Abstract
Objective:
To compare the efficacy and discontinuation of augmentation agents in adult patients with treatment-resistant depression (TRD). We conducted a systematic review and network meta-analyses (NMA) to combine direct and indirect comparisons of augmentation agents.
Methods:
We included randomized controlled trials comparing one active drug with another or with placebo following a treatment course up to 24 weeks. Nineteen agents were included: stimulants, atypical antipsychotics, thyroid hormones, antidepressants, and mood stabilizers. Data for response/remission and all-cause discontinuation rates were analyzed. We estimated effect-size by relative risk using pairwise and NMA with random-effects model.
Results:
A total of 65 studies (N = 12,415) with 19 augmentation agents were included in the NMA. Our findings from the NMA for response rates, compared to placebo, were significant for: liothyronine, nortriptyline, aripiprazole, brexpiprazole, quetiapine, lithium, modafinil, olanzapine (fluoxetine), cariprazine, and lisdexamfetamine. For remission rates, compared to placebo, were significant for: thyroid hormone(T4), aripiprazole, brexpiprazole, risperidone, quetiapine, and olanzapine (fluoxetine). Compared to placebo, ziprasidone, mirtazapine, and cariprazine had statistically significant higher discontinuation rates. Overall, 24% studies were rated as having low risk of bias (RoB), 63% had moderate RoB and 13% had high RoB.
Limitations:
Heterogeneity in TRD definitions, variable trial duration and methodological clinical design of older studies and small number of trials per comparisons.
Conclusions:
This NMA suggests a superiority of the regulatory approved adjunctive atypical antipsychotics, thyroid hormones, dopamine compounds (modafinil and lisdexamfetamine) and lithium. Acceptability was lower with ziprasidone, mirtazapine, and cariprazine. Further research and head-to-head studies should be considered to strengthen the best available options for TRD.
Keywords: Network Meta-analysis, Unipolar depression, Treatment resistant, Mood disorders, Efficacy
1. Introduction
Despite advances in pharmacological and non-pharmacological treatments for depression, strategies to improve treatment-resistant depression (TRD) continue to be inadequate, leading to poor outcomes and functional impairment (Fava, 2003; Fekadu et al., 2009; Malhi et al., 2005). Despite more than 25 FDA approved medications for major depressive disorder (MDD), TRD continues to be highly prevalent, with more than 30% of patients failing to achieve remission despite an adequate pharmacotherapeutic trial (Berlim and Turecki, 2007b; Rush et al., 2006). Current treatment guidelines recommend augmentation strategies after failure of two antidepressants or partial response with a primary antidepressant (Kennedy et al., 2016). However, consensus has not yet been established regarding an integrated definition for TRD (i.e. after trial of one or two antidepressant of same or different classes) (Berlim and Turecki, 2007a). Moreover, there has been a lack of consistency with recommendations for augmentation strategy in MDD not/partially responsive to first-line antidepressants (McIntyre et al., 2014). According to the Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines and American Psychiatric Association Practice guidelines, atypical antipsychotics (AA) have been reported as an effective augmentation strategy to antidepressants. Among other agents, mood stabilizers, combination of antidepressants, use of stimulants, thyroid hormones, and ketamine have been investigated (Gelenberg et al., 2010; Kennedy et al., 2016). Previous meta-analyses have shown evidence for the use of AAs, lithium, ketamine and esketamine (Carter et al., 2020; Strawbridge et al., 2019; Vázquez et al., 2021; Zhou et al., 2015).
Network meta-analysis (NMA) is a promising statistical method which allows to synthetize information from different clinical trials by combining direct and indirect evidence in the absence of head-to-head treatment comparisons. As compared to conventional meta analytical approaches; NMA yield estimates of the intervention compared to all others, using a higher degree of precision by rank probabilities which can have important implications for treatment decisions in clinical practice (Cipriani et al., 2013; Mavridis et al., 2015; Salanti, 2012). Previous studies utilizing NMA approaches have suggested the use of AAs (Zhou et al., 2015; Carter et al., 2020) as augmentation agents, although number of included trials and agents have varied across studies due to different inclusion criteria. Head-to-head trials comparing augmentation strategies in different phases of illness (acute versus maintenance), different treatment-resistant staging models, dose-response, and non-pharmacological interventions are needed. Carter et al. recently conducted a systematic review and NMA of pharmacological and psychological augmentation interventions for TRD (defined as two adequate treatments in current episode), however, no psychological trial could be included due to the absence of a common comparator (Carter et al., 2020). There are still controversies surrounding the relative efficacy of different augmentation agents [mood stabilizers (lithium, lamotrigine), thyroid hormones, or stimulants] in the daily clinical practice. Recent systematic reviews support the use of dopaminergic compounds (stimulants and stimulants-like compounds) both for unipolar and bipolar depression (McIntyre et al., 2017; Nunez et al., 2020; Szmulewicz et al., 2017). A comprehensive review comparing multiple interventions in a more diverse patient population would help provide important information to clinicians, especially for TRD patients who have failed one or more antidepressants. Therefore, we conducted an updated systematic review and NMA to appraise the efficacy and safety of augmentation agents in adult patients with unipolar TRD with the aim to improve clarity of pharmacological augmentation recommendations for TRD.
2. Methods
Our systematic review and NMA is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines.
2.1. Data sources and search
A comprehensive search of several databases from each database’s inception to May 29th of 2020, any language, was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Ovid PsycINFO, and Scopus. The search strategy was designed and conducted by an experienced librarian (LJP) with input from the study investigator. Controlled vocabulary supplemented with keywords (e.g. “major depression”, “refractory depression”, “response”, “remission”, “depression scores”, “affective disorders”, “augmentation treatments,) was used to identify the potential eligible studies. Search was performed focused on articles with English language. Search was limited to human studies and included randomized controlled trials (RCTs) which compared one active drug with another or placebo following an augmentation treatment course of up to 24 weeks and assessed change in severity of depressive symptoms. We included the following agents for augmentation treatments: stimulants/dopaminergic compounds (lisdexamfetamine, dextroamphetamine/amphetamine; methylphenidate; pramipexole; modafinil/armodafinil); thyroid hormones [specifically triiodothyronine (T3), levothyroxine (T4)], mood stabilizers (lithium, lamotrigine), atypical antipsychotics (olanzapine/olanzapine(fluoxetine), aripiprazole, brexpiprazole, quetiapine, risperidone, cariprazine, ziprasidone), antidepressants (mirtazapine, bupropion, venlafaxine nortriptyline, amitriptyline) and buspirone (a serotonin 5-HT1A partial agonist). We excluded case reports, longitudinal studies or conference abstracts or studies which included participants with bipolar disorder and non-resistant depression.
A manual search of references was performed in the included studies to identify additional studies. The actual strategy listing all search terms used and how they are combined is available in the Appendix 1, Table 1.
2.2. Study selection and eligibility criteria
Two reviewers, (NAN, MP) working independently, screened the titles and abstracts of potentially eligible articles. Subsequently, the full texts of eligible articles were reviewed separately by the same two reviewers. The study’s inclusion criteria were the following: (1) Adult MDD patients (18–65 years of age) refractory to one or more antidepressant therapies at an adequate dose; (2) RCTs; (3) Interventional studies evaluating the use of augmentation treatments; (4) Studies reporting outcome data on response or remission rates and all-cause discontinuation rates if available (details in the outcome measure section); (5) Articles published in English.
Exclusion criteria were: (1) Studies that evaluated only bipolar patients or MDD with psychotic features; patients with post-partum depression or prenatal depression or with serious medical illnesses; (2) Narrative reviews, letters or editorials; (3) Case series, case reports or conference abstracts; (4) Non-interventional or observational study design as well as prospective or non-randomized open label studies; (5) Multiple reports from the same data set (only the original research studies were included to prevent duplication of the data set).
Most studies had a study duration of 4 weeks or longer, however, we also included studies with a shorter duration (< 4 weeks) for lithium and thyroid as there is data suggesting faster efficacy for these two agents.
If a study included patients with both unipolar and bipolar disorders or psychotic depression, we included the study if data was available for patients with non-psychotic unipolar depression.
2.3. Data collection and risk of bias assessment
Data were extracted independently by three review authors (NAN, BJ, and MP) according to inclusion criteria. Any discrepancies were resolved by discussion and consensus by the group of investigators and the senior author (BS). The methodological quality of the RCTs was assessed using the Cochrane collaboration’s Risk Interventions Risk of Bias Tool (Higgins et al., 2011) reporting each of its five domains (adequate randomization and allocation, blinding, loss to follow-up, selective reporting, and other sources of potential bias) into a low risk of bias, high risk of bias, or unclear risk of bias. Additionally, studies domains were classified by a low, unclear and high risk of bias leading to an overall study level risk of bias (Higgins et al., 2011, 2019). For multiple treatment groups, we merged treatment groups into one when these groups used different doses of the same medication (Higgins et al., 2011). Similar to a previous study (Lorentzen et al., 2020), we interpreted the dose of 80 mg/day in the study by Fang et al. (2011) for thyroid hormones as T4.
We examined the publication bias by examining a symmetry of the funnel plot of the trials using Eggers tests and regression asymmetry tests (Begg and Mazumdar, 1994; Egger et al., 1997). In case of asymmetry we applied the trim and fill method for all outcome measures (Begg and Mazumdar, 1994).
2.4. Outcomes measures
Dichotomous data for response/remission and all cause discontinuation rates were analyzed. The primary outcome was response rates [(defined as a decrease of ≥ 50% of the validated behavioral scales for depression (e.g. HAMD or MADRS)]. Secondary outcomes included: remission rates, defined as a score below <10 for the MADRS or <7 on the HAMD or as per the study, and all cause discontinuation rates calculated for the patients who discontinued the trial. We elected to use all cause discontinuation (proportion of patients who discontinued due to side effects) as measure of overall acceptability considering that data was not widely available for all studies.
2.5. Statistical analyses
An NMA was conducted based on a multi treatment random-effects frequentist NMA for all comparisons within the trials. We estimated effect sizes by relative ratio (RR) with 95% CI for binary outcomes. Pairwise meta-analyses were conducted using the Der-Simonian and Laird random effects model (DerSimonian and Laird, 1986). To visualize the network geometry a network plot was produced. We created league tables to display the outcome (remission/response/all cause discontinuation) for all pair-wise comparisons of studied pharmacological agents. We used P-scores to rank pharmacological agents based on the studied outcome. For remission and response rates, P scores ranged from 0 to 1, with a lower P score indicating a favorable outcome compared to placebo. For all cause discontinuation, higher P scores were associated with lower discontinuation risk compared to placebo.
Heterogeneity between the studies was assessed using the I2 statistic (Higgins et al., 2003). The I2 statistic measures the percentage of variability that cannot be attributed to random error. A value of I2 = 0% to 50% was considered as low heterogeneity; 50% to 75% as moderate heterogeneity; and 75% to 90% as high heterogeneity. We produced a network plot by integrating all the randomized pharmacological treatments. Inconsistency between findings from pairwise meta-analyses (direct evidence) and NMA (indirect evidence) was evaluated by chi2 test using the “netheat” function (Krahn et al 2013) (Appendix 1, Table 2).
All the statistical analyses were conducted using the “meta” (version 4.9.6) “metafor” (version 2.1.0) and “netmeta” (version 0.9–7) packages of the R software for statistical computing (version 3.6.1) in R (R Core Team, 2019; Schwarzer, 2007; Team R, 2016; Viechtbauer, 2010). For statistical significance we used a p <0.05 and 95% CI not containing 1 were considered statistical significant. Meta-analysis code is provided in Appendix 2.
3. Results
A total of 6322 records were retrieved of which 69 studies were included (Fig. 1). To visualize the network geometry a network plot was produced (Fig. 2). Table 1 contains details of the trial characteristics: Nine studies (N = 1450) examined head to head drugs (Bauer et al., 2013; Cheon et al., 2017; Dorée et al., 2007; Dunner et al., 2007; Nierenberg et al., 2006; Raeisi et al., 2006; Schindler and Anghelescu, 2007; Shahal et al., 1996; Trivedi et al., 2006) with a treatment duration of up to 12 weeks. Fifty one studies (52 cohorts) evaluated medications in comparison to placebo (Abolfazli et al., 2011; Appelberg et al., 2001; Barbee et al., 2011; Barbosa et al., 2003; Bauer et al., 2009; Baumann et al., 1996; Berman et al., 2007; Browne et al., 1990,Carpenter et al., 2002; Chaput et al., 2008; Corrigan et al., 2000; Corya et al., 2006; Cusin et al., 2013; DeBattista et al., 2003; Dunlop et al., 2007; Durgam et al., 2016; Earley et al., 2018; El-Khalili et al., 2010; Fava et al., 2018, 2012, 2005; Gitlin et al., 1987; GlaxoSmithKline, 2009; Gulrez et al., 2012; Han et al., 2015; Heninger et al., 1983; Kamijima et al., 2013; Katona et al., 1995; Keitner et al., 2009; Kessler et al., 2018; Landén et al., 1998; Madhoo et al., 2014; Mahmoud et al., 2007; Marcus et al., 2008; McIntyre et al., 2007; Nierenberg et al., 2003; Normann et al., 2002; Papakostas et al., 2015; Patkar et al., 2006; Berman et al., 2009; Ravindran et al., 2008; Reeves et al., 2008; Richards et al., 2016; Santos et al., 2008; Schöpf et al., 1989; Stein and Bernadt, 1993; Thase et al., 2007, 2015b, 2015a; Trivedi et al., 2013; Zusky et al., 1988) with a sample size of N = 10,701, and the treatment duration ranging from 48 hours–16 weeks; and 9 studies were multi-arm studies (Fang et al., 2011; Franco-Chaves et al., 2013; Joffe et al., 1993; Joffe and Singer, 1990, 2006; Shelton et al., 2001; Shelton et al., 2005; Yoshimura et al., 2014, 2012) with a sample size of (N = 972), and treatment duration of 2–8 weeks.
Fig. 1.
Flow diagram.
Full-text articles were excluded due to the following reasons: (14) were open label studies; (18) were articles that did not report data on depressive symptoms focused on neuroimaging (10) wrong publication type, (5) were not treatment resistant and (7) articles fell under the exclusion criteria listed in the section above.
Fig. 2.
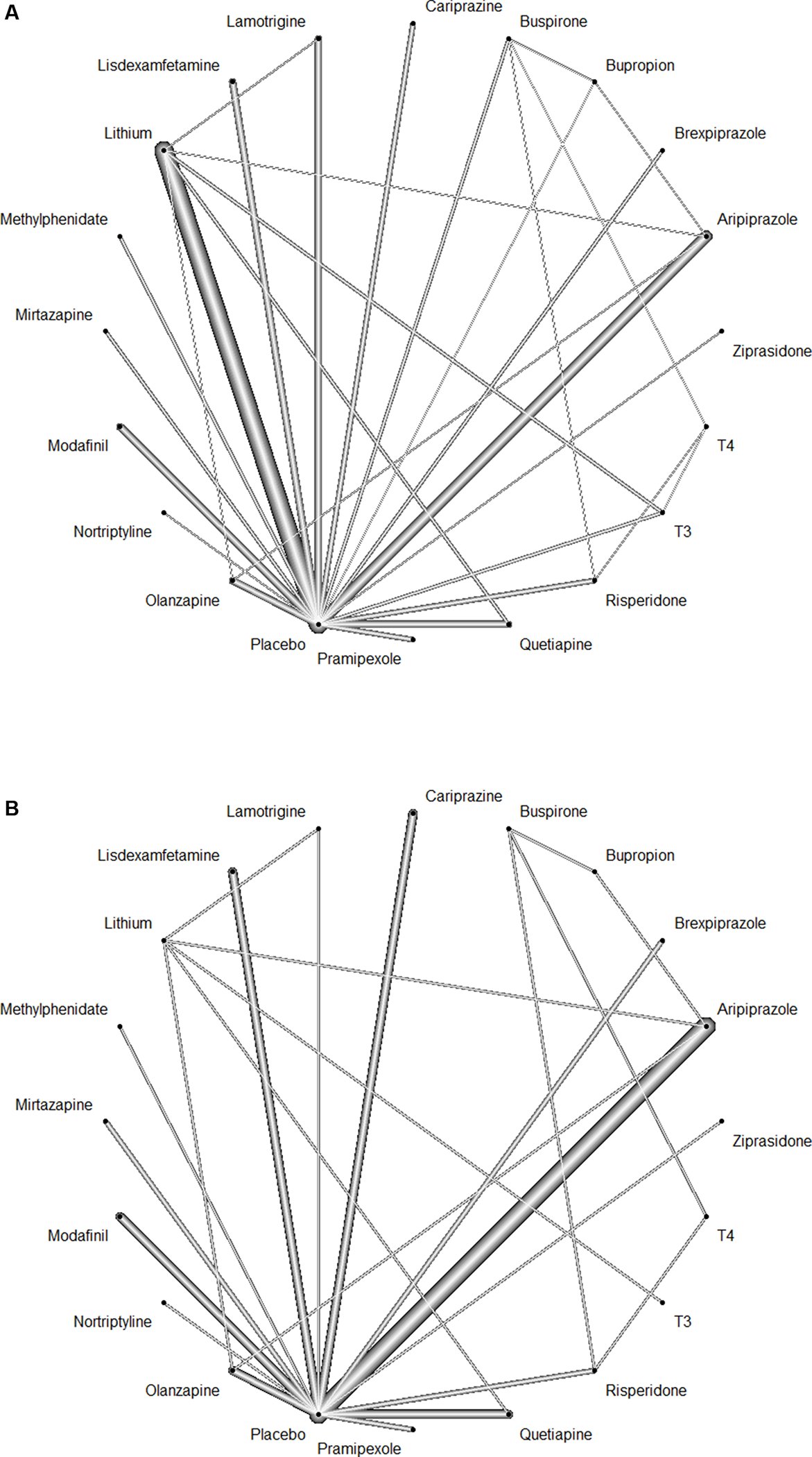
Network graph of the included studies to enable visualization of the geometry of the treatment network according to measured outcomes. (A) Response (B) Remission.
Each of the connected line represent a comparison between 2 interventions. The thickness of each line corresponds to the number of trials comparing every pair of treatments.
Table 1.
Socio-demographic and clinical characteristic of randomized clinical trials (RCTs) assessing the augmentation in TRD.
| Author, Year | Intervention/Stage of TRD | Sample size | Male: female ratio (M:F), Male (%) | Mean age ± SD (y)/range | Duration (weeks) | Primary Outcome Measure | Results |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Head-to-head randomized trials | |||||||
| Bauer et al. (2013) | Lithium vs. Quetiapine Stage I & II |
Total sample= 460 subjects QTP=231 subjects Li=229 subjects |
Not available. | 18–65 | 6 weeks | MADRS | Positive. Add-on QTP is not-inferior to add-on Li. |
| Cheon et al. (2017 | Aripiprazole vs Bupropion Stage I |
Total sample= 103 subjects ARI= 56 subjects BUP= 47 subjects |
ARI= 23:33; 41.1% BUP= 13:34; 27.7% |
ARI= 43.86 (16.63) BUP= 47.69 (16.05) |
6 weeks | MADRS HAM-D17 |
Positive. ARI and BUP augmentation therapies in combination with SSRI had comparable efficacy and tolerability. |
| Dorée et al. (2007) | Lithium vs. quetiapine Stage I & II |
Total sample= 20 subjects Li= 10 QTP= 10 |
Li= 3:7; 30% QTP= 5:5; 50% |
Li= 49.3 (9.4) QTP= 52.3 (8.1) |
8 weeks | HAM-D 17, MADRS | Positive. QTP efficacy in the sample of patients was greater than that of Li. Significant improvement was seen in both groups. |
| Dunner et al. (2007) | Ziprasidone vs. Sertraline Stage I & II | Total sample = 61 subjects ZIP=41 SER Mono=20 | ZIP= 22 (55%) SER Mono= 9 (45%) |
ZIP= 42.85 (11.35) SER= 46.3 (10.4) |
8 weeks | MADRS HAM-D 17 |
Positive. ZIP at higher doses were well tolerated and efficacious in improvement of depressive symptoms. |
| Nierenberg et al. (2006) | Lithium vs. Thyroid hormone (T3) Stage II |
Total sample= 142 subjects Li= 69 subjects T3= 73 subjects |
Li= 9:14; 39.13% T3= 32:41; 43.83% |
Li= 40.6 (12.2) T3= 43.2 (11.8) |
12 weeks | QIDS-C, HAMD-17 | Negative. Remission rates did not differ significantly. T3 had fewer side effects. |
| Raeisi et al. (2006) | Nortriptyline/Citalopram vs. Citalopram Stage II |
Total sample= 45 subjects NOR+CTP=23 subjects CTP=22 subjects |
NOR+CTP= 14:9; 60.86% CTP= 13:9; 59.09% |
NOR+CTP=33.63 (11.34) CTP=32.31 (9.97) |
8 weeks | HAM-D17 | Positive. Combo NOR+CTP was more effective than CTP alone. |
| Schindler and Anghelescu (2007) | Lamotrigine vs. Lithium Stage II |
Total sample= 34 subjects LAM=17 subjects Li= 17 subjects |
LAM= 8:9; 47.05% Li= 9:8; 52.94% |
LAM= 45.1(13.4) Li= 50.3(13.6) |
8 weeks | HAM-D 17 | Positive. LAM augmentation was efficacy and tolerability were comparable to Li augmentation. |
| Shahal et al. (1996) | Lithium/Imipramine vs. Imipramine Stage III |
Total subjects = 20 Li/IMI= 11 subjects IMI= 9 subjects |
Li/IMI= 4:7; 57.14% IMI= 5:4; 55.55% |
Li/IMI= 52.1 (17.2) IMI= 52.0 (14.9) |
5 weeks | HAM-D 17 CGI-I |
Negative. Li/IMI does not offer a therapeutic benefit. |
| Trivedi et al. (2006) | Bupropion vs buspirone Stage I | Total subjects = 565 BUP = 279 BUSP = 286 | BUP= 107/172 (38%) BUSP= 126/160 (44%) |
BUP= 40.8 (12.9) BUSP= 41.5 (12.6) |
12 weeks | HAMD 17, QIDS SR-16 | Positive. Augmentation of CPT with either BUP or BUSP appears to be useful. However, BUP had certain advantages compared to BUSP. |
| Placebo-controlled trials | |||||||
| Abolfazli et al. (2011) | Modafinil vs. Placebo Stage I |
Total sample= 46 subjects MOD+FLX= 23 subjects PLB+FLX= 23 subjects |
MOD+FLX= 11:12; 45.46% PLB+FLX = 11:11; 50% |
MOD+FLX= 33.13 (7.53) PLB+FLX = 33.27 (6.08) | 6 weeks | HAM-D 17 | Positive. No significant differences between groups in side effects. |
| Appelberg et al. (2001) | Buspirone vs. Placebo Stage I |
Total sample= 102 subjects BUSP= 51 subjects PLB= 51 subjects |
BUSP: 19:32, 37.25% PLB= 19:32, 37.25% |
BUSP= 44 PLB= 44 |
6 weeks | MADRS, CGI | Negative. Improvement with BUSP at first week only but no significant difference at endpoint. |
| Barbosa et al. (2003) | Lamotrigine vs. Placebo Stage I |
Total sample= 23 subjects LAM= 13 subjects PLB= 10 subjects (15 MDD; 8 BD-II) |
LAM: 8:5, 61.5% PLB= 2:3, 40% |
LAM=30.2 (8.4) PLB= 34.1 (6.9) |
6 weeks | HAM-D 17 MADRS CGI-S CGI-I |
Positive. Lam was superior to PLB by CGI at endpoint, however failed to separate statistically from PLB on HAM-D17 and MADRS.There was no statistically significant difference in outcomes between the MDD and BD-II patients. |
| Barbee et al. (2011) | Lamotrigine vs. Placebo Stage II |
Total sample= 96 subjects LAM= 48 subjects PLB= 48 subjects |
LAM: 5:11, 31.25% PLB= 5:11, 31.25% |
LAM= 44.59 (12.22) PLB= 45.83 (10.95) |
10 weeks | MADRS HAM-D 17 CGI-S/ CGI-I |
Negative. No significant difference between Lam and PLB; only on those more severely ill. |
| Bauer et al. (2009) | Quetiapine 300 mg/day, Quetiapine 150 mg/day vs. placebo Stage I |
Total sample = 487 subjects QTP 150= 166 subjects QTP 300= 161 subjects PLB= 160 subjects |
QTP 150= 51:115; 30.72% QTP 300= 51:110; 31.67% PLB= 7:13; 35% |
QTP 150= 46 (10.1) QTP 300= 45.5 (11.1) PLB= 44.8 (10.4) |
6 weeks | MADRS | Positive. QTP groups showed a significant difference from PLB from baseline to endpoint. |
| Baumann et al. (1996) | Lithium vs. Placebo Stage I |
Total sample= 24 subjects Li= 10 subjects PLB= 14 subjects | Li= 3:7; 30% PLB= 2:5; 28.57% |
Li= 40 (14) PLB= 43 (14) |
1 week | HAM-D 21 CGI |
Positive. CTP + Li was a safe and effective combination for TRD. |
| Berman et al. (2007) | Aripiprazole vs. Placebo Stage I-III |
Total sample= 362 subjects ARI= 184 subjects PLB= 178 subjects | ARI= 35:57; 38.5% PLB= 63:115; 35.8% |
ARI= 46.5 (10.6) PLB= 44.2 (10.9) |
6 weeks | MADRS | Positive. ARI was associated with a higher remission rate than PLB. |
| Berman et al. (2009) | Aripiprazole vs. Placebo Stage I -II |
Total sample= 349 subjects ARI= 177 subjects PLB= 172 subjects | ARI= 39:138; 22.0% PLB= 55:117; 32.0% |
ARI= 45.1 (10.6) PLB= 45.6 (11.3) | 6 weeks | MADRS | Positive. ARI was associated with a higher remission rate than PLB. |
| Browne et al. (1990) | Lithium vs. Placebo Stage I Cross-over study |
Total sample= 17 subjects Li= 7 subjects in phase-I, followed by 8 subjects PLB= 10 subjects in phase-I, followed by 4 subjects (MDD= 14; BDI=1 and BDII= 2) |
Total sample= 7:10; 41.2% | Total sample= 42.7 (25–66) | 48 hours | HAM-D 21 | Negative. Li was no more effective than placebo in producing rapid relief of symptoms. 4/13 unipolar depressed patients responded at 48 h, whereas only 1/11 unipolar depressed patient responded to placebo at 48 h. |
| Carpenter et al. (2002) | Mirtazapine vs. Placebo Stage I |
Total sample= 26 subjects Mirtazapine= 11 subjects PLB= 15 subjects (MDD= 23; BD-II= 3) |
MIRT= 5:6; 45.45% PLB= 2:3; 40% |
Total sample= 46.3 (9.4) MIRT= 45.9 (9.7) PLB= 46.6 (9.5) |
4 weeks | HAM-D 17 CGI-S |
Positive. Mirtazapine showed statistically significant superiority over placebo on all outcomes. |
| Chaput et al. (2008) | Quetiapine vs. Placebo Stage II |
Total sample= 22 subjects QTP=11 subjects PLB= 11 subjects |
QTP=1:3; 27.27% PLB= 1:3; 27.27% |
QTP=41.6 (13) PLB= 44.9 (10) |
12 weeks | HAM=D21 | Positive. QTP/CBT treatment showed significantly greater improvement in all measure scores. |
| Corrigan et al. (2000) | Pramipexole vs. Placebo Stage I |
Total sample= 139 subjects PPX= 104 subjects PLB= 35 subjects |
Not available. | Total sample= 42 | 8 weeks | MADRS, HAM-D 17 CGI-SI |
Positive. PPX significantly improved depressive symptoms at 1.0 mg and more in 5 mg groups. |
| Corya et al. (2006) | Olanzapine/FLX vs. FLX (placebo) Stage II |
Total sample= 483 subjects OLZ/FLX=302 subjects FLX/Placebo=60 subjects | M: F = 27.5:72.5 | 45.7 ± 10.8 | 12 weeks | MADRS | Negative. OLZ as an augmenter (with FLX) was not statistically superior to FLX, and venlafaxine at the end point. |
| Cusin et al. (2013) | Pramipexole vs. Placebo Stage I |
Total sample= 60 subjects PPX= 30 subjects PLB= 30 subjects |
PPX= 7:8; 46.7% PLB= 2:3; 40% |
PPX= 47.3 (12.9) PLB= 45.5 (1.8) |
8 weeks | MADRS | Positive. Modest statistically difference between groups favoring PPX. |
| DeBattista et al. (2003) | Modafinil vs. Placebo Stage I |
Total sample= 136 subjects MOD= 69 subjects PLB= 67 subjects |
MOD= 1:2; 33.33% PLB= 18:49; 26.86% |
MOD=45 (19–64) PLB=45 (23–64) |
6 weeks | HAM-D 21, HAM-D 17, CGI-S | Negative. No significant differences between adjunct Modafinil and placebo. |
| Dunlop et al. (2007) | Modafinil vs. Placebo Stage I |
Total sample= 72 subjects MODA= 36 subjects PLB= 36 subjects |
Total= 4:5; 44.44% | Total sample= 43.9 (10.3) | 6 weeks | HAM-D 21 MADRS |
Negative. No significant differences at end point between the treatment groups. Study had to discontinue due to new suicidal ideation. |
| Durgam et al. (2016) | Cariprazine vs. Placebo Stage I |
Total sample=819 Cariprazine=550 PLB=269 | Cariprazine = 158 (27%) PLB=76(28.5%) | Cariprazine= 45.3(11.6) PLB= 46.4 (11.6) |
8 weeks | MADRS | Positive. Cariprazine at higher dose was effective and well tolerated. |
| Earley et al. (2018) | Cariprazine vs Placebo Stage I-II |
Total sample=527 Cariprazine=269 PLB=258 | Cariprazine=95 (35.3%) PLB=88 (34.1%) |
Cariprazine= 44.2 (11.6) PLB= 43.8 (11.8) |
8 weeks | MADRS CGI-I | Negative. A greater percentage of subjects improved compared to placebo but differences were not significant. |
| El-Khalili et al. (2010) | Quetiapine 150 mg/day vs. Quetiapine 300 mg/day vs placebo Stage I |
Total sample= 432 subjects QTP 150= 143 subjects QTP 300= 146 subjects PLB= 143 subjects |
QTP 150 = 34:109; 23.8% QTP 300= 20:53; 23.8% PLB= 45:98; 31.5% | QTP 150= 45.0 (11.0) QTP 300= 44.3 (11.3) PLB= 46.2 (10.9) |
6 weeks | MADRS, HAM-D 17 | QTP 150 offers non-significant improvements from PLB at end-point. QTP 300 was effective at end-point. |
| Fava et al. (2005) | Modafinil vs. Placebo Stage I |
Total sample= 311 subjects MOD=158 subjects PLB=153 subjects | MOD= 47:111; 30% PLB= 43:110; 28% |
MOD=42.0 PLB= 42.3 |
8 weeks | CGI-I, HAM-D 17, MADRS | Positive. Significant improvement in CGI-I for MOD. |
| Fava et al. (2012) | Aripiprazole vs. placebo Stage I-III |
Total sample= 221 subjects ARI= 56 subjects PLB=169 subjects |
ARI= 19:56; 33.93% PLB= 61:169; 36.09% |
ARI= 45.36 (10.35) PLB= 45.06 (11.34) |
4 weeks | MADRS | Positive. Marginal efficacy of ARI compared to placebo. |
| Fava et al. (2018) | Cariprazine vs. Placebo Stage I |
Total sample=231 Cariprazine=150 Placebo=81 | Cariprazine= 49:100 (33.0%) Placebo=20:61 (24.7%) | Cariprazine=45.4(11.9) Placebo=45.2(10.2) |
8 weeks | MADRS CGI-I | Negative. No significant differences were seen on any measures between cariprazine and placebo. |
| Gitlin et al. (1987) | Liothyronine (T3) vs. placebo Stage I |
Total sample= 16 subjects T3= 8 subjects PLB= 8 subjects |
Not available. | 41(20–62) | 2 weeks | HAM-D 17 | Negative. No effect of T3 over imipramine. |
| GlaxoSmithKline (2009) | Bupropion vs. Placebo Stage I |
Total sample= 325 subjects BUP=166 subjects PLB=159 subjects |
BUP= 44:39; 53.01% PLB= 88:71; 55.34% |
BUP= 36.8 (9.28) PLB= 36.0 (8.91) |
12 weeks | HAM-D 17 | Negative. |
| Gulrez et al. (2012) | Bupropion vs. Placebo Stage I |
Total sample= 60 subjects Bup=30 subjects PLB=30 subjects |
BUP= 1:1; 50% PLB= 7:8; 46.66% |
BUP= 39.23 ± 2.21 PLB= 43.23 ± 2.67 |
4 weeks | HAM-D17, MADRS, ADI | Positive. Bupropion significantly improved scores of all measures. |
| Han et al. (2015) | Aripiprazole vs. Placebo Stage I |
Total sample= 95 subjects ARI= 50 subjects PLB= 46 subjects |
Not available. | ARI=47.9 (16.2) PLB= 50.3 (13.5) |
6 weeks | MADRS | Positive. ARI yielded potentially beneficial clinical outcomes compared to SW. |
| Heninger et al. (1983) | Lithium vs. Placebo Stage I |
Total sample= 15 subjects Li= 8 subjects PLB= 7 subjects |
Li= 1:7; 12.5% PLB= 2:5; 28.57% |
Li= 44.38 (15.85) PLB= 58.43 (6.35) |
12 days | HAM-D 17 | Positive. Li augmentation improved symptoms in nonresponding patients. |
| Kamijima et al. (2013) | Aripiprazole vs. Placebo Stage I-II |
Total sample= 592 subjects ARI=397 subjects PLB= 195 subjects |
ARI= 225:173; 56.67% PLB= 23:16; 58.97% |
ARI= 38.6 (9.35) PLB= 38.7 (9.2) |
6 weeks | MADRS | Positive. ARI augmentation at fixed or flexible dose was superior to antidepressant alone. |
| Katona et al. (1995) | Lithium vs. Placebo Stage I |
Total sample= 62 subjects Li= 29 subjects PLB= 33 subjects |
Li= 9:20; 31.03% PLB= 6:5; 54.54% |
Li= 40.05 (13.75) PLB= 40.5 (11.15) |
6 weeks | HAM-D 17 | Positive. Combination of Li and FLX improved symptoms at end-point. |
| Keitner et al. (2009) | Risperidone vs. Placebo Stage I |
Total sample= 97 subjects RSP= 64 subjects PLB= 33 subjects |
RSP= 57.8:42.2; 42.18% PLB= 54.4:45.5; 50% |
RSP= 45.5 (11.6) PLB= 44.6 (11.1) |
4 weeks | MADRS | Positive. Significantly higher and quicker rates of remission and odds of remission after 4 weeks for RSP groups compared to PLB. |
| Kessler et al. (2018) | Mirtazapine vs. Placebo Stage I |
Total sample= 480 subjects MIRT= 241 subjects PLB= 239 subjects |
MIRT= 73:168; 30% PLB= 75:164; 31% |
MIRT= 50.4 (13.8) PLB= 49.9 (12.5) |
12 weeks | BDI-II | Negative. No clinically important benefit with Mirtazapine addition to SSRI/SNRI. |
| Landén et al. (1998) | Buspirone vs. Placebo Stage I |
Total sample= 119 subjects BUSP= 58 subjects PLB= 61 subjects |
BUSP= 19:40; 32.75% PLB= 18:43; 29.5% |
BUSP= 44.9 PLB= 48.2 |
4 weeks | MADRS, CGI | Negative. No difference in efficacy was identified between augmentation with BUSP and PLB. |
| Madhoo et al. (2014) | Lisdexamfetamine vs. placebo Stage I |
Total sample= 143 subjects LDX= 71 subjects PLB= 72 subjects |
LDX= 20:51; 28.16% PLB= 1:2; 33.33% |
LDX=41.9 (9.79) PLB=39.5 (10.59) |
9 weeks | MADRS | Positive. LDX augmentation of SSRI significantly improved depressive symptoms. |
| Mahmoud et al. (2007) | Risperidone vs. Placebo Stage I |
Total sample= 268 subjects RSP= 137 subjects PLB= 131 subjects |
RSP= 29.8:70.8; 29.19% PLB= 23.7:76.3; 23.66% |
RSP= 45.9 (10.1) PLB= 46.4 (10.7) |
6 weeks | HAM-D 17 | Positive. RSP augmentation produced a statistically significant reduction in depression symptoms and increased remission and response compared to PLB. |
| Marcus et al. (2008) | Aripiprazole vs. Placebo Stage I |
Total sample = 381 subjects ARI= 191 subjects PLB= 190 subjects |
ARI= 17:33; 34.03% PLB= 32.6:67.4; 32.6% |
ARI= 44.6 (11.0) PLB= 44.4 (10.7) |
6 weeks | MADRS | Positive. ARI produced improvement in scores and higher response and remission rates compared to PLB. |
| McIntyre et al. (2007) | Quetiapine vs. Placebo Stage I |
Total sample= 58 subjects QTP= 29 subjects PLB= 29 subjects |
QTP= 10:19; 34.48% PLB= 14:15; 48.27% |
QTP= 44 (10) PLB= 45 (12) |
8 weeks | HAM-D 17 | Positive. QTP improved HAM-D total scores from baseline to Week 8 and symptoms of depression and anxiety compared to PLB. |
| Nierenberg et al. (2003) | Lithium vs. placebo Stage III |
Total sample= 35 subjects Li= 18 subjects PLB= 17 subjects |
Li= 1:1; 50% PLB= 10:7; 58.82% |
Li= 37.2 (8.3) PLB= 39.7 (11.9) |
6 weeks | HAM-D 17 | Negative. Lam augmentation for Ntp therapy showed no statistical difference when compared to PLB. |
| Normann et al. (2002) | Lamotrigine vs. Placebo Stage I |
Total sample= 40 subjects LAM= 20 subjects PLB= 20 subjects |
LAM= 3:7; 30% PLB= 7:13; 35% |
LAM=39.6 (3.4) PLB= 37.9 (1.9) |
9 weeks | HAM-D 17 | Negative. Lam was not found to be an efficient augmentation agent in treatment-resistant depression but accelerated onset of action of antidepressant. |
| Papakostas et al. (2015) | Ziprasidone vs. Placebo Stage I |
Total sample=139 ZIP=71 PLB=68 | ZIP=22(31%) PLB= 19 (28%) | ZIP=44.7(13.8) PLB=44.2(11.9) |
8 weeks | HAM-D 17 CGI-S |
Positive. Adjunctive ziprasidone to escitalopram resulted in a significant improvement in depression scores. |
| Patkar et al. (2006) | Methylphenidate vs. placebo Stage I |
Total sample= 60 subjects MPH= 30 subjects PLB= 30 subjects |
Total sample= 11:19; 37% | Total sample= 48.5 (10.3) | 4 weeks | HAM-D 21 | Negative. No significant differences in reduction of mean HAM-D 21 scores. |
| Ravindran et al. (2008) | Methylphenidate vs. placebo Stage I-II |
Total sample= 145 subjects MPH= 73 subjects PLB= 72 subjects |
MPH= 26:47; 35.61% PLB= 25:47; 34.72% |
MPH= 45.6 (10.8) PLB= 41.9 (10.9) | 5 weeks | MADRS | Negative. No statistical difference between groups at end-point. |
| Reeves et al. (2008) | Risperidone vs. Placebo Stage I-II |
Total sample= 23 subjects RSP=12 subjects PLB= 11 subjects |
RSP= 1:11; 8.33% PLB= 6:5; 54.54% |
RSP= 46.5 (12.1) PLB= 41.3 (12.6) |
8 weeks | MADRS | Positive. RSP significantly reduced suicidal ideation and overall effect was superior than PLB. |
| Richards et al. (2016) | Lisdexamfetamine vs. placebo Stage I |
Total sample= 402 subjects LDX= 201 subjects PLB= 201 subjects |
LDX= 24:43; 35.82% PLB= 68:133; 33.83% |
LDX= 42.0 (12.1) PLB= 41.8 (12.04) |
16 weeks | MADRS | Negative. LDX not superior to placebo in MDD. |
| Richards et al. (2016) | Lisdexamfetamine vs. placebo Stage I |
Total sample= 424 subjects LDX= 211 subjects PLB= 213 subjects |
LDX= 70:141; 33.17% PLB= 70:143; 32.86% |
LDX= 42.3 (11.4) PLB= 42.6 (11.41) |
16 weeks | MADRS | Negative. LDX not superior to placebo in MDD. |
| Santos et al. (2008) | Lamotrigine vs. Placebo Stage II |
Total sample= 34 subjects LAM= 17 subjects PLB= 17 subjects |
LAM= 1:5; 17.64% PLB= 6:11; 35.29% |
LAM= 26 PLB= 29 |
8 weeks | MADRS | Negative. Lam was not found to be an efficient augmentation agent in treatment-resistant depression. |
| Schöpf et al. (1989) | Lithium vs. Placebo Stage I-II |
Total sample= 27 subjects Li= 14 subjects PLB= 13 subjects |
Total sample= 8:19; 29.62% | Total sample= 54 | 2 weeks | HAM-D 17 | Positive. Combined therapy with Li may improve response in treatment resistant depression. |
| Stein and Bernadt (1993) | Lithium vs. Placebo Stage I (TCA) |
Total sample= 34 subjects Li= 16 subjects PLB= 18 subjects |
Li= 1:3; 25% PLB= 1:5; 16.66% |
Li= 47.2 (19.5) PLB= 47.1 (15.4) |
3 weeks | HAM-D 17 | Negative. Both groups improved; no statistical difference. |
| Thase et al. (2007) | Olanzapine/Fluoxetine vs. placebo (FLX) Stage II |
Total sample= 406 subjects OLZ/FLX=200 subjects PLB= 206 subjects | Not available. | OLZ/FLX=44.3(10.2) PLB=44.6(10.0) |
8 weeks | MADRS | Positive. OLZ/FLX combo was efficacious for patients with TRD with significant rapid onset of therapeutic benefits. |
| Thase et al. (2015a) | Brexpiprazole vs. placebo Stage I-II |
Total sample= 677 subjects BX1= 226 subjects BX3= 230 subjects PLB= 221 subjects |
BX1= 34:79; 30.08% BX3= 37:37; 32.17% PLB= 74:147; 33.48% |
BX1= 45.7 (11.6) BX3= 44.5 (11.2) PLB= 46.6 (11.0) |
6 weeks | MADRS | Positive. BX3 demonstrated higher efficacy when compared to BX1 and PLB. Both dosages well tolerated. |
| Thase et al. (2015b) | Brexpiprazole vs. placebo Stage I-II |
Total sample= 379 subjects BX2= 188 subjects PLB= 191 subjects |
BX2= 29:65; 30.85% PLB= 54:137; 28.27% |
BX2= 44.1 (11.6) PLB= 45.2 (11.3) |
6 weeks | MADRS | Positive. BX2 demonstrated greater efficacy compared to PLB. |
| Trivedi et al. (2013) | Lisdexamfetamine vs. placebo Stage I |
Total sample= 173 subjects LDX=88 subjects PLB= 85 subjects |
LDX= 35:53; 39.77% PLB= 31:54; 36.47% |
LDX= 39.4 (9.65) PLB= 38.6 (10.38) |
6 weeks | HAM-D 17 | Positive. Augmentation with LDX reduced depressive symptoms in patients with inadequate response to escitalopram. |
| Zusky et al. (1988) | Lithium vs. Placebo Stage I-II |
Total sample= 16 subjects Li= 8 subjects PLB= 8 subjects |
Li: 1:7; 14.28% PLB: 1:4; 25% |
Li= 46.8 PLB= 44.8 |
3 weeks | HAM-D 17 | Negative. Low dose Li was not different than placebo in terms of rate and degree of response. |
| Mixed treatment comparisons/Multi-arm trials | |||||||
| Fang et al. (2011) | Buspirone vs. risperidone vs. thyroid hormone vs. trazodone vs. sodium valproate Stage II |
Total sample= 225 subjects RSP= 45 subjects VPA= 39 subjects BUS= 46 subjects TRA= 47 subjects T4= 48 subjects |
Not available. | Not available. | 8 weeks | HAM-D 17, CGI-I | Positive. Augmentation with RSP, BUSP, TRA, VPA or T4 was effective with no statistical significance among treatment arms in remission rates. |
| Franco-Chaves et al. (2013) | Pramipexole vs. Escitalopram vs. combination Stage I |
Total sample= 39 subjects PPX= 13 subjects ESC= 13 subjects Combo= 13 subjects |
PPX= 6:7; 46.15% ESC= 4:9; 30.76% Combo= 3:10; 23.07% |
PPX= 44.9 (10.1) ESC= 45.6 (13.6) Combo= 46.1(12.5) |
6 weeks | MADRS | Negative. No significant difference in response rates or remission rates and not well tolerated. |
| Joffe et al. (1993) | Lithium vs. placebo vs. thyroid hormone Stage I |
Total sample= 50 subjects Li= 17 subjects T3= 17 subjects PLB= 16 subjects |
Li= 9:8; 52.94% T3= 5:13; 29.41% PLB= 3:5; 37.5% |
Total sample= 37.4 (11.2) | 2 weeks | HAM-D 17 | Positive. Li and T3 are both significantly better than placebo and both have comparable efficacy. |
| Joffe et al. (2006) | Lithium vs. thyroid hormone vs. combination (Li+T3) vs. placebo Stage I |
Total sample= 36 subjects Li= 9 subjects T3= 10 subjects Combo= 9 subjects PLB= 8 subjects |
Li= 1:8; 11.11% T3= 1:9; 10% Combo= 2:7; 22.22% PLB= 1:3; 25% |
Li= 38.3 (6.8) T3= 42.2 (6.8) PLB= 38.8 (7.4) Combo= 37.0 (6.7) |
2 weeks | HAM-D 17 | Negative. Similar efficacy across groups. No differences in combination vs either treatment alone or active treatment groups and PLB. |
| Joffe and Singer (1990) | T4 vs T3 augmentation of TCA (desipramine or imipramine) Stage I |
Total sample=40 T3= 17 subjects T4= 21 subjects |
T3= 8:9; 47% T4= 6:15; 28.5 | Total sample = 34.5 (10.5) | 3 weeks | HAM-D 17 | Positive. T3 is significantly more effective than T4. |
| Shelton et al. (2001) | Olanzapine vs. Fluoxetine vs. Olanzapine/Fluoxetine Stage II |
Total sample= 28 subjects FLX= 10 subjects OLZ= 8 subjects OLZ/FLX= 10 subjects |
Gender not specified | Age not specified | 8 weeks | MADRS | Positive. OLZ/FLX superior efficacy then either agent alone. |
| Shelton et al. (2005) | Olanzapine vs. placebo Stage II |
Total sample= 500 subjects OLZ (FLX)= 146 subjects FLX=142 subjects OLZ=144 subjects NOR=68 subjects | OLZ= 46 (32%) Placebo= 39 (27%) |
OLZ = 42.5 (10.7) Placebo = 41.7 (11.0) |
8 weeks | MADRS | Negative. OLZ augmentation of FLX was not superior to the other therapies at endpoint. |
| Yoshimura et al. (2012) | Aripripazole + Sertraline vs. Aripiprazole + Paroxetine Stage II |
Total sample= 24 subjects ARI+SER=13 subjects ARI+PAR=11 subjects |
ARI+SER= 6:7; 45.5% ARI+PAR= 5:6; 46.2% |
ARI+SER= 43.4 (10.9) ARI+PAR= 39.3 (9.41) |
4 weeks | HAM-D 17 | Positive. Adding ARI to Par or Ser-was equally effective and tolerated. |
| Yoshimura et al. (2014) | Lithium vs. olanzapine vs Aripiprazole (as augmentation for Paroxetine) Stage I |
Total sample= 30 subjects Li= 10 subjects OLZ= 10 subjects ARI= 10 subjects |
Li= 2:3; 40% OLZ= 1:2; 50% ARI= 3:7; 30% |
Li= 39 (8) OLZ= 42 (7) ARI= 40 (10) |
4 weeks | HAM-D 17 | Positive. OLZ and ARI could be used as alternatives to Li. |
AD, antidepressant; ARI, aripiprazole; CTP, citalopram; ESC, escitalopram; BUS, buspirone; FLX, fluoxetine; Li, Lithium; MIRT, Mirtazapine; OLZ/FLX, olanzapine/fluoxetine combination; OLZ, olanzapine; QTP, quetiapine; RSP, risperidone; T3, liothyronine; T4, levothyroxine; PAR, paroxetine; SER, sertraline; TRA, trazodone; PPX, pramipexole; LDX, lisdexamfetamine; MOD, modafinil; NOR, Nortriptyline; IMI, Imipramine; BUP, bupropion; LAM, lamotrigine; BX1, brexpiprazole 1 mg; BX2, brexpiprazole 2 mg; BX3, brexpiprazole 3 mg; ZIP, ziprasidone; TCA, Tricyclic antidepressants; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TRD, treatment-resistant depression; MADRS, Montgomery Asberg Depression rating scale; HAM-A, Hamilton anxiety rating scale; HAM-D, Hamilton depression rating scale; HAM-D17, 17-item Hamilton depression rating scale; HAM-D21, 21-item Hamilton Depression Rating scale; Clinical Global Impression- Improvement, CGI-I; Clinical Global Impression- Severity, CGI-S; SD, standard deviation; PLB, placebo. Staging of TRD was based on the Thase and Rush Model.
Primary analyses initially constituted comparisons of direct effect (response, remission, and discontinuation) for each pharmacological class compared with placebo (Appendix 3, Fig. 1a–c).
For response rates, 65 studies included data on 19 treatments [medications (N = 7669) and placebo (N = 4746)] comprising: 7 AAs (aripiprazole, n = 1147; brexpiprazole, n = 599; cariprazine, n = 963; olanzapine/olanzapine (fluoxetine) n = 668; quetiapine, n = 909; risperidone, n = 262 and ziprasidone, n = 71), 2 mood stabilizers (lithium, n = 469; lamotrigine, n = 115), buspirone (n =441), 3 antidepressants; (bupropion, n = 492; mirtazapine, n = 225; nortriptyline, n = 23), 4 dopaminergic compounds (pramipexole, n = 147; lisdexamfetamine, n = 568; modafinil, n = 284; and methylphenidate, n = 103), and thyroid hormones (T3, n = 114; T4, n = 69). In order of decreasing efficacy, T3, nortriptyline, aripiprazole, brexpiprazole, quetiapine, lithium, modafinil, olanzapine (fluoxetine), cariprazine and lisdexamfetamine were statistically significant compared to placebo, with RRs ranging from 1.18 (1.03–1.37) for lisdexamfetamine to 1.90 (1.16–3.11) for T3. Heterogeneity (I2=9.8%, p = 0.541) or inconsistency (p = 0.085) were not statistically significant. Two studies (Barbosa et al., 2003; Carpenter et al., 2002) had included patients with MDD (n=38) and bipolar-II disorder (n=11), without providing separate outcomes for MDD patients. We performed a sensitivty analysis by excluding one study and both studies respectively, to assess the outcomes. Overall, the findings remain fairly consistent with the main results (Appendix 3. Fig. 2 a & b).
In a sub-analysis, we reviewed lithium augmentation response rates of 7 included studies which were of ≤ 3 weeks of duration. Lithium showed a significantly higher response rate than placebo (RR=2.43, 95% CI:1.45–4.07, p<0.0007) in this subset (Appendix 3, Fig. 3). In a post-hoc subanalysis, just like lithium, we investigated thyroid augmentation response rates of three short-duration studies (≤ 3 weeks). The response rate with thyroid augmentation was not statistically significant compared to placebo (RR= 1.79; 0.48– 6.67).
In terms of remission rates, 39 studies (40 cohorts) which included 19 treatments [medications (N = 6279) and placebo (N = 3965)] comprised: 7 AAs (aripiprazole, n = 1123; brexpiprazole, n = 599; cariprazine, n = 963; olanzapine/olanzapine (fluoxetine), n = 658; quetiapine, n = 667; risperidone, n = 250 and ziprasidone, n = 71), 2 mood stabilizers (lithium, n = 106; lamotrigine, n = 30), 3 antidepressants (bupropion, n = 326; mirtazapine, n = 225; nortriptyline, n = 23), buspirone (n = 332), 4 dopaminergic compounds (pramipexole, n = 43; lisdexamfetamine, n = 497; modafinil, n = 215; and methylphenidate, n = 30), and thyroid hormones (T3, n = 73 and T4, n = 48). In order of efficacy, T4, aripiprazole, risperidone, quetiapine, olanzapine(fluoxetine), brexpiprazole were statistically significant compared to placebo with RRs ranging from 1.44 (1.00–2.08) for brexpiprazole to 1.91 (1.04–3.52) for T4. Heterogeneity (I2=2.4%, p = 0.473) or inconsistency (p = 0.306) were not statistically significant.
While examining all cause discontinuation, 53 studies (54 cohorts) which included 17 treatments were assessed [medications (N = 7223) and placebo (N = 4617)] with an overall of n = 1642 events comprising: 7 AAs (aripiprazole, n = 123; brexpiprazole, n = 22; cariprazine, n = 93; olanzapine(fluoxetine) n = 156; quetiapine, n = 177; risperidone, n = 47 and ziprasidone, n = 10), 2 mood stabilizers (lithium, n = 79; lamotrigine, n = 27), 2 antidepressants (bupropion, n = 102; mirtazapine, n = 51), buspirone (n = 69), 4 dopaminergic compounds (pramipexole, n = 10; lisdexamfetamine, n = 89; modafinil, n = 37; methylphenidate, n = 14), and thyroid hormones (T4, n = 3). Discontinuation data was not available for nortriptyline. Discontinuation rates were significantly higher compared to placebo for ziprasidone (RR= 20.12, 95% CI 1.17–344.58), mirtazapine (RR= 4.12, 95% CI, 1.97–8.63), and cariprazine (RR= 1.72 (95% CI 1.09–2.73). Heterogeneity was not statistically significant (I2=17.3%, p = 0.251) or inconsistency (p = 0.167).
In Tables 2a–2c we have synthetized the network of eligible comparisons for the measured outcomes represented by league tables.
Table 2a.
League table for response rates associated with studied pharmacological agents. For interpretation, a number larger than zero favors the column-defining treatment of a cell. Values are RR with associated 95% confidence intervals.
| Aripiprazole | |||||||||||||||||||
| 1.01 (0.72; 1.41) | Brexpiprazole | ||||||||||||||||||
| 1.37 (1.08; 1.74) | 1.36 (0.94; 1.97) | Bupropion | |||||||||||||||||
| 1.44 (1.11; 1.87) | 1.43 (0.98; 2.08) | 1.05 (0.84; 1.33) | Buspirone | ||||||||||||||||
| 1.31 (1.05; 1.64) | 1.30 (0.92; 1.84) | 0.96 (0.73; 1.26) | 0.91 (0.69; 1.21) | Cariprazine | |||||||||||||||
| 1.29 (0.91; 1.84) | 1.28 (0.82; 1.99) | 0.94 (0.64; 1.39) | 0.90 (0.60; 1.33) | 0.98 (0.68; 1.42) | Lamotrigine | ||||||||||||||
| 1.33 (1.08; 1.63) | 1.32 (0.94; 1.84) | 0.97 (0.75; 1.26) | 0.92 (0.71; 1.20) | 1.01 (0.81; 1.26) | 1.03 (0.72; 1.47) | Lisdexamfetamine | |||||||||||||
| 1.26 (0.97; 1.64) | 1.25 (0.86; 1.81) | 0.92 (0.68; 1.25) | 0.87 (0.64; 1.19) | 0.96 (0.73; 1.27) | 0.97 (0.67; 1.41) | 0.95 (0.73; 1.23) | Lithium | ||||||||||||
| 1.29 (0.88; 1.90) | 1.28 (0.81; 2.04) | 0.95 (0.62; 1.43) | 0.90 (0.59; 1.36) | 0.99 (0.67; 1.46) | 1.00 (0.62; 1.62) | 0.97 (0.67; 1.42) | 1.03 (0.68; 1.56) | Methylphenidate | |||||||||||
| 1.22 (0.90; 1.65) | 1.21 (0.81; 1.81) | 0.89 (0.63; 1.25) | 0.85 (0.60; 1.20) | 0.93 (0.68; 1.27) | 0.94 (0.62; 1.43) | 0.92 (0.68; 1.24) | 0.97 (0.69; 1.37) | 0.94 (0.61; 1.47) | Mirtazapine | ||||||||||
| 1.25 (1.00; 1.56) | 1.24 (0.88; 1.75) | 0.91 (0.70; 1.20) | 0.87 (0.66; 1.14) | 0.95 (0.75; 1.20) | 0.97 (0.67; 1.39) | 0.94 (0.76; 1.17) | 0.99 (0.75; 1.30) | 0.96 (0.65; 1.42) | 1.02 (0.75; 1.40) | Modafinil | |||||||||
| 0.77 (0.38; 1.56) | 0.76 (0.36; 1.62) | 0.56 (0.27; 1.16) | 0.53 (0.26; 1.11) | 0.58 (0.29; 1.20) | 0.59 (0.28; 1.28) | 0.58 (0.28; 1.17) | 0.61 (0.29; 1.26) | 0.59 (0.27; 1.29) | 0.63 (0.30; 1.32) | 0.61 (0.30; 1.26) | Nortriptyline | ||||||||
| 1.28 (1.00; 1.65) | 1.27 (0.88; 1.83) | 0.94 (0.70; 1.26) | 0.89 (0.66; 1.21) | 0.98 (0.75; 1.28) | 0.99 (0.68; 1.46) | 0.97 (0.75; 1.24) | 1.02 (0.76; 1.37) | 0.99 (0.66; 1.49) | 1.05 (0.75; 1.47) | 1.03 (0.79; 1.34) | 1.67 (0.81; 3.45) | Olanzapine | |||||||
| 1.17 (0.71; 1.92) | 1.16 (0.66; 2.03) | 0.85 (0.51; 1.44) | 0.81 (0.48; 1.37) | 0.89 (0.54; 1.47) | 0.90 (0.51; 1.61) | 0.88 (0.53; 1.44) | 0.93 (0.55; 1.57) | 0.90 (0.50; 1.63) | 0.96 (0.56; 1.65) | 0.94 (0.57; 1.55) | 1.52 (0.66; 3.53) | 0.91 (0.54; 1.53) | Pramipexole | ||||||
| 1.18 (0.95; 1.46) | 1.17 (0.83; 1.64) | 0.86 (0.66; 1.12) | 0.82 (0.62; 1.07) | 0.90 (0.71; 1.13) | 0.91 (0.64; 1.29) | 0.88 (0.72; 1.09) | 0.93 (0.77; 1.14) | 0.91 (0.62; 1.34) | 0.96 (0.71; 1.31) | 0.94 (0.75; 1.18) | 1.53 (0.75; 3.12) | 0.92 (0.71; 1.18) | 1.01 (0.61; 1.66) | Quetiapine | |||||
| 1.29 (0.96; 1.74) | 1.28 (0.86; 1.91) | 0.94 (0.69; 1.29) | 0.90 (0.67; 1.20) | 0.98 (0.72; 1.34) | 1.00 (0.66; 1.51) | 0.97 (0.72; 1.31) | 1.03 (0.73; 1.44) | 1.00 (0.64; 1.55) | 1.06 (0.73; 1.53) | 1.03 (0.76; 1.40) | 1.68 (0.80; 3.53) | 1.01 (0.72; 1.40) | 1.11 (0.64; 1.90) | 1.10 (0.81; 1.48) | Risperidone | ||||
| 0.83 (0.50; 1.39) | 0.82 (0.46; 1.47) | 0.61 (0.36; 1.03) | 0.58 (0.34; 0.98) | 0.63 (0.38; 1.07) | 0.64 (0.36; 1.15) | 0.62 (0.37; 1.05) | 0.66 (0.41; 1.06) | 0.64 (0.35; 1.18) | 0.68 (0.39; 1.19) | 0.66 (0.39; 1.12) | 1.08 (0.46; 2.54) | 0.65 (0.38; 1.10) | 0.71 (0.36; 1.41) | 0.71 (0.43; 1.16) | 0.64 (0.37; 1.11) | T3 | |||
| 1.32 (0.89; 1.94) | 1.30 (0.81; 2.09) | 0.96 (0.65; 1.42) | 0.91 (0.65; 1.28) | 1.00 (0.67; 1.50) | 1.02 (0.63; 1.65) | 0.99 (0.67; 1.46) | 1.04 (0.69; 1.58) | 1.02 (0.61; 1.69) | 1.08 (0.69; 1.69) | 1.05 (0.71; 1.57) | 1.71 (0.78; 3.76) | 1.02 (0.67; 1.56) | 1.13 (0.62; 2.05) | 1.12 (0.75; 1.66) | 1.02 (0.71; 1.46) | 1.58 (0.90; 2.78) | T4 | ||
| 0.92 (0.51; 1.68) | 0.91 (0.47; 1.76) | 0.67 (0.36; 1.25) | 0.64 (0.34; 1.19) | 0.70 (0.38; 1.28) | 0.71 (0.37; 1.38) | 0.69 (0.38; 1.26) | 0.73 (0.39; 1.36) | 0.71 (0.36; 1.40) | 0.75 (0.40; 1.43) | 0.74 (0.40; 1.35) | 1.20 (0.48; 2.96) | 0.72 (0.39; 1.33) | 0.79 (0.37; 1.67) | 0.78 (0.43; 1.43) | 0.71 (0.38; 1.34) | 1.11 (0.52; 2.38) | 0.70 (0.35; 1.39) | Ziprasidone | |
| 1.57 (1.36; 1.82) | 1.56 (1.15; 2.11) | 1.15 (0.93; 1.43) | 1.09 (0.87; 1.37) | 1.20 (1.01; 1.42) | 1.22 (0.88; 1.68) | 1.18 (1.03; 1.37) | 1.25 (1.00; 1.56) | 1.22 (0.85; 1.73) | 1.29 (0.99; 1.68) | 1.26 (1.07; 1.48) | 2.05 (1.02; 4.11) | 1.23 (1.00; 1.50) | 1.35 (0.84; 2.17) | 1.34 (1.14; 1.56) | 1.22 (0.94; 1.58) | 1.90 (1.16; 3.11) | 1.20 (0.83; 1.72) | 1.71 (0.96; 3.06) | Placebo |
Table 2c.
League table for all cause discontinuation associated with studied pharmacological agents. For interpretation, larger than zero favors the column-defining treatment a cell. Values are RR with associated 95% confidence intervals.
| Aripiprazole | |||||||||||||||||
| 0.43 (0.13; 1.49) | Brexpiprazole | ||||||||||||||||
| 1.07 (0.63; 1.82) | 2.47 (0.70; 8.81) | Bupropion | |||||||||||||||
| 0.83 (0.44; 1.55) | 1.91 (0.51; 7.12) | 0.77 (0.48; 1.23) | Buspirone | ||||||||||||||
| 0.70 (0.40; 1.22) | 1.62 (0.45; 5.83) | 0.66 (0.35; 1.23) | 0.85 (0.41; 1.74) | Cariprazine | |||||||||||||
| 1.43 (0.81; 2.52) | 3.31 (0.91; 11.97) | 1.34 (0.70; 2.54) | 1.73 (0.84; 3.59) | 2.04 (1.05; 3.96) | Lamotrigine | ||||||||||||
| 1.09 (0.69; 1.74) | 2.53 (0.73; 8.76) | 1.02 (0.59; 1.77) | 1.32 (0.69; 2.53) | 1.56 (0.88; 2.77) | 0.76 (0.42; 1.38) | Lisdexamfetamine | |||||||||||
| 0.92 (0.52; 1.61) | 2.13 (0.59; 7.69) | 0.86 (0.45; 1.63) | 1.11 (0.54; 2.30) | 1.31 (0.68; 2.54) | 0.64 (0.34; 1.23) | 0.84 (0.47; 1.51) | Lithium | ||||||||||
| 1.00 (0.40; 2.51) | 2.32 (0.53; 10.12) | 0.94 (0.36; 2.46) | 1.21 (0.43; 3.38) | 1.43 (0.54; 3.80) | 0.70 (0.26; 1.88) | 0.92 (0.36; 2.32) | 1.09 (0.41; 2.92) | Methylphenidate | |||||||||
| 0.29 (0.13; 0.65) | 0.68 (0.17; 2.76) | 0.27 (0.12; 0.64) | 0.36 (0.14; 0.89) | 0.42 (0.18; 1.00) | 0.20 (0.09; 0.49) | 0.27 (0.12; 0.61) | 0.32 (0.13; 0.77) | 0.29 (0.09; 0.91) | Mirtazapine | ||||||||
| 1.14 (0.63; 2.06) | 2.64 (0.72; 9.65) | 1.07 (0.55; 2.07) | 1.38 (0.65; 2.92) | 1.63 (0.82; 3.22) | 0.80 (0.40; 1.60) | 1.04 (0.57; 1.92) | 1.24 (0.62; 2.48) | 1.14 (0.42; 3.10) | 3.89 (1.59; 9.52) | Modafinil | |||||||
| 1.03 (0.66; 1.63) | 2.39 (0.69; 8.27) | 0.97 (0.56; 1.66) | 1.25 (0.66; 2.38) | 1.48 (0.84; 2.60) | 0.72 (0.41; 1.29) | 0.95 (0.59; 1.52) | 1.13 (0.63; 2.00) | 1.03 (0.41; 2.61) | 3.53 (1.57; 7.93) | 0.91 (0.50; 1.66) | Olanzapine | ||||||
| 1.45 (0.63; 3.35) | 3.35 (0.81; 13.97) | 1.36 (0.56; 3.30) | 1.76 (0.68; 4.56) | 2.07 (0.84; 5.11) | 1.01 (0.41; 2.53) | 1.33 (0.57; 3.11) | 1.58 (0.63; 3.92) | 1.45 (0.45; 4.64) | 4.95 (1.69; 14.48) | 1.27 (0.50; 3.22) | 1.40 (0.60; 3.26) | Pramipexole | |||||
| 1.06 (0.68; 1.66) | 2.45 (0.71; 8.47) | 0.99 (0.58; 1.70) | 1.28 (0.68; 2.44) | 1.51 (0.86; 2.66) | 0.74 (0.42; 1.31) | 0.97 (0.60; 1.56) | 1.15 (0.74; 1.79) | 1.06 (0.42; 2.67) | 3.62 (1.61; 8.12) | 0.93 (0.51; 1.70) | 1.02 (0.64; 1.63) | 0.73 (0.31; 1.70) | Quetiapine | ||||
| 0.95 (0.54; 1.69) | 2.20 (0.61; 7.97) | 0.89 (0.49; 1.61) | 1.15 (0.62; 2.15) | 1.36 (0.70; 2.64) | 0.66 (0.34; 1.31) | 0.87 (0.48; 1.57) | 1.03 (0.53; 2.03) | 0.95 (0.35; 2.56) | 3.24 (1.34; 7.84) | 0.83 (0.41; 1.68) | 0.92 (0.51; 1.64) | 0.66 (0.26; 1.64) | 0.90 (0.50; 1.61) | Risperidone | |||
| 2.73 (0.73; 10.25) | 6.32 (1.09; 36.55) | 2.56 (0.70; 9.36) | 3.31 (0.93; 11.83) | 3.90 (1.00; 15.26) | 1.91 (0.49; 7.53) | 2.50 (0.66; 9.46) | 2.98 (0.76; 11.69) | 2.73 (0.58; 12.85) | 9.33 (2.12; 41.05) | 2.40 (0.60; 9.54) | 2.64 (0.70; 9.94) | 1.89 (0.42; 8.47) | 2.58 (0.69; 9.71) | 2.88 (0.83; 9.98) | T4 | ||
| 0.06 (0.00; 1.05) | 0.14 (0.01; 3.03) | 0.06 (0.00; 0.99) | 0.07 (0.00; 1.31) | 0.09 (0.00; 1.52) | 0.04 (0.00; 0.75) | 0.05 (0.00; 0.96) | 0.07 (0.00; 1.16) | 0.06 (0.00; 1.17) | 0.20 (0.01; 3.86) | 0.05 (0.00; 0.94) | 0.06 (0.00; 1.01) | 0.04 (0.00; 0.79) | 0.06 (0.00; 0.99) | 0.06 (0.00; 1.13) | 0.02 (0.00; 0.50) | Ziprasidone | |
| 1.21 (0.88; 1.65) | 2.79 (0.85; 9.23) | 1.13 (0.74; 1.73) | 1.46 (0.84; 2.54) | 1.72 (1.09; 2.73) | 0.84 (0.52; 1.36) | 1.11 (0.78; 1.56) | 1.31 (0.82; 2.11) | 1.21 (0.51; 2.86) | 4.12 (1.97; 8.63) | 1.06 (0.64; 1.76) | 1.17 (0.84; 1.62) | 0.83 (0.38; 1.81) | 1.14 (0.82; 1.58) | 1.27 (0.78; 2.06) | 0.44 (0.12; 1.59) | 20.12 (1.17; 344.58) | Placebo |
3.1. Quality assessment and publication bias
The RoB assessment based on review authors’ judgements about each risk of bias item was determined. Overall, 15 (24%) studies were rated as having low risk of RoB, 40 (63%) had moderate RoB and 8 (13%) had high RoB (Appendix 4, Fig. 1). No indication of publication bias was found for all the outcomes (p-value >0.05) (Appendix 4, Fig. 2).
4. Discussion
This NMA of 19 agents showed that several pharmacotherapeutic agents are efficacious as adjunctive treatments for TRD. Our findings further strengthen the results from previous studies (Carter et al., 2020; Strawbridge et al., 2019; Vázquez et al., 2021; Zhou et al., 2015) and adds to the current evidence as it includes other agents such as AAs (brexpiprazole, cariprazine, ziprasidone), antidepressants (nortriptyline, mirtazapine), dopaminergic compounds (pramipexole, lisdexamfetamine, methylphenidate, and modafinil), and thyroid hormones. We found that T3, nortriptyline, aripiprazole, brexpiprazole, lithium, quetiapine, modafinil, olanzapine (fluoxetine), cariprazine and, lisdexamfetamine were more efficacious compared to placebo. Regarding all cause discontinuation, significant findings were found for ziprasidone, mirtazapine, and cariprazine compared to placebo. Similar to a previous NMA by Zhou et al. (2015), we did not include an open-label pilot study (Dunner et al., 2007) in the main analysis. However, as a post-hoc analysis, when we included the study by Dunner et al, the RR for ziprasidone response rate, remission rate, and discontinuation rate were 1.80 (1.06–3.08), 1.27 (0.81–2.00), and 2.53 (1.07, 5.98), respectively.
Our data support current pharmacological augmentation recommendations from several treatment guidelines for depression which recommend the use of AAs as first line agents, specifically the use of aripiprazole and quetiapine (Kennedy et al., 2016; NICE, 2010; Seshadri et al., 2021). The use of augmentation with AAs has been supported by many clinical studies for aripiprazole,(Berman et al., 2007; Marcus et al., 2008) olanzapine+fluoxetine (Corya et al., 2006; Shelton et al., 2010; Shelton and Papakostas, 2008; Thase et al., 2007), quetiapine (El-Khalili et al., 2010; McIntyre et al., 2007)) and risperidone (Keitner et al., 2009; Mahmoud et al., 2007). Additionally previous studies emphasize the use of these agents as a pivotal point to overcome treatment resistance (Nelson and Papakostas, 2009; Rafeyan et al., 2020).
Our study provides evidence for brexpiprazole and cariprazine as efficacious augmentation agents in TRD as well. Brexpiprazole, was FDA approved as an adjunctive treatment to oral antidepressants in MDD based on 3 trials which showed a significant reduction in depressive symptoms (Fava et al., 2016; Thase et al., 2015a; Thase et al., 2015b). Similarly, cariprazine currently FDA approved for treatment of schizophrenia and bipolar-type I disorder has been studied as an adjunctive agent for MDD showed a significant reduction in MADRS scores at week 8 compared to placebo (Durgam et al., 2016; Earley et al., 2018). Brexpiprazole trials were of slightly shorter duration compared to cariprazine (6 and 8 weeks respectively). There is a need for long term maintenance trials for these agents.
Aripiprazole and quetiapine are the two most studied AAs. Aripiprazole as an augmentation agent (mean dose,10.68 ± 3.1 mg/day) showed significant benefit and had low discontinuation rates, which aligns with previous studies (Seshadri et al., 2021; Strawbridge et al., 2019). In addition, quetiapine (mean dose,156.74 ± 97.6 mg/day) showed significant benefits for both response and remission rates compared to placebo, which is in accordance with current clinical recommendations for augmentation strategies for MDD (Cleare et al., 2015; Kennedy et al., 2016). Similar to Spielmans et al’s meta-analysis (Spielmans et al., 2016), we found that the olanzapine augmentation of SSRIs (primarily fluoxetine) (mean dose 8.5 mg/day± 3.9) had a higher remission rate compared to placebo/SSRI. Our results examining mood stabilizers suggested that lithium in combination with TCAs, SSRIs, or other antidepressants, dose range between 300 and 1200 mg, and duration between 1 and 12 weeks appears to be more effective than placebo. Prior studies have suggested a rapid onset of lithium’s therapeutic effects for depression despite variability in lithium dosages, population heterogeneity, and treatment durations among the studies. Therefore, in a subanalysis, that included 7 lithium augmentation studies of short duration (≤3 weeks), lithium’s response rate was significant compared to placebo (RR= 2.43; 1.45 −4.07). However, we should acknowledge that remission rates were not significant compared to placebo. Our results are consistent with previous NICE guidelines which showed a significantly better response rates but not remission rates (NICE, 2010). Importantly, these data provide further evidence of lithium’s antidepressant efficacy which is consistent with the results of a recent meta-analysis (Vázquez et al., 2021). This is also congruent with current recommendations for augmentation in patients with unipolar TRD by the World Federation of Societies of Biological Psychiatry Task Force which considers lithium as first-line agent and aripiprazole and quetiapine as alternatives (Grunze et al., 2018).
Our results showed that lamotrigine (100–400 mg daily dose) has limited efficacy compared to placebo. The lower efficacy of lamotrigine could be due to differences in the trial design, high placebo response rates, variations in mean doses, and dose titration regimens. In some studies, lamotrigine was administered for only 4 weeks, which may have been inadequate time to reach a response state. Lastly, we should also acknowledge the inconclusive evidence between association of lamotrigine serum levels and response which could contribute to inadequate therapeutic levels (Kumar et al., 2021).
Despite decades of presence of thyroid hormones amongst the affective disorders’ pharmacopeia, clinical recommendations have varied (Gelenberg et al., 2010; Kennedy et al., 2016). Thyroid hormones have shown evidence of antidepressant acceleration, particularly in women, as well as an effective augmentation strategy (Altshuler et al., 2001). Our results are in concordance with a previous NMA (Zhou et al., 2015) showing efficacy of thyroid hormones as an augmentation agent and in contrast to Lorentzen and colleagues who reported negative findings for the use of thyroid hormones in unipolar TRD (Lorentzen et al., 2020). In our study, we showed T3 to be significantly more effective than placebo and comparable to lithium. Nevertheless, in a post-hoc sub-analysis of only three short-term studies (≤3 weeks) we found that response rates were not significant as compared to placebo (RR= 1.79; 0.48–6.67). Plausible explanation for differences between our study and studies in the thyroid literature could be due to the difference in study population, study design, heterogeneity in TRD definitions and therapeutic dosages implemented in many of the studies included in previous meta-analysis.
This NMA provides data regarding a potential benefit of lisdexamfetamine and modafinil, mechanistically acting at vesicular monoamine transporter inhibition as well as dopamine reuptake inhibition respectively to improve depressive symptomatology. The importance of dopaminergic agents in depression has been suggested in both in unipolar and bipolar depression (McIntyre et al., 2017; Nunez et al., 2020; Szmulewicz et al., 2017) showing promising results as augmentation strategies. However, we should acknowledge that our results emphasize an improvement on response rates not remission. Clinical guidelines such as CANMAT suggest caution regarding the use of stimulant and stimulant like molecules and based on previous meta-analysis (Goss et al., 2013; Kennedy et al., 2016) recommend their utilization as second-third line adjunctive agents.
Our study has the following limitations: (a) We provided a single measure of all cause discontinuation as a general reflection of drugs acceptability and safety outcomes and did not include individualized side-effects associated to dropout rates due to data not being widely available for all studies. (b) We did not include comparisons of non-pharmacological treatments associated to augmentation strategies considering difficulties to standardize comparisons; plausibly, this could have overestimated the effect of a single medication or combination drug (c) Several studies comprised small sample sizes (e.g. thyroid hormones, nortriptyline, lithium, and stimulants) and had short follow-up time. Additionally, we should consider the possibility that reporting of side effects and dosing in the older trials (ie. thyroid, lithium) was not optimal due to trial design methodology at that time. This could contribute to wider confidence intervals and heterogeneity. (d) Different standards of regulations in development of clinical trials may have influenced reporting on adverse events or efficacy measures; (f) An overestimation of the results for the included studies which could be attributable indeed to the natural course of illness could not be ignored; (g) We did not include comparisons with NMDA antagonists, such as ketamine, since those have been addressed in a previous NMA (Carter et al., 2020) and has much faster action as compared to the conventional augmentation agents; (h) There was heterogeneity in the definition of TRD in the included studies which precludes assuming a homogenous population; (i) We cannot discount a dose-effect which could also contribute to sample heterogeneity; (j) We included 3 studies which reportedly included bipolar disorders patients however we tried to extract data for patients with unipolar depression where possible (one study) and conducted a sensitivty analysis after excluding the two studies where independent unipolar depression outcomes were not avilable. There may be a possibility that of some of those unipolar patients may have developed psychosis in their longitudinal course of the illness; (l) we defined TRD as failure of one or more antidepressants in the current episode of depression for our study. However, this definition has been used in prior systematic reviews and thus, the included patients could be less refractory than some other TRD studies requiring two failed antidepressants. The results may vary for patients with a higher level of treatment-refractoriness.
Additionally, we should consider some limitations inherent to NMAs. For example, while conducting a NMA it allows to estimate effectiveness of different treatments in the absence of direct comparison between trials (Cipriani et al., 2013), thus, issues regarding inconsistency between either direct or indirect evidence could arise which was assessed in our study and found to be noncontributing (Appendix 1, Table 2). Considering the extensive and highly heterogenous studies which were conducted in the TRD population we focussed on a strict inclusion and exclusion criteria aimed at a discreet phenotype which limited further analysis. We avoided the trials that included geriatric population medical comorbidities such as studies including stimulants for fatigue and somnolence in HIV clinical comorbidities. Moreover, we intended to include data on unipolar TRD patients excluding bipolar or depressive episodes with psychotic features. Importantly we should also consider the plausibility of the transitivity assumption as an essential factor while controlling for the individual biases that could occur in the selection of included studies, populations and environment in which the studies were conducted (LeLorier et al., 1997). Noteworthy is that the combination of trials from different decades, modifications in clinical trial design characteristics and methodologies, and a small number of trials per comparison (as we could see for the evidence for thyroid hormones) may have precluded a further control over some effect modifiers.
5. Conclusion
This study suggests an evidence base for considering atypical antipsychotics, dopaminergic compounds, lithium and thyroid hormones as effective augmentation agents for TRD. Lithium and thyroid hormones constitute an important augmentation strategy in the general pharmacopeia for TRD despite being relatively underutilized. Our data reflects that ziprasidone, mirtazapine, and cariprazine had higher risk for discontinuation. There is a need for long-term studies to further strengthen the current evidence.
Clinical recommendations should outweigh the potential risks and benefits of treatment interventions in the context of side-effect burden and patients’ preference to ameliorate depressive symptoms. Future directions in the design of clinical trials for TRD are needed and shouldinclude phenotypic variations (presence of comorbidities, psychotic features, atypical compared to melancholic depressions) as well as standardization in the development-methodology tailored by agent to effectively demonstrate real world plausible efficacy and tolerability of these compounds.
Supplementary Material
Table 2b.
League table for remission rates associated with studied pharmacological agents. For interpretation, larger than zero favors the column-defining treatment of a cell. Values are RR with associated 95% confidence intervals.
| Aripiprazole | |||||||||||||||||||
| 1.21 (0.81; 1.82) | Brexpiprazole | ||||||||||||||||||
| 1.33 (0.88; 2.01) | 1.10 (0.62; 1.93) | Bupropion | |||||||||||||||||
| 1.23 (0.79; 1.93) | 1.02 (0.57; 1.83) | 0.93 (0.72; 1.20) | Buspirone | ||||||||||||||||
| 1.52 (1.18; 1.96) | 1.26 (0.84; 1.89) | 1.14 (0.72; 1.82) | 1.23 (0.75; 2.02) | Cariprazine | |||||||||||||||
| 0.99 (0.35; 2.79) | 0.82 (0.28; 2.42) | 0.74 (0.25; 2.26) | 0.80 (0.26; 2.46) | 0.65 (0.23; 1.84) | Lamotrigine | ||||||||||||||
| 1.55 (1.18; 2.02) | 1.27 (0.84; 1.93) | 1.16 (0.72; 1.87) | 1.25 (0.76; 2.07) | 1.02 (0.77; 1.33) | 1.56 (0.55; 4.43) | Lisdexamfetamine | |||||||||||||
| 1.70 (0.83; 3.49) | 1.40 (0.64; 3.10) | 1.28 (0.56; 2.90) | 1.38 (0.60; 3.18) | 1.12 (0.54; 2.31) | 1.72 (0.62; 4.76) | 1.10 (0.53; 2.29) | Lithium | ||||||||||||
| 0.44 (0.05; 3.72) | 0.36 (0.04; 3.14) | 0.33 (0.04; 2.90) | 0.35 (0.04; 3.14) | 0.29 (0.03; 2.45) | 0.44 (0.04; 4.71) | 0.28 (0.03; 2.41) | 0.26 (0.03; 2.43) | Methylphenidate | |||||||||||
| 1.38 (0.96; 1.98) | 1.14 (0.70; 1.85) | 1.04 (0.61; 1.77) | 1.12 (0.64; 1.95) | 0.91 (0.63; 1.30) | 1.40 (0.48; 4.08) | 0.89 (0.62; 1.30) | 0.81 (0.38; 1.75) | 3.16 (0.37; 27.30) | Mirtazapine | ||||||||||
| 1.47 (1.09; 1.99) | 1.21 (0.78; 1.88) | 1.11 (0.67; 1.81) | 1.19 (0.71; 2.01) | 0.97 (0.71; 1.31) | 1.49 (0.52; 4.26) | 0.95 (0.69; 1.31) | 0.87 (0.41; 1.82) | 3.36 (0.39; 28.81) | 1.06 (0.71; 1.59) | Modafinil | |||||||||
| 0.17 (0.01; 2.85) | 0.14 (0.01; 2.40) | 0.12 (0.01; 2.20) | 0.13 (0.01; 2.38) | 0.11 (0.01; 1.87) | 0.17 (0.01; 3.43) | 0.11 (0.01; 1.85) | 0.10 (0.01; 1.82) | 0.38 (0.01; 13.23) | 0.12 (0.01; 2.09) | 0.11 (0.01; 1.95) | Nortriptyline | ||||||||
| 1.16 (0.84; 1.62) | 0.96 (0.61; 1.52) | 0.88 (0.52; 1.46) | 0.94 (0.55; 1.61) | 0.76 (0.55; 1.07) | 1.18 (0.41; 3.39) | 0.75 (0.53; 1.06) | 0.68 (0.32; 1.45) | 2.66 (0.31; 22.88) | 0.84 (0.55; 1.28) | 0.79 (0.55; 1.15) | 7.01 (0.40; 121.32) | Olanzapine | |||||||
| 1.41 (0.63; 3.19) | 1.17 (0.49; 2.80) | 1.06 (0.43; 2.62) | 1.14 (0.46; 2.87) | 0.93 (0.41; 2.10) | 1.43 (0.39; 5.23) | 0.92 (0.40; 2.08) | 0.83 (0.29; 2.40) | 3.23 (0.33; 31.51) | 1.02 (0.44; 2.41) | 0.96 (0.42; 2.21) | 8.51 (0.45; 162.16) | 1.21 (0.52; 2.82) | Pramipexole | ||||||
| 1.12 (0.85; 1.49) | 0.93 (0.60; 1.42) | 0.84 (0.52; 1.37) | 0.91 (0.55; 1.51) | 0.74 (0.55; 0.98) | 1.13 (0.40; 3.18) | 0.73 (0.54; 0.98) | 0.66 (0.33; 1.31) | 2.57 (0.30; 21.92) | 0.81 (0.55; 1.19) | 0.76 (0.55; 1.06) | 6.76 (0.39; 116.40) | 0.96 (0.68; 1.38) | 0.79 (0.35; 1.81) | Quetiapine | |||||
| 1.03 (0.68; 1.58) | 0.85 (0.49; 1.47) | 0.78 (0.48; 1.26) | 0.84 (0.52; 1.36) | 0.68 (0.44; 1.06) | 1.05 (0.35; 3.14) | 0.67 (0.43; 1.05) | 0.61 (0.27; 1.37) | 2.37 (0.27; 20.75) | 0.75 (0.45; 1.25) | 0.70 (0.44; 1.13) | 6.23 (0.35; 109.46) | 0.89 (0.54; 1.45) | 0.73 (0.30; 1.79) | 0.92 (0.58; 1.46) | Risperidone | ||||
| 1.10 (0.41; 2.95) | 0.91 (0.32; 2.57) | 0.83 (0.29; 2.40) | 0.89 (0.30; 2.62) | 0.72 (0.27; 1.95) | 1.11 (0.33; 3.78) | 0.71 (0.26; 1.93) | 0.65 (0.33; 1.27) | 2.51 (0.24; 26.27) | 0.80 (0.29; 2.22) | 0.75 (0.27; 2.05) | 6.62 (0.33; 133.12) | 0.94 (0.34; 2.60) | 0.78 (0.22; 2.74) | 0.98 (0.37; 2.58) | 1.06 (0.37; 3.06) | T3 | |||
| 0.91 (0.50; 1.68) | 0.75 (0.37; 1.54) | 0.69 (0.39; 1.21) | 0.74 (0.44; 1.26) | 0.60 (0.32; 1.14) | 0.92 (0.28; 3.04) | 0.59 (0.31; 1.13) | 0.54 (0.21; 1.36) | 2.09 (0.23; 19.23) | 0.66 (0.33; 1.31) | 0.62 (0.32; 1.20) | 5.50 (0.30; 100.31) | 0.79 (0.40; 1.54) | 0.65 (0.24; 1.76) | 0.81 (0.43; 1.56) | 0.88 (0.50; 1.55) | 0.83 (0.26; 2.63) | T4 | ||
| 1.42 (0.86; 2.35) | 1.17 (0.65; 2.12) | 1.07 (0.57; 2.02) | 1.15 (0.60; 2.22) | 0.93 (0.56; 1.54) | 1.44 (0.47; 4.43) | 0.92 (0.55; 1.53) | 0.84 (0.36; 1.94) | 3.25 (0.37; 28.86) | 1.03 (0.58; 1.81) | 0.97 (0.57; 1.64) | 8.55 (0.48; 151.80) | 1.22 (0.71; 2.11) | 1.00 (0.40; 2.53) | 1.27 (0.75; 2.13) | 1.37 (0.74; 2.55) | 1.29 (0.44; 3.82) | 1.55 (0.72; 3.35) | Ziprasidone | |
| 1.75 (1.47; 2.09) | 1.44 (1.00; 2.08) | 1.32 (0.86; 2.02) | 1.42 (0.89; 2.25) | 1.15 (0.96; 1.38) | 1.77 (0.63; 4.92) | 1.13 (0.93; 1.39) | 1.03 (0.51; 2.08) | 4.00 (0.47; 33.77) | 1.27 (0.92; 1.73) | 1.19 (0.93; 1.52) | 10.53 (0.62; 179.86) | 1.50 (1.14; 1.99) | 1.24 (0.56; 2.74) | 1.56 (1.25; 1.95) | 1.69 (1.13; 2.54) | 1.59 (0.60; 4.22) | 1.91 (1.04; 3.52) | 1.23 (0.77; 1.97) | Placebo |
Acknowledgements
We would like to thank Ms. Lori Solmonson for proofreading the manuscript and writing support.
Role of funding
There is no role of a funding source in this manuscript.
Footnotes
Declaration of Competing Interest
NA Nuñez is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health under award number T32 GM008685. MA Frye reports grant support from Assurex Health, Mayo Foundation and intellectual property licensed to Chymia LLC. with rights to receive future royalties. B Singh reports grant support from Mayo Clinic. All other authors report no financial relationships with commercial interests. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CRediT authorship contribution statement
Nicolas A Nuñez: Conceptualization, Visualization, Data curation, Formal analysis, Writing – review & editing. Boney Joseph: Conceptualization, Visualization, Data curation, Formal analysis, Writing – review & editing. Mehak Pahwa: Conceptualization, Visualization, Data curation, Writing – review & editing. Rakesh Kumar: Data curation, Writing – review & editing. Manuel Gardea Resendez: Data curation, Writing – review & editing. Larry J Prokop: Data curation, Writing – review & editing. Marin Veldic: Writing – review & editing. Ashok Seshadri: Writing – review & editing. Joanna M Biernacka: Writing – review & editing. Mark A Frye: Conceptualization, Visualization, Data curation, Writing – review & editing. Zhen Wang: Formal analysis, Writing – review & editing. Balwinder Singh: Conceptualization, Visualization, Data curation, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2021.12.134.
References
- Abolfazli R, Hosseini M, Ghanizadeh A, et al. , 2011. Double-blind randomized parallel-group clinical trial of efficacy of the combination fluoxetine plus modafinil versus fluoxetine plus placebo in the treatment of major depression. Depress. Anxiety 28, 297–302. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bauer M, Frye MA, et al. , 2001. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am. J. Psychiatry 158, 1617–1622. [DOI] [PubMed] [Google Scholar]
- Appelberg BG, Syvalahti EK, Koskinen TE, et al. , 2001. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J. Clin. Psychiatry 62, 448–452. [DOI] [PubMed] [Google Scholar]
- Barbee JG, Thompson TR, Jamhour NJ, et al. , 2011. A double-blind placebo-controlled trial of lamotrigine as an antidepressant augmentation agent in treatment-refractory unipolar depression. J. Clin. Psychiatry 72, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Barbosa L, Berk M, Vorster M, 2003. A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J. Clin. Psychiatry 64, 403–407. [DOI] [PubMed] [Google Scholar]
- Bauer M, Dell’Osso L, Kasper S, et al. , 2013. Extended-release quetiapine fumarate (quetiapine XR) monotherapy and quetiapine XR or lithium as add-on to antidepressants in patients with treatment-resistant major depressive disorder. J. Affect. Disord. 151, 209–219. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pretorius H, Constant E, et al. , 2009. Extended-Release Quetiapine as Adjunct to an Antidepressant in Patients with Major Depressive Disorder: Results of a Randomized, Placebo-Controlled, Double Blind Study. J. Clin Psychiatry 70 (4), 540–549. [DOI] [PubMed] [Google Scholar]
- Baumann P, Nil R, Souche A, et al. , 1996. A double-blind, placebo-controlled study of citalopram with and without lithium in the treatment of therapy-resistant depressive patients: a clinical, pharmacokinetic, and pharmacogenetic investigation. J. Clin. Psychopharmacol. 16, 307–314. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M, 1994. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. [PubMed] [Google Scholar]
- Berlim MT, Turecki G, 2007a. Definition, assessment, and staging of treatment—Resistant refractory major depression: a review of current concepts and methods. Can. J. Psychiatry 52, 46–54. [DOI] [PubMed] [Google Scholar]
- Berlim MT, Turecki G, 2007b. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur. Neuropsychopharmacol. 17, 696–707. [DOI] [PubMed] [Google Scholar]
- Berman RM, Fava M, Thase ME, et al. , 2009. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 14 (4), 197–206. [DOI] [PubMed] [Google Scholar]
- Berman RM, Marcus RN, Swanink R, et al. , 2007. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 68, 843–853. [DOI] [PubMed] [Google Scholar]
- Browne M, Lapierre Y, Hrdina P, et al. , 1990. Lithium as an adjunct in the treatment of major depression. Int. Clin. Psychopharmacol. 5, 103–110. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Yasmin S, Price LH, 2002. A double-blind, placebo-controlled study of antidepressant augementation with mirtazapine. Biol. Psychiatry 51, 183–188. [DOI] [PubMed] [Google Scholar]
- Carter B, Strawbridge R, Husain MI, et al. , 2020. Relative effectiveness of augmentation treatments for treatment-resistant depression: a systematic review and network meta-analysis. Int. Rev. Psychiatry 32, 477–490. [DOI] [PubMed] [Google Scholar]
- Chaput Y, Magnan A, Gendron A, 2008. The co-administration of quetiapine or placebo to cognitive-behavior therapy in treatment refractory depression: a preliminary trial. BMC Psychiatry 8, 1–8. 10.1186/1471-244X-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon EJ, Lee KH, Park YW, et al. , 2017. Comparison of the efficacy and safety of aripiprazole versus bupropion augmentation in patients with major depressive disorder unresponsive to selective serotonin reuptake inhibitors: a randomized, prospective, open-label study. J. Clin. Psychopharmacol. 37, 193–199. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Higgins JP, Geddes JR, et al. , 2013. Conceptual and technical challenges in network meta-analysis. Ann. Intern. Med. 159, 130–137. [DOI] [PubMed] [Google Scholar]
- Cleare A, Pariante CM, Young AH, et al. , 2015. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J. Psychopharmacol. 29, 459–525. [DOI] [PubMed] [Google Scholar]
- Corrigan MH, Denahan AQ, Wright CE, et al. , 2000. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress. Anxiety 11, 58–65. [DOI] [PubMed] [Google Scholar]
- Corya SA, Williamson D, Sanger TM, et al. , 2006. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depression. Depress. Anxiety 23, 364–372. [DOI] [PubMed] [Google Scholar]
- Cusin C, Iovieno N, Iosifescu DV, et al. , 2013. A randomized, double-blind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J. Clin. Psychiatry 74, 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBattista C, Doghramji K, Menza M, et al. , 2003. Adjunct modafinil for the short-term treatment of fatigue and sleepiness in patients with major depressive disorder: a preliminary double-blind, placebo-controlled study. J. Clin. Psychiatry 64, 1057–1064. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N, 1986. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. [DOI] [PubMed] [Google Scholar]
- Dorée JP, Rosiers JD, Lew V, et al. , 2007. Quetiapine augmentation of treatment-resistant depression: a comparison with lithium. Curr. Med. Res. Opin. 23, 333–341. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Crits-Christoph P, Evans DL, et al. , 2007. Coadministration of modafinil and a selective serotonin reuptake inhibitor from the initiation of treatment of major depressive disorder with fatigue and sleepiness: a double-blind, placebo-controlled study. J. Clin. Psychopharmacol. 27, 614–619. [DOI] [PubMed] [Google Scholar]
- Dunner DL, Amsterdam JD, Shelton RC, et al. , 2007. Efficacy and tolerability of adjunctive ziprasidone in treatment-resistant depression: a randomized, open-label, pilot study. J. Clin. Psychiatry 68, 1071–1077. [DOI] [PubMed] [Google Scholar]
- Durgam S, Earley W, Guo H, Li D, Németh G, Laszlovszky I, Fava M, Montgomery SA, et al. , 2016. Efficacy and safety of adjunctive cariprazine in inadequate responders to antidepressants: a randomized, double-blind, placebo-controlled study in adult patients with major depressive disorder. J Clin Psychiatry 77 (3), 371–378. [DOI] [PubMed] [Google Scholar]
- Earley WR, Guo H, Németh G, et al. , 2018. Cariprazine augmentation to antidepressant therapy in major depressive disorder: results of a randomized, double-blind, placebo-controlled trial. Psychopharmacol. Bull. 48, 62. [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, et al. , 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khalili N, Joyce M, Atkinson S, et al. , 2010. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int. J. Neuropsychopharmacol. 13, 917–932. [DOI] [PubMed] [Google Scholar]
- Fang Y, Yuan C, Xu Y, et al. , 2011. A pilot study of the efficacy and safety of paroxetine augmented with risperidone, valproate, buspirone, trazodone, or thyroid hormone in adult Chinese patients with treatment-resistant major depression. J. Clin. Psychopharmacol. 31, 638–642. [DOI] [PubMed] [Google Scholar]
- Fava M, 2003. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 53, 649–659. [DOI] [PubMed] [Google Scholar]
- Fava M, Durgam S, Earley W, et al. , 2018. Efficacy of adjunctive low-dose cariprazine in major depressive disorder: a randomized, double-blind, placebo-controlled trial. Int. Clin. Psychopharmacol. 33, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Ménard F, Davidsen CK, et al. , 2016. Adjunctive brexpiprazole in patients with major depressive disorder and irritability: an exploratory study. J. Clin. Psychiatry 77, 1695–1701. [DOI] [PubMed] [Google Scholar]
- Fava M, Mischoulon D, Iosifescu D, et al. , 2012. A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother. Psychosom. 81, 87–97. [DOI] [PubMed] [Google Scholar]
- Fava M, Thase ME, DeBattista C, 2005. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J. Clin. Psychiatry 66, 85–93. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Wooderson SC, Markopoulo K, et al. , 2009. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J. Affect. Disord. 116, 4–11. [DOI] [PubMed] [Google Scholar]
- Franco-Chaves JA, Mateus CF, Luckenbaugh DA, et al. , 2013. Combining a dopamine agonist and selective serotonin reuptake inhibitor for the treatment of depression: a double-blind, randomized pilot study. J. Affect. Disord. 149, 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg A, Freeman M, Markowitz J, et al. , 2010. American psychiatric association practice guidelines for the treatment of patients with major depressive disorder. Am. J. Psychiatry 167, 9–118. [Google Scholar]
- Gitlin MJ, Weiner H, Fairbanks L, et al. , 1987. Failure of T3 to potentiate tricyclic antidepressant response. J. Affect. Disord. 13, 267–272. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline, 2009. Study in patients with depression not responding to selective serotonin reuptake inhibitors. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT00296517.
- Goss AJ, Kaser M, Costafreda SG, et al. , 2013. Modafinil augmentation therapy in unipolar and bipolar depression: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Psychiatry 74, 1101–1107. [DOI] [PubMed] [Google Scholar]
- Grunze H, Vieta E, Goodwin GM, et al. , 2018. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: acute and long-term treatment of mixed states in bipolar disorder. World J. Biol. Psychiatry 19, 2–58. [DOI] [PubMed] [Google Scholar]
- Gulrez G, Badyal DK, Deswal RS, et al. , 2012. Bupropion as an augmenting agent in patients of depression with partial response. Basic Clin. Pharmacol. Toxicol. 110, 227–230. [DOI] [PubMed] [Google Scholar]
- Han C, Wang SM, Kwak KP, et al. , 2015. Aripiprazole augmentation versus antidepressant switching for patients with major depressive disorder: a 6-week, randomized, rater-blinded, prospective study. J. Psychiatr. Res. 66, 84–94. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Charney DS, Sternberg DE, 1983. Lithium carbonate augmentation of antidepressant treatment: an effective prescription for treatment-refractory depression. Arch. Gen. Psychiatry 40, 1335–1342. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, et al. , 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343 (d5928). 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thomas J, Chandler J, et al. , 2019. Cochrane Handbook For Systematic Reviews of Interventions. John Wiley & Sons. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, et al. , 2003. Measuring inconsistency in meta-analyses. BMJ Br. Med. J. 327, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe RT, Singer W, 1990. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res. 32, 241–251. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Singer W, Levitt AJ, MacDonald C, 1993. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch. Gen. Psychiatry 50, 387–393. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Sokolov ST, Levitt AJ, 2006. Lithium and triiodothyronine augmentation of antidepressants. Can. J. Psychiatry 51, 791–793. [DOI] [PubMed] [Google Scholar]
- Kamijima K, Higuchi T, Ishigooka J, et al. , 2013. Aripiprazole augmentation to antidepressant therapy in Japanese patients with major depressive disorder: a randomized, double-blind, placebo-controlled study (ADMIRE study). J. Affect. Disord. 151, 899–905. [DOI] [PubMed] [Google Scholar]
- Katona CL, Abou-Saleh MT, Harrison DA, et al. , 1995. Placebo-controlled trial of lithium augmentation of fluoxetine and lofepramine. Br. J. Psychiatry 166, 80–86. [DOI] [PubMed] [Google Scholar]
- Keitner GI, Garlow SJ, Ryan CE, et al. , 2009. A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. J. Psychiatr. Res. 43, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, et al. , 2016. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can. J. Psychiatry 61, 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DS, MacNeill SJ, Tallon D, et al. , 2018. Mirtazapine added to SSRIs or SNRIs for treatment resistant depression in primary care: phase III randomised placebo controlled trial (MIR). BMJ 363 (k4218). 10.1136/bmj.k4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn U, Binder H, König J, 2013. A graphical tool for locating inconsistency in network meta-analyses. BMC Med. Res. Methodol. 13, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Nunez N, Prokop L, et al. , 2021. Association of optimal lamotrigine serum levels and therapeutic efficacy in mood disorders: a systematic review. J. Clin. Psychopharmacol. 41, 681–686. [DOI] [PubMed] [Google Scholar]
- Landén M, Bjorling G, Agren H, et al. , 1998. A Randomized, Double Blind, Placebo Controlled Trial of Buspirone in Combination with an SSRI in patients with Treatment Refractory Depression. J Clin Psychiatry 59, 664–668. https://pubmed.ncbi.nlm.nih.gov/9921700. [PubMed] [Google Scholar]
- LeLorier J, Grégoire G, Benhaddad A, et al. , 1997. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N. Engl. J. Med. 337 (8), 536–542. [DOI] [PubMed] [Google Scholar]
- Lorentzen R, Kjær JN, Østergaard SD, et al. , 2020. Thyroid hormone treatment in the management of treatment-resistant unipolar depression: a systematic review and meta-analysis. Acta Psychiatr. Scand. 141, 316–326. [DOI] [PubMed] [Google Scholar]
- Madhoo M, Keefe RS, Roth RM, et al. , 2014. Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology 39, 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud RA, Pandina GJ, Turkoz I, et al. , 2007. Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann. Intern. Med. 147, 593–602. [DOI] [PubMed] [Google Scholar]
- Malhi G, Parker G, Crawford J, et al. , 2005. Treatment-resistant depression: resistant to definition? Acta Psychiatr. Scand. 112, 302–309. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, et al. , 2008. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J. Clin. Psychopharmacol. 28, 156–165. [DOI] [PubMed] [Google Scholar]
- Mavridis D, Giannatsi M, Cipriani A, et al. , 2015. A primer on network meta-analysis with emphasis on mental health. Evid. Based Ment. Health 18, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre A, Gendron A, McIntyre A, 2007. Quetiapine adjunct to selective serotonin reuptake inhibitors or venlafaxine in patients with major depression, comorbid anxiety, and residual depressive symptoms: a randomized, placebo-controlled pilot study. Depress. Anxiety 24, 487–494. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Filteau MJ, Martin L, et al. , 2014. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J. Affect. Disord. 156, 1–7. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Lee Y, Zhou AJ, et al. , 2017. The efficacy of psychostimulants in major depressive episodes: a systematic review and meta-analysis. J. Clin. Psychopharmacol. 37, 412–418. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI, 2009. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 166 (9), 980–991. 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- NICE, 2010. Depression: the Treatment and Management of Depression in Adults (updated edition). British Psychological Society. [PubMed] [Google Scholar]
- Nierenberg AA, Fava M, Trivedi MH, et al. , 2006. A comparison of lithium and T 3 augmentation following two failed medication treatments for depression: a STAR* D report. Am. J. Psychiatry 163, 1519–1530. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Papakostas GI, Petersen T, et al. , 2003. Lithium augmentation of nortriptyline for subjects resistant to multiple antidepressants. J. Clin. Psychopharmacol. 23, 92–95. [DOI] [PubMed] [Google Scholar]
- Normann C, Hummel B, Scharer LO, et al. , 2002. Lamotrigine as adjunct to paroxetine in acute depression: a placebo-controlled, double-blind study. J. Clin. Psychiatry 63, 337–344. [DOI] [PubMed] [Google Scholar]
- Nunez NA, Singh B, Romo-Nava F, et al. , 2020. Efficacy and tolerability of adjunctive modafinil/armodafinil in bipolar depression: a meta-analysis of randomized controlled trials. Bipolar Disord. 22, 109–120. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Fava M, Baer L, et al. , 2015. Ziprasidone augmentation of escitalopram for major depressive disorder: efficacy results from a randomized, double-blind, placebo-controlled study. Am. J. Psychiatry 172, 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Masand PS, Pae CU, et al. , 2006. A randomized, double-blind, placebo-controlled trial of augmentation with an extended release formulation of methylphenidate in outpatients with treatment-resistant depression. J. Clin. Psychopharmacol. 26, 653–656. [DOI] [PubMed] [Google Scholar]
- Core Team R, 2019. R: A language and Environment For Statistical computing. R Core Team. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- Raeisi F, Habibi N, Nasehi A, et al. , 2006. Combination of citalopram and nortriptyline in treatment of moderate to severe major depression: a double-blind, Placebo-controlled Trial. Iran J Psychiatry 1, 35–39. [Google Scholar]
- Rafeyan R, Papakostas G, Jackson W, et al. , 2020. Inadequate Response to Treatment in Major Depressive Disorder: Augmentation and Adjunctive Strategies. J Clin Psychiatry 81 (2), OT19037BR3. [DOI] [PubMed] [Google Scholar]
- Ravindran AV, Kennedy SH, O’Donovan MC, et al. , 2008. Osmotic-release oral system methylphenidate augmentation of antidepressant monotherapy in major depressive disorder: results of a double-blind, randomized, placebo-controlled trial. J. Clin. Psychiatry 69, 87–94. [DOI] [PubMed] [Google Scholar]
- Reeves H, Batra S, May RS, et al. , 2008. Efficacy of risperidone augmentation to antidepressants in the management of suicidality in major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. J. Clin. Psychiatry 69, 1228–1236. [DOI] [PubMed] [Google Scholar]
- Richards C, McIntyre R, Weisler R, et al. , 2016. Lisdexamfetamine dimesylate augmentation for adults with major depressive disorder and inadequate response to antidepressant monotherapy: results from 2 phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J. Affect. Disord. 206, 151–160. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, et al. , 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am. J. Psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Salanti G, 2012. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods 3, 80–97. [DOI] [PubMed] [Google Scholar]
- Santos MA, Rocha FL, Hara C, 2008. Efficacy and safety of antidepressant augmentation with lamotrigine in patients with treatment-resistant depression: a randomized, placebo-controlled, double-blind study. Prim. Care Companion J. Clin. Psychiatry 10, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler F, Anghelescu IG, 2007. Lithium versus lamotrigine augmentation in treatment resistant unipolar depression: a randomized, open-label study. Int. Clin. Psychopharmacol. 22, 179–182. [DOI] [PubMed] [Google Scholar]
- Schöpf J, Baumann P, Lemarchand T, et al. , 1989. Treatment of endogenous depressions resistant to tricyclic antidepressants or related drugs by lithium addition. Results of a placebo-controlled double-blind study. Pharmacopsychiatry 22, 183–187. [DOI] [PubMed] [Google Scholar]
- Schwarzer G, 2007. Meta: an R package for meta-analysis. R News 7 (3), 40–45. [Google Scholar]
- Seshadri A, Wermers ML, Habermann TJ, et al. , 2021. Long-term efficacy and tolerability of adjunctive aripiprazole for major depressive disorder: systematic review and meta-analysis. Prim. Care Companion CNS Disord. 23 (4) 10.4088/PCC.20r02799. [DOI] [PubMed] [Google Scholar]
- Shahal B, Piel E, Mecz L, et al. , 1996. Lack of advantage for imipramine combined with lithium versus imipramine alone in the treatment of major depression-a double-blind controlled study. Biol. Psychiatry 40, 1181–1183. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Osuntokun O, Heinloth AN, et al. , 2010. Therapeutic options for treatment-resistant depression. CNS Drugs 24, 131–161. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Papakostas GI, 2008. Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr. Scand. 117, 253–259. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tollefson GD, Tohen M, et al. , 2001. A novel augmentation strategy for treating resistant major depression. Am. J. Psychiatry 158, 131–134. [DOI] [PubMed] [Google Scholar]
- Shelton R, Williamson D, Corya S, et al. , 2005. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 66 (10), 1289–1297. [DOI] [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, Linardatos E, et al. , 2016. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. Focus 14, 244–265 (Madison). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G, Bernadt M, 1993. Lithium augmentation therapy in tricyclic-resistant depression: a controlled trial using lithium in low and normal doses. Br. J. Psychiatry 162, 634–640. [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Carter B, Marwood L, et al. , 2019. Augmentation therapies for treatment-resistant depression: systematic review and meta-analysis. Br. J. Psychiatry 214, 42–51. [DOI] [PubMed] [Google Scholar]
- Szmulewicz A, Angriman F, Samamé C, et al. , 2017. Dopaminergic agents in the treatment of bipolar depression: a systematic review and meta-analysis. Acta Psychiatr. Scand. 135, 527–538. [DOI] [PubMed] [Google Scholar]
- Team R, 2016. RStudio: Integrated Development Environment for R. RStudio Team. RStudio Inc, Boston, MA: http://www.rstudio.com/. [Google Scholar]
- Thase ME, Corya SA, Osuntokun O, et al. , 2007. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J. Clin. Psychiatry 68, 224–236. [DOI] [PubMed] [Google Scholar]
- Thase ME, Youakim JM, Skuban A, et al. , 2015a. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J. Clin. Psychiatry 76 (9), 1232–1240. [DOI] [PubMed] [Google Scholar]
- Thase ME, Youakim JM, Skuban A, et al. , 2015b. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J. Clin. Psychiatry 76, 1224–1231. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Cutler AJ, Richards C, et al. , 2013. A randomized controlled trial of the efficacy and safety of lisdexamfetamine dimesylate as augmentation therapy in adults with residual symptoms of major depressive disorder after treatment with escitalopram. J. Clin. Psychiatry 74, 802–809. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Fava M, Wisniewski SR, et al. , 2006. Medication augmentation after the failure of SSRIs for depression. N Engl J Med 354 (12), 1243–1252. [DOI] [PubMed] [Google Scholar]
- Vázquez GH, Bahji A, Undurraga J, et al. , 2021. Efficacy and tolerability of combination treatments for major depression: antidepressants plus second-generation antipsychotics vs. esketamine vs. lithium. J. Psychopharmacol. 35 (8), 890–900, 02698811211013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W, 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36 (3), 1–48. [Google Scholar]
- Yoshimura R, Hori H, Umene-Nakano W, et al. , 2014. Comparison of lithium, aripiprazole and olanzapine as augmentation to paroxetine for inpatients with major depressive disorder. Ther. Adv. Psychopharmacol. 4, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R, Kishi T, Hori H, et al. , 2012. Comparison of the efficacy between paroxetine and sertraline augmented with aripiprazole in patients with refractory major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 355–357. [DOI] [PubMed] [Google Scholar]
- Zhou X, Ravindran AV, Qin B, Del Giovane C, Li Q, Bauer M, Liu Y, Fang Y, da Silva T, Zhang Y, Fang L, Wang X, Xie P, 2015. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J. Clin. Psychiatry 76 (4), e487–e498. [DOI] [PubMed] [Google Scholar]
- Zusky PM, Biederman J, Rosenbaum JF, et al. , 1988. Adjunct low dose lithium carbonate in treatment-resistant depression: a placebo-controlled study. J. Clin. Psychopharmacol. 8 (2), 120–124. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


