Abstract
Psoriasis, which is regarded as a T-cell-mediated chronic inflammatory skin disease, is characterized by hyperproliferation and poor differentiation of epidermal keratinocytes. In this study, we aimed to determine the in vivo effect of a potentially probiotic strain, Lactobacillus pentosus GMNL-77, in imiquimod-induced epidermal hyperplasia and psoriasis-like skin inflammation in BALB/c mice. Oral administration of L. pentosus GMNL-77 significantly decreased erythematous scaling lesions. Real-time polymerase chain reaction showed that treatment with L. pentosus GMNL-77 significantly decreased the mRNA levels of proinflammatory cytokines, including tumor necrosis factor-alpha, interleukin (IL)-6, and the IL-23/IL-17A axis-associated cytokines (IL-23, IL-17A/F, and IL-22) in the skin of imiquimod-treated mice. In addition, we found that L. pentosus GMNL-77 decreased the spleen weights of the imiquimod-treated group and reduced the numbers of IL-17- and IL-22-producing CD4+ T cells in the spleen. In conclusion, the present study provides insight into the potential use of L. pentosus GMNL-77 in the future treatment of psoriasis.
Keywords: cytokines, imiquimod, inflammation, Lactobacillus pentosus GMNL-77, psoriasis, T cells
1. Introduction
Psoriasis is a chronic inflammatory skin disorder characterized by epidermal keratinocyte hyperplasia and massive leukocyte infiltration [1]. It affects approximately 2–3% of the world population [2]. However, the underlying pathogenic mechanisms of this condition have not been fully understood. Recently, numerous studies have confirmed that T helper 17 (Th17) cells and the immune-derived cytokines these cells produce, including interleukin (IL)-17, IL-22, and IL-23, were detected in psoriatic skin lesions and serum that were involved in and interacted as a network in the pathogenesis of psoriasis [3–10].
Probiotics are defined as “living microorganisms that, when administered in adequate amounts, confer health benefits to the host” [11]. Most microorganisms identified to date as probiotics belong to the genera Lactobacillus and Bifidobacterium, which have been extensively studied and commonly used for centuries in humans and animals. Modulation of the immune system is one of the most plausible mechanisms underlying the beneficial effects of probiotics on human health. Probiotics have been found to enhance innate immunity and modulate pathogen-induced inflammation via toll-like receptor-regulated signaling pathways [12]. Recent reports have shown that probiotic use is an effective preventive and therapeutic strategy for different diseases ranging from allergies to autoimmune diseases. Three interacting factors, including an aberrant intestinal microflora, a “leaky” intestinal mucosal barrier, and an altered intestinal immune responsiveness, have been suggested as being able to create a “perfect environment” for the development of autoimmune diseases. In addition to autoimmune diseases, regulation of the composition of intestinal microbiota by probiotics offers the possibility of influencing the development of mucosal and systemic immunity [13]. Probiotics have been reported to be efficacious against cancers, infections, allergies, inflammatory bowel diseases, and autoimmune diseases and it is important to explain how such multifunctional activities are generated. These findings can provide a theoretical basis for understanding the multifunctional activities of specific probiotics [14].
The actions of probiotics on the skin can be mediated by the modulation of both the innate and the adaptive immune responses in the host. Some probiotic strains display potent immune-modulatory properties in the skin. Recently, the ability of Lactobacillus paracasei CNCM-I 2116 (ST11) to modulate reactive skin-associated inflammatory mechanisms has been evaluated. ST11 was able to abrogate vasodilation, edema, mast cell degranulation, and tumor necrosis factor-alpha (TNF-α) release induced by substance P compared with controls. These results support a beneficial role of ST11 in key biological processes associated with barrier function and skin reactivity [15]. Oral administration of Lactobacillus casei can reduce antigen-specific skin inflammation by controlling the size of the CD8+ effector pool [16]. Oral administration of heat-killed Lactobacillus sakei proBio65, which are isolated from Kimchi, inhibited immunoglobulin E-mediated histamine and β-hexosaminidase release in NC/Nga mice. These results suggest that dead L. sakei proBio65 has an inhibitory effect on atopic dermatitis-like skin lesions; it is proposed to be a potential treatment for allergies [17]. In addition, Lactobacillus acidophilus cultured in a medium of taro waste hydrolysate showed immune-modulatory effects in luciferase-based nuclear factor-κB and cyclooxygenase-2 systems [18]. Orally administered L. casei DN-114 001 efficiently alleviates T-cell-mediated skin inflammation without causing immune suppression via mechanisms that include the control of CD8+ effector T cells and the involvement of regulatory CD4+ T cells. L. casei DN-114 001 may, thus, represent a probiotic of potential interest for the immunomodulation of T-cell-mediated allergic skin diseases in humans [19].
Experimental data show that the imiquimod (IMQ)-induced psoriasis model is highly correlated to human psoriasis lesions with regard not only to the clinical and histological characteristics, but also to the development of the lesions via the IL-23/IL-17A axis [20]. This study was designed to investigate the effect of Lactobacillus pentosus GMNL-77 (GMNL-77), a potent probiotic strain that is commercially available as a healthy food product in Taiwan, on an IMQ-induced psoriasis-like mouse model. The effect of GMNL-77 on immune responses was further investigated.
2. Methods
2.1. Mice
Male BALB/c mice (6–8 weeks; 20–25 g) were purchased from the National Laboratory Animal Center (Nangang District, Taipei). The mice were housed at the facility of National Chung Hsing University (South District, Taichung). All experimental animals were used in this study according to a protocol approved by the Institutional Animal Care and Use Committee of National Chung Hsing University.
2.2. Bacterial strain, media, and growth conditions
GMNL-77 was obtained from the culture collection of the GenMont Biotech Incorporation (Shanhua District, Tainan). GMNL-77 was statically grown in Man Rogosa Sharpe broth (Difco Laboratories, Detroit, MI, USA) at 37°C for 18 hours, collected by centrifugation at 3000 g for 5 minutes to remove the Man Rogosa Sharpe broth, and suspended in sterile water at a concentration of approximately 2 × 109 colony-forming units (CFU)/mL.
2.3. IMQ-induced psoriasis-like skin inflammation protocol
After their back skin was shaved, the mice received a topical application of 62.5 mg IMQ cream (Aldara; 3M Pharmaceuticals, St Paul, MN, USA) daily for 6 consecutive days. For the GMNL-77 treatment, the mice were fed orally with different doses of GMNL-77 (5 × 107 CFU/0.2 mL/d or 5 × 108 CFU/0.2 mL/ d) or with the vehicle control (distilled water) for 7 consecutive days, starting from 1 day before IMQ administration. The severity of inflammation of the back skin was evaluated using a modified target lesion psoriasis severity scoring system. Mice were monitored and graded daily with respect to back redness (erythema) and the presence of scales (scaling), on a scale from 0 (no alteration) to 4 (very distinct alteration).
2.4. Skin histology
On Experimental Day 6, the skin samples from the back lesions of mice were fixed in 4% formaldehyde and embedded in paraffin. Deparaffinized sections (6 μm) were stained with hematoxylin and eosin to study their microarchitecture.
2.5. Quantitative reverse transcriptase polymerase chain reaction
At 72 hours after starting the experiment, total RNA was extracted from the back skin lesions using the TRIzol (Invi-trogen, Carlsbad, CA, USA), and complementary DNA was generated using a Transcriptor First Strand cDNA Synthesis Kit (Roche, West Sussex, UK). The relative express levels of genes were assessed using SYBR Green Master Mix (Roche, West Sussex, UK) with the following primers: TNF-α, F: 5′-GGCTGCCCCGACTACGT-3′ and R: 5′-CTCCTGTGGTATGAGA-TAGCAAATC-3; IL-6, F: 5′-TGCCATTGCACAACTCTTTTCT-3′ and R: 5′-TCGGAGGCTTAATTACACATGTTC-3′; IL-23, F: 5′-GTATCCAGTGTGAAGATGGTTGTGA-3′ and R: 5′-CGGA TCCTTTGCAAGCAGAA-3′; IL-17A, F: 5′-TTTTCAGCAAG-GAATGTGGA-3′ and R: 5′-TTCATTGTGGAGGGCAGAC-3′; IL-17F, F: 5′-GAGGATAACACTGTGAGAGTTGAC-3′ and R: 5′-GAGTTCATGGTGCTGTCTTCC-3′; IL-22, F: 5′-GAAGGCTGAAG-GAGACAGTGAAA-3′ and R: 5′-GTTCCCCAATCGCCTTGA-3′; hypoxanthine guanine phosphoribosyl transferase 1 (HPRT), F: 5′-GTTGGATAAGGCCAGACTTTGTTG-3′ and R: 5′-GATT-CAACTTGCGCCATCTTAGGC-3; in Eco Real-Time PCR System (Illumina, San Diego, CA, USA). The HPRT gene was used as a reference to normalize the data. The relative expression level of the gene in the experimental group was compared with that of the water-treated mouse group.
2.6. Flow cytometry analysis
On Experimental Day 6, spleen cells of each group of mice were weighed with an electronic balance and were then extracted via mechanical disruption using 30 μm steel mesh screens in a fresh Petri dish to prepare single-cell suspensions. For intracellular detection of cytokines, 2 × 105 cells/well were stimulated with plate-bound anti-CD3 mAb [clone 145-2C11 (BD Pharmingen, Oxford, UK); 5 μg/mL] and soluble anti-CD28 (1 μg/mL) for 24 hours. Golgistop solution (BD Biosciences, San Diego, CA, USA) was put into the culture 4 hours before cell harvesting. The cells were then stained with phycoerythrin-conjugated antimouse CD4 (GK1.5; BioLegend), followed by treatment with Cytofix/Cytoperm Plus Kit (BD Biosciences) according to the manufacturer’s instructions (BD Biosciences). Cells were then stained intracellularly using the fluorescein isothiocyanate (FITC)-conjugated mAbs specific to murine IL-17A (TC11-18H10.1) and IL-22 (Poly5164), purchased from BioLegend. The stained cells were acquired on a BD Accuri C5 cytometer Cat. No. 657214 (BD Biosciences, San Jose, CA, USA) and analyzed with BD Accuri C6 software, version 1.0264.21.
2.7. Statistical analysis
The data were expressed as mean ± standard deviation. Statistical significance between groups was compared using the Mann–Whitney U test compared with water-treated IMQ control. All the statistical analyses were performed using GraphPad Prism software package, version 5.0 (GraphPad Software, San Diego, CA, USA). Significance was assigned at p < 0.05.
3. Results
3.1. GMNL-77 alleviates skin lesions in IMQ-induced psoriasis-like mice erythema (0.80 ± 0.40, p < 0.05) and scaling (0.75 ± 0.33, p < 0.05)
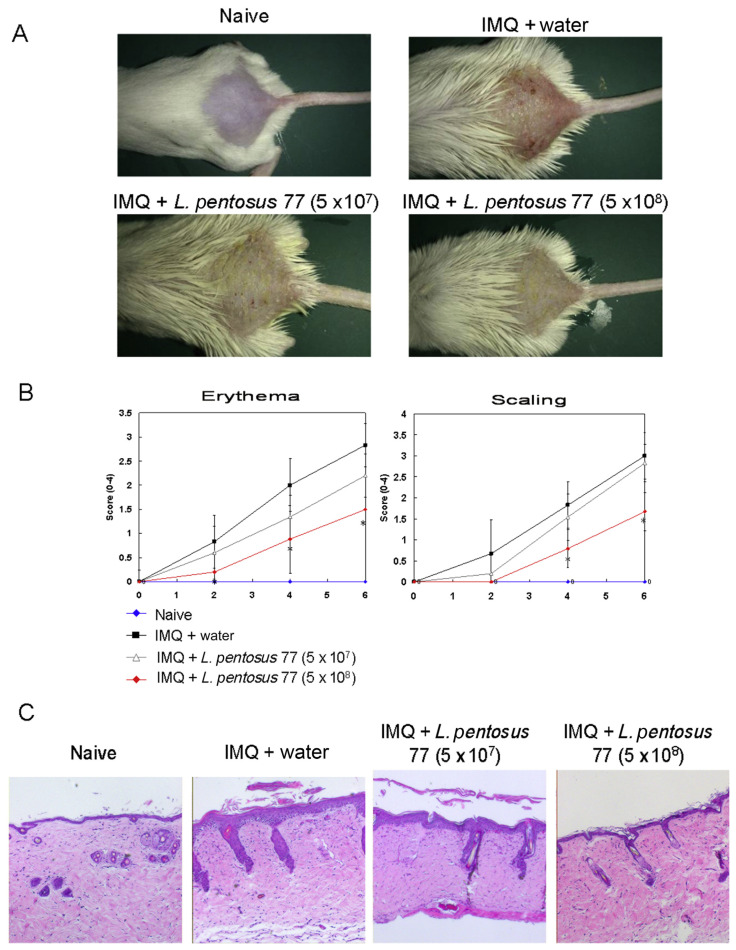
As shown in Figures 1A and 1B, mice developed apparent psoriasis-like lesions, including erythema and scaling of the lesion skin, after treatment with IMQ for 6 consecutive days. Meanwhile, mice fed 5 × 108 CFU/d of GMNL-77 showed significantly reduced lesions in the IMQ-treated group (p < 0.05) (Figures 1A and 1B). The erythema and scaling scores of the GMNL-77 (5 × 108 CFU/d) group were 2.13- and 2.26-fold less than the IMQ group at the end of the experiment. To further analyze the effects of GMNL-77, histological (hematoxylin and eosin) staining was also carried out on the skin lesions in mice on Day 6. The results showed that the epidermal thickness significantly increased in the IMQ-treated skin as a result of hyperproliferation of keratinocytes. However, a high dose of GMNL-77 (5 × 108 CFU/d) reduced the IMQ treatment-induced thickness of the epidermal tissue.
Figure 1.
GMNL-77 reduced psoriasis-like skin inflammation. (A) Phonotypical presentation of the effects of oral administration of GMNL-77 on IMQ-induced psoriasis-like skin lesions after 6 days of treatment. (B) Erythema and scaling of back skin scored daily on a scale from 0 to 4. Symbols represent mean score ± SD of six mice per group. Data shown are representative of three experiments. (C) Representative photomicrographs of hematoxylin and eosin-stained section of back skin tissue of the experimental mice. * p < 0.05 (Mann–Whitney U test) versus IMQ/water control group. GMNL-77 = L. pentosus GMNL-77; IMQ = imiquimod; SD = standard deviation.
3.2. GMNL-77 suppresses inflammatory cytokines in the skin lesions of IMQ-treated mice
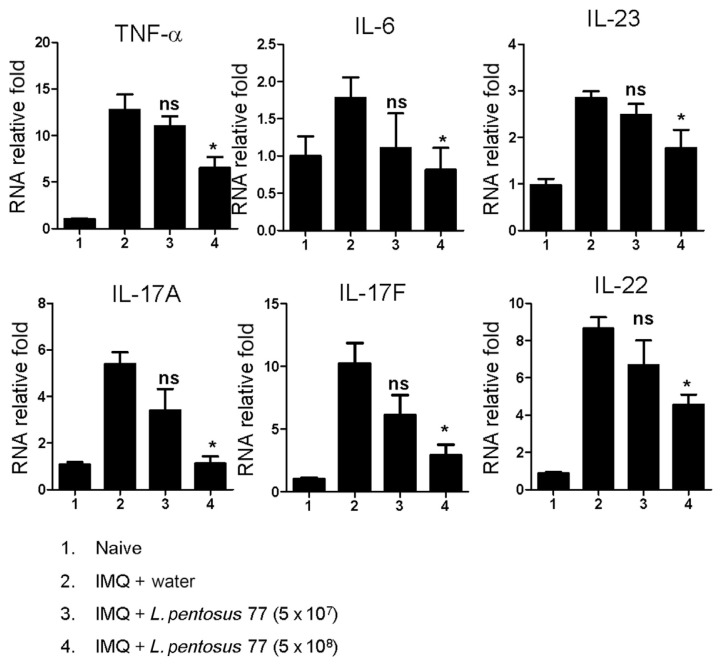
Recently, cytokines have also been found to be involved in the induction of IMQ-induced psoriasis-like inflammation [3,20–24]. Cytokines play important physiological and pathological roles in many diseases [25]. Therefore, to further explore whether GMNL-77 inhibits proinflammatory and Th17-associated cytokine production, we analyzed these psoriasis-related cytokine profiles in the skin lesions using quantitative reverse transcriptase polymerase chain reaction analysis. As shown in Figure 2, the levels of TNF-α (6.50 ± 2.38, p < 0.05), IL-6 (0.81 ± 0.59, p < 0.05), IL-23 (1.77 ± 0.78, p < 0.05), IL-17A (1.13 ± 0.61, p < 0.05), IL-17F (2.93 ± 1.67, p < 0.05), and IL-22 (4.55 ± 1.11, p < 0.05) were significantly reduced when mice were treated with a high dose of GMNL-77 (5 × 108 CFU/d).
Figure 2.
GMNL-77 reduced mRNA levels of skin inflammatory cytokines and IL-23/IL-17-associated cytokines in the back skin induced by IMQ. Skin was excised from different treatment groups at 72 hours, and RNA was extracted to analyze mRNA expression of TNF-α, IL-6, IL-23, IL-17A, IL-17F, and IL-22 by real-time RT-PCR. The HPRT gene was used as a reference to normalize the data. The relative expression level of the gene in the experimental group was compared with that of the water-treated mouse group. Data on the graph represents the mean ± SD, with six mice per group. Data shown are representative of three experiments. * p < 0.05 (Mann–Whitney U test) versus IMQ/water control group. GMNL-77 = L. pentosus GMNL-77; HPRT = hypoxanthine guanine phosphoribosyl transferase 1; IL = interleukin; IMQ = imiquimod; ns = not significant; RT-PCR = reverse transcriptase polymerase chain reaction; SD = standard deviation; TNF-α = tumor necrosis factor-alpha.
3.3. GMNL-77 reduces the number of Th17/Th22 T cells in the spleen of IMQ-treated mice
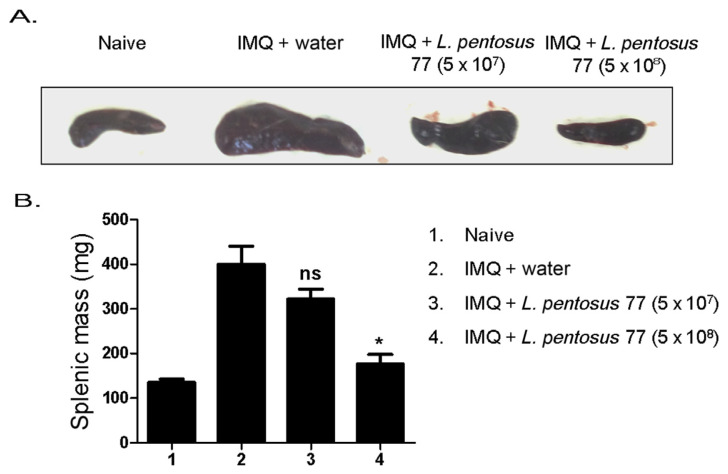
To further determine whether orally administered L. pentosus can affect systemic immune responses, we initially examined the spleen weights on Day 6 after IMQ treatment (Figure 3). IMQ was previously found to induce splenomegaly through systemic effects [20]. In this study, our data identified a consistently significant spleen enlargement following 6 days of IMQ treatment in mice. However, the average spleen weights of the mice in the IMQ-treated-group were reduced after these mice were fed with 5 × 108 CFU/d of GMNL-77 (399.8 ± 81.8 vs. 176.5 ± 43.0, p < 0.05) (Figure 4A).
Figure 3.
GMNL-77 reduced the size and weight of the spleen induced by IMQ. (A) Mice were sacrificed on Day 6, size of spleens with comparison in representative images direct-viewing. (B) The spleens were weighed when the mice were sacrificed. The bar graphs represent the mean ± SD, with six mice per group. Data shown are representative of three experiments. * p < 0.05 (Mann–Whitney U test) versus IMQ/water control group. GMNL-77 = L. pentosus GMNL-77; IMQ = imiquimod; SD = standard deviation.
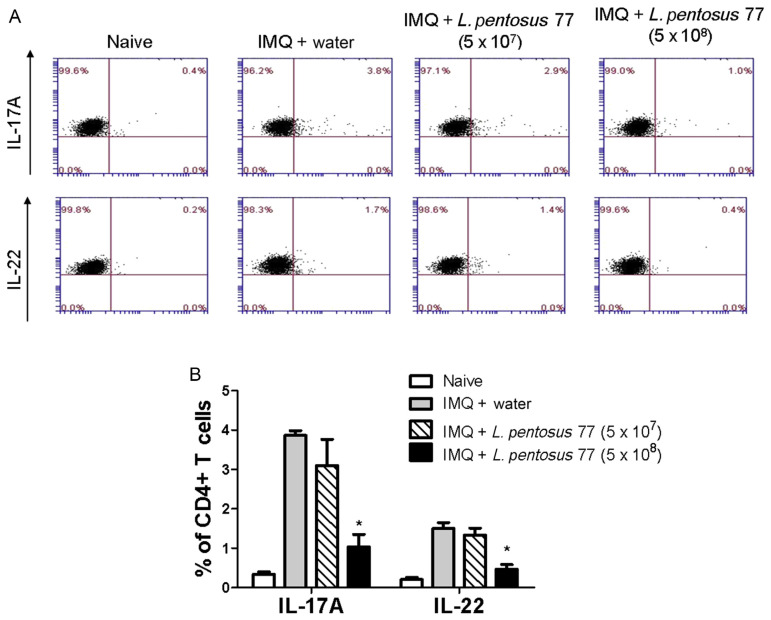
Figure 4.
GMNL-77 decreased the numbers of Th17 and Th22 cells of the mice spleen induced by IMQ treatment. Mice were sacrificed on Day 6, spleen cells were isolated, and in vitro stimulated for 24 hours by plate-bound anti-CD3 and anti-CD28 Ab. Data from flow cytometry were analyzed for the number of IL-17A +CD4 +Th17 and IL-22 +CD4 +Th22 cells. (A) The dot plots show data from one representative mouse of each group. (B) The bar graphs represent the mean ± SD with six mice per group. Data shown are representative of three experiments. * p < 0.05 (Mann–Whitney U test) versus IMQ/water control group. GMNL-77 = L. pentosus GMNL-77; IL = interleukin; IMQ = imiquimod; SD = standard deviation; Th = T helper cells.
By contrast, in several different chronic inflammatory conditions including psoriasis, IL-17 and IL-22 are secreted by CD4+ [20,26]. Therefore, we determined the effect of GMNL-77 on IL-17 and IL-22 production by CD4+ T cells in the spleen. Splenic cells were activated ex vivo by anti-CD3+ plus anti-CD28 Ab, stained intracellularly for IL-17 or IL-22 in gated CD4+ T cells and analyzed using flow cytometry. As shown in Figure 4B, the numbers of IL-17A-producing CD4+ T cells (Th17) and IL-22-producing CD4+ T cells (Th22) were increased following IMQ treatment. However, the percentages of Th17 and Th22 cells in the spleens of the GMNL-77 (5 × 108 CFU/d)-fed group were significantly lower than those of the IMQ-treated control group (Figure 4B). These results imply that GMNL-77 reduces the differentiation of CD4+ T cells to inhibit the systemic inflammatory response following IMQ treatment.
4. Discussion
IMQ, a kind of immunomodulating drug, was first approved by FDA for the topical treatment of external genital and perianal warts in 1997, and now is also used to treat superficial basal cell carcinoma and actinic keratosis [27–29]. However, IMQ may cause certain side effects related to inflammation, such as blisters, burning sensation, skin redness, skin flaking, or scaling. It has been reported that IMQ-induced skin inflammation of mice can be utilized as a model of human psoriatic lesions that exhibit similar phenotypic and histological characteristics, including erythema, epidermal thickening, scaling, and neoangiogenesis, as well as the inflammatory infiltrates of T cells, neutrophils, and dendritic cells [30]. In the present study, we investigated the effect of L. pentosus on skin lesions in IMQ-induced psoriasis-like mice model.
L. pentosus is a versatile lactic acid bacterium found in various environmental niches and in the gastrointestinal tract. It is widely used in the production of fermented foods, such as milk, breads, and vegetables, especially table olives [31]. Specific L. pentosus strains have been shown to elicit probiotic effects, including modulation of the intestinal microbiota [32], inhibition of bacterial and viral pathogens [33], and regulation of the host immune response [34]. In this study, GMNL-77 appears to inhibit IMQ-induced psoriasis-like inflammation in the skin of IMQ-treated mice based on the following roles of GMNL-77: (1) decreased skin erythema and scaling; (2) inhibited hyperplastic basal suprabasal keratinocytes; (3) suppressed mRNA expression of proinflammatory cytokines, including TNF-α, IL-6, and the IL-23–IL-17 cytokine axis (IL-23, IL-17A/F, IL-22) in skin lesions; (4) decreased the spleen weights; and (v) reduced the numbers of IL-17/IL-22-producing CD4+ T cells in the spleen of the IMQ-treated mice.
For the systemic action of IMQ, several weeks of topical treatment leads to psoriasis lesions, a severe side effect, not only at the site of application, but also at inaccessible sites [28,29]. The IMQ-induced skin inflammation model in mice helps illustrate this appearance. We found significant splenomegaly in IMQ-treated mice at the end of the experiment, and the spleen weight increased approximately threefold when compared with the naive group. The spleen is the largest immune organ reflecting systemic immune status in the human body and plays an important role in anti-infection and anticancer activities since it possesses various immunocompetent cytokines [35]. It is suggested that the increased spleen mass observed in the IMQ-induced psoriasis-like mice is due to a large increase in the number of cells in the spleen and may be a sign of increasing the immunoreaction in the body [30]. Enhancement of Th17/Th22 T cells in the spleen of IMQ-treated mice was confirmed in the present study. Oral administration of GMNL-77 (5 × 108 CFU/d) significantly reduced spleen mass in IMQ-treated mice, which suggests that GMNL-77 can decrease the number of Th17/Th22 T cells of the spleen and alleviate the inflammatory response.
Recent studies have demonstrated that IL-17- and IL-22-producing Th17 and Th22 cells serve as models for analyzing the pathogenic mechanisms associated with psoriasis-like dermatitis [21,22,36,37]. First, the results of the present study show that GMNL-77 can decrease the percentage of IL-17A-and IL-22-producing CD4+ T cells in the IMQ-treated mice (Figure 4). Although the mechanism for GMNL-77-suppressing T-cell activity in vivo is unclear, we propose the following two possibilities: (1) GMNL-77 may decrease the activity of the intestinal antigen-presenting cells, such as the CD103+ dendritic cells, which have been shown to play a critical role in modulating Tregs in the gastrointestinal tract, thus affecting cell proliferation or differentiation; and (2) GMNL-77 has a direct effect on the molecular mechanisms of the differentiation or proliferation of T cells.
In summary, we demonstrate that oral administration of GMNL-77 is an effective remedy for treating psoriasis in a mouse model. These effects were the result of inhibiting the differentiation and proliferation of keratinocytes reducing the levels of skin inflammatory cytokines and the differentiation of IL-17/IL-22-producing CD4+ T cells in the spleen. Data obtained from these studies are encouraging; however, the molecular and cellular mechanisms responsible for the multiple effects of GMNL-77, such as anti-inflammatory factor expression and the search for a new and efficient element to dampen the inflammation process, require further investigation.
Acknowledgments
This work was supported by the GenMont Biotech Incorporation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
REFERENCES
- 1. Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 2. Wagner EF, Schonthaler HB, Guinea-Viniegra J, Tschachler E. Psoriasis: what we have learned from mouse models. Nat Rev Rheumatol. 2010;6:704–14. doi: 10.1038/nrrheum.2010.157. [DOI] [PubMed] [Google Scholar]
- 3. El Malki K, Karbach SH, Huppert J, Zayoud M, Reisig S, Schuler R, Nikolaev A, Karram K, Munzel T, Kuhlmann CRW, Luhmann HJ, von Stebut E, Wortge S, Kurschus FC, Waisman A. An alternative pathway of imiquimod-induced psoriasis-like skin inflammation in the absence of interleukin-17 receptor a signaling. J Invest Dermatol. 2013;133:441–51. doi: 10.1038/jid.2012.318. [DOI] [PubMed] [Google Scholar]
- 4. Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–83. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mudigonda P, Mudigonda T, Feneran AN, Alamdari HS, Sandoval L, Feldman SR. Interleukin-23 and interleukin-17: importance in pathogenesis and therapy of psoriasis. Dermatol Online J. 2012;18:1. [PubMed] [Google Scholar]
- 6. Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, Teunissen MB. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One. 2010;5:e14108. doi: 10.1371/journal.pone.0014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tokura Y. Th17 cells and skin diseases. Nihon Rinsho Meneki Gakkai Kaishi. 2012;35:388–92. doi: 10.2177/jsci.35.388. [In Japanese] [DOI] [PubMed] [Google Scholar]
- 8. Wang WJ, Yin XY, Zuo XB, Cheng H, Du WD, Zhang FY, Yang S, Zhang XJ. Gene–gene interactions in IL23/Th17 pathway contribute to psoriasis susceptibility in Chinese Han population. J Eur Acad Dermatol Venereol. 2013;27:1156–62. doi: 10.1111/j.1468-3083.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 9. Yoo IS, Lee JH, Song ST, Kim JH, Lee HJ, Kang SW. T-helper 17 cells: the driving force of psoriasis and psoriatic arthritis. Int J Rheum Dis. 2012;15:531–7. doi: 10.1111/j.1756-185X.2012.01813.x. [DOI] [PubMed] [Google Scholar]
- 10. Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol. 2010;135:108–17. doi: 10.1016/j.clim.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 11. Joint FAO/WHO Expert Committee on Food Additives and World Health Organization Food & Agriculture Organization. Safety evaluation of certain mycotoxins in food. Issue 74 Meeting. 2001 [Google Scholar]
- 12. Yan F, Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27:496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Özdemir Ö. Any role for probiotics in the therapy or prevention of autoimmune diseases? Up-to-date review. J Complement Integr Med. 2013;10:229–50. doi: 10.1515/jcim-2012-0054. [DOI] [PubMed] [Google Scholar]
- 14. Shida K, Nanno M, Nagata S. Flexible cytokine production by macrophages and T cells in response to probiotic bacteria: a possible mechanism by which probiotics exert multifunctional immune regulatory activities. Gut Microbes. 2011;2:109–14. doi: 10.4161/gmic.2.2.15661. [DOI] [PubMed] [Google Scholar]
- 15. Gueniche A, Benyacoub J, Philippe D, Bastien P, Kusy N, Breton L, Blum S, Castiel-Higounenc I. Lactobacillus paracasei CNCM I-2116 (ST11) inhibits substance P-induced skin inflammation and accelerates skin barrier function recovery in vitro. Eur J Dermatol. 2010;20:731–7. doi: 10.1684/ejd.2010.1108. [DOI] [PubMed] [Google Scholar]
- 16. Chapat L, Chemin K, Dubois B, Bourdet-Sicard R, Kaiserlian D. Lactobacillus casei reduces CD8+ T cell-mediated skin inflammation. Eur J Immunol. 2004;34:2520–8. doi: 10.1002/eji.200425139. [DOI] [PubMed] [Google Scholar]
- 17. Kim JY, Pyo S. Oral administration of dead Lactobacillus sakei inhibits atopic dermatitis-like skin lesion in NC/Nga mice. FASEB J. 2012;26:lb364. [Google Scholar]
- 18. Hsieh SC, Liu JM, Pua XH, Ting Y, Hsu RJ, Cheng KC. Optimization of Lactobacillus acidophilus cultivation using taro waste and evaluation of its biological activity. Appl Microb Biotechnol. 2016;100:2629–39. doi: 10.1007/s00253-015-7149-1. [DOI] [PubMed] [Google Scholar]
- 19. Hacini-Rachinel F, Gheit H, Le Luduec JB, Dif F, Nancey S, Kaiserlian D. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS One. 2009;4:e4903. doi: 10.1371/journal.pone.0004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Fits L, Mourits S, Voerman JSA, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–45. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 21. Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–11. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 22. Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, Russell CB. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol. 2013;133:17–26. doi: 10.1038/jid.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 24. Flutter B, Nestle FO. TLRs to cytokines: mechanistic insights from the imiquimod mouse model of psoriasis. Eur J Immunol. 2013;43:3138–46. doi: 10.1002/eji.201343801. [DOI] [PubMed] [Google Scholar]
- 25. Sun Y, Shao Y, Zhang Z, Wang L, Mariga AM, Pang G, Geng C, Ho CT, Hu Q, Zhao L. Regulation of human cytokines by Cordyceps militaris. J Food Drug Anal. 2014;22:463–7. doi: 10.1016/j.jfda.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. γδ T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–7. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lacarrubba F, Nasca MR, Micali G. Advances in the use of topical imiquimod to treat dermatologic disorders. Ther Clin Risk Manag. 2008;4:87–97. doi: 10.2147/tcrm.s1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajan N, Langtry JA. Generalized exacerbation of psoriasis associated with imiquimod cream treatment of superficial basal cell carcinomas. Clin Exp Dermatol. 2006;31:140–1. doi: 10.1111/j.1365-2230.2005.01938.x. [DOI] [PubMed] [Google Scholar]
- 29. Fanti PA, Dika E, Vaccari S, Miscial C, Varotti C. Generalized psoriasis induced by topical treatment of actinic keratosis with imiquimod. Int J Dermatol. 2006;45:1464–5. doi: 10.1111/j.1365-4632.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- 30. Qin S, Wen J, Bai XC, Chen TY, Zheng RC, Zhou GB, Ma J, Feng JY, Zhong BL, Li YM. Endogenous n-3 polyunsaturated fatty acids protect against imiquimod-induced psoriasis-like inflammation via the IL-17/IL-23 axis. Mol Med Rep. 2014;9:2097–104. doi: 10.3892/mmr.2014.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hurtado A, Reguant C, Bordons A, Rozès N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012;31:1–8. doi: 10.1016/j.fm.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 32. Siggers RH, Siggers J, Boye M, Thymann T, Mølbak L, Leser T, Jensen BB, Sangild PT. Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs. J Nutr. 2008;138:1437–44. doi: 10.1093/jn/138.8.1437. [DOI] [PubMed] [Google Scholar]
- 33. Izumo T, Maekawa T, Ida M, Noguchi A, Kitagawa Y, Shibata H, Yasui H, Kiso Y. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int Immunopharmacol. 2010;10:1101–6. doi: 10.1016/j.intimp.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 34. Nonaka Y, Izumo T, Izumi F, Maekawa T, Shibata H, Nakano A, Kishi A, Akatani K, Kiso Y. Antiallergic effects of Lactobacillus pentosus strain S-PT84 mediated by modulation of Th1/Th2 immunobalance and induction of IL-10 production. Int Arch Allergy Immunol. 2008;145:249–57. doi: 10.1159/000109294. [DOI] [PubMed] [Google Scholar]
- 35. Liu CJ, Lin JY. Protective effects of strawberry and mulberry fruit polysaccharides on inflammation and apoptosis in murine primary splenocytes. J Food Drug Anal. 2014;22:210–9. [Google Scholar]
- 36. Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186:1495–502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- 37. SchOn MP, Detmar M, Parker CM. Murine psoriasis-like disorder induced by naive CD4+ T cells. Nat Med. 1997;3:183–8. doi: 10.1038/nm0297-183. [DOI] [PubMed] [Google Scholar]