Abstract
Aim
Risperidone is the most commonly prescribed antipsychotic drug to children and adolescents worldwide, but it is associated with serious side effects, including weight gain. This study assessed the relationship of risperidone and 9‐hydroxyrisperidone trough concentrations, maximum concentrations and 24‐hour area under the curves (AUCs) with body mass index (BMI) z‐scores in children and adolescents with autism spectrum disorder (ASD) and behavioural problems. Secondary outcomes were metabolic, endocrine, extrapyramidal and cardiac side effects and effectiveness.
Methods
Forty‐two children and adolescents (32 males) aged 6‐18 years were included in a 24‐week prospective observational trial. Drug plasma concentrations, side effects and effectiveness were measured at several time points during follow‐up. Relevant pharmacokinetic covariates, including medication adherence and CYP2D6, CYP3A4, CYP3A5 and P‐glycoprotein (ABCB1) genotypes, were measured. Nonlinear mixed‐effects modelling (NONMEM®) was used for a population pharmacokinetic analysis with 205 risperidone and 205 9‐hydroxyrisperidone concentrations. Subsequently, model‐based trough concentrations, maximum concentrations and 24‐hour AUCs were analysed to predict outcomes using generalized and linear mixed‐effects models.
Results
A risperidone two‐compartment model combined with a 9‐hydroxyrisperidone one‐compartment model best described the measured concentrations. Of all the pharmacokinetic parameters, higher risperidone sum trough concentrations best predicted higher BMI z‐scores during follow‐up (P < .001). Higher sum trough concentrations also predicted more sedation (P < .05), higher prolactin levels (P < .001) and more effectiveness measured with Aberrant Behavior Checklist irritability score (P < .01).
Conclusion
Our results indicate a therapeutic window exists, which suggests that therapeutic drug monitoring of risperidone might increase safety and effectiveness in children and adolescents with ASD and behavioural problems.
Keywords: adolescent, antipsychotic, autism spectrum disorder, body mass index, child, cytochrome P‐450, drug monitoring, prolactin, risperidone, weight gain
1. What is already known about this subject
Risperidone treatment in children and adolescents is associated with serious side effects, most importantly weight gain.
The risk of side effects increases with higher dosages, but the relationship of concentrations of risperidone and its active metabolite 9‐hydroxyrisperidone with side effects is unknown.
What this study adds
In children and adolescents with autism spectrum disorder (ASD), weight gain can be predicted by model‐based risperidone sum trough concentrations. The sum trough concentration is a better predictor than the maximum concentration or the 24‐hour area under the curves.
Higher risperidone sum trough concentrations predict higher body mass index z‐scores, but also more sedation, higher prolactin levels and, interestingly, more effectiveness.
Our findings indicate that a therapeutic window for effectiveness with acceptable weight gain in children and adolescents with ASD seems to exist, but more research is needed to establish the therapeutic reference range in this vulnerable population.
1. INTRODUCTION
Risperidone is the most frequently prescribed antipsychotic drug to children and adolescents worldwide, with a prevalence ranging from 1.1 to 6.6 per 1000 youths across different countries. 1 , 2 In this population risperidone is used for a broad range of mental health disorders, including disruptive behavioural disorders, schizophrenia and bipolar disorder. An important and increasing indication concerns irritability associated with autism spectrum disorder (ASD), with almost one in nine youths with ASD using risperidone. 3 For these and other indications, the short‐term efficacy of risperidone is well established and supported by numerous randomized controlled trials. 4 , 5
However, there are growing concerns about the side effects of risperidone in children and adolescents. Weight gain is the most important adverse effect, which is more pronounced in youths than in adults. 6 Children and adolescents gain several kilograms during the first weeks of risperidone treatment. 7 This results in serious long‐term health risks, including metabolic abnormalities and diabetes mellitus. 8 , 9
Other common side effects of risperidone include extrapyramidal symptoms (EPS), prolactin elevation and sedation. 10 During long‐term risperidone treatment, up to one in three youths experience mild to moderate EPS, and more than half demonstrate prolactin elevations, possibly leading to gynecomastia, galactorrhea and sexual dysfunction. 11 , 12 Risperidone‐induced sedation is significantly more prevalent in young patients than in adults. 13 Lastly, risperidone can increase the corrected QT (QTc) interval, although clinically relevant QTc prolongation is rare. 14
Several studies have shown that the risk of most of these side effects, including weight gain, increases with higher risperidone dosages in children and adolescents. 9 , 11 , 15 , 16 However, the relationship between individual exposure, reflected in risperidone plasma concentrations, and side effects remains unclear. A few studies have shown a correlation between prolactin elevation and plasma concentrations of risperidone or its active metabolite 9‐hydroxyrisperidone in youths, 16 , 17 , 18 , 19 , 20 but the relationship with weight gain and other side effects is unknown. This hampers the use of therapeutic drug monitoring to improve safety in this population.
The concentration‐effectiveness relationship of risperidone in children and adolescents has yet to be determined. One study showed no correlation between total plasma risperidone and 9‐hydroxyrisperidone concentrations and clinical response in a prospective cohort of children with ASD. 21 However, in that study, both trough and nontrough risperidone concentrations were analysed together, which prevents correct interpretation. Another study found a concentration‐effectiveness relationship in a sample of children and adolescents with different indications for risperidone use, but this study had limitations due to the naturalistic and retrospective study design. 22
Here we study for the first time the relationship between risperidone and 9‐hydroxyrisperidone plasma concentrations, weight gain, other side effects and effectiveness in a prospective cohort of children and adolescents with ASD. The primary aim is to investigate the relationship between model‐based individual pharmacokinetic parameters and body mass index (BMI) z‐scores. For this purpose, trough concentrations, maximum concentrations and 24‐hour area under the curves (AUCs) are analysed. The relationships between the most relevant pharmacokinetic parameter and EPS, sedation, metabolic abnormalities, prolactin elevation, QTc‐prolongation and effectiveness are also investigated. The influence of a large number of demographic and biochemical characteristics, including the cytochrome P450 enzymes CYP2D6, CYP3A4, CYP3A5 and P‐glycoprotein (ABCB1) genotypes, is taken into account.
The findings of this study should indicate whether there is a therapeutic window and rationale for therapeutic drug monitoring of risperidone to improve the safety and effectiveness in children and adolescents.
2. METHODS
2.1. Study population
Children aged 6‐18 years with the diagnosis of ASD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) IV 23 or 5 24 and using or starting risperidone for irritability were eligible for inclusion in this 24‐week observational prospective multicenter cohort study (Netherlands Trial Register 6050). Exclusion criteria were diabetes type I or II, congenital or acquired syndromes associated with changes in appetite, body weight or lipid profile (eg, Prader Willi), treatment with another antipsychotic drug within the last 6 months or known long QT syndrome. Patients were treated in one of the seven participating centres in the south‐west region of the Netherlands (two academic tertiary care centres and five psychiatric secondary care centres). They were prescribed risperidone as tablet formulation or oral solution in flexible dosing schemes by their treating physician according to standard clinical care. Patients were recruited between August 2016 and October 2018. All patients and/or their legal representatives gave written informed consent before entering the study. The study was approved by the medical ethics committee of the Erasmus Medical Center, the Netherlands (number MEC 2016‐124). The study was carried out in accordance with the Declaration of Helsinki and the Regulations on Medical Research with Human Subjects, the Netherlands.
2.2. Drug concentration measurement
Three blood samples were repeatedly collected for risperidone and 9‐hydroxyrisperidone quantification on two separate days. For patients who initiated risperidone treatment at the start of the study, blood samples were withdrawn at 12 and 24 weeks follow‐up. For patients who already used risperidone at the start of the study, blood samples were collected at the start of the study and at 24 weeks follow‐up. Blood samples were collected using venipuncture or the dried blood spot (DBS) method at random time points, with at least 1 hour between two samples. DBS sampling has experienced renewed interest in bioanalysis, as it requires only a simple finger prick and less blood than a venipuncture. DBS is regarded as less painful and stressful for the patient than conventional blood sampling, and can be collected in a home environment, which increases the feasibility of repeated sampling in children. 25 Time of sampling, time of risperidone intake in the prior 24 hours, risperidone dose and comedication were reported during sampling. Risperidone and 9‐hydroxyrisperidone plasma concentrations were measured with previously validated ultrahigh performance liquid chromatography‐mass spectrometry (LC‐MS/MS) methods for plasma and DBS. 26 , 27 , 28 The lower limit of quantification (LLOQ) for risperidone was 1 μg/L and for 9‐hydroxyrisperidone 0.7 μg/L for plasma and DBS samples. The lower limit of detection (LOD) for plasma risperidone samples was 0.02 μg/L and for plasma 9‐hydroxyrisperidone was 0.22 μg/L; LOD for the DBS risperidone samples was 0.9 μg/L and for the DBS 9‐hydroxyrisperidone samples was 0.5 μg/L.
DBS concentrations were converted to estimated plasma concentrations (EPC) using the following formulas with correction for hematocrit (ht) based on a previously performed clinical validation study 28 :
In this study hematocrit was standardly measured. When the hematocrit value was unknown and could not be extrapolated from a previous measurement, the median population value was used.
2.3. Assessment of outcomes
Side effects and effectiveness were prospectively recorded at the start of the study and at 24 weeks for all patients using and initiating risperidone treatment.
Patients who initiated risperidone treatment when starting the study had additional assessments of side effects and effectiveness at 4 and 12 weeks. For patients who already used risperidone at the start of the study, bodyweight, height, laboratory measurements and comedication since initiation of risperidone were retrospectively collected from the patient file.
2.3.1. Side effects
Bodyweight and height were measured at each visit. EPS were measured with the Abnormal Involuntary Movement Scale (AIMS), 29 filled in by treating physician, nurse or researcher. Sedation was assessed with the Epworth Sleepiness Scale 30 and filled in by parents. Laboratory indices measured were triglycerides, total cholesterol, high‐density lipoprotein (HDL)‐cholesterol, low‐density lipoproteins (LDL)‐cholesterol, glucose, hemoglobin A1C (HbA1C) and prolactin. Laboratory indices were measured at the start of the study and at 24 weeks follow‐up, with an additional measurement at 12 weeks for patients who had initiated risperidone treatment at the start of the study.
QT intervals were measured in triplicate from a 12‐lead ECG as described previously 31 at the start of the study and after 24 weeks of follow‐up. The QT interval was measured at steady‐state heart rates, using preferably lead II, from the beginning of the onset of the QRS complex to the end of the T‐wave. The measured QT intervals were corrected for heart rate using the Bazett's formula:
The QT times and RR intervals of the first 10 ECGs were measured by both a researcher and an experienced paediatric cardiologist who were blinded for study time point. As these measurements showed good agreement (<10% difference for all measurements), the researcher individually performed the remaining QTc measurements. If there was any doubt, the ECG was also reviewed by the paediatric cardiologist.
2.3.2. Effectiveness
The effectiveness of risperidone was assessed by parents and the treating physician. Parents filled in the Aberrant Behavior Checklist (ABC), 32 a 58‐item questionnaire that is sensitive to treatment effects in children with ASD. The ABC‐irritability subscale (ABC‐I) was used as measure for effectiveness; this scale reflects irritability symptoms with a maximum of 45 points. Treating physicians filled in the Clinical Global Impression Scale (CGI). 29 This scale describes the severity of psychopathology (CGI‐S) and its improvement (CGI‐I) by seven categories, rated by the treating physician. The CGI‐S describes the severity of illness relative to patients with the same diagnosis in ascending order, with 1 = normal and 7 = extremely ill. The CGI‐I rates the improvement in comparison to the original medication‐naive state of symptoms: 0 = not assessable, 1 = very much better, 2 = much better, 3 = moderately better, 4 = unchanged, 5 = minimally worse, 6 = much worse.
2.4. Assessment of covariates
Medication adherence was assessed with questionnaires (Medication Adherence Rating Scale MARS‐5, 33 filled in by parents, and a 100‐point visual analogue scale (VAS), filled in by parents and treating physician) and during the last month of follow‐up with an electronical monitoring system (MEMS). 34 A VAS score of 100, a MARS score of 25 or a MEMS adherence percentage of 100 represents optimal adherence on each scale. Comedication was retrieved from medical records and from pharmacy records. Comedication for ADHD was recorded for the following drugs which are known to influence weight: methylphenidate, amphetamine and atomoxetine.
Parents filled in a study questionnaire at every visit, including questions about the child's diet, physical activity, grapefruit juice use and over‐the‐counter self‐medication use (including St John's wort). Diet questions involved whether the child had visited a dietician and whether nutritional advice was followed. Physical activity questions involved the quantification of the child's high‐intensity (sports) and low‐intensity (walking or cycling) activity in hours per week. Familiar cardiometabolic risk was assessed after taking the family history at the start of the study, as previously defined. 35 If the family history was unknown, it was considered positive for cardiometabolic risk.
2.4.1. Laboratory measurements
Renal function (ureum, creatinine), liver function (aspartate aminotransferase [ASAT], alanine aminotransferase [ALAT], gamma glutamyl transpeptidase [GGT], alkalic fosfatase [AF], albumin) and haematocrit were assessed at baseline and during follow‐up.
All patients were genotyped for the following cytochrome P450 (CYP) enzymes that could influence pharmacokinetics: CYP3A4, CYP3A5, CYP2D6 and P‐glycoprotein PGP (ABCB1). The following single nucleotide polymorphisms were tested: for CYP3A4 *22, for CYP3A5 *3 and *6, for CYP2D6 *3, *4, *5, *41 and for ABCB1 (PgP) 3435C > T, using genomic DNA yeisolated from EDTA blood, and analysed using Taqman 5′ nuclease DME assays (ThermoFisher Scientific, Carlsbad, CA, USA). All pharmacogenetic testing was performed in the laboratory of the Erasmus MC, Rotterdam, the Netherlands.
2.5. Population pharmacokinetic analyses
Population pharmacokinetic analysis was performed by nonlinear mixed‐effects modelling using NONMEM version 7.4.2 (FOCE+I; ICON Development Solutions, Ellicott City, MD, USA) and PsN Version 4.7.0. Pirana software version 2.9.7 was used as an interface between NONMEM and R (version 3.4.4). Concentrations of 9‐hydroxyrisperidone were corrected for molecular weight of risperidone.
2.5.1. Base model development
One‐, two‐ and three‐compartment models were considered to describe the concentration‐time data based on visual inspection of the goodness‐of‐fit plots and a review of the literature. Typical values for lag‐time, first‐order absorption rate constant (k a), volume of distribution (V), clearance (CL), and intercompartmental clearance (Q) were estimated. As bioavailability (F) could not be quantified, certain parameters were estimated as ratios: CL/F, Q/F, and V/F. First, risperidone data were described and subsequently 9‐hydroxyrisperidone data were added. For each pharmacokinetic parameter interpatient variability (IPV) was evaluated and shrinkage was calculated for all parameters for which IPV was established. A shrinkage value below 25% was considered acceptable. 36 Allometric scaling was used to account for the influence of bodyweight on pharmacokinetic parameters, which was explored with a fixed exponent (0.75 for CL and Q, and 1 for V), with exponents estimated by the model. Residual variability was described with a combined (additive and proportional) error model, with extra errors for sampling method (DBS versus venipuncture) and concentrations below LLOQ. Concentrations measured below the LOD were set on half the value of the LOD in combination with an extra additional error of half the value of the LOD. Components of the error model estimated to approach zero were removed. Model selection criteria were a decrease in the NONMEM objective function value (OFV), goodness‐of‐fit plots and visual predictive checks (VPCs). A decrease in the OFV of 3.84 points was considered statistically significant (P < .05).
2.5.2. Covariate model development
The following covariates were considered as potential model covariates: sex, age, dose, dose per kilogram, bodyweight, height, BMI, CYP3A4 genotype, CYP3A5 genotype, CYP2D6 genotype, P‐glycoprotein (PGP) genotype, comedication, somatic comorbidities, haematocrit, renal function (ureum, creatinine), liver function (ASAT, ALAT, GGT, AF), albumin, medication adherence, grapefruit juice use, St John's wort use and smoking. First, the correlation between the covariates and IPV was evaluated graphically. Subsequently, covariates with a visual relationship with IPV were individually added to the model. Continuous covariates were described using an exponential function and categorical covariates using a proportional function. Covariates that significantly improved the model with the univariate analysis (P < .05) were selected for multivariate analysis. The forward inclusion‐backward elimination method was used. 37 During the backward elimination process, covariates that improved the model at a level of P < .001 were selected. A shark plot was generated for each covariate for case‐deletion diagnostics.
2.5.3. Internal model evaluation
First, a bootstrap analysis was performed with 1000 simulations. 38 The validity of the model was evaluated by comparing the bootstrap estimates and their 90th percentile range with the values generated using the original dataset. Second, the model was evaluated with a VPC stratified by compartment, using a set of 1000 simulated datasets to compare the observed concentrations with the distribution of the simulated concentrations. 39
2.5.4. Pharmacokinetic predictions
Model‐based individual pharmacokinetic predictions were used as trough concentration (C trough), maximum concentration (C max) and 24‐hour AUC (AUC24h) were not available for each patient because of sparse random sampling within the study. These pharmacokinetic parameters were predicted for risperidone and 9‐hydroxyrisperidone per subject for the days a BMI z‐score was known. The C trough prior to the first risperidone administration of the day was used. The C max was calculated for risperidone and was defined as the concentration at 40 minutes after risperidone administration, based on visual inspection of concentrations simulated by the final model. In case of multiple dosages per day, the highest C max on that day was used for the analyses.
2.6. Pharmacodynamic analyses
2.6.1. Primary outcome
The primary outcome was BMI adjusted for age and weight, the BMI z‐score. A BMI z‐score of ≥1 is considered overweight and a BMI z‐score ≥2 is considered obesity according to the World Health Organization (WHO). 40 BMI values were transformed into BMI z‐scores based on the WHO BMI‐for age reference values (5‐19 years). 41
2.6.2. Secondary outcomes
The following secondary outcomes were analysed: EPS, sedation, triglycerides level, total cholesterol level, HDL‐cholesterol level, LDL‐cholesterol level, glucose level, HbA1C level, prolactin level, QTc‐time, CGI‐improvement score and ABC‐irritability (ABC‐I) score. EPS, sedation and CGI‐improvement score were considered categorical outcomes. EPS was defined positive if at least two times mild or one time moderate was scored with the AIMS. Sedation was defined as an ESS total score of 1 or higher. Only children for whom a baseline measurement (before start of risperidone treatment) of the concerning outcome was available were included in the analyses.
2.7. Statistical analyses
Generalized and linear mixed‐effects models were used to analyse the longitudinal data. Random effects were employed to capture the heterogeneity between the patients. The BMI z‐score was considered the primary outcome. Each pharmacokinetic parameter of risperidone was separately analysed as predictor versus BMI z‐score. Potential relevant covariates (duration of risperidone use, sex, age, somatic comorbidities, comedication, IQ, prematurity, physical activity, diet) were tested in each model and the best model was selected using backwards variable selection. The final model among all the best models with different pharmacokinetic parameters of risperidone was chosen by Akaike information criterion. 42
The secondary analyses were performed with the pharmacokinetic parameter that was selected for the final model of the primary outcome. This pharmacokinetic parameter was entered as predictor in univariable models and, if significant, relevant covariates were added in a stepwise manner. On top of the covariates for the primary analyses, psychiatric comorbidities, psychotropic treatment before start of risperidone, nonpharmacological treatment of parent and/or child, and familiar cardiometabolic risk were checked when relevant.
The correlation between model‐based pharmacokinetic parameters was analysed with Pearson's correlation. Changes in BMI z‐scores between baseline and the last visit was assessed with the Wilcoxon signed‐rank test. The explained variance by the final model was calculated with the conditional pseudo‐R2. In all analyses P < .05 was considered significant and all analyses were performed in R 43 (version 3.4.4).
3. RESULTS
3.1. Study sample
Forty‐two patients were included, of whom 31 initiated risperidone treatment at the time they were included in the study and 11 already used risperidone before inclusion in the study. The baseline characteristics of the study sample are presented in Table 1. The median (interquartile range, IQR) follow‐up time since start of risperidone therapy was 5.7 (4.8) months. The median (IQR) risperidone daily dose at the end of follow up was 1.0 (0.5) mg and 0.02 (0.02) mg/kg. The majority of children had one or more comorbid psychiatric disorders besides ASD (64.3%), ie, attention deficit/hyperactivity disorder (ADHD, 52.4%), oppositional defiant disorder (11.9%), mood disorder (7.1%), post‐traumatic stress disorder (4.8%) or anxiety disorder (2.4%).
TABLE 1.
Baseline characteristics
| Characteristic | |
|---|---|
| Male, n (%) | 32 (76.2) |
| Age a (years) | 9.7 (5.3) |
| Bodyweight a (kg) | 32.4 (18.3) |
| Height a (m) | 1.42 (0.34) |
| Body mass index a (kg m−2) | 16.18 (4.06) |
| Body mass index z‐score a | −0.32 (1.69) |
| Ethnicity, n (%) | |
| Both parents Dutch origin | 33 (78.6) |
| Otherb | 8 (19.0) |
| Unknown | 1 (2.3) |
| Metabolic laboratory measurements a | |
| Triglycerides (mmol/L) | 0.57 (0.42) |
| Total cholesterol (mmol/L) | 4.00 (1.00) |
| HDL‐cholesterol (mmol/L) | 1.50 (0.49) |
| LDL‐cholesterol (mmol/L) | 2.33 (0.79) |
| Glucose (mmol/L) | 4.90 (0.40) |
| HbA1C (mmol/Mol) | 33 (4) |
| Prolactin (U/L) | 0.12 (0.10) |
| Genotype, n (%)c | |
| CYP2D6 | |
| Poor metabolizer | 0 (0) |
| Intermediate metabolizer | 27 (64.3) |
| Normal metabolizer | 14 (33.3) |
| CYP3A4 | |
| Poor metabolizer | 1 (2.4) |
| Intermediate metabolizer | 6 (14.3) |
| Normal metabolizer | 34 (81.0) |
| CYP3A5 | |
| Expressor | 8 (19.0) |
| Non‐expressor | 33 (78.6) |
| ABCB1 | |
| Poor metabolizer | 14 (33.3) |
| Normal metabolizer | 27 (64.3) |
| Unknown genotype | 1 (2.4) |
| QTc time (ms)a | 387 (31) |
| Clinical global impression scale (CGI‐s) score a | 5 (2) |
| Comorbid psychiatric disorders other than ASD, n (%) | 27 (64.3) |
| Comedication ADHD drugs, n (%)d | 10 (23.8) |
| IQ a | 100 (40) |
| Treatment setting, n (%) | |
| Outpatient | 37 (88.1) |
| Inpatient | 5 (11.9) |
| Prior psychotropic treatment, n (%) | 25 (59.5) |
| Physical activity a | |
| High intensity (hours/week) | 2.5 (3) |
| Low intensity (hours/week) | 2 (2) |
| Increased familiar cardiometabolic risk, n (%)e | 18 (42.9) |
| Formulation of risperidone administration, n (%) | |
| Tablet | 31 (73.8) |
| Oral solution | 11 (26.2) |
All patients were diagnosed with autism spectrum disorder. The values represent start of risperidone treatment unless otherwise specified. Values represent total sample of n = 42 patients, except for triglycerides (n = 29), total cholesterol (n = 28), HDL‐cholesterol (n = 28), LDL‐cholesterol (n = 28), glucose (n = 30), HbA1C (n = 23), prolactin (U/L), CGI (n = 29), IQ (n = 40), physical activity (n = 11), QTc (n = 25).
Abbreviations: ADHD, attention deficit/hyperactivity disorder; CYP, cytochrome P450; HbA1C, hemoglobin A1C; HDL, high‐density lipoprotein; LDL, low‐density lipoproteins; PGP, P‐glycoprotein; QTc, corrected QT; 1
Presented as median and interquartile range (IQR) for continuous variables.
Seven children had one or two parents of non‐European descent. Of these, five children had one or two parents with African descent.
Metabolizing status within the sample was defined as follows: CYP2D6: poor = 2 inactive alleles (eg, *4/*4); intermediate = 1 active and 1 inactive allele (*1/*3, *1/*4, *1/*5) OR 1 inactive and 1 decreased activity allele (*4/*41); normal = 2 active alleles (*1/*1) OR 1 active and 1 decreased activity allele (eg, *1/*41); CP3A4: poor = *22/*22, intermediate = *1/*22, normal = *1*1; CYP3A5: expressor = at least 1 active (*1) allele (*1/*3, *1/*6); non‐expressor = 2 inactive alleles (*3/*3, *3/*6); ABCB1: poor = 3435TT, normal = 3435CT, 3435CC.
Includes methylphenidate, amphetamine, atomoxetine.
As defined by American Academy of Pediatrics. 35
One‐hundred and fifty‐four DBS samples and 72 plasma samples were collected. Twenty‐one DBS samples (13.6%) were of insufficient quality for drug quantification, resulting in a total of 205 risperidone and 205 9‐hydroxyrisperidone concentrations. The median (IQR) measured risperidone concentration was 1.72 (6.19) μg/L in plasma and 3.57 (3.55) μg/L in DBS. The median (IQR) measured 9‐hydroxyrisperidone concentration was 7.02 (6.13) μg/L in plasma and 6.39 (6.72) μg/L in DBS. Haematocrit was known for 85 DBS measurements in 32 patients, with a median (IQR) haematocrit of 0.40 (0.08) L/L.
The medication adherence was generally high: the median (IQR) MEMS adherence percentage was 96% (62%), VAS score filled in by treating physician 100 (4), VAS score filled in by parents 100 (2) and MARS score filled in by parents 24 (1).
3.2. Population pharmacokinetic analyses
3.2.1. Base model
The data was best described using a two‐compartment model for risperidone combined with a one‐compartment model for 9‐hydroxyrisperidone. IPV on CL of risperidone and CL of 9‐hydroxyrisperidone significantly improved the model. The residual error was described with additional and proportional errors for risperidone and 9‐hydroxyrisperidone, concentrations below the LOD, concentrations >LOD and <LLOQ, and DBS samples. Pharmacokinetic parameters are presented in Table 2 and estimates of residual variability are presented in Supporting Information Table S1.
TABLE 2.
Pharmacokinetic parameter estimates of the final model and bootstrap analysis
| Parameter | Estimate (shrinkage) | Bootstrap median (90th percentile) b |
|---|---|---|
| t lag (h) | 0.42 | 0.42 (0.40‐0.50) |
| K a (L/h) | 18.6 | 34.2 (4.0‐2587.1) |
| V c/F a (L/70 kg) | 107 | 107.2 (85.5‐142.1) |
| V p/F a (L/70 kg) | 46.2 | 426.3 (39.1‐9671.2) |
| Q/F a (L/h/70 kg) | 3.31 | 4.4 (1.6‐14.7) |
| CL/F a (L/h/70 kg) | 23.9 | 24.2 (19.5‐32.1) |
| IPV CL | 80% (6%) | 82% (66‐103) |
| V CM/F a (L/70 kg) | 111 | 101.4 (75.2‐181.9) |
| CLM/F a (L/h/70 kg) | 5.19 | 5.2 (4.6‐5.7) |
| IPV CLM | 28% (12%) | 25% (17‐34) |
3.2.2. Covariate analysis
No covariates remained significant in multivariate analyses after backward elimination except for bodyweight, which was best described using fixed exponents with allometric scaling.
3.2.3. Evaluation of the final model
The bootstrap analysis yielded similar values to the model‐based parameter estimates, except for a larger estimate for the peripheral V (V p). The model‐based estimate of V p, however, was within the bootstrap 90th percentile range, indicating the stability of the model. The goodness‐of‐fit‐plots showed that the model adequately described the observed concentrations (Supporting Information Figure S1). The VPC showed a good predictive performance, although the lower 90th percentile of the simulated concentrations was slightly lower than the measured concentrations (Supporting Information Figure S2).
3.2.4. Pharmacokinetic predictions
For the time points for which a BMI z‐score was known, 270 model‐based individual pharmacokinetic predictions for C trough, C max and AUC24h of risperidone and 9‐hydroxyrisperidone were calculated. The correlation between the predicted pharmacokinetic parameters was moderate to strong (r > 0.5, data not shown), except for risperidone C trough and 9‐hydroxyrisperidone AUC24h, which had a weak correlation (r = 0.39).
3.3. Pharmacodynamic analyses
3.3.1. Primary outcome: BMI z‐scores
Two hundred and seventy BMI z‐scores were available in 42 patients; for three patients no baseline BMI z‐score was available. The mean BMI z‐score increased significantly during follow‐up from −0.28 ± 1.34 at baseline to 0.26 ± 1.24 at end of follow‐up (P < .001).
A higher risperidone and 9‐hydroxyrisperidone exposure significantly predicted higher BMI z‐scores during follow up for all pharmacokinetic parameters (C trough, C max and AUC24h P < .001). Significant covariates in multivariate analyses were risperidone duration of use (months) and comedication for ADHD (P < .05).
The sum C trough most strongly predicted BMI z‐scores during follow‐up, together with the significant covariates: sum C trough (β = 0.042, P < .001), duration of use (β = −0.009, P < .001) and ADHD comedication (β = −0.340, P = .004) with an intercept of −0.040 (P = .836). The relationship between sum C trough and BMI z‐scores is shown in Figure 1 and Table 3.
FIGURE 1.
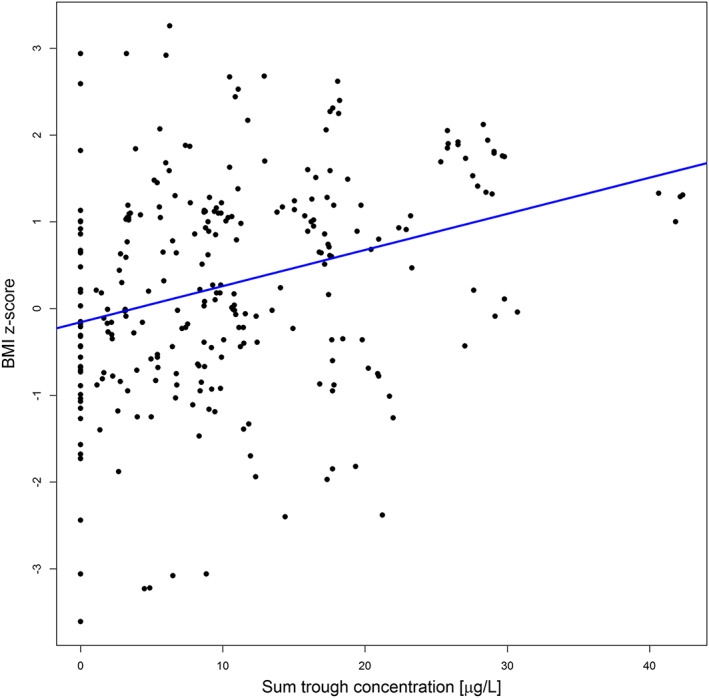
Sum trough concentration versus BMI z‐scores. BMI, body mass index; sum, risperidone and 9‐hydroxyrisperidone
TABLE 3.
Association between trough concentrations of risperidone + 9‐hydroxyrisperidone, BMI z‐score and secondary effectiveness outcomes
| Variable | n (obs) | Estimate | Standard error | P value |
|---|---|---|---|---|
| Primary outcome | ||||
| BMI z‐score | 42 (270) | |||
| Sum C trough | 0.042 | 0.005 | <.001 | |
| Duration of use | −0.009 | 0.002 | <.001 | |
| Comedication ADHD | −0.340 | 0.116 | .004 | |
| Secondary outcomes: effectiveness | ||||
| CGI – response | 29 (107) | |||
| Sum C trough | 0.300 | 0.158 | .057 | |
| Duration of use | 0.445 | 0.183 | .015 | |
| ABC – Irritability | 42 (121) | |||
| Sum C trough | −0.281 | 0.093 | .003 | |
| Age at start | −1.390 | 0.398 | .001 | |
The median (IQR) predicted sum C trough was 10.07 (11.54) μg/L. The influence of diet and physical activity could not be analysed due to too many missing values.
Abbreviations: ABC‐I, Aberrant Behavior Checklist – irritability score; BMI, body mass index; CGI, Clinical Global Impression Scale; obs, number of observations.
The explained variance in BMI z‐scores of the final model with sum C trough was 90.4%, which was higher than with risperidone dose at time of measurement (89.3%).
3.3.2. Secondary outcomes
Sum C trough significantly predicted sedation, prolactin levels and ABC‐irritability score with correction for relevant covariates. No association was found between sum C trough and EPS, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, glucose, HBA1C, prolactin or QTc time and response based on CGI‐improvement score. The results of the effectiveness analyses are presented in Table 3 and the results of the secondary side‐effects analyses are presented in Supporting Information Table S2.
3.3.3. Therapeutic window
Based on the estimated coefficients for BMI z‐scores, ABC‐I score and the relevant covariates, a plausible therapeutic window for a child 10 years of age, with 3 months of risperidone treatment and without ADHD comedication is visualized in Figure 2. Assuming a BMI z‐score <1 and an ABC‐I score <11 as relevant cut‐offs for treatment success with acceptable weight gain, the theoretical therapeutic window of the sum C trough would be between 15 and 25 μg/L.
FIGURE 2.
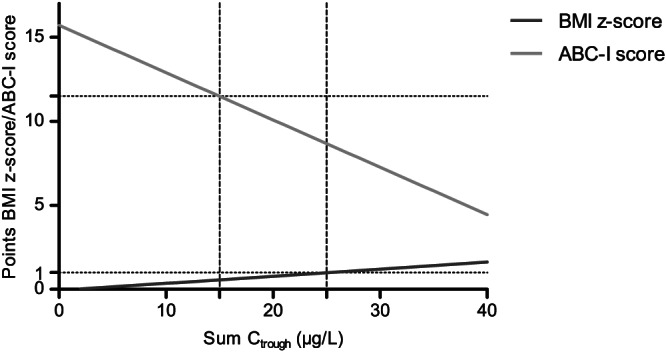
Example of theoretical therapeutic window. The relationship between BMI z‐scores, ABC‐I scores and sum C trough for a child 10 years old, with 3 months of risperidone treatment and without ADHD comedication. The grey lines indicate a theoretical therapeutic window (15‐25 μg/L) for a BMI z‐score <1 and a ABC‐I score <11. BMI, body mass index; ABC‐I, Aberrant Behavior Checklist – Irritability scale; sum C trough, sum of trough concentrations of risperidone and 9‐hydroxyrisperidone
4. DISCUSSION
This study demonstrates that higher risperidone and 9‐hydroxyrisperidone plasma concentrations are associated with more weight gain, more sedation, higher prolactin levels and increased effectiveness in children and adolescents with ASD and behavioural problems. The sum trough concentration of risperidone and 9‐hydroxyrisperidone was the most predictive pharmacokinetic parameter.
The risperidone and 9‐hydroxyrisperidone population pharmacokinetic parameters found in this study are comparable to previously described values in children and adolescents. 44 , 45 After adjusting for bodyweight, these pharmacokinetic parameters are similar to adults. 45 The effects of demographic and biochemical characteristics on risperidone plasma concentrations in youths have not been studied extensively. While several studies did not find an influence of sex, 44 , 45 , 46 some found higher total plasma concentrations in girls than boys 47 and others the opposite. 48 Within our sample, no influence of sex was found, but our sample mainly consisted of boys. The activity of the CYP2D6 enzyme has repeatedly been shown to strongly affect risperidone clearance in children and adolescents, 44 , 45 , 49 but was not found as significant covariate due to the absence of poor and extensive metabolizers in our study population. The influence of polymorphisms in other cytochrome P450 enzymes CYP3A4, CYP3A5 and the transport protein PGP on risperidone concentrations has not been previously studied in children and adolescents, despite evidence for this influence in adults. 50 , 51 Our study is the first to examine these polymorphisms for risperidone in youths, but did not find any significant effect of polymorphisms in these genes on risperidone pharmacokinetics.
Higher risperidone and 9‐hydroxyrisperidone concentrations predicted higher BMI z‐scores during risperidone treatment in children and adolescents. The sum trough concentration of risperidone and 9‐hydroxyrisperidone, also referred to as the “active moiety”, was found to be the most predictive pharmacokinetic parameter. This confirms the recommendation in current therapeutic drug monitoring guidelines, 52 but was not self‐evident, as for other drugs, including antibiotic drugs, it is known that maximum concentrations or AUCs can predict outcomes better than trough concentrations. 53 Two other studies have investigated the relationship between both risperidone and 9‐hydroxyrisperidone concentrations and BMI z‐scores in children and adolescents. One of these two studies did find higher risperidone metabolite and sum concentrations in children and adolescents with higher BMI z‐scores, 48 the other did not find any association. 54 However, both studies had a cross‐sectional design without baseline measurement, and were not able to take weight gain into account. In adults, to date no studies have investigated the relationship between risperidone plasma concentrations and weight gain. 55
The analysis of the relationship between risperidone plasma concentrations and weight gain is complicated, as this relationship is bidirectional. Risperidone exposure may influence bodyweight in a pharmacodynamic way, but bodyweight also moderates risperidone exposure by pharmacokinetic processes. Higher body weight is expected to result in lower risperidone concentrations with an unaltered dose, as volume of distribution and clearance increase with bodyweight as a result of allometric scaling. Therefore, the pharmacodynamic explanation of the relationship is favoured, as in our study higher concentrations were found with higher bodyweights. Still, it is unknown how well the established allometric scaling exponents fit paediatric populations with overweight. 56 For risperidone, a relatively lipophilic drug, the volume of distribution is likely to increase with increasing fat mass, but for 9‐hydroxyrisperidone, the more hydrophilic metabolite, this is more unlikely. Further research is needed to clarify the relationship between measured risperidone concentrations, rather than predicted concentrations, and overweight in paediatric patients.
Higher risperidone sum trough concentrations were also related to more sedation, prolactin elevation and effectiveness in our sample. In adults, the risperidone exposure‐response relationship has been studied almost exclusively in schizophrenia patients, yielding conflicting results. While some studies found a higher risperidone plasma concentrations predicting better response, others found the opposite. 55 The positive correlation with extrapyramidal symptoms is better established, although different plasma thresholds are reported, including >40 μg/L, 57 >180 μg/L 58 or > 74 μg/L. 55 As a result, a therapeutic reference range of risperidone in adults has not yet been clearly established, but is proposed as 20‐60 μg/L for schizophrenia by the AGNP consensus guideline. 52
For children and adolescents, no therapeutic reference range is known, although it is suggested that optimal risperidone concentrations in children with impulsive‐aggressive symptoms are lower than in adults with schizophrenia. 22 Our findings confirm this, and show that optimal concentrations might depend on the child's age at start of risperidone therapy, duration of treatment and comedication with ADHD drugs. When accounting for these variables, a therapeutic window of risperidone in children and adolescents with autism and behavioural problems seems to exist. For example, for a child 10 years old, after 3 months of risperidone treatment without ADHD comedication, the theoretical therapeutic range would be 15‐25 μg/L, when a BMI z‐score <1 and ABC irritability score <11 are considered as optimal treatment outcomes. This response corresponds to a 25% reduction in ABC irritability score, which has been previously defined as clinically relevant. 59
Although this study demonstrates a rationale to explore the added value of therapeutic drug monitoring in this population, more research is needed to better define the therapeutic reference range of risperidone in these youths. Future studies should assess measured rather than predicted sum trough concentrations and focus on their predictive value in an early treatment phase for weight gain during later follow‐up. Eventually, a randomized controlled trial should evaluate the added value of risperidone titration towards optimal concentrations versus treatment as usual. These efforts should be made before therapeutic drug monitoring of risperidone in youths can be routinely used as standard care.
The findings of this study should be interpreted in the light of its limitations. First, the study had a sparse sampling design with different sampling methods, including venous sampling and dried blood spot sampling. This design was chosen to minimalize the patient burden and increase the study feasibility, as has been previously advised for paediatric pharmacokinetic trials. 25 This, however, resulted in a higher variability in measured and predicted pharmacokinetic concentrations, further enhanced by the relatively large share of the measured risperidone concentrations below the LOD. We have reduced these variabilities in the analyses with the development of an extensive pharmacokinetic residual error model, although this resulted in less accuracy in the estimation of V p/F. Second, the analysis of the relationship between risperidone exposure and effects was done with model‐based concentrations rather than the measured concentrations themselves. Although this is suboptimal compared to really measured exposure, the model‐based concentrations showed a good fit with measured concentrations. Moreover, this allowed for an analysis of not only trough concentrations, but also peak concentrations and AUC24h. Third, due to a limited sample size, relatively few patients had high risperidone sum trough concentrations. These patients had a quite large impact on the regression analyses. However, the mg/kg doses and characteristics of these patients were within the range of the total sample, thus reflecting average patients. Fourth, the sample size was not powered for the secondary outcomes, thus possibly leading to nonsignificant results. Fifth, this study has the typical limitations of naturalistic study designs in analysing the exposure‐response relationship, 60 as placebo‐responders and patients with side effects are likely to receive lower dosages, while nonresponders are likely to receive higher dosages. This might have led to an over‐ or underestimation of the exposure‐response relationship. Lastly, although a large panel of pharmacogenetic polymorphisms was tested for its pharmacokinetic influence, candidate genes that might be relevant for pharmacodynamic outcomes were currently not tested; these, however, are of interest for future research. 61
This is the first study that prospectively investigated the relationship between risperidone pharmacokinetic parameters, side effects and effectiveness in children and adolescents with autism spectrum disorder and severe behavioural problems. The finding that the risperidone sum trough concentration predicts weight gain, other side effects and response indicates that therapeutic drug monitoring might improve the safety and efficacy of risperidone treatment in this vulnerable population.
COMPETING INTERESTS
S.K., B.D. and B.K. received grant research support from The Netherlands Organization for Health Research and Development (ZonMW), number 836041011. TG has received lecture fees and study grants from Chiesi and Astellas, in addition to consulting fees from Roche Diagnostics, Vitaeris, Astellas, Aurinia Pharma and Novartis.
CONTRIBUTORS
B.D., B.C.P.K., T.G., G.C.D. and S.M.K. designed the research; S.M.K., B.C.M.W. and K.N. analyzed the data; S.M.K., B.D., B.C.P.K. wrote the manuscript; B.C.M., C.G.R., M.E.J.K., M.M.J.K., E.D., W.A.E., R.R., D.A., B.B., R.H.N.S., K.N., M.H.J.H., and T.G. commented on the manuscript; C.G.R., M.E.J.K., M.M.J.K., E.D., W.A.E., R.R., D.A., B.B., B.D. and B.C.P.K. performed the research.
Supporting information
Supporting Information Table S1 Residual variability of the final model and bootstrap analysis
Supporting Information Table S2 Association between trough concentrations of risperidone + 9‐hydroxyrisperidone and secondary side‐effect outcomes
Supporting Information Figure S1a Goodness‐of‐fit plot of final model. Measured concentrations versus individual predictions for risperidone
Supporting Information Figure S1b (b) Goodness‐of‐fit‐plot of final model. Measured concentrations versus individual predictions for 9‐hydroxy‐risperidone
Supporting Information Figure S2 Visual predictive check (VPC). Left, VPC of risperidone; right, VPC of 9‐hydroxyrisperidone
ACKNOWLEDGEMENTS
We thank Soma Bahmany of the Erasmus MC Rotterdam for the quantification of the risperidone and 9‐hydroxyrisperidone concentrations. S.K., B.D. and B.K. received grant research support from The Netherlands Organization for Health Research and Development (ZonMW), number 836041011. TG has received lecture fees and study grants from Chiesi and Astellas, in addition to consulting fees from Roche Diagnostics, Vitaeris, Astellas, Aurinia Pharma and Novartis.
Kloosterboer SM, de Winter BCM, Reichart CG, et al. Risperidone plasma concentrations are associated with side effects and effectiveness in children and adolescents with autism spectrum disorder. Br J Clin Pharmacol. 2021;87:1069–1081. 10.1111/bcp.14465
The authors confirm that the Principal Investigators for this paper are Dr. Birgit C.P. Koch and Dr. B. Dierckx and that they had direct clinical responsibility for patients.
Footnotes
CGI‐s, Clinical Global Impression Severity Scale: 1 = normal; 2 = borderline; 3 = mildly ill; 4 = moderately ill; 5 = markedly ill; 6 = severely ill; 7 = extremely ill.
St Johns wort and grapefruit juice were not tested as covariates, as no children used these during follow‐up.
Abbreviations: t lag, lag time; K a, absorption rate constant; V, volume of distribution; CL, clearance; IPV, interpatient variability; M, metabolite, 9‐hydroxyrisperidone; P, peripheral; C, central; RSE, relative standard error.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Halfdanarson O, Zoega H, Aagaard L, et al. International trends in antipsychotic use: a study in 16 countries, 2005‐2014. Eur Neuropsychopharmacol. 2017;27:1064‐1076. [DOI] [PubMed] [Google Scholar]
- 2. Kloosterboer SM, Schuiling‐Veninga CCM, Bos JHJ, et al. Antipsychotics in Dutch youth: prevalence, dosages, and duration of use from 2005 to 2015. J Child Adolesc Psychopharmacol. 2018;28(3):173‐2179. [DOI] [PubMed] [Google Scholar]
- 3. Park SY, Cervesi C, Galling B, et al. Antipsychotic use trends in youth with autism spectrum disorder and/or intellectual disability: a meta‐analysis. J Am Acad Child Adolesc Psychiatry. 2016;55(6):456‐468. e4 [DOI] [PubMed] [Google Scholar]
- 4. Jensen PS, Buitelaar J, Pandina GJ, Binder C, Haas M. Management of psychiatric disorders in children and adolescents with atypical antipsychotics: a systematic review of published clinical trials. Eur Child Adolesc Psychiatry. 2007;16(2):104‐120. [DOI] [PubMed] [Google Scholar]
- 5. Lee ES, Vidal C, Findling RL. A focused review on the treatment of pediatric patients with atypical antipsychotics. J Child Adolesc Psychopharmacol. 2018;28(9):582‐605. [DOI] [PubMed] [Google Scholar]
- 6. Safer DJ. A comparison of risperidone‐induced weight gain across the age span. J Clin Psychopharmacol. 2004;24(4):429‐436. [DOI] [PubMed] [Google Scholar]
- 7. De Hert M, Dobbelaere M, Sheridan EM, Cohen D, Correll CU. Metabolic and endocrine adverse effects of second‐generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26(3):144‐158. [DOI] [PubMed] [Google Scholar]
- 8. Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA. Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bobo WV, Cooper WO, Stein CM, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiat. 2013;70(10):1067‐1075. [DOI] [PubMed] [Google Scholar]
- 10. Cohen D, Bonnot O, Bodeau N, Consoli A, Laurent C. Adverse effects of second‐generation antipsychotics in children and adolescents: a Bayesian meta‐analysis. J Clin Psychopharmacol. 2012;32(3):309‐316. [DOI] [PubMed] [Google Scholar]
- 11. Laita P, Cifuentes A, Doll A, et al. Antipsychotic‐related abnormal involuntary movements and metabolic and endocrine side effects in children and adolescents. J Child Adolesc Psychopharmacol. 2007;17(4):487‐502. [DOI] [PubMed] [Google Scholar]
- 12. Roke Y, van Harten PN, Boot AM, Buitelaar JK. Antipsychotic medication in children and adolescents: a descriptive review of the effects on prolactin level and associated side effects. J Child Adolesc Psychopharmacol. 2009;19(4):403‐414. [DOI] [PubMed] [Google Scholar]
- 13. Liu XI, Schuette P, Burckart GJ, et al. A comparison of pediatric and adult safety studies for antipsychotic and antidepressant drugs submitted to the United States Food and Drug Administration. J Pediatr. 2019;208:236‐242. e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen KG, Juul K, Fink‐Jensen A, Correll CU, Pagsberg AK. Corrected QT changes during antipsychotic treatment of children and adolescents: a systematic review and meta‐analysis of clinical trials. J Am Acad Child Adolesc Psychiatry. 2015;54(1):25‐36. [DOI] [PubMed] [Google Scholar]
- 15. Hoekstra PJ, Troost PW, Lahuis BE, et al. Risperidone‐induced weight gain in referred children with autism spectrum disorders is associated with a common polymorphism in the 5‐hydroxytryptamine 2C receptor gene. J Child Adolesc Psychopharmacol. 2010;20(6):473‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roke Y, Buitelaar JK, Boot AM, Tenback D, van Harten PN. Risk of hyperprolactinemia and sexual side effects in males 10‐20 years old diagnosed with autism spectrum disorders or disruptive behavior disorder and treated with risperidone. J Child Adolesc Psychopharmacol. 2012;22(6):432‐439. [DOI] [PubMed] [Google Scholar]
- 17. Duval F, Guillon MS, Mokrani MC, Crocq MA, Garcia DF. Relationship between prolactin secretion, and plasma risperidone and 9‐hydroxyrisperidone concentrations in adolescents with schizophreniform disorder. Psychoneuroendocrinology. 2008;33(2):255‐259. [DOI] [PubMed] [Google Scholar]
- 18. Migliardi G, Spina E, D'Arrigo C, et al. Short‐ and long‐term effects on prolactin of risperidone and olanzapine treatments in children and adolescents. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1496‐1501. [DOI] [PubMed] [Google Scholar]
- 19. Ngamsamut N, Hongkaew Y, Vanwong N, et al. 9‐Hydroxyrisperidone‐induced hyperprolactinaemia in Thai children and adolescents with autism spectrum disorder. Basic Clin Pharmacol Toxicol. 2016;119(3):267‐272. [DOI] [PubMed] [Google Scholar]
- 20. Troost PW, Lahuis BE, Hermans MH, et al. Prolactin release in children treated with risperidone: impact and role of CYP2D6 metabolism. J Clin Psychopharmacol. 2007;27(1):52‐57. [DOI] [PubMed] [Google Scholar]
- 21. Gagliano A, Germano E, Pustorino G, et al. Risperidone treatment of children with autistic disorder: effectiveness, tolerability, and pharmacokinetic implications. J Child Adolesc Psychopharmacol. 2004;14(1):39‐47. [DOI] [PubMed] [Google Scholar]
- 22. Klampfl K, Taurines R, Preuss A, et al. Serum concentrations, therapeutic response and side effects in children and adolescents with impulsive‐aggressive symptoms during risperidone therapy. Pharmacopsychiatry. 2010;43(02):58‐65. [DOI] [PubMed] [Google Scholar]
- 23. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Editon ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 24. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th Editon ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 25. Patel P, Mulla H, Tanna S, Pandya H. Facilitating pharmacokinetic studies in children: a new use of dried blood spots. Arch Dis Child. 2010;95(6):484‐487. [DOI] [PubMed] [Google Scholar]
- 26. Wijma RA, van der Nagel BC, Dierckx B, et al. Identification and quantification of the antipsychotics risperidone, aripiprazole, pipamperone and their major metabolites in plasma using ultra‐high performance liquid chromatography‐mass spectrometry. Biomed Chromatogr. 2016;30(6):794‐801. [DOI] [PubMed] [Google Scholar]
- 27. Tron C, Kloosterboer SM, van der Nagel BCH, et al. Dried blood spots combined with ultra‐high‐performance liquid chromatography‐mass spectrometry for the quantification of the antipsychotics risperidone, aripiprazole, pipamperone, and their major metabolites. Ther Drug Monit. 2017;39(4):429‐440. [DOI] [PubMed] [Google Scholar]
- 28. Kloosterboer SM, de Winter BCM, Bahmany S, et al. Dried blood spot analysis for therapeutic drug monitoring of antipsychotics: drawbacks of its clinical application. Ther Drug Monit. 2018;40(3):344‐350. [DOI] [PubMed] [Google Scholar]
- 29. W G. Ecdeu Assessment Manual for Psychopharmacologyrevised edition Editon. Bethesda, MD: US Department of Health, Education and Welfare; 1976. [Google Scholar]
- 30. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540‐545. [DOI] [PubMed] [Google Scholar]
- 31. Postema PG, De Jong JS, Van der Bilt IA, Wilde AA. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm. 2008;5(7):1015‐1018. [DOI] [PubMed] [Google Scholar]
- 32. Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89:485‐491. [PubMed] [Google Scholar]
- 33. Horne R, Weinman J. Self‐regulation and self‐management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non‐adherence to preventer medication. Psychol Health. 2002;17(1):17‐32. [Google Scholar]
- 34. Aardex . MEMS 6 Monitor (MEMS 6 Track Cap) Zug. Switzerland, 2005,2006.
- 35. Daniels SR, Greer FR. Committee on N. lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198‐208. [DOI] [PubMed] [Google Scholar]
- 36. Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82(1):17‐20. [DOI] [PubMed] [Google Scholar]
- 37. Jonsson EN, Karlsson MO. Automated covariate model building within NONMEM. Pharm Res. 1998;15(9):1463‐1468. [DOI] [PubMed] [Google Scholar]
- 38. Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol. 1997;37(6):486‐495. [DOI] [PubMed] [Google Scholar]
- 39. Comets E, Brendel K, Mentre F. Computing normalised prediction distribution errors to evaluate nonlinear mixed‐effect models: the npde add‐on package for R. Comput Methods Programs Biomed. 2008;90(2):154‐166. [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization . Growth reference 5‐19 years. In: World Helath Organization, 2019.
- 41. WHO . Growth reference 5‐19 years In: World Health Organization, 2006.
- 42. Akaike H. Information Theory and an Extension of the Maximum Likelihood Principle. In: FC BNP , ed. Proceedings of the 2nd International Symposium on Information Theory. Budapest: Akademiai Kiado; 1973:267‐281. [Google Scholar]
- 43. Douglas Bates MM, Bolker B, Walker S. Fitting linear mixed‐effects models using lme4. J Stat Softw. 2015;67:1‐48. [Google Scholar]
- 44. Sherwin CM, Saldana SN, Bies RR, Aman MG, Vinks AA. Population pharmacokinetic modeling of risperidone and 9‐hydroxyrisperidone to estimate CYP2D6 subpopulations in children and adolescents. Ther Drug Monit. 2012;34(5):535‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thyssen A, Vermeulen A, Fuseau E, Fabre MA, Mannaert E. Population pharmacokinetics of oral risperidone in children, adolescents and adults with psychiatric disorders. Clin Pharmacokinet. 2010;49(7):465‐478. [DOI] [PubMed] [Google Scholar]
- 46. Pozzi M, Cattaneo D, Baldelli S, et al. Therapeutic drug monitoring of second‐generation antipsychotics in pediatric patients: an observational study in real‐life settings. Eur J Clin Pharmacol. 2016;72(3):285‐293. [DOI] [PubMed] [Google Scholar]
- 47. Aichhorn W, Marksteiner J, Walch T, et al. Age and gender effects on olanzapine and risperidone plasma concentrations in children and adolescents. J Child Adolesc Psychopharmacol. 2007;17(5):665‐674. [DOI] [PubMed] [Google Scholar]
- 48. Calarge CA, del Miller D. Predictors of risperidone and 9‐hydroxyrisperidone serum concentration in children and adolescents. J Child Adolesc Psychopharmacol. 2011;21(2):163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vanwong N, Ngamsamut N, Medhasi S, et al. Impact of CYP2D6 polymorphism on steady‐state plasma levels of risperidone and 9‐Hydroxyrisperidone in Thai children and adolescents with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(2):185‐191. [DOI] [PubMed] [Google Scholar]
- 50. Gunes A, Spina E, Dahl ML, Scordo MG. ABCB1 polymorphisms influence steady‐state plasma levels of 9‐hydroxyrisperidone and risperidone active moiety. Ther Drug Monit. 2008;30(5):628‐633. [DOI] [PubMed] [Google Scholar]
- 51. Ragia G, Dahl ML, Manolopoulos VG. Influence of CYP3A5 polymorphism on the pharmacokinetics of psychiatric drugs. Curr Drug Metab. 2016;17(3):227‐236. [DOI] [PubMed] [Google Scholar]
- 52. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51:e1. [DOI] [PubMed] [Google Scholar]
- 53. de Velde F, Mouton JW, de Winter BCM, van Gelder T, Koch BCP. Clinical applications of population pharmacokinetic models of antibiotics: challenges and perspectives. Pharmacol Res. 2018;134:280‐288. [DOI] [PubMed] [Google Scholar]
- 54. Dos Santos‐Junior A, Tamascia ML, Lorenzetti R, et al. Serum concentration of risperidone and adverse effects in children and adolescents. J Child Adolesc Psychopharmacol. 2017;27(2):211‐212. [DOI] [PubMed] [Google Scholar]
- 55. Mauri MC, Paletta S, Di Pace C, et al. Clinical pharmacokinetics of atypical antipsychotics: an update. Clin Pharmacokinet. 2018;57(12):1493‐1528. [DOI] [PubMed] [Google Scholar]
- 56. Eleveld DJ, Proost JH, Absalom AR, Struys MM. Obesity and allometric scaling of pharmacokinetics. Clin Pharmacokinet. 2011;50(11):751‐753. discussion 55‐6 [DOI] [PubMed] [Google Scholar]
- 57. Vandenberghe F, Guidi M, Choong E, et al. Genetics‐based population pharmacokinetics and pharmacodynamics of risperidone in a psychiatric cohort. Clin Pharmacokinet. 2015;54(12):1259‐1272. [DOI] [PubMed] [Google Scholar]
- 58. Spina E, Avenoso A, Facciola G, et al. Relationship between plasma risperidone and 9‐hydroxyrisperidone concentrations and clinical response in patients with schizophrenia. Psychopharmacology (Berl). 2001;153(2):238‐243. [DOI] [PubMed] [Google Scholar]
- 59. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta‐analysis. Pediatrics. 2016;137(Suppl 2):S124‐S135. [DOI] [PubMed] [Google Scholar]
- 60. Hiemke C. Concentration‐effect relationships of psychoactive drugs and the problem to calculate therapeutic reference ranges. Ther Drug Monit. 2019;41(2):174‐179. [DOI] [PubMed] [Google Scholar]
- 61. Correia CT, Almeida JP, Santos PE, et al. Pharmacogenetics of risperidone therapy in autism: association analysis of eight candidate genes with drug efficacy and adverse drug reactions. Pharmacogenomics J. 2010;10(5):418‐430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1 Residual variability of the final model and bootstrap analysis
Supporting Information Table S2 Association between trough concentrations of risperidone + 9‐hydroxyrisperidone and secondary side‐effect outcomes
Supporting Information Figure S1a Goodness‐of‐fit plot of final model. Measured concentrations versus individual predictions for risperidone
Supporting Information Figure S1b (b) Goodness‐of‐fit‐plot of final model. Measured concentrations versus individual predictions for 9‐hydroxy‐risperidone
Supporting Information Figure S2 Visual predictive check (VPC). Left, VPC of risperidone; right, VPC of 9‐hydroxyrisperidone
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


