Abstract
Point-of-care (POC) biochemical sensors have found broad applications in areas ranging from clinical diagnosis to environmental monitoring. However, POC sensors often suffer from poor sensitivity. Here, we synthesized a metal-organic framework, where the ligand is the aggregation-induced emission luminogen (AIEgen), which we call metal-AIEgen frameworks (MAFs), for use in the ultrasensitive POC biochemical sensors. MAFs process a unique luminescent mechanism of structural rigidity-enhanced emission to achieve a high quantum yield (~99.9%). We optimized the MAFs to show 102- to 103-fold enhanced sensitivity for a hydrogel-based POC digital sensor and lateral flow immunoassays (LFIA). MAFs have a high affinity to directly absorb proteins, which can label antibodies for immunoassays. MAFs-based LFIA with enhanced sensitivity shows robust serum detection for POC clinical diagnosis.
Photoluminescent mesoporous metal-AIEgen frameworks boost the sensitivity and convenience of biochemical point-of-care sensors.
INTRODUCTION
Point-of-care (POC) biochemical sensors constitute a great advance for the growing demand for health care, food/water safety, and antibiotic/drug tests (1–7). POC sensors offer convenience and efficiency in both time and cost, but they often sacrifice the required sensitivity. To increase sensitivity, the enzyme/nanozyme-linked amplification (8–12), surface-enhanced Raman scattering (13), Cas endonuclease–mediated assays (14), and spin-enhanced nanodiamond techniques (15) are developed to cooperate with traditional POC sensors. These ultrasensitive improvements typically have to resort to auxiliary operations, reactions, or devices, to compromise the convenience and time/cost-efficiency. POC sensors face the limitation to balance sensitivity and convenience. An ultrasensitive and convenient POC sensor is the desired choice but a great challenge.
Metal-organic frameworks (MOFs), one of the most versatile materials for broad applications in chemistry and biomedicine (16–27), may provide a solution to the problem of sensitivity in POC sensors by fluorescent MOFs. Although lanthanide-based fluorescent MOFs are broadly reported (28–30), they use rare-earth metals that are costly. Fluorophores conjugating with metal ions that are not rare-earth metals constitute strong candidates for fluorescent MOF sensors. Unfortunately, so far, the fluorescent MOF sensors are limited owing to the low quantum yield (QY) and partially because of the lack of systematic investigation of nanoscale synthesis of particles. Existing MOFs are typically not sensitive enough for POC sensors. The low QY is restricted by aggregation-caused quenching (ACQ) and ligand-to-metal charge transfer (31, 32). To de novo design fluorescent MOFs, aggregation-induced emission luminogens (AIEgens) are the ideal ligands (33, 34). Recent studies have explored AIEgen-based MOF to some extent (35–41). Unlike fluorophores undergoing ACQ, AIEgens are brightly emissive in an aggregated state (42, 43). Their luminescent mechanism perfectly fits MOFs, which have metal ions–anchored rigid state to confine the intramolecular rotation/vibration, thus generating strongly fluorescent metal-AIEgen frameworks (MAFs). QY of MAFs is high, even close to 99.9% (33). However, MAFs, as a powerful light-emitting material, have not been valued, especially in the context of POC sensors.
Here, we exploited the mesoporous MAFs to develop sensitive and convenient POC sensors, e.g., hydrogel digital sensors (HDSs) and lateral flow immunoassays (LFIAs). The successful integration of MAFs with HDS and LFIA enables fast POC detection of ions and macromolecular clinical biomarkers with 102- to 103-fold enhanced sensitivity. We used 1,1,2,2-tetra(4-carboxylphenyl)ethylene (TCPE, a typical AIEgen) and zirconium chloride to synthesize the bright MAFs and modulated the morphology and particle size to improve their detection properties. Using the blue-emissive mesoporous MAFs (B-MAFs) and commercial red-emissive quantum dots (R-QDs) to integrate with a custom-designed hydrogel complex, we fabricated the white-emissive POC HDS. HDS shows a white-to-red fluorescence color transition signal, which is more readable for naked eyes at a low analyte concentration and quantitive digital detection using smartphone-based red-green-blue (RGB) analysis. Using MAFs for LFIA, we found a high affinity between MAFs and antibodies (Ab). Without stabilizing polymers or covalent linking agents, MAFs can directly absorb Abs to form fluorescent labeling particles. MAFs-based LFIAs show excellent sensitivity, selectivity, and reliability for real clinical diagnosis.
RESULTS
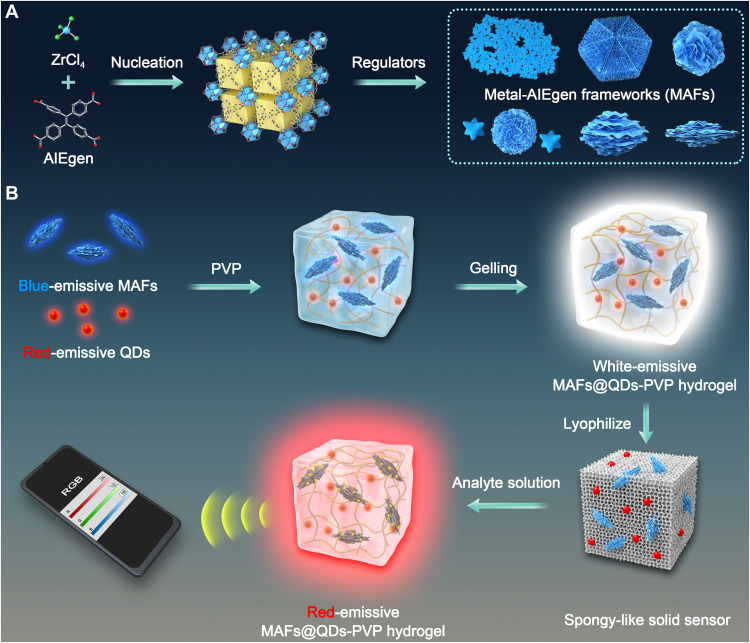
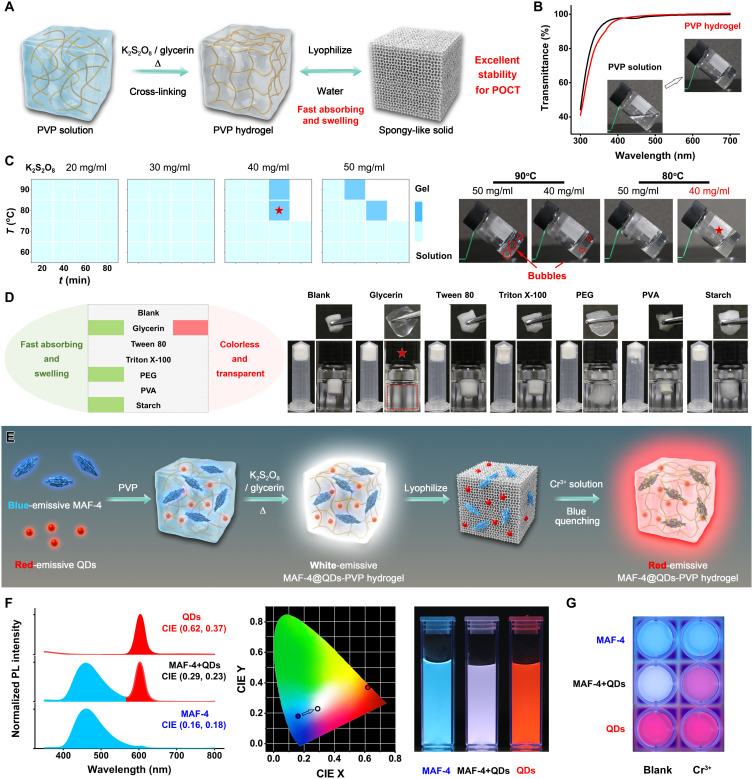
For POC detection using HDS, we designed a cross-linked polyvinylpyrrolidone (PVP) hydrogel to support the analyte-triggered reaction. By introducing the B-MAFs and R-QDs, we developed the white-emissive MAFs@QDs-PVP hydrogel complex (Fig. 1). After freeze-drying, the MAFs@QDs-PVP hydrogel complex becomes spongy-like HDS, which is highly stable and storable. Once an analyte solution is added, the solid sensor absorbs water fast, swells, and turns transparent. Using the white-to-red fluorescence color transition signal, we realized fast and sensitive POC detection with naked eyes and quantitive digital RGB analysis within a few minutes. This quantitive HDS strategy can be versatile by replacing MAFs with any other stimuli-responsive MOFs or other materials.
Fig. 1. Schematic illustrations.
(A) The synthesis of different MAFs. (B) The fabrication of MAFs@QDs-PVP hydrogel complex for HDS and the digital sensing strategy.
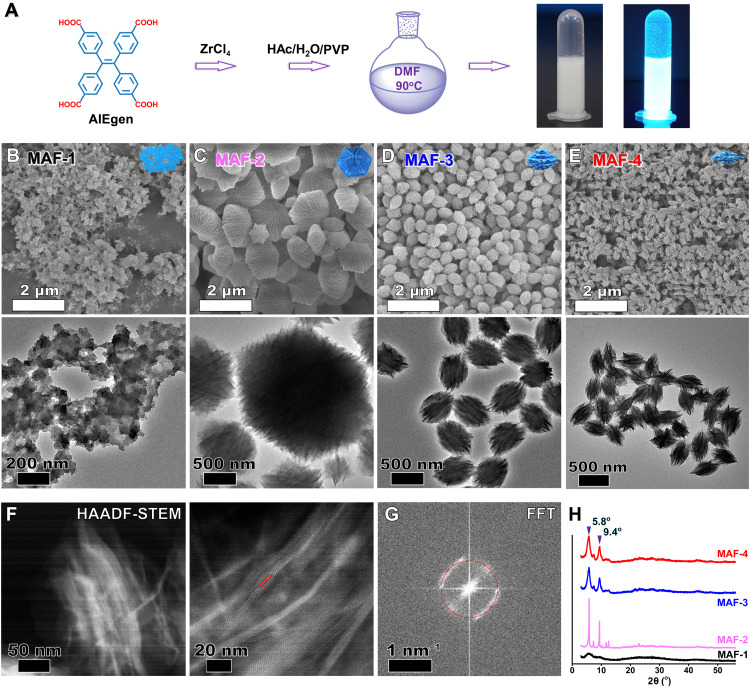
We synthesized different MAFs by heating a mixture of TCPE and ZrCl4 in the presence of different regulators [N,N′-dimethylformamide (DMF), 90°C; Fig. 2A]. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images indicate an amorphous MAF-1 without using regulators (Fig. 2B). Acetic acid (HAc), the regulator to cause a slow deprotonation effect, strongly affects the nucleation process and the kinetic growth of MOFs to facilitate the oriented crystal (44, 45). Here, HAc-caused slow deprotonation effect generates a highly structured hexagonal starfish-like MAF-2 (Fig. 2C). Water as a coregulator normally changes the polarity of the surface of MOFs to alter their morphology (46). Using HAc and water as regulators, we obtained a monodisperse olive-like MAF-3 (Fig. 2D). Water plays an essential role in the unique morphology. Either less or excess water produces nonuniform MAFs (fig. S1). Simply adding water without HAc results in amorphous products (fig. S2). Adjusting the mass ratio of TCPE:ZrCl4 strongly regulates MAFs. A preferable ratio of 1:1 produces monodisperse MAF-3 with uniform size and mesoporous morphology. Other ratios yield MAFs with bad control of size and shape (fig. S3).
Fig. 2. Characterization of MAFs.
(A) Illustration of the synthesis of high-fluorescent MAFs. Inset: Photographs of MAF-4 under daylight and UV light. (B to E) SEM (top) and TEM (bottom) images of MAF-1 without regulator during the synthesis, MAF-2 with HAc, MAF-3 with HAc/H2O, and MAF-4 with HAc/H2O/PVP as regulators. (F) HAADF-STEM images of MAF-4. The lattice spacing of 1.52 nm is marked with red lines. (G) The corresponding selected-area fast Fourier transform (FFT) pattern. The electron diffraction spots are marked with the red circle. (H) PXRD profiles of different MAFs.
Surfactants regulate the kinetic growth of nanomaterials with preferential crystal orientation and morphology (47, 48). We further introduced PVP, polyethylene glycol (PEG), or cetyltrimethylammonium bromide (CTAB). CTAB and PEG regulate MAFs into a mixture of spherical and spiky particles (fig. S4). PVP undergoes dynamic adsorption and desorption on surfaces of MAFs through the interactions between Zr4+ and pyrrolidinone group. The dynamic process affects the kinetic growth of MAFs to generate the mesoporous shuttle-like MAF-4 with smaller size than MAF-3 (Fig. 2E). This inhibition effect of PVP is concentration dependent. A low concentration of PVP does not affect their morphology (fig. S5). MAFs show bright sky-blue fluorescence under ultraviolet (UV) light (e.g., MAF-4; Fig. 2A). High-resolution TEM images of MAF-4 reveal a layer-by-layer stacking structure (fig. S6). High-angle annular dark-field scanning TEM (HAADF-STEM) images of MAF-4 depict the refined crystalline texture with a lattice spacing of 1.52 nm (Fig. 2, F and G, and fig. S7). Powder x-ray diffraction (PXRD) tests further prove the amorphous MAF-1 (no distinct 2θ peaks) and crystalline MAF-2/MAF-3/MAF-4 (Fig. 2H). The 2θ peak at 5.8o is consistent with TEM-measured lattice spacing.
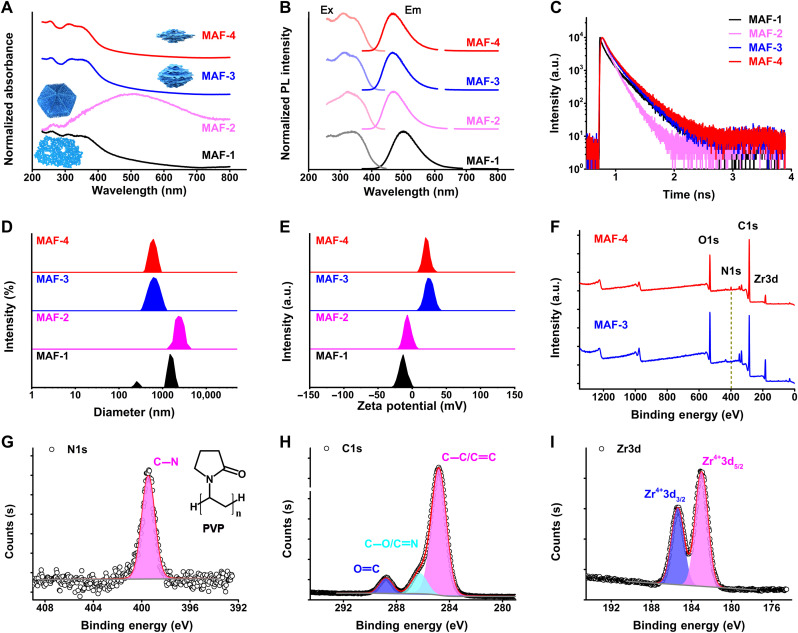
MAFs have three absorption bands at 250 to 350 nm, except for MAF-2, which displays a broad extinction band at 505 nm (Fig. 3A). From MAF-1 to MAF-4, the excitation/emission peaks shift to a shorter wavelength (MAF-4, 310/465 nm; Fig. 3B and table S1). QY of MAF-4 in solution is above 78%. Fluorescent lifetime (τ) tests indicate the longest value of 3.5 ns for MAF-4 (Fig. 3C). The monodisperse MAF-4 has a diameter of 500 ± 100 nm (Fig. 3D). The surface of MAF particles consists of TCPE ligands and Zr4+ ions. Their surface zeta-potential values are determined by the relative amount of TCPE/Zr4+ exposed to the solution. MAF-1 and MAF-2 are negatively charged owing to more TCPE ligands exposed on the surface of particles. In comparison, introducing water during the synthesis altered the distribution to expose more Zr4+ on the surface of particles. Thus, MAF-3 and MAF-4 are positively charged (Fig. 3E). Fourier transform infrared (FTIR) spectra reveal that the stretching vibration νC=O (1690 cm−1) of TCPE nearly disappears in MAF-4, indicating the strong coordination of metal ions and TCPE (fig. S8) (49, 50). X-ray photoelectron spectroscopy (XPS) analysis of MAF-4 proves the surface coating of PVP (the C-N band matched to alkylpyrrole groups; Fig. 3, F to I).
Fig. 3. Characterization of MAFs.
(A) Normalized UV-visible absorption spectra of different MAFs. (B) Normalized excitation (Ex) and emission (Em) spectra of different MAFs. PL, photoluminescence. (C) Fluorescent lifetime profiles of different MAFs. a.u., arbitrary units. (D) Dynamic light scattering (DLS) size distribution profiles of different MAFs. (E) Zeta-potential profiles of different MAFs. (F) XPS binding energy profiles of MAF-3 and MAF-4. (G to I) High-resolution XPS binding energy profiles of N1s, C1s, and Zr3d of MAF-4.
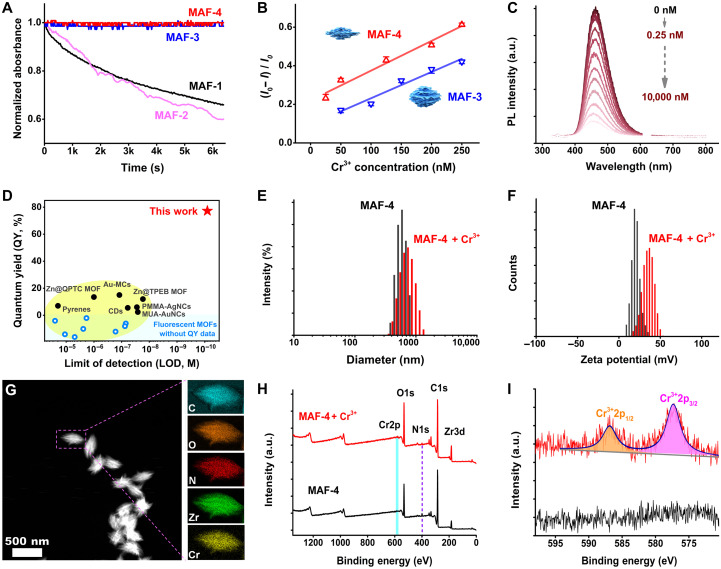
We chose different MAFs according to their properties. MAF-1/MAF-2 with micrometer size are not stable regarding dispersity. They precipitate from the solution within an hour (Fig. 4A). Their negative surface charges may cause nonspecific aggregation through electrostatic binding with metal ions. Positively charged MAF-3/MAF-4 are stable because of their small size and monodisperse distribution. The positive surface charge helps avoid nonspecific electrostatic binding when incubated with metal ions. The hydrophilic PVP can disperse MAF-4 in aqueous solutions compared to MAF-3. At low concentrations of Cr3+, MAF-4 is more sensitive than MAF-3, showing a bigger fluorescence change (Fig. 4B). We tested their responses to metal ions. MAFs (0.5 μg/ml) fit the best performance of the spectrophotometer. We tested the stability by suffering hypersaline, organic, and highly acidic conditions. MAF-4 has excellent tolerance to salinity (against saturated NaCl solution; fig. S9), most organic solvents (fig. S10), and acid (against 1 M HCl solution; fig. S11).
Fig. 4. Analytical performance of MAFs.
(A) Stability tests by monitoring precipitation of different MAFs from aqueous solution using absorbance decrease as the signal. (B) Sensitivity tests of Cr3+-induced quenching of MAF-3 and MAF-4. The error bars represent three independent tests. (C) Fluorescence spectra of MAF-4 in the presence of different concentrations of Cr3+ (0, 0.25, 0.5, 2.5, 5, 25, 50, 125, 200, 250, 500, 2500, 5000, and 10,000 nM). (D) LOD and QY information of different fluorescent sensors (detailed information seen in table S2). (E) DLS profiles of MAF-4 in the absence and presence of Cr3+. (F) Zeta-potential profiles of MAF-4 in the absence and presence of Cr3+. (G) HAADF-STEM image and elemental mapping images of MAF-4 after incubation with Cr3+. (H and I) XPS profiles of MAF-4 before and after incubation with Cr3+.
Cr3+-induced quenching of MAFs is a fast reaction. Within 2 min, the fluorescence change reaches a maximum level (fig. S12). Figure 4C and fig. S13 show the concentration-dependent fluorescence spectra and the intensity change ratio curve. A good linear detection range is 2.5 to 500 nM [adjusted coefficient of determination (Adj. R2) > 0.97]. A calculated limit of detection (LOD) value is about 0.1 nM (3σ per slope), which is more sensitive than current MOF sensors and other sensors (Fig. 4D and table S2) (51–63). Cr3+-incubated MAF-4 has a slight increase of hydrodynamic diameter (Fig. 4E), which indicates Cr3+ attaching on surfaces of MAF-4. The massive Cr3+ ions change the surface charge of MAF-4 (~21 to ~36 mV; Fig. 4F). After incubation, MAF-4 maintains its intact morphology and size (Fig. 4G). The XRD tests do not reveal any distinct 2θ shift (fig. S14A). No evident structural ion exchange of Zr4+/Cr3+ and structural damage were observed. A uniform distribution of chromium in elemental mapping reveals the homogeneous interaction between mesoporous MAF-4 and Cr3+. XPS analysis further proves that the chromium is Cr3+ (2p1/2/2p3/2, 586.9 eV/577.3 eV; Fig. 4, H and I). Comparing 19 kinds of metal ions, the Cr3+ ion–induced quenching has good selectivity (fig. S15). The optical spectra reveal the notable overlap between the absorption spectrum of Cr3+ and the fluorescence emission spectrum of MAF-4 (fig. S14B). However, other weakly interfering ions, such as Fe2+/Cu2+/Ag+, do not show significant spectral overlap. It may suggest that the energy transfer between MAF-4 and the absorbed Cr3+ causes selective fluorescence quenching. We tested the potential application in biological fluids, such as saliva, urine, and serum. A slight fluorescence increase of these biological samples is probably due to biological fluorescence (fig. S16). For the spiked tests, Cr3+-induced quenching is robust with a recovery range of 103 to 110%.
To achieve the proposed HDS, we designed a smart hydrogel to load MAFs. The hydrogel-based detection is highly stable for the operation to avoid interferences and storage problems. The optical sensing requires hydrogel substrate to be nonemissive, colorless, and transparent to visible light. PVP hydrogel meets these criteria (64–66). We synthesized the cross-linked PVP hydrogel using K2S2O8 as the promotor and glycerin as a dopant (Fig. 5A). After being freeze-dried, the hydrogel becomes a white spongy-like solid. Once immersed into water, the spongy-like solid rapidly swells upon water absorption to become a colorless and transparent hydrogel. This solid is ultrastable and storable for at least 1 year (fig. S17).
Fig. 5. Characterization of MAF-4@QDs-PVP hydrogel complex.
(A) Schematic illustration of the cross-linked PVP hydrogel. POCT, point-of-care test. (B) UV-visible transmittance spectra of PVP solution and PVP hydrogel. (C) Optimization of the cross-linking conditions of temperature, time, and K2S2O8 concentration. (D) Dopant effects on the water-absorbing and swelling ability of PVP hydrogel. (E) Schematic illustration of the fabrication of MAF-4@QDs-PVP hydrogel complex and the analyte-induced white-to-red fluorescence color transition strategy for POC HDS. (F) Normalized fluorescence spectra, CIE value graph, and corresponding photograph (UV light) of MAF-4@PVP, QDs@PVP, and MAF-4@QDs-PVP hydrogel complex. (G) The photograph of MAF-4@PVP, QDs@PVP, and MAF-4@QDs-PVP hydrogel complex response to Cr3+ (200 nM).
PVP solution (5%) has high transmittance at the range of 400 to 700 nm (>97%). After cross-linking, the PVP hydrogel is colorless and transparent without transmission loss (<1%; Fig. 5B). The high-transmittance area covers the emission of MAFs, which is advanced for fluorometric detection. The optimized cross-linking conditions are time (1 hour), temperature (80°C), and K2S2O8 (40 mg/ml) (Fig. 5C). Low temperature and short reaction time do not cause gelation. The higher temperature and concentration of K2S2O8 generate bubbles. Overreaction will produce a yellow gel (fig. S18). Immersing the freeze-dried hydrogel in water, the white solid slowly absorbs water for a few hours. Many air bubbles fill the pores, and the hydrogel turns back to being colorless and transparent. Increasing the hydroexpansivity helps to eliminate the bubbles. A fast water absorption and swelling will squeeze out the bubbles to accelerate the conversion into a colorless and transparent hydrogel. We tested different dopants, including glycerin, Tween 80, Triton X-100, PEG, polyvinyl alcohol (PVA), and soluble starch (Fig. 5D and fig. S19). Except for PVA, other dopants promote the water absorption. PEG and soluble starch enhance the swelling. Glycerin show strongly enhanced water absorption and swelling to achieve the desired conversion. The freeze-dried PVP hydrogel with glycerin dopant rapidly absorbs water and swells to squeeze out air bubbles. This promotion is concentration dependent. The more glycerin dopant, the faster the conversion is. However, at high concentrations, glycerin breaks the gelation (fig. S20).
Aiming to POC detection with an easy readout signal, we modulated the fluorescence quenching signal into a fluorescence color transition signal by cooperating with R-QDs because red is the most recognizable color for naked eyes. By in situ embedding the B-MAFs and R-QDs into the designed PVP hydrogel, we obtained the white-emissive MAF-4@QDs-PVP hydrogel complex (Fig. 5E). In this complex, the analyte-induced quenching of blue fluorescence turns into the fluorescence color transition from white to red. The fluorescence intensity change signal is not easy to discriminate when the analyte is at low concentrations. The white-to-red fluorescence color change is easy to be read by naked eyes and digital analysis. To fit the excitation at 310 nm, we modulated MAF-4 [emission, 465 nm; Commission Internationle de L’Eclairage (CIE) color coordinates, 0.16 and 0.18] and QD (emission, 604 nm; CIE, 0.62 and 0.37; fig. S21) components with optimized fluorescence intensity (i.e., concentration) in hydrogel to formulate the composite white fluorescence (CIE, 0.29 and 0.23; Fig. 5F). The equal fluorescence intensity of MAF-4 and QDs in HDS enables that, once the blue fluorescence of MAF-4 decreases, the white-emissive HDS changes to display the red fluorescence of QDs. Immersing the MAF-4@QDs-PVP hydrogel complex in Cr3+ solution, we can easily observe the fluorescence color change with naked eyes (Fig. 5G). In contrast, the fluorescence intensity change of the MAF-4-PVP hydrogel complex is hardly readable.
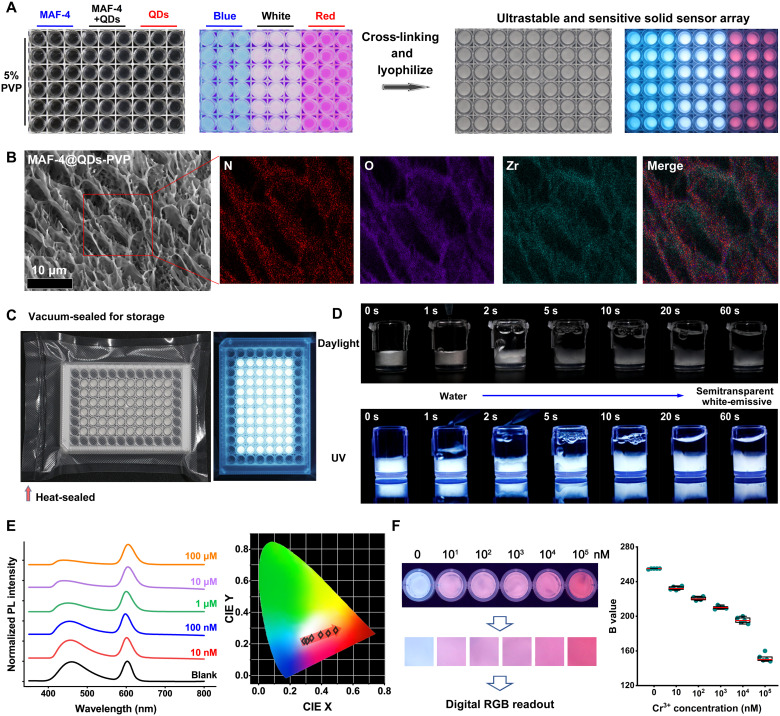
For the scale-up fabrication, we used a commercial 96-well plate to manufacture the HDS arrays (Fig. 6A). Each well is filled with 100 μl of 5% PVP solution containing MAF-4, QDs, 2% glycerin, and 4% K2S2O8. The plate is coated with a food wrapper and placed at 80°C for 1 hour to gelate. After freeze-drying, we obtained the white HDS arrays. SEM images reveal a cellular structure of the hydrogel complex (Fig. 6B and fig. S22). The overlap of N and Zr in elemental mapping images indicates that MAF-4 is attached to or embedded in the hydrogel frameworks. The HDS arrays can be stored after being vacuum-sealed (Fig. 6C). We took videos to monitor the fast water-absorbing and swelling behavior of the HDS (Fig. 6D and movies S1 and S2). Once exposed to water, the HDS immediately absorbs water and swells to squeeze out air bubbles. Within 1 min, the HDS becomes a colorless and transparent hydrogel with consistent white fluorescence.
Fig. 6. MAFs-based HDS.
(A) Photographs of PVP solutions and the cross-linked hydrogel complex (after freeze-drying) containing MAF-4, MAF-4@QDs, and QDs under daylight and UV light. (B) SEM image and elemental mapping images of the freeze-dried MAF-4@QDs-PVP hydrogel complex. (C) Photographs of the vacuum-sealed freeze-dried MAF-4@QDs-PVP hydrogel complex in the commercial 96-well plate after storage for 1 year, under daylight and UV light. (D) The time-dependent photographs of the fast water-absorbing and swelling behavior of the freeze-dried MAF-4@QDs-PVP hydrogel complex under daylight and UV light. (E) The normalized fluorescence spectra and corresponding CIE value graph of the freeze-dried MAF-4@QDs-PVP hydrogel complex after incubation with different concentrations of Cr3+ solution. (F) The photograph and extracted images of the freeze-dried MAF-4@QDs-PVP hydrogel complex in the presence of different concentrations of Cr3+. Right: The captured B value change via different concentrations of Cr3+. The data represent five independent tests.
We tested the HDS on responding to aqueous Cr3+ ions. Chromium is a trace element necessary for life. Cr3+ disorder (either deficiency or excess) is correlated to diabetes, cardiovascular diseases, and kidney toxicity (67–69). The gold standard of chromium (Cr3+/6+) in drinking water set by the U.S. Environmental Protection Agency is less than 100 μg/liter (or 1.92 μM). The World Health Organization/European Community/Chinese national standard is less than 50 μg/liter (or 0.96 μM). As the concentration of Cr3+ increases, the blue fluorescence of MAF-4 decreases. The red fluorescence of QDs does not change (Fig. 6E). The corresponding CIE chromaticity coordinates shift from the white area to the red area. This change is recognizable at 10 nM Cr3+ by naked eyes (Fig. 6F). Cr6+ can be easily reduced to Cr3+ by ascorbic acid. The HDS fully satisfies the water quality standards and has big potential for water/food safety applications. For the POC digital detection, we captured the RGB values from the photographs using the smartphone and used the B value as a readout signal. The color images are extracted from the central area of photographs. The captured B value reduces as the concentration of Cr3+ increases (Fig. 6F). The full dynamic response range covers five orders of magnitude. A good linear quantitive digital detection range is 10 to 10,000 nM with Adj.R2 of 0.99 (fig. S23).
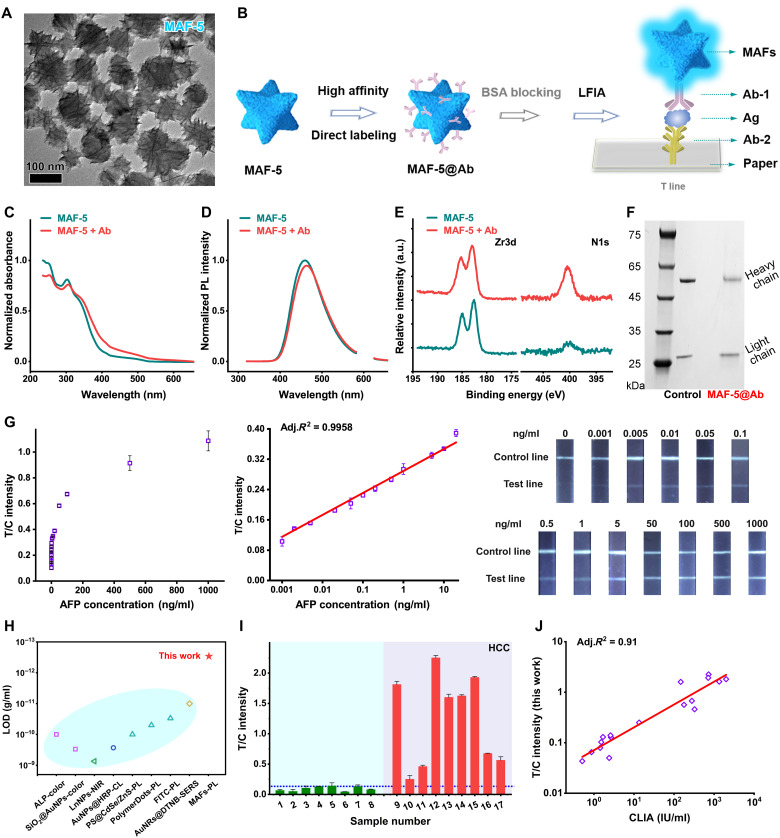
We demonstrated that MAFs-based HDS has advantages in fluorescent POC detection. Besides the potential application in water/food safety, the sensitive protein labeling and immunodetection for clinical macromolecular diagnosis is another powerful application of strongly fluorescent MAFs. We used MAFs to label Abs for ultrasensitive fluorescent LFIA. To fit the immunochromatography in the nitrocellulose membrane, we further synthesized negatively charged MAF-5 particles with a size of ~100 nm and spiky morphology (Fig. 7A). The negatively charged, relatively small particles help avoid the nonspecific binding (false signal) and reduce the flow time. The multivalent Zr4+ ions on the surface of particles are favored to bind proteins through electrostatic interactions and coordinations (70–72). MAF-5 can directly label Abs without using polymer coating or covalent linking agents (Fig. 7B). Compared to conventional QDs-based LFIA (fig. S24), it greatly simplifies the fabrication and reduces the cost but retains the required sensitivity.
Fig. 7. MAFs-based LFIA.
(A) TEM image of MAF-5. (B) Schematic illustration of MAF-5 for direct labeling Abs and the classic “Sandwich” structure in LFIA. BSA, bovine serum albumin. (C) The normalized absorption spectra and (D) the normalized fluorescence spectra of MAF-5 in the absence and presence of Abs. (E) The high-resolution XPS binding energy profiles of MAF-5 before and after incubation with Abs. (F) The SDS–polyacrylamide gel electrophoresis image of Ab@MAF-5 lysate. The protein is stained with Coomassie brilliant blue. (G) From left to right: The concentration-dependent curve of fluorescence ratio of test line/control line responding to different concentrations of AFP, the corresponding linear fitting line at a range of 1 pg/ml to 20 ng/ml, and the corresponding fluorescence images of LFIA strips. (H) The LOD information of different paper-based immunoassays (detailed information seen in table S3). (I) The clinical serum sample testing results using the MAFs-based LFIA. (J) The correlation studies of the MAFs-based LFIA and the clinically used CLIA.
We selected alpha-fetoprotein (AFP), a serum marker for clinical diagnosis of hepatocellular carcinoma (HCC) and the therapeutic monitoring, as a target to establish MAFs-based LFIA. During incubation with excess Abs, the zeta-potential change indicates strong binding between MAF-5 and primary Abs (AFP-Ab1; fig. S25). The absorption spectra have a slight red shift (Fig. 7C), while the fluorescence intensity of MAF-5@AFP-Ab1 has no significant decrease (Fig. 7D). MAF-5 is stable to label proteins for LFIA or other applications. XPS profiles reveal the appearance of N element, which confirms the attached proteins (Fig. 7E and fig. S26). To confirm AFP-Ab1 on surfaces of MAF-5, we carried out SDS–polyacrylamide gel electrophoresis tests. The mixture was centrifuged to gather the MAF-5@AFP-Ab1 complex. The complex was suspended in loading buffer and boiled to detach and denature proteins. After electrophoresis and staining with Coomassie brilliant blue, we observed two protein fragments near 25 and 55 kDa (Fig. 7F). They are exactly the same as the control (pure Abs) where the light chain and heavy chain of the Abs appear as separate blots on the gel.
We fabricated the LFIA strips following the optimized conditions of AFP-Ab1 (8 μg/ml), secondary Ab (2 mg/ml, AFP-Ab2), and anti-Ab (2 mg/ml) (fig. S27). A balanced running buffer [phosphate-buffered saline (PBS) with 1% Triton X-100 (pH 7.4)] ensured the fast immunochromatography within 15 min and minimized the nonspecific signal (fig. S28). For the standard curve tests, we prepared a gradient dilution in PBS. As the AFP amount increases, the test line becomes bright with blue fluorescence under UV light (Fig. 7G). The linear detection range is 1 pg/ml to 20 ng/ml (Adj. R2 > 0.99). A calculated LOD value is about 0.6 pg/ml (3σ per slope). MAFs-based LFIA has excellent sensitivity (102- to 103-fold enhancement) compared to the traditional QDs-based LFIA (Fig. 7H and table S3) (12, 73–79). MAFs-based LFIA is even more sensitive than some surface-enhanced Raman spectroscopy–based LFIA. The good selectivity is shown against other proteins (fig. S29). A highly sensitive and selective detection method is important and practical. It makes sense of a lower serum volume for testing and is thus friendly to reducing sample volume requirement for the sick by using only fingertip blood tests (instead of venous blood sampling). We tested the standard curve in spiked serum (fig. S30). A clinical cutoff line was set to 0.12 (IT/IC) according to the gold standard value of 20 ng/ml. Seventeen human serum samples were divided into HCC and non-HCC groups (9 versus 8; detailed sample information is seen in table S6). For the HCC group, nine cases are positive, containing one weakly positive case (no. 10; Fig. 7I). At the same time, all non-HCC cases are negative. To verify the reliability of MAFs-based LFIA, we compared it to the clinically used chemiluminescence immunoassay (CLIA) method. There is a highly correlated relationship between MAFs-based LFIA and the CLIA (Adj. R2 > 0.91; Fig. 7J). Besides the serum tests, we further evaluated the MAFs-based LFIA in clinical biofluids such as urine. Eight urine samples from patients with HCC are tested, showing positive signals (fig. S31 and table S7). MAFs-based LFIA robustly works in different real biofluids for the clinical macromolecular biomarker diagnosis. Considering the achieved sensitivity of MAFs-based LFIA, we believed it would be possible to apply it to different clinical macromolecular biomarkers, such as the prostate-specific antigen, procalcitonin, interleukin, and so on.
DISCUSSION
In conclusion, we demonstrated the rational design and manipulation of MAFs for ultrasensitive and convenient POC detections based on HDS and LFIA. Combining the high QY and synthetic modulation, the sensitivity of POC sensors increases a hundredfold to a thousandfold and avoids sacrificing the required convenience and cost-efficiency. It has the potential to develop smart sensors for small molecules and macromolecules by designing specific responsive MAFs with molecular engineering. Combining with metal nanoparticles/clusters, microfabrication, and barcode technologies, MAFs may greatly facilitate POC sensors.
MATERIALS AND METHODS
Instruments and characterizations
Scanning electron microscope characterizations are performed using a Regulus8200 cold-field emission scanning electron microscope (Hitachi, Japan). Samples are prepared by drop-casting on the conductive tape, dried, and then plasma sputtering–coated with gold. Transmission electron microscope, HAADF-STEM, and elemental mapping characterizations are performed using a Tecnai G2 F20 U-TWIN field-emission transmission electron microscope (FEI, USA) equipped with EDAX Genesis 2000 XMS accessory. Samples are prepared by drop-casting on the carbon-coated copper grid. UV-visible optical spectra are recorded using a UV-2450 spectrophotometer (Shimadzu, Japan). Excitation and emission spectra are recorded using an RF-5301PC fluorescence spectrophotometer (Shimadzu, Japan). Hydrodynamic diameter and zeta-potential distribution profiles are measured by a Zetasizer Nano ZS instrument (Malvern, UK). Fluorescent lifetime profiles and absolute QY of different MAFs are measured by an FLS980 transient steady-state fluorescence spectrometer (Edinburgh Instruments, UK). PXRD characterizations are performed using a D/MAX-TTRIII (CBO) instrument (Rigaku, Japan). XPS characterizations are performed using an ESCALAB 250Xi instrument (Thermo Fisher Scientific, USA). FTIR spectra are measured by a Spectrum One instrument (PerkinElmer, USA).
Synthesis of Zr@TCPE MAFs without regulators (MAF-1)
Typically, 23 mg of ligand TCPE and 23 mg of anhydrous ZrCl4 are dissolved in 6 ml of DMF solvent. After sonication for 5 min, the mixture is transferred into a glass bottle (Φ 3.25 cm by 15 cm) and sealed and then immersed into the oil bath at 90°C. The mixture solution is clear liquor until it is fully heated. During the reaction, white precipitates reveal the formation of MAFs. The reaction without regulators takes much more time to form Zr@TCPE MAFs. Over 24 hours, there is no apparent increase of precipitates. The precipitates are centrifuged (5000 rpm, 5 min) and washed twice with DMF and ethanol. The final product is suspended in ethanol and placed in the refrigerator (4°C) or at room temperature.
Synthesis of Zr@TCPE MAFs with regulators (MAF-2, MAF-3, and MAF-4)
All procedures and operations are the same as described above, except for the addition of different regulators of HAc, H2O, and PVP. For the synthesis of MAF-2, 2 ml of HAc is added before undergoing the oil bath. For the synthesis of MAF-3, 2 ml of HAc is added before undergoing the oil bath. Then, 0.1 ml of H2O is added when the mixture turns to clear liquor under heating. For the synthesis of MAF-4, 230 mg of PVP is dissolved in the DMF mixture, which contains 23 mg of ligand TCPE and 23 mg of anhydrous ZrCl4. HAc (2 ml) is added before undergoing the oil bath. H2O (0.1 ml) is added when the mixture turns to clear liquor. Regulators (HAc, H2O, and PVP) can efficiently speed up reactions to save lots of time. For example, in the synthesis of product MAF-4, a necessary synthetic time is greatly reduced from over 24 hours to 5 hours.
Synthesis of cross-linked PVP hydrogel
Typically, 5% PVP solution containing K2S2O8 (40 mg/ml) is gelated at 80°C for 1 hour. For the glycerin-dopped PVP hydrogel, 5% PVP solution containing K2S2O8 (40 mg/ml) and 2% glycerin is gelated at 80°C for 1 hour.
Fabrication of freeze-dried hydrogel solid
Typically, the above PVP hydrogel is frozen at −80°C and then freeze-dried overnight to obtain the white, spongy-like solid.
Synthesis of MAF-4@QDs@PVP hydrogel complex
Typically, the appropriate amounts of MAF-4 and QDs are added into the 5% PVP solution, which contains K2S2O8 (40 mg/ml) and 2% glycerin, and mixed. Then, the mixture is gelated at 80°C for 1 hour.
Fabrication of MAF-4@QDs@PVP hydrogel complex sensor array
For the freeze-dried hydrogel solid sensor array, the mixture solution of MAF-4, QDs, 5% PVP, K2S2O8 (40 mg/ml), and 2% glycerin is piped into the commercial 96-well plate for 100 μl per well. The plate is covered with the food wrapper and placed at 80°C for 1 hour. The hydrogel complex is frozen at −80°C and then freeze-dried overnight to obtain the white, spongy-like solid sensor array.
Synthesis of Zr@TCPE MAFs for LFIA (MAF-5)
For the synthesis of small-sized MAF-5, we increased the mass ratio of TCPE:ZrCl4 with HAc as regulators. TCPE (96 mg), 23 mg of ZrCl4, and 2 ml of HAc were added into 6 ml of DMF and heated under vigorous stirring. Other procedures and operations are the same as MAF-1, except for the centrifuge condition of 8000 rpm for 15 min.
Synthesis of Ab@MAF-5 complex for LFIA
Typically, MAF-5 with appropriate dilution was incubated with Ab (10 μg/ml). The mixture was placed on the shaker for 3 hours. Then, a blocking solution of bovine serum albumin (BSA) was added into the mixture with a concentration of 10 mg/ml. The mixture was further incubated for 1 hour on the sharker. At last, the mixture was centrifuged (8000 rpm, 15 min) to obtain the precipitates of the Ab@MAF-5 complex. The precipitates were resuspended using a PBS buffer containing 1% BSA and placed in the refrigerator (4°C) for usage.
Clinical samples
The used clinical serum and urine samples were collected from the Department of Hepatobiliary and Pancreas Surgery, Shenzhen People’s Hospital (The First Affiliated Hospital of Southern University of Science and Technology), and approved by the institutional ethics committee (LL-KT-2018207).
Acknowledgments
We thank SUSTech Core Research Facilities for the material characterizations. We thank Wuhan Jiayuan Quantumdots Co. LTD (Wuhan, China) for providing the QDs.
Funding: This work was supported by the National Key R&D Program of China (2018YFA0902600), the National Natural Science Foundation of China (22104049, 22005195, and 81730051), Shenzhen Science and Technology Program (KQTD20190929172743294, JCYJ20200109110608167, and JCYJ20210324105006017), the Chinese Academy of Sciences (QYZDJ-SSW-SLH039), Guangdong Innovative and Entrepreneurial Research Team Program (2019ZT08Y191), Shenzhen Key Laboratory of Smart Healthcare Engineering (ZDSYS20200811144003009), Guangdong Provincial Key Laboratory of Advanced Biomaterials (2022B1212010003), and Tencent Foundation through the XPLORER PRIZE.
Author contributions: J.Z., Y.L., B.Z.T., and X.J. designed the research. J.Z. and F.C. performed the research. Q.L. helped with the TEM studies. D.W. helped with the fluorescence studies. L.L. collected the clinical samples. J.Z., Y.L., F.C., L.L., and X.J. analyzed the data. J.Z., B.Z.T., and X.J. wrote the paper.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S31
Tables S1 to S7
Other Supplementary Material for this manuscript includes the following:
Movies S1 and S2
REFERENCES AND NOTES
- 1.Yang M., Liu Y., Jiang X., Barcoded point-of-care bioassays. Chem. Soc. Rev. 48, 850–884 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Quesada-González D., Merkoçi A., Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 47, 4697–4709 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Xianyu Y., Jiang X., Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 50, 310–319 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Mou L., Jiang X., Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 11, 923–936 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., Ma Y., Jiao X., Li T., Lv Z., Yang C. J., Zhang X., Wen Y., Control of capillary behavior through target-responsive hydrogel permeability alteration for sensitive visual quantitative detection. Nat. Commun. 10, 1036 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkowska McConnell W., Davis C., Sabir S. R., Garrett A., Bradley-Stewart A., Jajesniak P., Reboud J., Xu G., Yang Z., Gunson R., Thomson E. C., Cooper J. M., Paper microfluidic implementation of loop mediated isothermal amplification for early diagnosis of hepatitis C virus. Nat. Commun. 12, 6994 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M., Zhang W., Yang J., Hu B., Cao F., Zheng W., Chen Y., Jiang X., Skiving stacked sheets of paper into test paper for rapid and multiplexed assay. Sci. Adv. 3, eaao4862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xianyu Y., Wu J., Chen Y., Zheng W., Xie M., Jiang X., Controllable assembly of enzymes for multiplexed lab-on-a-chip bioassays with a tunable detection range. Angew. Chem. Int. Ed. Engl. 57, 7503–7507 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Ho N. R. Y., Lim G. S., Sundah N. R., Lim D., Loh T. P., Shao H., Visual and modular detection of pathogen nucleic acids with enzyme-DNA molecular complexes. Nat. Commun. 9, 3238 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Sun J., Xianyu Y., Yin B., Niu Y., Wang S., Cao F., Zhang X., Wang Y., Jiang X., A dual-readout chemiluminescent-gold lateral flow test for multiplex and ultrasensitive detection of disease biomarkers in real samples. Nanoscale 8, 15205–15212 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Loynachan C. N., Thomas M. R., Gray E. R., Richards D. A., Kim J., Miller B. S., Brookes J. C., Agarwal S., Chudasama V., McKendry R. A., Stevens M. M., Platinum nanocatalyst amplification: Redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 12, 279–288 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J., Yang M., Wu J., Zhang W., Jiang X., A self-contained chemiluminescent lateral flow assay for point-of-care testing. Anal. Chem. 90, 9132–9137 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Tran V., Walkenfort B., König M., Salehi M., Schlücker S., Rapid, quantitative, and ultrasensitive point-of-care testing: A portable SERS reader for lateral flow assays in clinical chemistry. Angew. Chem. Int. Ed. Engl. 58, 442–446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gootenberg Jonathan S., Abudayyeh Omar O., Kellner Max J., Joung J., Collins James J., Zhang F., Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 360, 439–444 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller B. S., Bezinge L., Gliddon H. D., Huang D., Dold G., Gray E. R., Heaney J., Dobson P. J., Nastouli E., Morton J. J. L., McKendry R. A., Spin-enhanced nanodiamond biosensing for ultrasensitive diagnostics. Nature 587, 588–593 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Furukawa H., Cordova K. E., O’Keeffe M., Yaghi O. M., The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Yuan S., Feng L., Wang K., Pang J., Bosch M., Lollar C., Sun Y., Qin J., Yang X., Zhang P., Wang Q., Zou L., Zhang Y., Zhang L., Fang Y., Li J., Zhou H.-C., Stable metal-organic frameworks: Design, synthesis, and applications. Adv. Mater. 30, 1704303 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Mason J. A., Oktawiec J., Taylor M. K., Hudson M. R., Rodriguez J., Bachman J. E., Gonzalez M. I., Cervellino A., Guagliardi A., Brown C. M., Llewellyn P. L., Masciocchi N., Long J. R., Methane storage in flexible metal-organic frameworks with intrinsic thermal management. Nature 527, 357–361 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Li L., Lin R.-B., Krishna R., Li H., Xiang S., Wu H., Li J., Zhou W., Chen B., Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 362, 443–446 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Wu X., Yue H., Zhang Y., Gao X., Li X., Wang L., Cao Y., Hou M., An H., Zhang L., Li S., Ma J., Lin H., Fu Y., Gu H., Lou W., Wei W., Zare R. N., Ge J., Packaging and delivering enzymes by amorphous metal-organic frameworks. Nat. Commun. 10, 5165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M., Long Z., Dong R., Wang L., Zhang J., Li S., Zhao X., Hou X., Shao H., Jiang X., Titanium incorporation into Zr-porphyrinic metal-organic frameworks with enhanced antibacterial activity against multidrug-resistant pathogens. Small 16, 1906240 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Zhao M., Yuan K., Wang Y., Li G., Guo J., Gu L., Hu W., Zhao H., Tang Z., Metal-organic frameworks as selectivity regulators for hydrogenation reactions. Nature 539, 76–80 (2016). [DOI] [PubMed] [Google Scholar]
- 23.An B., Li Z., Song Y., Zhang J., Zeng L., Wang C., Lin W., Cooperative copper centres in a metal-organic framework for selective conversion of CO2 to ethanol. Nat. Catal. 2, 709–717 (2019). [Google Scholar]
- 24.Balachandran Y. L., Li X., Jiang X., Integrated microfluidic synthesis of aptamer functionalized biozeolitic imidazolate framework (BioZIF-8) targeting lymph node and tumor. Nano Lett. 21, 1335–1344 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Zhou J., Lin Z., Penna M., Pan S., Ju Y., Li S., Han Y., Chen J., Lin G., Richardson J. J., Yarovsky I., Caruso F., Particle engineering enabled by polyphenol-mediated supramolecular networks. Nat. Commun. 11, 4804 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Q., Aguila B., Perman J., Ivanov A. S., Bryantsev V. S., Earl L. D., Abney C. W., Wojtas L., Ma S., Bio-inspired nano-traps for uranium extraction from seawater and recovery from nuclear waste. Nat. Commun. 9, 1644 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo J., Ping Y., Ejima H., Alt K., Meissner M., Richardson J. J., Yan Y., Peter K., von Elverfeldt D., Hagemeyer C. E., Caruso F., Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew. Chem. Int. Ed. Engl. 53, 5546–5551 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Cui Y., Yue Y., Qian G., Chen B., Luminescent functional metal-organic frameworks. Chem. Rev. 112, 1126–1162 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Lustig W. P., Mukherjee S., Rudd N. D., Desai A. V., Li J., Ghosh S. K., Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 46, 3242–3285 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Yan B., Lanthanide-functionalized metal-organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 50, 2789–2798 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Huang R.-W., Wei Y.-S., Dong X.-Y., Wu X.-H., Du C.-X., Zang S.-Q., Mak T. C. W., Hypersensitive dual-function luminescence switching of a silver-chalcogenolate cluster-based metal-organic framework. Nat. Chem. 9, 689–697 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y., Wang J., Zhu W., Liu L., Pei R., The modulation effect of charge transfer on photoluminescence in metal-organic frameworks. Nanoscale 13, 4505–4511 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Wei Z., Gu Z.-Y., Arvapally R. K., Chen Y.-P., McDougald R. N., Ivy J. F., Yakovenko A. A., Feng D., Omary M. A., Zhou H.-C., Rigidifying fluorescent linkers by metal-organic framework formation for fluorescence blue shift and quantum yield enhancement. J. Am. Chem. Soc. 136, 8269–8276 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Shustova N. B., McCarthy B. D., Dincă M., Turn-on fluorescence in tetraphenylethylene-based metal-organic frameworks: An alternative to aggregation-induced emission. J. Am. Chem. Soc. 133, 20126–20129 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Zhu Z.-H., Ni Z., Zou H.-H., Feng G., Tang B. Z., Smart metal-organic frameworks with reversible luminescence/magnetic switch behavior for HCl vapor detection. Adv. Funct. Mater. 31, 2106925 (2021). [Google Scholar]
- 36.Hu Z., Huang G., Lustig W. P., Wang F., Wang H., Teat S. J., Banerjee D., Zhang D., Li J., Achieving exceptionally high luminescence quantum efficiency by immobilizing an AIE molecular chromophore into a metal-organic framework. Chem. Commun. 51, 3045–3048 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Zhang M., Feng G., Song Z., Zhou Y.-P., Chao H.-Y., Yuan D., Tan T. T. Y., Guo Z., Hu Z., Tang B. Z., Liu B., Zhao D., Two-dimensional metal-organic framework with wide channels and responsive turn-On fluorescence for the chemical sensing of volatile organic compounds. J. Am. Chem. Soc. 136, 7241–7244 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Shang W., Zhu X., Liang T., Du C., Hu L., Li T., Liu M., Chiral reticular self-assembly of achiral AIEgen into optically pure metal-organic frameworks (MOFs) with dual mechano-switchable circularly polarized luminescence. Angew. Chem. Int. Ed. 59, 12811–12816 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Dong J., Shen P., Ying S., Li Z.-J., Yuan Y. D., Wang Y., Zheng X., Peh S. B., Yuan H., Liu G., Cheng Y., Pan Y., Shi L., Zhang J., Yuan D., Liu B., Zhao Z., Tang B. Z., Zhao D., Aggregation-induced emission-responsive metal-organic frameworks. Chem. Mater. 32, 6706–6720 (2020). [Google Scholar]
- 40.Wu X.-H., Luo P., Wei Z., Li Y.-Y., Huang R.-W., Dong X.-Y., Li K., Zang S.-Q., Tang B. Z., Guest-triggered aggregation-induced emission in silver chalcogenolate cluster metal-organic frameworks. Adv. Sci. 6, 1801304 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y. Y., Zhang X., Li K., Peng Q. C., Qin Y. J., Hou H. W., Zang S. Q., Tang B. Z., Restriction of intramolecular vibration in aggregation-induced emission luminogens: Applications in multifunctional luminescent metal-organic frameworks. Angew. Chem. Int. Ed. 60, 22417–22423 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Mei J., Leung N. L. C., Kwok R. T. K., Lam J. W. Y., Tang B. Z., Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 115, 11718–11940 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Alam P., Leung N. L. C., Zhang J., Kwok R. T. K., Lam J. W. Y., Tang B. Z., AIE-based luminescence probes for metal ion detection. Coord. Chem. Rev. 429, 213693 (2021). [Google Scholar]
- 44.Guo H., Zhu Y., Wang S., Su S., Zhou L., Zhang H., Combining coordination modulation with acid-base adjustment for the control over size of metal-organic frameworks. Chem. Mater. 24, 444–450 (2012). [Google Scholar]
- 45.Wang S., McGuirk C. M., d’Aquino A., Mason J. A., Mirkin C. A., Metal-organic framework nanoparticles. Adv. Mater. 30, 1800202 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Fu Y., Kang Z., Yin J., Cao W., Tu Y., Wang Q., Kong X., Duet of acetate and water at the defects of metal-organic frameworks. Nano Lett. 19, 1618–1624 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Zhao M., Wang Y., Ma Q., Huang Y., Zhang X., Ping J., Zhang Z., Lu Q., Yu Y., Xu H., Zhao Y., Zhang H., Ultrathin 2D metal-organic framework nanosheets. Adv. Mater. 27, 7372–7378 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Bakshi M. S., How surfactants control crystal growth of nanomaterials. Cryst. Growth Des. 16, 1104–1133 (2016). [Google Scholar]
- 49.Chen M., Dong R., Zhang J., Tang H., Li Q., Shao H., Jiang X., Nanoscale metal-organic frameworks that are both fluorescent and hollow for self-indicating drug delivery. ACS Appl. Mater. Interfaces 13, 18554–18562 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Zhang J., Jia Y., Qi J., Yan W., Jiang X., Four-in-one: Advanced copper nanocomposites for multianalyte assays and multicoding logic gates. ACS Nano 14, 9107–9116 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Yin Y. B., Coonrod C. L., Heck K. N., Lejarza F., Wong M. S., Microencapsulated photoluminescent gold for ppb-level chromium(VI) sensing. ACS Appl. Mater. Interfaces 11, 17491–17500 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Sun J., Zhang J., Jin Y., 11-Mercaptoundecanoic acid directed one-pot synthesis of water-soluble fluorescent gold nanoclusters and their use as probes for sensitive and selective detection of Cr3+ and Cr6+. J. Mater. Chem. C 1, 138–143 (2013). [Google Scholar]
- 53.Liu S., Lu F., Zhu J.-J., Highly fluorescent Ag nanoclusters: Microwave-assisted green synthesis and Cr3+ sensing. Chem. Commun. 47, 2661–2663 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Liu L., Li Y., Zhan L., Liu Y., Huang C., One-step synthesis of fluorescent hydroxyls-coated carbon dots with hydrothermal reaction and its application to optical sensing of metal ions. Sci. China Chem. 54, 1342–1347 (2011). [Google Scholar]
- 55.Lu H., Xu S., Liu J., One pot generation of blue and red carbon dots in one binary solvent system for dual channel detection of Cr3+ and Pb2+ based on ion imprinted fluorescence polymers. ACS Sens. 4, 1917–1924 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Rasheed L., Yousuf M., Youn I. S., Yoon T., Kim K.-Y., Seo Y.-K., Shi G., Saleh M., Hur J.-H., Kim K. S., Turn-on ratiometric fluorescent probe for selective discrimination of Cr3+ from Fe3+ in aqueous media for living cell imaging. Chem. A Eur. J. 21, 16349–16353 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Li W., Zhang Z., Zhou W., Liu J., Kinetic discrimination of metal ions using DNA for highly sensitive and selective Cr3+ detection. ACS Sens. 2, 663–669 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Jia X.-X., Yao R.-X., Zhang F.-Q., Zhang X.-M., A fluorescent anionic MOF with Zn4(trz)2 Chain for highly selective visual sensing of contaminants: Cr(III) ion and TNP. Inorg. Chem. 56, 2690–2696 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Gu T.-Y., Dai M., Young D. J., Ren Z.-G., Lang J.-P., Luminescent Zn(II) coordination polymers for highly selective sensing of Cr(III) and Cr(VI) in water. Inorg. Chem. 56, 4668–4678 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Guo X.-Y., Zhao F., Liu J.-J., Liu Z.-L., Wang Y.-Q., An ultrastable zinc(II)–organic framework as a recyclable multi-responsive luminescent sensor for Cr(III), Cr(VI) and 4-nitrophenol in the aqueous phase with high selectivity and sensitivity. J. Mater. Chem. A 5, 20035–20043 (2017). [Google Scholar]
- 61.Li H., Li D., Qin B., Li W., Zheng H., Zhang X., Zhang J., Turn-on fluorescence in a stable Cd(II) metal-organic framework for highly sensitive detection of Cr3+ in water. Dyes Pigm. 178, 108359 (2020). [Google Scholar]
- 62.Guo Q., Ma T., Zhou L., Ma J.-X., Yang J., Yang Q., Efficient detection of Cr3+ and Cr2O72− using a Zn(II) luminescent metal-organic framework. New J. Chem. 44, 7293–7299 (2020). [Google Scholar]
- 63.Lv R., Wang J., Zhang Y., Li H., Yang L., Liao S., Gu W., Liu X., An amino-decorated dual-functional metal-organic framework for highly selective sensing of Cr(III) and Cr(VI) ions and detection of nitroaromatic explosives. J. Mater. Chem. A 4, 15494–15500 (2016). [Google Scholar]
- 64.Ming Z., Ruan X., Bao C., Lin Q., Yang Y., Zhu L., Micropatterned protein for cell adhesion through phototriggered charge change in a polyvinylpyrrolidone hydrogel. Adv. Funct. Mater. 27, 1606258 (2017). [Google Scholar]
- 65.Nizamoglu S., Gather M. C., Humar M., Choi M., Kim S., Kim K. S., Hahn S. K., Scarcelli G., Randolph M., Redmond R. W., Yun S. H., Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat. Commun. 7, 10374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Oliveira M. J. A., Villegas G. M. E., Motta F. D., Fabela-Sánchez O., Espinosa-Roa A., Fotoran W. L., Peixoto J. C., Tano F. T., Lugão A. B., Vásquez P. A. S., Influence of gamma radiation on Amphotericin B incorporated in PVP hydrogel as an alternative treatment for cutaneous leishmaniosis. Acta Trop. 215, 105805 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Chen W., Cao F., Zheng W., Tian Y., Xianyu Y., Xu P., Zhang W., Wang Z., Deng K., Jiang X., Detection of the nanomolar level of total Cr[(III) and (VI)] by functionalized gold nanoparticles and a smartphone with the assistance of theoretical calculation models. Nanoscale 7, 2042–2049 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Zhang S.-R., Li J., Du D.-Y., Qin J.-S., Li S.-L., He W.-W., Su Z.-M., Lan Y.-Q., A multifunctional microporous anionic metal-organic framework for column-chromatographic dye separation and selective detection and adsorption of Cr3+. J. Mater. Chem. A 3, 23426–23434 (2015). [Google Scholar]
- 69.Du J., Ge H., Gu Q., Du H., Fan J., Peng X., Gold nanoparticle-based nano-probe for the colorimetric sensing of Cr3+ and Cr2O72− by the coordination strategy. Nanoscale 9, 19139–19144 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Xia M., Li Z., Chen Y., Xiang Y., Yang L., Zhao X., Guo A., Chen H., Tan C., Hu Y., Protein self-assembly via Zr4+ ions on spore-based microspheres for immunoassays. Sensor. Actuat. B Chem. 254, 166–176 (2018). [Google Scholar]
- 71.Lin Z., Zhou J., Cortez-Jugo C., Han Y., Ma Y., Pan S., Hanssen E., Richardson J. J., Caruso F., Ordered mesoporous metal-phenolic network particles. J. Am. Chem. Soc. 142, 335–341 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Wang S., Chen Y., Wang S., Li P., Mirkin C. A., Farha O. K., DNA-functionalized metal-organic framework nanoparticles for intracellular delivery of proteins. J. Am. Chem. Soc. 141, 2215–2219 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu X., Mei T., Guo Q., Zhou W., Li X., Chen J., Zhou X., Sun N., Fang Z., Improved performance of lateral flow immunoassays for alpha-fetoprotein and vanillin by using silica shell-stabilized gold nanoparticles. Microchim. Acta 186, 2 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Preechakasedkit P., Siangproh W., Khongchareonporn N., Ngamrojanavanich N., Chailapakul O., Development of an automated wax-printed paper-based lateral flow device for alpha-fetoprotein enzyme-linked immunosorbent assay. Biosens. Bioelectron. 102, 27–32 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Liu Q., Cheng S., Chen R., Ke J., Liu Y., Li Y., Feng W., Li F., Near-infrared lanthanide-doped nanoparticles for a low interference lateral flow immunoassay test. ACS Appl. Mater. Interfaces 12, 4358–4365 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Xiao R., Lu L., Rong Z., Wang C., Peng Y., Wang F., Wang J., Sun M., Dong J., Wang D., Wang L., Sun N., Wang S., Portable and multiplexed lateral flow immunoassay reader based on SERS for highly sensitive point-of-care testing. Biosens. Bioelectron. 168, 112524 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Jiao Y., Du C., Zong L., Guo X., Han Y., Zhang X., Li L., Zhang C., Ju Q., Liu J., Yu H.-D., Huang W., 3D vertical-flow paper-based device for simultaneous detection of multiple cancer biomarkers by fluorescent immunoassay. Sensor. Actuat. B Chem. 306, 127239 (2020). [Google Scholar]
- 78.Zhou C., Mao M., Yuan H., Shen H., Wu F., Ma L., Li L. S., Fluorescent QDs-polystyrene composite nanospheres for highly efficient and rapid protein antigen detection. J. Nanopart. Res. 15, 1901 (2013). [Google Scholar]
- 79.Fang C.-C., Chou C.-C., Yang Y.-Q., Wei-Kai T., Wang Y.-T., Chan Y.-H., Multiplexed detection of tumor markers with multicolor polymer dot-based immunochromatography test strip. Anal. Chem. 90, 2134–2140 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S31
Tables S1 to S7
Movies S1 and S2