Abstract
Plant-based drugs of abuse are as old as recorded human history. Although traditional addictive substances, such as opium, cannabis and coca, have been controlled by the United Nations anti-drug conventions, many, if not most, natural plants with addictive or abuse liability remain elusive. Therefore, the United Nations Office on Drugs and Crime (UNODC) has warned the emerging threat from new psychoactive substances (NPS), which are mostly derived or modified from the constituents of natural origin. For example, synthetic cannabinoids and synthetic cathinones are derived from the cannabis and khat plant, respectively. In this review, we briefly discussed the chemistry, pharmacology and toxicology of five common NPS of natural origin, i.e., khat, kratom, salvia, magic mushroom and mandrake. Through the review, we hope that professionals and general public alike can pay more attention to the potential problems caused by natural NPS, and suitable control measures will be taken.
Keywords: New psychoactive substances (NPS), Khat, Kratom, Salvia, Magic mushroom, Mandrake
1. Introduction
Abuse of plant-based drugs is as old as humans with opium (the coagulated juice of the opium poppy, Papaver somniferum L.), cannabis (the flowering or fruiting tops of any plant of the genus Cannabis, including C. sativa and its varieties) and cocaine (the major addictive ingredient in Erythroxylon coca and several species in the genus Erythroxylum) that were used for pain relief and in religious rituals [1]. However, it was not until the profound aftermath of opium smoking during the Ching Dynasty of China in the late 19th Century that eventually led to the enactment of the 1961 Single Convention on Narcotic Drugs [2]. Besides the 1961 Single Convention that dealt with the control of traditional narcotic drugs, the 1971 Convention on Psychotropic Substances and the 1988 Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances were subsequently promulgated mainly for synthetic drug control [3,4]. Although these three Conventions still serve as the basis for international drug control, many other psychoactive substances are elusive. They are collectively defined as New Psychoactive Substances (NPS) by the United Nations Office on Drugs and Crime (UNODC) [5].
NPS, with no comprehensively scientific studies on their toxicity and abuse liability, may pose a threat to public health. According to the UNODC Early Warning Advisory (EWA) on NPS, these substances have been indicated a global phenomenon with over 100 countries and territories from all regions of the world. More than 600 substances have been reported as of December 2015 [6]. In the market, NPS have been known by terms such as ‘designer drugs’, ‘research chemicals’, ‘legal highs’, ‘herbal highs’ and ‘bath salts’ [7]. While the term ‘legal highs’ or ‘herbal highs’ implies that NPS offer as a legal alternative to controlled drugs, ‘designer drugs’, ‘research chemicals’, and ‘bath salts’ are often used to describe the NPS that are chemically synthesized or structurally modified [8].
Although most NPS are synthetic chemicals, many of them are derived from natural products. For example, synthetic cannabinoids and synthetic cathinones, which constitute a majority of NPS items, are analogues of Δ9-tetrahydrocannabinol and natural cathinone from cannabis and khat, respectively. In other words, the plant-based substances are usually the origin of NPS but their toxicity remains to be elucidated. Chemical classification of some natural toxins with abuse or dependence liability indicates that most of the active ingredients in the natural NPS are alkaloids(Table 1). To prevent redundancy, the traditional plant-based drugs, such as opium, coca and cannabis, which have been thoroughly explored, will not be reviewed. Instead, this review will focus on plant-based NPS to discuss their chemistry, pharmacology, and toxicology.
Table 1.
Chemical classification of some plant and fungal toxins with abuse or dependence liability.
| Chemical Category | Genera | Examples |
|---|---|---|
| Alkaloids | Atropa & Erythroxylum | Tropanes (e.g., Atropine and Cocaine) |
| Catha | Cathinone | |
| Mitragyna | Mitragynine | |
| Papaver | Isoquinolines (e.g., Morphine) | |
| Psilocybe | Psilocybin, Psilocin | |
| Resins and Resinoids | Cannabis | Tetrahydrocannabinol |
| Terpenoids | Salvia | Salvinorin A |
2. Materials and methods
The NPS of natural origin, or plant-based NPS, is one of the 9 NPS groups as defined by UNODC [5]. In order to review the NPS of natural origin, similar keywords such as plant-based drugs, natural origin drugs, new psychoactive substances and addicted/abuse were selected and searched from literature on the Pubmed, Web of Science (WOS), DrugBank and other websites. Studies were included if they were plant-based NPS according to UNODC classifications. Five common NPS of natural origin, including khat, kratom, salvia, magic mushroom and mandrake, were systematically explored by chemistry, pharmacology and toxicology in this study.
3. Chemistry, pharmacology and toxicology of NPS of natural origin
3.1. Khat
Khat (Catha edulis, Celastraceae), whose traditional names include kat, Kafta, qat, qaad, ghat, chat, tschat, miraa, Abessinischer Tee, Abyssinian Tea, Somali Tea and Arabian Tea, is a plant native to the Horn of Africa and the Arabian Peninsula [6]. A ‘katin’ alkaloid was identified first in 1887, ‘cathine’ in 1930 and ‘cathinone’ in 1975 [7]. In Yemen, people usually consume khat by chewing its leaf and shoot [8]. After chewing, cathinone and cathine alkaloids can be released for psychoactive effects [9]. In recent years, the alcoholic extracts of khat sold as ‘herbal highs’ has also been reported [10].
According to the report of EU Member States from 2009 to 2012, khat was the second most popular plant-based substance after Salvia divinorum in Europe [10]. In the past, khat was usually consumed by migrant communities from Ethiopia, Kenya, Somalia and Yemen. But recently, its use has distributed over these communities. In 2009, Canada, Ireland, Italy, New Zealand, Norway, the United States and Hong Kong reported khat emerged in their illicit drug markets. Khat is under national control in some, if not most, countries although its ingredients, cathinone and cathine, are listed in schedules I and III, respectively, in the 1971 Convention [10].
3.1.1. Chemistry
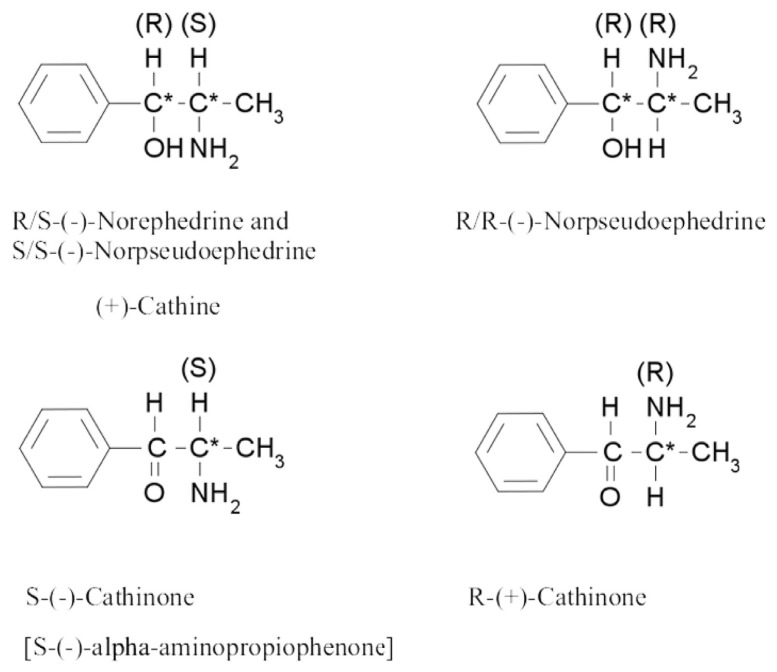
There are dozens of alkaloids as well as tannins, flavonoids, glycosides, non-cyclic nitrogen containing compounds, and other compounds identified in khat [11–15]. Of these chemical constituents, the most important group contributing to the stimulant activity of khat is the phenylalkylamines, which have structural resemblance with the neurotransmitters dopamine and norepinephrine [12]. The khat phenylalkylamines constitute at least one cathinone [S-(−)-cathinone], and two diastereomers cathine [1S, 2S-(+)-norpseudoephedrine] and norephedrine [1R, 2S-(−)-norephedrine] (Fig. 1). The structures of these compounds are related to amphetamine and noradrenaline. Khat only contains the (−)-enantiomer of cathinone, whose absolute stereochemistry is identical to that of S-(+)-amphetamine. Besides phenylalkylamines, the other major alkaloid group is cathedulins, which are based on a poly-hydroxylated sesquiterpene framework and are basically polyesters of euonyminol. Sixty-two cathedulins have been identified from fresh khat leaves [16].
Fig. 1.
Chemical structures of cathinone and cathine.
3.1.2. Pharmacology
The major active constituents in khat could be extracted via chewing and dissolved in the saliva, then absorbed through the buccal mucosa and gastro-intestinal (GI) tract [17,18]. Due to the greater lipophilicity, cathinone is more predominant as a psychoactive agent than cathine or norephedrine [19]. The mechanism of cathinone on neurotransmission is to trigger presynaptic dopamine release and restrain the reuptake of dopamine, which is similar to the effect of amphetamine [20,21]. Cathinone may bind to dopamine and 5-HT receptor sites, although it has the highest affinity for norepinephrine receptors [18,22]. Furthermore, cathinone has also been shown to induce serotonin release and inhibit its reuptake [23].
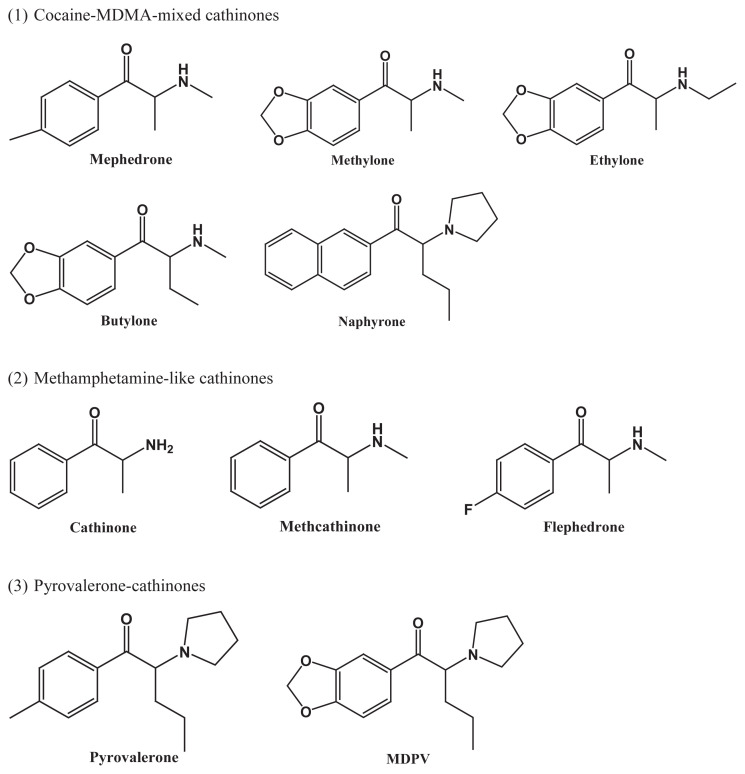
All cathinones, including natural cathinone and its synthetic derivatives, are inhibitors of the monoamine transporters, but their selectivity for the 5-HT transporter (SERT), norepinephrine transporter (NET) and dopamine transporter (DAT) varies substantially [24]. In addition, most of the cathinones are substrate releasers. However, the molecular pharmacology of cathinones has not yet been fully elucidated. With radioligand binding assays performed in cultured HEK cells, Simmler et al. classified the cathinones into three groups, according to their relative potency to inhibit SERT, NET and DAT, as well as their action as substrate releasers: (1) cocaine-MDMA-mixed cathinones, which are relatively nonselective monoamine uptake inhibitors similar to cocaine; (2) methamphetamine-like cathinones, which are preferential catecholamine inhibitors and DA releasers; and (3) pyrocalerone-cathinones, which are very potent and selective catecholamine uptake blockers but not substrate releasers [24]. As exemplified in Fig. 2, all listed cathinones share the same basic structure of ß-ketoamphetamines, but it would be difficult to correlate the different functions of these three groups with modification of their structures. Further study on the structure-activity relationship of cathinone is needed.
Fig. 2.
Three groups of cathinone structures according to their relative potency to act as SERT, NET and DAT inhibitors, and their action as substrate releasers (modified from Simmler et al., [24]).
3.1.3. Toxicology
Due to various compounds present in khat, khat chewing may have miscellaneous effects, especially on the GI system and the nervous system. In general, effects on peripheral nervous system could include constipation, urine retention and acute cardiovascular effects, while effects on the central nervous system could include heightened alertness, tolerance, dependence and psychiatric symptoms [12].
There is still some debate about whether khat use may cause dependence [18]. Kassim et al. assessed 204 khat chewers in Yemen and defined a third of those interviewed met the criteria for dependence [24]. The majority of them had experienced cravings for retaining the drug or trying to reduce their intake. Abstinence can lead to a withdrawal syndrome that includes sleep disorder, depression and sleepiness, as well as temporary hypotension [19]. Because most of the individuals who tried to withdraw or reduce intake of the drug relapsed into their khat habit, it was concluded that khat use caused dependence similar to other drugs of abuse [25].
Khat use may also cause psychological problems. Banjaw et al. [26] reported that khat extract increased baseline aggression in rats, indicating khat induced psychotic episodes [12]. Previous study also found these psychotic symptoms occur rarely and would disappear upon abstain the consumption of khat [18]. However, previously diagnosed or hidden psychiatric conditions may be exacerbated by chewing khat [12,18]. A typical session of khat chewing would result in absorption of effective components that is similar to the effect of 5 mg amphetamine [27]. The pharmacological effects of heightened alertness, euphoria, hyperthermia, anorexia may also increase respiration rate, heart rate, and blood pressure [28], but fatalities associated with khat consumption have not been reported. However, long-term use of khat has been associated with adverse effects from psychiatric disorder to harm of major body organs, and also similar to neurological disorders caused by amphetamine or cocaine use [29].
3.2. Kratom
Kratom (Mitragyna speciosa Korth., Rubiaceae), also known as Kas Biak-Biak or Ketum in Malaysia and Kratom, Kakuam, Kraton, Ithang or Thom in Thailand [30–34]. Kratom, a tropical tree with 4–16 m high, is native to South East Asia, the Philippines and New Guinea, and is also cultivated elsewhere now. People often use fresh kratom leaves to alleviate pain. Depending upon the dose consumption, the effects of psycho-stimulant and opiate-like efficacy have been reported [35,36]. Kratom are consumed by chewing at a dosage of normally 10 to 30 fresh leaves per day, while for the dry powdered leaves it is often swallowed or brewed, but seldom smoked [33]. During long working hours, low kratom dosage has provocative effects against lassitude. However, it can have sedative-anesthetic effects at higher dosages. Kratom is also regarded as an opium substitute in traditional medicine. Phytochemicals, including over 40 structurally related alkaloids and several flavonoids, polyphenols, and various glycosides, have been isolated from various parts of kratom [37]. In the leaves, the primary psychoactive constituents are mitragynine and 7-hydroxymitragynine, both being found only in kratom [38].
In Europe, products labeled as ‘kratom acetate’ or ‘mitragynine acetate’ has been available in the early 2000s [10]. Of the kratom products, a brand under the name ‘krypton’ has been found to be a blend of caffeine and O-desmethyltramadol, an active metabolite of tramadol. In recent years, products containing kratom have been sold as ‘incense’ for their psychoactive effects, but concentrations of these active components vary depending on the variety of kratom used, the circumstance and the time of harvesting. According to the internet surveys by EMCDDA in 2008 and 2011, kratom was one of the most widely supplied NPS [38]. And the answers to UNODC questionnaire on NPS also revealed kratom was on the list of top three plant-based substances, along with khat and S. divinorum. Because kratom was not often under surveillance in national drug abuse surveys, the information of the prevalence of kratom use has been limited. Kratom and its active alkaloids are not listed under the 1961 and 1971 Conventions, but several countries have made control policies on kratom, mitragynine and 7-hydroxymitragynine.
3.2.1. Chemistry
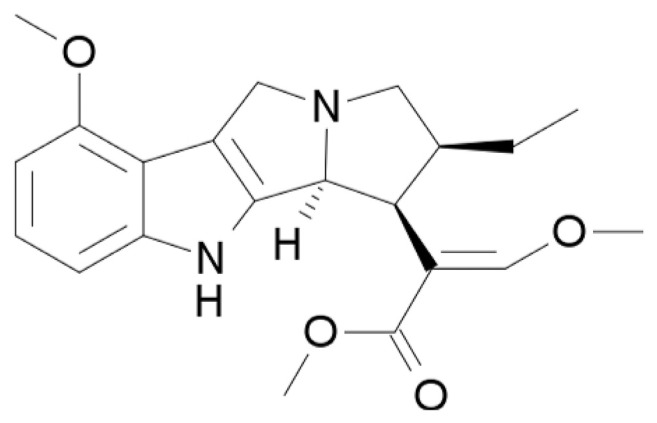
There are more than 40 active compounds in kratom [30]. Mitragynine (Fig. 3) is the major alkaloid, which can constitute up to 66% in the leaf extract of kratom. Mitragynine and its analogues, such as mitraphylline and 7-hydroxymitragynine, are indole alkaloids of the Corynanthe-type possessing a monoterpene (iridoid) moiety [33]. Other alkaloids in kratom also include raubasine and some yohimbe alkaloids [39–41]. Kratom contains at least one alkaloid that can block calcium channel and reduces current induced by N-methyl-d-aspartate (NMDA) [42,43]. The mitragynine contents in kratom vary with location and season. When kratom tree is grown in Southeast Asia, the content of mitragynine tends to be higher, but in elsewhere it tends to be low or non-existent [44]. Using liquid chromatography-electrospray ionization mass spectrometry (LC-ESI-MS) to analyze the products marketed as kratom leaf, mitragynine was found to contain 1–6% and 7-hydroxymirtrogynine 0.01–0.04% [45].
Fig. 3.
Chemical structure of mitragynine.
3.2.2. Pharmacology
Kratom leaves are traditionally used to treat intestinal diseases, muscle pain, coughing and diarrhea in Malaysia and Thailand [32,36,46–48]. Quite a few studies recommended that kratom products have the effects of analgesic, antipyretic, antitussive, antidiarrheal, euphoric, antidepressant, and anxiolytic [38]. They may act as immune booster, lower blood pressure, and have anti-viral, anti-diabetes and appetite suppressing effects [49,50].
The pharmacokinetics of kratom in humans has not been well studied. Various pharmacokinetic factors such as metabolic half-life, protein binding property, elimination and metabolism are not yet known [44]. In animal models, kratom has been found with addiction potential when mitragynine and 7-hydroxymitragynine were given orally for 5 days [51]. In terms of analgesic activity, mitragynine is around 13 times more effective than morphine while 7-hydroxymitragynine is four times more effective than mitragynine [36,51]. Alkaloids in kratom can interact with opioid and monoaminergic receptors but they differ from opioids in structure [52]. Mitragynine is an agonist of multiple receptors that binds not only mu- and kappa-opioid receptors, but also additional receptors that might augment its effectiveness at mitigating opioid withdrawal [53,54]. Kratom's opioid-like effects at lower doses may be comparable to morphine's equivalent effect at lower doses [30]. Effects are dose dependent, beginning 5–10 min after using, and continuing for 1 h after exposure [46]. In addition, a variety of chemicals separated from kratom have been shown to present opioid-like activity on smooth muscle systems and in ligand-binding studies [35,37,55]. Most distinctly, many regions of the central nervous system are sensible to the external effects of these kratom originated substances and the effects can be interfered by opioid antagonists [35,37,41,51,55–57].
3.2.3. Toxicology
Although the use of kratom is increasing, scientific research of its adverse effects and toxicity is still scarce. As mentioned above, the pharmacologic effects of kratom leaves and their constituents are dose-dependent. Low to moderate dosages (1–5 g) can offer light stimulant effects to help workers against fatigue, and moderate to high dosages (5–15 g) may have opioid-like effects [36]. Therefore, kratom can be used for controlling pain, diarrhea, and opioid withdrawal symptoms [32,46,50,58,59]. But kratom also has stimulant effects at high dosages (>15 g) [42,46,60]. Some individuals may have anxiety, irritability, and enhanced aggression due to stimulant effects, and long-term, high-dose consumption has been related to several atypical effects [36]. Hyperpigmentation of the cheeks, tremor, anorexia, weight loss, and psychosis have been noted in individuals with long-term addiction [46].
In the past 5 years, there are nine fatal cases of intoxication associated with the use of kratom-based product known as “Krypton”, a mixture of mitragynine and O-desmethyltramadol [61,62]. However, the mortalities have been ascribed to the addition of O-desmethyltramadol to the dried kratom leaves [61]. Thus, it can be concluded that the use of kratom is not negligible, more clinical, pharmacological, socio-anthropological, and toxicological studies are needed to tackle this marginally explored issue [63].
3.3. S. divinorum
Salvia (S. divinorum, Lamiaceae) is a perennial herb native to Oxaca, Mexico. Traditionally it was used by the Mazatec Indians for religious practices and medical purposes, although there is no approved medicinal use for S. divinorum or its active ingredient salvinorin A. Its street names include “Psychedelic Sage,” “Diviner's Sage,” “Mystic Sage,” “Salvia,” “Sally-D”, and “Magic Mint” etc. In 2009, UN Member Sates identified S. divinorum as the most common plant-based NPS, and in 2012 it was the third, after khat and kratom. The active component for psychoactive effects of S. divinorum is salvinorin A, which is a diterpene, the first non-alkaloidal hallucinogen. The concentrations in the leaves of S. divinorum depend on the growing conditions and preparation procedures. S. divinorum is usually sold as seeds or leaves, but a combination of dried leaves and extracts of salvinorin A is also available in the market [35]. S. divinorum is also one of the most widely marketed recreational plants available through the Internet [64]. Many advertisements on websites mentioned it as a “legal” hallucinogen to promote experimentation with a combination of dried leaves and extracts of salvinorin A as “the freshman selection” or the “starter pack” [65]. Traditional consumption of S. divinorum is via sucking and chewing the fresh leaves or alternatively grinding the fresh leaves to make a drinkable liquid. Many users self-reported to inhale vaporized salvinorin A extract, or smoke the dried leaves to have short but strong hallucinations.
While there are more than 500 species of salvia, S. divinorum has been identified for its hallucinogenic characteristic for centuries. Both adults and adolescents internationally regard S. divinorum as a creational substance. In a latest research of innovative adolescent drug users, 25% of the investigated adolescents expressed they obtained information about S. divinorum via the Internet [66]. Although S. divinorum and salvinorin A are not controlled by any international conventions so far, they are increasingly controlled due to the growing use in some countries [35].
3.3.1. Chemistry
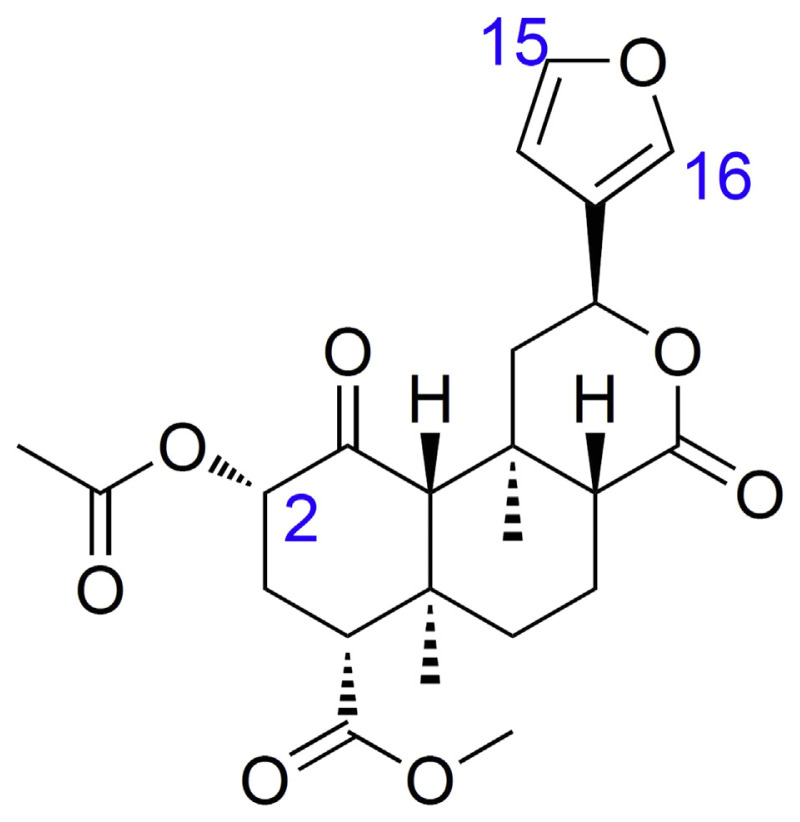
Salvinorin A (Fig. 4) is unique from other naturally occurring hallucinogens since it is not an alkaloid but a neoclerodane diterpene. Although there are several structurally related salvinorins found in the S. divinorum plant, it seems to be the only active naturally occurring salvinorin.
Fig. 4.
Chemical structure of salvinorin A.
3.3.2. Pharmacology
Salvinorin A is a potent and highly selective agonist of the kappa opioid receptor (KOR) that is present in the spinal cord and brain [67]. Studies on the structure–activity relationship of salvinorin A with KOR have shown that the potency and duration of action can be increased if its structure is modified at C-2 (Fig. 4) [67–70]. Transformation of other compounds at C-2 can also extensively affect functional selectivity at the μ (mu) opioid receptor (μ-OR) [71]. In contrast, while small substituent at C-16 has hardly any effect, transformations at other positions, such as C-1 ketone, C-8 epimer, C-12 furan, and C-17 lactone, result in substantial reduction or elimination of affinity for opioid receptors [67, 70]. The affinity for κ-OR is significantly reduced by most transformations of the furan ring [67, 70].
Psychotomimesis, diuresis and spinal analgesia can be induced by stimulation of the kappa-receptor, but without respiratory depression. Results of the research on the pharmacological activity of salvinorin A are consistent with the effects of KOR agonists such as analgesia, sedation, inhibition of GI transit, and depressant effects [72–77]. The mechanism on how KOR agonists initiate hallucinations is unknown, but a role in perception disorders, including schizophrenia and depression, has been proposed [78]. S. divinorum could be used as a probe to verify the molecular basis of psychiatric conditions due to selectivity of salvinorin A for the KOR [79]. Salvinorin A is absorbed rapidly via the buccal mucosa; both extract and leaf forms can produce psychoactive effects within seconds to minutes, and last for up to 1 h. Inhalation can induce psychoactive effects within seconds, which last up to 20–30 min. Threshold dosages of S. divinorum to produce hallucinations vary with the routes of administration [80]. The threshold dosage of salvinorin A by inhalation is 200 mg for hallucinations, while 10 mg fails to induce hallucinations. Studies of salvinorin A absorption via the intravenous administration are lacking in humans but have been performed in animal models [81]. There are three subsets of the hallucinogens, including psychedelics, dissociative, and deliriants. Salvinorin A has been classified into the dissociative, in pace with phencyclidine (PCP, angel dust), ketamine, dextromethorphan, ibogaine, and nitrous oxide [81].
3.3.3. Toxicology
S. divinorum has been indicated with low toxicity and low addictive potential from animal studies [82]. Comparing with other hallucinogens, especially amphetamine derivatives such as MDMA, salvinorin A has rarely been reported for its toxicity [83]. There are limited studies that reports about acute or chronic toxicity associated with S. divinorum in humans, but psychosis in vulnerable individuals has been observed in clinic. So far, there are no fatality case reports due to use of S. divinorum.
3.4. Hallucinogenic (or magic) mushrooms
Hallucinogenic mushrooms, also called “magic mushrooms”, are the psychotropic fungus containing psilocybin and psilocin (Fig. 5) [84]. They have a huge difference in potency depending on the sorts or species of use, place of origin, growing conditions, and age. At low dosage, the main effects of magic mushrooms are perceptual distortions and changes of thought or mood. The majority of them are sold by heads or smart shops in the Netherlands, and the consumption and possession of magic mushrooms have become illegal as List II in the Dutch Narcotic Law along with cannabis since 2008.
Fig. 5.
Chemical structures of (a) psilocybin (b) psilocin.
Although their names consist of “hallucinogenic”, hallucination occurs rarely after using them. They are categorized into three major subsets based on their chemical structures, including indolealkylamines or tryptamines (e.g. psilocybin and psilocin), phenethylamines such as mescaline and methylenedioxymethamphetamine (MDMA) [84].
3.4.1. Chemistry
Psilocybe cubensis and Psilocybe semilanceata (or “psilocybe)” that are commonly known as “liberty caps” contain 10 mg psilocybin per gram of dried mushroom weight (1% w/w). Some species like Psilocybe bohemica contain a little more psilocybin. The average dosage of psilocybin could induce hallucinogenic effects is 4–10 mg [85] or 50–300 g/kg body weight [86]. Therefore, the minimum dosage to get the desired recreational effect is around 1 g of dried magic mushrooms or 10 g of fresh magic mushrooms. Interestingly, psilocin and psilocybin, the psychoactive substances, are more stable in dried mushrooms than in fresh ones. However, following 4 weeks storage of the fresh magic mushrooms, the Netherlands Food and Consumer Product Safety Authority could only find traces of psilocin and psilocybin [87].
Psilocybin is converted into the pharmacologically active compound psilocin in the body by a dephosphorylation reaction through alkaline phosphatases. Psilocybin is a tryptamine derivative, and is structurally similar to the neurotransmitter serotonin. Phenylethylamine, a sympathomimetic amine structurally related to amphetamines, was also found in some mushrooms [85], and might induce the cardiovascular effects like tachycardia and other adverse reactions such as nausea and anxiety.
3.4.2. Pharmacology
The major adverse effects of magic mushrooms are associated with the central nervous system and sympathomimetic effects vary greatly between individuals and the duration time of use [88]. People may have the feelings of relaxation, dizziness, euphoria, visual disturbances, delusions, illusion, or real hallucinations. Other adverse effects include restlessness, incoordination, anxiety, and faint judgments of time or distance etc. but in general, body temperature remains normal. Users may use 'bad trips' to describe their panic reactions and psychosis-like states. However, apparent physical symptoms have been recorded such as severe stomach pain, persistent vomiting, and diarrhea [84].
3.4.3. Toxicology
The physiological side effects, such as nausea, vomiting, trepidation, abdominal pain and dilation of pupils (mydriasis), were often observed after consumption of magic mushrooms, and the common finding of intoxication was tachycardia [89]. At an oral dose of 0.2 mg/kg psilocybin, mild-to-moderate increase in breathing frequency, heart rate, and systolic and diastolic blood pressure (+25, and +10 mm Hg, respectively) was reported [88, 89]. The temporary increase in blood pressure may be a risk factor for users' cardiovascular conditions, especially hypertension without any treatments [86].
The duration of a ‘trip’ usually lasts 2–6 h. Moderate adverse effects such as sleep problems may persist up to 12 h. In 2004, there were high rates of anxiety (32%) and paranoia (35%) among 174 magic mushroom users reported in a UK survey [90]. Recently, with a web-based survey, magic mushrooms were regarded as benefits with low harm potential among hallucinogenic drug users [91].
3.5. Mandrake
Mandrake is the general name for any herbal, perennial plants included in the genus Mandragora [90]. The most well-known mandrake is Mandragora officinarum [92]. Mandrake is native to the eastern Mediterranean region and distributed in southern Europe, the Middle East and northern Africa, and also Himalayas [92]. The name was Latinized from two Sanskrit words: ‘Mandros’ (sleep) and ‘Agora’ (substance), meaning ‘sleep producing drug’. The word “mandrake” is also used frequently for plants' roots with poisonous alkaloids. Mandrake is known for its aphrodisiac, healing, hallucinogenic, and poisonous characteristics. Its fruits are also called love apples that were thought to increase fertility [90].
3.5.1. Chemistry
In ancient times, mandrake had been used as an anodyne and a sedative to anesthetize patients during surgery, but an overdose can be fatal. All parts of mandrake contain active alkaloids, especially in fresh and dried mandrake root. Mandrake contains 0.3–4.0% tropane-based alkaloids, such as atropine, hyoscyamine (daturine), and scopolamine (hyoscine). Tropane alkaloids make the root and leaves poisonous via anticholinergic, hallucinogenic, and hypnotic effects. In the Solanaceae family, the concentration of tropane alkaloids is not as high as some other plants, but if a person had high mandrake consumption or had an allergic reaction, fatality could occur [92–94].
Atropine i.e. (±)-hyoscyamine, obtained from deadly nightshade is a poisonous crystalline alkaloid. It can inhibit the autonomic nervous system, speed a slow heart rate, relieve spasms, decrease secretions, exert anticholinergic effects, and is used to dilate the pupil of the eye locally [94–96].
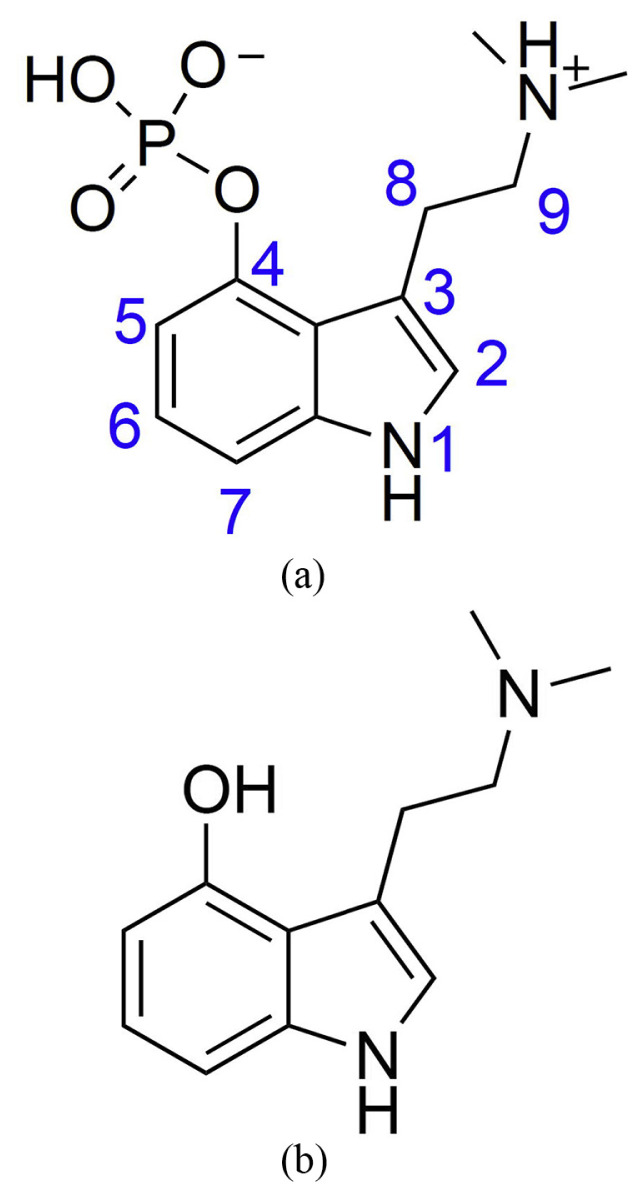
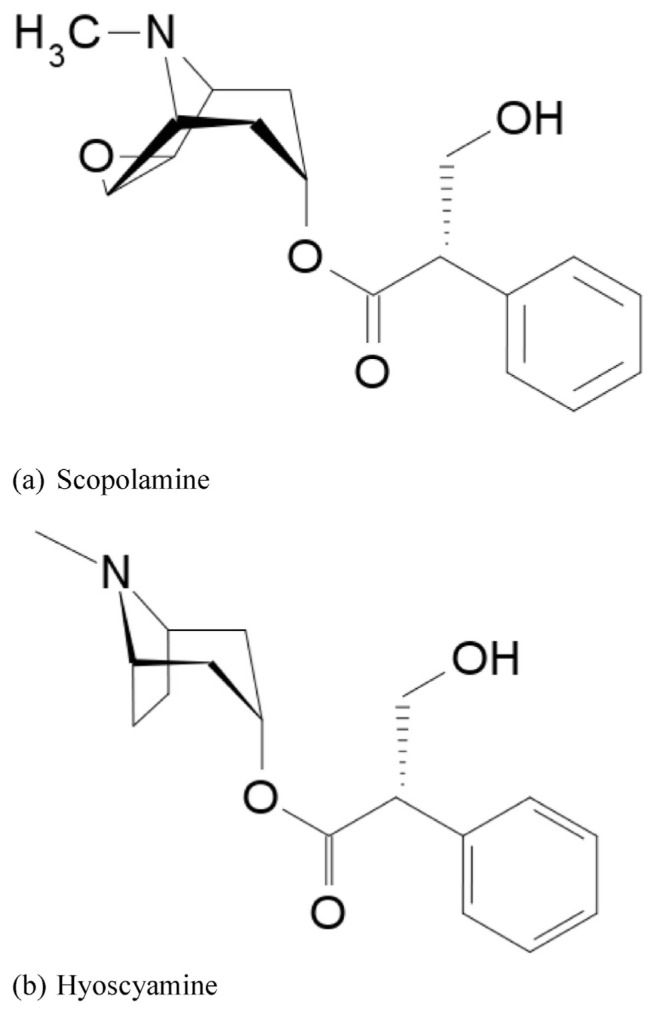
Scopolamine (also called hyoscine, Fig. 6a) is a colorless, water-soluble alkaloidal poison. The primary use is as a sedative and mydriatic to prevent the symptoms of motion disease; it is also taken as an anticholinergic and sedative. (−)-Hyoscyamine (Fig. 6b) is a poison obtained from henbane and other Solanaceous plants. It is a white crystalline alkaloid, and used as a sedative, analgesic, mydriatic, and antispasmodic. Relative to atropine, (−)-hyoscyamine has similar uses but more potent effects [92].
Fig. 6.
Chemical Structures of (a) scopolamine (b) hyoscyamine.
3.5.2. Pharmacology
Mandrake has been well known as anticholinergic, therefore it could restrain the neurological signals transmitted by the endogenous neurotransmitter, acetylcholine. But there are adverse effects including mouth dryness, ataxia, urinary retention, hallucinations, coma, and even death.
Tropane alkaloids, esters of the tropines with various acids, have been used for their sedative, analgesic, antispasmodic and mydriatic effects. Hyoscyamine and atropine in comparatively high doses could cause central effects from stimulation of the cerebrum, midbrain and medulla oblongata, leading to coma and depression [95]. Hyoscine is the other active principle of mandragoras to affect the memory and cause predominantly central respiratory depression in high doses [96,97].
3.5.3. Toxicology
Mandrake's medicinal uses can date back to ancient times. Before surgery, it was given to patients by chewing pieces of root. The root is toxic, but also can be used as a painkiller to relieve and alleviate pain for its soporific characteristics. It has been used to induce vomiting and bowel movements [98].
The effects of mandrake consumption include severe symptoms similar to atropine poisoning, such as blurred vision, mydriasis, and dryness of the mouth, difficulty in urinating, dizziness, vomiting, and tachycardia. The majority of patients also have hyperactivity and hallucination [99].
4. Conclusion
This work reviewed plant-based substances of NPS that have recently emerged on the recreational drugs market. As mentioned above, there were numerous NPS or so-called ‘designer drugs’ used in non-medical purposes to be synthetic alternatives for illicit drugs of abuse [100]. NPS are easily available, and would not be detected by standard screening tests so far [88]. And because the structures of NPS can be chemically modified easily to alter its properties, the items of new synthetic NPS can be expected to increase very quickly.
The Natural NPS, which are often the origin of synthetic NPS, therefore deserve further scrutiny. In this review, khat, kratom, salvia, magic mushroom and mandrake are all easily available to general public. Some of them have been consumed as traditional medicines or ritual substances but their abuse or addictive potentials are not fully elucidated. In addition, their adverse effects could even be fatal if improperly consumed.
Thus, it is deemed necessary to develop a standard and sensitive screening mechanism to early detect NPS for the purposes of prevention, policy-making and regulation.
Acknowledgements
This work was supported in part by grants from the Ministry of Science and Technology, Taiwan (MOST 103-2923-B-037-001-MY2) and Kaohsiung City Government (T105004).
Funding Statement
This work was supported in part by grants from the Ministry of Science and Technology, Taiwan (MOST 103-2923-B-037-001-MY2) and Kaohsiung City Government (T105004).
Footnotes
Conflict of interests
The authors declare no conflict of interests.
REFERENCES
- 1. Musto DF. Opium, cocaine and marijuana in American history. Sci Am. 1991;265:40–7. doi: 10.1038/scientificamerican0791-40. [DOI] [PubMed] [Google Scholar]
- 2. Single convention on narcotic drugs. 1961 [Google Scholar]
- 3. Convention on psychotropic substances. 1971 [Google Scholar]
- 4. Convention against illicit. Traffic in narcotic drugs and psychotropic substances. 1988 [Google Scholar]
- 5.Global synthetics monitoring: analyses, reporting and trends (SMART) programme. Vienna: 2013. The challenge of new psychoactive substances. [Google Scholar]
- 6.UNODC early warning advisory (EWA) on new psychoactive substances (NPS) United Nations Office on Drugs and Crime; Available at: https://www.unodc.org/LSS/Home/NPS. [Google Scholar]
- 7. Szendrei K. The chemistry of khat. Bull Narc. 1980;32:5–35. [PubMed] [Google Scholar]
- 8. Nichols T, Khondkar P, Gibbons S. The psychostimulant drug khat (Catha edulis): a mini-review. Phytochem Lett. 2015;13:127–33. [Google Scholar]
- 9. Sawair FA, Al-Mutwakel A, Al-Eryani K, Al-Surhy A, Maruyama S, Cheng J, et al. High relative frequency of oral squamous cell carcinoma in Yemen: qat and tobacco chewing as its aetiological background. Int J Env Health Res. 2007;17:185–95. doi: 10.1080/09603120701254813. [DOI] [PubMed] [Google Scholar]
- 10.Khat drug profile. European monitoring centre for drugs and drug addiction. Available at: http://www.emcdda.europa.eu/publications/drug-profiles/khat.
- 11. Aziz HA, Tan YTF, Peh KK, Yam MF. Direct effect of khat and garlic extracts on blood lipids contents: preliminary in vitro study. Obes Res Clin Pract. 2010;4:E247–52. doi: 10.1016/j.orcp.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 12. Nasir Tajure W. Chemistry, pharmacology, and toxicology of khat (catha edulis forsk): a review. Addict Health. 2011;3:137–49. [PMC free article] [PubMed] [Google Scholar]
- 13. Glenice C, Hagen R. Adverse effects of khat: a review. Adv Psychiatr Treat. 2003;9:456–63. [Google Scholar]
- 14. Nencini P, Ahmed AM. Khat consumption - a pharmacological review. Drug Alcohol Depend. 1989;23:19–29. doi: 10.1016/0376-8716(89)90029-x. [DOI] [PubMed] [Google Scholar]
- 15. Kalix P, Braenden O. Pharmacological aspects of the chewing of khat leaves. Pharmacol Rev. 1985;37:149–64. [PubMed] [Google Scholar]
- 16. Sikiru L. Flower of paradise (khat: catha edulis): psychosocial, health and sports perspect. Afr J Health Sci. 2012;24:69–83. [Google Scholar]
- 17. Krizevski R, Dudai N, Bar E, Lewinsohn E. Developmental patterns of phenylpropylamino alkaloids accumulation in khat (Catha edulis, Forsk.) J Ethnopharmacol. 2007;114:432–8. doi: 10.1016/j.jep.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 18. Molham A-H. The potential adverse effects of habitual use of Catha edulis (khat) Expert Opin Drug Saf. 2005;4:1145–54. doi: 10.1517/14740338.4.6.1145. [DOI] [PubMed] [Google Scholar]
- 19. Kalix P. Pharmacological properties of the stimulant khat. Pharmacol Ther. 1990;48:397–416. doi: 10.1016/0163-7258(90)90057-9. [DOI] [PubMed] [Google Scholar]
- 20. Feyissa AM, Kelly JP. A review of the neuropharmacological properties of khat. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1147–66. doi: 10.1016/j.pnpbp.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 21. Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: the current situation and directions for future research. J Ethnopharmacol. 2010;132:540–8. doi: 10.1016/j.jep.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 22. Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, et al. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther. 2003;307:138–45. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- 23. Jones S, Fileccia EL, Murphy M, Fowler MJ, King MV, Shortall SE, et al. Cathinone increases body temperature, enhances locomotor activity, and induces striatal c-fos expression in the Siberian hamster. Neurosci Lett. 2014;559:34–8. doi: 10.1016/j.neulet.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 24. Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Brit J Pharmacol. 2013;168:458–70. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kassim S, Croucher R, al'Absi M. Khat dependence syndrome: a crosssectional preliminary evaluation amongst UK-resident Yemeni khat chewers. J Ethnopharmacol. 2013;146:835–41. doi: 10.1016/j.jep.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 26. Banjaw MY, Miczek K, Schmidt WJ. Repeated Catha edulis oral administration enhances the baseline aggressive behavior in isolated rats. J Neural Transm. 2006;113:543–56. doi: 10.1007/s00702-005-0356-7. [DOI] [PubMed] [Google Scholar]
- 27. Ishraq D, Jiri S. Khat habit and its health effect. A natural amphetamine. Biomed Pap. 2004;148:11–5. [PubMed] [Google Scholar]
- 28. Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–53. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- 29. Hoffman R, Al'Absi M. Khat use and neurobehavioral functions: suggestions for future studies. J Ethnopharmacol. 2010;132:554–63. doi: 10.1016/j.jep.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adkins JE, Boyer EW, McCurdy CR. Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. Curr Top Med Chem. 2011;11:1165–75. doi: 10.2174/156802611795371305. [DOI] [PubMed] [Google Scholar]
- 31. Hassan Z, Muzaimi M, Navaratnam V, Yusoff NHM, Suhaimi FW, Vadivelu R, et al. From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev. 2013;37:138–51. doi: 10.1016/j.neubiorev.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 32. Jansen KLR, Prast CJ. Ethnopharmacology of kratom and the mitragyma alkaloids. J Ethnopharmacol. 1988;23(1):115–9. doi: 10.1016/0378-8741(88)90121-3. [DOI] [PubMed] [Google Scholar]
- 33. Ingsathit A, Woratanarat P, Anukarahanonta T, Rattanasiri S, Chatchaipun P, Wattayakorn K, et al. Prevalence of psychoactive drug use among drivers in Thailand: a roadside survey. Accid Anal Prev. 2009;41:474–8. doi: 10.1016/j.aap.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 34. Maruyama T, Kawamura M, Kikura-Hanajiri R, Takayama H, Goda Y. The botanical origin of kratom (Mitragyna speciosa; Rubiaceae) available as abused drugs in the Japanese markets. J Nat Med. 2009;63:340–4. doi: 10.1007/s11418-009-0325-9. [DOI] [PubMed] [Google Scholar]
- 35. Babu KM, McCurdy CR, Boyer EW. Opioid receptors and legal highs: salvia divinorum and Kratom. Clin Tox. 2008;46:146–52. doi: 10.1080/15563650701241795. [DOI] [PubMed] [Google Scholar]
- 36. Vicknasingam B, Narayanan S, Beng GT, Mansor SM. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21:283–8. doi: 10.1016/j.drugpo.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 37. Matsumoto K, Hatori Y, Murayama T, Tashima K, Wongseripipatana S, Misawa K, et al. Involvement of muopioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63–70. doi: 10.1016/j.ejphar.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Kratom (Mitragyna speciosa) drug profile. European Monitoring Centre for Drugs and Drug Addiction; Available at: http://www.emcdda.europa.eu/publications/drug-profiles/kratom. [Google Scholar]
- 39. Chittrakarn S, Keawpradub N, Sawangjaroen K, Kansenalak S, Janchawee B. The neuromuscular blockade produced by pure alkaloid, mitragynine and methanol extract of kratom leaves (Mitragyna speciosa Korth.) J Ethnopharmacol. 2010;129:344–9. doi: 10.1016/j.jep.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 40. Walter CP, Jateen KJ, Shridhar VA. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Asso. 2012;112:792–9. [PubMed] [Google Scholar]
- 41. Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, et al. Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands. J Med Chem. 2002;45:1949–56. doi: 10.1021/jm010576e. [DOI] [PubMed] [Google Scholar]
- 42. Administration USDoJDE. Micro bull – heroin-fentanyl mixtures in Cleveland, Ohio. 2006 [Google Scholar]
- 43. James BH, James JS. Mitragyna alkaloids: the structure of stipulatine. Tetrahedron Lett. 1963;4:929–35. [Google Scholar]
- 44. Ward J, Rosenbaum C, Hernon C, McCurdy CR, Boyer EW. Herbal medicines for the management of opioid addiction safe and effective alternatives to conventional pharmacotherapy? CNS Drugs. 2011;25:999–1007. doi: 10.2165/11596830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45. Kikura-Hanajiri R, Kawamura M, Maruyama T, Kitajima M, Takayama H, Goda Y. Simultaneous analysis of mitragynine, 7-hydroxymitragynine, and other alkaloids in the psychotropic plant “kratom” (Mitragyna speciosa) by LC-ESI-MS. Forensic Toxicol. 2009;27:67–74. [Google Scholar]
- 46. Sangun S. A study of kratom eaters in Thailand. Bull Narc. 1975;27:21–7. [PubMed] [Google Scholar]
- 47. Said IM, Chun NC, Houghton PJ. Ursolic acid from mitragyna-speciosa. Planta Med. 1991;57:398. doi: 10.1055/s-2006-960132. [DOI] [PubMed] [Google Scholar]
- 48. Watanabe K, Yano S, Horie S, Yamamoto LT. Inhibitory effect of mitragynine, an alkaloid with analgesic effect from Thai medicinal plant Mitragyna speciosa, on electrically stimulated contraction of isolated Guinea-pig ileum through the opioid receptor. Life Sci. 1997;60:933–42. doi: 10.1016/s0024-3205(97)00023-4. [DOI] [PubMed] [Google Scholar]
- 49.Burkill IH, Birtwistle W, Foxworthy FW, Scrivenor JB, Watson JG.A dictionary of the economic products of the malay Peninsula: governments of the straits settlements and federated malay states by the crown agents for the colonies. 2009.
- 50. Macko E, Weisbach J, Douglas B. Some observations on the pharmacology of mitragynine. Arch Int Pharmacodyn Thér. 1972;198:145–61. [PubMed] [Google Scholar]
- 51. Matsumoto K, Horie S, Takayama H, Ishikawa H, Aimi N, Ponglux D, et al. Antinociception, tolerance and withdrawal symptoms induced by 7-hydroxymitragynine, an alkaloid from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2005;78:2–7. doi: 10.1016/j.lfs.2004.10.086. [DOI] [PubMed] [Google Scholar]
- 52. Stolt AC, Schroder H, Neurath H, Grecksch G, Hollt V, Meyer MR, et al. Behavioral and neurochemical characterization of kratom (Mitragyna speciosa) extract. Psychopharmacol. 2014;231:13–25. doi: 10.1007/s00213-013-3201-y. [DOI] [PubMed] [Google Scholar]
- 53. Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth) Addiction. 2008;103:1048–50. doi: 10.1111/j.1360-0443.2008.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Megan AR, Elisabeth D, Jacqueline MCD, Laura O, Elizabeth G. New drugs of abuse. Pharmacother. 2015;35:189–97. [Google Scholar]
- 55. Matsumoto K, Yamamoto LT, Watanabe K, Yano S, Shan J, Pang PKT, et al. Inhibitory effect of mitragynine, an analgesic alkaloid from Thai herbal medicine, on neurogenic contraction of the vas deferens. Life Sci. 2005;78:187–94. doi: 10.1016/j.lfs.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 56. Thongpradichote S, Matsumoto K, Tohda M, Takayama H, Aimi N, Sakai S, et al. Identification of opioid receptor subtypes in antinociceptive actions of supraspinally-administered mitragynine in mice. Life S. C. 1998;62:1371–8. doi: 10.1016/s0024-3205(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 57. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull. 2004;52:916–28. doi: 10.1248/cpb.52.916. [DOI] [PubMed] [Google Scholar]
- 58. Assanangkornchai S, Muekthong A, Sam-Angsri N, Pattanasattayawong U. The use of Mitragynine speciosa (“Krathom”), an addictive plant, in Thailand. Subst Use Misuse. 2007;42:2145–57. doi: 10.1080/10826080701205869. [DOI] [PubMed] [Google Scholar]
- 59. Shellard EJ. Ethnopharmacology of kratom and the mitragyna alkaloids. J Ethnopharmacol. 1989;25(1):123–4. doi: 10.1016/0378-8741(89)90053-6. [DOI] [PubMed] [Google Scholar]
- 60. Grewal KS. Observations on the pharmacology of mitragynine. J Pharmacol Exp Ther. 1932;46:251–71. [Google Scholar]
- 61. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-Desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35:242–7. doi: 10.1093/anatox/35.4.242. [DOI] [PubMed] [Google Scholar]
- 62. Arndt T, Claussen U, Gussregen B, Schrofel S, Sturzer B, Werle A, et al. Kratom alkaloids and O-desmethyltramadol in urine of a “Krypton” herbal mixture consumer. Forensic Sci Int. 2011;208:47–52. doi: 10.1016/j.forsciint.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 63. Cinosi E, Martinotti G, Simonato P, Singh D, Demetrovics Z, Roman-Urrestarazu A, et al. Following “the roots” of kratom (mitragyna speciosa): the evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in western countries. Biomed Res Int. 2015;11 doi: 10.1155/2015/968786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dennehy CE, Tsourounis C, Miller AE. Evaluation of herbal dietary supplements marketed on the internet for recreational use. Ann Pharmacother. 2005;39:1634–9. doi: 10.1345/aph.1G185. [DOI] [PubMed] [Google Scholar]
- 65. Prisinzano TE. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci. 2005;78:527–31. doi: 10.1016/j.lfs.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 66. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115:302–5. doi: 10.1542/peds.2004-1199. [DOI] [PubMed] [Google Scholar]
- 67. Christopher WC, Richard BR, Thomas EP. Neuropharmacology of the naturally occurring κ-opioid hallucinogen Salvinorin A. Pharmacol Rev. 2011;63:316–47. doi: 10.1124/pr.110.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hooker JM, Munro TA, Béguin C, Alexoff D, Shea C, Xu Y, et al. Salvinorin A and derivatives: protection from metabolism does not prolong short-term, whole-brain residence. Neuropharmacol. 2009;57:386–91. doi: 10.1016/j.neuropharm.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peet MM, Baker LE. Salvinorin B derivatives, EOM-Sal B and MOM-Sal B, produce stimulus generalization in male Sprague-Dawley rats trained to discriminate salvinorin A. Behav Pharmacol. 2011;22:450–7. doi: 10.1097/FBP.0b013e328349fc1b. [DOI] [PubMed] [Google Scholar]
- 70.Munro TA, Xu W, Ho DM, Liu-Chen L-Y, Cohen BM. Studies toward bivalent κ opioids derived from salvinorin A: heteromethylation of the furan ring reduces affinity. In: Dickschat JS, editor. Beilstein J Org Chem. Vol. 9. 2013. pp. 2916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lamb K, Tidgewell K, Simpson DS, Bohn LM, Prisinzano TE. Antinociceptive effects of herkinorin, a MOP receptor agonist derived from salvinorin A in the formalin test in rats: new concepts in mu opioid receptor pharmacology: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121:181–8. doi: 10.1016/j.drugalcdep.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol Biochem Behav. 2006;83:109–13. doi: 10.1016/j.pbb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 73. Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, et al. Neoclerodane diterpenes as a novel scaffold for mu opioid receptor ligands. J Med Chem. 2005;48:4765–71. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 74. Capasso R, Borrelli F, Capasso F, Siebert DJ, Stewart DJ, Zjawiony JK, et al. The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A inhibit enteric cholinergic transmission in the Guinea-pig ileum. Neurogastroenterol Motil. 2006;18:69–75. doi: 10.1111/j.1365-2982.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 75. Fantegrossi WE, Kugle KM, Valdes LJ, Koreeda M, Woods JH. Kappa-opioid receptor-mediated effects of the plant-derived hallucinogen, salvinorin A, on inverted screen performance in the mouse. Behav Pharmacol. 2005;16:627–33. doi: 10.1097/00008877-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 76. Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology. 2005;179:551–8. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- 77. Carlezon WA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–7. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 78. Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, et al. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Nat Acad Sci. 2002;99:11934–9. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hanes KR. Antidepressant effects of the herb salvia divinorum: a case report. J Clin Psychopharmacol. 2001;21:634–5. doi: 10.1097/00004714-200112000-00025. [DOI] [PubMed] [Google Scholar]
- 80. Daniel JS. Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43:53–6. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 81. Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, et al. Pharmacokinetics of the plant-derived kappa-opioid hallucinogen salvinorin a in nonhuman primates. Synapse. 2005;58:208–10. doi: 10.1002/syn.20191. [DOI] [PubMed] [Google Scholar]
- 82. Mowry M, Mosher M, Briner W. Acute physiologic and chronic histologic changes in rats and mice exposed to the unique hallucinogen salvinorin a. J Psychoact Drugs. 2003;35:379–82. doi: 10.1080/02791072.2003.10400021. [DOI] [PubMed] [Google Scholar]
- 83. Wolowich WR, Perkins AM, Cienki JJ. Analysis of the psychoactive terpenoid salvinorin A content in five Salvia divinorum herbal products. Pharmacotherapy. 2006;26:1268–72. doi: 10.1592/phco.26.9.1268. [DOI] [PubMed] [Google Scholar]
- 84.Hallucinogenic mushrooms drug profile. European monitoring centre for drugs and drug addiction; Available at: http://www.emcdda.europa.eu/publications/drug-profiles/mushrooms. [Google Scholar]
- 85. Beck O, Helander A, Karlson-Stiber C, Stephansson N. Presence of phenylethylamine in hallucinogenic Psilocybe mushroom: possible role in adverse reactions. J Anal Toxicol. 1998;22:45–9. doi: 10.1093/jat/22.1.45. [DOI] [PubMed] [Google Scholar]
- 86. Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145–56. doi: 10.1007/s00213-003-1640-6. [DOI] [PubMed] [Google Scholar]
- 87. Wittmann M, Carter O, Hasler F, Cahn BR, Grimberg U, Spring P, et al. Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol. 2007;21:50–64. doi: 10.1177/0269881106065859. [DOI] [PubMed] [Google Scholar]
- 88. Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, et al. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers - results of an experimental double blind placebo controlled study. Psychopharmacology. 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- 89. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423–9. doi: 10.1016/j.yrtph.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 90. Riley SCE, Blackman G. Between prohibitions: patterns and meanings of magic mushroom use in the UK. Subst Use Misuse. 2008;43:55–71. doi: 10.1080/10826080701772363. [DOI] [PubMed] [Google Scholar]
- 91. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15:283–300. [Google Scholar]
- 92. Hanus LO, Rezanka T, Spizek J, Dembitsky VM. Substances isolated from Mandragora species. Phytochemistry. 2005;66:2408–17. doi: 10.1016/j.phytochem.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 93. Vlachos P, Poulos L. A case of mandrake poisoning. J Toxicol Clin Toxicol. 1982;19:521–2. doi: 10.3109/15563658208992510. [DOI] [PubMed] [Google Scholar]
- 94. Frasca T, Brett AS, Yoo SD. Mandrake toxicity. A case of mistaken identity. Arch Intern Med. 1997;157:2007–9. doi: 10.1001/archinte.157.17.2007. [DOI] [PubMed] [Google Scholar]
- 95. Ramoutsaki IA, Askitopoulou H, Konsolaki E. Pain relief and sedation in roman byzantine texts: mandragoras officinarum, hyoscyamos Niger and atropa belladonna. Int Congr Ser. 2002;1242:43–50. [Google Scholar]
- 96.Hintzenstern Uv. Anaesthesia with mandrake in the tradition of Dioscorides and its role in classical antiquity. In: Atkinson RS, Boulton TB, editors. The history of anaesthesia. london: the royal society of medicine services and the parthenon publishing group; 1989. pp. 38–45. [Google Scholar]
- 97.Bowes JB. In: Mandrake in the history of anaesthesia. Atkinson RS, Boulton TB, editors. London: The History of Anaesthesia The Royal Society of Medicine Services and The Parthenon Publishing Group; 1989. pp. 26–8. [Google Scholar]
- 98.Blakemore C, Jennett S. The oxford companion to the body. Oxford University Press; 2003. [Google Scholar]
- 99. Askitopoulou H, Ramoutsaki IA, Konsolaki E. Analgesia and anesthesia: etymology and literary history of related Greek words. Anesth Analg. 2000;91:486–91. doi: 10.1097/00000539-200008000-00048. [DOI] [PubMed] [Google Scholar]
- 100. Baumann MH, Solis E, Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL. Baths salts, spice, and related designer drugs: the science behind the headlines. J Neurosci. 2014;34:15150–8. doi: 10.1523/JNEUROSCI.3223-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]