Abstract
Cystic fibrosis-related diabetes (CFRD) is one the most common comorbidities in cystic fibrosis (CF). Pancreatic oxidative stress has been postulated in the pathogenesis of CFRD, but no studies have been done to show an association. The main obstacle is the lack of suitable animal models and no immediate availability of pancreas tissue in humans. In the CF porcine model, we found increased pancreatic total glutathione (GSH), glutathione disulfide (GSSG), 3-nitrotyrosine- and 4-hydroxynonenal-modified proteins, and decreased copper zinc superoxide dismutase (CuZnSOD) activity, all indicative of oxidative stress. CF pig pancreas demonstrated increased DHE oxidation (as a surrogate marker of superoxide) in situ compared to non-CF and this was inhibited by a SOD-mimetic (GC4401). Catalase and glutathione peroxidase activities were not different between CF and non-CF pancreas. Isolated CF pig islets had significantly increased DHE oxidation, peroxide production, reduced insulin secretion in response to high glucose and diminished secretory index compared to non-CF islets. Acute treatment with apocynin or an SOD mimetic failed to restore insulin secretion. These results are consistent with the hypothesis that CF pig pancreas is under significant oxidative stress as a result of increased O2●− and peroxides combined with reduced antioxidant defenses against reactive oxygen species (ROS). We speculate that insulin secretory defects in CF may be due to oxidative stress.
Keywords: Oxidative stress, Pancreas, Cystic fibrosis, Islet cells, Glutathione, Superoxide, Peroxides, 4-hydroxynonenal
Introduction
Cystic fibrosis (CF) is a disease with high morbidity and mortality. It is caused by mutations in the gene that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) [1–3]. Lungs are typically involved and individuals with CF usually succumb to complications of pulmonary disease [4]. In the gastrointestinal tract, CF primarily involves the pancreas, where it causes exocrine pancreatic insufficiency and later diabetes. With advancement in the care of pulmonary complications and extended life expectancy of people with CF, gastrointestinal manifestations and diabetes began to emerge and impact patient care and quality of life. Cystic fibrosis related diabetes (CFRD) is one of the most significant co-morbidities, affecting ~50% of CF patients > 30 years of age [5,6]. The development of diabetes in persons with CF is associated with a rapid decline in pulmonary function as well as higher risk for morbidity and mortality ensue [7,8]. Even before diabetes is apparent, patients demonstrate insulin secretory defects, with diminished and/or delayed insulin responses to oral glucose.
The pathophysiology leading to CFRD is largely unknown. The main barriers to understanding the disease mechanisms have been the inaccessibility of pancreas in humans and the absence of significant pancreatic disease in CF mice [9,10]. CF pigs develop multi-organ disease similar to humans with CF, including severe exocrine pancreatic disease and a CFRD phenotype [11–15]. At birth, CF pigs exhibit hyperglycemia in response to glucose challenge and diminished insulin secretion despite relative sparing of insulin-positive cell mass [16], features common to humans with CF. These traits make the CF pig an attractive model for studying physiologically relevant CFRD and its mechanistic underpinnings.
Oxidative stress is caused by excesses in reactive oxygen species (ROS) and/or decreases in antioxidant capacity that lead to cell injury and loss of critical intracellular functions, which are associated with development of diabetes [17–23]. In general, ROS can be generated through hyperglycemia [24,25]. In type 1 diabetes, invading immune cells within pancreas would expose beta-cells to ROS [26]. Free radicals derived from superoxide, produced in the pancreas can impact islet structure and function [27,28] and inhibit insulin secretion [29]. Significant advances have been made in understanding the impact of ROS on beta-cell function [17–23]. ROS can negatively impact beta-cell function, though this depends on the source, nature, and cellular localization of the ROS. Beta-cells have limited capacity for SOD-based neutralization of ROS [30–33]. Oxidative stress has been postulated to play a similar role in the development of CFRD [34,35]. Glutathione (GSH), the main redox buffer of mammalian cells, is depleted in CF lung disease [36–41], possibly due to diminished transport caused by defective CFTR [39]. Lastly, the blood levels of 4-hydroxynonenal-modified proteins (indicative of oxidative stress within lipid compartments) are elevated in children with CF and their concentrations correlate with the degree of impaired glucose tolerance [42]. However, there are no published studies demonstrating that CF pancreas and/or islets are experiencing oxidative stress.
We hypothesized that CF pancreata and/or islets would demonstrate indications of oxidative stress relative to their normal counterparts. To test this hypothesis, we assayed for antioxidant enzyme activity, levels of superoxide using DHE oxidation, response to high glucose, and GSH, GSSG status in both normal and CF pancreas models. We found increased levels of DHE oxidation in frozen sections of the newborn CF pig pancreas, which was inhibited by the SOD-mimetic (GC4401), suggesting the involvement of superoxide. We then utilized immunohistochemical and immunoblotting approaches to demonstrate the presence of protein oxidative damage in the form of 3-nitrotyrosine- and 4-hydroxynonenal-modified proteins in the CF pig pancreata relative to non-CF controls. Cultured CF pig islets had diminished insulin secretion in response to high glucose, reduced secretory index as well as evidence of superoxide and peroxide production in comparison to non-CF islets. However, acute treatment with inhibitors of superoxide and NADPH oxidase production failed to restore insulin secretion. These results are consistent with the hypothesis that newborn CF pig pancreas and islet cells are under significant oxidative stress, which may be a chronic contributing factor to insulin secretory defects in people with CF.
Materials and methods
Animals.
Animal experiments were reviewed and approved by the University of Iowa Institutional Animal Care and Use Committee. CF (CFTR−/−, CFTRΔF508/ΔF508) and non-CF (CFTR+/+, CFTR+/−, CFTR+/ΔF508) piglets were obtained from Exemplar Genetics (Sioux Center, IA, USA), and studied within 24 h after birth.
Pancreas sample processing for enzyme activity assays.
Following euthanasia, the pancreas was quickly prosected and fresh pancreas tissue (~100 mg) was frozen at −80 °C until use. Samples were thawed on ice, homogenized in 500 μl cold phosphate buffer (50 mM, pH 7.0) and centrifuged briefly at 12,000 × g. The supernatant was used for protein quantification and enzyme activity assays. Protein concentration was0020measured using BCA kit (Sigma Aldrich, St. Louis, MO, #QPBCA) according to the manufacturer’s protocol.
Neonatal islet cultures.
CF and non-CF islets were isolated and cultured using a previously described protocol for neonatal pig islets [43]. Briefly, newborn pancreata from CF or non-CF neonatal pigs were minced into 1- to 2-mm pieces and digested in HBSS containing 0.5% BSA, 1% penicillin/streptomycin, and 2.5 mg/ml collagenase for 4 min at 37 °C. Following digestion, the tissue fragments were washed 3 times in medium without collagenase and then cultured in 24-well plates with 2 ml Ham’s F12 medium (containing 10 mM glucose) supplemented with 0.5% BSA, 50 μM 3-isobutyl-1-methylxanthine (IBMX), 10 mM nicotinamide, 1.67 mM l-glutamine, and 100 U/ml penicillin and 100 μg/ml streptomycin. The medium was changed on the second day after isolation and every other day thereafter. In some experiments, 10 μM of GC4401 was added to the medium and continued through insulin secretion studies.
Glutathione assay.
The glutathione assay is based on the following coupled redox reaction: GSH is oxidized by 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) to give glutathione disulfide (GSSG) with stoichiometric formation of 5-thio-2-nitrobenzoic acid (TNB), and GSSG is reduced to GSH by glutathione reductase and NADPH [44,45]. The rate of change in absorbance of TNB formation is then recorded at 412 nm and it is proportional to the total GSH present. The addition of 2-vinylpyridine, which binds any reduced GSH, in a separate aliquot of sample was used to determine GSSG only. GSH (Sigma Aldrich, St. Louis, MO #G4251) and GSSG (Sigma Aldrich, St. Louis, MO #G4626) standard curves were performed along with pancreas extract samples. Reduced GSH was determined via subtracting GSSG from total GSH. Pancreas tissue was minced and deproteinized with 5% 5-sulfosalicylic acid. Total GSH and GSSG were measured and normalized to the total amount of protein in each pellet. Samples were mixed with 150 mM potassium phosphate buffer containing 1 mg/ml DTNB (Sigma Aldrich, St. Louis, MO #D8130), 0.25 mg/ml NADPH and 0.4 U/ml glutathione reductase (Sigma Aldrich St. Louis, MO, #3664). The absorbance was recorded at 412 nm for 5 min with 30 s interval. Reduced GSH was determined via subtracting GSSG from total GSH.
4-hydroxynonenal (HNE) modified proteins assay.
Pancreas was digested in 10 mM DETAPAC/RIPA (Sigma Aldrich, St. Louis, MO, #R0278)/50 μM Butylated Hydroxytoluene (Sigma Aldrich, St. Louis, MO, #B1378)/Roche Mini Protease inhibitor buffer (Sigma Aldrich, St. Louis, MO # 11836153001). Protein concentration was measured using the Pierce BCA Protein Assay (Thermo Fisher Scientific, Waltham MA, #23227). Protein was loaded into Hoefer PR648 slot blot manifold (Hoefer Inc Holliston, MA, #PR648) after assembly according to the manufacturer’s instruction. Briefly, the bottom block was put together with the membrane support block. A Whatman filter paper 100×150 mm (GE Healthcare, Chicago, IL #GB003), pre-soaked in Tris-Buffered Saline (TBS) (50mM Tris, 150mM NaCl, pH 7.6) was placed into the membrane recess, followed by an activated membrane (1-minute methanol incubation, 5-minute 20% methanol / 80% 100 mM 3-(N-morpholino) propanesulfonic acid (MOPS), 100% MOPS until ready for application). The top block was then placed on top of the membrane support block, where it was screwed in following the manufacturer’s instructions. The manifold was attached to an adjustable vacuum, followed by a 10 s vacuum initiation by applying 25 cm Hg. The samples were applied and run through the apparatus at 25 cm Hg followed by a wash with TBS at 50 cm Hg.
A positive control sample for the 4-HNE modified protein antibody was generated using a 50 μM spike of 4-HNE (Cayman Chemical Company, Ann Arbor, MI, #32100) into a control sample (randomly selected wildtype) at 0.2 μg/mL protein concentration, followed by incubation at 37 °C for 30 min. Standard curves were generated by utilizing various protein concentrations from the spiked control (30, 25, 15, 10, 5 μg) alongside a negative addition standard utilizing increased protein concentrations from a non-spiked wildtype (30, 60, 90, 120, 150 μg). Equal protein (30 μg) was applied to the membrane for each experimental sample. After removal of the membrane from the apparatus and drying followed by reactivation with methanol for one minute, and incubation in 80% of 100 mM MOPS/ 20% methanol for 5 min, the membrane was incubated in 250 mM sodium borohydride (Sigma Aldrich, St. Louis, MO, # 480886) at room temperature for 15 min to stabilize Schiff bases and Michael adducts. The membrane was then washed three times in dIH2O and once in PBS before the membrane was blocked in 5% BSA/ TBST for one hour at room temperature. Following wash cycle with TBST the membrane was incubated with rabbit anti – 4-HNE [1:2,000] (Millipore, Burlington, MA #ABN249) in 5% BSA/ TBST overnight at 4 °C. The membrane was then incubated with Anti-Rabbit IgG – peroxidase [1:25,000] (Sigma Aldrich, St. Louis, MO, #A6154) for one hour at room temperature after another wash cycle. Following another wash cycle, the membrane was incubated in Pierce ECL 2 Western Blotting Substrate (Thermo Fisher Scientific, Waltham MA # 80196) for five min and visualized on x-ray film (Sigma Aldrich, St. Louis, MO, #Z363006). Densitometry was done on ImageJ using an integrated density measurement after subtracting background and results were normalized to total protein using Ponceau S staining (0.5% Ponceau S/ 0.1% Acetic Acid) (Thermo Fisher Scientific, Waltham, MA #BP103-10).
Catalase activity assay.
Catalase activity was measured as the decomposition of H2O2 monitored spectrophotometrically as previously described [46]. Pancreas homogenates were mixed in 50 mM phosphate buffer (pH 7) following addition of 10 mM H2O2 (Thermo Fisher Scientific, Waltham, MA, #H-325). The raw absorbance data was recorded at 240 nm for 60 s and plotted, then the linear part of data to generate a slope was used to calculate the activity.
Superoxide dismutase activity assay.
The measurement of SOD activity is based on the inhibition of nitroblue tetrazolium (NBT) reduction by O2•− [47,48]. Pancreas homogenates were incubated in 50 mM potassium phosphate buffer (pH 7.8) containing 1 mM diethylen-etriaminepentaacetic acid (DETAPAC) (Sigma Aldrich, St. Louis, MO #D6518), 0.13% BSA, 1 unit/ml catalase (Sigma Aldrich, St. Louis, MO, #C-40), 56 μM NBT (Sigma Aldrich St. Louis, MO, #N6876), 0.1 mM xanthine (Sigma Aldrich St. Louis, MO, #X0125), and 50 μM bathocuproine disulfonic acid (Sigma Aldrich, St. Louis, MO, #146625) for 30 min. Xanthine oxidase (Sigma Aldrich St. Louis, MO, #X187) was added to give an NBT reduction rate of 0.015–0.025/min at 560 nm. One minute after sample solution was mixed with 0.01 units/ml xanthine oxidase, the absorbance was measured at 560 nm for 2 min at 15 s intervals. To assay MnSOD, 5 mM NaCN (Sigma Aldrich, St. Louis, MO, #380970) was added to the buffer and allowed to incubate for 1 hr. One unit of SOD activity was defined as the quantity of protein required for half of the maximal inhibition of NBT reduction.
Glutathione peroxidase (GPx) activity assay.
The measurement of GPx activity [49] is based on the coupled reaction of GPx with reduction of oxidized glutathione by glutathione reductase consuming nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), and oxidation of NADPH to NADP+ that results in decrease of absorbance at 340 nm. Pancreas homogenates were mixed with 50 mM potassium phosphate buffer (pH 7) containing 1 mM ethylenediaminetetraacetic acid (EDTA) (Sigma Aldrich, St. Louis, MO, #E9884), 1 mM sodium azide (Sigma Aldrich St. Louis, MO, #2002), 0.2 mM NADPH (Sigma Aldrich, St. Louis, MO, #N-6505), 1 E.U./ml glutathione reductase (Sigma Aldrich, St. Louis, MO, #G-4759), 1 mM GSH (Sigma Aldrich, St. Louis, MO, #G-4759) and 0.25 mM H2O2 (Fisher Scientific, Waltham, MA, #H-325). The absorbance of the reaction mix was recorded at 340 nm for 5 min with 30 s intervals. Glutathione peroxidase activity was expressed as Unit/mg tissue or Unit/μg protein where 1 unit of GPx was equal to the amount of protein required to oxidize 1 μM NADPH/min.
In situ measurement of superoxide (O2•−).
This technique measures oxidation of dihydroethidium (DHE) (Thermo Fisher Scientific, Waltham, MA, #D11347) to its fluorescent product as a surrogate marker for superoxide [50,51]. Frozen pancreas tissue slides (thickness 6 μm) or islets cultures were used. The neonatal islets were handpicked and cultured in glass bottom dishes coated with collagen at 37 °C 5% CO2 overnight, then moved to room temperature (about 25 °C) without additional CO2. After addition of 10 μM of DHE, slides were allowed to incubate 30 min at 37 C in a humidified chamber, then rinsed with cold PBS and images were immediately acquired at excitation/emission wavelength of 405/570 nm using a Zeiss 710 confocal microscope. The fluorescent intensity of image was quantified with ImageJ. To confirm that the DHE signal was due to superoxide, frozen pancreas tissue slides were also exposed to 500 nM of the selective SOD mimetic GC4401 or its inactive analogue GC4404 (courtesy of Galera Therapeutics, Creve Coeur, MO) before staining with DHE [52].
Neonatal islet culture and insulin secretion studies.
On the ninth day of culture, approximately 500–600 islets were selectively removed with a small glass pipette, washed, and divided into 2 equal-sized matched groups for each genotype and then incubated in RPMI 1640 containing 1.67 mM glucose, 2 mM l-glutamine, and 0.5% BSA for 1 hour at 37 °C. To initiate the insulin secretion experiment, islets were then transferred into 1 ml of fresh RPMI 1640 medium containing 1.67 mM or 16.7 mM glucose for 1 hour at 37 °C. The media and islets were then collected, and total insulin was quantified in both by ELISA. Insulin was measured using an ELISA kit that is specific for bovine and porcine fully processed insulin and does not cross-react with proinsulin or C-peptide. The amount of insulin secreted into the media during the 1 hour and that remaining in the islets was used to calculate the percent insulin secretion (% insulin secretion = secreted insulin in the media/total insulin in the media and islets at the end of the experiment). Secretory index (SI) was calculated as percent insulin secretion at 16.7 mM glucose divided by percent insulin secretion at 1.67 mM glucose. For apocynin studies, matured islets were treated for 24 hours with 0.2 mM apocynin + 0.1% DMSO or 0.1% DMSO alone. Hand-picked islets were moved to a perifusion device (Biorep Technologies, Miami Lakes, FL), using 500–600 islets per channel and a flow rate of 0.12 mL/min [53]. Islets were perifused for 52 min in KRB buffer containing 1.67 mmol/L glucose, followed thereafter by 16.7 mmole/L glucose. Insulin content in the eluate was normalized to the total islet insulin content collected from the channel.
Immunohistochemical staining for 3-nitrotyrosine-modified proteins.
CF and non-CF pig pancreas pieces cut ~4 mm3 were placed into 4% paraformaldehyde for a minimum of 2–4 days. Tissues were processed with standard IHC protocols. Testicular hyaluronidase (H3884: Sigma Aldrich, St. Louis, MO) was used for antigen retrial as previously described [54] and blocked in blocking solution (1% BSA with 1 % goat serum and 0.5% tween 20 in PBS). Slides were incubated with primary antibody, anti-3NT (06–284: Sigma Aldrich, St. Louis, MO) overnight at 4 °C. Next, goat anti-rabbit IgG (H+L) biotinylated (BA-1000: Vector Laboratories, Burlingame, CA) secondary antibody was added for 40 min followed by additional of avidin-biotin complex with a ABC-HRP kit (PK-4000: Vector Laboratories, Burlingame, CA) for 30 min. Samples were developed with a DAB kit (SK-4100: Vector Laboratories, Burlingame, CA). A common color threshold was used to extract the brown staining of the DAB kit in the images and the hue saturation over each pixel in the total tissue area was averaged.
Amplex Red assay.
The oxidation sensitive fluorescent probe, Amplex Red (Invitrogen #A12222) was utilized to estimate production of peroxides by islets. This assay is based on the oxidation of 10-acetyl-3,7 dihydroxypenoxazine, which is catalyzed by horseradish peroxidase (HRP) to produce a red florescent oxidation product, resorufin, at 1:1 ratio [55]. The reaction buffer (pH=7.2) contained 129 mM NaCl, 2.4 mM K2HPO4, 0.6 mM K2H2PO4, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.67 mM glucose, 20 μm Amplex Red, 5 units/ml horseradish peroxidase (Sigma #P8375–1KU). After culturing for 9 days, islets were hand-picked and placed in 96 well optical plates (Thermo Scientific #165305), mixed with the reaction buffer. The fluorescence intensity of all wells was measured at excitation/emission wavelength of 560/590 nm using a plate reader (Molecular Devices, Spectra Max M2).
Statistical Analysis.
Statistical analysis was performed by Mann-Whitney test for all tests except analysis of islet perifusion studies was done using 2-way ANOVA to assess the impact of CF-status, antioxidant treatment, and their interaction. When appropriate unpaired Student’s t test or one-way ANOVA were used. Unless otherwise specified, statistical significance was determined as p<0.05.
Results
CF pig pancreas demonstrates oxidative stress.
To determine whether oxidative stress could be present in newborn CF pig pancreas, pancreatic GSH and glutathione disulfide (GSSG), the major soluble thiol redox couple that is very sensitive to changes indicative of oxidative stress was assessed. Total GSH and GSSG levels were higher but the GSH:GSSG ratio was not significantly decreased in CF pig pancreas compared to non-CF (Fig. 1), supporting the presence of oxidative stress in a major thiol redox pool that was compensated for by increased GSH synthesis and metabolism.
Fig. 1.
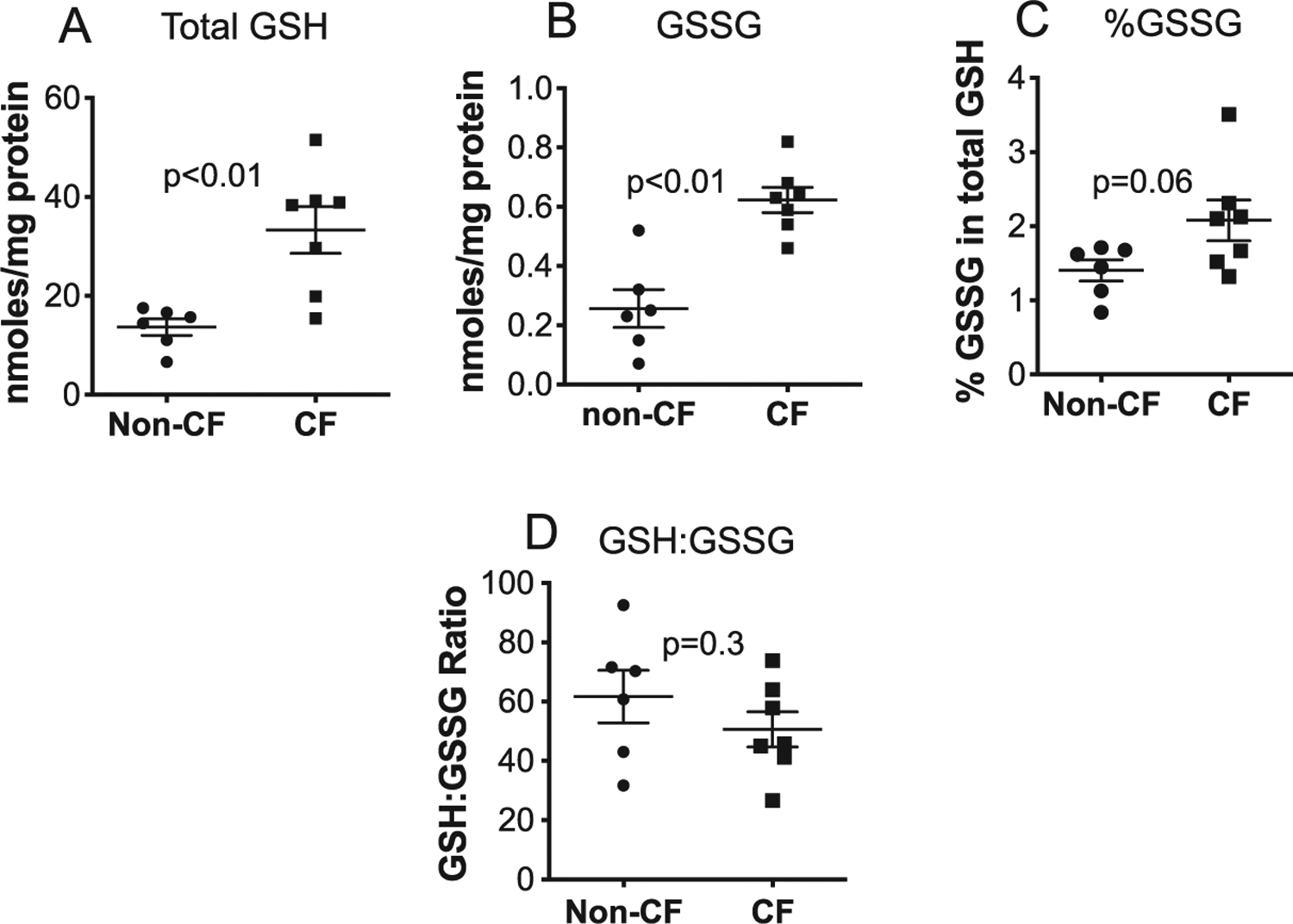
GSH is oxidized to GSSG in neonatal CF pig pancreas. (A) Total GSH, (B) total GSSG, (C) %GSSG and (D) GSH:GSSG ratio were measured in newborn pig pancreas homogenates using spectrophotometric detection of GSH oxidation by the sulfhydryl reagent 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) forming the yellow derivative 5′-thio-2-nitrobenzoic acid (TNB). Values for all graphs show the mean +/− SEM from independent animals. P values are shown (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
CF pig pancreas has decreased antioxidant activities.
The activities of catalase, glutathione peroxidase (GPx1) and SOD (total, CuZnSOD and MnSOD) (Table 1) were assessed to determine if any of the major antioxidant enzymes responsible for superoxide and hydrogen peroxide were compromised. All these antioxidant enzymes activities were significantly lower in CF pancreas compared to non-CF if measured per mg of tissue wet weight, probably because of significant parenchymal loss in CF. Once samples were normalized to μg protein per sample, the most significant changes were the decreases in total SOD and CuZnSOD activities in the CF pancreas.
Table 1.
Antioxidant Enzyme Activities in CF and non-CF Pancreas.
| Enzyme | Genotype | Enzyme Activity (unit/mg tissue wet weight) | P value | Enzyme Activity (unit/μg protein) | P value |
|---|---|---|---|---|---|
| Catalase | Non-CF | 0.26 | 0.17 | ||
| CF | 0.15 | p = 0.008 | 0.19 | p = 0.24 | |
| GPx1 | Non-CF | 0.0072 | 0.39 × 10−4 | ||
| CF | 0.0027 | p = 0.001 | 0.36 × 10−4 | p = 0.74 | |
| Total | Non-CF | 27.6 | 0.19 | ||
| SOD | CF | 8.7 | p < 0.0001 | 0.11 | p = 0.003 |
| MnSOD | Non-CF | 6.8 | 0.045 | ||
| CF | 3.27 | p = 0.005 | 0.044 | p = 0.89 | |
| CuZnSOD | Non-CF | 20.9 | 0.14 | ||
| CF | 5.7 | p < 0.0001 | 0.07 | p = 0.002 |
CuZnSOD: Copper Zinc superoxide dismutase; MnSOD: Manganese superoxide dismutase; GPX1: glutathione peroxidase; n=5 for each separate enzyme activity experiment.
CF pig pancreas shows evidence of increased pro-oxidant production and oxidative stress.
In-situ generation of pro-oxidants was assessed in the fresh frozen neonatal CF pig pancreas using fluorescent confocal microscopy. DHE oxidation was used as a surrogate marker of superoxide levels and was significantly increased in CF pig pancreas samples at birth compared to non-CF (Fig. 2). The DHE oxidation signal was also inhibited by the SOD mimetic GC4401, but not by the inactive analogue GC4404 (Fig. 3), confirming that the increased DHE oxidation was derived from increased levels of superoxide in the CF pig pancreas samples. To explore further whether CF pancreas led to oxidative stress in vivo, we measured 4-HNE-modified protein content, a common lipid peroxidation by-product that reacts with proteins by Michael addition reactions and represents a stable mark of oxidative damage. As demonstrated in Fig. 4A, 4-HNE modified proteins were significantly elevated in CF pancreas homogenates compared to non-CF when measured using slot blotting techniques. Using immunohistochemistry, we also observed a significant increase in 3-nitrotyrosine-modified proteins in CF pancreas compared to non-CF (Fig 4B–D). These data demonstrate significant oxidative damage and evidence for superoxide and nitric oxide mediated oxidative stress in the CF pancreas.
Fig. 2.
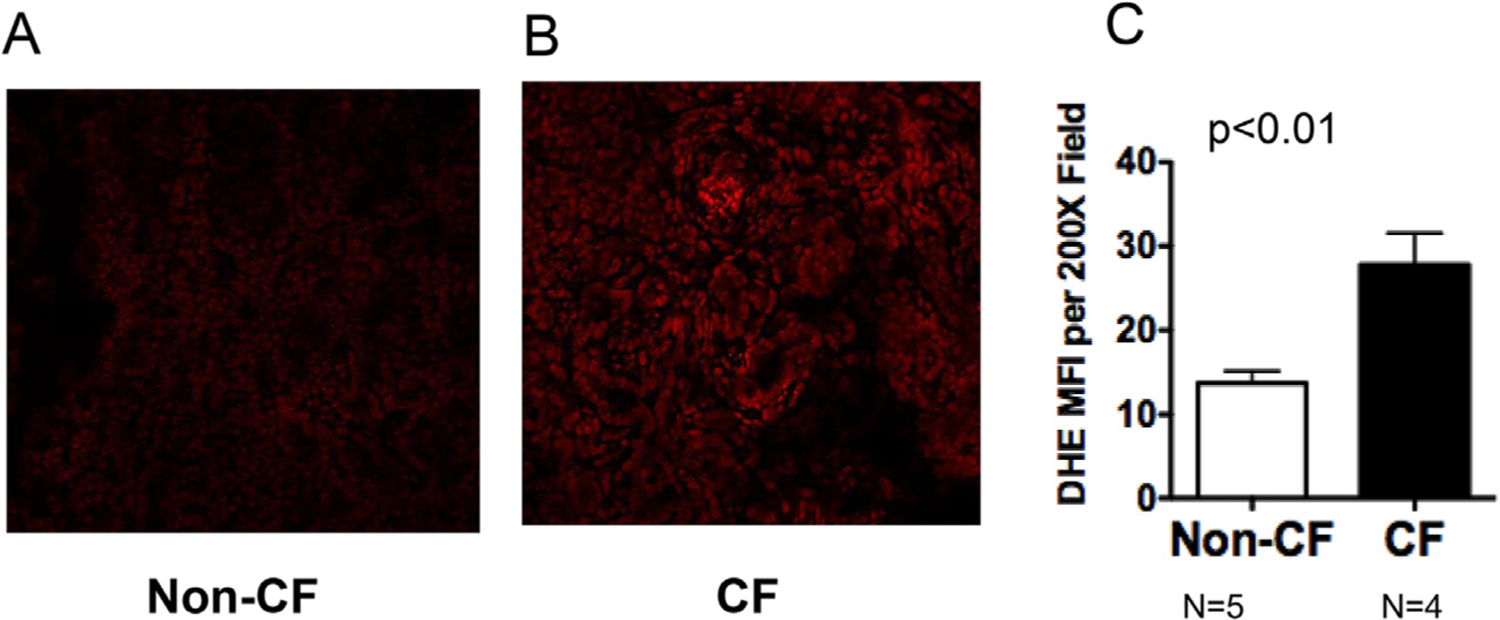
CF pancreas shows increased levels of ROS in situ. Pig pancreata were harvested within 12 h after birth and immediately frozen in OCT compound. Samples were cryo-sectioned onto microscope slides and stained with 5 μM dihydroethidium for 30 min at 37 °C. At least three images per tissue section (average 4 sections per tissue) were captured at 200x and 630x using a Zeiss 710 confocal microscope. Image fluorescence intensity was quantified using Image J. Pictures shown in (A) and (B) are representative of quantified data shown in (C). P value is shown.
Fig. 3.
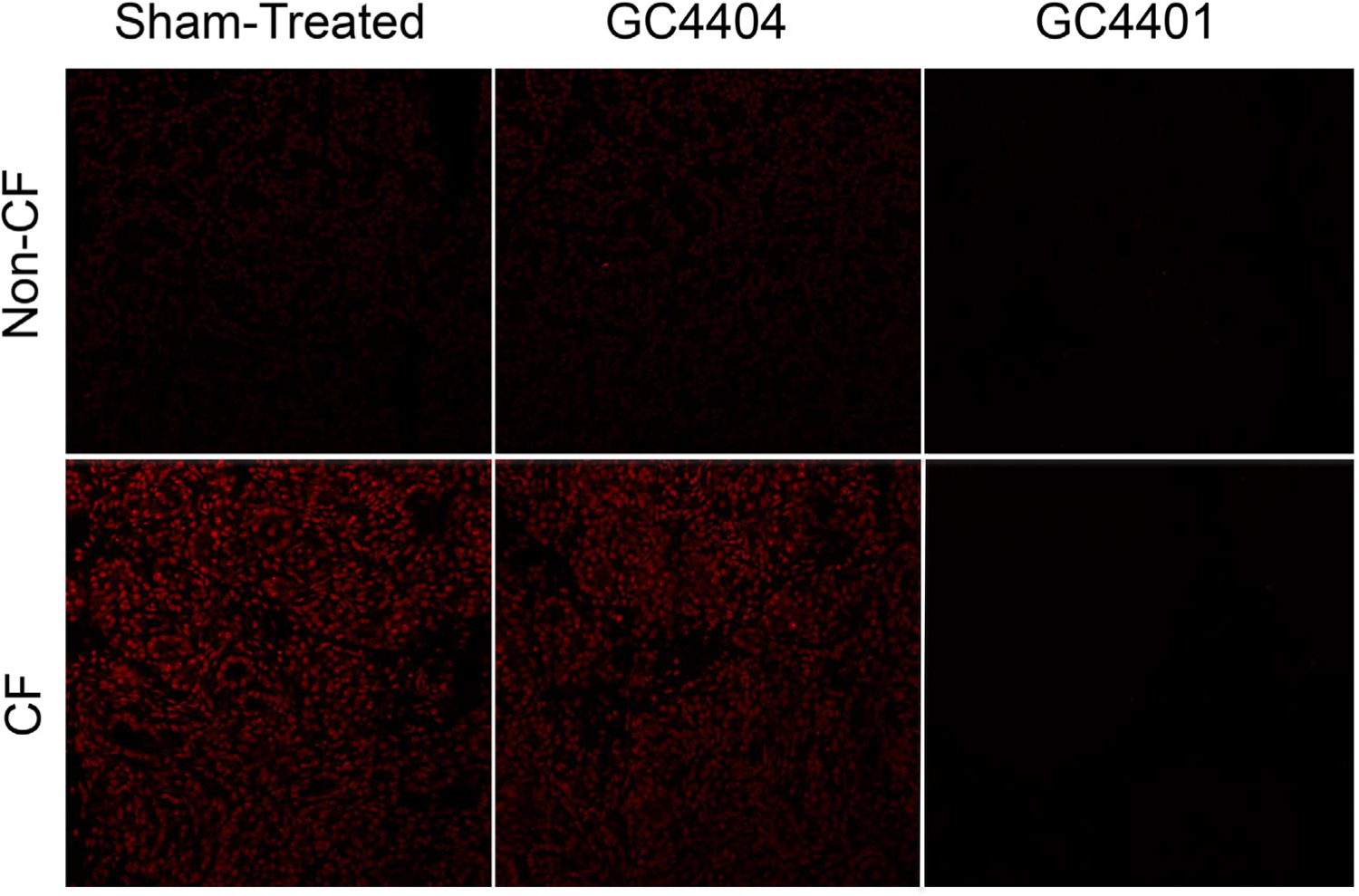
Increased levels of ROS in situ in CF pancreas can be inhibited with SOD mimetic compounds. Pig pancreata were harvested and cryo-sectioned as in Fig. 2, but some specimens had 500 nM GC4401, an SOD mimetic, or GC4404, an inactive analogue, included in the reaction during the DHE staining. Cells were then analyzed as in Fig. 2. GC4401 was able to inhibit greater than 75% of the signal from DHE oxidation under these conditions, supporting the hypothesis that superoxide levels are increased in CF pancreas.
Fig. 4.
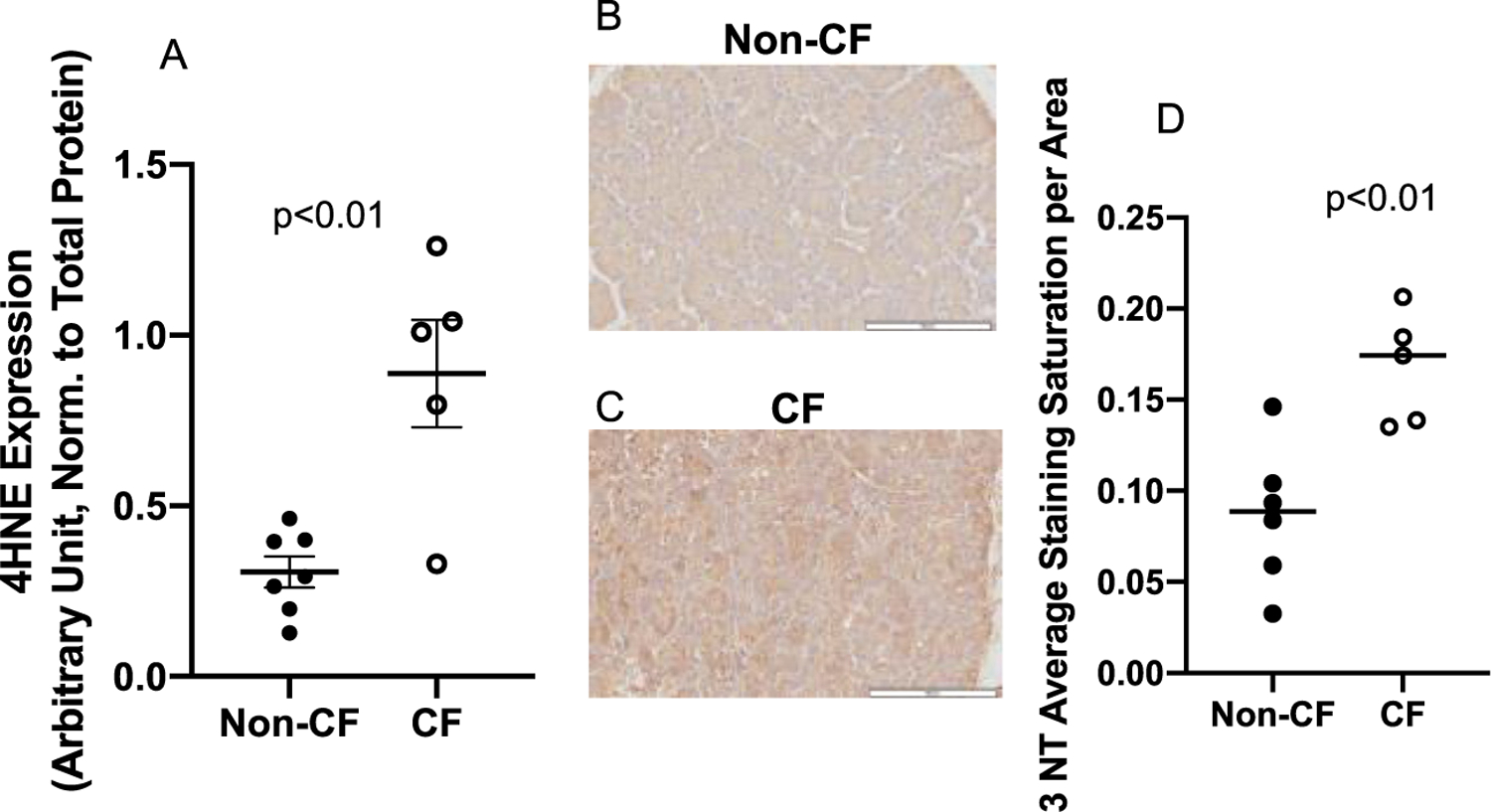
4-Hydroxynonenal modified proteins and 3-Nitrotyrosine as determined by immuno-blotting in pancreatic tissue. (A) Pancreatic tissue from pigs with non-CF (N = 7), or CF (N = 5) were probed for 4-HNE expression using immuno-slot-blotting. 4-HNE was significantly elevated in CF pig pancreas relative to non-CF (p<0.01). Data shown were the average staining of samples from three separate blots normalized to total protein via ponceau staining and reported with SEM. Micrographs of pancreatic tissue from (B) non-CF (N = 5) and (C) CF (N = 4) pigs probed for 3-NT-modified protein using immunohistochemistry and (D) quantified according to staining intensity per area. CF pig pancreas displayed significantly increased (p < 0.01) 3-NT staining throughout the pancreas. Data shown are the average of at least 6 sites selected randomly from each tissue section and reported with SD.
CF islets have increased pro-oxidant production.
When isolated and cultured porcine pancreatic islet cells were assessed for DHE oxidation on the ninth day of culture, CF islets demonstrated significantly increased DHE oxidation as compared to non-CF islets (Fig. 5). This demonstrates an increased level of pro-oxidants in cultured CF islets compared to non-CF, similar to what was seen in the experiments with pancreas frozen sections in Fig. 3.
Fig. 5.
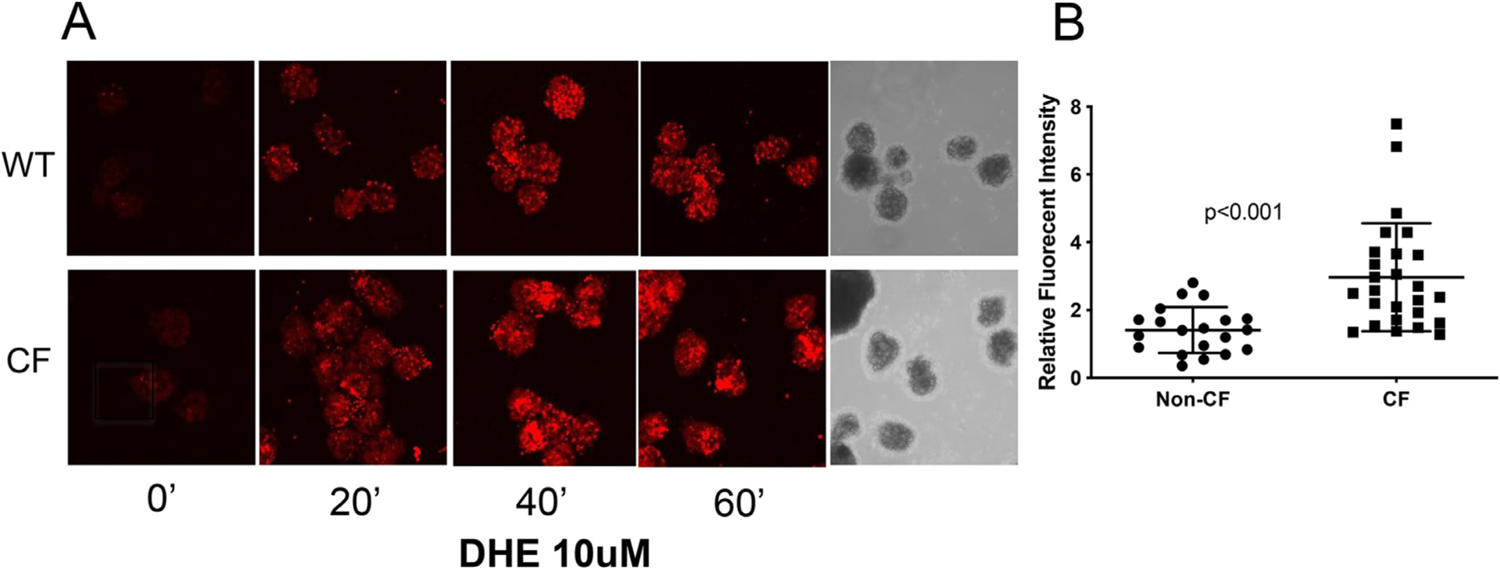
CF Islets have increased levels of ROS. (A) Islets were isolated and cultured in glass bottom dishes overnight at 37 °C, 5% CO2. The images were acquired immediately after addition of 10 μM of DHE at room temperature (about 25 °C) without additional CO2, at excitation/emission wavelength of 405/570 nm, using a Zeiss 710 confocal microscope. Images for WT and CF islets were obtained at the exact same gain setting on the microscope. (B) The fluorescent intensity of the images was quantified using ImageJ. Data shown are representative of three separate experiments.
Insulin secretion is impaired in CF islets.
To determine whether CF pig islets demonstrated impaired glycemic responses, glucose-stimulated insulin secretion (GSIS) studies were conducted on cultured islets. Following stimulation with 16.7 mM glucose, non-CF islets demonstrated a significant induction in percent insulin secretion (p < 0.001), while the percent insulin secretion for CF islets was not significantly different (p > 0.05) (Fig. 6). Interestingly, insulin hyposecretion in CF islets was not reversed by GC4401, an SOD mimetic (Fig. 7).
Fig. 6.
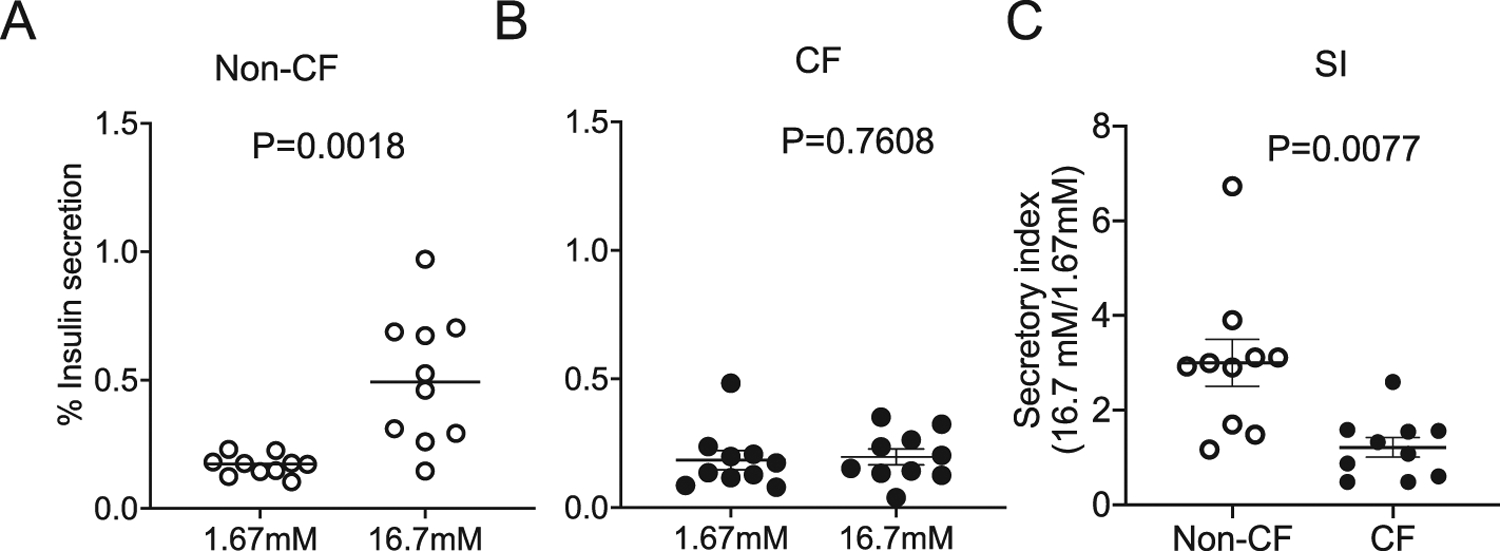
Glucose stimulated insulin secretion (GSIS) from CF and non-CF pig islets. Insulin secretion from cultured neonatal (A) non-CF and (B) CF pig islets at 1.67 mM to 16.7 mM glucose. (C) A reduction in the insulin secretory index (SI) was observed in CF as compared to non-CF cultured neonatal pig islets. Values for all graphs show the mean +/− SEM from independent animals. P values are shown.
Fig. 7.
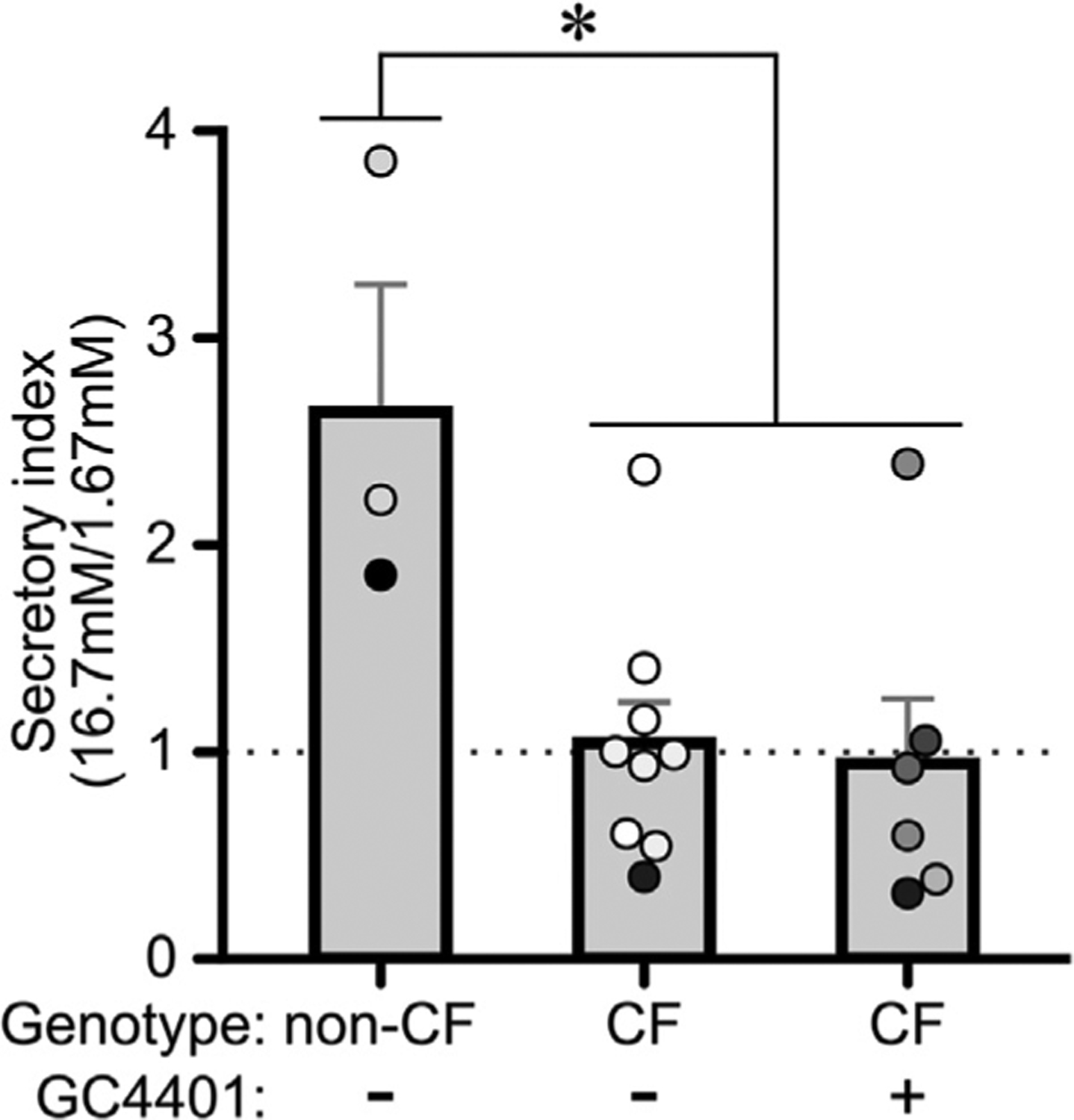
GC4401 does not reverse insulin secretory defects in CF islets. The insulin secretory index at 1.67 mM versus 16.7 mM glucose is shown. CF and non-CF pig islets were treated with GC4401 where indicated. The mean +/− SEM from independent animals is shown. The dots indicate independent islet preparations, with differing colors indicating separate animals. * P<0.05 for both CF groups versus non-CF, as determined by ANOVA followed by Tukey’s posthoc testing.
Apocynin does not reverse insulin secretion in perfused islets.
We next examined whether a NADPH oxidase-2 inhibitor, apocynin would reverse the insulin secretory defects in CF islets. As expected, insulin secretion was impaired in CF islets compared to non-CF islets. However, incubation with apocynin for 24 hours in culture did not ameliorate the insulin secretion defects in CF islets (Fig. 8).
Fig. 8.
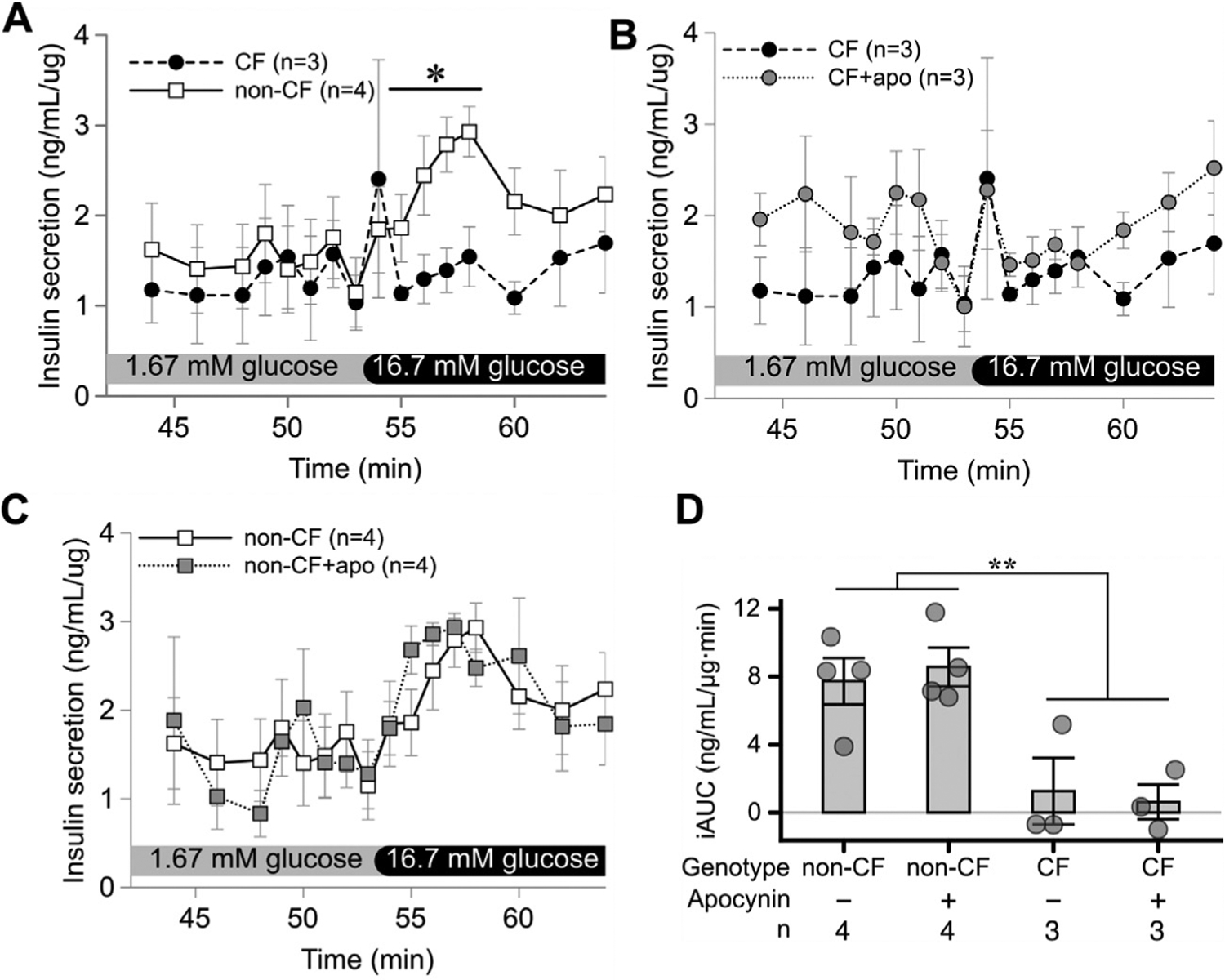
Apocynin does not reverse insulin secretory defects in perifused CF islets. Glucose stimulated insulin secretion was impaired in CF compared to non-CF cultured neonatal islets (A). 24 h culture with apocynin (apo) failed to impact insulin secretion in CF islets (B) or non-CF islets (C). Likewise, glucose stimulated insulin secretion as measured as incremental area under the curve (iAUC) was impaired in CF versus non-CF animals, but was not impacted by apocynin (D). Values for all graphs show the mean +/− SEM from independent animals. * p<0.05 for indicated time points (A) or iAUC (D), as assessed by 2-way ANOVA.
Peroxide levels are increased in CF islets.
We hypothesized that the lack of response to an SOD mimetic and apocynin may be due to peroxide production from a non-apocynin sensitive source in CF islets. To determine whether the CF islets have increased production of peroxides, we performed the Amplex Red assay as a surrogate marker for peroxides. Amplex red oxidation was linear over time in CF islets, with no increase in non-CF (Fig. 9A). Compared to non-CF, there was a significantly increased oxidation of Amplex red in CF islets at 120 min (Fig. 9B).
Fig. 9.
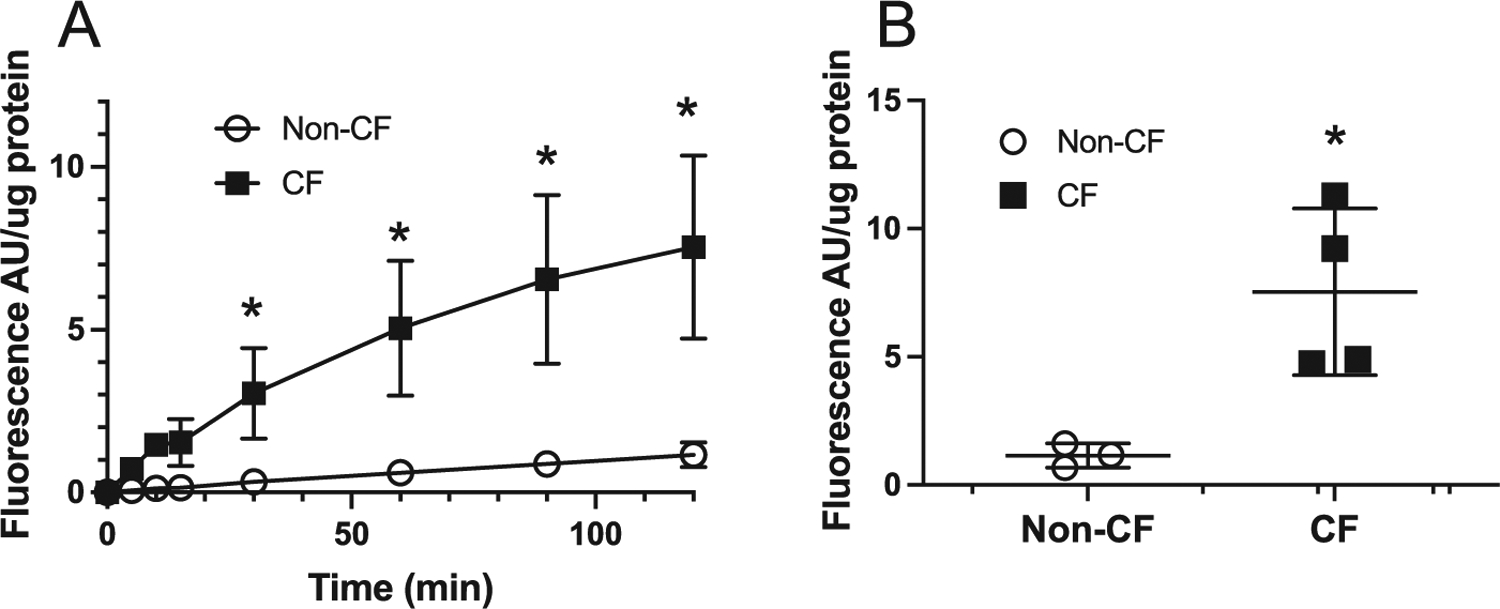
CF islets have increased peroxide production. (A) Time course of the change in the fluorescence of 20 μM Amplex Red at 590 nm of CF and non-CF islets following 9 days in culture. (B) Fluorescence measurements taken at 2h after exposing islet cultures to Amplex Red. The data points are the mean ± SD of individual experiments isolated from independent animals, n = 3; Non-CF, n = 4 CF; * p <0.05 (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Discussion
The current results demonstrated significantly decreased CuZnSOD activity, increased pro-oxidant production (presumably superoxide and other peroxides derived from reactions of superoxide) and increased oxidatively modified proteins in the pancreas of a well-established CF porcine model. Increased pancreatic DHE oxidation levels were seen in frozen CF pancreatic tissue sections and staining for this was inhibited by a selective SOD-mimetic, suggesting the signal was generated by superoxide. Increased levels of pro-oxidants (presumably superoxide and peroxides derived from superoxide), also occurred in cultured newborn CF pig islets while insulin secretion and secretory index were significantly reduced compared to non-CF. These results suggest increased oxidative stress in CF pig pancreas and islet cells that may be contributing to glycemic changes and insulin secretion defects in people with CF.
Oxidant-related damage and disruptions in glutathione metabolism has previously been shown in the lungs of humans with CF as well as other model systems [56,57], but not the pancreatic tissue. Oxidative stress was postulated as playing a role in CFRD because the blood levels of 4-HNE-modified proteins were elevated in children with CF and their concentrations correlated with the degree of impaired glucose tolerance [42]. Our study shows for the first time, the direct evidence of oxidative stress in CF pancreas in a well-established large animal model. This study suggests that oxidative stress may be playing a role in CFRD pathogenesis, as it is recognized as a major factor in the development of impaired insulin secretion in type 2 diabetes [58–66] as well as a tumor suppressor in cancer development [67].
GSH, a major redox buffer in cells, is particularly important for oxidative and immunological responses in CF. GSH is low in the airway surface liquid (ASL) and plasma of patients with CF [68–72] possibly because of abnormal GSH transport due to defective CFTR [39]. A low GSH found in the ASL of children with CF, irrespective of oxidation supports an inherent glutathione deficiency in CF [41]. Our study demonstrates an increase in total GSH (GSH+GSSG) and an increase the level GSSG in CF pancreas, all indicative of a redox stress and compensatory response by the glutathione synthetic pathways. Thiol redox stress and alterations in GSH metabolism in CF may be directly linked to CFTR activity and abnormal antioxidant defenses as well as priming and perpetuating excessive inflammation in CF.
The pancreatic β-cells may be susceptible to oxidative stress because of their low enzymatic antioxidant capacity (CuZnSOD, MnSOD, catalase, GPx) to detoxify excess superoxide and hydrogen peroxide [30–33]. This may explain why the degree of insulin secretion impairment correlates with the concentrations of oxidative damage markers in type 2 diabetes [58,73,74]. We found significant pro-oxidant generation in the CF pancreas that could be inhibited by the antioxidant enzyme, SOD. suggesting increased production of O2●−. CuZnSOD activity was significantly decreased in CF pancreas, suggesting that the ability to detoxify superoxide in the cytosol and mitochondrial inter-membrane space was impaired. We found no difference in pancreatic catalase, GPx and MnSOD between CF and non-CF pancreas. Superoxide dismutase catalyzes dismutation of superoxide to H2O2. Hydrogen peroxide is then detoxified by catalase or GPx to oxygen and water. We found increased generation of peroxides in CF islets suggesting production of H2O2, lipid hydroperoxides and peroxynitrite [75,76], all implicated in CF disease pathogenesis [77,78]. These results suggest that the detoxification reactions of superoxide in the cytosol and intermembrane space of the mitochondria may be compromised and accumulated products may cause oxidative stress. Also superoxide reacts with Fe and Cu to generate increased levels of both pools and redox cycling metal ions as well as causes the release of Fe from Ferritin which could exacerbate oxidative damage through Fenton chemistry [79–83]. These previous observations taken together with the data in the current report on pro-oxidant production, antioxidant capacity, and oxidative damage markers are consistent with the hypothesis that Fenton chemistry involving hydroperoxides could contribute to injury in islets from pigs expressing CFTR mutations.
Early exocrine pancreatic disease is a risk factor for CFRD [84], thus it is expected that the exocrine pancreas will have an impact on islet cell function in CF. We previously demonstrated impaired insulin secretion in CF pigs using intravenous glucose tolerance test (IV-GTT) [16]. In this study, insulin secretory defects were confirmed in cultured CF newborn islets. With high levels of superoxide, and peroxides and no compensatory increase in GPx or catalase activity to inhibit H2O2, CF islets are at risk for the deleterious effects of pro-oxidants. Low levels of pro-oxidants likely help mediate and regulate GSIS, but at higher concentrations, they inhibit insulin secretion from the islets, probably by directly or indirectly disturbing the integrity and physiological function of cellular macromolecules, such as DNA, lipids and proteins [85–90]. Consistent with this, prior in vitro studies of cultured CF islets have concluded that their damage and dysfunction originate from factors produced by exocrine pancreas cell types [91–93].
A dysfunctional CFTR and aberrant GSH function may be another source of the oxidative stress observed. Other sources to consider are ROS generated by tissue infiltrating inflammatory cells as is the case with CF lungs [94,95]. NADPH oxidase is likely not the source of ROS generation in CF islets because the insulin secretory defects were not reversed with apocynin. The source of ROS may be islet cells or ductal epithelial cells as the islet cultures may contain CFTR-expressing ductal cells [91,92] and are devoid of inflammatory cells. Free radicals released in the pancreas can cause islet cell damage [27,28] but at least in the newborn period, there was no evidence of widespread islet cell loss in CF pigs [16].
Conclusions
To the best of our knowledge, this is the first study demonstrating the direct evidence of oxidative stress in CF pancreas with a loss of cytosolic and mitochondrial intermembrane space SOD1 activity. Although short term treatments with two ROS-inhibitors failed to restore islet dysfunction, whether pro-oxidants contribute to long term islet dysfunction in CF remains to be determined. Our study suggests that oxidative stress, specifically peroxides formed from peroxidases, may contribute to the CF pathology. Further investigation into the ROS species, source and impact on pancreatic function is warranted.
Acknowledgments
We would like to Dr. Dennis Riley from Galera Therapeutics, Inc. for providing the GC4401 SOD mimetic and GC4404 inactive analogue under a material transfer agreement. We also acknowledge the support of CA086862, CA078586, and CA217797 as well as the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (AR070914) which supported some of the oxidation measures shown.
Funding
This work was supported by the National Institutes of Health NIH DK090490, DK097820, DK096518, DK118752, DK115791, RC2 DK124207, and U01 DK108334.
Abbreviations:
- CFRD
Cystic fibrosis-related diabetes
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
Footnotes
Declaration of Competing Interest
None.
References
- [1].Quinton PM, Cystic fibrosis: lessons from the sweat gland, Physiology. 22 (2007) 212–225 (Bethesda.). [DOI] [PubMed] [Google Scholar]
- [2].Wilschanski M, Durie PR, Patterns of GI disease in adulthood associated with mutations in the CFTR gene, Gut 56 (8) (2007) 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Borowitz D, Durie PR, Clarke LL, Werlin SL, Taylor CJ, Semler J, De Lisle RC, Lewindon P, Lichtman SM, Sinaasappel M, Baker RD, Baker SS, Verkade HJ, Lowe ME, Stallings VA, Janghorbani M, Butler R, Heubi J, Gastrointestinal outcomes and confounders in cystic fibrosis, J. Pediatr.Gastroenterol.Nutr 41 (3) (2005) 273–285. [DOI] [PubMed] [Google Scholar]
- [4].Dodge JA, Lewis PA, Stanton M, Wilsher J, Cystic fibrosis mortality and survival in the UK: 1947–2003, Eur.Respir.J 29 (3) (2007) 522–526. [DOI] [PubMed] [Google Scholar]
- [5].Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W, Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality, Diabetes Care 32 (9) (2009) 1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stecenko AA, Moran A, Update on cystic fibrosis-related diabetes, Curr. Opin. Pulm. Med 16 (6) (2010) 611–615. [DOI] [PubMed] [Google Scholar]
- [7].Milla CE, Warwick WJ, Moran A, Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline, Am.J.Respir.Crit. Care Med 162 (3 Pt 1) (2000) 891–895. [DOI] [PubMed] [Google Scholar]
- [8].Chamnan P, Shine BS, Haworth CS, Bilton D, Adler AI, Diabetes as a determinant of mortality in cystic fibrosis, Diabetes Care. 33 (2) (2010) 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH, An animal model for cystic fibrosis made by gene targeting, Science 257 (5073) (1992) 1083–1088. [DOI] [PubMed] [Google Scholar]
- [10].Clarke LL, Grubb BR, Gabriel SE, Smithies O, Koller BH, Boucher RC, Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis, Science 257 (5073) (1992) 1125–1128. [DOI] [PubMed] [Google Scholar]
- [11].Abu-El-Haija M, Sinkora M, Meyerholz DK, Welsh MJ, McCray PB, Butler J, Uc A, An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis, Pancreatology 11 (5) (2011) 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, Rogan MP, Davis GJ, Dohrn CL, Wohlford-Lenane C, Taft PJ, Rector MV, Hornick E, Nassar BS, Samuel M, Zhang Y, Richter SS, Uc A, Shilyansky J, Prather RS, McCray PB, Zabner J, Welsh MJ, Stoltz DA, The DeltaF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs, Sci. Transl. Med 3 (74) (2011) 74ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Zabner J, Welsh MJ, Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth, Sci.Transl.Med 2 (29) (2010) 29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Zabner J, Prather RS, Welsh MJ, Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs, Science 321 (5897) (2008) 1837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ, Pathology of gastrointestinal organs in a porcine model of cystic fibrosis, Am. J. Pathol 176 (3) (2010) 1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Uc A, Olivier AK, Griffin MA, Meyerholz DK, Yao J, Abu-El-Haija M, Buchanan KM, Vanegas Calderon OG, Abu-El-Haija M, Pezzulo AA, Reznikov LR, Hoegger MJ, Rector MV, Ostedgaard LS, Taft PJ, Gansemer ND, Ludwig PS, Hornick EE, Stoltz DA, Ode KL, Welsh MJ, Engelhardt JF, Norris AW, Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass, Clin. Sci 128 (2) (2015) 131–142 (Lond.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roma LP, Jonas JC, Nutrient metabolism, subcellular redox state, and oxidative stress in pancreatic islets and beta-cells, J. Mol. Biol 432 (5) (2020) 1461–1493. [DOI] [PubMed] [Google Scholar]
- [18].Broniowska KA, Oleson BJ, McGraw J, Naatz A, Mathews CE, Corbett JA, How the location of superoxide generation influences the beta-cell response to nitric oxide, J. Biol. Chem 290 (12) (2015) 7952–7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stancill JS, Broniowska KA, Oleson BJ, Naatz A, Corbett JA, Pancreatic beta–cells detoxify H2O2 through the peroxiredoxin/thioredoxin antioxidant system, J. Biol. Chem 294 (13) (2019) 4843–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stancill JS, Happ JT, Broniowska KA, Hogg N, Corbett JA, Peroxiredoxin 1 plays a primary role in protecting pancreatic beta-cells from hydrogen peroxide and peroxynitrite, Am. J. Physiol. Regul. Integr. Comp. Physiol 318 (5) (2020) R1004–R1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Urbano F, Bugliani M, Filippello A, Scamporrino A, Di Mauro S, Di Pino A, Scicali R, Noto D, Rabuazzo AM, Averna M, Marchetti P, Purrello F, Piro S, Atorvastatin but not pravastatin impairs mitochondrial function in human pancreatic islets and rat beta-cells. direct effect of oxidative stress, Sci. Rep 7 (1) (2017) 11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang J, Yang X, Zhang J, Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic beta cells, Cell Signal 28 (8) (2016) 1099–1104. [DOI] [PubMed] [Google Scholar]
- [23].Newsholme P, Keane KN, Carlessi R, Cruzat V, Oxidative stress pathways in pancreatic beta-cells and insulin-sensitive cells and tissues: importance to cell metabolism, function, and dysfunction, Am. J. Physiol. Cell Physiol 317 (3) (2019) C420–C433. [DOI] [PubMed] [Google Scholar]
- [24].Kuroki T, Isshiki K, King GL, Oxidative stress: the lead or supporting actor in the pathogenesis of diabetic complications, J. Am. Soc. Nephrol 14 (2003) S216–S220 8 Suppl 3. [DOI] [PubMed] [Google Scholar]
- [25].Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H, Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection, Diabetes 52 (3) (2003) 581–587. [DOI] [PubMed] [Google Scholar]
- [26].Mandrup-Poulsen T, Helqvist S, Wogensen LD, Molvig J, Pociot F, Johannesen J, Nerup J, Cytokine and free radicals as effector molecules in the destruction of pancreatic beta cells, Curr. Top. Microbiol. Immunol 164 (1990) 169–193. [DOI] [PubMed] [Google Scholar]
- [27].Burkart V, Koike T, Brenner HH, Kolb H, Oxygen radicals generated by the enzyme xanthine oxidase lyse rat pancreatic islet cells in vitro, Diabetologia 35 (11) (1992) 1028–1034. [DOI] [PubMed] [Google Scholar]
- [28].Fehsel K, Jalowy A, Qi S, Burkart V, Hartmann B, Kolb H, Islet cell DNA is a target of inflammatory attack by nitric oxide, Diabetes 42 (3) (1993) 496–500. [DOI] [PubMed] [Google Scholar]
- [29].Corbett JA, Sweetland MA, Wang JL, Lancaster JR, McDaniel ML, Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans, Proc. Natl. Acad. Sci. USA 90 (5) (1993) 1731–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Asayama K, Kooy NW, Burr IM, Effect of vitamin E deficiency and selenium deficiency on insulin secretory reserve and free radical scavenging systems in islets: decrease of islet manganosuperoxide dismutase, J. Lab. Clin. Med 107 (5) (1986) 459–464. [PubMed] [Google Scholar]
- [31].Grankvist K, Marklund SL, Taljedal IB, CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse, Biochem. J 199 (2) (1981) 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tiedge M, Lortz S, Drinkgern J, Lenzen S, Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells, Diabetes 46 (11) (1997) 1733–1742. [DOI] [PubMed] [Google Scholar]
- [33].Malaisse WJ, Malaisse-Lagae F, Sener A, Pipeleers DG, Determinants of the selective toxicity of alloxan to the pancreatic B cell, Proc. Natl. Acad. Sci. USA 79 (3) (1982) 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nathan BM, Laguna T, Moran A, Recent trends in cystic fibrosis-related diabetes, Curr. Opin. Endocrinol. Diabetes Obes 17 (4) (2010) 335–341. [DOI] [PubMed] [Google Scholar]
- [35].Hunt WR, Hansen JM, Stecenko AA, Glucose ingestion in cystic fibrosis induces severe redox imbalance: a potential role in diabetes, J. Cyst. Fibros 19 (3) (2020) 476–482. [DOI] [PubMed] [Google Scholar]
- [36].Hudson VM, New insights into the pathogenesis of cystic fibrosis: pivotal role of glutathione system dysfunction and implications for therapy, Treat. Respir. Med 3(6) (2004) 353–363. [DOI] [PubMed] [Google Scholar]
- [37].Montuschi P, Kharitonov SA, Ciabattoni G, Corradi M, van Rensen L, Geddes DM, Hodson ME, Barnes PJ, Exhaled 8-isoprostane as a new non-invasive biomarker of oxidative stress in cystic fibrosis, Thorax 55 (3) (2000) 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kettle AJ, Chan T, Osberg I, Senthilmohan R, Chapman AL, Mocatta TJ, Wagener JS, Myeloperoxidase and protein oxidation in the airways of young children with cystic fibrosis, Am. J. Respir. Crit. Care Med 170 (12) (2004) 1317–1323. [DOI] [PubMed] [Google Scholar]
- [39].Gao L, Kim KJ, Yankaskas JR, Forman HJ, Abnormal glutathione transport in cystic fibrosis airway epithelia, Am. J. Physiol 277 (1999) L113–L118 1 Pt 1. [DOI] [PubMed] [Google Scholar]
- [40].Linsdell P, Hanrahan JW, Glutathione permeability of CFTR, Am. J. Physiol 275 (1998) C323–C326 1 Pt 1. [DOI] [PubMed] [Google Scholar]
- [41].Dickerhof N, Pearson JF, Hoskin TS, Berry LJ, Turner R, Sly PD, Kettle AJ, Arest CF, Oxidative stress in early cystic fibrosis lung disease is exacerbated by airway glutathione deficiency, Free Radic. Biol. Med 113 (2017) 236–243. [DOI] [PubMed] [Google Scholar]
- [42].Ntimbane T, Krishnamoorthy P, Huot C, Legault L, Jacob SV, Brunet S, Levy E, Gueraud F, Lands LC, Comte B, Oxidative stress and cystic fibrosis-related diabetes: a pilot study in children, J. Cyst.Fibros 7 (5) (2008) 373–384. [DOI] [PubMed] [Google Scholar]
- [43].Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV, Large scale isolation, growth, and function of porcine neonatal islet cells, J. Clin. Invest 97 (9) (1996) 2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Morinville VD, Barmada MM, Lowe ME, Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: is greater awareness among physicians responsible? Pancreas 39 (1) (2010) 5–8. [DOI] [PubMed] [Google Scholar]
- [45].Griffith OW, Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine, Anal. Biochem 106 (1) (1980) 207–212. [DOI] [PubMed] [Google Scholar]
- [46].Olney KE, Du J, van ‘t Erve TJ, Witmer JR, Sibenaller ZA, Wagner BA, Buettner GR, Cullen JJ, Inhibitors of hydroperoxide metabolism enhance ascorbate-induced cytotoxicity, Free Radic. Res 47 (3) (2013) 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Spitz DR, Oberley LW, Measurement of MnSOD and CuZnSOD activity in mammalian tissue homogenates, Curr. Protoc. Toxicol (2001) Chapter 7: Unit7.5. [DOI] [PubMed] [Google Scholar]
- [48].Spitz DR, Oberley LW, An assay for superoxide dismutase activity in mammalian tissue homogenates, Anal. Biochem 179 (1) (1989) 8–18. [DOI] [PubMed] [Google Scholar]
- [49].Lawrence RA, Burk RF, Glutathione peroxidase activity in selenium-deficient rat liver, Biochem. Biophys. Res. Commun 71 (4) (1976) 952–958. [DOI] [PubMed] [Google Scholar]
- [50].Li WG, Miller FJ, Zhang HJ, Spitz DR, Oberley LW, Weintraub NL, H2O2-induced O2 production by a non-phagocytic NAD(P)H oxidase causes oxidant injury, J. Biol. Chem 276 (31) (2001) 29251–29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhu Y, Kalen AL, Li L, Lehmler HJ, Robertson LW, Goswami PC, Spitz DR, Aykin-Burns N, Polychlorinated-biphenyl-induced oxidative stress and cytotoxicity can be mitigated by antioxidants after exposure, Free Radic. Biol. Med 47 (12) (2009) 1762–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Coleman MC, Olivier AK, Jacobus JA, Mapuskar KA, Mao G, Martin SM, Riley DP, Gius D, Spitz DR, Superoxide mediates acute liver injury in irradiated mice lacking sirtuin 3, Antioxid. Redox. Signal 20 (9) (2014) 1423–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu S, Promes JA, Harata M, Mishra A, Stephens SB, Taylor EB, Burand AJ, Sivitz WI, Fink BD, Ankrum JA, Imai Y, Adipose triglyceride lipase is a key lipase for the mobilization of lipid droplets in human beta cells and critical for the maintenance of syntaxin1a level in beta cells, Diabetes 69 (6) (2020) 1178–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Akkiraju H, Bonor J, Nohe A, An improved immunostaining and imaging methodology to determine cell and protein distributions within the bone environment, J. Histochem. Cytochem 64 (3) (2016) 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mohanty JG, Jaffe JS, Schulman ES, Raible DG, A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative, J. Immunol. Methods 202 (2) (1997) 133–141. [DOI] [PubMed] [Google Scholar]
- [56].Kleme ML, Levy E, Cystic fibrosis-related oxidative stress and intestinal lipid disorders, Antioxid. Redox. Signal 22 (7) (2015) 614–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hudson VM, Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation, Free Radic. Biol. Med 30 (12) (2001) 1440–1461. [DOI] [PubMed] [Google Scholar]
- [58].Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, Del Prato S, Marchetti P, Functional and molecular defects of pancreatic islets in human type 2 diabetes, Diabetes 54 (3) (2005) 727–735. [DOI] [PubMed] [Google Scholar]
- [59].Shah S, Iqbal M, Karam J, Salifu M, McFarlane SI, Oxidative stress, glucose metabolism, and the prevention of type 2 diabetes: pathophysiological insights, Antioxid. Redox. Signal 9 (7) (2007) 911–929. [DOI] [PubMed] [Google Scholar]
- [60].Lim S, Rashid MA, Jang M, Kim Y, Won H, Lee J, Woo JT, Kim YS, Murphy MP, Ali L, Ha J, Kim SS, Mitochondria-targeted antioxidants protect pancreatic beta-cells against oxidative stress and improve insulin secretion in glucotoxicity and glucolipotoxicity, Cell Physiol. Biochem 28 (5) (2011) 873–886. [DOI] [PubMed] [Google Scholar]
- [61].Robertson RP, Oxidative stress and impaired insulin secretion in type 2 diabetes, Curr. Opin. Pharmacol 6 (6) (2006) 615–619. [DOI] [PubMed] [Google Scholar]
- [62].Zraika S, Aston-Mourney K, Laybutt DR, Kebede M, Dunlop ME, Proietto J, Andrikopoulos S, The influence of genetic background on the induction of oxidative stress and impaired insulin secretion in mouse islets, Diabetologia 49 (6) (2006) 1254–1263. [DOI] [PubMed] [Google Scholar]
- [63].Wang W, Guo Y, Xu M, Huang HH, Novikova L, Larade K, Jiang ZG, Thayer TC, Frontera JR, Aires D, Ding H, Turk J, Mathews CE, Bunn HF, Stehno-Bittel L, Zhu H, Development of diabetes in lean Ncb5or-null mice is associated with manifestations of endoplasmic reticulum and oxidative stress in beta cells, Biochim. Biophys. Acta 1812 (11) (2011) 1532–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jastroch M, Unraveling the molecular machinery that promotes pancreatic beta–cell dysfunction during oxidative stress: focus on “Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic beta-cells: evidence for regulation by Rac1”, Am. J. Physiol. Regul. Integr. Comp. Physiol 300 (1) (2011) R9–11. [DOI] [PubMed] [Google Scholar]
- [65].Gier B, Krippeit-Drews P, Sheiko T, Aguilar-Bryan L, Bryan J, Dufer M, Drews G, Suppression of KATP channel activity protects murine pancreatic beta cells against oxidative stress, J. Clin. Invest 119 (11) (2009) 3246–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Numazawa S, Sakaguchi H, Aoki R, Taira T, Yoshida T, Regulation of the susceptibility to oxidative stress by cysteine availability in pancreatic beta-cells, Am. J. Physiol. Cell Physiol 295 (2) (2008) C468–C474. [DOI] [PubMed] [Google Scholar]
- [67].Than BL, Linnekamp JF, Starr TK, Largaespada DA, Rod A, Zhang Y, Bruner V, Abrahante J, Schumann A, Luczak T, Walter J, Niemczyk A, O’Sullivan MG, Medema JP, Fijneman RJ, Meijer GA, Van den Broek E, Hodges CA, Scott PM, Vermeulen L, Cormier RT, CFTR is a tumor suppressor gene in murine and human intestinal cancer, Oncogene 35 (32) (2016) 4179–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG, Systemic deficiency of glutathione in cystic fibrosis, J. Appl. Physiol 75 (6) (1993) 2419–2424. [DOI] [PubMed] [Google Scholar]
- [69].Bishop C, Hudson VM, Hilton SC, Wilde C, A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis, Chest 127 (1) (2005) 308–317. [DOI] [PubMed] [Google Scholar]
- [70].Duijvestijn YC, Brand PL, Systematic review of N-acetylcysteine in cystic fibrosis, Acta Paediatr. 88 (1) (1999) 38–41. [DOI] [PubMed] [Google Scholar]
- [71].Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis, Proc. Natl. Acad. Sci. USA 103 (12) (2006) 4628–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kelly-Aubert M, Trudel S, Fritsch J, Nguyen-Khoa T, Baudouin-Legros M, Moriceau S, Jeanson L, Djouadi F, Matar C, Conti M, Ollero M, Brouillard F, Edelman A, GSH monoethyl ester rescues mitochondrial defects in cystic fibrosis models, Hum. Mol. Genet 20 (14) (2011) 2745–2759. [DOI] [PubMed] [Google Scholar]
- [73].Lupi R, Del Guerra S, Mancarella R, Novelli M, Valgimigli L, Pedulli GF, Paolini M, Soleti A, Filipponi F, Mosca F, Boggi U, Del Prato S, Masiello P, Marchetti P, Insulin secretion defects of human type 2 diabetic islets are corrected in vitro by a new reactive oxygen species scavenger, Diabetes Metab. 33 (5) (2007) 340–345. [DOI] [PubMed] [Google Scholar]
- [74].Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, Bugliani M, Boggi U, Vistoli F, Mosca F, Del Prato S, Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin, J. Clin. Endocrinol. Metab 89 (11) (2004) 5535–5541. [DOI] [PubMed] [Google Scholar]
- [75].Debski D, Smulik R, Zielonka J, Michalowski B, Jakubowska M, Debowska K, Adamus J, Marcinek A, Kalyanaraman B, Sikora A, Mechanism of oxidative conversion of Amplex(R) Red to resorufin: Pulse radiolysis and enzymatic studies, Free Radic. Biol. Med 95 (2016) 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bhattacharya A, Muller FL, Liu Y, Sabia M, Liang H, Song W, Jang YC, Ran Q, Van Remmen H, Denervation induces cytosolic phospholipase A2-mediated fatty acid hydroperoxide generation by muscle mitochondria, J. Biol. Chem 284 (1) (2009) 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dominguez C, Gartner S, Linan S, Cobos N, Moreno A, Enhanced oxidative damage in cystic fibrosis patients, Biofactors 8 (1–2) (1998) 149–153. [DOI] [PubMed] [Google Scholar]
- [78].Ricciardolo FL, Di Stefano A, Sabatini F, Folkerts G, Reactive nitrogen species in the respiratory tract, Eur. J. Pharmacol 533 (1–3) (2006) 240–252. [DOI] [PubMed] [Google Scholar]
- [79].Aliaga ME, Carrasco-Pozo C, Lopez-Alarcon C, Olea-Azar C, Speisky H, Superoxide-dependent reduction of free Fe(3+) and release of Fe(2+) from ferritin by the physiologically-occurring Cu(I)-glutathione complex, Bioorg. Med. Chem 19 (1) (2011) 534–541. [DOI] [PubMed] [Google Scholar]
- [80].Ding H, Zhao Y, Yu X, Chen L, Han J, Feng J, Tolerable upper intake level of iron damages the liver of weaned piglets, J. Anim. Physiol. Anim. Nutr 105 (4) (2021) 668–677 (Berl.). [DOI] [PubMed] [Google Scholar]
- [81].Liochev SL, The role of iron-sulfur clusters in in vivo hydroxyl radical production, Free Radic. Res 25 (5) (1996) 369–384. [DOI] [PubMed] [Google Scholar]
- [82].Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM, Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury, J. Clin. Invest 103 (7) (1999) 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, Sandhu S, Carlisle TL, Smith MC, Abu Hejleh T, Berg DJ, Zhang J, Keech J, Parekh KR, Bhatia S, Monga V, Bodeker KL, Ahmann L, Vollstedt S, Brown H, Shanahan Kauffman EP, Schall ME, Hohl RJ, Clamon GH, Greenlee JD, Howard MA, Schultz MK, Smith BJ, Riley DP, Domann FE, Cullen JJ, Buettner GR, Buatti JM, Spitz DR, Allen BG, O2(−) and H2O2-Mediated Disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate, Cancer Cell 31 (4) (2017) 487–500 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Soave D, Miller MR, Keenan K, Li W, Gong J, Ip W, Accurso F, Sun L, Rommens JM, Sontag M, Durie PR, Strug LJ, Evidence for a causal relationship between early exocrine pancreatic disease and cystic fibrosis-related diabetes: a Mendelian randomization study, Diabetes 63 (6) (2014) 2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Maechler P, Jornot L, Wollheim CB, Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells, J. Biol. Chem 274 (39) (1999) 27905–27913. [DOI] [PubMed] [Google Scholar]
- [86].Evans JL, Goldfine ID, Maddux BA, Grodsky GM, Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 52 (1) (2003) 1–8. [DOI] [PubMed] [Google Scholar]
- [87].Nakazaki M, Kakei M, Koriyama N, Tanaka H, Involvement of ATP-sensitive K+ channels in free radical-mediated inhibition of insulin secretion in rat pancreatic beta-cells, Diabetes 44 (8) (1995) 878–883. [DOI] [PubMed] [Google Scholar]
- [88].Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S, Reactive oxygen species as a signal in glucose-stimulated insulin secretion, Diabetes 56 (7) (2007) 1783–1791. [DOI] [PubMed] [Google Scholar]
- [89].Singh A, Kukreti R, Saso L, Kukreti S, Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes, Molecules 27 (3) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Leloup C, Magnan C, Benani A, Bonnet E, Alquier T, Offer G, Carriere A, Periquet A, Fernandez Y, Ktorza A, Casteilla L, Penicaud L, Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing, Diabetes 55 (7) (2006) 2084–2090. [DOI] [PubMed] [Google Scholar]
- [91].Norris AW, Ode KL, Merjaneh L, Sanda S, Yi Y, Sun X, Engelhardt JF, Hull RL, Survival in a bad neighborhood: pancreatic islets in cystic fibrosis, J. Endocrinol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sun X, Yi Y, Xie W, Liang B, Winter MC, He N, Liu X, Luo M, Yang Y, Ode KL, Uc A, Norris AW, Engelhardt JF, CFTR influences beta cell function and insulin secretion through non-cell autonomous exocrine-derived factors, Endocrinology 158 (10) (2017) 3325–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hart NJ, Aramandla R, Poffenberger G, Fayolle C, Thames AH, Bautista A, Spigelman AF, Babon JAB, DeNicola ME, Dadi PK, Bush WS, Balamurugan AN, Brissova M, Dai C, Prasad N, Bottino R, Jacobson DA, Drumm ML, Kent SC, MacDonald PE, Powers AC, Cystic fibrosis-related diabetes is caused by islet loss and inflammation, JCI insight 3 (8) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mitri C, Xu Z, Bardin P, Corvol H, Touqui L, Tabary O, Novel Anti-inflammatory approaches for cystic fibrosis lung disease: identification of molecular targets and design of innovative therapies, Front. Pharmacol 11 (2020) 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nichols DP, Chmiel JF, Inflammation and its genesis in cystic fibrosis, Pediatr. Pulmonol 50 (2015) S39–S56 Suppl 40. [DOI] [PubMed] [Google Scholar]


