ABSTRACT
Despite improvements in the therapeutic arsenal and the recommendations of guidelines, low rates of prescribing osteoporosis medications are being reported worldwide for patients surviving a hip fracture, and important geographical variation remain. We aimed to describe trends in the proportion of patients that receive osteoporosis medication after hip fracture and to analyze the geographical variation in the prescription of drug therapy and its associated factors in the region of Valencia, Spain. We studied a population‐based retrospective cohort of 30,965 patients aged 65 years and older, discharged from hospital after a hip fracture from January 2008 to December 2015, who were followed up for 3 months after discharge to identify the presence of any prescription of osteoporosis medication. We conducted a multilevel multiple logistic regression analysis with two levels (individuals and health departments [HD]) to determine which individual covariates were associated with receiving a prescription of osteoporosis medication in the 3 months after discharge, as well as the importance of the HD of hospitalization. The percentage of patients treated in the region decreased from a maximum of 28.9% in 2009 to 16.4% in 2015. By sex, the proportion of women treated reached a maximum of 33.4% in 2009 and declined to 19% in 2015, while the proportion of men reached a maximum of 14% in 2011 and reduced to 8.1% in 2015. By health department, there was a noticeable variability in the rate of patients treated, ranging from 40.9% to 11.1% in the whole period (intraclass correlation coefficient [ICC] = 7.54%; median odds ratio [MOR] = 1.64). Proportion of treated patients decreased in 20 of the 24 HDs. Variability could be also observed with regard to choice of medication by HD. This situation pressingly demands action (both at the organizational and professional levels) focused on populations at a higher risk (such as hip fracture patients) that particularly address underutilization and unwarranted variation.
Keywords: OSTEOPOROSIS, HEALTH SERVICES RESEARCH, FRACTURE PREVENTION, THERAPEUTICS, STATISTICAL METHODS
Introduction
Osteoporotic hip fracture is associated with important morbidity, elevated risk of recurrent falls and refracture, as well as with increased mortality.1, 2, 3 After hip fracture, effective treatment with osteoporosis medication is available and recommended by every clinical guideline in order to prevent subsequent fractures.4 Significant advances in osteoporosis pharmacological treatment to reduce fracture risk5 have occurred in the last 13 years. Taking into account the clear clinical indication to hip fracture patients, it would be reasonable to expect high rates of treatment in the real world, although the frailty and comorbidities that often come along with hip fracture could also contribute inversely to lowering the rates of treatment observed. However, and despite progress in pharmacological therapies and the recommendations of guidelines,6 real‐world evidence shows that translating research evidence and guidance into routine clinical care is insufficient, with low rates of prescribing being reported worldwide for patients surviving a hip fracture. Evidence of this post‐fracture gap in care is abundant and comes from multiple health care settings, countries, and populations.7, 8, 9, 10, 11, 12 Accordingly, there is growing public health concern in that fewer individuals with previous osteoporotic hip fracture are receiving effective drug treatment to prevent further osteoporotic fractures.13
Evidence from the US, both in Medicare and commercially insured patients, states that in 2011 only 21% of patients received effective drug therapy after a hip fracture.7 A cross‐national study in the US found even lower rates (11% Medicare, 13% commercial) and there are higher but still concerning rates in Korea (39%) and Spain (25%).14 More recently, Desai and colleagues15 echoed these previous reports in a large US population of commercially insured individuals by reporting rates of post‐hip fracture treatment and associated treatment effectiveness. The rate of effective drug treatment in the 180 days after hip fracture was 9.8% in 2004, decreasing to 3.3% in 2015, with additional analyses showing that low treatment rates were even worse in men. In the UK, Klop and colleagues found that 32% of patients received medication in the year after the hip fracture in the period 2000 to 2010,16 whereas Shah and colleagues provided data of patients treated in the 4 months after the fracture and found that the percentage of patients treated was as low as 9% in 1999, rising to 51% in 2011 and decreasing to 39% in 2013.17
Interestingly, Shah and colleagues also point out the existence of important geographical variations in the prescribing of antiosteoporotic drugs after a hip fracture.17 Geographic variability in the provision of health care has been used to inform health care policy,18 as any variation that remains after adjustment for demographic factors is unlikely to be due to differences in disease prevalence or patient characteristics or preferences, with health services being responsible for inequalities in access to health care services and treatments.
The aim of this study is to describe trends in the proportion of patients that receive osteoporosis medication after hip fracture during the period 2008 to 2015 and to analyze the geographical variation in the prescription of osteoporosis drug therapy and its associated factors in the region of Valencia, Spain.
Materials and Methods
Design
The retrospective study includes a population‐based cohort of all patients aged 65 years and older, discharged from hospital after hip fracture from January 1, 2008, to December 31, 2015. Patients were followed up for 3 months after discharge to identify the presence of any prescription of osteoporosis medication.
Setting
The study was conducted in the Valencia Health System (VHS), a comprehensive structure of hospitals, primary care facilities, and other public resources managed by the government of the region of Valencia in Spain (more than 5 million inhabitants registered in 2010) providing free, universal health care services (besides drug cost‐sharing) to 97% of the region's population The VHS is organized territorially into health departments (HDs). Each HD is composed of one hospital and several primary care centers serving a population of between 150,000 and 250,000 people. The number of HDs increased from 22 in 2008 to 23 in 2009 and to 24 for the rest of the study period, due to the opening of two new hospitals in the region.
Population
We included all patients aged 65 years and older discharged from VHS hospitals after a hip fracture (International Classification of Diseases 9th revision Clinical Modification [ICD9CM] codes: 820.xx and 733.14) and without a diagnosis of road accident, multiple fracture, or active bone cancer (Supplemental Table S1) between January 1, 2008, and December 31, 2015, and we followed them up for 3 months after the date of discharge to identify those with at least one osteoporotic treatment prescribed (either by primary care doctors or any hospital specialist) in that period. We excluded patients who died within the first 3 months after the index date, non‐residents in the region, or those excluded from pharmaceutical coverage (Fig. 1).
Figure 1.
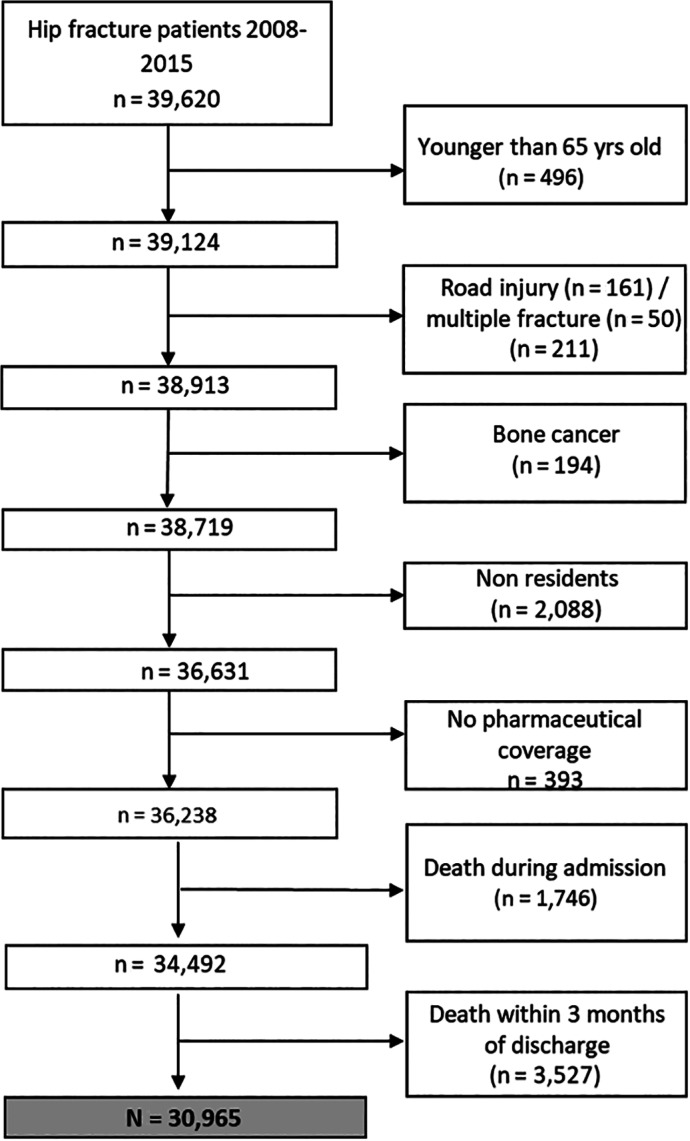
Flowchart of participants.
Data sources
Data were obtained from the Valencia Health System Integrated Database (VID). The VID is the result of the linkage, by means of a single personal identification number, of a set of publicly owned population‐wide health care, clinical, and administrative electronic databases in the region of Valencia, Spain, which has provided comprehensive information about the region's 5 million inhabitants since 2008. The VID includes sociodemographic and administrative information (sex, age, nationality, etc.) and health care information such as diagnoses, procedures, lab data, pharmaceutical prescriptions and dispensations, hospitalizations, mortality, health care utilization, and public health data. It also includes a set of specific associated databases with population‐wide information on significant care areas such as cancer, rare disease, vaccines, or imaging data.19
Study endpoints
The primary outcome was the individual level binary outcome (yes/no) of receiving at least one prescription for osteoporosis medication in the 3 months after discharge for an osteoporotic hip fracture in the period 2008 to 2015, for the whole region, by sex and by HD. We further described the choice of osteoporosis medication (bisphosphonates, calcitonin, denosumab, parathyroid hormone [PTH, 1–34 and 1–84], raloxifene, or strontium ranelate) for treated patients by year and HD (Supplemental Table S1).
Covariates
Potentially relevant variables with regard to the risk of hip fracture and to the use of osteoporosis medication were included, with a look‐back period of 365 days before the index hospital discharge. These variables included sociodemographic information, comorbidities, use of osteoporosis medication and other concomitant treatments before the fracture, and health care resources use (Supplemental Table S2).
Ethics approval and consent to participate
Our study was observational and used retrospective data pseudo‐anonymized before being transferred to the research team in accordance with Spanish laws on data protection for health research (Act 3/2018 transposing the 2015 European Data Protection Regulation). It was approved by the Ethics Committee for Clinical Research of the General Directorate of Public Health and the Centre for Public Health Research (approval resolution numbers 20160224 and 20160930).
Analysis
We first described baseline patient characteristics for patients treated and untreated after a hip fracture. Second, we described the yearly percentage of patients treated within 3 months of discharge for the whole region, by sex, and for the 24 HDs. We also performed a sensitivity analysis using a 6‐month window instead of a 3‐month window. Third, we conducted two multilevel multiple logistic regression analyses with two levels, individuals and HDs: an empty model (a model considering only the general contextual effect of the HD level on treatment initiation, which informs about the variability of treatment attributable to the HD component without the interaction of other variables), and a second model including both the HD and individual‐level variables, which informs about the variability in treatment initiation attributable to the interaction of the individual and HD levels. We further used the model with individual variables to determine which individual covariates were associated with receiving a prescription of osteoporosis medication in the 3 months after discharge, as well as the importance of the HD of hospitalization. We estimated these associations using regression coefficients expressed as odds ratios (ORs) with 95% confidence intervals (CIs) for individual covariates, and the median odds ratio (MOR) and the intraclass correlation coefficient (ICC) for the effect of the HDs. The MOR translates the HD variance into the OR scale, which makes the MOR comparable with the OR of individual covariates. The MOR can be interpreted as the amount by which the odds of receiving a prescription by a randomly selected patient would increase (in average) if this patient moved to a HD with a higher probability of being treated. At MOR = 1, there would be no differences between HDs in the odds of being treated after hip fracture. The ICC informs on the proportion of total variance in the probability of being treated after hip fracture that is attributable to the HD level. An ICC = 0 would reflect that the HD level does not affect the individual probability of being treated, and thus there would be no general contextual effects.20, 21, 22, 23, 24 We also provided the area under the receiver‐operating characteristic (ROC) curve to inform about the discriminatory accuracy of the models. Fourth, we plotted adjusted differences between HDs in the percentage of patients treated obtained from the multilevel logistic regression analysis. These differences were expressed as ORs and 95% CIs. Fifth, we described the trends in the choice of osteoporosis drugs in those patients actually receiving medication within 3 months of discharge, per year and by HD. Zoledronic acid is not included in figures because inpatient medication is not recorded in the electronic medical record. The same applies to over‐the‐counter medication and other treatments prescribed by private doctors that are not publicly reimbursed. All the analyses were performed using the Stata 13.0 (StataCorp, College Station, TX, USA) statistical software. We used the command from Stata xtmelogit to run the model in Stata and the command xtmrho to compute the ICC and MOR.22
Results
Patient characteristics
After taking into account the exclusion criteria, we ended up with a cohort of 30,965 patients who were discharged alive after a hip fracture in the whole period. From those, 6938 (22.4%) received an osteoporosis medication within 3 months of discharge. Treated patients were younger than untreated patients (81.9 versus 83.4 years old, p < 0.001), were more likely to be female (87.9% versus 72.9%, p < 0.001), had used health services less in the year before the fracture, had fewer comorbidities except for osteoporosis, rheumatoid arthritis, and depression (p < 0.001), but had received more previous medication (opioids, hypnotics, corticoids, anxiolytics, and cardiovascular drugs) overall (p < 0.001; Table 1).
Table 1.
Baseline Characteristics in the 365 Days Before the Index Hip of Patients Who Received Pharmacologic Treatment for Osteoporosis at 3 Months from Index Date
| Characteristics | Treated (n = 6938) | Untreated (n = 24,027) | Total (n = 30,965) | p Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | |||
| Sociodemographics | |||||||
| Sex | Women | 6097 | 87.9 | 17,508 | 72.9 | 23,605 | 0.000 |
| Men | 841 | 12.1 | 6519 | 27.1 | 7360 | ||
| Age (years) | 65–74 | 1053 | 15.2 | 3061 | 12.7 | 4114 | 0.000 |
| 75–84 | 3557 | 51.3 | 10,354 | 43.1 | 13,911 | ||
| ≥85 | 2328 | 33.6 | 10,612 | 44.2 | 12,940 | ||
| Mean (SD) | 81.9 (6.5) | 83.4 (7.0) | 83.1 (6.9) | 0.000 | |||
| Use of health services | |||||||
| Primary care visits | 0–4 | 2644 | 38.1 | 10,008 | 41.7 | 12,652 | 0.000 |
| 5–12 | 2912 | 42.0 | 9855 | 41.0 | 12,767 | ||
| 13 | 1382 | 19.9 | 4164 | 17.3 | 5546 | ||
| ER visits | 0–1 | 4408 | 63.5 | 15,413 | 64.2 | 19,821 | 0.348 |
| ≥2 | 2530 | 36.5 | 8614 | 35.9 | 11,144 | ||
| Hospital admission | Yes | 1302 | 18.8 | 5006 | 20.8 | 6308 | 0.000 |
| No | 5636 | 81.2 | 19,021 | 79.2 | 24,657 | ||
| Polypharmacy | 0–5 | 3289 | 47.4 | 12,782 | 53.2 | 16,071 | 0.000 |
| 6–12 | 3592 | 51.8 | 11,126 | 46.3 | 14,718 | ||
| ≥13 | 57 | 0.8 | 119 | 0.5 | 176 | ||
| Mean (SD) | 5.88 (4.3) | 4.74 (4.4) | 4.99 (4.4) | 0.000 | |||
| Comorbidities | |||||||
| Previous fracture | 1734 | 25.0 | 4959 | 20.6 | 6693 | 0.000 | |
| Osteoporosis | 1717 | 24.8 | 2424 | 10.1 | 4141 | 0.000 | |
| Parkinson’s | 408 | 5.9 | 1523 | 6.3 | 1931 | 0.165 | |
| Dementia | 1445 | 20.8 | 6677 | 27.8 | 8122 | 0.000 | |
| Diabetes | 2030 | 29.3 | 7710 | 32.1 | 9740 | 0.000 | |
| Rheumatoid arthritis | 322 | 4.6 | 642 | 2.7 | 964 | 0.000 | |
| Stroke | 687 | 9.9 | 3118 | 13.0 | 3805 | 0.000 | |
| Myocardial infarction | 778 | 11.2 | 3209 | 13.4 | 3987 | 0.000 | |
| Heart failure | 750 | 10.8 | 3092 | 12.9 | 3842 | 0.000 | |
| Depression | 1492 | 21.5 | 4468 | 18.6 | 5960 | 0.000 | |
| Cancer | 975 | 14.1 | 3851 | 16.0 | 4826 | 0.000 | |
| Malnutrition | 98 | 1.4 | 434 | 1.8 | 532 | 0.026 | |
| Medication use | |||||||
| Opioid treatment | 2482 | 35.8 | 6021 | 25.0 | 8503 | 0.000 | |
| Hypnotic treatment | 1204 | 17.4 | 4233 | 17.6 | 5437 | 0.611 | |
| Oral corticoids | 794 | 11.4 | 2261 | 9.4 | 3055 | 0.000 | |
| Antipsychotics | 1112 | 16.0 | 4570 | 19.0 | 5682 | 0.000 | |
| Anxiolytics | 3409 | 49.1 | 10,503 | 43.7 | 13,912 | 0.000 | |
| Antiarrythmics | 325 | 4.7 | 117 | 4.9 | 1495 | 0.526 | |
| Antihypertensive treatment | 5255 | 75.7 | 17,612 | 73.3 | 22,867 | 0.000 | |
| Diuretic treatment | 2330 | 33.6 | 8201 | 34.1 | 10,531 | 0.395 | |
| Osteoporosis treatment | 3805 | 54.8 | 1453 | 6.1 | 5258 | 0.000 | |
ER = emergency room.
Trends of treatment after hip fracture
The percentage of patients treated in the region declined from a maximum of 28.9% in 2009 to 16.4% in 2015. By sex, women treated reached a maximum of 33.4% in 2009 to 19% in 2015, whereas men reached a maximum of 14% in 2011 to 8.1% in 2015 (Fig. 2). By health department, there was a noticeable variability in the rate of patients treated, ranging from 40.9% to 11.1% in the whole period. Proportion of treated patients decreased from 2008 to 2015 in 20 of the 24 HDs. In 2008, 15 HDs were treating more than 20% of the patients at discharge (six of them treating more than 30%, with three treating more than 40% of the patients), whereas in 2015 only six HDs treated more than 20% of the patients (and only one HD more than 40% of patients). In nine of the 24 HDs, the percentage of patients treated fell by more than 50% (Fig. 3). Proportion of treatment at 6 months was only marginally higher (Supplemental Fig. S1).
Figure 2.
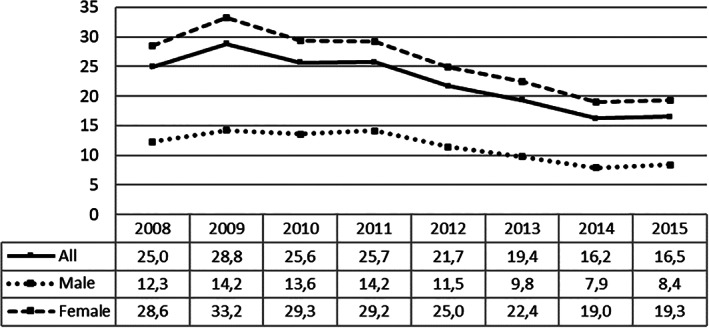
Percentage of patients treated in the region, for the whole cohort and by sex, 2008 to 2015.
Figure 3.
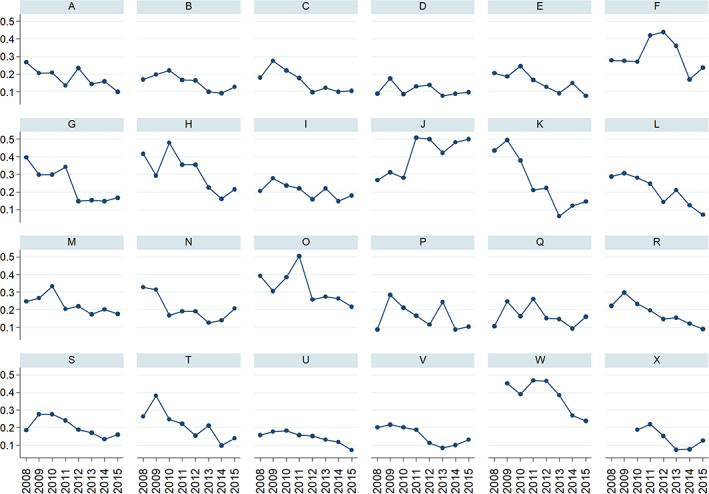
Percentage of patients treated per health department, 2008 to 2015.
Factors associated with receiving treatment
Table 2 presents the results of the two multilevel models, showing the specific associations between contextual (HDs) characteristics and treatment within 3 months of hip fracture (empty model) and between individual and contextual characteristics and treatment (model with HD and individual variables), as well as the analysis of variance (MOR) and the values of the intraclass correlation statistic (ICC). ICC, informing on the proportion of total variance in treatment initiation attributable to the HD component, was 5.33% in the empty model and 7.57% in the model with individual variables, showing that variability among HDs was statistically significant. The median odds ratio, that translates the area level variance in the widely used odds ratio scale, was 1.51 for the empty model and 1.64 for the model with covariates, showing differences in the probability of receiving treatment between HDs.
Table 2.
Factors Associated with Receiving Treatment: Multilevel Analysis Results
| Model with individual variables | ||||
|---|---|---|---|---|
| Empty model | OR | 95% CI | ||
| Sociodemographics | ||||
| Female | 1.80 | 1.64–1.98 | ||
| Aged 85 years and older | 0.76 | 0.68–0.84 | ||
| Osteoporosis treatment | 17.12 | 15.80–18.56 | ||
| Year of discharge | ||||
| 2009 | 1.14 | 1.01–1.29 | ||
| 2010 | 0.86 | 0.76–0.97 | ||
| 2012 | 0.64 | 0.56–0.73 | ||
| 2013 | 0.57 | 0.50–0.65 | ||
| 2014 | 0.53 | 0.46–0.61 | ||
| 2015 | 0.56 | 0.49–0.64 | ||
| Comorbidities | ||||
| Prior stroke | 0.88 | 0.79–0.99 | ||
| Osteoporosis | 1.31 | 1.19–1.44 | ||
| Dementia | 0.72 | 0.66–0.78 | ||
| Rheumatoid arthritis | 1.23 | 1.03–1.47 | ||
| Medication use | ||||
| Antipsychotics | 0.83 | 0.75–0.91 | ||
| Antiarrythmics | 0.83 | 0.71–0.98 | ||
| Diuretics | 0.90 | 0.83–0.98 | ||
| Anxiolytics | 0.92 | 0.86–0.99 | ||
| Oral corticoids | 1.13 | 1.01–1.27 | ||
| Hypnotics | 0.88 | 0.80–0.96 | ||
| Use of health services | ||||
| Hospital admission | 0.82 | 0.75–0.90 | ||
| Random effects | ||||
| Intraclass correlation (ICC) | 5.33% | 7.54% | ||
| Median odds ratio (MOR) | 1.51 | 1.64 | ||
| Area under ROC curve (AUC) | 0.62 | 0.84 | ||
OR = odds ratio; CI = confidence interval.
Only significant variables are shown. Nonsignificant variables: aged 75 to 84 years, year 2011, previous fracture, heart failure, diabetes, Parkinson’s, cancer, depression, malnutrition, myocardial infarction, use of opioids, use of antihypertensives, emergency room visits, polypharmacy.
Regarding discriminatory accuracy, the area under the ROC curve (AUC) in the empty model was 0.62. Information provided by individual variables resulted in an increase of the AUC to a value of 0.84. In the model with individual covariates, patients treated within 3 months of hip fracture discharge were more likely to be female (OR = 1.80, 95% CI 1.64–1.98), to have had previous osteoporosis treatment (OR = 17.12, 95% CI 15.80–18.56), to have a diagnosis of osteoporosis (OR = 1.31, 95% CI 1.19–1.44) and rheumatoid arthritis (OR = 1.23, 95% CI 1.03–1.48), and to use oral corticoids (OR = 1.13, 95% CI 1.01–1.27). Patients at an increased risk of remaining untreated after a hip fracture were more prone to being older than 85 years (versus 65 years and younger, OR = 0.76, 95% CI 0.68–0.84), having a prior stroke (OR = 0.88, 95% CI 0.79–0.99) and dementia (OR = 0.72, 95% CI 0.66–0.78), and were more likely to use antipsychotic drugs, antiarrhythmic drugs, diuretics, anxiolytics, and hypnotics (OR = 0.83, 95% CI 0.76–0.92; OR = 0.82, 95% CI 0.68–0.98; OR = 0.86, 95% CI 0.78–0.94; OR = 0.92, 95% CI 0.86–0.99; OR = 0.88, 95% CI 0.80–0.96, respectively), as well as to have a previous hospital admission for any cause (OR = 0.82, 95% CI 0.75–0.90). In 10 HDs, the probability of being treated was below the regional average, 8 were above it, and 6 showed no difference with the regional average (Fig. 4). With respect to year 2008, patients were more likely to be treated in 2009 (OR = 1.14, 95% CI 1.01–1.30) but less likely during other years (p < 0.001), except for 2011 (Table 2).
Figure 4.
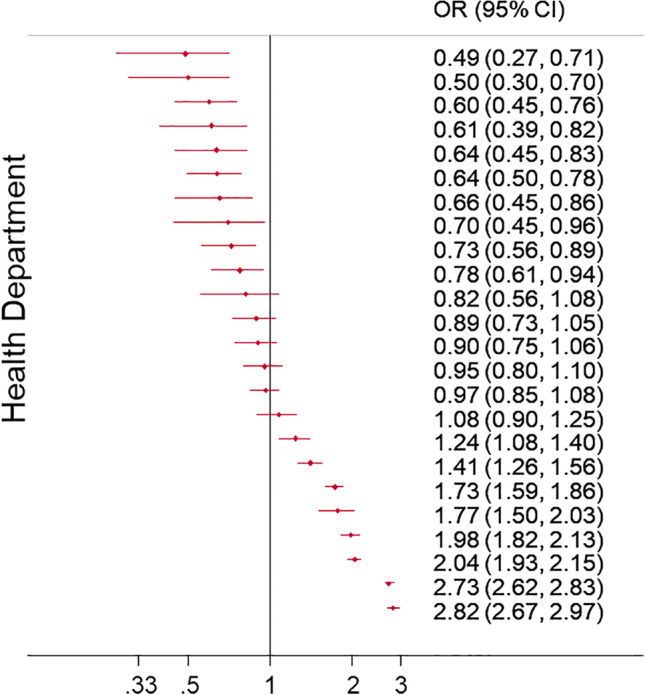
Ranking of adjusted differences between health departments. Differences are expressed as odds ratios (ORs), and 95% confidence intervals (CIs) are shown.
Choice of medication according to health departments
In 2008, patients receiving bisphosphonates in the 22 HDs existing that year ranged from 38.1% to 6.7% of patients treated. For PTH, percentages ranged from 14.3% to 0% and from 10.8% to 0% for strontium ranelate. In 2015, with 24 HDs, 44.4% to 3.2% patients received a bisphosphonate depending on the HD of discharge, 7.3% to 0% were treated with PTH, and 6.9% to 0% were treated with denosumab. Overall, among treated patients, an important variability between HDs can be observed in the percentage of use of bisphosphonates (from 81.4% to 47.7%), PTH (from 26.7% to 4.2%), or denosumab (from 11.3% to 0.8%; Supplemental Fig. S2).
Discussion
Our results showed that secondary prevention after an osteoporotic hip fracture in the region of Valencia was not only suboptimal but followed a deteriorating trend in the period 2008 to 2015. Only 1 in 4 patients were treated at the beginning of the follow‐up, whereas only 1 in 6 were treated at the end of the period. Systematically, men were treated less than women throughout the study period. Moreover, ICC indicates that significant differences between HDs exist with regard to percentage of patients treated and the choice of drug.
Our study provides relevant and original information on the temporal evolution of secondary prevention of hip fracture in a large, population‐based cohort, combined with the analysis of the general contextual effect of HD variation and the individual level variables associated with receiving treatment. We showed that HDs are contextual factors that influence significantly patterns of treatment in patients after a hip fracture, irrespective of individual characteristics. The HD of discharge was one of the most prominent factors related to the decision to treat, as well as some individual variables, and our model showed a good discriminative ability. At patient level, being a female and having a previous osteoporosis diagnosis, previous osteoporosis treatment, as well as rheumatoid arthritis were associated with receiving post‐discharge treatment, whereas factors associated with a lower chance of being treated were older age, patients with prior stroke and dementia, use of antipsychotics, antiarrhythmic drugs, diuretics, anxiolytics, or hypnotics, and previous hospitalization for any reason. With regard to the choice of drug by health department, important variations could be observed, with an almost twofold difference in the use of bisphosphonates, a sevenfold difference in the use of PTH, or a 14‐fold difference in the use of denosumab.
A series of elements, mainly supply‐side factors, may be associated with such different behaviors among HDs: physician preferences based on clinical experience, risk aversion, or safety warning awareness, HD‐specific health care policies and protocols (or their absence) influencing both specialist and primary care prescription, or different promotional intensity over time and districts. Qualitative research is warranted to identify specific HD characteristics that may explain such differential trends.
The overall decline in the use of medication after a hip fracture is consistent with the observed downward trend of osteoporosis medication in many countries such as the UK, Australia, or the US.13, 15, 25, 26, 27, 28 This trend has been linked to the safety warning issued in 2010 by the FDA with regard to the association between long‐term use of bisphosphonates and atypical fractures. In Spain, the Spanish Agency for Medicines and Medicinal Products did not publish the warning on atypical fractures (simultaneously with the European Medicines Agency [EMA]) until mid‐2011. That may explain why the drop observed in the consumption of osteoporosis drugs happened at a later moment—2011—in Spain. In 2009, a previous decline can be also observed, coinciding in time with the EMA safety warning on osteonecrosis of the jaw. However, in a recent study evaluating the impact of these warnings on the use of osteoporosis medication for primary and secondary prevention, we did not find a significant impact of the 2009 warning on drug consumption.29 In that study, we found that the decline in medication use was similar for both high‐ and low‐risk patients in the same period, which is consistent with our current results based on a cohort of high‐risk patients, where the general trend is clearly downward. Only in four of the 24 health departments in the region of Valencia does the percentage of treated patients increase over time. Finally, a further drop can be observed around 2012, coinciding with a change in the pharmaceutical cost‐sharing system, which we showed in the aforementioned study as impacting on the overall consumption of anti‐osteoporotic drugs in the region.29
Our study is subject to some limitations. First, VID databases gather real‐world clinical practice data and contain information as registered by health professionals during routine clinical practice; these are not specifically prepared for research. In this sense, studies based on real‐world clinical information like VID may be subject to well‐known biases such a differential recording, misclassification bias, or missing data. This is a common feature in studies based on real‐word data. However, diagnostic accuracy for hospital discharge diagnoses due to acute surgical conditions (such as hip fracture, our main inclusion criterion) is expected to be very high, and prescription and dispensation information (the essential data to define our main endpoint) is also very accurate as it is used for billing purposes. Second, we lack information on zoledronic acid, which is provided in‐hospital in Spain. Even if some patients may be prescribed this drug (resulting in an underestimation of the proportion of patients treated in our study), international evidence suggest that zoledronic acid consumption has declined in the same way as other bisphosphonates.30 Third, we analyzed the simplest possible multilevel structure of individuals within health departments, but we recognize that other care levels or contexts, for instance primary care zones or physicians, may influence patterns of prescription. Notwithstanding, this design is commonly used in multilevel health care studies,20, 21, 22, 23, 24, 31 and our analyses included appropriate variables for evaluating differences in the prescription of anti‐osteoporotic drugs. Fourth, despite using many relevant patient variables, we cannot discard the existence of missing clinical factors relevant for treatment decisions, such as treatment contraindications. Fifth, we have not analyzed the impact of the decrease in prescription on clinical outcomes, but current evidence shows relevant benefits of osteoporosis treatment after hip fracture, reducing refracture rates by about 50%.32, 33, 34 Finally, the generalization of our results to other settings outside, or even to other regions within, the Spanish National Health System should be done with great caution, especially considering the importance of contextual factors.
The main findings of this study are the acknowledgment of a suboptimal and worrying trend in the treatment of patients after a hip fracture in the region of Valencia between 2008 and 2015 and that the decision regarding treatment was influenced by provider factors. The identification of factors that are not related to clinical appropriateness but are conditioning choices regarding initiation of treatment brings to light important management issues. Although we have evidence that an important problem of osteoporosis treatment overuse in primary prevention in low‐risk populations still remains in Spain,29 the problem of underuse in high‐risk populations is aggravated. This situation pressingly demands action (both at the organizational and professional levels) focused on populations at a higher risk (such as hip fracture patients) that particularly address underutilization.
Disclosures
The FISABIO Foundation (a nonprofit research institution depending on the Valencia Ministry of Health) had a collaboration agreement with Amgen SA to conduct nonconditioned independent research on osteoporosis (ESOSVAL and PREV2FO cohorts) during 2014.
Supporting information
Supplemental Fig. S1. Percentage of patients treated using a 3‐month window versus a 6‐month window.
Supplemental Fig. S2. Choice of medication per health department in 2008 and 2015 (%).
Supplemental Table S1. ATC Codes Used for Medication.
Supplemental Table S2. ICD9MC Codes Used to Define Comorbidities.
Supplemental Table S3. ICD9MC Codes Used to Define Criteria.
Acknowledgments
The PREV2FO cohort study was funded by Spanish Ministry of Science, Innovation, and Universities (grants PI14/00993 and PI18/01675, cofinanced by the European Regional Development Fund), and awarded with the Merck Research Grant 2019 in health outcomes and research from Merck Salud Foundation. CR was partially funded by grants RD12/0001/0005 and RD16/0001/0011 (Spanish health services research on chronic diseases network, REDISSEC) from the Instituto de Salud Carlos III, Spanish Ministry of Science, Innovation, and Universities, cofinanced by the European Regional Development Fund. None of the aforementioned institutions/firms played any role in this study. The views presented are those of the authors and not necessarily those of the FISABIO Foundation, the Valencia Ministry of Health, the Instituto de Salud Carlos III, or any other institution/firm.
Authors' roles: Study design: AG, GS, IH, and SP. Study conduct: IH and AG. Data collection: IH. Data analysis: IH, AG, SP, and GS. Data interpretation: AG, IH, SP, and GS. Drafting manuscript: AG. Revising manuscript content: SP, GS, AG, and IH. Approving final version of the manuscript: AG, IH, SP, GS, CR, and JS.
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4028.
References
- 1. Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20(10):1633–50. [DOI] [PubMed] [Google Scholar]
- 2. Cooper C, Mitchell P, Kanis JA. Breaking the fragility fracture cycle. Osteoporos Int. 2011;22(7):2049–50. [DOI] [PubMed] [Google Scholar]
- 3. Johnell O, Kanis JA, Oden A, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15(3):175–9. [DOI] [PubMed] [Google Scholar]
- 4. Kanis JA, Cooper C, Rizzoli R, Reginster JY. Scientific advisory board of the European Society for clinical and economic aspects of osteoporosis (ESCEO) and the committees of scientific advisors and national societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ström O, Borgström F, Kanis JA, et al. Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2011;6:59–155. [DOI] [PubMed] [Google Scholar]
- 6. Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29(9):1929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaile J, Sullivan L, Bennett C, Bleasel J. First fracture project: addressing the osteoporosis care gap. Intern Med J. 2007;37(10):717–20. [DOI] [PubMed] [Google Scholar]
- 9. Nayak S, Roberts MS, Greenspan SL. Factors associated with diagnosis and treatment of osteoporosis in older adults. Osteoporos Int. 2009;20(11):1963–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elliot‐Gibson V, Bogoch ER, Jamal SA, Beaton DE. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int. 2004;15(10):767–78. [DOI] [PubMed] [Google Scholar]
- 11. Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006;35(5):293–305. [DOI] [PubMed] [Google Scholar]
- 12. Balasubramanian A, Tosi LL, Lane JM, Dirschl DR, Ho PR, O'Malley CD. Declining rates of osteoporosis management following fragility fractures in the U.S., 2000 through 2009. J Bone Joint Surg Am. 2014;96(7):e52. [DOI] [PubMed] [Google Scholar]
- 13. Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996‐2012: an ecological analysis. J Bone Miner Res. 2015;30(12):2179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim SC, Kim MS, Sanfélix‐Gimeno G, et al. Use of osteoporosis medications after hospitalization for hip fracture: a cross‐national study. Am J Med. 2015;128(5):519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desai RJ, Mahesri M, Abdia Y, et al. Association of osteoporosis medication use after hip fracture with prevention of subsequent nonvertebral fractures: an instrumental variable analysis. JAMA Netw Open. 2018;1(3):e180826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klop C, Gibson‐Smith D, Elders PJ, et al. Anti‐osteoporosis drug prescribing after hip fracture in the UK: 2000‐2010. Osteoporos Int. 2015;26(7):1919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah A, Prieto‐Alhambra D, Hawley S, et al. Geographic variation in secondary fracture prevention after a hip fracture during 1999‐2013: a UK study. Osteoporos Int. 2017;28(1):169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peiró S, Maynard A. Variations in health care delivery within the European Union. Eur J Public Health. 2015;25(Suppl 1):1–2. [DOI] [PubMed] [Google Scholar]
- 19. García‐Sempere A, Orrico‐Sánchez A, Muñoz‐Quiles C, et al. Data resource profile: the Valencia health system integrated database (VID). Int J Epidemiol. 2020;pii: dyz266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med. 2017;36(20):3257–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–8. [DOI] [PubMed] [Google Scholar]
- 23. Hjerpe P, Ohlsson H, Lindblad U, Bostrom KB, Merlo J. Understanding adherence to therapeutic guidelines: a multilevel analysis of statin prescription in the Skaraborg primary care database. Eur J Clin Pharmacol. 2011;67(4):415–23. [DOI] [PubMed] [Google Scholar]
- 24. Ohlsson H, Librero J, Sundquist J, Sundquist K, Merlo J. Performance evaluations and league tables: Do they capture variation between organizational units? An analysis of 5 Swedish pharmacological performance indicators. Med Care. 2011;49(3):327–31. [DOI] [PubMed] [Google Scholar]
- 25. Peeters G, Tett SE, Duncan EL, Mishra GD, Dobson AJ. Osteoporosis medication dispensing for older Australian women from 2002 to 2010: influences of publications, guidelines, marketing activities and policy. Pharmacoepidemiol Drug Saf. 2014;23(12):1303–11. [DOI] [PubMed] [Google Scholar]
- 26. van der Velde RY, Wyers CE, Teesselink E, et al. Trends in oral anti‐osteoporosis drug prescription in the United Kingdom between 1990 and 2012: variation by age, sex, geographic location and ethnicity. Bone. 2017;94:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balkhi B, Seoane‐Vazquez E, Rodriguez‐Monguio R. Changes in the utilization of osteoporosis drugs after the 2010 FDA bisphosphonate drug safety communication. Saudi Pharm J. 2018;26:238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim SC, Kim DH, Mogun H, et al. Impact of the U.S. Food and Drug Administration's safety‐related announcements on the use of bisphosphonates after hip fracture. J Bone Miner Res. 2016;31(8):1536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hurtado‐Navarro I, García‐Sempere A, Rodríguez‐Bernal C, Sanfélix‐Genovés J, Peiró S, Sanfélix‐Gimeno G. Impact of drug safety warnings and cost‐sharing policies on osteoporosis drug utilization in Spain: a major reduction but with the persistence of over and underuse. Data from the ESOSVAL cohort from 2009 to 2015. Front Pharmacol. 2019;10:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wysowski DK, Greene P. Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002‐2012. Bone. 2013;57(2):423–8. [DOI] [PubMed] [Google Scholar]
- 31. Diez Roux AV. The study of group‐level factors in epidemiology: rethinking variables, study designs, and analytical approaches. Epidemiol Rev. 2004;26:104–11. [DOI] [PubMed] [Google Scholar]
- 32. Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333:1437–43. [DOI] [PubMed] [Google Scholar]
- 33. Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–41. [DOI] [PubMed] [Google Scholar]
- 34. Harris ST, Watts NB, Genant HK, et al. Effect of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. JAMA. 1999;282:1344–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1. Percentage of patients treated using a 3‐month window versus a 6‐month window.
Supplemental Fig. S2. Choice of medication per health department in 2008 and 2015 (%).
Supplemental Table S1. ATC Codes Used for Medication.
Supplemental Table S2. ICD9MC Codes Used to Define Comorbidities.
Supplemental Table S3. ICD9MC Codes Used to Define Criteria.


