1. Introduction
Cardiovascular complications from cancer therapy remain a significant and growing health challenge. Nowhere is this more apparent than in the rising population of cancer survivors who are at an increased risk of cardiovascular complications. In the US today, there are ~17 million pediatric and adult cancer survivors, a nearly 1.2-fold increase from ~14 million in 2012 and projected to increase to ~22 million by 2030 [1–3]. This positive trend of survival is welcome news for cancer patients and clearly demonstrates the dramatic improvements in efficacy of many anti-cancer treatments in recent years [4]. However, improved survival is associated with a parallel rise in the incidence of cancer treatment-related cardiovascular toxicity and increased morbidity and mortality in survivors [5–8]. Longer survival duration together with age-related comorbidities and newer therapies with unknown cardiotoxicity potential in pediatric and adult cancer patients may result in further increases in cardiotoxicity risk in survivors. Anti-cancer therapies continue to improve and expand rapidly from the traditional cytotoxic chemotherapies and radiation to targeted immunomodulatory agents and newer immuno-based therapies, such as immune checkpoint inhibitors (ICI) and chimeric antigen receptor T-cell (CAR-T) immunotherapies [9–11]. Increasingly, these therapies are used in multiple combined treatment regimens across numerous cancer types at time of diagnosis, progression, and relapse. As new cancer therapies continue to emerge and expand rapidly, as treatment regimens become more complex, and as cancer survival continues to improve, we can expect cancer treatment-induced cardiotoxicity to remain a highly significant health challenge.
Cardiotoxicity generally refers to all forms of cardiovascular complications. Although cardiotoxicity has been an established consequence of anthracyclines such as doxorubicin, the explosion of approved anti-cancer agents has led to a re-thinking of the full spectrum of cardiovascular adverse effects that can result from targeting new pathways, stimulating the immune system, and revising radiation strategies. Cardiovascular sequelae may manifest as acute, chronic, or late-onset adverse events. Acute cardiotoxicity that occurs during or immediately after therapy may lead to dose modifications, dose delays, and even cessation of cancer therapy. Patients on chronic cancer therapy, with or without co-existing cardiovascular disease or co-morbidities, may develop chronic or cumulative cardiovascular complications. Late-onset cardiotoxicity that occurs years or decades after cancer treatments may lead to increased morbidity and early mortality due to what is thought to mimic an accelerated treatment-induced cardiovascular disease progression [12–14]. While the methods of diagnosis and monitoring may be similar, the management strategies and implementation of cardioprotective interventions for acute, chronic, and late-onset cardiotoxicity require distinctly different approaches, implying that cardiovascular complications can be progressive and may involve a highly non-uniform or complex pathogenic process.
Risk assessment tools, which aid prevention and management of cardiotoxicity risk, are currently lacking for many cancer treatments. The risk of cardiovascular toxicity depends greatly on the treatment regimen. In addition, other factors such as cardiovascular health status, age, genetics, social determinants of health, and environmental stress may further add to an individual’s risk of cardiovascular complications. Unfortunately, how these risk factors combine to determine the timing of onset, clinical and subclinical presentations, and clinical outcomes are not fully understood for most anti-cancer treatment regimens. As a result, it has been challenging to critically assess the individual risk profile of cardiotoxicity and develop effective management strategies for prevention, treatment, and monitoring. These challenges are hindering improvements in clinical outcomes and quality of life in cancer survivors. Furthermore, clinical guidelines for managing risk of cardiotoxicity from specific cancer treatment regimens have focused primarily on managing the cardiovascular adverse effects from Adriamycin and radiation [15–20]. To date, limited evidence exists to support clinical guidelines for other cancer treatment regimens. Enhanced research collaborations between cardiologists and oncologists could generate the evidence needed to support more comprehensive guidelines.
Development of new diagnostic and cardioprotective approaches requires advances in mechanistic understanding of cardiovascular injury. Despite undergoing extensive safety testing during development, nearly all anti-cancer therapeutic agents have the potential to cause cardiovascular complications [21, 22]. The known adverse cellular and tissue impacts of cancer therapies include dysfunction of the cardiac myocytes and other cells of the cardiovascular system, such as vascular endothelial cells, cardiac macrophages, cardiac fibroblasts, and myofibroblasts; injury to the vasculature; cardiac valvular dysfunction; hemodynamic flow alterations; thrombotic events; and radiation-mediated tissue damage [9, 23]. However, the molecular mechanisms of the injury remain unclear. This uncertainty is illustrated by the case for anthracyclines, one of the earliest and most common classes of anti-cancer therapeutic agents. Reported molecular mechanisms of anthracycline (e.g., doxorubicin) cardiotoxicity include topoisomerase 2β (Top2b) mediated DNA damage; mitochondrial dysfunction; alterations of calcium, cell survival and metabolic signaling pathways; excessive reactive oxygen species (ROS); dysregulation of neuregulin-1 and endothelin-1 signaling; and ultimately, autophagy and apoptosis [24]. While such research advances have contributed to new diagnostics and cardioprotective approaches, anthracycline cardiotoxicity remains an active research area and new research findings continue to reveal additional mechanisms by which these therapeutic agents cause cardiovascular complications. For other more recent pharmacologic, biologic, targeted therapeutic agents, radiation therapy, or combination treatment regimens, much less is known about the cellular and molecular mechanisms that lead to cardiovascular injury.
The goals of research advances in cancer treatment-induced cardiotoxicity are to optimize cancer treatment efficacy while minimizing cardiotoxicity risk. This broad research field spans risk assessment, prevention, diagnosis, monitoring, treatment, and management. As cancer treatments continue to improve and expand, early assessment of whether the treatments pose cardiotoxicity sequelae might support more effective integration of preventive and cardioprotective approaches. Recently, several pathogenic mechanisms have been recognized as common elements in cancer and cardiac disorders; thus, studies of cardiovascular effects of anti-cancer agents have the potential to inform basic and translational mechanisms in cardiovascular disease [25]. Although NIH-supported research continues to support significant scientific advances, major research gaps remain in areas such as mechanistic understanding of cardiovascular injury, preclinical risk evaluations, preclinical disease models, chronic and late-onset mechanisms, and development and assessment of preventive and cardioprotective approaches.
2. NIH Workshops and Funding Opportunity Announcements
To address the growing research challenges and gaps in cancer treatment-related cardiotoxicity, the NCI and NHLBI co-sponsored a workshop entitled ‘Cancer Treatment-Related Cardiotoxicity: Understanding the Current State of Knowledge and Future Research Priorities’ in March 2013 [26]. The goals of the workshop were to identify knowledge gaps and research barriers, and to help determine how best to prioritize research opportunities, allocate resources, and establish needed infrastructure. While recognizing the broad scope of multiple cardiotoxicity disorders, this initial workshop focused primarily on hypertension and heart failure and included all cancer treatment modalities in use at that time. Over forty scientific knowledge gaps and research opportunities were identified by the workshop [26]. In addition, several resources and infrastructure for the research community were identified. The workshop helped increase collaborations among the cardiovascular and cancer research investigators who are involved in cardiotoxicity research.
An early concern regarding cardiotoxicity that became evident during the discussions at the workshop was the nonuniform and poorly defined cardiotoxicity terms used to record the various cardiovascular complications across different clinical studies at the time. To address this issue, two working groups were established following the workshop that were facilitated by the NCI: one for adverse event reporting and another for data collection in randomized clinical trials. These working groups have continued to refine, standardize, and harmonize cardiotoxicity terms in clinical data across the different adverse event reporting and clinical trial studies and this effort has enhanced the sharing and utility of the collected data [27]. Additionally, the recognition of this issue by the cardio-oncology research community subsequently led to the revision of the clinical care guidelines for pediatric and adult cancer survivors which was published by the American Society of Cardio-Oncology in 2017 [15, 27] and by the European Society of Medical Oncology in 2020 [28].
A follow-on workshop entitled ‘Changing Hearts and Minds: Improving Outcomes in Cancer Treatment-Related Cardiotoxicity’ was held in June 2018 to evaluate progress, reassess research gaps, identify emerging areas of research opportunities, and update the scientific priorities since the 2013 workshop [29]. This workshop included all forms of cardiotoxicity as defined by cardiac and/or vascular specific common terminology criteria for adverse events. Significant research challenges remained despite the substantial progress since the first workshop, such as an expanded cardiotoxicity portfolio of NIH research awards, establishment of interdisciplinary working groups within cardiology and oncology, initiation of several prevention and management trials and development of cardio-oncology specific clinical guidelines. These challenges were, in part, due to the proliferation of new and improved cancer treatments and continued improvement in survival duration. Over twenty additional research gaps and emerging research opportunities were identified by the second workshop [29]. Together with the research gaps identified by the earlier workshop, the updated knowledge gaps and research priorities provide a comprehensive and detailed roadmap of research opportunities in the field and serve as a useful resource of information for researchers who are interested in pursuing cardio-oncology research.
In November 2015, NCI and NHLBI released the first set of two Funding Opportunity Announcements (FOAs) aimed at addressing the broad research gaps in cancer treatment-related cardiotoxicity (Table 1). The reason for having two FOAs were to allow both exploratory (i.e., R21, PA16-036) as well as standard (i.e., R01, PA16-035) research project grant proposals. The goal of the solicitations was to encourage collaborative applications from cardiology and oncology researchers to focus on identification and characterization of patients at risk of developing cancer treatment-related cardiotoxicity and developing mitigation/prevention strategies to minimize cardiovascular dysfunction while optimizing cancer outcomes. Innovative methods designed to evaluate cardiac risk prior to treatment and integrate evidence-based cancer treatment regimens with screening, diagnostic, and/or management strategies were encouraged. These FOAs were subsequently renewed twice, first in November 2017 (PA18-003 and PA18-013) and again in December 2018 (PA19-111 and PA19-112) and covered eleven grant application receipt cycles over a period of nearly 5 years (Table 1).
Table 1:
Workshops and Funding Opportunity Announcements
| NIH Workshops | |||
|---|---|---|---|
| Title | Date | Reference | |
| Cancer Treatment-Related Cardiotoxicity: Understanding the Current State of Knowledge and Developing Future Research Priorities | 3/20–21/2013 |
https://epi.grants.cancer.gov/events/cardiotoxicity/ Publication with summary of future research opportunities: [26] |
|
| Changing Hearts and Minds: Improving Outcomes in Cancer Treatment-Related Cardiotoxicity | 6/25–26/2018 |
https://epi.grants.cancer.gov/events/cardiotoxicity/improving-outcomes.html Publication with summary of future research opportunities: [29] |
|
| Funding Opportunity Announcements | |||
| Title | Number | Date | Website address |
| Improving Outcomes in Cancer Treatment-Related Cardiotoxicity (R01 & R21) | R01: PA16–035 R21: PA16–036 |
11/17/2015–1/24/2018 |
https://grants.nih.gov/grants/guide/pa-files/PA-16-035.html
https://grants.nih.gov/grants/guide/pa-files/PA-16-036.html |
| Improving Outcomes in Cancer Treatment-Related Cardiotoxicity (R01 & R21, Clinical Trial Optional) | R01: PA-18–003 R21: PA-18–013 |
11/2/2017–12/17/2018 |
https://grants.nih.gov/grants/guide/pa-files/PA-18-003.html
https://grants.nih.gov/grants/guide/pa-files/PA-18-013.html |
| Improving Outcomes in Cancer Treatment-Related Cardiotoxicity (R01 & R21, Clinical Trial Optional) | R01: PA-19–112 R21: PA-19–111 |
12/17/2018–1/8/2022 |
https://grants.nih.gov/grants/guide/pa-files/PA-19-112.html
https://grants.nih.gov/grants/guide/pa-files/PA-19-111.html |
3. Spectrum of NIH-Supported Research Projects
To date, NCI and NHLBI have supported 33 research project grants through the above FOAs amounting to nearly $26M1 in research support (data accessed on August 4, 2021). Four of the funded exploratory R21 research awards led to subsequent funding of the larger R01 research awards. The research awards have supported the careers of five early-stage investigators and two new investigators. In addition, the FOAs have resulted in the funding of several clinical trials. Most of the awarded research projects have begun recently and thus the research work is ongoing. The topics of research supported by these R21 and R01 awards are included below under the research categories.
Beyond the FOAs, NIH has been supporting cancer treatment-related cardiotoxicity research through the investigator-initiated research projects. To get an idea of the approximate extent of research support and potential research gaps and trends, a keyword search was conducted on the database of NIH-funded research projects over a 10-year period, from fiscal year 2011 to 2020 [30]. The search was limited to the title, abstract and specific aims sections of the awarded grant applications. Only new or competing renewal projects were counted to avoid duplicate counting of multi-year awarded projects. The output of the search was verified to ensure that the resulted list of grant awards contained the keywords used for the search in those sections. The list was then adjudicated by the authors to ensure that at least one or more aims of the projects were focused on cancer treatment-related cardiotoxicity. Duplicates, intramural projects or inadvertent grants were removed, and the grants were separated into three broad categories: basic and translational, clinical trials and interventions, and observational. For the majority of awards, the categorization was unanimous, for a few where there was disagreement, discussion among the reviewers facilitated consensus. It should be noted that the keyword search strategy may miss or overestimate the actual number of research projects that focus on cancer treatment-related cardiotoxicity. Additionally, the categorization is subjective. The list of the funded research projects identified here thus is a representative spectrum of NIH support on this topic rather than a comprehensive portfolio analysis.
Over the 10-year period, the search yielded a set of 102 awarded research projects amounting to a cumulative total of approximately $94M in NIH grant research support (Figure 1). The NIH uses three-character activity codes to indicate the type of award mechanism (https://grants.nih.gov/grants/funding/ac_search_results.htm). The research projects in the list fell into four activity types: research project grants (activity codes: R01, R21, R56, R03, R15), training/career grants (activity codes: F32, K01, K08, K23, K24, K99, L30), small-business innovation research (SBIR) and technology transfer (STTR) grants (activity codes: R41, R43, U42, R44), and an ‘other’ type to indicate exploratory resource-related grants (activity code: P20). The types of research projects and their respective funding are shown in Figure 1A. It shows that, in terms of the number of projects, the largest percentage was research project grants (70%), followed by training (16%), small business SBIR/STTR (14%), and other (1%). The respective funding supports were 90% for the research project grants, 4% for training, 5% for small business SBIR/STTR, and 0.3% for the other grant award. The NHLBI and NCI each supported the largest numbers of research projects numbering 59 and 33, respectively, followed by the National Institute of General Medical Sciences (NIGMS), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institute of Environmental Health Sciences (NIEHS) supporting five, three, one, and one awards, respectively (Figure 1B). The majority of the projects were categorized as basic and translational (N=63, 62%), followed by observational (N=30, 29%), and clinical trials and interventions (N=9, 9%) (Figure 1C).
Figure 1: NIH-Supported Research on Cancer Treatment Related Cardiotoxicity.
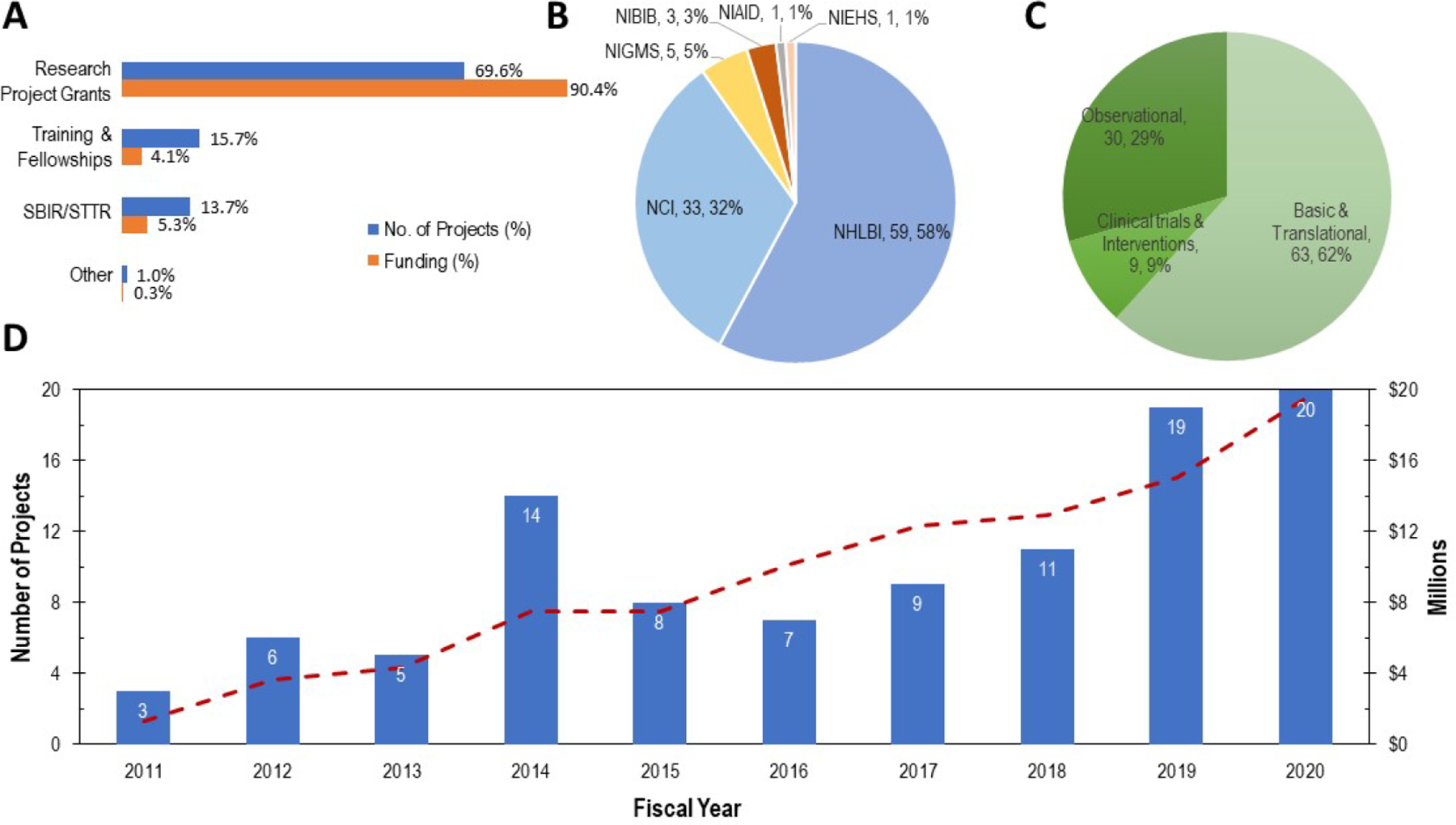
Representative spectrum of awarded research projects over a 10-year period (fiscal year 2011–2020) obtained by a keyword search of the title, abstract, and specific aims sections of the awarded grants from the NIH database of funded projects [30]. Total number of projects and funding were 102 and $94M, respectively. A. In terms of the proportion of the types of research projects and respective funding, the majority of the projects and funding were for research project grants (70%, 90%), followed by training (16%, 4%), small business (SBIR/STTR) (14%, 5%), and other (1%, 0.3%, e.g., resource) grants. B. Number of projects and their corresponding Institute assignments. NHLBI and NCI supported the largest numbers of research projects, 59 and 32, respectively, followed by, NIGMS, NIBIB, NIAID and NIEHS supporting 5, 3, 1, and 1, respectively. C. Funded projects were categorized, based on the research aims, into basic and translational, clinical trials and interventions, and observational. As indicated, the majority were basic and translational (N=63, 62%), followed by observational (N=30, 29%) and clinical trials and interventions (N=9, 9%). D. The number of projects (bar chart, left y-axis) and approximate support amounts (dotted line, right vertical axis) for each year over the ten-year period. The numbers within the bars indicate the total number of awarded projects in that year. The number of projects began modestly between years 2011–2013, jumped in year 14, then decreased again and remained at 11 or below in years 2015–2018. The number of projects have recently risen significantly as seen in years 2019 and 2020. The corresponding funding support shows a gradual rise over the ten-year period, likely due to the multi-year funding periods (e.g., 2–4 years) of the research projects.
The number of funded projects and the funding amounts for each year over the ten-year period are shown in Figure 1D. The numbers inside the bars in Figure 1D indicate the total number of awards in that year. Interestingly, although only a few awards were made every year between 2011 to 2013, the number of awards in 2014 increased dramatically to 14 awards, coinciding with a one-year period following the first workshop held in 2013. The number of awards dropped to 11 or less thereafter in years 2015–2018, but rose again significantly in years 2019 and 2020 (Figure 1D). Research funding shows a gradual increase in all years, likely due to the multi-year research funding period which typically ranges from two to four years for each research project (Figure 1D). The recent increase in the number of funded research awards and corresponding funding amounts reflect the potential positive impact of the second workshop augmented by the grants funded by the recent FOAs. The data indicate that research interest in cancer treatment related cardiotoxicity in recent years has increased.
3.1. Basic and Translational Studies
In the basic and translational category, there were 37 grants with activity codes R01 and R21, which are the activity codes for primary research-oriented projects. The aims of these research projects ranged from mechanistic studies and research model development to biomarkers of risk, development of clinical imaging methods for early subclinical cardiovascular injury, and novel cardioprotective therapeutic strategies. The focus of these basic research studies included understanding of cardiovascular injury mechanisms primarily related to anthracyclines (e.g., doxorubicin) and a few related to radiation injury or adverse effects of other agents such as trastuzumab and tyrosine kinase inhibitors. Additional basic studies included influence of sex differences, cancer cachexia, late-onset mechanisms, clonal hematopoiesis, and innate immune mechanisms. Several studies explored mitochondrial dysfunction and mechanistic understanding of metabolic or hypoxia-related cellular and molecular signaling pathways involved in anthracycline cardiotoxicity (e.g., Top2b, CCR5, BAG3) and potential cardioprotective or therapeutic strategies to mitigate such cardiotoxicity. A few projects focused on the development of new in vitro human induced pluripotent stem cell-derived cardiomyocyte (iPSC-CM) models and animal models (e.g., swine models for clinical imaging, cross-species validation, etc.). Biomarker-related research included genetic factors, DNA methylation, biomarkers for risk assessment, and development of iPSC-CM-based in vitro methods for risk screening. Clinical imaging methods included improvements of imaging probes and imaging methods such as PET, SPECT-CT and cardiac MRI for early injury evaluation. Cardioprotective and therapeutic studies included a few novel therapeutic agents such as small molecule inhibitor of the serine-threonine protein kinase, p90RSK, new chemical analogs of doxorubicin, neuregulin-1 directed therapy to improve potency of doxorubicin and alleviate its cardiotoxicity, a novel small peptide therapeutic to reduce radiation injury, a liposome-encapsuled doxorubicin for ultrasound activated treatment and exercise.
In addition to the research project grants, there were 12 small business SBIR/STTR research projects in the basic and translational category. The focus of these projects included the development of cardiac imaging probes for early detection of doxorubicin-induced cardiotoxicity, a cardiac MRI image-analysis tool to assess subclinical cardiac injury, a method to develop mature human iPSC-CMs for pre-clinical safety screening, a real-time microfluidic biosensor-based assay to monitor chemotherapy levels in blood, and several new therapeutic agents to reduce cardiotoxicity.
3.2. Clinical Trials and Interventional Studies
There were a total of eight R01 and R21 research projects in the clinical trials and interventional studies category as shown in Table 2. The projects included the following:
Table 2:
Clinical Trials and Interventions
| Title, NCT# | Intervention | Goals | Study Population | Enrollment | Dates |
|---|---|---|---|---|---|
| Cardiac Sparing Whole Lung Intensity Modulated Radiation Therapy (IMRT) in Children and Young Adults with Lung Metastases, NCT01586104 | Whole lung intensity modulated radiation therapy (WL-IMRT) | Demonstrate feasibility, dosimetric advantages, and short-term efficacy of WL-IMRT over standard of care whole lung irradiation. | Children and young adults with lung metastases from Wilms tumor, rhabdomyosarc oma and Ewing Sarcoma. | 20 participants | 2/2011–9/2015 |
| Preventing Anthracycline Cardiovascular Toxicity with Statins (PREVENT), NCT01988571 | Atorvastatin (a generic statin) | Double-blind, randomized, placebo controlled trial to determine if atorvastatin administration attenuates deterioration in left ventricular ejection fraction (LVEF) in women receiving adjuvant anthracycline-based chemotherapy for breast cancer. | Women receiving anthracycline-based chemotherapy for triple negative breast cancer. | 279 participants | 2/2014–9/2020 |
| Prevention of Heart Failure induced by Doxorubicin with Early Administration of Dexrazoxane (PHOENIX1), NCT03930680 | Dexrazoxane | Determine whether reduced dose and early administration of dexrazoxane prevents doxorubicin-induced cardiotoxicity. | Non-metastatic, HER2-negative, breast cancer patients HER2-negative, stage I-III female breast cancer patients. | 25 participants | 6/2021–6/2023 |
| Reducing risk of Anthracycline-related heart failure after childhood cancer (PREVENT-HF), NCT02717507 | Carvedilol (beta-blocker) | Randomized, placebo-controlled trial of low-dose carvedilol (beta-blocker) to determine the impact of a two-year course of carvedilol on left ventricular function. | Asymptomatic childhood cancer survivors with prior exposure to high-dose anthracyclines (≥300mg/m2). | 182 participants | 4/2016–7/2022 |
| Statins to prevent Cardiotoxicity from Anthracyclines (STOP-CA), NCT02943590 | Atorvastatin (a generic statin) | Randomized placebo-controlled trial to determine whether statins preserve LVEF twelve months after the initiation of chemotherapy. | Patients with non-Hodgkin’s Lymphoma undergoing anthracycline-based chemotherapy. | 270 participants | 1/2017–10/2023 |
| Strategies in Cardiotoxicity Risk Reduction with Breast Cancer Therapy, NCT04023110 | Risk-guided Carvedilol (beta-blocker) | A randomized, placebo controlled pilot study to determine if a risk-guided treatment strategy that initiates carvedilol in high risk patients prior to cancer therapy will be feasible, safe, well-tolerated, and result in decreases in cardiotoxicity measures, compared to placebo | Patients from Penn Cardiotoxicity of Cancer Therapy (Penn CCT) study population, a longitudinal cohort study of 630 breast cancer patients. | 110 participants | 8/2019–12/2023 |
| The Feasibility of a Biomarker Guided Strategy in Anthracycline Cardiotoxicity, NCT04737265 | Biomarker (NT-proBNP) guided therapy strategy | Pilot study to determine if a biomarker guided strategy (NT-proBNP) to identify and treat high CV risk patients is feasible, well-tolerated and superior to usual care in breast cancer or lymphoma patients treated with doxorubicin. | Patients from Penn Cardiotoxicity of Cancer Therapy (Penn CCT) study population, a longitudinal cohort study of 630 breast cancer patients. | 100 participants | 3/2021–3/2025 |
| Trastuzumab Cardiomyopathy Therapeutic Intervention with Carvedilol (TACTIC) Trial, NCT03879629 | Carvedilol (beta-blocker) | Determine best management strategy (i.e., pre-emptive or preventive) for initiation and duration of beta-blocker carvedilol treatment to reduce cardiotoxicity in breast cancer patients undergoing treatment with trastuzumab. | Adult breast cancer patients undergoing trastuzumab therapy. | 450 participants | 8/2019–9/2025 |
An evaluation of cardiac-sparing whole lung intensity modulated radiation therapy (IMRT) aimed at reducing cardiovascular injury of radiotherapy treatment of lung metastases in young adults;
Two randomized trials testing the effectiveness of statins in preserving left ventricular function following anthracycline treatment - one for triple negative breast cancer and the other for non-Hodgkin’s lymphoma;
A trial evaluating whether early timing of and reduced-dose dexrazoxane is effective in reducing doxorubicin-induced cardiotoxicity in HER2-negative breast cancer patients;
A pilot study to determine if a cardioprotective strategy guided by the biomarker, NT-proBNP, in high-risk breast cancer patients or lymphoma patients treated with doxorubicin is feasible, well-tolerated and superior to usual care;
- Three trials on carvedilol:
- A randomized, placebo-controlled trial to evaluate the cardioprotective effect of carvedilol on high-dose anthracycline treated childhood cancer survivors;
- A randomized, placebo-controlled pilot trial to assess whether a risk-guided prior treatment with carvedilol is safe, feasible, well-tolerated and effective in reducing cardiotoxicity in high-risk breast cancer patients;
- A trial to determine optimal preventive treatment strategy with carvedilol to reduce cardiomyopathy in breast cancer patients undergoing trastuzumab therapy.
The IMRT radiotherapy and one of the earlier statin studies have concluded while the remaining six studies are ongoing (Table 2). NCI sponsors several clinical trials networks to evaluate novel anti-cancer agents, compare standard versus experimental cancer treatment regimens, address cancer treatment toxicity via symptom mitigation research, and evaluate the effectiveness of cancer care delivery. In these networks, it is possible to integrate cardiotoxicity assessments as well as develop trials to answer cardiotoxicity questions [31]
3.2. Observational Studies
There were a total of 19 R21 and R01 research project grants in the observational category. The majority were focused on evaluation and validation of early cardiovascular risk assessment or detection of subclinical cardiovascular injury utilizing ongoing patient trial data or data from cohorts of survivors following cancer therapies in order to inform and improve clinical outcomes in survivors. These include, cardiac magnetic resonance (CMR) imaging, CMR-based algorithms, echocardiography or echocardiography-derived parameters, serum biomarkers, or combined imaging and serum biomarkers, maximum VO2 as a measure of cardiac reserve, CMR-derived aortic stiffness as a measure of early subclinical injury to the vasculature, and automated electronic health record (EHR) based or genetics based risk management tools. Several of the studies were ancillary studies that utilized clinical data from recent or ongoing clinical trials such as the AH-HA [32], the RadComp [33], the PREVENT trial (Table 2), or prior cohort studies such as the Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) [34]. The remaining few research projects were focused on identifying radiotherapies with reduced cardiotoxicity risk (i.e., photon vs. proton), assessing long-term impact of dexrazoxane cardioprotective strategy, studies of sex differences, and racial differences in venous thromboembolism risk. The observational category also included two small business SBIR/STTR grants and both were focused on the development of a clinical tool to analyze cardiac MRI images for detection of subclinical cardiac injury and to provide clinical assessments as a service to clinicians.
4. Research Training and Fellowships
Support for research training of the next generation of scientists is an important component of the NIH mission. Nearly 16% of the awarded grants were related to research training which included a loan repayment, two fellowships and 13 mentored career development awards over the ten-year period. The awards supported the research training of 15 early career scientists and accounted for about 4% of the total funding. The majority of these awards were supported by the NHLBI, two by the NCI, and one by the NIBIB. In terms of the categories, one was clinical trial/interventional study, six were observational studies, and nine were basic/translational research projects. The topics of the supported research are included under the categories above. Existing training-related funding opportunities were used to apply for these awards and the recipients included both basic researchers and physician scientists whose stated long-term career goals were cardio-oncology research. The number of awards per year were low for years 2011–2016, varying between zero and one, except for the year 2014 which had three awards. The number of awards per year increased to two in years 2017–2019 and four in the year 2020. Five of the earlier awardees have been successful in receiving subsequent investigator-initiated R01 research project grants. The data show a growing interest in cardio-oncology research by early career scientists. Their success in obtaining the training and subsequent research grants demonstrates that cardio-oncology is emerging as an important investigative field at the intersection of cardiovascular disease, cancer biology, and therapeutics, with excellent opportunities to make impactful research advances in all three research areas [35].
5. Summary and Future Directions
As cancer survival outlook continues to improve with expanded and improved treatments, it is reasonable to expect that the significance and potential risk of cardiovascular complications for survivors will remain high in the near future. The goal of cancer treatment-related cardiotoxicity research is to mitigate cardiovascular dysfunction of cancer therapy without compromising its therapeutic efficacy and thereby improve patient outcomes. Since most cancer treatments carry potential risk of cardiovascular complications and complete elimination of such risk may not be feasible, it may be helpful to consider a cardiotoxicity risk management strategy that optimizes the balance of cardiovascular risk with cancer therapy benefit (Figure 2). Such strategy would require, for every cancer treatment regimen, an assessment of cardiovascular risk factors, including existing history of cardiovascular disease risk and potential co-morbidities, evaluation of the treatment’s potential impact on cardiovascular health, and implementation of appropriate cardioprotective interventions. Significantly, the different cardiotoxicity stages (i.e., acute, chronic, and late-onset) may require different mitigation approaches. It is also important to recognize that cardiovascular health, cancer disease, and cancer therapy are not independent entities, but are interconnected and changes in any one of these entities may impact the other two (Figure 2). Cancer therapies continue to evolve rapidly from pharmacotherapies and molecularly targeted agents to immunotherapies, expanding treatment options greatly, but also bringing potentially unknown cardiotoxicity risks. Recognizing the opportunities to address critical research gaps in this field with increasing importance, NIH has supported and continues to support a broad portfolio of FOA-solicited and investigator-initiated research projects on cardiotoxicity.
Figure 2: Research Strategy: Optimize the Balance of Cardiotoxicity with Cancer-Therapy Benefit.
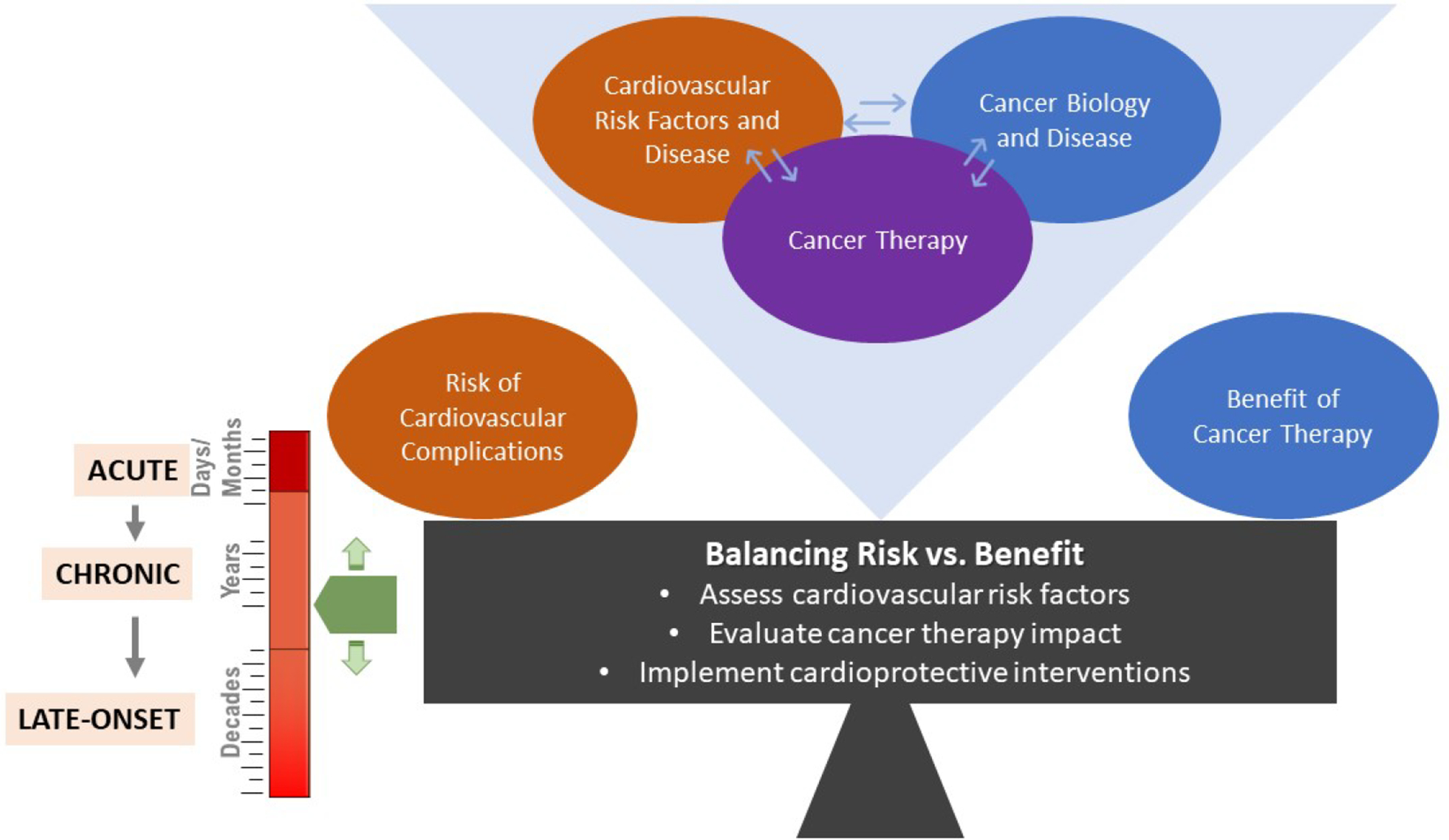
The goal of cancer treatment-related cardiotoxicity research is to reduce cardiovascular complications of cancer therapy without compromising efficacy and thereby improve patient outcomes. Since all cancer therapies carry potential risk of cardiovascular complications and complete elimination of such risk may not be achievable, a cardiotoxicity management strategy that optimizes the risk-benefit balance, as depicted, may offer the best practical approach. For every cancer therapy, this would require assessment of cardiovascular risk factors (including existing disease and co-morbidities), evaluation of potential cardiotoxicity impact of cancer therapy and implementation of appropriate cardioprotective interventions. Cardiotoxicity stage, i.e., acute, chronic, or late-onset, is an important consideration in this approach as it may impact the risk-benefit balance. It is also important to recognize that cardiovascular health, cancer disease and cancer therapy are interconnected and changes in any one may impact the other two.
It is important to recognize that the burdens of cancers, cardiovascular diseases and cancer treatment-related cardiotoxicity remain highly disproportionate among minority and under-resourced populations [36,37]. For example, Black and under-represented minority populations have higher risk and higher prevalence of cancers and cardiovascular diseases compared to their non-Hispanic White counterparts [38–40]. Similarly, although the data remains limited, the incidence of cancer-treatment related cardiotoxicity following anthracycline or trastuzumab treatments is higher in Black patients compared to White patients [41–43]. Disproportionately higher risk for poor cancer prognosis coupled with a higher burden of cardiovascular disease before cancer treatment likely contribute to the disparities in post-treatment cardiotoxicity outcomes. Some of the key recognized barriers to health equity are related to structural racism and include under representation in research, mistrust, socioeconomic and cultural factors, and lack of access to specialty care [37]. Consistent with the NIH’s commitment to address broader health inequities and significant recent efforts aimed towards a vision of inclusive excellence [44], future research in cancer treatment-related cardiotoxicity needs to recognize and prioritize potential health inequities early and address the barriers effectively.
The participants of the two NIH workshops identified a comprehensive list of potential gap area topics [26, 29], and the recent FOAs and investigator-initiated research projects are beginning to address many of these identified research gaps. However, many research challenges remain and research questions related to new cancer treatments and potential impact of co-morbidities associated with longer survival duration remain unaddressed. Research discoveries and advances in cardiotoxicity are highly amenable to translation into human applications and disease management across populations; such opportunities remain underexplored [45, 46]. Since research in this field is informative and conducive to both cardiovascular and cancer research advances, the crosstalk between cardiovascular diseases and cancer biology is of particular interest.
NIH-supported research projects over the previous ten years indicate that the majority of research projects were focused on anthracycline or anthracycline-combined therapies and cancers that require these therapies such as breast cancer. By contrast, relatively few research projects were focused on other treatments and other cancers. While anthracycline and anthracycline-combined cardiotoxicity remains an important investigation area and the focus on anthracyclines may have been in part due to the substantial prior understanding of anthracycline related cardiotoxicity or practical convenience, there is a persistent gap and opportunity to address the treatment effects of other therapies; for example, immune modulators, checkpoint inhibitors, radiation, immunotherapy, and dual/multi-agent therapies, which can have synergistic adverse effects. Knowledge of the underlying mechanisms of cardiotoxicities for these more recent therapies is lagging. Within the basic and translational research category, most research has centered on cardiac myocytes and relatively less effort has been focused on the other potential cells, such as vascular endothelial cells, cardiac fibroblasts and myofibroblasts, macrophages, vascular and cardiac epithelial cells, among other potential cellular targets. Additionally, research has focused on cellular signaling or molecular signaling of remodeling and less on metabolic, energetic and microvascular injury mechanisms.
Investigators submitting NIH research applications in this area should note that the NIH Center for Scientific Review (CSR) assigns grant applications to individual NIH Institutes and Centers (ICs) by matching research aims to IC mission. CSR also assigns the review study sections by matching the review requirements of the proposals with review focus of the standing study sections. Because this research spans the missions of both NCI and NHLBI, the assignments are often not straightforward, especially if the aims overlap with the missions of both Institutes. Typically, if the proposed research aims focus on risk assessment/modeling, treatments and interventions, survivorship including clinical trials, they are likely to be assigned to NCI, whereas if the research focus is more on fundamental mechanistic understanding of cardiotoxicity, they are assigned to NHLBI. However, Institute assignment is not straightforward and both NCI and NHLBI support all three categories of grant proposals. Since Institute and study section assignments may have potential impact on review and funding of the proposal, researchers proposing aims that span the missions of the two Institutes may benefit from early advice by the respective NIH program officers who oversee this research area to ensure that the applications are assigned to the appropriate Institute and study section.
Cancer treatment-related cardiotoxicity is a secondary effect of treatment or a derived condition from post-treatment. Relevant research requires an approach that combines the research expertise of not only cardiologists and oncologists, but potentially others, such as toxicologists, pharmacists, radiologists, and those with translational research experience, including, potentially, from the pharmaceutical industry. Recognizing the potential benefits of a multidisciplinary and collaborative research approach, the NCI and NHLBI are thus addressing this health challenge by close coordination, leveraging staff expertise and resources of both Institutes, to bring oncology and cardiology researchers together to more effectively advance cardio-oncology.
Key Points.
Although survival from cancer is improving dramatically with newer and improved therapies, the incidence and impact of cancer treatment-related cardiotoxicity also has grown. In response, the National Institutes of Health (NIH) is supporting a broad range of basic/translational, clinical/interventional, and observational studies in cardio-oncology. The National Cancer Institute (NCI) and the National Heart, Lung, and Blood Institute (NHLBI) jointly held workshops to identify gaps and future research opportunities and released funding opportunity announcements to advance the science in this area.
With the improvements of cancer treatments and survival, research opportunities exist to better predict, mitigate, and manage the acute, chronic, and late-onset cardiovascular effects of cancer therapy and improve the quality of life for cancer survivors.
A review of recently funded cardio-oncology research during the past decade shows that NIH research support on cardiotoxicity has been increasing. Most studies have focused on anthracyclines or anthracycline-containing treatment regimens (e.g., with trastuzumab and/or radiotherapy). Future research could also address the potential cardiovascular complications of emerging therapeutics and treatment regimens.
Understanding, predicting, and managing cancer treatment-induced cardiovascular adverse events could be addressed by a collaborative and multidisciplinary research approach to synergize research advances across cardiovascular and oncology science.
Synopsis.
Advances in cancer treatments have led to nearly 17 million survivors in the US today. Cardiovascular complications attributed to cancer treatments are the leading cause of morbidity and mortality in cancer survivors. In response, NCI and NHLBI held two workshops and issued funding opportunities to strengthen research on cardiotoxicity. A representative portfolio of NIH grants categorizing basic, interventional, and observational projects is presented. Compared to anthracyclines, research on radiation therapy and newer treatments is underrepresented. Multidisciplinary collaborative research that considers cardiotoxicity stage and optimizes the balance between cardiovascular risk and cancer-treatment benefit might support continued improvements in cancer outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer:
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Cancer Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Disclosure Statement:
For all authors: “The Authors have nothing to disclose.”
All dollar values reported in this paper are in 2021 dollars, converted using the Biomedical Research and Development Price Index (BRDPI): https://officeofbudget.od.nih.gov/gbipriceindexes.html Accessed, August 27, 2021
Contributor Information
Bishow B. Adhikari, Division of Cardiovascular Sciences, NHLBI, NIH.
Scarlet Shi, Division of Cardiovascular Sciences, NHLBI, NIH.
Eileen P. Dimond, Division of Cancer Prevention, NCI, NIH.
Nonniekaye Shelburne, Division of Cancer Control and Population Sciences, NCI, NIH.
Patrice Desvigne-Nickens, Division of Cardiovascular Sciences, NHLBI, NIH.
Lori M. Minasian, Division of Cancer Prevention, NCI, NIH.
References:
- 1.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69(5):363–85 doi: 10.3322/caac.21565[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019–2021. Atlanta: American Cancer Society; 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf [Google Scholar]
- 3.Miller KD, Fidler-Benaoudia M, Keegan TH, et al. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin 2020;70(6):443–59. 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol 2015;2(5):e186–93 doi: 10.1016/S2352-3026(15)00048-4[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med 2016;374(9):833–42 doi: 10.1056/NEJMoa1510795[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol 2001;19(13):3163–72 doi: 10.1200/JCO.2001.19.13.3163[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309(22):2371–81 doi: 10.1001/jama.2013.6296[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2008;100(19):1368–79 doi: 10.1093/jnci/djn310[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrmann J Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol 2020;17(8):474–502 doi: 10.1038/s41569-020-0348-1[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L, Kitsis RN. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest 2021;131(5) doi: 10.1172/JCI145186[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J Am Coll Cardiol 2019;74(25):3099–108 doi: 10.1016/j.jacc.2019.10.038[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen K, Levitt GA, Bull C, Dorup I, Sullivan ID. Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer 2003;97(8):1991–8 doi: 10.1002/cncr.11274[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Scully RE, Lipshultz SE. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc Toxicol 2007;7(2):122–8 doi: 10.1007/s12012-007-0006-4[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 14.Ewer SM, Pham DD. Late-onset heart failure after treatment for breast cancer. Cancer 2020;126(1):19–21 doi: 10.1002/cncr.32483[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 15.Armenian SH, Lacchetti C, Barac A, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology 2017;35(8):893–U144 doi: 10.1200/Jco.2016.70.5400[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37(36):2768–801 doi: 10.1093/eurheartj/ehw211[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 17.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol 2016;34(6):611–35 doi: 10.1200/JCO.2015.64.3809[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Miller JM, Meki MH, Ou Q, et al. Heart slice culture system reliably demonstrates clinical drug-related cardiotoxicity. Toxicol Appl Pharmacol 2020;406:115213 doi: 10.1016/j.taap.2020.115213[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe JI, Yusuf SW, Deswal A, Herrmann J. Cardio-Oncology: Learning From the Old, Applying to the New. Front Cardiovasc Med 2020;7:601893 doi: 10.3389/fcvm.2020.601893[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armenian SH, Lacchetti C, Lenihan D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. Journal of Oncology Practice 2017;13(4):270–75 doi: 10.1200/jop.2016.018770[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 21.Oikonomou E, Anastasiou M, Siasos G, et al. Cancer Therapeutics-Related Cardiovascular Complications. Mechanisms, Diagnosis and Treatment. Curr Pharm Des 2018;24(37):4424–35 doi: 10.2174/1381612825666190111101459[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 22.Lamore SD, Kohnken RA, Peters MF, Kolaja KL. Cardiovascular Toxicity Induced by Kinase Inhibitors: Mechanisms and Preclinical Approaches. Chem Res Toxicol 2020;33(1):125–36 doi: 10.1021/acs.chemrestox.9b00387[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 23.Herrmann J Vascular toxic effects of cancer therapies. Nat Rev Cardiol 2020;17(8):503–22 doi: 10.1038/s41569-020-0347-2[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anjos M, Fontes-Oliveira M, Costa VM, Santos M, Ferreira R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci 2021;280:119760 doi: 10.1016/j.lfs.2021.119760[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 25.de Boer RA, Hulot JS, Tocchetti CG, et al. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the Translational Research Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22(12):2272–89 doi: 10.1002/ejhf.2029[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shelburne N, Adhikari B, Brell J, et al. Cancer treatment-related cardiotoxicity: current state of knowledge and future research priorities. J Natl Cancer Inst 2014;106(9) doi: 10.1093/jnci/dju232[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2015;16(3):e123–36 doi: 10.1016/s1470-2045(14)70409-7[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31(2):171–90 doi: 10.1016/j.annonc.2019.10.023[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shelburne N, Simonds NI, Adhikari B, et al. Changing Hearts and Minds: Improving Outcomes in Cancer Treatment-Related Cardiotoxicity. Curr Oncol Rep 2019;21(1):9 doi: 10.1007/s11912-019-0751-0[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 30.The search terms and method: (“cardiac toxicit*” OR “cardiovascular toxicity”~4 OR cardiotoxicity OR “cardiovascular adverse event*”~4 OR “cardiac complication*”~4) AND (chemotherap* OR radiothera* OR “cancer treatment*”~4 OR “cancer therap*”~4 OR “cancer radiation”~8). OR and AND are logical operators and terms within the quotations are word combinations. The ‘*’ indicate a wild card allowing any words after the text. The ‘~’ and number following it allow the preceding words within the quotations to be searched in any order along the text at a word length of upto the number indicated. The search was conducted on the ‘Title’, ‘Abstract’, and ‘Specific Aims’ fields of funded grants that are available in the NIH RePORTER database (https://reporter.nih.gov/) over the period 2011–2020 using an NIH in-house developed tool. Date accessed 7/29/2021.
- 31.NCTN: NCI’s National Clinical Trials Network. Secondary NCTN: NCI’s National Clinical Trials Network. https://www.cancer.gov/research/infrastructure/clinical-trials/nctn Date accessed August 18, 2021.
- 32.AH- HA. Secondary AH-HA. https://clinicaltrials.gov/ct2/show/NCT03935282 Date accessed August 18, 2021.
- 33.RADCOMP. Secondary RADCOMP. https://clinicaltrials.gov/ct2/show/NCT02603341. Date accessed August 18, 2021.
- 34.WHI LILAC Study. Secondary WHI LILAC Study. https://sp.whi.org/studies/LILAC/Pages/Home.aspx Date accessed August 18, 2021.
- 35.Asnani A, Moslehi JJ, Adhikari BB, et al. Preclinical Models of Cancer Therapy-Associated Cardiovascular Toxicity: A Scientific Statement From the American Heart Association. Circ Res 2021;129(1):e21–e34 doi: 10.1161/res.0000000000000473[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohman RE, Yang EH, Abel ML. Inequity in Cardio-Oncology: Identifying Disparities in Cardiotoxicity and Links to Cardiac and Cancer Outcomes. J Am Heart Assoc 2021;10(24):e023852. 10.1161/JAHA.121.023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fazal M, Malisa J, Rhee JW, et al. Racial and Ethnic Disparities in Cardio-Oncology: A Call to Action. JACC CardioOncol 2021;3(2):201–4. 10.1016/j.jaccao.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carnethon MR, Pu J, Howard G, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation 2017;136(21):e393–423. 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 39.Swenson CJ, Trepka MJ, Rewers MJ, et al. Cardiovascular disease mortality in Hispanics and non-Hispanic whites. Am J Epidemiol 2002;156(10):919–28. 10.1093/aje/kwf140. [DOI] [PubMed] [Google Scholar]
- 40.Society. AC. Cancer Facts & Figures for Hispanics/Latinos 2018-2020. 2018–2020.
- 41.Cousin L, Roper N, Nolan TS. Cardio-Oncology Health Disparities: Social Determinants of Health and Care for Black Breast Cancer Survivors. Clin J Oncol Nurs 2021;25(5):36–41. 10.1188/21.Cjon.S1.36-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasan S, Dinh K, Lombardo F, et al. Doxorubicin cardiotoxicity in African Americans. J Natl Med Assoc 2004;96(2):196–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Litvak A, Batukbhai B, Russell SD, et al. Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer. Cancer 2018;124(9):1904–11. 10.1002/cncr.31260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ending Structural Racism. Secondary Ending Structural Racism. Available at: https://www.nih.gov/ending-structural-racism/health-equity-research.
- 45.Fort DG, Herr TM, Shaw PL, Gutzman KE, Starren JB. Mapping the evolving definitions of translational research. J Clin Transl Sci 2017;1(1):60–66 doi: 10.1017/cts.2016.10[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westfall JM, Mensah GA. T4 Translational Moonshot: Making Cardiovascular Discoveries Work for Everyone. Circ Res 2018;122(2):210–12 doi: 10.1161/CIRCRESAHA.117.312273[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]


