Abstract
Endoplasmic reticulum (ER) stress and inflammation can induce hyperglycemia. Long-chain bases (LCBs) from sea cucumber exhibit antihyperglycemic activities. However, their effects on ER stress and inflammation are unknown. We investigated the effects of LCBs on ER stress and inflammatory response in high-fat, fructose diet-induced obesity mice. Reactive oxygen species and free fatty acids were measured. Inflammatory cytokines in serum and their mRNA expressions in epididymal adipose tissues were investigated. Hepatic ER stress-related key genes were detected. c-Jun NH2-terminal kinase and nuclear factor κB inflammatory pathways were also evaluated in the liver. Results showed that LCBs reduced serum and hepatic reactive oxygen species and free fatty acids concentrations. LCBs decreased serum proinflammatory cytokines levels, namely interleukin (IL)-1β, tumor necrosis factor-α, IL-6, macrophage inflammatory protein 1, and c-reactive protein, and increased anti-inflammatory cytokine IL-10 concentration. The mRNA and protein expressions of these cytokines in epididymal adipose tissues were regulated by LCBs as similar to their circulatory contents. LCBs inhibited phosphorylated c-Jun NH2-terminal kinase and inhibitor κ kinase β, and nuclear factor κB nuclear translocation. LCBs also inhibited mRNA expression of ER stress markers glucose regulated protein, activating transcription factor 6, double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase, and X-box binding protein 1, and phosphorylation of eukaryotic initiation factor-α and inositol requiring enzyme 1α. These results indicate that LCBs can alleviate ER stress and inflammatory response. Nutritional supplementation with LCBs may offer an adjunctive therapy for RE stress-associated inflammation.
Keywords: long-chain bases, sea cucumber, endoplasmic reticulum stress, inflammation, cytokines
1. Introduction
Low-grade inflammation in adipose tissue is considered an important procedure in the development of obesity-related comorbidities, including diabetes mellitus, hyperglycemia, and insulin resistance [1,2]. The chronic inflammation in adipose tissue is powerfully augmented through the secretion of free fatty acids (FFAs) and deleterious inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor α (TNF-α) [3]. Elevated proinflammatory cytokines and excessive oxidative stress maintain a proinflammatory environment, and firstly cause tissue damage in the liver and lead to further activation of inhibitor κ kinase β (IκKβ), c-Jun NH2-terminal kinase (JNK), and other serine kinases [4]. These serine kinases, in turn, lead to the production of IL-1β, TNF-α, FFAs, reactive oxygen species (ROS), etc. [5,6]. Recent studies suggested that endoplasmic reticulum (ER) stress was an important contributor to chronic tissue inflammation [7,8]. ER is the organelle for the synthesis, folding and trafficking of secretory and membrane proteins [9]. Disruption of ER homeostasis results in an adaptive unfolded protein response (UPR) triggered by glucose regulated protein (GRP78/Bip) pathway, intended to restore the capacity of ER and alleviate this stress [10]. Three distinct ER transmembrane proteins initiate the canonical UPR: activating transcription factor 6 (ATF6), inositol requiring enzyme 1 (IRE1), and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) [4,11]. Once the UPR is unable to prevent the accumulation of unfolded proteins, ER stress response occurs. JNK and nuclear factor (NF) κB pathways are directly involved in ER stress-induced inflammatory response. For example, JNK and NFκB can be activated by IRE1 through the proinflammatory cytokine, TNF-α [12]. phosphoenolpyruvate kinase/eukaryotic initiation factor α (eIF2α)-triggered ER stress directly promotes NFκB nuclear translocation [13], which subsequently increases the productions of TNF-α and IL-6 [14].
Long-chain bases (LCBs), also called sphingoid bases, are the simplest members in the family glycosphingolipids. Current studies showed that LCBs possessed several bioactivities, such as antioxidation, antitumor, improvement in type 2 diabetes, and inhibition of keratinocyte differentiation [15–18]. LCBs from marine organisms exhibit better bio-activities because of their special environment [19]. Therefore, raising contents have been drawn in LCBs extracted from sea cucumbers (SC-LCBs). SC-LCBs were reported to induce apoptosis in human hepatoma HepG2 cells through phosphorylated protein kinase B and death receptor-5 [20]. SC-LCBs also ameliorated glucose tolerance and hepatic triglyceride content in obese mice [21,22]. However, the effects of LCBs on ER stress associated with inflammatory response have not been verified. Sphingoid bases from plants were proved to inhibit TNF-α and IL-8 levels in human endothelial cells, but the mechanism is not understood [23]. Therefore, the present study was conducted to evaluate whether LCBs could influence ER stress and inflammation or not. In addition, the molecular mechanism by which LCBs altered ER stress-induced inflammatory signal transduction was also investigated.
2. Methods
2.1. Preparation of LCBs
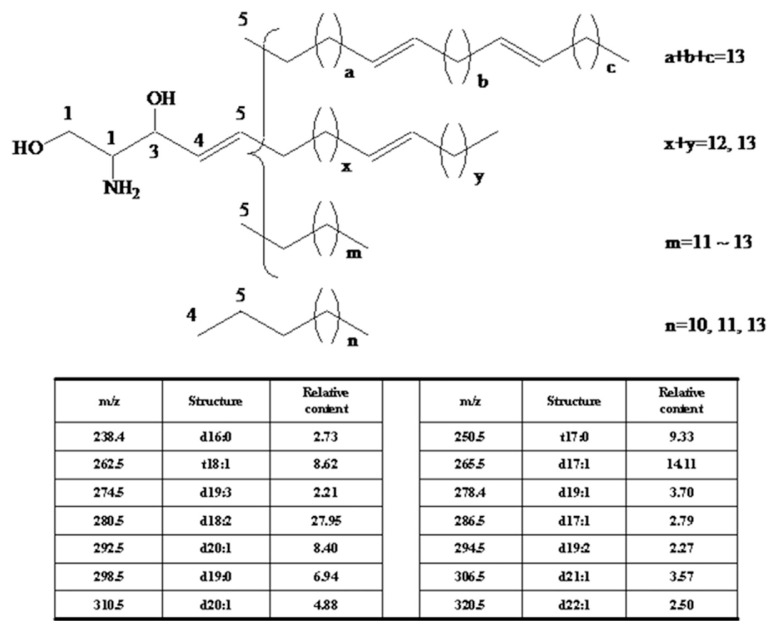
Dried sea cucumber, Cucumaria frondosa, was purchased from Nanshan Aquatic Products Market (Qingdao, China). LCBs were extracted and analyzed as the previous reports [24]. Briefly, total lipids were extracted from sea cucumber using chloroform–methanol (2:1 v/v); 4M KOH in methanol was added into the total lipids and performed 2 hours at 37°C. Extraction was subsequently performed with chloroform–methanol–distilled water (2:1:0.9 v/v/v), and the chloroform layer was collected. After vacuum concentration, the lipids were under HCl acidolysis 16 hours at 80°C, and then was extracted using n-hexane and subsequently diethyl ether, respectively. The crude LCBs were obtained from diethyl ether. To gain pure LCBs, HPLC was performed using an Agilent 1100 HPLC system (Santa Clara, CA, USA) with diode array detection, and a TSK gel ODS-80Ts. The purity of LCBs was 96.4% using HPLC system with a diode array detector. The yield of LCBs was about 1.47%. Their molecular weights were in the range of 238.4–320.5 analyzed by the electrospray ionization-MS method. The components and the main chemical structure of C. frondosa LCBs are shown in Figure 1.
Figure 1.
The structures of long-chain bases from the sea cucumber, Cucumaria frondosa.
2.2. Animal experiments
Five-week-old male C57BL/6J mice (licensed ID: SCXK2011-0011) were obtained from Vital River Laboratory Animal Center (Beijing, China). Animals (n = 12/group) were housed in individual cages under a 12-hour light/dark cycle at 23 ± 1°C daily. The animals were assigned to four groups: control group (maintained on a control diet for 12 weeks); HFFD group [fed high-fat fructose diet (HFFD) diet for 12 weeks]; and low and high dosage of LCBs groups (fed HFFD diet for 4 weeks, and then continuously fed HFFD diet with LCBs at a diet supplement dosage of 0.008% and 0.025% for 8 weeks, respectively). The control diet consisted of 20% protein, 5% fat, and no fructose, whereas the HFFD consisted of 20% protein, 25% fat, and 20% fructose. All experimental protocols used in this study were approved by animal ethics committee as per the guidelines of the Standards for Laboratory Animals of China (GB 14922-94, GB 14923-94, and GB/T 14925-94).
2.3. Fasting serum ROS, FFAs, and inflammatory factors assay
Serum ROS, FFAs, TNF-α, C-reactive protein (CRP), macrophage inflammatory protein 1 (MIP-1), IL-1β, IL-6, and IL-10 levels were assessed with their corresponding enzyme-linked immunosorbent assay kits (Invitrogen, Carlsbad, CA, USA).
2.4. Hepatic ROS and FFAs concentrations analysis
Hepatic ROS and FFAs levels were detected as in our previous study [4]. Briefly, the liver was homogenized in hydroxyethylpiperazine ethane sulfonic acid (HEPES) buffered saline (containing 140mM NaCl, 5mM KCl, 1mM CaCl2, 1mM MgCl2, 10mM HEPES, and 140mM glucose, pH 7.4). The supernatant for ROS and FFA analysis was obtained by centrifugation at 12,000 × g for 30 minutes. ROS and FFA concentrations were tested using their corresponding enzyme-linked immunosorbent assay kits (Invitrogen, Carlsbad, CA, USA).
2.5. Quantitative real-time polymerase chain reaction analysis
RNA in the liver and epididymal adipose tissues was extracted using TRIzol regent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized from 1 μg of RNA using a polymerase chain reaction (PCR) thermocycler (iQ5; Bio-Rad, Hercules, CA, USA). PCR was tested by amplification of 15 ng cDNA in a 25-μL reaction containing SYBR-Green mix (Invitrogen, Carlsbad, CA, USA) using iQ5 Real-Time PCR System. The mixtures were incubated for a predenaturation at 95°C for 10 minutes, followed by 45 PCR cycles: 15 seconds at 95°C, 20 seconds at 60°C, 30 seconds at 72°C. Supplementary Table 1 shows the primers used in this study. Data were analyzed using the software of iCycler iQ5. The levels of expression of inflammatory cytokines genes and ER stress marker genes were normalized to that of β-actin.
2.6. Obtainment of cytosolic and nuclear fractions
For detecting NFκB, cytosolic and nuclear fractions in epididymal adipose tissue were obtained according to our previous study [25]. Briefly, epididymal adipose tissue was homogenized in extraction buffer (250mM NaCl, 20mM NaOH, 50mM HEPES, 40mM sucrose, and protease inhibitor cocktail) and centrifuged repeatedly to obtain the supernatant, which contained the cytosolic fraction. The other liver was homogenized in buffer A (containing 25mM Tris-HCl, 130mM NaCl, and 5mM KCl, pH 7.4) and centrifuged to gain a pellet. The pellet was lysed in buffer B (containing 10mM KCl, 10mM ethylene diamine tetraacetic acid, 10mM HEPES, 0.1mM ethylene glycol tetraacetic acid, 1mM phenylmethanesulfonyl fluoride (PMSF), and 1mM dithiothreitol, pH 7.9), incubated on ice for 20 minutes, centrifuged to obtain the rough nuclei. The rough nuclei was re-extracted with buffer C (containing 400mM NaCl, 1mM ethylene diamine tetraacetic acid, 20mM HEPES, 1mM ethylene glycol tetraacetic acid, 1mM phenylmethanesulfonyl fluoride, and 1mM dithiothreitol, pH 7.9), incubated on ice for 2 hours, and then centrifuged to obtain the supernatant nuclear fraction.
2.7. Western blot analysis
Cellular protein in the liver or epididymal adipose tissue was obtained using IP lysis buffer. The proteins were resolved by 10% polyacrylamide gels containing sodium dodecyl sulfate, transferred to polyvinylidene fluoride membranes, and blotted with 5% bovine serum albumin. Protein was incubated overnight at 4°C with primary antibody (Promega, Madison, WI, USA), and subsequently incubated for 2 hours with horseradish peroxidase-conjugated secondary antibody (Cell Signaling, Danvers, MA, USA). Immunodetection was carried out using electrochemical luminescence kit and then normalized with β-actin or the total corresponding protein for the phosphorylation, and proliferating cell nuclear antigen for nuclear NFκB protein.
2.8. Statistical analysis
For all experiments, statistical analysis using SPSS version 17.0 software was performed by a one-way ANOVA followed by Tukey’s test. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. LCBs reduce body weight in obesity mice
HFFD caused a significant increase in body weight gain in C57BL/6J mice. Although a 16.1% reduction in body weight gain was exhibited in low dosage of LCBs group (Table 1), this was not statistically significant compared with HFFD-fed mice (p > 0.05). High dosage of LCBs significantly decreased body weight gain by 35.0% in HFFD-fed mice. Meanwhile, epididymal adipose weight was lowered by 48.0% in the high dose of LCBs group compared with the HFFD group.
Table 1.
Effects of LCBs on body weight, serum inflammatory cytokines, and serum and hepatic ROS and FFAs concentrations in obese mice.
| Control | HFFD | 0.008% LCBs | 0.025% LCBs | |
|---|---|---|---|---|
| Body weight gain (g) | 15.5 ± 1.7 | 28.6 ± 2.1## | 24.0 ± 2.3 | 18.6 ± 1.1** |
| Epididymal adipose weight (g) | 0.554 ± 0.083 | 1.67 ± 0.20## | 1.35 ± 0.22 | 0.868 ± 0.127** |
| Serum TNF-α (pg/mL) | 287 ± 13 | 536 ± 30## | 479 ± 25* | 304 ± 19** |
| Serum CRP (ng/mL) | 92.3 ± 6.6 | 137 ± 18## | 124 ± 10 | 102 ± 8* |
| Serum MIP-1 (pg/mL) | 28.9 ± 1.4 | 56.3 ± 3.4## | 50.4 ± 2.1 | 36.6 ± 2.4** |
| Serum IL-1β (pg/mL) | 54.3 ± 6.1 | 71.8 ± 4.4## | 67.5 ± 3.7 | 62.2 ± 4.1* |
| Serum IL-6 (pg/mL) | 32.9 ± 3.7 | 52.5 ± 4.4## | 47.7 ± 3.6 | 41.1 ± 2.4** |
| Serum IL-10 (pg/mL) | 122 ± 7 | 70.1 ± 5.7## | 102 ± 7* | 91.8 ± 8.5** |
| Serum ROS (U/mL) | 164 ± 8 | 258 ± 13## | 233 ± 11 | 189 ± 6** |
| Serum FFAs (μmol/L) | 236 ± 14 | 376 ± 20## | 315 ± 12* | 270 ± 10** |
| Hepatic ROS (U/mg) | 48.0 ± 3.1 | 97.2 ± 6.7## | 83.8 ± 6.2 | 60.4 ± 5.3** |
| Hepatic FFAs (μmol/mg) | 0.449 ± 0.016 | 1.38 ± 0.09## | 1.16 ± 0.11 | 0.735 ± 0.039** |
The obesity model mice were established by fed HFFD. Data are presented as mean ± standard deviation (n = 12/group). Multiple comparisons were done using one way ANOVA.
p < 0.01 versus control;
p < 0.05,
p < 0.01 versus HFFD.
CRP = C-reactive protein; FFA = free fatty acid; HFFD = high-fat fructose diet; IL = interleukin; LCBs = long-chain bases; MIP = macrophage inflammatory protein; ROS = reactive oxygen species; TNF = tumor necrosis factor-α.
3.2. LCBs decrease ROS and FFAs concentrations
Table 1 shows the effects of LCBs on ROS and FFAs concentrations in serum and liver. In HFFD group, mice showed significantly higher levels of ROS and FFAs concentrations compared to control group. High dosage of LCBs addition to HFFD-fed mice significantly reduced ROS and FFAs concentrations by 26.7% and 28.2% in serum and by 37.9% and 46.7% in the liver, respectively. Moreover, treatment with high dosage of LCBs also lowered serum FFAs concentration (p < 0.05).
3.3. LCBS inhibit inflammatory response
TNF-α, CRP, MIP-1, IL-1β, and IL-6 are the main proinflammatory cytokines, and IL-10 is an anti-inflammatory factor. As shown in Table 1, high dosage of LCBs caused an increase by 31.0% in serum IL-10 and reductions in serum TNF-α, CRP, MIP-1, IL-1β, and IL-6 by 43.3%, 25.5%, 35.0%, 13.4%, and 21.7%, respectively. Low dosage of LCBs also significantly increased serum IL-10 level and reduced TNF-α concentration (p < 0.05). These results suggest that LCBs can alleviate inflammatory response.
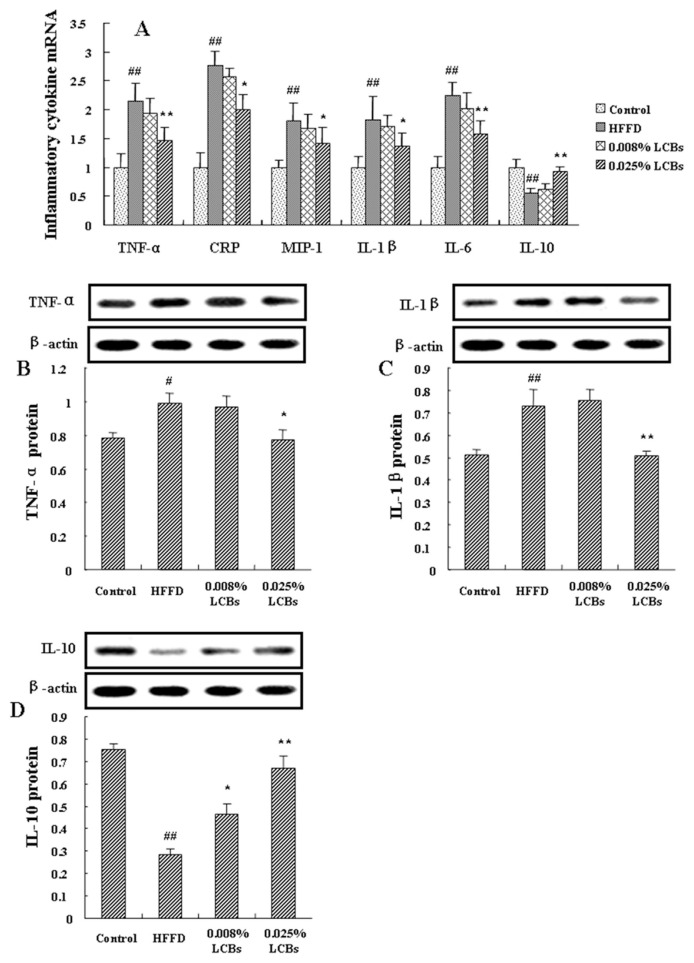
3.4. LCBs regulate inflammatory cytokines expressions
We next detected the mRNA and protein expressions of inflammatory cytokines in epididymal adipose tissue of LCBs-treated mice. Figure 2A showed 68.5% increase in IL-10 mRNA expression in high dosage of LCBs-treated mice compared with HFFD animals. TNF-α, CRP, MIP-1, IL-1β, and IL-6 gene mRNA expressions were significantly decreased in high dosage of LCBs-treated mice (p < 0.05, p < 0.01). Meanwhile, as shown in Figure 2B and C, high dosage of LCBs significantly lowered the protein expressions of TNF-α and IL-1β (p < 0.05, p < 0.01). Both low and high dosage of LCBs promoted IL-10 protein expression in epididymal adipose tissue of obesity mice (p < 0.05, p < 0.01). These indicate that LCBs alleviate inflammation via normalization of inflammatory cytokine productions.
Figure 2.
Effects of long-chain bases on inflammatory cytokines mRNA and protein expression in epididymal adipose tissue of obesity mice. (A) inflammatory cytokines mRNA; (B) tumor necrosis factor-α protein; (C) interleukin-1β protein; (D) |interleukin-10 protein. Data are expressed as mean ± standard deviation (n =12/group). Multiple comparisons were done using one-way ANOVA analysis followed by Tukey test. ## p < 0.01 versus control; * p < 0.05, ** p < 0.01 versus high-fat fructose diet (HFFD).
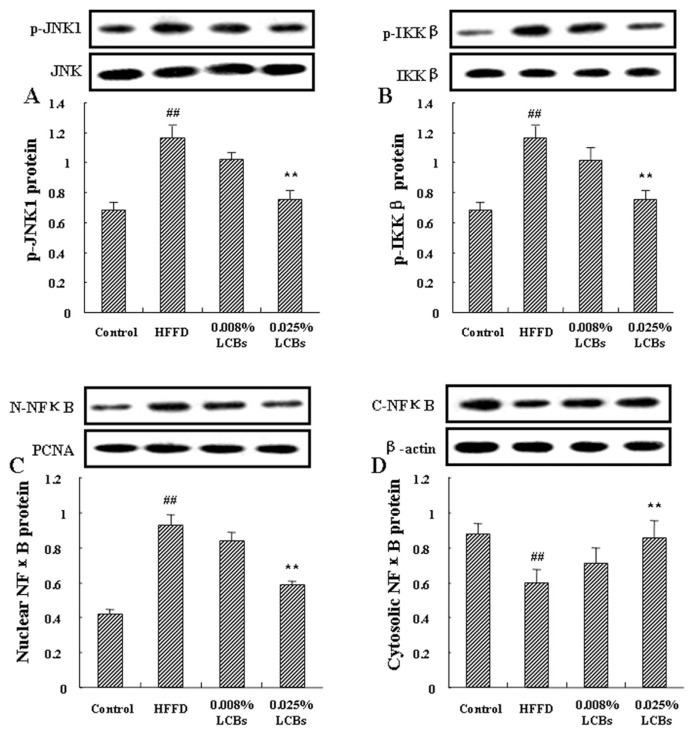
3.5. LCBs activate JNK and NFκB pathways
Inflammatory signal is transferred through a series of inflammation-related signal cascades, in which JNK and IκKβ/ NFκB pathways are the main signals. Aforementioned data suggest that LCBs could improve inflammatory response. Therefore, we subsequently tested the effects of LCBs on JNK and IκKβ/NFκB inflammatory pathways in epididymal adipose tissue. As shown in Figure 3A, high dosage of LCBs caused a significant reduction in JNK1 phosphorylation (p < 0.01). High dosage of LCBs also lowered the p-IκKβ protein abundance (p < 0.01; Figure 3B). Figure 3C and D show the nuclear and cytoplasmic protein expression of NFκB in epididymal adipose tissue of obesity mice. Higher NFκB expression in the cytoplasmic fraction and lower in the nuclear fraction were exhibited in the LCBs supplementary groups than in the obesity mice, suggesting that LCBs induced NFκB translocation and activation. These results indicate that inactivation of JNK and IκKβ/NFκB pathways by LCBs may be the signal response of improvement of inflammatory response.
Figure 3.
Effects of long-chain bases on c-Jun NH2-terminal kinase and inhibitor κ kinase β/nuclear factor (NF) κB pathways in epididymal adipose tissue of obesity mice. (A) p-c-Jun NH2-terminal kinase 1 protein expression; (B) p-inhibitor κ kinase β protein expression; (C) nuclear NFκB protein expression; (D) cytoplasmic NFκB protein expression. Data are expressed as mean ± standard deviation (n =12/group). Multiple comparisons were done using one way ANOVA analysis followed by Tukey’s test. ## p < 0.01 versus control; * p < 0.05, ** p < 0.01 versus high-fat fructose diet (HFFD).
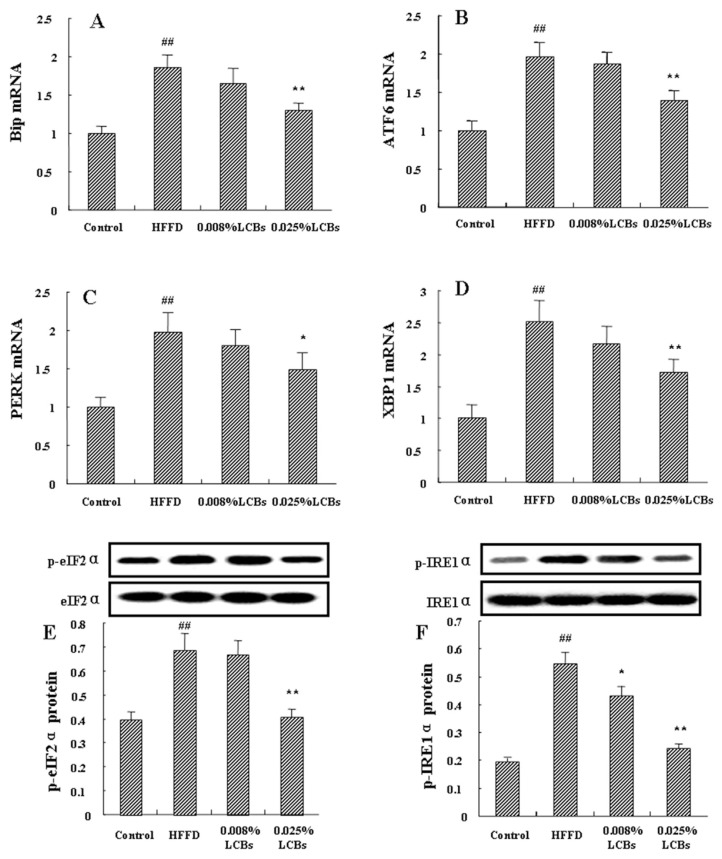
3.6. LCBs improve ER stress
Prolonged or unmitigated activity of UPR can induce inflammation. As the inflammatory signaling was inhibited by LCBs in HFFD-fed mice, we next determined LCBs-influenced ER stress. The initiated gene for UPR, Bip, can cope with the interrupted ER homeostasis through a series of stressors. As shown in Figure 4A, increased Bip mRNA expression was observed in obesity mice, and the elevation was significantly reduced in the liver obtained from LCBs-treated mice (p < 0.01). Three arms are directly involved in initiation of the canonical UPR: ATF6, PERK, and IRE1. ATF6 is released and activated by dissociated Bip from the luminal side of ER. High dosage of LCBs caused a significant decrease in ATF6 mRNA expression in HFFD-fed mice (p < 0.01; Figure 4B). Figure 4C showed that the mRNA expression of PERK was also reduced by LCBs (p < 0.05). Autophosphorylated PERK can activate eIF2α, the target gene to attenuate the rate of translation initiation. Treatment with high dosage of LCBs significantly promoted phosphorylation of eIF2α (p < 0.01), and this tendency was also observed in low dosage of LCBs-treated mice (p < 0.05; Figure 4E). IRE1α is activated through autophosphorylation and specifically splices XBP1 mRNA, which induces UPR target genes. As shown in Figure 4D and F, in the LCBs-fed mice, phosphorylated IRE1α and XBP1 mRNA expression were significantly reduced (p < 0.01). These results suggest that LCBs can alleviate ER stress through inhibition of Bip-triggered UPR.
Figure 4.
Effects of long-chain bases on ER stress in the liver of obesity mice. (A) Bip mRNA expression; (B) ATF6 mRNA expression; (C) phosphoenolpyruvate kinase mRNA expression; (D) XBP1 mRNA expression; (E) p-eIF2α protein expression; (F) p-IRE1α protein expression. Data are expressed as mean ± standard deviation (n =12/group). Multiple comparisons were done using one-way ANOVA analysis followed by Tukey’s test. ## p < 0.01 versus control; * p < 0.05, ** p < 0.01 versus high-fat fructose diet (HFFD).
4. Discussion
Sustained inflammation and ER stress can result in hyperglycemia [11,26]. In the previous studies, LCBs from C. frondosa could reduce blood glucose levels in obesity mice [21,22]. Here, the effects of LCBs on alleviation of hepatic ER stress and systemic inflammation were further investigated. The results showed that LCBs reduced body weight and the levels of stressors of ER stress and inflammation, ROS and FFAs. LCBs also mitigated ER stress and inflammatory response in the liver of obesity mice.
We showed that LCBs significantly lowered body weight gain and epididymal adipose weight, suggesting an antiobesity effects of LCBs. High-calorie diet feeding-induced obesity causes obvious inflammatory response [27]. Adipose tissues are familiarized as the production and secretion of cytokines, which are interrelated with inflammation [28]. Our results showed that LCBs inhibited proinflammatory mRNA expressions and increased anti-inflammatory mRNA expression in epididymal adipose tissue of obesity mice, suggesting that LCBs-reduced epididymal adipose weight may cause the decreases in TNF-α, CRP, MIP-1, IL-1β, and IL-6, and the increase in IL-10. Circulated cytokines concentrations in HFFD-fed mice were also regulated toward to the levels in control group when supplemented with LCBs, which indicates that LCBs mitigate inflammatory response through regulation of inflammatory cytokines.
Inflammatory response occurs in part through IκKβ/NFκB pathway [29]. Abnormal stimulation by ROS, FFAs, or proinflammatory cytokines induces the inhibitor of NFκB phosphorylation and subsequently promotes NFκB nuclear translocation [30]. The nuclear NFκB then regulates the transcription of numerous inflammatory genes, such as TNF-α, MIP-1, and IL-6 [29,31]. In this study, LCBs significantly inhibited the activation of IκKβ and suppressed the nuclear translocation of NFκB, suggesting that LCBs can alleviate inflammatory response through inhibition of the IκKβ/NFκB pathway. JNK signaling is another pathway of progression of chronic low-grade systemic inflammation. Phosphorylated JNK can result in inflammation and its complication [32]. Our results showed that phosphorylated JNK1 was decreased by LCBs supplementary in obesity mice. This indicates that LCBs-deactivated JNK signal also exerts an important effect on the alleviation of inflammatory response.
Studies have proposed ER stress to be involved in the pathophysiology of inflammation [7,33]. Anabatic ER chaperones Bip mRNA expression, and the amplified mRNA levels of ATF6, phosphoenolpyruvate kinase, and XBP1 and phosphorylation status of eIF2α and IRE1α suggest obvious ER stress in the liver of high-calorie diet-fed mice. When treated with LCBs from C. frondosa, the obesity mice exhibited hypoactive ER stress, showing the significant decreases in the mRNA and phosphorylated protein expressions of the aforementioned UPR-related genes. The inhibition of ER stress was coupled with an improvement in inflammatory response by LCBs. Kim et al [34] reported that when interrupted ER stress response, TNF-α and IL-1β mRNA expressions were reduced through the adynamic JNK and NFκB pathways in hepG2 cells. Pan et al [35] showed that the lower response of ER stress resulted in lower mRNA and protein expression of TNF-α and IL-1β in the liver of high-fat diet-fed rats. In the present study, LCBs-reduced ER stress response also caused decreases in proinflammatory cytokines levels and increase in IL-10 level. This indicates that LCBs can improve inflammatory response through intervention of ER stress.
In the earlier stages of ER stress conditions, increased level of mitochondrial calcium alters metabolism and eventually ROS production [10]. The elevation of ROS in the mitochondria induces ER to release calcium continuously through a channel in the membrane of ER, sending a feedback signal [36]. The increased ROS triggers inflammatory response through several complex mechanisms, including phosphorylation of JNK and NFκB nuclear translocation [37,38]. Li et al [4] confirmed that alleviation of ROS-associated ER stress resulted in a reduction in IL-1β secretion. Bhuvaneswari et al [31] also showed that impaired ER stress response can lead to the reduction in intracellular ROS production, and subsequently alleviate inflammation through JNK and NFκB pathways in HFFD-fed mice. Our results showed that LCBs reduced circulating ROS and hepatic ROS production associated with alleviation on ER stress in obesity mice. Meanwhile, the JNK and NFκB pathways were also blocked by LCBs, suggesting that suppression of ROS production by LCBs can counterbalance the deleterious outcome of ER stress and its response to inflammation.
FFAs typically exacerbate ER stress-triggered inflammation [6]. Tampakakis et al [39] found that both peripheral blood mononuclear cells and vascular endothelial cells showed severe ER stress, when suffered with FFAs. FFAs can also activate IκKβ pathways and finally lead to inflammation in adipocytes [40]. Neacsu et al [41] showed that TNF-α and activated JNK proteins were downregulated when the FFAs levels decreased. Moreover, overloaded FFAs and proinflammatory cytokines can increase ER stress-induced mitochondrial ROS product, which finally activates NFκB and JNK pathways [42]. In the present study, high-calorie diet-fed mice exhibited high levels of ROS in serum and liver, and interestingly, the elevations were decreased by LCBs supplementary. These observations indicate that LCBs mitigate ER stress and inflammation through negative induction of FFAs and ROS.
5. Conclusions
This study showed that LCBs were capable of alleviation of ER stress and inflammatory response in high-calorie feeding-induced obesity mice. Downregulated Bip mRNA and the blocking of three branches of UPR (IRE1α/XBP1, PERK/eIF2α, and ATF6 signaling) explained the underlying mechanism of LCBs-mitigated ER stress. Inactivation of JNK and IκK-β/NFκB pathways caused the attenuation of inflammatory response by LCBs through regulating inflammatory cytokine production. To the best of our knowledge, this was the first studying in the existing literature on this topic and it provided important information on the utilization of LCBs from sea cucumber against inflammation.
Acknowledgements
This study was financially supported by Zhejiang Provincial Natural Science Foundation of China (LQ16C200004) and the research startup foundation of Zhejiang Ocean University (Q1442 & Q1443). We also appreciate Professor Yulin Liao for the identification of the species of the sea cucumber, C. frondosa.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2016.10.011.
Funding Statement
This study was financially supported by Zhejiang Provincial Natural Science Foundation of China (LQ16C200004) and the research startup foundation of Zhejiang Ocean University (Q1442 & Q1443).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Majdoubi A, Kishta OA, Thibodeau J. Role of antigen presentation in the production of pro-inflammatory cytokines in obese adipose tissue. Cytokine. 2016;82:112–21. doi: 10.1016/j.cyto.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 2. Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;15:61–7. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3. Rius B, López-Vicario C, González-Périz A, Morán-Salvador E, García-Alonso V, Clária J, Titos E. Resolution of inflammation in obesity-induced liver disease. Front Immunol. 2012;3:257. doi: 10.3389/fimmu.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li S, Jiang W, Hu S, Song W, Ji L, Wang Y, Cai L. Fucosylated chondroitin sulphate from Cusumaria frondosa mitigates hepatic endoplasmic reticulum stress and inflammation in insulin resistant mice. Food Funct. 2015;6:1547–56. doi: 10.1039/c4fo01153h. [DOI] [PubMed] [Google Scholar]
- 5. Liu SL, Peng BJ, Zhong YL, Liu YL, Song Z, Wang Z. Effect of 5-caffeoylquinic acid on the NF-κB signaling pathway, peroxisome proliferator-activated receptor gamma 2, and macrophage infiltration in high-fat diet-fed Sprague–Dawley rat adipose tissue. Food Funct. 2015;6:2779–86. doi: 10.1039/c5fo00626k. [DOI] [PubMed] [Google Scholar]
- 6. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446–62. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liong S, Lappas M. Endoplasmic reticulum stress regulates inflammation and insulin resistance in skeletal muscle from pregnant women. Mol Cell Endocrinol. 2016;425:11–25. doi: 10.1016/j.mce.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 8. Salvadó L, Barroso E, Gómez-Foix AM, Palomer X, Michalik L, Wahli W, Vázquez-Carrera M. PPAR β/δ prevents endoplasmic reticulum stress-associated inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia. 2014;57:2126–35. doi: 10.1007/s00125-014-3331-8. [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Yang J, Chen MH, Wang Q, Qin MJ, Zhang T, Chen XQ, Liu BL, Wen XD. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol Res. 2015;99:101–15. doi: 10.1016/j.phrs.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 10. Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim OK, Jun W, Lee J. Mechanism of ER stress and inflammation for hepatic insulin resistance in obesity. Ann Nutr Metab. 2015;67:218–27. doi: 10.1159/000440905. [DOI] [PubMed] [Google Scholar]
- 12. Greene MW, Ruhoff MS, Burrington CM, Garofalo RS, Oreña SJ. TNFalpha activation of PKCdelta, mediated by NFkappaB and ER stress, cross-talks with the insulin signaling cascade. Cell Signal. 2010;22:274–84. doi: 10.1016/j.cellsig.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 13. Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–8. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–62. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimajiri J, Shiota M, Hosokawa M, Miyashita K. Synergistic antioxidant activity of milk sphingomyeline and its sphingoid base with α-tocopherol on fish oil triacylglycerol. J Agric Food Chem. 2013;61:7969–75. doi: 10.1021/jf401788j. [DOI] [PubMed] [Google Scholar]
- 16. Alden KP, Dhondt-Cordelier S, McDonald KL, Reape TJ, Ng CK, McCabe PF, Leaver CJ. Sphingolipid long chain base phosphates can regulate apoptotic-like programmed cell death in plants. Biochem Biophys Res Commun. 2011;410:574–80. doi: 10.1016/j.bbrc.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 17. Wei N, Pan J, Pop-Busui R, Othman A, Alecu I, Hornemann T, Eichler FS. Altered sphingoid base profiles in type 1 compared to type 2 diabetes. Lipids Health Dis. 2014;13:161. doi: 10.1186/1476-511X-13-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sigruener A, Tarabin V, Paragh G, Liebisch G, Koehler T, Farwick M, Schmitz G. Effects of sphingoid bases on the sphingolipidome in early keratinocyte differentiation. Exp Dermatol. 2013;22:677–9. doi: 10.1111/exd.12231. [DOI] [PubMed] [Google Scholar]
- 19. Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs. 2011;9:1761–805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hossain Z, Sugawara T, Hirata T. Sphingoid bases from sea cucumber induce apoptosis in human hepatoma HepG2 cells through p-AKT and DR5. Oncol Rep. 2013;29:1201–7. doi: 10.3892/or.2013.2223. [DOI] [PubMed] [Google Scholar]
- 21. Gao Z, Zhou X, Hu X, Xue C, Xu J, Wang Y. Effects of sea cucumber cerebroside and its long-chain base on lipid and glucose metabolism in obese mice. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012;41:60–4. [In Chinese, English abstract] [PubMed] [Google Scholar]
- 22. Liu X, Xu J, Xue Y, Gao Z, Li Z, Leng K, Wang J, Xue C, Wang Y. Sea cucumber cerebrosides and long-chain bases from Acaudina molpadioides protect against high fat diet-induced metabolic disorders in mice. Food Funct. 2015;6:3428–36. doi: 10.1039/c5fo00602c. [DOI] [PubMed] [Google Scholar]
- 23. Rozema E, Binder M, Bulusu M, Bochkov V, Krupitza G, Kopp B. Effects on inflammatory responses by the sphingoid base 4:8-sphingadienine. Int J Mol Med. 2012;30:703–7. doi: 10.3892/ijmm.2012.1035. [DOI] [PubMed] [Google Scholar]
- 24. Sugawara T, Zaima N, Yamamoto A, Sakai S, Noguchi R, Hirata T. Isolation of sphingoid bases of sea cucumber cerebrosides and their cytotoxicity against human colon cancer cells. Biosci Biotechnol Biochem. 2006;70:2906–12. doi: 10.1271/bbb.60318. [DOI] [PubMed] [Google Scholar]
- 25. Hu S, Jiang W, Li S, Song W, Ji L, Cai L, Liu X. Fucosylated chondroitin sulphate from sea cucumber reduces hepatic endoplasmic reticulum stress-associated inflammation in obesity mice. J Funct Food. 2015;16:352–63. doi: 10.1039/c4fo01153h. [DOI] [PubMed] [Google Scholar]
- 26. Okin D, Medzhitov R. The effect of sustained inflammation on hepatic mevalonate pathway results in hyperglycemia. Cell. 2016;165:343–56. doi: 10.1016/j.cell.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xia J, Yuan J, Xin L, Zhang Y, Kong S, Chen Y, Yang S, Li K. Transcriptome analysis on the inflammatory cell infiltration of nonalcoholic steatohepatitis in bama minipigs induced by a long-term high-fat, high-sucrose diet. PLoS One. 2014;9:e113724. doi: 10.1371/journal.pone.0113724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jung CC, Tsai YS, Chang CC, Cheng TJ, Chang CW, Liu PY, Chiu YJ, Su HJ. Allergen exposure induces adipose tissue inflammation and insulin resistance. Int Immunopharmacol. 2014;23:104–12. doi: 10.1016/j.intimp.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 29. Murtaza G, Sajjad A, Mehmood Z, Shah SH, Siddiqi AR. Possible molecular targets for therapeutic applications of caffeic acid phenethyl ester in inflammation and cancer. J Food Drug Anal. 2015;23:11–8. doi: 10.1016/j.jfda.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Q, Engelhardt JF. Interleukin-1beta induction of NFkappaB is partially regulated by H2O2-mediated activation of NFkappaB-inducing kinase. J Biol Chem. 2006;281:1495–505. doi: 10.1074/jbc.M511153200. [DOI] [PubMed] [Google Scholar]
- 31. Bhuvaneswari S, Yogalakshmi B, Sreeja S, Anuradha CV. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-κB-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones. 2014;19:183–91. doi: 10.1007/s12192-013-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li CB, Li XX, Chen YG, Gao HQ, Bu PL, Zhang Y, Ji XP. Huang-lian-jie-du-tang protects rats from cardiac damages induced by metabolic disorder by improving inflammation-mediated insulin resistance. PLoS One. 2013;8:e67530. doi: 10.1371/journal.pone.0067530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghosh AK, Garg SK, Mau T, O’Brien M, Liu J, Yung R. Elevated endoplasmic reticulum stress response contributes to adipose tissue inflammation in aging. J Gerontol A Biol Sci Med Sci. 2015;70:1320–9. doi: 10.1093/gerona/glu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim OK, Jun W, Lee J. Effect of Cudrania tricuspidata and kaempferol in endoplasmic reticulum stress-induced inflammation and hepatic insulin resistance in HepG2 cells. Nutrients. 2016;8 doi: 10.3390/nu8010060. pii:E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pan QR, Ren YL, Liu WX, Hu YJ, Zheng JS, Xu Y, Wang G. Resveratrol prevents hepatic steatosis and endoplasmic reticulum stress and regulates the expression of genes involved in lipid metabolism, insulin resistance, and inflammation in rats. Nutr Res. 2015;35:576–84. doi: 10.1016/j.nutres.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 36. Bhandary B, Marahatta A, Kim HR, Chae HJ. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. 2012;14:434–56. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guichard C, Moreau R, Pessayre D, Epperson TK, Krause KH. NOX family NADPH oxidases in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochem Soc Trans. 2008;36:920–9. doi: 10.1042/BST0360920. [DOI] [PubMed] [Google Scholar]
- 38. Remya S, Chikku AM, Renjith RS, Arunima S, Rajamohan T. Coconut kernel protein in diet protects the heart by beneficially modulating endothelial nitric oxide synthase, tumor necrosis factor-alpha, and nuclear factor-kappaB expressions in experimental myocardial infarction. J Food Drug Anal. 2013;21:325–31. [Google Scholar]
- 39. Tampakakis E, Tabit CE, Holbrook M, Linder EA, Berk BD, Frame AA, Bretón-Romero R, Fetterman JL, Gokce N, Vita JA, Hamburg NM. Intravenous lipid infusion induces endoplasmic reticulum stress in endothelial cells and blood mononuclear cells of healthy adults. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002574. pii: e002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiao P, Ma J, Feng B, Zhang H, Diehl JA, Chin YE, Yan W, Xu H. FFA-induced adipocyte inflammation and insulin resistance: involvement of ER stress and IKKβ pathways. Obesity. 2011;19:483–91. doi: 10.1038/oby.2010.200. [DOI] [PubMed] [Google Scholar]
- 41. Neacsu O, Cleveland K, Xu H, Tchkonia TT, Kirkland JL, Boney CM. IGF-I attenuates FFA-induced activation of JNK1 phosphorylation and TNFα expression in human subcutaneous preadipocytes. Obesity. 2013;21:1843–9. doi: 10.1002/oby.20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Egnatchik RA, Leamy AK, Jacobson DA, Shiota M, Young JD. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol Metab. 2014;3:544–53. doi: 10.1016/j.molmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]