28 | <0.001 | 22.19±2.99 | 23.67±3.26 | 24.50±3.85 | 25.94±3.47 | <0.001 |
| Wc‐hc | 0.86±0.07 | 0.87±0.07 | 0.88±0.08 | 0.89±0.07 | <0.001 | 0.87±0.07 | 0.88±0.08 | 0.89±0.06 | 0.90±0.07 | <0.001 | 0.88±0.06 | 0.89±0.06 | 0.90±0.07 | 0.91±0.06 | <0.001 |
| AST‐ALT | 1.61±0.52 | 1.50±0.50 | 1.38±0.47 | 1.26±0.46 | <0.001 | 1.68±0.88 | 1.50±0.53 | 1.39±0.50 | 1.27±0.47 | <0.001 | 1.59±0.82 | 1.39±0.55 | 1.33±0.52 | 1.19±0.41 | <0.001 |
| TG,mmol/L | 1.12±0.74 | 1.33±0.84 | 1.55±1.04 | 1.91±1.27 | <0.001 | 1.24±0.82 | 1.58±1.06 | 1.87±1.38 | 2.06±1.36 | <0.001 | 1.43±1.36 | 1.57±1.33 | 1.78±1.17 | 2.21±1.78 | <0.001 |
| CHOL,mmol/L | 4.77±1.15 | 4.91±1.15 | 4.97±1.19 | 5.05±1.22 | <0.001 | 4.73±1.21 | 4.92±1.19 | 4.95±1.21 | 5.04±1.24 | <0.001 | 4.69±1.10 | 4.81±1.12 | 4.87±1.22 | 5.02±1.23 | <0.001 |
| LDL,mmol/L | 2.76±0.88 | 2.90±0.88 | 2.96±0.94 | 3.02±0.95 | <0.001 | 2.72±0.89 | 2.89±0.90 | 2.88±0.90 | 2.98±0.97 | <0.001 | 2.69±0.78 | 2.79±0.82 | 2.85±0.95 | 2.94±0.95 | <0.001 |
| HDL,mmol/L | 1.41±0.37 | 1.35±0.36 | 1.29±0.33 | 1.22±0.34 | <0.001 | 1.36±0.39 | 1.28±0.33 | 1.23±0.33 | 1.19±0.31 | <0.001 | 1.29±0.34 | 1.26±0.32 | 1.22±0.35 | 1.17±0.29 | <0.001 |
| GFR(mL/min*1.73m²) | 99.37±21.90 | 97.40±21.34 | 97.25±20.94 | 96.46±21.59 | <0.001 | 101.28±23.58 | 98.29±20.77 | 96.43±20.28 | 95.37±20.40 | <0.001 | 98.46±24.73 | 98.20±19.79 | 96.30±22.51 | 94.23±22.01 | 0.004 |
| Ln(UACR/Median) | ‐0.14±1.00 | ‐0.13±0.99 | ‐0.17±1.04 | ‐0.08±1.05 | 0.07 | ‐0.13±0.96 | ‐0.09±0.89 | ‐0.05±0.88 | ‐0.01±0.94 | <0.001 | 0.04±0.93 | 0.19±0.95 | 0.13±1.13 | 0.31±1.11 | <0.001 |
| Sex(male) | 31.4 (1531) | 24.2(927) | 23.4 (580) | 23.3 (234) | <0.001 | 46.2(603) | 30.0(469) | 26.8(475) | 25.8(404) | <0.001 | 53.8(112) | 51.5(174) | 44.1(249) | 41.1(478) | <0.001 |
| Smoking habits,% | <0.001 | <0.001 | 0.0386 | ||||||||||||
| Never smoker | 81.8(3947) | 86.4(3289) | 88.2(2167) | 87.6(872) | 77.8(1008) | 85.4(1327) | 88.2(1544) | 87.3(1351) | 76.3(158) | 76.6(256) | 79.9(448) | 79.4(914) | |||
| Sometimes smoker | 2.2(105) | 2.0(78) | 2.9(72) | 2.4(24) | 3.7(48) | 3.0(47) | 2.7(47) | 3.2(50) | 2.9(6) | 4.8(16) | 4.6(26) | 3.3(38) | |||
| Regular smoker | 16.1(775) | 11.5(438) | 8.9(219) | 10.0(100) | 18.5(240) | 11.5(179) | 9.1(160) | 9.5(147) | 20.8(43) | 18.6(62) | 15.5(87) | 17.3(199) | |||
| Drinking habits,% | <0.001 | <0.001 | 0.483 | ||||||||||||
| Never drinker | 70.5(3414) | 73.3(2786) | 75.6(1853) | 75.8(755) | 66.4(860) | 72.5(1126) | 76.4(1343) | 76.3(1185) | 70.7(145) | 71.9(241) | 75.2(422) | 74.6(862) | |||
| Sometimes drinker | 22.6(1093) | 22.0(834) | 20.3(497) | 19.6(195) | 22.9(297) | 21.2(329) | 19.8(348) | 18.9(294) | 20.5(42) | 22.1(74) | 18.4(103) | 17.9(207) | |||
| Regular drinker | 6.9(334) | 4.7(179) | 4.2(102) | 4.6(46) | 10.7(138) | 6.4(99) | 3.9(68) | 4.8(74) | 8.8(18) | 6.0(20) | 6.4(36) | 7.5(87) | |||
Note: Data were mean ± SD or median (IQR) for skewed variables or numbers (proportions) for categorical variables.
AST‐ALT, aspartate aminotransferase to alanine aminotransferase rate; BMI, body mass index; CHOL, cholesterol; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL‐C, high density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hypertension (HT, SBP ≥140 mmHg or DBP ≥90 mmHg); IQR, interquartile range; LDL‐C, low density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglycerides; UACR, urinary albumin‐creatinine ratio; Wc‐hc,waist‐to‐height ratio.
2. Logistical correlation analysis: There was a positive correlation between HOMA‐IR and UACR in the total sample of participants and in the hypertension with diabetes group (P < 0.05, Table 3, Figure 1), and a negative correlation existed between GFR and HOMA‐IR (P < 0.05, Table 4, Figure 1). The correlation was adjusted for only age and sex or was fully adjusted for sex, age, BMI, WC‐HC, AST‐ALT, SBP, DBP, TGs, GGT, TC, HDL‐C, LDL‐C, heart rate (HR), tumor history, myocardial infarction history, stroke history, smoking habits, and alcohol consumption habits.
Table 3.
Correlation between UACR and IR in total participants or HT with DM group
| OR | LnHOMA‐IR(1) (Quartile one) | LnHOMA‐ IR(2) (Quartile two) | LnHOMA‐ IR(3) (Quartile three) | LnHOMA‐IR(4) (Quartile four) | P value | |
|---|---|---|---|---|---|---|
| total | Age and sex adjusted | 0.59 | 0.63 | 0.71 | 1 | <0.001 |
| (n = 34 136) | Multivariate adjusted | 0.63 | 0.65 | 0.72 | 1 | <0.001 |
|
HT with DM group (n = 4459) |
Age and sex adjusted | 0.61 | 0.68 | 0.75 | 1 | <0.001 |
| Multivariate adjusted | 0.65 | 0.75 | 0.79 | 1 | <0.003 |
Figure 1.
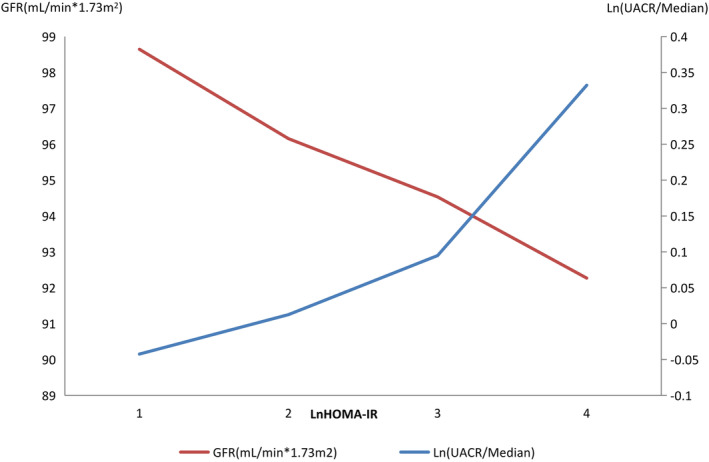
Relationship between UACR and GFR and HOMA‐IR in total participants; UACR, urinary albumin‐creatinine ratio; GFR, glomerular filtration rate; HOMA‐IR, homeostatic model assessment of insulin resistance
Table 4.
Correlation between GFR and IR in total participants or HT with DM group
| OR | LnHOMA‐IR(1) (Quartile one) | LnHOMA‐ IR(2) (Quartile two) | LnHOMA‐ IR(3) (Quartile three) | LnHOMA‐IR(4) (Quartile four) | P value | |
|---|---|---|---|---|---|---|
|
total (n = 34 136) |
Age and sex adjusted | 1.5 | 1.28 | 1.15 | 1 | <0.001 |
| Multivariate adjusted | 1.5 | 1.27 | 1.16 | 1 | <0.001 | |
|
HT with DM group (n = 4459) |
Age and sex adjusted | 2.02 | 1.3 | 1.18 | 1 | 0.014 |
| Multivariate adjusted | 2.04 | 1.22 | 1.17 | 1 | 0.047 |
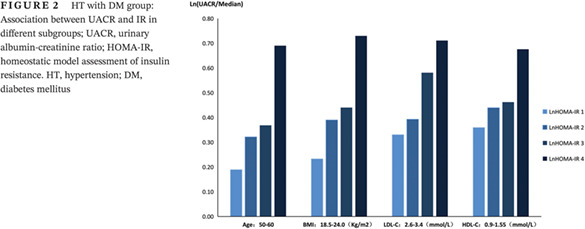
3. Diverse subgroups in the HT with DM group: Our study showed that in the HT with diabetes group, in individuals with a BMI from 18.5‐24.0 kg/m2, age from 50‐60 years old, LDL‐C from 2.6‐3.4 mmol/L or HDL‐C from 0.9‐1.55 mmol/L, HOMA‐IR was positively associated with UACR (P < 0.05, Table 5). The higher the IR index, the higher the UACR (Figure 2). There was also a negative correlation between GFR and HOMA‐IR in the hypertension with diabetes group, in which the BMI was 18.5‐24.0 kg/m2 or age was over 65 years old (P < 0.05, Table 6,Figure 3).
Table 5.
HT with DM group: the relationship between UACR and IR in diverse subgroup
| Variable | LnHOMA‐IR(1) | LnHOMA‐IR(2) | LnHOMA‐IR(3) | LnHOMA‐IR(4) | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR | P value | OR | P value | OR | P value | OR | P value | ||
| TG(mmol/L) | <0.56 | 1.05 | >0.05 | 27.22 | >0.05 | 8.16 | >0.05 | 1 | — |
| 0.56‐1.7 | 0.7 | 0.022 | 1.22 | >0.05 | 0.82 | >0.05 | 1 | — | |
| >1.7 | 0.74 | >0.05 | 1.46 | 0.015 | 0.81 | 0.048 | 1 | — | |
| BMI(Kg / m2) | <18.5 | 6.1 | >0.05 | 8.91 | >0.05 | 1.98 | >0.05 | 1 | — |
| 18.5–24.0 | 0.46 | 0 | 0.6 | 0.003 | 0.68 | 0.01 | 1 | — | |
| 24.0‐27.9 | 0.81 | >0.05 | 0.8 | >0.05 | 0.92 | >0.05 | 1 | — | |
| ≥28.0 | 0.62 | >0.05 | 0.91 | >0.05 | 0.68 | 0.02 | 1 | — | |
| Sex | Male | 0.71 | 0.049 | 0.68 | 0.007 | 0.82 | >0.05 | 1 | — |
| Female | 0.68 | 0.041 | 0.89 | >0.05 | 0.81 | 0.026 | 1 | — | |
| Age(years old) | ≤50 | 2.22 | >0.05 | 0.93 | >0.05 | 0.55 | 0.048 | 1 | — |
| 50–60 | 0.48 | 0.001 | 0.6 | 0.003 | 0.71 | 0.011 | 1 | — | |
| 60‐70 | 0.97 | >0.05 | 0.82 | >0.05 | 0.8 | >0.05 | 1 | — | |
| >70 | 0.47 | 0.004 | 0.86 | >0.05 | 1.04 | >0.05 | 1 | — | |
| LDL‐C(mmol/L) | <1.8 | 1.07 | >0.05 | 0.92 | >0.05 | 1.29 | >0.05 | 1 | — |
| 1.8‐2.6 | 0.68 | >0.05 | 0.67 | 0.05 | 0.76 | >0.05 | 1 | — | |
| 2.6–3.4 | 0.49 | 0.004 | 0.65 | 0.015 | 0.82 | >0.05 | 1 | — | |
| ≥3.4 | 0.65 | >0.05 | 0.76 | >0.05 | 0.7 | 0.05 | 1 | — | |
| HDL‐C(mmol/L) | <0.9 | 0.7 | >0.05 | 0.87 | >0.05 | 0.95 | >0.05 | 1 | — |
| 0.9–1.55 | 0.66 | 0.006 | 0.76 | 0.018 | 0.79 | 0.005 | 1 | — | |
| >1.55 | 0.66 | >0.05 | 0.66 | >0.05 | 0.76 | >0.05 | 1 | — | |
| Regionalism | south | 0.56 | 0.002 | 0.74 | 0.046 | 0.85 | >0.05 | 1 | — |
| north | 0.79 | >0.05 | 0.76 | 0.037 | 0.79 | 0.012 | 1 | — | |
BMI, body mass index; DM, diabetes mellitus; HDL‐C, high density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL‐C, low density lipoprotein cholesterol OR, odds ratio; TG, triglycerides; UACR, urinary albumin‐creatinine ratio.
Figure 2.
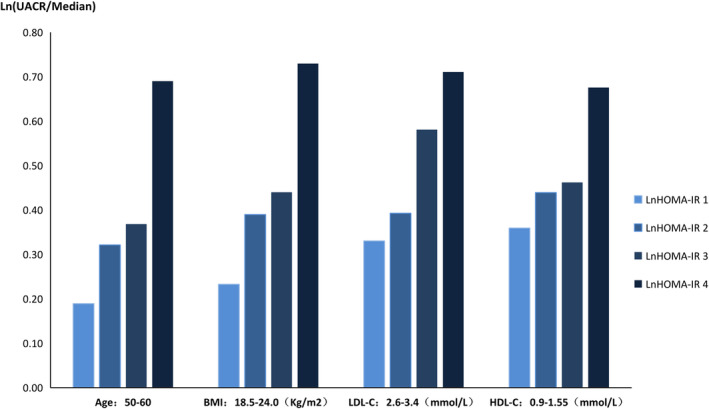
HT with DM group: Association between UACR and IR in different subgroups; UACR, urinary albumin‐creatinine ratio; HOMA‐IR, homeostatic model assessment of insulin resistance. HT, hypertension; DM, diabetes mellitus
Table 6.
HT with DM group: the relationship between GFR and IR in diverse subgroup
| Variable | LnHOMA‐IR(1) | LnHOMA‐IR(2) | LnHOMA‐IR(3) | LnHOMA‐IR(4) | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR | P value | OR | P value | OR | P value | OR | P value | ||
| BMI(Kg / m2) | <18.5 | 6515 | >0.05 | 758 | >0.05 | 696 623 | >0.05 | 1 | — |
| 18.5–24.0 | 3.3 | 0 | 1.48 | 0.02 | 1.34 | 0.046 | 1 | — | |
| 24.0‐27.9 | 1.39 | >0.05 | 1.21 | >0.05 | 1.15 | >0.05 | 1 | — | |
| ≥28.0 | 2.01 | >0.05 | 1.06 | >0.05 | 1.21 | >0.05 | 1 | — | |
| Sex | Male | 1.74 | 0.002 | 1.26 | >0.05 | 0.95 | >0.05 | 1 | — |
| Female | 2.39 | 0 | 1.16 | >0.05 | 0.35 | 0.002 | 1 | — | |
| Age(years old) | <45 | 0.87 | >0.05 | 0.22 | >0.05 | 0.5 | >0.05 | 1 | — |
| 45‐55 | 1.28 | >0.05 | 0.81 | >0.05 | 1.13 | >0.05 | 1 | — | |
| 55‐65 | 2.09 | >0.05 | 1.24 | >0.05 | 1.08 | >0.05 | 1 | — | |
| ≥65 | 2.28 | 0 | 1.49 | 0.011 | 1.29 | 0.037 | 1 | — | |
| LDL‐C(mmol/L) | <1.8 | 2.47 | 0.026 | 0.92 | >0.05 | 1.29 | >0.05 | 1 | — |
| 1.8–2.6 | 2.53 | 0 | 1.09 | >0.05 | 0.95 | >0.05 | 1 | — | |
| 2.6–3.4 | 1.51 | >0.05 | 1.49 | 0.02 | 1.05 | >0.05 | 1 | — | |
| ≥3.4 | 1.77 | 0.02 | 1.18 | >0.05 | 1.48 | 0.002 | 1 | — | |
| HDL‐C(mmol/L) | <0.9 | 2.86 | 0.032 | 1.02 | >0.05 | 0.87 | >0.05 | 1 | — |
| 0.9–1.55 | 1.92 | 0 | 1.2 | >0.05 | 1.12 | >0.05 | 1 | — | |
| >1.55 | 2.69 | 0.001 | 1.45 | >0.05 | 1.69 | 0.014 | 1 | — | |
| Regionalism | south | 2.22 | 0 | 1.21 | >0.05 | 1.26 | >0.05 | 1 | — |
| north | 2.15 | 0 | 1.32 | 0.039 | 1.13 | >0.05 | 1 | — | |
BMI, body mass index; DM, diabetes mellitus; GFR, glomerular filtration rate; HDL‐C, high density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL‐C, low density lipoprotein cholesterol; OR, odds ratio.
Figure 3.
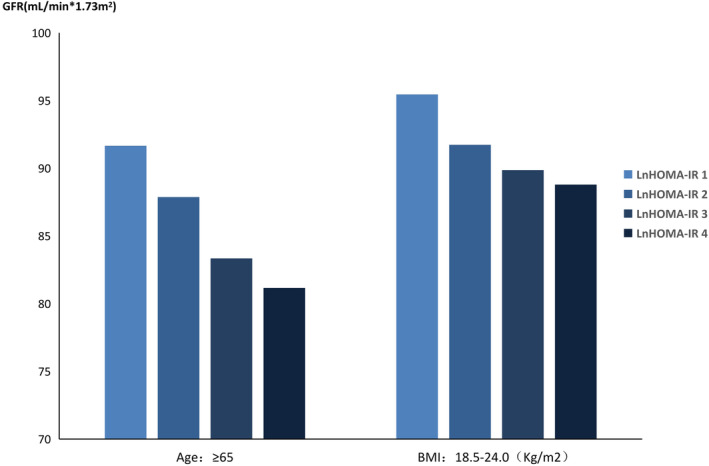
HT with DM group: Association between GFR and IR in different subgroups; GFR, glomerular filtration rate; HOMA‐IR, homeostatic model assessment of insulin resistance; HT, hypertension; DM, diabetes mellitus
4. DISCUSSION
As early as 1974, Weisinger et al first reported that high levels of albuminuria were detected in patients with severe obesity, and the corresponding pathological biopsy suggested kidney damage. Subsequent similar cases were summarized, and this disease was defined as obesity‐related nephropathy (BMI > 30 kg/m2).17 So far, the pathogenesis of the disease remains unclear, although many investigators are committed to studying the disease. Obese patients are often associated with IR, which can damage renal podocytes and cause renal hemodynamic disturbances by affecting the podocyte insulin pathway. Numerous studies have shown that IR is an independent risk factor for kidney damage and ultimately renal failure.18, 19 Clinical studies have indicated that insulin sensitizers or insulin‐sensitizing hypoglycemic drugs can increase the body's sensitivity to insulin while improving endothelial function, having a significant protective effect on the cardiovascular system and kidneys, but these drugs can also significantly reduce the urinary albumin excretion rate.20 The United States Kidney Disease Outcome Quality Initiative (K/DOQI) recommended screening for microalbuminuria in people with a high risk of kidney disease and cardiovascular disease. This index has good stability. Chen et al and Srivastava et al reported that CKD was positively associated with HOMA‐IR in the United States and India.21, 22 In Korean adults with or without T2DM, insulin resistance was positively associated with CKD.23 In contrast, Satirapoj et al and Trirogoff et al reported that HOMA‐IR had no significant correlation with GFR in the United States and the United Kingdom.24, 25 It was reported that in the Indian CKD patient group, estimated GFR did not correlate with the degree of IR.21 In the present study, the relationship between IR and microalbuminuria in groups with different blood pressure and glucose metabolism statuses was studied by grouping individuals by blood pressure (SBP ≥140 mmHg or DBP ≥90 mmHg) and glucose tolerance according to the results from a 75 g OGTT. The association between albuminuria and IR was mainly analyzed in the HT with diabetes group due to the significant correlation. Our study showed that HOMA‐IR was significantly associated with age, sex, BMI, WC‐HC, GFR, ALT/AST, TGs, CHOL, LDL‐C, and HDL‐C. In participants with prediabetes and diabetes, UACR and IR had statistically significant differences, independent of blood pressure. Thus, UACR was not associated with IR in individuals with NGT. IR most often occurs in patients with MetS and is the central component of the pathogenesis of MetS. In regard to BMI, WC‐HC ratio, and blood lipids, our study's results are consistent with those of previous reports. A large prospective longitudinal survey using community‐based risk of atherosclerosis (Atherosclerosis Risk in Communities, ARIC) showed that MetS and HOMA‐IR quintiles were correlated with the incidence of CKD in adults without diabetes after controlling for the development of DM and HT.26 However, our research showed no correlation between HOMA‐IR and UACR in NGT patients, which is not in accordance with the conclusion of the aforementioned study. This difference may be related to the prediabetes population, that is, the population of individuals with impaired glucose tolerance and impaired fasting glucose but not meeting the criteria for the standard diagnosis of diabetes. HOMA‐IR is associated with UACR in these individuals, suggesting that IR should be observed in people with impaired glucose tolerance and diabetes. The occurrence and aggravation of IR may predict the progression of microalbuminuria. In addition, the findings also showed that there was a significant correlation between HOMA‐IR and GFR in the total sample of participants, which suggested that IR may affect GFR levels in individuals with different glucose tolerance and blood pressure statuses.
Recent research has demonstrated that IR may promote the development of microalbuminuria among type 1 diabetic patients,27 type 2 diabetic patients,28 and hypertensive patients.29 In this study, stratified regression analysis of hypertensive patients with DM by age, sex, BMI, TGs, LDL‐C, HDL‐C, and geographical region showed that in the individuals in whom BMI was 18.5‐24.0 kg/m2 or age ranged from 50 to 60 years old, HOMA‐IR and UACR both exhibited positive correlations and regression relationships. Thus, this finding indicated that, in this part of the population, the lower the insulin sensitivity or the higher the resistance index, the higher the urine microalbumin, which is associated with kidney damage risk. In general, a HOMA‐IR interquartile range greater than 75% is considered insulin resistance. This study showed that among the hypertensive population with diabetes, when the BMI remained at 18.5‐24.0 kg/m2, the risk of developing microalbuminuria decreased by at least 32% in the population without insulin resistance compared to the population with IR. Similarly, the risk was at least 29% lower in people aged 50‐60 years. In terms of BMI, our findings were different from those of previous studies. IR was associated with the incidence of CKD in ARIC research, and this relationship was not associated with BMI. In previous studies, all participants were of black or white race and aged 45‐64.26 Other studies have shown that, although IR and MetS are common in the Chinese population with normal weight, they are not related to CKD. IR is associated with the development of CKD in obese and overweight people.12 However, our study demonstrated that HOMA‐IR is positively associated with the occurrence of microalbuminuria in hypertensive Chinese participants with normal body mass and diabetes, and this relationship was independent of weight loss and obesity/overweight status. In the diverse subgroup analysis, GFR and IR were also affected by different BMI status. In the normal BMI participants, the more severe the IR, the lower the GFR. This finding indicated that the population with a BMI of 18.5‐24 kg/m2 could reduce or delay the progression of microalbuminuria and GFR by managing the development of IR and MetS. The differences in these studies may be related to race, age, and selected population.
Previous studies have shown that abdominal obesity, elevated triglycerides, decreased levels of HDL‐C, elevated blood pressure, and elevated fasting glucose levels are risk factors for CKD.26 Hypertensive renal blood flow in patients with automatic regulation of dysfunction leads to glomerular long‐term high perfusion and reduces the charge of the filtration membrane, thus causing substantial albumin leakage and the formation of proteinuria. Hyperinsulinemia, diabetes, etc., promote the development of kidney disease through the worsening of renal hemodynamics, the release of inflammatory cytokines and the stress of the renal endoplasmic reticulum. This study attempted to find a clear and meaningful point among these risk factors that had indications for the management of CKD risk. The results showed that when the blood lipid level was controlled at 2.6 mmol/L ≤ LDL‐C<3.4 mmol/L or 0.9 mmol/L ≤ HDL‐C ≤ 1.55 mmol/L in individuals with hypertension and diabetes, HOMA‐IR was positively associated with urinary microalbumin, which suggested that in this numerical range, the risk of urinary microalbumin can be reduced by decreasing IR. Urine microalbumin in the early diagnosis and prevention of CKD has important value.30
In conclusion, HOMA‐IR was significantly associated with basic clinical parameters (BMI, WC‐HC, GFR, ALT‐AST, TGs, TC, LDL‐C, and HDL‐C) in the general population. HOMA‐IR was positively associated with UACR, but this relationship was not found in the NGT group. In normotensive patients with DM, IR had no correlation with smoking habits or alcohol consumption habits. In different blood pressure and glycometabolism situations, the positive correlation between UACR and HOMA‐IR was affected by BMI, age, LDL‐C, and HDL‐C levels. Similarly, the negative correlation between GFR and HOMA‐IR was affected by BMI and age. Above all, the IR index is associated with UACR and GFR in normal weight individuals with HT and diabetes. In the HT with DM group, the early detection and intervention of IR and its related risk factors in patients with a BMI from 18.5‐24.0 kg/m2 may reduce the occurrence of microalbuminuria and delay the progression of CKD, resulting in more clinical benefits.
AUTHORS' CONTRIBUTIONS
Shi GU analyzed the data and wrote the manuscript. Anping WANG and Linxi ZHANG performed statistical analysis. Guang NING and Yiming MU searched data. Yiming MU reviewed the manuscript. All authors read and approved the final manuscript.
DISCLOSURE OF INTEREST
The authors declare that they have no competing interests.
AVAILABILITY OF DATA AND MATERIALS
The datasets used to support this study are not freely available in view of participants' privacy protection.
CONSENT FOR PUBLICATION
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the Committee on Human Research at Rui‐Jin Hospital affiliated with the School of Medicine, Shanghai Jiao Tong University. Written informed consents were obtained from all participants before data collection.
ACKNOWLEDGEMENT
We would like to thank the participants and their families for participating in this study.
Gu S, Wang A, Ning G, Zhang L, Mu Y. Insulin resistance is associated with urinary albumin‐creatinine ratio in normal weight individuals with hypertension and diabetes: The REACTION study. Journal of Diabetes. 2020;12:406–416. 10.1111/1753-0407.13010
Funding information Present work was supported by Chinese Society of Endocrinology, the Key Laboratory for Endocrine and Metabolic Diseases of Ministry of Health (1994DP131044), the National Key New Drug Creation and Manufacturing Program of Ministry of Science and Technology (2012ZX09303006‐001), the National High Technology Research and Development Program of China (863 Program, 2011AA020107). National Science and Technology Major Project 288 (2011ZX09307‐001‐08).
Footnotes
Note: Multivariate adjusted: Adjusted for sex, age, BM, Wc‐hc, AST‐ALT, SBP, DBP, TG, GGT, CHOL, HDL‐C, LDL‐C, HR, smoking, drinking, history of oncology, history of myocardial infarction, history of stroke.
AST‐ALT, aspartate aminotransferase to alanine aminotransferase rate; BM, body mass; CHOL, cholesterol; DBP, diastolic blood pressure; DM, diabetes mellitus; GGT, gamma‐glutamyl transpeptidase; HDL‐C, high density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; HR, heart rate; HT, hypertension; LDL‐C, low density lipoprotein cholesterol; OR, odds ratio; SBP, systolic blood pressure; TG, triglycerides; UACR, urinary albumin‐creatinine ratio; Wc‐hc,waist‐to‐height ratio.
Note: Multivariate adjusted: Adjusted for sex, age, BM, Wc‐hc, AST‐ALT, SBP, DBP, TG, GGT, CHOL, HDL‐C, LDL‐C, HR, smoking, drinking, history of oncology, history of myocardial infarction, history of stroke.
AST‐ALT, aspartate aminotransferase to alanine aminotransferase rate; BMI, body mass index; CHOL, cholesterol; DBP, diastolic blood pressure; DM, diabetes mellitus; GFR, glomerular filtration rate; GGT, gamma‐glutamyl transpeptidase; HDL‐C, high density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; HR, heart rate; HT, hypertension; LDL‐C, low density lipoprotein cholesterol; OR, odds ratio; SBP, systolic blood pressure; TG, triglycerides; UACR, urinary albumin‐creatinine ratio; Wc‐hc,waist‐to‐height ratio.
REFERENCE
- 1. Jha V, Garcia‐Garcia G, Iseki K, Li Z, Yang C‐W. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260‐272. [DOI] [PubMed] [Google Scholar]
- 2. Kuritzky L, Toto R, Van Buren P. Identification and management of albuminuria in the primary care setting. J Clin Hypertens. 2011;13:438‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995‐2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414‐1431. [DOI] [PubMed] [Google Scholar]
- 4. Chen J, Wildman RP, Hamm LL, et al. Association between inflammation and insulin resistance in US nondiabetic adults. Diabetes Care. 2004;27:2960‐2965. [DOI] [PubMed] [Google Scholar]
- 5. Rasmussen JB, Thomsen PR, Parkinson S, et al. Diabetes mellitus, hypertension and albuminuria in rural Zambia: a hospital‐based survey. Trop Med Int Health. 2013;18:1080‐1084. [DOI] [PubMed] [Google Scholar]
- 6. Pilz S, Rutters F, Nijpels G, Stehouwer CDA, et al. Insulin sensitivity and albuminuria: the RISC Study. Diabetes Care. 2014;37:1597‐1603. [DOI] [PubMed] [Google Scholar]
- 7. Bagby SP. Obesity‐initiated metabolic syndrome and the kidney: a recipe for chronic kidney disease? J Am Soc Nephrol. 2004;15:2775‐2791. [DOI] [PubMed] [Google Scholar]
- 8. Mykkänen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, et al. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793‐800. [DOI] [PubMed] [Google Scholar]
- 9. De Cosmo S, Trevisan R, Minenna A, Vedovato M, Viti R, et al. Insulin resistance and the cluster of abnormalities related to the metabolic syndrome are associated with reduced glomerular filtration rate in patients with type 2 diabetes. Diabetes Care. 2006;29:432‐434. [DOI] [PubMed] [Google Scholar]
- 10. De Cosmo S, Menzaghi C, Prudente S, Trischitta V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant. 2012;28:29‐36. [DOI] [PubMed] [Google Scholar]
- 11. Lin ZQ, Guang‐ping S, Li LO, et al. Factors related to insulin resistance in IgA nephropathy. Chin Gen Pract. 2016;19:3270‐3274. [Google Scholar]
- 12. Chen S, Chen Y, Liu X, Li M, Wu B, et al. Association of insulin resistance with chronic kidney disease in non‐diabetic subjects with normal weight. PLoS One. 2013;8:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryu S, Chang Y, Woo HY, et al. Time‐dependent association between metabolic syndrome and risk of CKD in Korean men without hypertension or diabetes. Am J Kidney Dis. 2009;53:59‐69. [DOI] [PubMed] [Google Scholar]
- 14. Johns BR, Pao AC, Kim SH. Metabolic syndrome, insulin resistance and kidney function in non‐diabetic individuals. Nephrol Dial Transplant. 2012;27:1410‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15‐E26. [DOI] [PubMed] [Google Scholar]
- 17. Moriyama T, Tanaka K, Iwasaki C, et al. Prognosis in IgA nephropathy: 30‐year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui MJ, Zhang BH, Xiao QF, et al. The relationship between hyperuricaemia and clinic pathology of IgA nephropathy. Chin J Int Med. 2011;50:659‐663. (In Chinese). [PubMed] [Google Scholar]
- 19. SHI Y, CHEN W, JALAL D, et al. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney Blood Press Res. 2012;35:153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D C C, COPPO R, et al. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, The Oxford classification of IgA nephropathy: rationale,clinicopathological correlations,and classification. Kidney Int. 2009;76:534‐545. [DOI] [PubMed] [Google Scholar]
- 21. Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, et al. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol. 2003;14:469‐477. [DOI] [PubMed] [Google Scholar]
- 22. Srivastava N, Singh RG, Usha KA, Singh S. Insulin resistance in predialytic, nondiabetic, chronic kidney disease patients: a hospital‐based study in Eastern Uttar Pradesh, India. Saudi J Kidney Dis Transpl. 2017;28:36‐43. [DOI] [PubMed] [Google Scholar]
- 23. Kim GS, Kim SG, Kim HS, Hwang EY, Lee JH, et al. The relationship between chronic kidney function and homeostasis model assessment of insulin resistance and beta cell function in Korean adults with or without type 2 diabetes mellitus. Endocr J. 2017;64:1181‐1190. [DOI] [PubMed] [Google Scholar]
- 24. Satirapoj B, Supasyndh O, Boonyavarakul A, Luesutthiviboon L, Choovichian P. The correlation of insulin resistance and renal function in non diabetic chronic kidney disease patients. J Med Assoc Thail. 2005;88:S97‐S104. [PubMed] [Google Scholar]
- 25. Trirogoff ML, Shintani A, Himmelfarb J, Ikizler TA. Body mass index and fat mass are the primary correlates of insulin resistance in nondiabetic stage 3–4 chronic kidney disease patients. Am J Clin Nutr. 2007;86:1642‐1648. [DOI] [PubMed] [Google Scholar]
- 26. Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134‐2140. [DOI] [PubMed] [Google Scholar]
- 27. Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburg Epidemiology of Diabetes Complications Study. Kidney Int. 2002;62:963‐970. [DOI] [PubMed] [Google Scholar]
- 28. Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and micoralbuminuria a cross‐sectional, casecontrol study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456‐1462. [DOI] [PubMed] [Google Scholar]
- 29. Agewall S, Fagerberg B, Attvall S, et al. Microalbuminuria, insulin sensitivity and haemostatic factors in non‐diabetic treated hypertensive men risk factor intervention study group. J Intern Med. 1995;237:195‐203. [DOI] [PubMed] [Google Scholar]
- 30. Rezi W, Budula A, Xue S. Value analysis of urine microalbumin in the early diagnosis and prevention of hypertensive nephropathy. Mod Med Health Res. 2017;1:138‐138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used to support this study are not freely available in view of participants' privacy protection.


