Abstract
Background:
There is limited information on the prevalence of SARS-CoV-2 infection in obstetric settings in Canada, beyond the first wave of the COVID-19 pandemic (February to June 2020). We sought to describe the prevalence of SARS-CoV-2 infection in pregnant people admitted to triage units at a tertiary care hospital in Ottawa, Canada.
Methods:
We conducted a descriptive study of pregnant people admitted to obstetric triage assessment units at The Ottawa Hospital between Oct. 19 and Nov. 27, 2020 (second local wave of the COVID-19 pandemic). Participants underwent SARS-CoV-2 polymerase chain reaction (PCR) (via naso- or oropharyngeal swabs) and serology testing upon admission. We excluded individuals younger than 18 years, those who did not speak English or French, those who enrolled in conflicting studies, those admitted for pregnancy termination and those triaged between 11:31 pm and 7:29 am. Swab and serology samples were analyzed using digital droplet PCR and enzyme-linked immunosorbent assays, respectively. We defined SARS-CoV-2 seropositivity as a positive result for immunoglobulin (Ig) G, either alone or in combination with IgM or IgA.
Results:
Of the 632 eligible patients, 363 (57.4%) consented to participation and 362 collectively provided 284 swab and 352 blood samples eligible for analysis. Common reasons for declining participation included feeling overwhelmed or anxious, being worried about repercussions of testing, pain or discomfort with testing or disinterest in research. Participants were mostly multiparous (53.9%) and in their third trimester upon admission (88.4%). In all, 18 (4.9%) participants had evidence of SARS-CoV-2 exposure; 2 (0.7%) of 284 were positive for SARS-CoV-2 by PCR and 16 (4.5%) of 352 were positive for IgG antibodies to SARS-CoV-2.
Interpretation:
During the second local wave of the COVID-19 pandemic, the prevalence of active SARS-CoV-2 infection among obstetric patients in Ottawa was 0.7% and seroprevalence was 4.5%. Our low participation rate highlights the need for improvements in patient education and public health messaging on the benefits of SARS-CoV-2 testing programs.
The onset of the COVID-19 pandemic saw the widespread implementation of policies in clinical care settings to protect vulnerable patient populations. 1 Among these populations are obstetric patients, who are at increased risk for severe complications related to COVID-19, including admission to hospital and to the intensive care unit.2 Pregnant individuals require regular and frequent access to health care services and most are ultimately admitted to the hospital for delivery.3
Temporary adoption of universal COVID-19 screening and testing approaches in some obstetric departments provided important insight for local hospital administrators and public health authorities on the prevalence of SARS-CoV-2 infection in obstetric patients. Early estimates of SARS-CoV-2 prevalence among pregnant individuals are highly variable, ranging from 0.012% to 37%.4,5 Variability in these estimates reflects differences in population density across settings, socioeconomic composition, available technology for laboratory testing, timing and duration of testing programs, and local community infection rates.6 Furthermore, cautious and appropriate interpretation of serologic assays are necessary when estimating seroprevalence, given the emerging evidence on serologic cross-reactivity and antibody decay,7,8 particularly in populations with low levels of exposure.9
There is limited information on the prevalence of SARS-CoV-2 infection from obstetric settings in Canada, and existing reports are limited to the first pandemic wave (about February to June 2020). In a single-centre investigation from Montréal, all obstetric patients (n = 803) admitted between March and July 2020 were tested for SARS-CoV-2; of these, 5% tested positive.10 A subsequent study from Toronto reported 0.9% prevalence of SARS-CoV-2 infection among 446 pregnant individuals tested between April and May 2020.11 Serologic analysis of more than 1000 antenatal maternal serum samples, collected from multiple provincial health units in British Columbia from March to June 2020, suggested that the seroprevalence of SARS-CoV-2 antibodies in this obstetric sample was less than 1%.12
Ongoing surveillance among obstetric patients is warranted to help characterize the burden of disease across different pandemic waves and inform local public health measures for future disease outbreaks. At the time of our study, Ontario was at the height of Canada’s second pandemic wave (September 2020 to February 2021) and vaccinations were not yet available to the general public.13 Our objective was to describe the prevalence of SARS-CoV-2 infection in pregnant individuals admitted to obstetric triage units at a tertiary care hospital in Ottawa, Ontario.
Methods
Study design and setting
We conducted a descriptive study of pregnant individuals admitted to obstetric triage units at The Ottawa Hospital. The Ottawa Hospital is a tertiary care centre with 2 obstetric triage units located at different campuses (General Campus and Civic Campus). Participant SARS-CoV-2 virus and serology testing was conducted over 5.5 weeks during the second local wave of the pandemic,13 between Oct. 19 and Nov. 27, 2020. The COVID-19 restrictions that were in place during this time allowed only 1 support person to accompany the birthing mother and this was limited to admissions to the birthing unit (i.e., during active labour).14
Study population
Participants were pregnant individuals who were admitted to 1 of 2 obstetric triage units (generally ≥ 20 weeks’ gestation, unless the circumstances are exceptional). Patients with or without symptoms of COVID-19 were eligible to participate. We excluded individuals who did not speak English or French, were younger than 18 years, were enrolled in conflicting research studies or were admitted for a pregnancy termination. Given the necessity of having research staff available to collect consent, individuals who presented to triage units overnight (i.e., between the hours of 11:31 pm and 7:29 am) were not eligible to participate.
Sample collection
We invited participants to provide both a blood sample and a naso- or oropharyngeal swab sample; however, provision of both sample types was not a requirement for participation. Thus, participants in our study could provide both a swab sample and a blood sample, a swab sample alone or a blood sample alone.
Samples were collected at the participants’ first presentation to the triage unit. If participants visited the triage unit more than once during the study period, subsequent samples were collected only if the participant was suspected to have COVID-19 based on the presence of symptoms, at-risk contact history or travel history, as determined from a Febrile Respiratory Illness Screening Tool used at hospital entry (Appendix 1, Section 1, available at www.cmajopen.ca/content/10/3/E643/suppl/DC1). For participants who provided samples across multiple study visits, we looked at all polymerase chain reaction (PCR) and serology results. We used results from the most recent triage visit in this analysis, unless positive PCR or serology findings were detected in the first round of samples but not the second round. In such scenarios, we used PCR and serology results from the first round of collected samples.
For participants who provided samples at multiple visits, we looked at all results. If there was a positive PCR or serology finding, we used this. If not, we used the results from the most recent visit.
Naso- and oropharyngeal samples were collected using sterile swabs (Norgen CM-96000 or Norgen CY-93050, respectively) and placed in viral transport media (Norgen Total Nucleic Acid Preservation Tubes Dx). Blood samples were collected as peripheral whole blood via venipuncture or as dried blood spots via finger prick.
SARS-CoV-2 virus and antibody testing
All viral and antibody testing occurred at a containment level 2 facility at the University of Ottawa. Swab samples were analyzed for SARS-CoV-2 RNA using digital droplet PCR assays (Bio-Rad QX200 Droplet Reader) targeting the orf1ab region (nsp14), the nucleocapsid gene and the envelope gene.15 Participants who tested positive for SARS-CoV-2 infection through this research study were notified by their clinical care team and instructed to follow public health guidelines.16
Blood samples were evaluated for antibodies specific to SARS-CoV-2 (immunoglobulin [Ig] A, IgG, IgM) using enzyme-linked immunosorbent assays (ELISAs). Assays were run on automated high-throughput instruments (Hamilton Microlab STAR Liquid Handling System), to quantify antibody levels against the full-length viral spike protein and nucleoprotein. Assay signals were used as correlates of detected antibody levels in each patient sample.
A positive cut-off value was established for each assay plate as a value equal to the mean of negative control signal values (manual ELISA) or mean of negative samples (automated ELISA) plus 3 times the standard deviation (SD) of the optical density value distribution from plasma or serum negative for SARS-CoV-2. Antibody levels in positive samples were correlated to SARS-CoV-2 control antibodies for IgA, IgG and IgM (CR3022 antibody). We used a signal to cut-off ratio to classify serology results as positive or negative.
We required a positive result for antibody reactivity against both the full-length viral spike protein and the nucleoprotein for a sample to be classified as seropositive. A positive serologic result was determined to be a positive IgG antibody result alone, or a positive IgG result in combination with a positive IgM or IgA result. Full laboratory methodology is presented in the supplementary materials (Appendix 1, Section 2).
Data sources
To minimize the burden of this study on both participants and health care workers at The Ottawa Hospital, we linked individual laboratory results from SARS-CoV-2 virus and antibody testing to secondary data sources to ascertain participant sociodemographic, obstetric and medical information.
We used the Better Outcomes Registry & Network (BORN) Ontario,17 Ontario’s provincial birth registry, to ascertain data on participant demographics (i.e., maternal age, neighbourhood median family income quintile), medical history (i.e., parity, prepregnancy body mass index, preexisting conditions), lifestyle behaviours (i.e., substance use in pregnancy), pregnancy characteristics and complications (i.e., maternal anxiety or depression in pregnancy, gestational diabetes, hypertensive disorder in pregnancy, number of fetuses, antenatal health care provider type) and delivery and newborn outcomes (i.e., mode of delivery, gestational age, preterm birth, live or stillbirth). Using this provincial birth registry, we had complete ascertainment of pregnancy and newborn outcomes. We also used BORN Ontario to obtain participant results from community SARS-CoV-2 testing during the study period. These data were derived from the Case and Contact Management System, which captures case-specific information on reportable diseases, including COVID-19, from public health units across Ontario. Case and Contact Management data on pregnant individuals are integrated into the BORN Ontario database on a monthly basis.
We used The Ottawa Hospital’s electronic medical record system — Electronic Privacy Information Centre — to ascertain participant responses for the Febrile Respiratory Illness Screening Tool, administered at hospital entry (i.e., suspected COVID-19 symptoms, travel history, close contacts), and to verify results from in-hospital SARS-CoV-2 tests. We also used the Electronic Privacy Information Centre to verify information not yet posted or missing from BORN Ontario records (i.e., participant ethnicity, date of delivery).
We used The Ottawa Hospital Data Warehouse, a relational database that consolidates records from across the hospital’s information systems, to determine the total number of pregnant people who presented to obstetric triage units during the study period and to estimate the study recruitment rate. More information on these data sources and how they were used are detailed in Appendix 1, Section 3.
Study outcomes
The primary outcome was the prevalence of SARS-CoV-2 infection. For the purpose of this study, SARS-CoV-2 infection was defined as either active, based on the presence of measurable SARS-CoV-2 virus in maternal samples at the time of presentation to the triage unit, or recovered, based on laboratory-confirmed history of infection earlier in pregnancy or positive serology results from maternal samples at the time of presentation to the triage unit.
Statistical analysis
We managed statistical analyses in the BORN Ontario environment. We linked results from SARS-CoV-2 PCR and serology testing, as well as medical records from the Electronic Privacy Information Centre and The Ottawa Hospital Data Warehouse, to records in BORN Ontario using participant medical record numbers. In BORN Ontario, we mapped unique participant identifiers and medical record numbers to unique BORN Ontario identifiers to generate a single analytical file.
We calculated descriptive summary statistics for sociodemographic information, obstetric histories and delivery details for the overall study population. We summarized data using frequency distributions for categorical variables, and means and SDs or medians and interquartile ranges (IQRs) for continuous variables. We performed all analyses using SAS version 9.4 (SAS Institute).
Ethics approval
This study was approved by the Ottawa Health Science Network Research Ethics Board (20200640-01H). All participants provided informed consent at the time of enrolment.
Results
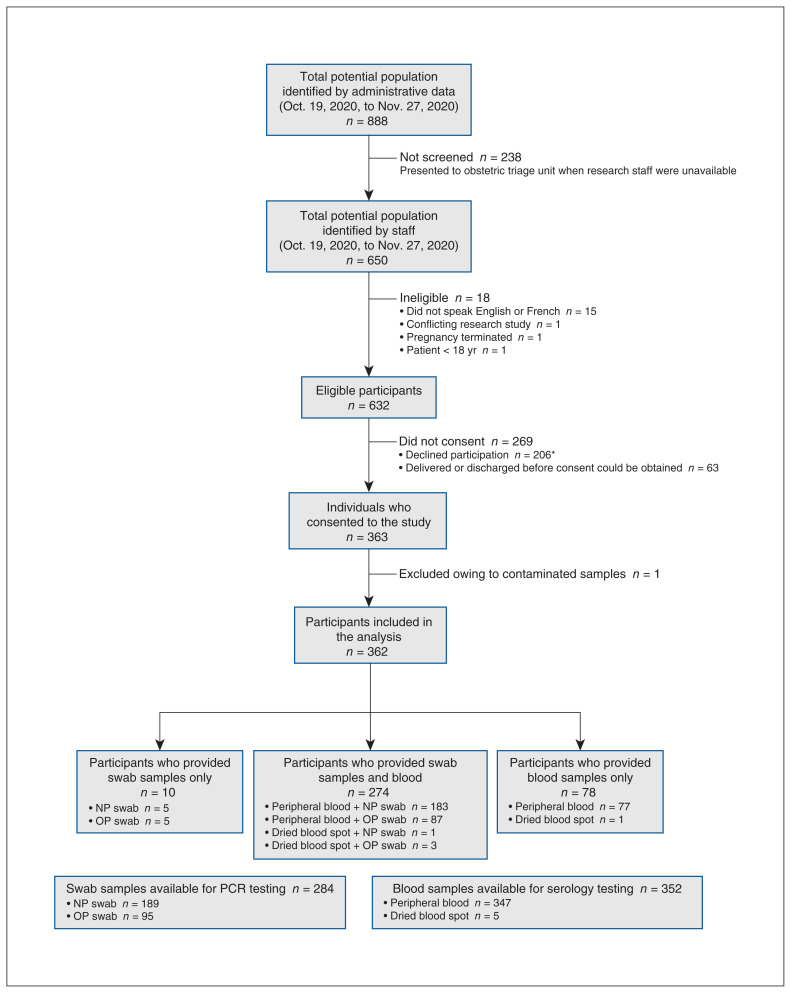
From Oct. 19 to Nov. 27, 2020, 888 pregnant people presented to an obstetric triage unit at The Ottawa Hospital. Of these, 650 arrived while research staff were on site and were screened for eligibility. A total of 18 people were ineligible (2.8%), 206 declined participation (31.7%) and 63 were discharged before consent could be obtained (9.7%). Thus, 363 (57.4%) of 632 eligible patients consented to sample collection, including 6 who provided 2 samples each. One participant was excluded after consent owing to sample contamination. Common reasons for declining participation included feeling too overwhelmed or anxious to participate, being unwilling to undergo the pain or discomfort of sample collection, being worried about the SARS-CoV-2 test results and generally not wanting to learn about the research study (Appendix 1, Section 4).
In total, the samples of 362 unique participants were included in the analysis (Figure 1), providing 284 swab samples and 352 blood samples. About three-quarters of participants (274/362) provided both sample types, and the remainder (88/362) provided only 1 type (either blood or swab).
Figure 1:
Flow chart of study enrolment and sample collection. Note: NP = nasopharyngeal, OP = oropharyngeal, PCR = polymerase chain reaction. *Common reasons for nonparticipation are summarized in Appendix 1, Section 4.
Participant characteristics
The sociodemographic, clinical and pregnancy characteristics of the study cohort are summarized in Table 1. Most participants (n = 320, 88.4%) were in their third trimester of pregnancy, multiparous (n = 195, 53.9%) and did not have preexisting health conditions (n = 306, 84.5%). The average maternal age was 32.4 (SD 4.9) years. Participants were evenly distributed across neighbourhood median family income quintiles. The median gestational age at delivery was 39.0 (IQR 38.0–40.0) weeks, and 141 (38.9%) participants gave birth by cesarean delivery. A total of 57 (15.7%) participants had symptoms consistent with COVID-19 upon presentation to triage.
Table 1:
Sociodemographic, clinical and pregnancy characteristics of study participants
| Characteristic | No. (%) of participants* n = 362 |
|---|---|
| Trimester at triage visit† | |
| 1st (< 14 weeks’ gestation) | < 6 (S) |
| 2nd (14–28 weeks’ gestation) | 41 (11.3) |
| 3rd (≥ 28 weeks’ gestation) | 320 (88.4) |
| Symptoms consistent with COVID-19 upon admission to triage‡ | 57 (15.7) |
| SARS-CoV-2 infection in pregnancy documented in medical chart§ | 5 (1.4) |
| Maternal age, yr, mean ± SD | 32.4 ± 4.9 |
| Parity | |
| 0 | 166 (45.9) |
| ≥ 1 | 195 (53.9) |
| Missing | < 6 (S) |
| Neighbourhood median family income quintile | |
| 1 (lowest) | 51 (14.1) |
| 2 | 80 (22.1) |
| 3 | 73 (20.2) |
| 4 | 80 (22.1) |
| 5 (highest) | 76 (21.0) |
| Missing | < 6 (S) |
| Race and ethnicity¶ | |
| White | 203 (56.1) |
| Asian | 28 (7.7) |
| Black | 26 (7.2) |
| Other | 45 (12.4) |
| Missing | 60 (16.6) |
| Prepregnancy BMI, median (IQR) | 25.6 (21.9–30.3) |
| Obese (BMI > 30.0)** | |
| No | 228 (63.0) |
| Yes | 86 (23.8) |
| Missing | 48 (13.3) |
| Substance use during pregnancy††,‡‡ | |
| No | 323 (89.2) |
| Yes | 35 (9.7) |
| Missing | < 6 (S) |
| Anxiety‡‡ | |
| No | 277 (76.5) |
| Yes | 85 (23.5) |
| Depression‡‡ | |
| No | 311 (85.9) |
| Yes | 51 (14.1) |
| Pre-existing maternal health conditions | |
| None | 306 (84.5) |
| Asthma | 44 (12.2) |
| Chronic hypertension | 7 (1.9) |
| Diabetes | 7 (1.9) |
| Gestational diabetes | |
| No | 324 (89.5) |
| Yes | 38 (10.5) |
| Hypertensive disorder in pregnancy§§ | |
| No | 314 (86.7) |
| Yes | 48 (13.3) |
| Antenatal health care provider | |
| Family physician only | 24 (6.6) |
| Obstetrician only | 252 (69.6) |
| Family physician and obstetrician | 44 (12.2) |
| Midwife | 31 (8.6) |
| Other provider | 8 (2.2) |
| No provider | < 6 (S) |
| Missing | < 6 (S) |
| Number of fetuses | |
| Singleton | 345 (95.3) |
| Multiple | 17 (4.7) |
| Gestational age at delivery, wk, median (IQR) | 39.0 (38.0–40.0) |
| Preterm delivery (< 37 weeks’ gestation) | |
| No | 310 (85.6) |
| Yes | 51 (14.1) |
| Missing | < 6 (S) |
| Mode of delivery | |
| Vaginal | 221 (61.0) |
| Cesarean | 141 (39.0) |
| Missing | < 6 (S) |
| Birth outcome | |
| Live birth | 361 (99.7) |
| Intrapartum stillbirth | < 6 (S) |
Note: BMI = body mass index, IQR = interquartile range, S = suppressed due to small cell size, SD = standard deviation.
Unless indicated otherwise.
Trimester of triage visit was calculated using the date that the patient was first enrolled in the study.
Participant reported symptoms consistent with COVID-19 on Febrile Respiratory Illness Screening Tool used at hospital entry.
Participant had documentation of a positive SARS-CoV-2 laboratory test result that was reported during pregnancy and within time period of study.
Information on race and ethnicity was obtained from the Ontario birth registry (Better Outcomes Registry & Network) and supplemented with medical chart review wherever possible. Racial and ethnic designations were assigned by the provider, sometimes through self-declaration by patients.
BMI values below 10.3 and above 79.9 were excluded as outliers and set to missing for BMI grouping and obesity.
Substance use during pregnancy is defined as alcohol, drug or smoking during pregnancy.
Self-reported variables obtained from the Ontario birth registry (Better Outcomes Registry & Network).
Conditions include eclampsia, gestational hypertension, HELLP (hemolysis, elevated liver enzymes and low platelets), preeclampsia and pre-existing hypertension with superimposed preeclampsia.
Prevalence of SARS-CoV-2 infection
Among the 284 participants who provided a swab sample, 2 (0.7%) tested positive for SARS-CoV-2 by PCR.
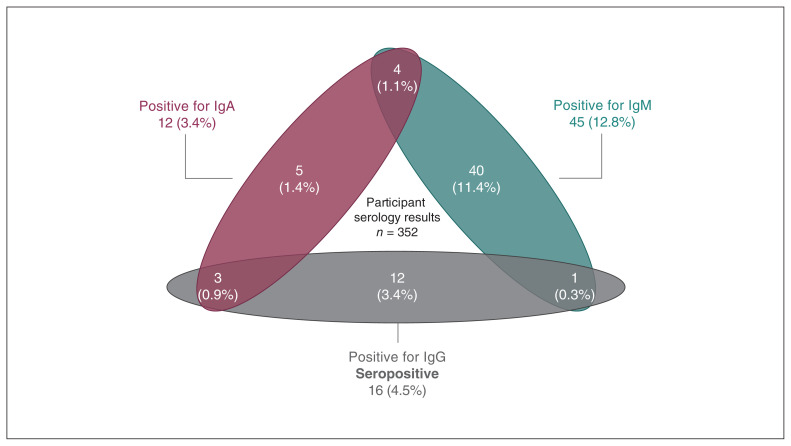
Serology test results are summarized in Figure 2. Among the 352 participants who provided a blood sample, 45 (12.8%) had IgM antibodies against SARS-CoV-2 and 12 (3.4%) had IgA antibodies. However, based on the presence of IgG antibodies, 16 (4.5%) met the criteria for seropositivity for SARS-CoV-2. Of the 16 seropositive participants, 3 (18.8%) were also positive for IgA and 1 (6.2%) was also positive for IgM. Three of the seropositive participants had received a positive PCR result through community testing before the study; the remaining 13 had not previously tested positive for SARS-CoV-2. Neither of the 2 PCR-positive participants were seropositive.
Figure 2:
Serology results of participants (n = 352). Participants with immunoglobulin (Ig) G antibodies against SARS-CoV-2 were considered seropositive.
Overall, 4.9% (18/362) participants had evidence of current or previous SARS-CoV-2 infection, based on PCR or antibody testing. Five were symptomatic (2/2 PCR-positive participants and 3/16 seropositive participants) and 13 were asymptomatic at the time of testing. Participant-reported symptoms included dry cough, shortness of breath, congestion, nausea, vomiting, abdominal pain and unexplained fatigue.
Interpretation
In this descriptive study of patients admitted to obstetric triage units at The Ottawa Hospital during the second local wave of the COVID-19 pandemic, the prevalence of active SARS-CoV-2 infection was 0.7%. Seroprevalence based on the presence of IgG antibodies was 4.5%; 3.4% of serology samples exhibited IgG alone, whereas 1.1% exhibited IgG in combination with either IgA or IgM.
Our findings are similar to those from a study conducted in Toronto during the first wave of the pandemic, where the prevalence of active SARS-CoV-2 infection in the obstetric population was estimated at 0.9%. However, seroprevalence in our sample was substantially higher than what was reported in BC during the first wave (< 1%).12 This may, in part, reflect the later time period during which our study was conducted; the larger second wave of the pandemic in Canada began after a period of relaxation of public health measures during the late summer and early fall of 2020, and it follows that the number of individuals with a history of SARS-CoV-2 infection increased with time.
Antibodies to SARS-CoV-2 may develop days or weeks after onset of clinical illness.18,19 Immunoglobulin G antibodies may persist for several months or more after infection,20 whereas IgM and IgA antibodies decay more rapidly.21 For this reason, we based our estimates of seroprevalence on the presence of IgG antibodies. The presence of IgM and IgA antibodies without IgG in 13.6% of our samples is likely the result of cross-reactivity in our assays with seasonal coronavirus strains.22,23 Our finding that most seropositive participants exhibited IgG antibodies alone suggests that they had likely contracted SARS-CoV-2 weeks, if not months, before the study.
Interestingly, just 18.7% (3/16) of seropositive cases had documentation of a positive SARS-CoV-2 test in their medical records. At the time this study was conducted, PCR tests were available through local pharmacies and clinical assessment centres; however, access to PCR testing was limited in the early period of the pandemic, which may have contributed to the lack of testing among these cases. Alternatively, these individuals could have refrained from testing owing to asymptomatic presentation. Rapid tests were not widely available to the public.
Our study has several strengths. We determined active SARS-CoV-2 infection by digital droplet PCR, a more sensitive technique than traditional PCR methods (sensitivity 87.4%–97.6% v. 70%).24,25 We employed serologic testing, a service that was not available through the Ontario publicly funded health care system or private clinics at the time of the study. In secondary waves of disease outbreak and spread, serologic data provide essential complementary information to PCR data.19 Indeed, this approach allowed us to identify participants with a history of SARS-CoV-2 infection, who otherwise would not have been identified through conventional PCR testing.
Although the prevalence data presented in this study alludes to the burden of COVID-19 in the local obstetric population, this study also provides insights on implementation of virus and serology testing of patients at our tertiary care centre. Testing was offered free of charge and provided participants with information as to whether they were currently infected or had recently been infected with SARS-CoV-2; however, about a third of eligible people declined participation. Leading reasons for not wanting to participate, as indicated during the consent process, included desire to avoid the physical discomfort caused by testing, and anxiety regarding possible care and lifestyle implications of testing positive.
Stigma and discrimination against individuals who have had COVID-19 are well documented26,27 and are shown to influence individuals’ decisions to get tested or seek treatment. 28,29 Where SARS-CoV-2 testing continues to be a predominantly elective process, more work is needed to examine and address individual-, community- and system-level barriers that inhibit SARS-CoV-2 test-seeking behaviours.30 Implementation of universal testing protocols at our hospital centre may have improved our study’s enrolment rate; however, the logistics of implementing such protocols are complex. Whether universal testing programs are a justifiable use of resources remains a subject of debate,6,31 and such programs have only been adopted elsewhere as temporary measures early in the first wave of the pandemic.4,6,32–34 For these programs to be effective, patients and visitors must be willing to submit to testing, testing methods need to be reliable, and staff and laboratory resources must be readily available to accommodate timely reporting of results. Finally, given the high financial costs, there must be substantive benefits to both patients and the health system.
Limitations
As our study was a single-centre initiative, our findings may not be generalizable to obstetric populations elsewhere. However, our sample was derived from a large patient population accessing the largest hospital network in Ottawa–Gatineau and thus provides meaningful insight into the burden of COVID-19 in obstetric patients from Canada’s National Capital Region. Further, our sample is well characterized, with detailed information on sociodemographic, clinical and pregnancy characteristics, facilitating comparison with other populations. As noted earlier, we did not test all patients who presented to the obstetric triage unit, and a large number of potential participants were missed or declined participation. It is therefore possible that our participants differ from the obstetric population of The Ottawa Hospital as a whole. The proportion of our study population that gave birth by cesarean delivery (39%) was slightly higher than what has been previously reported by The Ottawa Hospital (about 30%). It is possible that patients delivering via cesarean were more likely to participate in the study than those in active labour; however, it is unknown whether this selection bias would differ by SARS-CoV-2 infection status.35 The presence or absence of COVID-19 symptoms was self-reported by participants at the time of consent, and we were unable to ascertain symptoms participants had outside of their triage visit. This limited our ability to capture information on symptoms that may have been experienced by recovered participants to determine if they were symptomatic or asymptomatic at the time of infection. It also limited our ability to capture symptom information from presymptomatic cases.
Conclusion
The prevalence of SARS-CoV-2 infection among obstetric patients who presented to obstetric triage units during Ottawa’s second pandemic wave was 0.7% for active infection and 4.5% for recovered infection. Our low participation rate highlights opportunities to improve patient education and public health messaging about the benefits of testing programs.
Supplementary Material
Acknowledgements
The authors acknowledge the nurses, obstetricians, family physicians, midwives and clerks at the Birthing Unit and Mother Baby Unit, as well as the laboratory technicians, within The Ottawa Hospital for their support and assistance with this research study. They also gratefully acknowledge the patients who participated in the study. Lastly, they acknowledge several individuals for their assistance in study recruitment and coordination including Chantal Horth, Marisa Murray, Joseph Cyr and Kathryn Denize.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Romina Fakhraei, Malia Murphy, Alysha Dingwall-Harvey, Ruth Rennicks White, Deshayne Fell, Marc-André Langlois and Darine El-Chaâr contributed to the conception and design of the work. Romina Fakhraei, Erica Erwin, Kameela Alibhai, Sheryll Dimanlig-Cruz, Rosemary LaRose, Kimberly Grattan, Jian-Jun Jia, George Liu, Corey Arnold, Yannick Galipeau, Khatereh Shir-Mohammadi, Gillian Alton, Jessica Dy and Darine El-Chaâr contributed to the acquisition, analysis and interpretation of data. Romina Fakhraei, Erica Erwin, Kameela Alibhai and Malia Murphy drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/3/E643/suppl/DC1.
Funding: This research was supported by the Canadian Institutes of Health Research (no. VR4-172760).
Data sharing: The data are not publicly available owing to their containing information that could compromise the privacy of research participants. The data that support the findings of this study are available on request from the corresponding author with the permission of the Better Outcomes Registry & Network Ontario.
References
- 1.de Oliveira Menezes M, Andreucci CB, Nakamura-Pereira M, et al. Universal COVID-19 testing in the obstetric population: impacts on public health [article in Portuguese] Cad Saude Publica. 2020;36:e00164820. doi: 10.1590/0102-311x00164820. [DOI] [PubMed] [Google Scholar]
- 2.Wei SQ, Bilodeau-Bertrand M, Liu S, et al. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–8. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gagliardi L, Danieli R, Suriano G, et al. Universal severe acute respiratory syndrome coronavirus 2 testing of pregnant women admitted for delivery in 2 Italian regions. Am J Obstet Gynecol. 2020;223:291–2. doi: 10.1016/j.ajog.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naqvi M, Burwick RM, Ozimek JA, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) universal testing experience on a Los Angeles labor and delivery unit. Obstet Gynecol. 2020;136:235–6. doi: 10.1097/AOG.0000000000003987. [DOI] [PubMed] [Google Scholar]
- 5.Maru S, Patil U, Carroll-Bennett R, et al. Universal screening for SARS-CoV-2 infection among pregnant women at Elmhurst Hospital Center, Queens, New York. PLoS One. 2020;15:e0238409. doi: 10.1371/journal.pone.0238409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaCourse SM, Kachikis A, Blain M, et al. Low prevalence of severe acute respiratory syndrome coronavirus 2 among pregnant and postpartum patients with universal screening in Seattle, Washington. Clin Infect Dis. 2021;72:869–72. doi: 10.1093/cid/ciaa675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoyama R, Kurano M, Morita Y, et al. Validation of a new automated chemiluminescent anti-SARS-CoV-2 IgM and IgG antibody assay system detecting both N and S proteins in Japan. PLoS One. 2021;16:e0247711. doi: 10.1371/journal.pone.0247711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383:1085–7. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitman JD, Hiatt J, Mowery CT, et al. Test performance evaluation of SARS-CoV-2 serological assays. medRxiv. 2020 May 17; doi: 10.1101/2020.04.25.20074856. [DOI] [Google Scholar]
- 10.Trahan M-J, Mitric C, Malhamé I, et al. Screening and testing pregnant patients for SARS-CoV-2: first-wave experience of a designated COVID-19 hospitalization centre in Montréal. J Obstet Gynaecol Can. 2021;43:571–5. doi: 10.1016/j.jogc.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei-Dan E, Satkunaratnam A, Cahan T, et al. Questionnaire-based vs universal PCR testing for SARS-CoV-2 in women admitted for delivery. Birth. 2021;48:96–103. doi: 10.1111/birt.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClymont E, Elwood C, Sekirov I, et al. Population SARS-CoV-2 seroprevalence using antenatal serum samples in British Columbia, Canada. J Obstet Gynaecol Can. 2021;43:1242–3. doi: 10.1016/j.jogc.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 cases and deaths in Ottawa [daily COVID-19 dashboard] Ottawa: Ottawa Public Health; 2020. [accessed 2021 Mar. 23]. Available: https://www.arcgis.com/home/item.html?id=6bfe7832017546e5b30c5cc6a201091b. Login required to access content. [Google Scholar]
- 14.COVID-19 guidance: labour, delivery and newborn care - version 3. Ottawa: Ontario Ministry of Health; 2020. Nov 10, [accessed 2022 June 22]. Available: https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/2019_labour_delivery_newborn_guidance.pdf. [Google Scholar]
- 15.Government of Canada invests in new research to address COVID-19 variants [news release] Ottawa: Canadian Institutes of Health Research; 2021. Mar 26, [accessed 2021 June 23]. Available: https://www.canada.ca/en/institutes-health-research/news/2021/03/government-of-canada-invests-in-new-research-to-address-covid-19-variants.html. [Google Scholar]
- 16.Information for those who have symptoms, test positive for COVID-19 and high-risk contacts. Ottawa: Ottawa Public Health; [accessed 2021 Aug. 24]. Available: https://www.ottawapublichealth.ca/en/public-health-topics/self-isolation-instructions-for-novel-coronavirus-covid-19.aspx. [Google Scholar]
- 17.Murphy MSQ, Fell DB, Sprague AE, et al. Data resource profile: Better Outcomes Registry & Network (BORN) Ontario. Int J Epidemiol. 2021;50:1416–7h. doi: 10.1093/ije/dyab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina LP, Chow S-K, Nickel A, et al. Prolonged detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in an obstetric patient with antibody seroconversion. Obstet Gynecol. 2020;136:838–41. doi: 10.1097/AOG.0000000000004086. [DOI] [PubMed] [Google Scholar]
- 19.Galipeau Y, Greig M, Liu G, et al. Humoral responses and serological assays in SARS-CoV-2 infections. Front Immunol. 2020;11:610688. doi: 10.3389/fimmu.2020.610688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5:eabe0367. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyara M, Saichi M, Sterlin D, et al. Pre-COVID-19 immunity to common cold human coronaviruses induces a recall-type IgG response to SARS-CoV-2 antigens without cross-neutralisation. Front Immunol. 2022;13:790334. doi: 10.3389/fimmu.2022.790334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov A, Semenova E. Long-term monitoring of the development and extinction of IgA and IgG responses to SARS-CoV-2 infection. J Med Virol. 2021;93:5953–60. doi: 10.1002/jmv.27166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong L, Zhou J, Niu C, et al. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta. 2021;224:121726. doi: 10.1016/j.talanta.2020.121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suo T, Liu X, Feng J, et al. ddPCR: a more sensitive and accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg Microbes Infect. 2020;9:1259–68. doi: 10.1080/22221751.2020.1772678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor S, Landry CA, Rachor GS, et al. Fear and avoidance of healthcare workers: an important, under-recognized form of stigmatization during the COVID-19 pandemic. J Anxiety Disord. 2020;75:102289. doi: 10.1016/j.janxdis.2020.102289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukumbang FC. Pervasive systemic drivers underpin COVID-19 vulnerabilities in migrants. Int J Equity Health. 2021;20:146. doi: 10.1186/s12939-021-01487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID-19: reducing stigma. Gatineau (QC): Indigenous Services Canada; [accessed 2022 Mar. 8]. modified 2021 July 28. Available: https://www.sac-isc.gc.ca/eng/1627070714082/1627070737178. [Google Scholar]
- 29.Stigma and prejudice: How to combat the rise in discrimination that has been sparked by the COVID-19 pandemic. Toronto: Centre for Addiction and Mental Health; [accessed 2022 Mar. 8]. Available https://www.camh.ca/en/health-info/mental-health-and-covid-19/stigma-and-prejudice. [Google Scholar]
- 30.Embrett M, Sim SM, Caldwell HAT, et al. Barriers to and strategies to address COVID-19 testing and testing hesitancy: a rapid scoping review. BMC Public Health. 2022;22:750. doi: 10.1186/s12889-022-13127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metz TD. Is universal testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) needed on all labor and delivery units? Obstet Gynecol. 2020;136:227–8. doi: 10.1097/AOG.0000000000003972. [DOI] [PubMed] [Google Scholar]
- 32.Goldfarb IT, Diouf K, Barth WH, et al. Universal SARS-CoV-2 testing on admission to the labor and delivery unit: low prevalence among asymptomatic obstetric patients. Infect Control Hosp Epidemiol. 2020;41:1095–6. doi: 10.1017/ice.2020.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochiai D, Kasuga Y, Iida M, et al. Universal screening for SARS-CoV-2 in asymptomatic obstetric patients in Tokyo, Japan. Int J Gynaecol Obstet. 2020;150:268–9. doi: 10.1002/ijgo.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obstetric anesthesiology. Ottawa: The Ottawa Hospital; 2017. Jan 6, [accessed 2022 June 22]. Available: https://www.ottawahospital.on.ca/en/clinical-services/deptpgrmcs/departments/anesthesiology/obstetric-anesthesiology/#:~:text=Our%20caesarean%20section%20rate%20is,9%20epidurals%20in%20a%20day. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.