Abstract
The existence of presynaptic, release‐regulating NMDA receptors in the CNS has been long matter of discussion. Most of the reviews dedicated to support this conclusion have preferentially focussed on the results from electrophysiological studies, paying little or no attention to the data obtained with purified synaptosomes, even though this experimental approach has been recognized as providing reliable information concerning the presence and the role of presynaptic release‐regulating receptors in the CNS. To fill the gap, this review is dedicated to summarising the results from studies with synaptosomes published during the last 40 years, which support the existence of auto and hetero NMDA receptors controlling the release of transmitters such as glutamate, GABA, dopamine, noradrenaline, 5‐HT, acetylcholine and peptides, in the CNS of mammals. The review also deals with the results from immunochemical studies in isolated nerve endings that confirm the functional observations.
Keywords: GluN subunit, glutamate, glycine, presynaptic NMDA receptor, synaptosomes, transmitter release

Abbreviations
- [3H]DA
[3H]dopamine
- 5,7‐Cl‐Kyna
5‐7‐dichloro kynurenic acid
- 7‐Cl‐Kyna
7‐chloro kynurenic acid
- CCK‐LI
cholecystokinin
- CP‐101,606
(1S,2S)‐1‐(4‐hydroxy‐phenyl)‐2‐(4‐hydroxy‐4‐phenylpiperidino)‐1‐propanol
- CPP
3‐(2‐carboxypiperazin‐4‐yl)propyl‐1‐phosphonic acid
- D‐AP5
D‐2‐amino‐5‐phosphonovaleric acid
- D‐AP7
D‐(−)‐2‐amino‐7‐phosphonoheptanoic acid
- DL‐tBOA
DL‐threo‐b‐Benzyloxyaspartate
- GluN
glutamate NMDA
- GlyT1
glycine transporter type 1
- GS91755
cis‐4‐[phosphomethyl]‐piperidine‐2‐carboxylic acid
- HA‐966
(±)3‐amino‐1‐hydroxy‐pyrrolidin‐2‐one
- mGlu receptor
metabotropic glutamate receptor
- NAc
nucleus accumbens
- NFPS
(N‐[3‐(4¢‐fluorophenyl)‐3‐(4‐phenylphenoxy)propyl]) sarcosine hydrochloride
- PKC
protein kinase C
- RO25‐6981
(αR,βS)‐α‐(4‐hydroxyphenyl)‐β‐methyl‐4‐(phenylmethyl)‐1‐piperidinepropanol maleate
- SRIF‐LI
somatostatin
- TTx
tetrodotoxin
1. INTRODUCTION
The amount of transmitter released from nerve terminals does not exclusively depend on the efficiency of the mechanism of exocytosis but also on the activation by ambient ligands of receptors located presynaptically on nerve terminals (Langer, 1997, 2008; Raiteri, 2006). When focussing on the role of glutamate as a presynaptic modulator of transmitter release, it emerges that both metabotropic (mGlu) and ionotropic glutamate receptors mediate this function. However, although the presynaptic, release‐regulating mGlu receptors have been largely described in term of functions and pharmacological profiles, the existence and the role of presynaptic ionotropic glutamate receptors, particularly the NMDA receptor subtypes, still remains matter of debate. First proposed as almost pure postsynaptic receptors, evidence was then provided supporting their presence also at the presynaptic level.
The first report suggesting the existence of presynaptic release‐regulating NMDA receptors appeared in the late '70s, when Spencer (1976) showed that ligands of these receptors can modify transmitter outflow. More than 40 years later, the knowledge of these receptors has greatly increased, and there is, nowadays, a consensus on their existence and role in synaptic communication.
In recent years, several reviews were dedicated to the NMDA receptors located presynaptically and to their role as release‐regulating receptors, but almost all of them focussed on the electrophysiological observations supporting this conclusion, paying few attentions, if ever, to the data from isolated nerve endings (synaptosomes; Bouvier et al., 2018; MacDermott et al., 1999; Rodríguez‐Moreno et al., 2010). To fill the gap, this review emphasizes the functional observations obtained with this tissue preparation that, starting from the late '80s, support the existence of release‐regulating presynaptic NMDA receptors in the CNS. I apologize for omissions in the coverage of the existing literature.
2. EXPERIMENTAL APPROACHES TO DETECT PRESYNAPTIC RELEASE‐REGULATING NMDA RECEPTORS
As already mentioned, most of the articles seeking to prove the existence of the presynaptic NMDA receptors deal with electrophysiological studies measuring the effects of NMDA antagonists on the spontaneous and/or the miniature excitatory/inhibitory postsynaptic currents in slices (Banerjee et al., 2016; Corlew et al., 2008; Duguid, 2013; Pinheiro & Mulle, 2008). These studies provided evidence of the presence of presynaptic release‐regulating NMDA receptors at defined subsets of synapses, mainly located in the cortex and the hippocampus (Buchanan et al., 2012; Crozier et al., 2007; Froemke et al., 2005; Larsen et al., 2014; McGuinness et al., 2010). The main shortcoming of this experimental approach, however, is that the NMDA‐mediated responses also could involve (as related to presynaptic events) the signalling of postsynaptic NMDA receptors. The results are therefore correctly interpretable only if the postsynaptic receptors are blocked in advance (Berretta & Jones, 1996; Corlew et al., 2007; Brasier & Feldman, 2008; Woodhall et al., 2001; but see Rodríguez‐Moreno & Paulsen, 2008).
Apart from slice preparations, preparations of purified nerve endings (synaptosomes), isolated from selected regions of the CNS were also used to confirm the existence of presynaptic NMDA receptors controlling transmitter exocytosis. The first findings from these experiments date from the late 80's/early 90's (Desce et al., 1992; Fink et al., 1989; Pittaluga & Raiteri, 1990). In general, in these studies, dynamic conditions (e.g., the up‐down superfusion of a thin layer of synaptosomes; Raiteri et al., 1974) were applied to monitor the transmitter release from synaptosomes in the absence or in the presence of receptor ligands. These experimental conditions largely avoid the effects of the extracellular biophase, decreasing some of the disadvantages that mainly affect electrophysiological recordings (see for further technical information Raiteri & Raiteri, 2000; Pittaluga, 2016, 2019; Figure 1). This approach also permits the differentiation of spontaneous from depolarization‐evoked, release of transmitters, a discrimination that cannot be easily achieved with other approaches. However, synaptosomes also suffer from some technical limitations mainly due to the heterogeneity of the preparation. The synaptosomal suspension isolated from a selected CNS region consists of particles isolated from synaptic processes of different neuronal subpopulations that innervate the structure. Although monitoring the release of a selected transmitter allows the isolation, from a functional point of view, of a specific synaptosomal subpopulation, this heterogeneity limits (e.g., when the synaptosomal subpopulation represents a low percentage of the entire population) the use of immunochemical approaches to confirm the presence of the receptor proteins in a defined synaptosomal subpopulation. Actually, as for electrophysiological studies, the functional results from superfused synaptosomes should be accompanied by immunochemical results to confirm the presence of the receptor proteins in the superfused particles. Unfortunately, this is sometimes difficult, if even impossible, not only when monitoring the release of transmitter from a synaptosomal subpopulation poorly expressed within the entire synaptosomal suspension (as above introduced, but see Paragraph 4.2) but also when trying to purify synaptosomes from certain physically small‐sized, subregions of the CNs, such as the L3/L4 layer of the somatosensory motor cortex).
FIGURE 1.

The up‐down superfusion of a thin layer of synaptosomes as first proposed by Maurizio Raiteri and colleagues in 1974. (a) The continuous flowing of the superfusion medium assures the quick removal of any endogenous substance released by the superfused particles, then minimizing the shortcomings due to the presence of the biophase (which has major effects on electrophysiological recordings in slices). (b) In this dynamic condition, presynaptic release‐regulating receptors are activated by receptor ligands exogenously added to the superfusion medium. By acting at the respective binding sites (e.g., on the respective GluN subunits), the orthosteric agonists added to the superfusion medium (e.g., glutamate and glycine for the NMDA receptors) influence the molecular events controlling vesicular exocytosis, then causing significant changes to transmitter overflow that could be quantified and correlated with the activation of the presynaptic receptors. Notably, the technique also permits distinguishing between the spontaneous and the depolarization‐evoked release of a selected transmitter, a discrimination that cannot be easily achieved in electrophysiological studies (see for technical information Raiteri & Raiteri, 2000)
3. PRESYNAPTIC NMDA HETERORECEPTORS CONTROLLING DOPAMINE RELEASE
3.1. Making short a long story: An historical perspective
The existence of presynaptic NMDA receptors controlling dopamine release emerged while dissecting the cortical glutamatergic projections to the dopaminergic striatal neurons. The iontophoretic application of glutamate to striatal neurons caused an excitatory response that was suppressed by l‐glutamate diethylester, a compound with generalized antagonistic activity towards ionotropic glutamate receptors (Spencer, 1976). Soon later, Glowinski and collaborators (Giorguieff et al., 1977) and Roberts and Sharif (1978) demonstrated that glutamate stimulated [3H]dopamine release in rat striatal slices through a tetrodotoxin (TTx)‐resistant mechanism, suggesting that the cortico‐striatal afferent terminals impinge directly onto the dopaminergic ones. Comparable results were obtained in slices containing dopaminergic nerve terminals (Cheramy et al., 1986; Marien et al., 1983; Snell & Johnson, 1986) or in dendritic processes of cultured cells (e.g., those isolated from the substantia nigra; Cheramy et al., 1981; Marien et al., 1983). Again, the releasing activity was TTx insensitive, indicating that voltage‐sensitive Na+ channels were not involved. As a whole, the data were interpreted as indicating the existence of release‐regulating glutamatergic receptors, although they did not allow these receptors to be located definitely on nerve terminals or on dendritic processes.
Trying to gain information on the pharmacological profile of the glutamate receptor(s), Roberts and Anderson (1979) (but see also Marien et al., 1983) found that NMDA mimicked glutamate in controlling the release of preloaded [3H]dopamine in striatal slices. This observation led to propose the existence of NMDA heteroreceptors, presynaptically located on striatal dopaminergic processes. A few years later, this hypothesis was definitively proved by Glowinski's group (Desce et al., 1992; Krebs et al., 1991). The authors demonstrated that the release of newly synthetized [3H]dopamine from striatal synaptosomes superfused with a medium lacking Mg2+ ions (to favour activation of NMDA receptors) was significantly augmented when synaptosomes were exposed to micromolar NMDA in the presence of saturating glycine. Glycine potentiated the releasing activity in a strychnine‐insensitive manner, and the releasing activity was TTx resistant but prevented by dizocilpine or physiological amounts of magnesium ions in the biophase ([Mg2+]out). Comparable conclusions were described by Johnson and Jeng (1991) and by Wang (1991), confirming that striatal dopaminergic nerve endings are endowed with Mg2+‐sensitive, NMDA heteroreceptors controlling dopamine exocytosis (Tables 1 and 3). These studies also demonstrated the relevance of the sensitivity to the Mg2+ ions as a pharmacological tool, to associate functional responses to the activation of NMDA receptors. Indeed, sensitivity to this divalent cation and to dizocilpine (MK‐801) represents a major discriminating feature of the pharmacological properties of the NMDA receptors, first introduced by Meguro et al. (1992) and Mori et al. (1992).
TABLE 1.
Effects of NMDA, glycine or NMDA+glycine on the spontaneous and the depolarization‐evoked transmitter release
| Spontaneous release | Depolarization‐evoked release | |||||
|---|---|---|---|---|---|---|
| NMDA | Glycine | NMDA/glycine | NMDA | Glycine | NMDA/glycine | |
| Dopamine | ↑ | n.e. | ↑ ↑ | n.d. | n.d. | n.d. |
| Noradrenaline | ↑ | n.e. | ↑ ↑ | n.d. | n.d. | n.d. |
| Acetylcholine | n.e. | n.e. | n.e. | ↑ | n.e. | n.d. |
| GABA | n.d. | n.d. | ↑ | n.d. | n.d. | n.d. |
| CCK‐LI/SRIF‐LI | n.e. | n.e. | n.e. | ↑ | ↑ | ↑; n.a. |
| Glutamate | ↑ | n.e. | ↑ ↑ | n.d. | n.d. | n.d. |
Note. ↑/↑↑, increased transmitter release; n.d., not determined; n.e., no effect; n.a., not additive.
TABLE 3.
Effects of ions, pH and receptor antagonists on the NMDA auto and heteroreceptors
| Mg2+ ions | Dizocilpine | D‐AP5CGS19755 | 7‐Cl‐Kyna | Zn2+ ions | (RS)‐CPP | Ifenprodil | pH 6.0 | pH 8.0 | |
|---|---|---|---|---|---|---|---|---|---|
| NMDA heteroreceptors | |||||||||
| Dopamine (striatum) | ↓ | ↓ | ↓ | ↓. | n.e. | n.d. | ↓ | n.e. | n.e. |
| Dopamine (NAc) | ↓ | ↓ | ↓ | ↓. | n.e. | n.e. | ↓ | n.e. | n.e. |
| Noradrenaline | ↓ | ↓ | ↓ | ↓. | n.e. | n.d. | ↓ | ↓ | ↑ |
| Acetylcholine | n.e. | ↓ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| GABA | ↓ | ↓ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| CCK‐LI/SRIF‐LI | n.e. | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| NMDA autoreceptors | |||||||||
| Hippocampus | n.e. | ↓ | ↓ | ↓ | ↓ | n.d. | ↓ | ↓ | ↑ |
| Nucleus accumbens | ↓ | ↓ | n.d. | ↓ | n.d. | ↓ | n.e. | n.d. | n.d. |
| Cortex | n.e. | ↓ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Spinal cord | ↓ | ↓ | n.d. | n.d. | ↓ | n.d. | ↓ | ↓ | n.d. |
Note. ↓, decreased transmitter release; n.d., not determined; n.e., no effect.
3.2. Distribution in the CNS
Presynaptic NMDA heteroreceptors controlling dopamine release are not limited to striatal synaptosomal preparations (Chéramy, Godeheu, L'Hirondel, & Glowinski, 1996; Desce et al., 1994; Keita et al., 1997; L'hirondel et al., 1999; Livingstone et al., 2010; Mennini et al., 1997; Pittaluga et al., 2001; Risso et al., 2004; Salamone et al., 2014; Whittaker et al., 2008), but also can be found in other CNS regions. In the hippocampus, a significant increase of the spontaneous release of [3H]dopamine was observed when hippocampal synaptosomes were exposed to NMDA/glycine in the absence of Mg2+ ions (Malva et al., 1994). Again, the marked sensitivity to dizocilpine suggested the involvement of presynaptic release‐regulating NMDA receptors. Similarly, NMDA concentration dependently released [3H]dopamine from synaptosomes isolated from the prefrontal cortex of adult rats, in a Mg2+ and dizocilpine‐dependent fashion, proving the involvement of the NMDA receptor subtype (Grilli et al., 2009). Notably, the existence of presynaptic NMDA receptors on dopaminergic striatal synaptosomes was also confirmed with non‐functional approaches. In 2014, Salamone and colleagues described the immunocytochemical co‐localization of the dopamine transporter (DAT; used as a selective marker of dopaminergic terminals), of the GluN1 receptor subunit (to highlight the presence of NMDA receptors) and of synaptophysin (used as marker of presynaptic particles) in synaptosomes isolated from the nucleus accumbens (NAc) of adult rats.
3.3. The pharmacological profile
Over the years, selective NMDA receptor agonists/antagonists acting at the different GluN subunits became available and were used to define the subunit composition of the NMDA receptors. These compounds allowed the characterization of the presynaptic NMDA receptors controlling dopamine release, and also allowed the association of particular GluN subunits with the presynaptic receptors.
The involvement of the GluN1 subunit was first proposed based on the high sensitivity of the receptor to ambient glycine. Low nanomolar glycine, unable by itself to elicit dopamine release, was required to permit the response mediated by NMDA, while increasing its concentration to micromolar level almost doubled the NMDA‐induced signal (Krebs et al., 1991; Pittaluga et al., 2001). The allosteric activity of glycine was mimicked, although less efficiently, by d‐serine (Table 2), it was insensitive to strychnine but prevented by selective antagonist(s) at the strychnine‐insensitive, glycine‐sensitive allosteric binding site of the NMDA receptors (Table 3), including 7‐chlorokynurenic acid (7‐Cl‐Kyna), 5,7‐dichlorothiokynurenic acid (5,7‐Cl‐Kyna) and (±)3‐amino‐1‐hydroxy‐pyrrolidin‐2‐one (HA‐966; Krebs et al., 1991; Mennini et al., 1997; Pittaluga et al., 2001). Notably, 7‐Cl‐Kyna also prevented the release elicited by NMDA alone (in the absence of exogenously added glycine, Krebs et al., 1991), stressing a major role for ambient, contaminating glycine in disclosing the NMDA‐mediated control of dopamine release.
TABLE 2.
Effects of glutamatergic and glycinergic agonists on the presynaptic release‐regulating NMDA receptors
| NMDA | Quinolinic acid | Glycine | d‐serine | |
|---|---|---|---|---|
| Dopamine | Full agonist | n.e. | Co‐agonist | Co‐agonist |
| Noradrenaline | Full agonist | Full agonist | Co‐agonist | Co‐agonist |
| Acetylcholine | Full agonist | n.d. | n.e. | n.d. |
| GABA | Full agonist | n.d. | Co‐agonist | n.d. |
| CCK‐LI/SRIF‐LI | Full agonist | n.d. | Full agonist | Full agonist |
| Glutamate | Full agonist | Full agonist | Co‐agonist | Partial agonist |
Note. n.d., not determined; n.e., no effect.
GluN1 subunits exist in eight different splice variants, depending on the presence or the absence of the extracellular N1 cassette and of the intraterminal C1 and C2 sequences (Zukin & Bennett, 1995). The N1 sequence controls the pH sensitivity of the NMDA receptor. It blocks the pH sensor, limiting the sensitivity of the receptor to ambient protons. Studies dedicated to monitor the pH sensitivity of the NMDA receptors controlling dopamine exocytosis in striatal synaptosomes showed that the releasing activity was unmodified when the pH of the external milieu was either reduced to 6.6 or augmented to 8.0 (Pittaluga et al., 2001; Table 3). The observations seemed most consistent with the involvement of a GluN1 subunit containing the N1 sequence. As far as the C1 and C2 sequences are concerned, these cassettes contain protein kinase C (PKC)‐sensitive phosphorylation sites. Unfortunately, selective ligands for these sites are not available, and PKC inhibitors cannot be used to predict their presence, because PKC‐dependent phosphorylation also occurs in the GluN2 subunits. It was therefore generically proposed that N1‐containing GluN1 subunits (the GluN1+xx one; see, for the GluN1 subunit classification, Zukin & Bennett, 1995) were involved in the expression of these receptors, at least in striatal nerve endings (Pittaluga et al., 2001).
Besides the GluN1 subunits, NMDA heteroreceptors on dopaminergic nerve endings also consist of GluN2 subunits, as suggested by the finding that NMDA is pivotal to trigger the releasing activity. Accordingly, broad spectrum GluN2 antagonists (Table 3) including D‐2‐amino‐5‐phosphonovaleric acid (D‐AP5) and cis‐4‐[phosphomethyl]‐piperidine‐2‐carboxylic acid (CGS19755, selfotel) prevented release (Chéramy et al., 1996; Mennini et al., 1997; Pittaluga et al., 2001). The NMDA receptors controlling dopamine release are insensitive to quinolinic acid and only slightly affected by kynurenic acid (Pittaluga et al., 2001). Furthermore, they were unaffected by nanomolar zinc ions (Paoletti & Neyton, 2007) or (RS)‐3‐(2‐carboxypiperazin‐4‐yl)propyl‐1‐phosphonic acid [(RS)‐CPP], two GluN2A‐preferring NMDA antagonists, but they were sensitive to ligands acting at the Glu2B subunits (viz., infenprodil, (1S,2S)‐1‐(4‐hydroxy‐phenyl)‐2‐(4‐hydroxy‐4‐phenylpiperidino)‐1‐propanol; CP‐101,606, traxoprodil) and (αR,βS)‐α‐(4‐hydroxyphenyl)‐β‐methyl‐4‐(phenylmethyl)‐1‐piperidinepropanol maleate, Ro 25‐6981; Mennini et al., 1997; Pittaluga et al., 2001; Salamone et al., 2014; Table 3). As for the GluN2C and GluN2D subunits, the lack of selective ligands for these two subunits limited at that time the study of their participation in the NMDA receptor assembly. The involvement of the GluN2C, however, seems unlike considering its almost negligible expression in several CNS regions, including the striatum (Harney et al., 2008; Misra et al., 2000). However, the GluN2D subunit is developmentally regulated in the striatum, becoming almost undetectable at 49 days postnatally. Furthermore, if present, it preferentially locates extrasynaptically (in axonal and dendritic processes, far from the synaptic active zone), being absent from synapses in striatal neurons (Dunah et al., 1996), indirectly ruling out its participation in the presynaptic NMDA heteroreceptor.
Whether the NMDA heteroreceptors controlling dopamine release also involve the GluN3 subunit was not investigated, but the available data allow some speculations. Our knowledge of the GluN3 subunit has greatly increased in recent years and, today, we know that this subunit also exists presynaptically (Larsen et al., 2011; Musante et al., 2011; Olivero, Vergassola, Cisani, Usai, & Pittaluga, 2019). The GluN3‐containing NMDA receptors were proposed to preferentially consist of GluN1/GluN3 and of GluN1/GluN2/ GluN3 assemblies. The former assembly is activated by glycine alone, whereas the latter also requires glutamate to exert its function and it is potentiated by HA‐966, a mimic of glycine (Al‐Hallaq et al., 2002; Perez‐Otano et al., 2001; Pérez‐Otaño et al., 2016; Smothers & Woodward, 2007). Taking into consideration that NMDA receptor‐evoked release of dopamine involves a glutamate‐sensitive receptor whose releasing activity is antagonized by HA‐966 (Mennini et al., 1997), it seems reasonable to exclude the participation of the GluN3 subunit in the NMDA heteroreceptors.
3.4. The sensitivity to Mg2+ ions
The NMDA receptors on dopaminergic terminals are highly sensitive to the presence of Mg2+ in the external medium, because physiological concentrations of the divalent cation blocks their releasing activity (Krebs et al., 1991; Risso et al., 2004). The question therefore arises whether and how these receptors could modulate dopaminergic transmission in physiological conditions, for example, in the presence of high [Mg2+]out. It is known that the concomitant application of a depolarizing stimulus, such as that elicited by co‐localized non‐NMDA receptors (Desce et al., 1992) as well as other non‐glutamatergic ionotropic receptors (viz., nicotinic receptors; Risso et al., 2004; Marchi & Grilli, 2010), allows the ionic block to be overcome, restoring the releasing activity. Several reviews already focused on these aspects will be not further discussed in this review (see Marchi & Grilli, 2010; Marchi et al., 2015).
4. PRESYNAPTIC NMDA HETERORECEPTORS CONTROLLING NORADRENALINE RELEASE
4.1. A non‐synaptic release‐regulating NMDA receptor
By the end of the 1980s, two groups provided functional evidence of the existence of presynaptic NMDA heteroreceptors controlling noradrenaline release from hippocampal nerve terminals (Fink et al., 1989; Pittaluga & Raiteri, 1990). These results were soon later confirmed by other groups (Malva et al., 1994; Wang et al., 1992), which also extended the study to other CNS regions, including the olfactory bulb (Wang et al., 1992), the spinal cord (Klarica et al., 1996; Nankai et al., 1998; Sundström et al., 1998 and the cortex of rodents (mice and rats; Fink et al., 1989; Wang et al., 1992; Pittaluga et al., 1996; Gemignani et al., 2004; Longordo et al., 2006) and humans (Pittaluga & Raiteri, 1994). Because noradrenergic processes from the locus coeruleus do not make synaptic contact in the regions they innervate, these presynaptic NMDA receptors would be better defined as extrasynaptic receptors (Langer, 2008) that sense glutamate reaching the noradrenergic nerve endings and varicosities by the mechanisms of ‘volume diffusion’ (Pittaluga, 2016; Vizi et al., 2004) to modulate noradrenaline exocytosis.
4.2. The subunit composition and the pharmacological profile
At a first glance, the pharmacological profile of the NMDA receptors controlling noradrenaline release largely resembles that of the NMDA receptors on dopaminergic terminals (see Table 1, 2, 3). In both cases,
the releasing activity is detectable in basal condition by omitting external Mg2+ ions (Fink et al., 1989; Pittaluga et al., 2000; Pittaluga & Raiteri, 1990, 1992a);
unavoidable contamination of glycine in the experimental solutions allows receptor activation (low nM; Longordo et al., 2006; Pittaluga & Raiteri, 1990; Raiteri et al., 1992, 1993, 2000), whereas higher, micromolar, concentrations reinforce the NMDA‐mediated releasing activity (Pittaluga et al., 1993, 1996; Pittaluga & Raiteri, 1992b);
channel blockers including dizocilpine and memantine prevent, although to a different extent, the releasing activity (Pittaluga et al., 1996; Pittaluga & Raiteri, 1994);
the analysis of the subunit composition suggested the involvement of both the GluN1 and GluN2 receptor subunits (Malva et al., 1994; Pittaluga et al., 2001).
The NMDA receptors on noradrenergic terminals, however, differ from those in dopaminergic nerve endings in their GluN1 composition. 7‐Cl‐Kyna and HA‐966 behaved as receptor antagonists, whereas d‐serine and d‐cycloserine potentiated, although less efficiently than glycine, the NMDA‐induced effect. (Longordo et al., 2006; Pittaluga & Raiteri, 1990, 1992b; Table 2). The marked sensitivity to glycine led Wang and colleagues to hypothesize that the presynaptic NMDA receptors on noradrenergic terminals could consist of an oligomeric complex of GluN1 subunits (Wang et al., 1992; Wang & Thukral, 1996). This hypothesis, however, contrasted with the agonist‐like activity of glutamate and glutamate agonist(s) reported by other groups (Fink et al., 1989; Malva et al., 1994; Pittaluga & Raiteri, 1990). Conflicting results were also obtained when studying the proton sensitivity of these NMDA receptors. Wang provided evidence suggesting a low sensitivity to external protons (Wang et al., 1992). However, we found a marked dependency on the external pH, the releasing activity of these heteroreceptors being reduced at pH ≅ 6.6, but significantly potentiated at pH ≅ 8.0 (Pittaluga et al., 2001), most consistent with the presence of GluN1 subunits bearing the N1 cassette (the GluN1−xx, Zukin & Bennett, 1995), which makes the receptors in noradrenergic terminals different from that controlling dopamine exocytosis (Table 3).
As far as the GluN2 subunit is concerned, the participation of this subunit was postulated because either NMDA or quinolinic acid elicits a dizocilpine‐sensitive noradrenaline release that was prevented by broad spectrum GluN2 antagonists (e.g., kynurenic acid, D‐AP5 and CGS19755). To note, selective GluN2B antagonists (e.g., ifenprodil and CP‐101,606) mimicked D‐AP5, whereas nanomolar Zn2+ ions, which selectively block GluN2A subunits, did not, suggesting that GluN2B rather than the GluN2A subunits are most likely to be involved in the receptor composition (Table 3; Malva et al., 1994; Pittaluga & Raiteri, 1992b; Pittaluga et al., 2001). The proposed composition in GluN subunits, however, cannot be confirmed with immunochemical studies because of the low percentage (less than the 1%) of the noradrenergic terminals in the hippocampal synaptosomal preparation. Furthermore, there are no published data concerning the possible participation of GluN2C and D subunits.
An unexpected finding was that the potency and the efficiency of GluN2B antagonists and channel blockers as well, mainly depend on the mode of activation of the NMDA heteroreceptors. As already discussed for the NMDA receptors on dopaminergic terminals (paragraph 3.4), the Mg2+ blockade of the NMDA receptors can be overcome by applying a concomitant depolarizing stimulus that permit the opening of the voltage‐dependent ion channel associated with the NMDA receptors. The local depolarization could be achieved by activating AMPA receptors co‐localized with NMDA receptors. Such AMPA‐induced activation of NMDA receptors, however, triggers conformational changes to the NMDA receptors in noradrenergic terminals that are expressed as increased affinity of GluN2 antagonists (e.g., D‐AP5) and of dizocilpine at the receptors (Luccini, Musante, Neri, Brambilla Bas et al., 2007; Pittaluga & Raiteri, 1992a, 1992b; Raiteri et al., 1992). These pharmacodynamic adaptations were not observed in dopaminergic synaptosomes, further strengthening the conclusion that the presynaptic NMDA receptors in noradrenergic and dopaminergic terminals have different pharmacological and functional profiles. The detailed mechanism(s) underlying these conformational changes remains so far unexplored, although modifications in the GluN compositions as well as in the phosphorylation of the GluN subunits could play a role (Dorville et al., 1992; Zuo et al., 1993).
5. PRESYNAPTIC NMDA HETERORECEPTORS CONTROLLING 5‐HT RELEASE
The existence of presynaptic release‐regulating NMDA receptors in 5‐hydroxytryptaminergic nerve endings has been poorly investigated. In 1995, Manfred Gothert and colleagues demonstrated that in cortical slices pre‐incubated with [3H]5‐HT and superfused with a solution from which Mg2+ was removed, NMDA elicited the release of radioactive tracer in a concentration‐dependent fashion (Fink et al., 1995). The NMDA‐mediated releasing activity was prevented by the (E)‐2‐amino‐4‐methyl‐5‐phosphonopent‐3‐enoic acid (CGP37894), an antagonist acting at the glutamate GluN2 subunit, by 5,7‐Cl‐Kyna and by dizocilpine. Furthermore, it was largely insensitive to TTx, suggesting that it was located in nerve endings. Finally, omission of external Ca2+ ions almost totally prevented the releasing activity, compatible with the conclusion that the NMDA releasing activity relies on the influx of external Ca2+ ions (the authors speculated through a voltage‐dependent associated ionic channel located near the glutamate receptor). These results led the authors to propose the existence of presynaptic release‐regulating NMDA receptors in cortical 5‐hydroxytryptaminergic nerve endings. Because the NMDA‐induced releasing activity was largely prevented by ifenprodil and eliprodil, the authors also hypothesized that GluN1/GluN2B receptor assemblies mainly account for these NMDA receptors (Fink et al., 1995, 1996). To the best of my knowledge, however, other results supporting the existence of NMDA controlling 5‐HT release from synaptosomes have not been published.
6. PRESYNAPTIC NMDA HETERORECEPTORS CONTROLLING GABA RELEASE
The existence of presynaptic release‐regulating NMDA receptors in GABAergic axon terminals was first inferred with electrophysiological studies. Bath application of NMDA increased the frequency of spontaneous inhibitory synaptic currents (Farrant & Cull‐Candy, 1991; Llano et al., 1991) and this effect was prevented by D‐AP5, but it was resistant to TTx (Glitsch & Marty, 1999). The conclusion was also supported by results obtained in cultured cells from the retina and the cortex, showing that NMDA elicites a Na+‐dependent, Ca2+‐insensitive release of [3H]GABA release from a cytosolic intracellular pool (Belhage et al., 1993; Duarte et al., 1993). The releasing activity was proposed to rely on Na+ ions flowing through the ionic channel associated with the NMDA receptor, which might have caused a local depolarization of plasma membranes, thus favouring the reversal of the GABA transporters and the release of GABA from the cytosolic pool (Levi & Raiteri, 1993).
Soon after, it was proposed that presynaptic NMDA receptors can enhance GABA release at cerebellar‐interneurons Purkinje cells synapses (Duguid & Smart, 2004). The activation of these presynaptic NMDA receptors elicited a long‐lasting increase in GABA release from stellate neurons that enhanced the frequency of spontaneous inhibitory postsynaptic currents through a mechanism involving the Ca2+‐induced Ca2+‐ release from intra‐terminal stores (Rossi et al., 2012; see Moreau & Kullmann, 2013). NMDA receptors were also proposed to have a role in the facilitation of GABA release between multipolar interneurons and pyramidal cells (De‐May & Ali, 2013).
As far as the results with synaptosomes are concerned, the existence and the role of the NMDA receptors controlling GABA release is still a matter of debate. In 1993, we could not observe an NMDA‐mediated releasing activity in cortical synaptosomes (Bonanno et al., 1993) which, on the contrary, was described by Breukel and colleagues in synaptosomes isolated from the hippocampus (Breukel et al., 1998). Exposure of hippocampal synaptosomes in superfusion to NMDA in the presence of saturating glycine but in the absence of external Mg2+ significantly increased the spontaneous release of endogenous GABA in a dizocilpine‐sensitive manner (Tables 1, 2, 3). The releasing activity was independent on external Ca2+ ions but dependent on the reversal of the amino acid transporter. Further studies are needed to investigate the cellular events accounting for the release of the inhibitory amino acid elicited by the NMDA receptors.
7. PRESYNAPTIC NMDA HETERORECEPTORS CONTROLLING ACETYLCHOLINE RELEASE
The existence of presynaptic release‐regulating NMDA heteroreceptors controlling acetylcholine release first originated from studies in slices (Lehmann et al., 1983; Lehmann & Scatton, 1982; Ransom & Deschenes, 1989; Scatton & Lehmann, 1982). Evidence was then provided showing that presynaptic glutamate receptors belonging to the NMDA receptor subtype are present at the developing neuromuscular synapses of Xenopus, where they promote the release of acetylcholine (Fu et al., 1995). Soon after these findings, Morari et al. (1998) proved the existence of presynaptic NMDARs controlling the release of [3H]acetylcholine from striatal synaptosomes. The authors demonstrated that NMDA did not affect the basal release of [3H]acetylcholine but significantly potentiated the overflow of ACh elicited by a mild depolarizing stimulus (Table 1). Such NMDA‐induced effects occurred in a concentration‐dependent manner, and they were blocked by low micromolar concentrations of dizocilpine, despite the presence of Mg2+ ions (Table 3). The NMDA‐mediated facilitation of [3H]acetylcholine exocytosis was controlled by co‐localized metabotropic mGlu2 / 3 receptors (Mela et al., 2006; Marti et al., 1999; Marti et al., 2003), further supporting the proposed presynaptic location of the NMDA receptors under study and the exocytotic nature of the transmitter release.
As to the subunit composition of these NMDA receptors, the available data do not allow any conclusion. Morari et al. (1998) demonstrated that GluN1 proteins exist in striatal synaptosomes and that GluN1 co‐immuno‐precipitate with GluN2B subunits. However, the acetylcholine exocytosis evoked by low micromolar NMDA was unaffected by exogenous glycine (Table 2) posing the question of the functional role of the GluN1 protein, if present, in dictating the function of the presynaptic NMDA receptors (Morari et al., 1998). Furthermore, data concerning the effects of GluN2‐selective ligands are so far lacking.
8. PRESYNAPTIC NMDA HETERORECEPTORS CONTROLLING THE RELEASE OF PEPTIDES: A NON‐CONVENTIONAL PRESYNAPTIC NMDA HETERORECEPTOR
The available data in the literature suggests the existence of presynaptic NMDA receptors controlling the release of cholecystokinin (CCK‐LI) and somatostatin (SRIF‐LI) in cortical synaptosomes (Gemignani et al., 2000; Paudice et al., 1998) having comparable pharmacological profiles.
NMDA as well as glycine failed to modify, by themselves, the spontaneous release of both peptides but significantly potentiated their exocytosis elicited by a mild depolarizing stimulus. These observations were best interpreted by assuming that both the somatostatinergic and the cholecystokinergic terminals express presynaptic NMDA receptors whose activation increases the availability of Ca2+ ions in the cytosol to a level insufficient, per se, to trigger peptide overflows, but sufficient to reinforce the depolarization‐evoked, Ca2+‐dependent exocytosis. NMDA or glycine‐mediated effects occurred in a concentration‐dependent manner but were not additive (Tables 1 and 2).
The NMDA‐ or glycine‐induced facilitation of peptides exocytosis was significantly reduced by antagonists acting at either the glutamate binding site (D‐AP5 or CGS19755) or the glycine binding site (7‐Cl‐Kyna), respectively, which could suggest the presence of a classic GluN1/GluN2 heteromeric receptor assembly. Furthermore, the agonist‐evoked facilitation of peptides exocytosis was prevented by ifenprodil and Zn2+ ions, compatible with the participation of both GluN2A and GluN2B subunits to the NMDA complex (Table 3). Data on the effects of selective GluN2C or GluN2D antagonists are not available, but the low expression of these subunits in the cortex of adult mice would limit their involvement in the NMDA receptor assemblies.
These NMDA heteroreceptors were insensitive to the presence of Mg2+ ions but were inhibited by non‐competitive antagonists acting at the associated voltage‐dependent ionic channel (e.g., (RS)‐CPP, dizocilpine, memantine). d‐serine mimicked glycine in potentiating the exocytosis of CCK‐LI and SRIF‐LI from cortical synaptosomes in an ifenprodil and Zn2+ ions‐sensitive manner (Table 2). Finally, lowering the external pH to 6.4 significantly reduced the NMDA‐evoked facilitation of peptide exocytosis. Unexpectedly, the external alkalinisation at pH = 8.0 also reduced the facilitation elicited by NMDA or by d‐serine (Table 3), leaving unsolved the question on the involvement of GluN1 subunits lacking the N1 cassette (e.g., the GluN1‐1a subunit for instance) in the NMDA receptor assembly (Gemignani et al., 2004). Based on these observations, it was proposed that cortical nerve terminals releasing SRIF‐LI and CCK‐LI possess ‘non‐conventional’ NMDA receptors typified by a tri‐heteromeric GluN1/GluN2A/GluN2B assembly that can be operated by glycinergic agonists in the apparent absence of the glutamate co‐agonist, but in the presence of physiological amounts of Mg2+ ions (Paudice et al., 1998).
At that time, the involvement of the GluN3 subunits in the expression of NMDARs was not taken in consideration, but the available data, particularly the fact that glycine acts as a full agonist, allow some speculation on this regard. As already mentioned (see Paragraph 3.3), the GluN3‐containing NMDA receptors are roughly subdivided in GluN1‐GluN2‐GluN3 subunits assembly, (the so‐called GluN3‐NMDA receptors) and in GluN1‐GluN3 subunit complexes (Al‐Hallaq et al., 2002; Pachernegg et al., 2012; Perez‐Otano et al., 2001, 2016; Smothers & Woodward, 2007). As far as the stoichiometry of the GluN3‐NMDA receptor subtypes is concerned, it is generally accepted that it can vary depending on the neuronal subpopulations and the CNS regions involved (Paoletti, 2011; Pilli & Kumar, 2012; Stroebel et al., 2017). In general, however, these receptors are typified by low Ca2+ permeability and are relatively insensitive to external Mg2+ ions and glycine, and some glycine agonists act as full agonists at the receptor in a 5,7‐Cl‐Kyna‐sensitive manner. However, the GluN1‐GluN3 complexes are not activated by NMDA or glutamate, they are insensitive to D‐AP5 and to the channel blockers (Mg2+, dizocilpine, memantine, (RS)‐CPP), and they behave as glycine‐gated receptors, glycine acting as a full agonist and d‐serine as a partial agonist (Chatterton et al., 2002; Henson et al., 2012; Pachernegg et al., 2012). Finally, protons potentiate the glycine‐mediated responses at the GluN1/GluN3 complexes but inhibit the GluN3‐NMDA receptors (Cummings & Popescu, 2015).
The possibility that the NMDA receptors controlling SRI‐LI and CCK‐LI exocytosis could belong to the ‘glycine‐sensitive’ GluN1/ GluN3A NMDAR subtype seems unlikey because (i) it is operated by NMDA and (ii) the external acidification would be expected to potentiate instead of inhibit the receptor‐mediated releasing activity, as indeed observed. Rather, the pharmacological profile of the NMDA receptors controlling SRIF‐LI and CCK‐LI exocytosis, particularly their sensitivity to glycine and the inhibitory effect exerted by protons, seems most consistent with the conclusion that these receptors belong to the GluN3‐ NMDA receptor subtypes. In line with this view, the GluN3A subunit expression is high in somatostatin‐containing GABAergic interneurons in the cortex, to a level that makes the Grin3a mRNA an efficient marker, distinguishing these cells from other interneurons in this CNS region (Matsuda et al., 2003; Wee et al., 2008). Further studies are needed to verify the hypothesis.
9. PRESYNAPTIC NMDA AUTORECEPTORS CONTROLLING THE RELEASE OF GLUTAMATE FROM NERVE ENDINGS
9.1. Presynaptic NMDA autoreceptors in the hippocampus
The first data suggesting the existence of presynaptic release‐regulating NMDA autoreceptors originated from studies in rat hippocampal CA1 slices (Chapman & Bowker, 1987; Connick & Stone, 1988; Crowder et al., 1987; Martin et al., 1991; Nadler et al., 1990) showing that NMDA antagonists reduce the exocytosis of glutamate/aspartate elicited by high‐K+ , while NMDA did not evoke any measurable release under depolarized or basal conditions, despite the omission of Mg2+ ions in the external milieu. It was proposed that the endogenous glutamate could have saturated the respective binding site at the NMDA receptors, disabling exogenous NMDA agonists to activate the autoreceptors, but permitting the competition of NMDA antagonists at the agonist binding site, indirectly showing the activity of the endogenous amino acid as enhancer of its own release through NMDA autoreceptors. Starting from these findings, several electrophysiological recordings in hippocampal slices (Mameli et al., 2005; Madara & Levine, 2008; McGuinness et al., 2010; reviewed by Duguid, 2013; Pinheiro & Mulle, 2008) supported the existence of NMDA receptors in different hippocampal subregions (Dalby & Mody, 2003; Madara & Levine, 2008; Mameli et al., 2005; Martin et al., 1991; McGuinness et al., 2010; Nadler et al., 1990; see Duguid, 2013; Pinheiro & Mulle, 2008).
The presence of functional NMDA receptors in CA1 glutamatergic terminals was then confirmed by using synaptosomes (Table 1). The release of aspartate, but not glutamate, elicited by a mild depolarizing stimulus from these terminals was reinforced by NMDA in a D‐AP5‐sensitive manner (Zhou et al., 1995). Next, Breukel et al. (1998) provided evidence of a Ca2+‐independent NMDA‐mediated release of both aspartate and glutamate from a non‐vesicular, cytosolic pool. The authors proposed that the NMDA autoreceptors could be located near glutamate transporters and that the proximity of the two proteins would permit their functional crosstalk, leading to an NMDA‐induced reversal of the carrier and to the Ca2+‐independent outflow of the amino acid from the cytosolic pools. The existence of the NMDA autoreceptors was then confirmed in both rat and mice hippocampal synaptosomes (Luccini, Musante, Neri, Raiteri, & Pittaluga, 2007; Musante et al., 2011; Olivero, Vergassola, Cisani, Usai, & Pittaluga, 2019; Sequeira et al., 2001). The results indicated that NMDA in the presence of saturating glycine potentiated the spontaneous release of glutamate in a concentration‐dependent manner through different molecular events strictly dependent on the concentration of the agonist. Low micromolar NMDA caused a transporter‐independent, Ca2+‐ and Na+‐dependent overflow of glutamate, whereas higher concentrations led to the reversal of the glutamate transporter and to the carrier‐mediated DL‐threo‐b‐benzyloxyaspartate (DL‐tBOA)‐sensitive glutamate overflow (Luccini, Musante, Neri, Raiteri, & Pittaluga, 2007).
9.1.1. The glutamate binding site
The involvement of the GluN2 subunit in the expression of the NMDA autoreceptors was shown by the results with selective GluN2 ligands. Quinolinic acid released glutamate, although less efficiently than NMDA, whereas broad‐spectrum antagonists acting at the glutamate binding site (D‐AP5, D‐(−)‐2‐amino‐7‐phosphonoheptanoic acid, D‐AP7) inhibited the NMDA‐evoked glutamate exocytosis from hippocampal slices and synaptosomes (Tables 2 and 3; Chapman & Bowker, 1987; Connick & Stone, 1988; Crowder et al., 1987; Luccini, Musante, Neri, Raiteri, & Pittaluga, 2007). Furthermore, low (nM) concentration of Zn2+ ions (which selectively block the GluN2A subunit) and ifenprodil (that specifically targets the GluN2B subunits) prevented the NMDA‐evoked glutamate exocytosis almost to a comparable extent (Table 3; Luccini, Musante, Neri, Raiteri, & Pittaluga, 2007; Musante et al., 2011; Olivero, Vergassola, Cisani, Usai, & Pittaluga, 2019). Comparable effects were also observed when incubating synaptosomes with antibodies recognizing the outer sequence of the GluN2A and of the GluN2B receptor proteins (which mimic antagonists at the respective GluN subunit in an experimental approach called the ‘immunopharmacological approach’ (see Di Prisco et al., 2012; Olivero et al., 2017; Olivero, Vergassola, Cisani, Roggeri, & Pittaluga, 2019; Olivero, Vergassola, Cisani, Usai, & Pittaluga, 2019).
Western blot analysis and confocal microscopy confirmed the presence of the GluN2A and the GluN2B subunits in both rats and mice hippocampal synaptosomes (Henson et al., 2012; Musante et al., 2011; Olivero, Vergassola, Cisani, Usai, & Pittaluga, 2019), particularly in the presynaptic component of the synaptic active zone that was isolated by applying a technique of subfractioning of synaptosomal lysates (see Feligioni et al., 2006; Phillips et al., 2001). Notably, in this fraction, the GluN2A and the GluN2B subunits were shown to physically associate, consistent with their co‐participation in the NMDA autoreceptor (Musante et al., 2011). The possible participation of the GluN2C and the GluN2D subunits was not investigated due to their low, almost undetectable, expression in the hippocampal synaptosomal lysates (Olivero, Vergassola, Cisani, Usai, & Pittaluga, 2019).
9.1.2. The glycine binding site
The participation of the GluN1 subunit (e.g., the GluN11xx; Zukin & Bennett, 1995) in the expression of the NMDA autoreceptors in hippocampal synaptosomes is supported by the finding that (i) glycine reinforces the NMDA‐evoked release of glutamate in a concentration‐dependent fashion; (ii) d‐serine mimics, although less efficiently, glycine; (iii) 7‐Cl‐Kyna totally prevented the NMDA‐mediated releasing activity, independently of the concentration of the co‐administered glycine; (iv) increasing the concentration of external protons hampers the NMDA‐evoked release efficiency (Tables 2 and 3; Luccini, Musante, Neri, Raiteri, & Pittaluga, 2007; Musante et al., 2011; Olivero, Vergassola, Cisani, Usai, & Pittaluga, 2019). These observations, however, also questioned the possible involvement of GluN3 subunits in the expression of the NMDA autoreceptors. In particular, the dual effect exerted by d‐serine at these receptors (Table 2, e.g.; it potentiated NMDA‐induced exocytosis in the presence of low concentration of glycine but significantly reduced that in the presence of saturating glycine; Musante et al., 2011; Smothers & Woodward, 2007) seemed to indicate participation of this subunit in the receptor complex.
In line with this hypothesis, incubation of hippocampal synaptosomes with an antibody recognizing the outer NH2‐terminal sequence of the GluN3A protein significantly reduced the NMDA‐evoked release of glutamate, supporting evidence for the presence of this subunit in the receptor complex. Western blot analysis of the total hippocampal synaptosomal lysates and of the presynaptic synaptosomal fraction disclosed the presence of the GluN1 and GluN3A, but not of the GluN3B, immunoreactivities in both rats and mouse hippocampal preparations (Musante et al., 2011). Furthermore, the anti‐GluN2B immune precipitate from the presynaptic component of the hippocampal synaptosomes was immuno‐positive for GluN1 and for GluN3A subunits, consistent with the physical interaction linking the GluN1 and the GluN3A proteins with the GluN2B one. Finally, confocal microscopy showed that mouse hippocampal synaptosomes immuno‐positive for the vesicular glutamate transporter 1 (VGLUT1) and syntaxin‐1A, also displayed immunolabelling for the GluN1 and the GluN3A proteins (Henson et al., 2012; Musante et al., 2011; Olivero, Vergassola, Cisani, Usai, & Pittaluga, 2019). Overall, these findings seemed best interpreted by assuming that the NMDA autoreceptors consist of GluN1‐GluN2‐GluN3 subunits. How GluN3A subunits fit into the NMDAR assembly remains, however, an unanswered question, although the possibility should be considered that GluN1 subunits associate with GluN2 subunits to form a tetra‐heterodimeric complex (GluN1/GluN2A/GluN2B, Stroebel et al., 2017), which further evolve to a multimeric form including GluN3 subunits (Stys & Lipton, 2007).
9.1.3. The sensitivity of the hippocampal NMDA autoreceptors to external cations
In 1998, Breukel and colleagues demonstrated that NMDA efficiently released glutamate and aspartate from CA1 synaptosomes despite the presence in the superfusion medium of physiological concentrations of Mg2+ ions. The authors proposed that the voltage‐dependent ionic channel associated with the NMDA receptors could have structural characteristics that permit the influx of cations having low mass (e.g., Na+ ions), favouring a local depolarization of the plasma membranes and the consequent reversal of the glutamate carrier‐mediated functions. More than a decade later, Musante et al. (2011) confirmed the low sensitivity of the NMDA autoreceptor to external Mg2+ ions and its modulation by Na+ and Ca2+ ions in the external milieu (Breukel et al., 1998; Luccini, Musante, Neri, Raiteri, & Pittaluga, 2007; Musante et al., 2011). The authors however provided evidence that the Na+ dependency of the releasing activity also relied on mechanisms of ‘metamodulation’ of the presynaptic NMDA receptors triggered by the activation of co‐localized glycine transporter type 1 (GlyT1; Musante et al., 2011) that was permissive for the NMDA‐mediated releasing activity.
In general, it is proposed that GlyT1s are located near NMDA receptors and modulate its function by maintaining the concentration of [glycine]out at sub‐saturating levels (Aragón & López‐Corcuera, 2005; Raiteri & Raiteri, 2010). GlyT1, however, is an electrogenic transporter, which provides a continuous influx of 2 Na+ and 1 Cl− ions, while taking up glycine. Therefore, beyond controlling the concentration of external glycine, the transporter also depolarizes plasma membranes because of the net influx of positive charges that parallels its activity. We found that the NMDA‐evoked releasing effect was abolished by the concomitant presence of the GlyT1 blocker N‐[3‐(4¢‐fluorophenyl)‐3‐(4‐phenylphenoxy)propyl] (NFPS, sarcosine hydrochloride), consistent with the view that GlyT1 ‘metamodulates’ NMDA autoreceptors, favouring its releasing activity (Musante et al., 2011).
9.2. Presynaptic release‐regulating NMDA autoreceptors in other region of the CNS
Presynaptic release‐regulating NMDA autoreceptors were reported to exist in several regions of the CNS (Banerjee et al., 2016; Bouvier et al., 2015; Bouvier et al., 2018; Corlew et al., 2008; Fan et al., 2014; Pinheiro & Mulle, 2008), and the studies involving synaptosomes support the existence of presynaptic NMDA autoreceptors in rat NAc (Zappettini et al., 2014), in mouse cortex (Marcelli et al., 2019; Nisticò et al., 2015) and spinal cord (Fariello et al., 2014).
As to the NMDA autoreceptors in glutamatergic nerve endings isolated from the NAc (Zappettini et al., 2014), these receptors co‐localize with nicotinic receptors that are permissive to the NMDA‐mediated releasing activity in physiological conditions (the presence of physiological Mg2+ ions). In this case, the results from release studies were supported by data showing a significant increase of Ca2+ ions in the synaptosomes exposed to NMDA and glycine, as well as by immunocytochemical data showing the co‐localization of the GluN1 subunit protein and VGLUT1. In contrast to the NMDA receptors in hippocampal nerve endings, those in synaptosomes from the NAc were sensitive to (RS)‐CPP and Ro256981, two GluN2A‐preferring antagonists, but insensitive to ifenprodil (Zappettini et al., 2014), suggesting the existence of autoreceptors composed of the GluN1/GluN2A assembly in these terminals (Table 3).
However, the NMDA autoreceptors in spinal cord nerve endings consisted of a tri‐heteromeric assembly of GluN1, GluN2A and the GluN2B subunits, as observed in the hippocampal terminals. Quite interestingly, in this case, lowering the external pH to 6.8 drastically reduced the releasing activity, while increasing it to 8.0 did not affected the receptor‐mediated event. Whether these results could suggest the possible involvement of a GluN3 subunit remains to be investigated (Table 3; Fariello et al., 2014).
10. CONCLUSION
In recent years, two reviews, the first from Pinheiro and Mulle (2008) and the second from Duguid (2013), suggested criteria that, if satisfied, could help to define the existence of presynaptic release‐regulating NMDA receptors. Interestingly, some of these criteria can be successfully satisfied also by using the synaptosomal preparation, as briefly summarised below.
An important criterion to support the existence of presynaptic NMDA receptors is that NMDA agonist(s) can efficiently modulate the transmitter release probability as well as the Ca2+ dynamics in nerve terminals (Table 1). Most of the functional data described in this review satisfy this criterion because it emerges that (i) the exposure of synaptosomes (which are pinched‐off resealed nerve endings) to NMDA agonists affects transmitter release and (ii) the releasing activity is paralleled by Ca2+ ions movements within the synaptosomal particles specialized for the release of the transmitter under study (e.g., glutamate; Zappettini et al., 2014; Nisticò et al., 2015).
Another criterion that is largely satisfied in release studies from superfused synaptosomes is that NMDA antagonists should prevent or reduce the agonist‐induced effects. In almost all the reported studies (with the exception of the antagonist profile of the NMDA heteroreceptor controlling 5‐HT and acetylcholine release), antagonists specific for selected subunits were found to modulate the NMDA/glycine‐evoked transmitter release. Notably, as already discussed in the review, the results obtained with these ligands are attributed to their ability to target selected GluN subunits, and therefore, besides confirming the existence of presynaptic release‐regulating NMDA receptors, they also aloow suggestions of the subunit composition of the receptor involved and the main feature of the associated ionic channel (Table 3).
Finally, another criterion to support the existence of presynaptic NMDA receptors is the presence of GluN subunit proteins at the presynaptic structures. The review reports data from both immunocytochemical analysis and confocal microscopy demonstrating the presence of selected GluN subunits in synaptosomal particles specialized for the release of selected transmitters and in particular in the presynaptic fraction of the synaptosomal plasma membranes (e.g., the fraction corresponding to the presynaptic component of the synaptic active zone; Phillips et al., 2001). Furthermore, in some cases, the studies also show a clear correlation between the subunits and the transmitter specialization of the synaptosomal particles (Musante et al., 2011; Nisticò et al., 2015; Olivero, Vergassola, Cisani, Usai, & Pittaluga, 2019; Salamone et al., 2014; Zappettini et al., 2014).
Starting from these considerations, the review describes a complex scenario that includes several NMDA receptors having different localization, different subunit composition and different conditions of activation. The complexity is consistent with a heterogenous pharmacological profile, heighted by using selective ligands for the glutamate and the glycine binding sites as well as compounds acting at the voltage dependent associated channel (see Figure 2 for an iconographic illustration).
FIGURE 2.
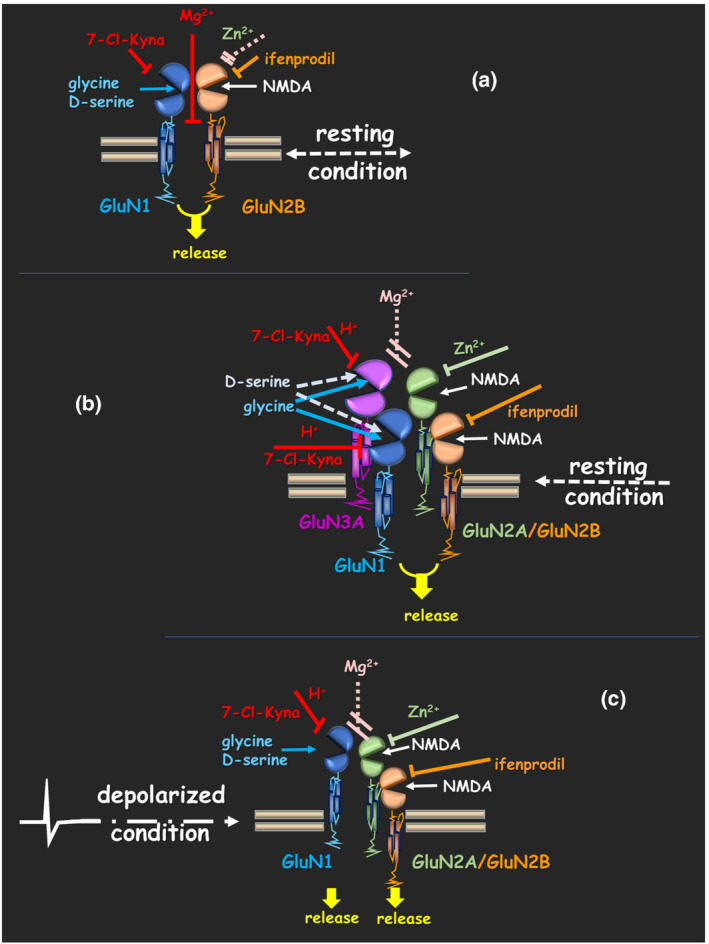
Heterogeneity of the presynaptic release‐regulating NMDA receptors. The pharmacological profile of the receptors was obtained using selective ligands for the GluN subunits, as well as compounds acting at the voltage dependent associated channel. The results are consistent with the presence of (a) dimeric GluN1/GluN2B assemblies in the dopaminergic and the noradrenergic nerve endings and of heteromeric GluN associations typified by a high level of complexity in (b) peptidergic and (c) glutamatergic terminals. The NMDA receptors also act differently on the transmitter outflow. (a and c) Presynaptic NMDA receptors controlling dopamine, noradrenaline and glutamate potentiate the spontaneous release of these transmitters, whereas (b) the presynaptic NMDA receptors controlling the release of CCK‐LI and SRIF‐LI significantly reinforce the exocytosis of the two peptides elicited by a mild depolarizing stimulus. Straight line with arrow: full agonist; dotted line with arrow: partial agonist; straight line with cap: antagonist/channel blockers; dotted line with breaks: antagonist/channel blockers inactive at the receptor
In particular, analysis of the available data suggests the presence of dimeric GluN1/GluN2B assemblies in the dopaminergic and the noradrenergic nerve endings (Figure 2a) and of heteromeric GluN associations typified by a high level of complexity in peptidergic (Figure 2b) and glutamatergic (Figure 2c) terminals, possibly consisting of GluN3‐containing GluN1/GluN2 NMDA heterocomplexes. There are however some NMDA receptors (e.g., those controlling acetylcholine and GABA release) whose subunit composition is poorly investigated, and others (e.g., the one controlling 5‐HT release) whose existence depends on data from experimental approaches other than synaptosomes, but not confirmed in isolated nerve endings.
As already mentioned, the ‘superfusion of synaptosomes’ allows discrimination between basal and depolarized transmitter release and how these two events are controlled by autoheteroreceptors. By using this technique, it emerged that the presynaptic NMDA receptors affect differently the release of different transmitters. Presynaptic NMDA receptors controlling dopamine, noradrenaline and glutamate potentiate the spontaneous release of the transmitters (Table 1 and Figure 2) but display a different sensitivity towards the external Mg2+ ions, which possibly reflects different subunit composition (including the participation of the GluN3 subunit to the receptor assembly; Table 3 and Figure 2). Other presynaptic NMDA receptors fail to affect the spontaneous release of transmitters but significantly augment their exocytosis elicited by a mild depolarizing stimulus (as in the case of the NMDA receptors controlling the release of acetylcholine and of peptides; Table 1 and Figure 2), and in most cases, the efficiency of the control of transmitter release slightly depends on the presence of Mg2+ ions (e.g., the NMDA receptors controlling glutamate, peptides and acetylcholine release; Table 3 and Figure 2).
The NMDA‐mediated modulation of transmitter release generally involves the influx of positive charges to the cytosolic compartments but also relies on mechanisms involving the reversal of co‐localized transporters (e.g., the GLYT1; Musante et al., 2011) as well as the concomitant activation of co‐localized receptors, for example, ionotropic (e.g., AMPA and nicotinic receptors; Pittaluga & Raiteri, 1992a, 1992b; Risso et al., 2004) and metabotropic (mGlu1 receptors, somatostatin type 5 receptors, CXCR4 chemokine receptors; Di Prisco et al., 2016) receptors that are permissive to the opening of the associated ionic channels.
Finally, the reciprocal role of glutamate and glycine at the presynaptic release‐regulating receptors varies depending on the synaptosomal subpopulations and the transmitter involved (Table 2). For most of the presynaptic NMDA receptors described in this review, glutamate is essential for receptor activation, whereas glycine preferentially acts as a co‐agonist, being a fine tuner of the releasing activity (it is permissive when added at nanomolar concentration, but allosteric at micromolar level, it is the case of the NMDA receptors controlling the release of catecholamines, glutamate and perhaps GABA). There are, however, NMDA receptors activated by glycine alone (e.g., the presynaptic release‐regulating NMDA receptors controlling peptide exocytosis; Paudice et al., 1998) or receptors that are almost insensitive to the presence of glycine (e.g., those controlling the release of acetylcholine; Morari et al., 1998).
In summary, the data reviewed in this article manuscript substantiate the conclusion that presynaptic release‐regulating NMDARs exist in selected regions of the CNS and control synaptic strength and efficiency, playing a major role in the neuronal network. Most of the functional properties of NMDA receptors relies on the subunit composition and on the co‐localized receptors/transporters. Of relevance are the results concerning the effectsof agonists, antagonists and allosteric modulators on the presynaptic NMDA receptors because these results promise new opportunities to dissect the pre from the post NMDA‐mediated component in synaptic transmission as well as to targeting therapeutically the presynaptic receptors.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019; Alexander, Kelly et al., 2019; Alexander, Mathie et al., 2019;).
CONFLICT OF INTEREST
The author declares no conflicts of interest.
ACKNOWLEDGEMENTS
The review is dedicated to Professor Maurizio Raiteri and Professor Mario Marchi. Thanks for sharing the fascinating story of the presynaptic release‐regulating NMDA receptors.
Pittaluga A. Presynaptic release‐regulating NMDA receptors in isolated nerve terminals: A narrative review. Br J Pharmacol. 2021;178:1001–1017. 10.1111/bph.15349
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176, S397–S493. 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … the CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176(Suppl 1), S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Hallaq, R. A. , Jarabek, B. R. , Fu, Z. , Vicini, S. , Wolfe, B. B. , & Yasuda, R. P. (2002). Association of NR3A with the N‐methyl‐D‐aspartate receptor NR1 and NR2 subunits. Molecular Pharmacology, 62(5), 1119–1127. 10.1124/mol.62.5.1119 [DOI] [PubMed] [Google Scholar]
- Aragón, C. , & López‐Corcuera, B. (2005). Glycine transporters: crucial roles of pharmacological interest revealed by gene deletion. Trends in Pharmacological Sciences, 26(6), 283–286. 10.1016/j.tips.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Banerjee, A. , Larsen, R. S. , Philpot, B. D. , & Paulsen, O. (2016). Roles of presynaptic NMDA receptors in neurotransmission and plasticity. Trends in Neurosciences, 39(1), 26–39. 10.1016/j.tins.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhage, B. , Hansen, G. H. , & Schousboe, A. (1993). Depolarization by K+ and glutamate activates different neurotransmitter release mechanisms in GABAergic neurons: Vesicular versus non‐vesicular release of GABA. Neuroscience, 54(4), 1019–1034. 10.1016/0306-4522(93)90592-4 [DOI] [PubMed] [Google Scholar]
- Berretta, N. , & Jones, R. S. (1996). Tonic facilitation of glutamate release by presynaptic N‐methyl‐D‐aspartate autoreceptors in the entorhinal cortex. Neuroscience, 75(2), 339–344. 10.1016/0306-4522(96)00301-6 [DOI] [PubMed] [Google Scholar]
- Bonanno, G. , Pittaluga, A. , Fedele, E. , Fontana, G. , & Raiteri, M. (1993). Glutamic acid and gamma‐aminobutyric acid modulate each other's release through heterocarriers sited on the axon terminals of rat brain. Journal of Neurochemistry, 61(1), 222–230. 10.1111/j.1471-4159.1993.tb03558.x [DOI] [PubMed] [Google Scholar]
- Bouvier, G. , Bidoret, C. , Casado, M. , & Paoletti, P. (2015). Presynaptic NMDA receptors: Roles and rules. Neuroscience, 311, 322–340. 10.1016/j.neuroscience.2015.10.033 Epub 2015 Oct 24 [DOI] [PubMed] [Google Scholar]
- Bouvier, G. , Larsen, R. S. , Rodríguez‐Moreno, A. , Paulsen, O. , & Sjöström, P. J. (2018). Towards resolving the presynaptic NMDA receptor debate. Current Opinion in Neurobiology, 51, 1–7. 10.1016/j.conb.2017.12.020 [DOI] [PubMed] [Google Scholar]
- Brasier, D. J. , & Feldman, D. E. (2008). Synapse‐specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. Journal of Neuroscience, 28(9), 2199–2211. 10.1523/JNEUROSCI.3915-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukel, A. I. , Besselsen, E. , Lopes da Silva, F. H. , & Ghijsen, W. E. (1998). A presynaptic N‐methyl‐D‐aspartate autoreceptor in rat hippocampus modulating amino acid release from a cytoplasmic pool. European Journal of Neuroscience, 10(1), 106–114. 10.1046/j.1460-9568.1998.00008.x [DOI] [PubMed] [Google Scholar]
- Buchanan, K. A. , Blackman, A. V. , Moreau, A. W. , Elgar, D. , Costa, R. P. , Lalanne, T. , … Sjöström, P. J. (2012). Target‐specific expression of presynaptic NMDA receptors in neocortical microcircuits. Neuron, 75(3), 451–466. 10.1016/j.neuron.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, A. G. , & Bowker, H. M. (1987). Inhibition of hippocampal 3H‐D‐aspartate release by 2‐ABP, 2‐APV, and 2‐APH. In Hick T. P., Lodge D., & McLennan H. (Eds.), Neurology and neurobiology: Excitatory amino acid transmission (Vol. 24) (pp. 165–168). New York: Liss. [Google Scholar]
- Chatterton, J. E. , Awobuluyi, M. , Premkumar, L. S. , Takahashi, H. , Talantova, M. , Shin, Y. , … Tong, G. (2002). Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature, 415(6873), 793–798. 10.1038/nature715 [DOI] [PubMed] [Google Scholar]
- Cheramy, A. , Leviel, V. , & Glowinski, J. (1981). Dendritic release of dopamine in the substantia nigra. Nature, 289(5798), 537–542. 10.1038/289537a0 [DOI] [PubMed] [Google Scholar]
- Cheramy, A. , Romo, R. , & Glowinski, J. (1986). The relative roles of neuronal activity and direct presynaptic mechanisms in controlling the release of dopamine from the cat caudate nucleus. Annals of the New York Academy of Sciences, 473, 80–91. 10.1111/j.1749-6632.1986.tb23606.x [DOI] [PubMed] [Google Scholar]
- Chéramy, A. , Godeheu, G. , L'Hirondel, M. , & Glowinski, J. (1996). Cooperative contributions of cholinergic and NMDA receptors in the presynaptic control of dopamine release from synaptosomes of the rat striatum. The Journal of Pharmacology and Experimental Therapeutics, 276, 616–625. [PubMed] [Google Scholar]
- Connick, J. H. , & Stone, T. W. (1988). Excitatory amino acid antagonists and endogenous aspartate and glutamate release from rat hippocampal slices. British Journal of Pharmacology, 93, 863–867. 10.1111/j.1476-5381.1988.tb11473.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew, R. , Brasier, D. J. , Feldman, D. E. , & Philpot, B. D. (2008). Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. The Neuroscientist, 14, 609–625. 10.1177/1073858408322675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew, R. , Wang, Y. , Ghermazien, H. , Erisir, A. , & Philpot, B. D. (2007). Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long‐term depression. The Journal of Neuroscience, 27, 9835–9845. 10.1523/JNEUROSCI.5494-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder, J. M. , Croucher, M. J. , Bradford, H. F. , & Collins, J. F. (1987). Excitatory amino acid receptors and depolarization‐induced Ca2+ influx into hippocampal slices. Journal of Neurochemistry, 48, 1917–1924. 10.1111/j.1471-4159.1987.tb05756.x [DOI] [PubMed] [Google Scholar]
- Crozier, R. A. , Wang, Y. , Liu, C. H. , & Bear, M. F. (2007). Deprivation‐induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proceedings of the National Academy of Science U S a, 104, 1383–1388. 10.1073/pnas.0609596104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, K. A. , & Popescu, G. K. (2015). Glycine‐dependent activation of NMDA receptors. Journal of General Physiology, 145, 513–527. 10.1085/jgp.201411302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby, N. O. , & Mody, I. (2003). Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. Journal of Neurophysiology, 90, 786–797. 10.1152/jn.00118.2003 [DOI] [PubMed] [Google Scholar]
- De‐May, C. L. , & Ali, A. B. (2013). Involvement of pre‐ and postsynaptic NMDA receptors at local circuit interneuron connections in rat neocortex. Neuroscience, 228, 179–189. 10.1016/j.neuroscience.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desce, J. M. , Godeheu, G. , Galli, T. , Artaud, F. , Chéramy, A. , & Glowinski, J. (1992). L‐glutamate‐evoked release of dopamine from synaptosomes of the rat striatum: involvement of AMPA and N‐methyl‐D‐aspartate receptors. Neuroscience, 47, 333–339. 10.1016/0306-4522(92)90249-2 [DOI] [PubMed] [Google Scholar]
- Desce, J. M. , Godeheu, G. , Galli, T. , Glowinski, J. , & Chéramy, A. (1994). Opposite presynaptic regulations by glutamate through NMDA receptors of dopamine synthesis and release in rat striatal synaptosomes. Brain Research, 640, 205–214. 10.1016/0006-8993(94)91874-0 [DOI] [PubMed] [Google Scholar]
- Di Prisco, S. , Olivero, G. , Merega, E. , Bonfiglio, T. , Marchi, M. , & Pittaluga, A. (2016). CXCR4 and NMDA receptors are functionally coupled in rat hippocampal noradrenergic and glutamatergic nerve endings. Journal of Neuroimmune Pharmacology, 11, 645–656. 10.1007/s11481-016-9677-6 [DOI] [PubMed] [Google Scholar]
- Di Prisco, S. , Summa, M. , Chellackudam, V. , Rossi, P. I. , & Pittaluga, A. (2012). RANTES‐mediated control of excitatory amino acid release in mouse spinal cord. Journal of Neurochemistry, 121, 428–437. 10.1111/j.1471-4159.2012.07720.x [DOI] [PubMed] [Google Scholar]
- Dorville, A. , McCort‐Tranchepain, I. , Vichard, D. , Sather, W. , Maroun, R. , Ascher, P. , & Roques, B. P. (1992). Preferred antagonist binding state of the NMDA receptor: synthesis, pharmacology, and computer modeling of (phosphonomethyl)phenylalanine derivatives. Journal of Medicinal Chemistry, 35(14), 2551–2562. 10.1021/jm00092a005 [DOI] [PubMed] [Google Scholar]
- Duarte, C. B. , Ferreira, I. L. , Santos, P. F. , Oliveira, C. R. , & Carvalho, A. P. (1993). Glutamate increases the [Ca2+]i but stimulates Ca(2+)‐independent release of [3H]GABA in cultured chick retina cells. Brain Research, 611, 130–138. 10.1016/0006-8993(93)91784-p [DOI] [PubMed] [Google Scholar]
- Duguid, I. C. (2013). Presynaptic NMDA receptors: Are they dendritic receptors in disguise? Brain Research Bullettin, 93, 4–9. 10.1016/j.brainresbull.2012.12.004 [DOI] [PubMed] [Google Scholar]
- Duguid, I. C. , & Smart, T. G. (2004). Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron‐Purkinje cell synapses. Nature Neuroscience, 7, 525–533. 10.1038/nn1227 Epub 2004. Apr 18 [DOI] [PubMed] [Google Scholar]
- Dunah, A. W. , Yasuda, R. P. , Wang, Y. H. , Luo, J. , Dávila‐García, M. , Gbadegesin, M. , … Wolfe, B. B. (1996). Regional and ontogenic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit‐specific antibody. Journal of Neurochemistry, 67, 2335–2345. 10.1046/j.1471-4159.1996.67062335.x [DOI] [PubMed] [Google Scholar]
- Fan, X. , Jin, W. Y. , & Wang, Y. T. (2014). The NMDA receptor complex: a multifunctional machine at the glutamatergic synapse. Frontiers in Cellular Neuroscience, 8, 1–9. 10.3389/fncel.2014.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariello, R. G. , Ghelardini, C. , Di Cesare Mannelli, L. , Bonanno, G. , Pittaluga, A. , Milanese, M. , … Farina, C. (2014). Broad spectrum and prolonged efficacy of dimiracetam in models of neuropathic pain. Neuropharmacology, 81, 85–94. 10.1016/j.neuropharm.2014.01.029 [DOI] [PubMed] [Google Scholar]
- Farrant, M. , & Cull‐Candy, S. G. (1991). Excitatory amino acid receptor‐channels in Purkinje cells in thin cerebellar slices. Proceedings in Biological Science., 244, 179–184. 10.1098/rspb.1991.0067 [DOI] [PubMed] [Google Scholar]
- Feligioni, M. , Holman, D. , Haglerød, C. , Davanger, S. , & Henley, J. M. (2006). Ultrastructural localisation and differential agonist‐induced regulation of AMPA and kainate receptors present at the presynaptic active zone and postsynaptic density. Journal of Neurochemistry, 99, 549–560. 10.1111/j.1471-4159.2006.04087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink, K. , Böing, C. , & Göthert, M. (1996). Presynaptic 5‐HT autoreceptors modulate N‐methyl‐D‐aspartate‐evoked 5‐hydroxytryptamine release in the guinea‐pig brain cortex. European Journal of Pharmacology, 300, 79–82. 10.1016/0014-2999(96)00042-8 [DOI] [PubMed] [Google Scholar]
- Fink, K. , Göthert, M. , Molderings, G. , & Schlicker, E. (1989). N‐Methyl‐D‐aspartate (NMDA) receptor‐mediated stimulation of noradrenaline release, but not release of other neurotransmitters, in the rat brain cortex: Receptor location, characterization and desensitization. Naunyn Schmiedebergs Archieve of Pharmacology., 339(5), 514–521. 10.1007/BF00167254 [DOI] [PubMed] [Google Scholar]
- Fink, K. , Schmitz, V. , Böing, C. , & Göthert, M. (1995). Stimulation of serotonin release in the rat brain cortex by activation of ionotropic glutamate receptors and its modulation via alpha 2‐heteroreceptors. Naunyn Schmiedebergs Archieve of Pharmacology., 352, 394–401. 10.1007/BF00172776 [DOI] [PubMed] [Google Scholar]
- Froemke, R. C. , Poo, M. M. , & Dan, Y. (2005). Spike‐timing‐dependent synaptic plasticity depends on dendritic location. Nature, 434, 221–225. 10.1038/nature03366 [DOI] [PubMed] [Google Scholar]
- Fu, W. M. , Liou, J. C. , Lee, Y. H. , & Liou, H. C. (1995). Potentiation of neurotransmitter release by activation of presynaptic glutamate receptors at developing neuromuscular synapses of Xenopus. The Journal of Physiology, 489, 813–823. 10.1113/jphysiol.1995.sp021094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemignani, A. , Paudice, P. , Longordo, F. , & Raiteri, M. (2004). External pH changes affect NMDA‐evoked and spontaneous release of cholecystokinin, somatostatin and noradrenaline from rat cerebrocortical nerve endings. Neurochemistry International, 45(5), 677–685. 10.1016/j.neuint.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Gemignani, A. , Paudice, P. , Pittaluga, A. , & Raiteri, M. (2000). The HIV‐1 coat protein gp120 and some of its fragments potently activate native cerebral NMDA receptors mediating neuropeptide release. The European Journal of Neuroscience, 12, 2839–2846. 10.1046/j.1460-9568.2000.00172.x [DOI] [PubMed] [Google Scholar]
- Giorguieff, M. F. , Kemel, M. L. , & Glowinski, J. (1977). Presynaptic effect of l‐glutamic acid on dopamine release in rat striatal slices. Neuroscience Letters, 6(1), 73–77. 10.1016/0304-3940(77)90068-4 [DOI] [PubMed] [Google Scholar]
- Glitsch, M. , & Marty, A. (1999). Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. Journal of Neuroscience, 19(2), 511–519. 10.1523/JNEUROSCI.19-02-00511.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli, M. , Pittaluga, A. , Merlo‐Pich, E. , & Marchi, M. (2009). NMDA‐mediated modulation of dopamine release is modified in rat prefrontal cortex and nucleus accumbens after chronic nicotine treatment. Journal of Neurochemistry, 108(2), 408–416. 10.1111/j.1471-4159.2008.05792.x [DOI] [PubMed] [Google Scholar]
- Harney, S. C. , Jane, D. E. , & Anwyl, R. (2008). Extrasynaptic NR2D‐containing NMDARs are recruited to the synapse during LTP of NMDAR‐EPSCs. The Journal of Neuroscience, 28(45), 11685–11194. 10.1523/JNEUROSCI.3035-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson, M. A. , Larsen, R. S. , Lawson, S. N. , Pérez‐Otaño, I. , Nakanishi, N. , Lipton, S. A. , & Philpot, B. D. (2012). Genetic deletion of NR3A accelerates glutamatergic synapse maturation. PLoS ONE, 7(8), e42327. 10.1371/journal.pone.0042327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. M. , & Jeng, Y. J. (1991). Pharmacological evidence for N‐methyl‐D‐aspartate receptors on nigrostriatal dopaminergic nerve terminals. Canadian Journal of Physiology and Pharmacology, 69(10), 1416–1421. 10.1139/y91-212 [DOI] [PubMed] [Google Scholar]
- Keita, H. , Lepouse, C. , Henzel, D. , Desmonts, J. M. , & Mantz, J. (1997). Riluzole blocks dopamine release evoked by N‐methyl‐D‐aspartate, kainate, and veratridine in the rat striatum. Anesthesiology, 87(5), 1164–1171. 10.1097/00000542-199711000-00021 [DOI] [PubMed] [Google Scholar]
- Klarica, M. , Fage, D. , & Carter, C. (1996). Pharmacology of N‐methyl‐D‐aspartate‐evoked [3H]noradrenaline release in adult rat spinal cord. European Journal of Pharmacology, 308(2), 135–144. 10.1016/0014-2999(96)00287-7 [DOI] [PubMed] [Google Scholar]
- Krebs, M. O. , Desce, J. M. , Kemel, M. L. , Gauchy, C. , Godeheu, G. , & Cheramy, A. (1991). Glutamatergic control of dopamine release in the rat striatum: Evidence for presynaptic N‐methyl‐D‐aspartate receptors on dopaminergic nerve terminals. Journal of Neurochemistry, 56(1), 81–85. 10.1111/j.1471-4159.1991.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Langer, S. Z. (1997). 25 years since the discovery of presynaptic receptors: Present knowledge and future perspectives. Trends in Pharmacological Sciences, 18(3), 95–99. 10.1016/s0165-6147(96)01034-6 [DOI] [PubMed] [Google Scholar]
- Langer, S. Z. (2008). Presynaptic autoreceptors regulating transmitter release. Neurochemistry International, 52(1–2), 26–30. 10.1016/j.neuint.2007.04.031 [DOI] [PubMed] [Google Scholar]
- Larsen, R. S. , Corlew, R. J. , Henson, M. A. , Roberts, A. C. , Mishina, M. , Watanabe, M. , … Philpot, B. D. (2011). NR3A‐containing NMDARs promote neurotransmitter release and spike timing‐dependent plasticity. Nature Neuroscience, 14(3), 338–344. 10.1038/nn.2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, R. S. , Smith, I. T. , Miriyala, J. , Han, J. E. , Corlew, R. J. , Smith, S. L. , & Philpot, B. D. (2014). Synapse‐specific control of experience‐dependent plasticity by presynaptic NMDA receptors. Neuron, 83(4), 79–93. 10.1016/j.neuron.2014.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, J. , & Scatton, B. (1982). Characterization of the excitatory amino acid receptor‐mediated release of [3H]acetylcholine from rat striatal slices. Brain Research, 252(1), 77–89. 10.1016/0006-8993(82)90980-5 [DOI] [PubMed] [Google Scholar]
- Lehmann, J. , Schaefer, P. , Ferkany, J. W. , & Coyle, J. T. (1983). Quinolinic acid evokes [3H]acetylcholine release in striatal slices: mediation by NMDA‐type excitatory amino acid receptors. European Journal of Pharmacology, 96(1–2), 111–115. 10.1016/0014-2999(83)90536-8 [DOI] [PubMed] [Google Scholar]
- Levi, G. , & Raiteri, M. (1993). Carrier‐mediated release of neurotransmitters. Trends in Neurosciences, 16(10), 415–419. 10.1016/0166-2236(93)90010-j [DOI] [PubMed] [Google Scholar]
- L'hirondel, M. , Chéramy, A. , Artaud, F. , Godeheu, G. , & Glowinski, J. (1999). Contribution of endogenously formed arachidonic acid in the presynaptic facilitatory effects of NMDA and carbachol on dopamine release in the mouse striatum. The European Journal of Neuroscience, 11(4), 1292–1300. 10.1046/j.1460-9568.1999.00534.x [DOI] [PubMed] [Google Scholar]
- Livingstone, P. D. , Dickinson, J. A. , Srinivasan, J. , Kew, J. N. , & Wonnacott, S. (2010). Glutamate‐dopamine crosstalk in the rat prefrontal cortex is modulated by Alpha7 nicotinic receptors and potentiated by PNU‐120596. Journal of Molecular Neuroscience, 40(1–2), 172–176. 10.1007/s12031-009-9232-5 [DOI] [PubMed] [Google Scholar]
- Llano, I. , Marty, A. , Armstrong, C. M. , & Konnerth, A. (1991). Synaptic‐ and agonist‐induced excitatory currents of Purkinje cells in rat cerebellar slices. The Journal of Physiology, 434, 183–213. 10.1113/jphysiol.1991.sp018465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longordo, F. , Feligioni, M. , Chiaramonte, G. , Sbaffi, P. F. , Raiteri, M. , & Pittaluga, A. (2006). The human immunodeficiency virus‐1 protein transactivator of transcription up‐regulates N‐methyl‐D‐aspartate receptor function by acting at metabotropic glutamate receptor 1 receptors coexisting on human and rat brain noradrenergic neurones. The Journal of Pharmacology and Experimental Therapeutics, 317(3), 1097–1105. 10.1124/jpet.105.099630 [DOI] [PubMed] [Google Scholar]
- Luccini, E. , Musante, V. , Neri, E. , Brambilla Bas, M. , Severi, P. , Raiteri, M. , & Pittaluga, A. (2007). Functional interactions between presynaptic NMDA receptors and metabotropic glutamate receptors co‐expressed on rat and human noradrenergic terminals. British Journal of Pharmacology, 151(7), 1087–1094. 10.1038/sj.bjp.0707280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccini, E. , Musante, V. , Neri, E. , Raiteri, M. , & Pittaluga, A. (2007). N‐Methyl‐D‐aspartate autoreceptors respond to low and high agonist concentrations by facilitating, respectively, exocytosis and carrier‐mediated release of glutamate in rat hippocampus. Journal of Neuroscience Research, 85(16), 3657–3665. 10.1002/jnr.21446 [DOI] [PubMed] [Google Scholar]
- MacDermott, A. B. , Role, L. W. , & Siegelbaum, S. A. (1999). Presynaptic ionotropic receptors and the control of transmitter release. Annual Review of Neuroscience, 22, 443–485. 10.1146/annurev.neuro.22.1.443 [DOI] [PubMed] [Google Scholar]
- Madara, J. C. , & Levine, E. S. (2008). Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain‐derived neurotrophic factor on synaptic transmission. Journal of Neurophysiology, 100(6), 3175–3184. 10.1152/jn.90880.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malva, J. O. , Carvalho, A. P. , & Carvalho, C. M. (1994). Modulation of dopamine and noradrenaline release and of intracellular Ca2+ concentration by presynaptic glutamate receptors in hippocampus. British Journal of Pharmacology, 113(4), 1439–1447. 10.1111/j.1476-5381.1994.tb17158.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli, M. , Carta, M. , Partridge, L. D. , & Valenzuela, C. F. (2005). Neurosteroid‐induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. Journal of Neuroscience, 25(9), 2285–2294. 10.1523/JNEUROSCI.3877-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli, S. , Iannuzzi, F. , Ficulle, E. , Mango, D. , Pieraccini, S. , Pellegrino, S. , … Feligioni, M. (2019). The selective disruption of presynaptic JNK2/STX1a interaction reduces NMDA receptor‐dependent glutamate release. Scientific Reports, 9(1), 1–12. 10.1038/s41598-019-43709-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi, M. , & Grilli, M. (2010). Presynaptic nicotinic receptors modulating neurotransmitter release in the central nervous system: Functional interactions with other coexisting receptors. Progress in Neurobiology, 92(2), 105–111. 10.1016/j.pneurobio.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Marchi, M. , Grilli, M. , & Pittaluga, A. M. (2015). Nicotinic modulation of glutamate receptor function at nerve terminal level: A fine‐tuning of synaptic signals. Frontiers in Pharmacology, 6, 1–10. 10.3389/fphar.2015.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien, M. , Brien, J. , & Jhamandas, K. (1983). Regional release of [3H]dopamine from rat brain in vitro: Effects of opioids on release induced by potassium, nicotine, and l‐glutamic acid. Canadian Journal of Physiology and Pharmacology, 61(1), 43–60. 10.1139/y83-005 [DOI] [PubMed] [Google Scholar]
- Marti, M. , Paganini, F. , Stocchi, S. , Mela, F. , Beani, L. , Bianchi, C. , & Morari, M. (2003). Plasticity of glutamatergic control of striatal acetylcholine release in experimental parkinsonism: Opposite changes at group‐II metabotropic and NMDA receptors. Journal of Neurochemistry, 84(4), 792–802. 10.1046/j.1471-4159.2003.01569.x [DOI] [PubMed] [Google Scholar]
- Marti, M. , Sbrenna, S. , Fuxe, K. , Bianchi, C. , Beani, L. , & Morari, M. (1999). In vitro evidence for increased facilitation of striatal acetylcholine release via pre‐ and postsynaptic NMDA receptors in hemiparkinsonian rats. Journal of Neurochemistry, 72(2), 875–878. 10.1046/j.1471-4159.1999.720875.x [DOI] [PubMed] [Google Scholar]
- Martin, D. , Bustos, G. A. , Bowe, M. A. , Bray, S. D. , & Nadler, J. V. (1991). Autoreceptor regulation of glutamate and aspartate release from slices of the hippocampal CA1 area. Journal of Neurochemistry, 56(5), 1647–1655. 10.1111/j.1471-4159.1991.tb02063.x [DOI] [PubMed] [Google Scholar]
- Matsuda, K. , Fletcher, M. , Kamiya, Y. , & Yuzaki, M. (2003). Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors. The Journal of Neuroscience, 23(31), 10064–10073. 10.1523/JNEUROSCI.23-31-10064.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness, L. , Taylor, C. , Taylor, R. D. , Yau, C. , Langenhan, T. , Hart, M. L. , … Emptage, N. J. (2010). Presynaptic NMDARs in the hippocampus facilitate transmitter release at theta frequency. Neuron, 68(6), 1109–1127. 10.1016/j.neuron.2010.11.023 [DOI] [PubMed] [Google Scholar]
- Meguro, H. , Mori, H. , Araki, K. , Kushiya, E. , Kutsuwada, T. , Yamazaki, M. , … Mishina, M. (1992). Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature, 357(6373), 70–74. 10.1038/357070a0.Mela [DOI] [PubMed] [Google Scholar]
- Mela, F. , Marti, M. , Fiorentini, C. , Missale, C. , & Morari, M. (2006). Group‐II metabotropic glutamate receptors negatively modulate NMDA transmission at striatal cholinergic terminals: Role of P/Q‐type high voltage activated Ca++ channels and endogenous dopamine. Molecular Cellular Neuroscience, 31(2), 284–292. 10.1016/j.mcn.2005.09.016 [DOI] [PubMed] [Google Scholar]
- Mennini, T. , Mancini, L. , Reggiani, A. , & Trist, D. (1997). GV 150526A, 7‐Cl‐kynurenic acid and HA 966 antagonize the glycine enhancement of N‐methyl‐D‐aspartate‐induced [3H]noradrenaline and [3H]dopamine release. European Journal of Pharmacology, 336(2–3), 275–281. 10.1016/s0014-2999(97)01260-0 [DOI] [PubMed] [Google Scholar]
- Misra, C. , Brickley, S. G. , Wyllie, D. J. , & Cull‐Candy, S. G. (2000). Slow deactivation kinetics of NMDA receptors containing NR1 and NR2D subunits in rat cerebellar Purkinje cells. Journal of Physiology, 525(Pt 2), 299–305. 10.1111/j.1469-7793.2000.t01-1-00299.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morari, M. , Sbrenna, S. , Marti, M. , Caliari, F. , Bianchi, C. , & Beani, L. (1998). NMDA and non‐NMDA ionotropic glutamate receptors modulate striatal acetylcholine release via pre‐ and postsynaptic mechanisms. Journal of Neurochemistry, 71(5), 2006–2017. 10.1046/j.1471-4159.1998.71052006.x [DOI] [PubMed] [Google Scholar]
- Moreau, A. W. , & Kullmann, D. M. (2013). NMDA receptor‐dependent function and plasticity in inhibitory circuits. Neuropharmacology, 74, 23–31. 10.1016/j.neuropharm.2013.03.004 [DOI] [PubMed] [Google Scholar]
- Mori, H. , Masaki, H. , Yamakura, T. , & Mishina, M. (1992). Identification by mutagenesis of a Mg(2+)‐block site of the NMDA receptor channel. Nature, 358(6388), 673–675. 10.1038/358673a0 [DOI] [PubMed] [Google Scholar]
- Musante, V. , Summa, M. , Cunha, R. A. , Raiteri, M. , & Pittaluga, A. (2011). Pre‐synaptic glycine GlyT1 transporter—NMDA receptor interaction: Relevance to NMDA autoreceptor activation in the presence of Mg2+ ions. Journal of Neurochemistry, 117(3), 516–527. 10.1111/j.1471-4159.2011.07223.x [DOI] [PubMed] [Google Scholar]
- Nadler, J. V. , Martin, D. , Bustos, G. A. , Burke, S. P. , & Bowe, M. A. (1990). Regulation of glutamate and aspartate release from the Schaffer collaterals and other projections of CA3 hippocampal pyramidal cells. Progress in Brain Research, 83, 115–130. 10.1016/s0079-6123(08)61245-5 [DOI] [PubMed] [Google Scholar]
- Nankai, M. , Klarica, M. , Fage, D. , & Carter, C. (1998). The pharmacology of native N‐methyl‐D‐aspartate receptor subtypes: Different receptors control the release of different striatal and spinal transmitters. Progress in Neuropsychopharmacology and Biological Psychiatry, 22(1), 35–64. 10.1016/s0278-5846(97)00180-2 [DOI] [PubMed] [Google Scholar]
- Nisticò, R. , Florenzano, F. , Mango, D. , Ferraina, C. , Grilli, M. , Di Prisco, S. , … Marchi, M. (2015). Presynaptic c‐Jun N‐terminal kinase 2 regulates NMDA receptor‐dependent glutamate release. Scientific Reports, 5, 1–14. 10.1038/srep09035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivero, G. , Bonfiglio, T. , Vergassola, M. , Usai, C. , Riozzi, B. , Battaglia, G. , … Pittaluga, A. (2017). Immuno‐pharmacological characterization of group II metabotropic glutamate receptors controlling glutamate exocytosis in mouse cortex and spinal cord. British Journal of Pharmacology, 174(24), 4785–4796. 10.1111/bph.14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivero, G. , Vergassola, M. , Cisani, F. , Roggeri, A. , & Pittaluga, A. (2019). Presynaptic release‐regulating metabotropic glutamate receptors: An update. Current Neuropharmacology, 18(7), 655–672. 10.2174/1570159X1766619112711233933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivero, G. , Vergassola, M. , Cisani, F. , Usai, C. , & Pittaluga, A. (2019). Immuno‐pharmacological characterization of presynaptic GluN3A‐containing NMDA autoreceptors: Relevance to anti‐NMDA receptor autoimmune diseases. Molecular Neurobiology, 56(9), 6142–6155. 10.1007/s12035-019-1511-8 [DOI] [PubMed] [Google Scholar]
- Pachernegg, S. , Strutz‐Seebohm, N. , & Hollmann, M. (2012). GluN3 subunit‐containing NMDA receptors: Not just one‐trick ponies. Trends in Neurosciences, 35(4), 240–249. 10.1016/j.tins.2011.11.010 [DOI] [PubMed] [Google Scholar]
- Paoletti, P. (2011). Molecular basis of NMDA receptor functional diversity. The European Journal of Neuroscience, 33(8), 1351–1365. 10.1111/j.1460-9568.2011.07628.x [DOI] [PubMed] [Google Scholar]
- Paoletti, P. , & Neyton, J. (2007). NMDA receptor subunits: Function and pharmacology. Current Opinion in Pharmacology, 7(1), 39–47. 10.1016/j.coph.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Paudice, P. , Gemignani, A. , & Raiteri, M. (1998). Evidence for functional native NMDA receptors activated by glycine or d‐serine alone in the absence of glutamatergic coagonist. The European Journal of Neuroscience, 10(9), 2934–2944. 10.1046/j.1460-9568.1998.00302.x [DOI] [PubMed] [Google Scholar]
- Perez‐Otano, I. , Schulteis, C. T. , Contractor, A. , Lipton, S. A. , Trimmer, J. S. , Sucher, N. J. , & Heinemann, S. F. (2001). Assembly with the NR1 subunit is required for surface expression of NR3A‐containing NMDA receptors. Journal of Neuroscience, 21(4), 1228–1237. 10.1523/JNEUROSCI.21-04-01228.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Otaño, I. , Larsen, R. S. , & Wesseling, J. F. (2016). Emerging roles of GluN3‐containing NMDA receptors in the CNS. Nature Reviews. Neuroscience, 17(10), 623–635. 10.1038/nrn.2016.92 [DOI] [PubMed] [Google Scholar]
- Phillips, G. R. , Huang, J. K. , Wang, Y. , Tanaka, H. , Shapiro, L. , Zhang, W. , … Colman, D. R. (2001). The presynaptic particle web: Ultrastructure, composition, dissolution, and reconstitution. Neuron, 32(1), 63–77. 10.1016/s0896-6273(01)00450-0 [DOI] [PubMed] [Google Scholar]
- Pilli, J. , & Kumar, S. S. (2012). Triheteromeric N‐methyl‐D‐aspartate receptors differentiate synaptic inputs onto pyramidal neurons in somatosensory cortex: involvement of the GluN3A subunit. Neuroscience, 222, 75–88. 10.1016/j.neuroscience.2012.07.020 [DOI] [PubMed] [Google Scholar]
- Pinheiro, P. S. , & Mulle, C. (2008). Presynaptic glutamate receptors: Physiological functions and mechanisms of action. Nature Reviews. Neuroscience, 9(6), 423–436. 10.1038/nrn2379 [DOI] [PubMed] [Google Scholar]
- Pittaluga, A. (2016). Presynaptic release‐regulating mGlu1 receptors in central nervous system. Frontiers in Pharmacology, 7, 1–15. 10.3389/fphar.2016.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittaluga, A. (2019). Acute Functional Adaptations in Isolated Presynaptic Terminals Unveil Synaptosomal Learning and Memory. International Journal of Molecular Sciences, 20(15), 1–16. 10.3390/ijms20153641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittaluga, A. , Bonfanti, A. , & Raiteri, M. (2000). Somatostatin potentiates NMDA receptor function via activation of InsP(3) receptors and PKC leading to removal of the Mg(2+) block without depolarization. British Journal of Pharmacology, 130(3), 557–566. 10.1038/sj.bjp.0703346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittaluga, A. , Fedele, E. , Risiglione, C. , & Raiteri, M. (1993). Age‐related decrease of the NMDA receptor‐mediated noradrenaline release in rat hippocampus and partial restoration by d‐cycloserine. European Journal of Pharmacology, 231(1), 129–134. 10.1016/0014-2999(93)90693-c [DOI] [PubMed] [Google Scholar]
- Pittaluga, A. , Pattarini, R. , Feligioni, M. , & Raiteri, M. (2001). N‐Methyl‐D‐aspartate receptors mediating hippocampal noradrenaline and striatal dopamine release display differential sensitivity to quinolinic acid, the HIV‐1 envelope protein gp120, external pH and protein kinase C inhibition. Journal of Neurochemistry, 76(1), 139–148. 10.1046/j.1471-4159.2001.00057.x [DOI] [PubMed] [Google Scholar]
- Pittaluga, A. , Pattarini, R. , Severi, P. , & Raiteri, M. (1996). Human brain N‐methyl‐D‐aspartate receptors regulating noradrenaline release are positively modulated by HIV‐1 coat protein gp120. Aids, 10(5), 463–468. 10.1097/00002030-199605000-00003 [DOI] [PubMed] [Google Scholar]
- Pittaluga, A. , & Raiteri, M. (1990). Release‐enhancing glycine‐dependent presynaptic NMDA receptors exist on noradrenergic terminals of hippocampus. European Journal of Pharmacology, 191(2), 231–234. 10.1016/0014-2999(90)94153-o [DOI] [PubMed] [Google Scholar]
- Pittaluga, A. , & Raiteri, M. (1992a). N‐Methyl‐D‐aspartic acid (NMDA) and non‐NMDA receptors regulating hippocampal norepinephrine release. I. Location on axon terminals and pharmacological characterization. The Journal of Pharmacology and Experimental Therapeutics, 260, 232–237. [PubMed] [Google Scholar]
- Pittaluga, A. , & Raiteri, M. (1992b). N‐Methyl‐D‐aspartic acid (NMDA) and non‐NMDA receptors regulating hippocampal norepinephrine release. III. Changes in the NMDA receptor complex induced by their functional cooperation. The Journal of Pharmacology and Experimental Therapeutics, 263, 327–333. [PubMed] [Google Scholar]
- Pittaluga, A. , & Raiteri, M. (1994). HIV‐1 envelope protein gp120 potentiates NMDA‐evoked noradrenaline release by a direct action at rat hippocampal and cortical noradrenergic nerve endings. The European Journal of Neuroscience, 6(11), 1743–1749. 10.1111/j.1460-9568.1994.tb00566.x [DOI] [PubMed] [Google Scholar]
- Raiteri, L. , & Raiteri, M. (2000). Synaptosomes still viable after 25 years of superfusion. Neurochemistry Research, 25(9–10), 1265–1274. 10.1023/a:1007648229795 [DOI] [PubMed] [Google Scholar]
- Raiteri, L. , & Raiteri, M. (2010). Functional 'glial' GLYT1 glycine transporters expressed in neurons. Journal of Neurochemistry, 114(3), 647–653. 10.1111/j.1471-4159.2010.06802.x [DOI] [PubMed] [Google Scholar]
- Raiteri, M. (2006). Functional pharmacology in human brain. Pharmacological Reviews, 58(2), 162–193. 10.1124/pr.58.2.5 [DOI] [PubMed] [Google Scholar]
- Raiteri, M. , Angelini, F. , & Levi, G. (1974). A simple apparatus for studying the release of neurotransmitters from synaptosomes. European Journal of Pharmacology, 25(3), 411–414. 10.1016/0014-2999(74)90272-6 [DOI] [PubMed] [Google Scholar]
- Raiteri, M. , Garrone, B. , & Pittaluga, A. (1992). N‐Methyl‐D‐aspartic acid (NMDA) and non‐NMDA receptors regulating hippocampal norepinephrine release. II. Evidence for functional cooperation and for coexistence on the same axon terminal. The Journal of Pharmacology and Experimental Therapeutics, 260(1), 238–242. [PubMed] [Google Scholar]
- Ransom, R. W. , & Deschenes, N. L. (1989). Glycine modulation of NMDA‐evoked release of [3H]acetylcholine and [3H]dopamine from rat striatal slices. Neuroscience Letters, 96(3), 323–328. 10.1016/0304-3940(89)90399-6 [DOI] [PubMed] [Google Scholar]
- Risso, F. , Parodi, M. , Grilli, M. , Molfino, F. , Raiteri, M. , & Marchi, M. (2004). Chronic nicotine causes functional upregulation of ionotropic glutamate receptors mediating hippocampal noradrenaline and striatal dopamine release. Neurochemistry International, 44(5), 293–301. 10.1016/s0197-0186(03)00173-6 [DOI] [PubMed] [Google Scholar]
- Roberts, P. J. , & Anderson, S. D. (1979). Stimulatory effect of l‐glutamate and related amino acids on [3H]dopamine release from rat striatum: An in vitro model for glutamate actions. Journal of Neurochemistry, 32(5), 1539–1545. 10.1111/j.1471-4159.1979.tb11096.x [DOI] [PubMed] [Google Scholar]
- Roberts, P. J. , & Sharif, N. A. (1978). Effects of l‐glutamate and related amino acids upon the release of [3H]dopamine from rat striatal slices. Brain Research, 157(2), 391–395. 10.1016/0006-8993(78)90048-3 [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Moreno, A. , Banerjee, A. , & Paulsen, O. (2010). Presynaptic NMDA receptors and spike timing‐dependent depression at cortical synapses. Frontiers in Synaptic Neuroscience, 2, 1–16. 10.3389/fnsyn.2010.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Moreno, A. , & Paulsen, O. (2008). Spike timing‐dependent long‐term depression requires presynaptic NMDA receptors. Nature Neuroscience, 11(7), 744–745. 10.1038/nn.2125 Epub 2008 May 30 [DOI] [PubMed] [Google Scholar]
- Rossi, B. , Ogden, D. , Llano, I. , Tan, Y. P. , Marty, A. , & Collin, T. (2012). Current and calcium responses to local activation of axonal NMDA receptors in developing cerebellar molecular layer interneurons. PLoS ONE, 7(6), e39983. 10.1371/journal.pone.0039983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone, A. , Zappettini, S. , Grilli, M. , Olivero, G. , Agostinho, P. , Tomé, A. R. , … Marchi, M. (2014). Prolonged nicotine exposure down‐regulates presynaptic NMDA receptors in dopaminergic terminals of the rat nucleus accumbens. Neuropharmacology, 79, 488–497. 10.1016/j.neuropharm.2013.12.014 [DOI] [PubMed] [Google Scholar]
- Scatton, B. , & Lehmann, J. (1982). N‐Methyl‐C‐aspartate‐type receptors mediate striatal 3H‐acetylcholine release evoked by excitatory amino acids. Nature, 297(5865), 422–424. 10.1038/297422a0 [DOI] [PubMed] [Google Scholar]
- Sequeira, S. M. , Malva, J. O. , Carvalho, A. P. , & Carvalho, C. M. (2001). Presynaptic N‐methyl‐D‐aspartate receptor activation inhibits neurotransmitter release through nitric oxide formation in rat hippocampal nerve terminals. Brain Research Molecular Brain Reserach, 89(1–2), 111–118. 10.1016/s0169-328x(01)00069-9 [DOI] [PubMed] [Google Scholar]
- Smothers, C. T. , & Woodward, J. J. (2007). Pharmacological characterization of glycine‐activated currents in HEK 293 cells expressing N‐methyl‐D‐aspartate NR1 and NR3 subunits. Journal of Pharmacol and Experimental Therapeutics, 322(2), 739–748. 10.1124/jpet.107.123836 [DOI] [PubMed] [Google Scholar]
- Snell, L. D. , & Johnson, K. M. (1986). Characterization of the inhibition of excitatory amino acid‐induced neurotransmitter release in the rat striatum by phencyclidine‐like drugs. The Journal of Pharmacology and Experimental Therapeutics, 238(3), 938–946. [PubMed] [Google Scholar]
- Spencer, H. J. (1976). Antagonism of cortical excitation of striatal neurons by glutamic acid diethyl ester: Evidence for glutamic acid as an excitatory transmitter in the rat striatum. Brain Research, 102(1), 91–101. 10.1016/0006-8993(76)90577-1 [DOI] [PubMed] [Google Scholar]
- Stroebel, D. , Casado, M. , & Paoletti, P. (2017). Triheteromeric NMDA receptors: From structure to synaptic physiology. Current Opinion in Physiology, 2, 1–12. 10.1016/j.cophys.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys, P. K. , & Lipton, S. A. (2007). White matter NMDA receptors: An unexpected new therapeutic target? Trends in Pharmacological Sciences, 28(11), 561–566. 10.1016/j.tips.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Sundström, E. , Holmberg, L. , & Souverbie, F. (1998). NMDA and AMPA receptors evoke transmitter release from noradrenergic axon terminals in the rat spinal cord. Neurochemical Research, 23(12), 1501–1507. 10.1023/a:1020967601813 [DOI] [PubMed] [Google Scholar]
- Vizi, E. S. , Kiss, J. P. , & Lendvai, B. (2004). Nonsynaptic communication in the central nervous system. Neurochemistry International, 45(4), 443–451. 10.1016/j.neuint.2003.11.016 [DOI] [PubMed] [Google Scholar]
- Wang, J. K. (1991). Presynaptic glutamate receptors modulate dopamine release from striatal synaptosomes. Journal of Neurochemistry, 57(3), 819–822. 10.1111/j.1471-4159.1991.tb08224.x [DOI] [PubMed] [Google Scholar]
- Wang, J. K. , Andrews, H. , & Thukral, V. (1992). Presynaptic glutamate receptors regulate noradrenaline release from isolated nerve terminals. Journal of Neurochemistry, 58(1), 204–211. 10.1111/j.1471-4159.1992.tb09297.x [DOI] [PubMed] [Google Scholar]
- Wang, J. K. , & Thukral, V. (1996). Presynaptic NMDA receptors display physiological characteristics of homomeric complexes of NR1 subunits that contain the exon 5 insert in the N‐terminal domain. Journal of Neurochemistry, 66(2), 865–868. 10.1046/j.1471-4159.1996.66020865.x [DOI] [PubMed] [Google Scholar]
- Wee, K. S. , Zhang, Y. , Khanna, S. , & Low, C. M. (2008). Immunolocalization of NMDA receptor subunit NR3B in selected structures in the rat forebrain, cerebellum, and lumbar spinal cord. The Journal of Comparative Neurology, 509(1), 118–135. 10.1002/cne.21747 [DOI] [PubMed] [Google Scholar]
- Whittaker, M. T. , Gibbs, T. T. , & Farb, D. H. (2008). Pregnenolone sulfate induces NMDA receptor dependent release of dopamine from synaptic terminals in the striatum. Journal of Neurochemistry, 107(2), 10–21. 10.1111/j.1471-4159.2008.05627.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhall, G. , Evans, D. I. , Cunningham, M. O. , & Jones, R. S. (2001). NR2B‐containing NMDA autoreceptors at synapses on entorhinal cortical neurons. Journal of Neurophysiology, 86(4), 1644–1651. 10.1152/jn.2001.86.4.1644 [DOI] [PubMed] [Google Scholar]
- Zappettini, S. , Grilli, M. , Olivero, G. , Chen, J. , Padolecchia, C. , Pittaluga, A. , … Marchi, M. (2014). Nicotinic α7 receptor activation selectively potentiates the function of NMDA receptors in glutamatergic terminals of the nucleus accumbens. Frontiers in Cellular Neuroscience, 8, 1–10. 10.3389/fncel.2014.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M. , Peterson, C. L. , Lu, Y. B. , & Nadler, J. V. (1995). Release of glutamate and aspartate from CA1 synaptosomes: Selective modulation of aspartate release by ionotropic glutamate receptor ligands. Journal of Neurochemistry, 64(4), 1556–1566. 10.1046/j.1471-4159.1995.64041556.x [DOI] [PubMed] [Google Scholar]
- Zukin, R. S. , & Bennett, M. V. (1995). Alternative spliced isoforms of the NMDARI receptor subunit. Trends in Neurosciences, 18(7), 306–313. 10.1016/0166-2236(95)93920-s [DOI] [PubMed] [Google Scholar]
- Zuo, P. , Ogita, K. , Suzuki, T. , Han, D. , & Yoneda, Y. (1993). Further evidence for multiple forms of an N‐methyl‐D‐aspartate recognition domain in rat brain using membrane binding techniques. Journal of Neurochemistry, 61(5), 1865–1873. 10.1111/j.1471-4159.1993.tb09828.x [DOI] [PubMed] [Google Scholar]


