Abstract
The objective of this study was to assess the occurrence of drug residues in the raw milk collected from individual farms and milk collection points during 2009–2010 in six different major regions of Kosovo (Prishtinë, Gjilan, Mitrovicë, Pejë, Gjakovë, Prizren). In the present study, a total of 1734 raw milk samples were collected, and qualitatively screened with two different tests, the Delvotest SP assay and an enzyme-linked receptor-binding assay (SNAP). Overall, 106 (6.11%) out of 1734 samples examined with Delvotest SP contained possible drug residues (5.12% and 7.51% of samples from 2009 and 2010, respectively). All suspect samples were further analyzed by three distinct enzyme-linked receptor-binding assays specific for β-lactams (new β-lactam test), tetracyclines (SNAP tetracycline test), and sulfonamides (SNAP sulfamethazine test). Only the new SNAP βlactam test detected residues in 40 out of 52 samples in 2009 and 54 out of 54 suspect samples in 2010. A confirmatory method based on liquid chromatography-tandem mass spectrometry was used to confirm the presence of β-lactam drug residues in samples detected by the enzyme-linked receptor-binding assay. Amoxicillin, penicillin G, and cloxacillin were the most frequently detected residues and were in a concentration range between 2.1 μg/kg and 1973 μg/kg. Seventeen of the positive samples exceeded the maximum residue levels for one or more β-lactam drug. The highest number of positive milk samples came from the Pejë Region (58.8%) and Gjakovë Region (23.5%), and the lowest number of positive samples originated from Gjilan (5.88%), with no positive samples detected in two regions, Mitrovicë and Prizren.
Keywords: antibacterial residues, detection method, Kosovo, milk, public health
1. Introduction
Antibiotic residues in milk are of great concern to dairy farmers, milk processors, regulatory agencies, and consumers. In lactating cows, antimicrobial agents are used mostly for the therapy of mastitis but are used to treat other diseases as well. Today, antimicrobial drugs are used to control, prevent, and treat infection, and to enhance animal growth and feed efficiency [1]. Currently, approximately 80% of all food-producing animals receive medication for part or most of their lives [2]. The most likely cause of violative drug residues is the failure to observe proscribed withdrawal times [3–6]. The presence of antimicrobial residues in milk can engender drug hypersensitivity reactions in milk consumers, manifested as dermal reactions, asthma, or anaphylactic shock [7–12]. Antimicrobial drugs can also interfere with the manufacture of dairy products, decrease acid and flavor production associated with butter manufacture, reduce the curdling of milk, and cause improper ripening of cheeses [13,14]. Finally, the use of antibiotics can give rise to an increase in antibiotic resistance of pathogenic bacteria and contribute to a global health crisis [15,16].
In many countries, governmental authorities have established monitoring programs to determine the antibiotic levels in food and set a maximum residue level (MRL) for these drugs. In the European Union, veterinary drug residue monitoring is enforced according to the requirements set down in Council directive 96/23/EC [18] and Commission Decision 97/747/EC [19], and the MRLs were fixed according to Regulation 470/2009/CE [20] and Regulation 37/2010/UE [21]. The Kosovo program of monitoring residues in live animals and animal products has been in place since 2005. Various analytical methods in detecting antibiotic residues in milk have been reported in the literature [17,22]. Microbiological growth inhibition, enzyme-linked immunosorbent assays, and chromatographic methods are the most commonly used [23,24].
Kosovo’s dairy sector is one of the key sectors in the development of agriculture and continues to recover after the war in 1999, when at least half of livestock production was depleted. Milk production is widespread throughout Kosovo, with more than 25 dairy processing companies in operation [25]. These dairies produce some 381,896 tons of milk annually, and 58,563.45 tons are imported. The market value of locally produced milk was €35,934,158 and from imports it was €32,463,988 [26]. In Kosovo, there is currently no monitoring of drug residues in milk. Hence, there are no data concerning the presence of antibiotic residues in milk produced and marketed in Kosovo. The present study was therefore designed to assess the presence of antimicrobial drug residues in raw milk marketed at different regions of Kosovo.
2. Methods
2.1. Samples
A total of 1734 milk samples from individual farms and milk collection points were collected over a 2-year period (April–October 2009 and February–November 2010) from six major regions of Kosovo (Prishtinë, Gjilan, Mitrovicë, Pejë, Gjakovë, Prizren). In 2009, a total of 1015 milk samples were collected, 826 samples from milk collection points and 189 samples from individual farms. In 2010, in total of 719 milk samples were collected, 635 samples from milk collection points and 84 samples from individual farms. All milk samples were stored at 4°C until analysis. For additional investigations, drug-positive milk samples were stored at –20°C for 3 weeks.
2.2. Screening methods
Antimicrobial drug screening tests were performed at the Kosovo Food and Veterinary Agency in Prishtina, Kosovo. The screening tests used were the Delvotest SP assay supplied by DSM (DSM Food Specialities, Dairy Ingredients, Delft, The Netherlands), and enzyme-linked receptor-binding assays (SNAP tests) provided by IDEXX Lab. Inc. (Westbrook, ME, USA). All drug-positive samples detected by the Delvotest SP were checked with enzyme-linked receptor-binding assays specific for β-lactams, tetracycline, and sulfonamides. Positive samples confirmed by SNAP test were further quantitatively analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
2.2.1. Reagents and standard solutions for screening tests
Penicillin G (PNG) potassium salt and sulfamethazine were obtained from Fluka (St. Louis, MO, USA), Tetracycline hydrochloride was obtained from Sigma (St. Louis, MO, USA). For the preparation of negative control, drug-free milk from cows that had not been treated with an antibiotic for at least 30 days was collected. The milk was collected from the experimental farm of the Agriculture and Veterinary Faculty (Prishtina, Kosovo).
A stock solution of PNG potassium was prepared in a 100-mL volumetric flask, adding 11.17 mg penicillin and distilled water to the target volume. From this solution, 1 mL (100 μg penicillin/mL) was diluted 100-fold with distilled water (i.e., to a final concentration of 1 μg penicillin/mL).
For preparation of drug-spiked milk samples, drug concentrations of ≥ 40 μg/L of milk were prepared by adding the appropriate amount of stock solution directly to milk samples. The equivalent volume of milk was removed prior to adding the appropriate volume of stock solution. The amount added was always ˂ 0.5% of the total volume. Penicillin-G potassium was present at a final concentration of 4 μg/L for the positive control sample, whereas tetracycline and sulfamethazine were added to milk to achieve final concentrations of 60 μg/L and 100 μg/L, respectively.
2.2.2. Delvotest SP microbial test
The qualitative analysis of PNG residues in milk was performed using the Delvotest SP assay as described by Suhren and Beukers [27]. This method is based on the susceptibilities of bacteria to different antibiotics. The method was carried out according to the instructions by the manufacturer.
2.2.3. Enzyme-linked receptor-binding assays (SNAP tests)
Positive samples found by Delvotest SP were subjected to further testing with enzyme-linked receptor-binding assays (SNAP tests). The New SNAP Beta-lactam Test Kit, SNAP tetracycline test, and SNAP sulfamethazine test were used to screen antibiotic residues in milk. The SNAP tests were performed in accordance with the manufacturer’s instructions.
2.3. Confirmatory methods
2.3.1. Reagents and analytical standards
High performance liquid chromatography (HPLC)-grade methanol, acetonitrile, n-hexane, and acetic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). Water was HPLC grade and was prepared with a Milli-Q system (Millipore, Bedford, MA, USA). Sodium phosphate buffer (0.05M, pH 7.5) was prepared by dissolving 0.73 g of NaH2PO4 dihydrate and 3.61 g of Na2HPO4 monohydrate (Sigma-Aldrich) in 500 mL water. Ammoniumacetate (0.05M) was prepared by dissolving 1.92 g of ammonium acetate in 500 mL water and adjusted to pH 7.5 by addition of ammonium hydroxide.
Analytical standard-grade (VETRANAL) amoxicillin trihydrate (purity 99.3%), ampicillin trihydrate (purity 99.7%,), PNG sodium salt (purity 99.4%,), cloxacillin sodium salt monohydrate (purity 98.9%), and dicloxacillin sodium salt monohydrate (purity 99.4%) were purchased from Sigma-Aldrich. Individual stock solutions of amoxicillin (AMX), ampicillin (AMP), PNG, cloxacillin (CXA) and dicloxacillin (DCX) were prepared to achieve a final concentration of 1000 μg/mL by dissolving each exactly weighed drug (10 mg) in 10 mL of water/acetonitrile (80:20, v/v; AMX and AMP) or water/acetonitrile (50:50, v/v; PNG, CXA, DCX). All solutions were stored at −20°C in amber glass containers.
Intermediate standard mixture solutions containing 10 μg/mL for AMX, AMP, PNG, and 75 μg/mL for CXA and DCX were prepared by mixing the appropriate amount of each stock standard solution in water/acetonitrile (50:50, v/v) with storage at 4°C.
Working standard mixture solutions containing 0.1 μg/mL for AMX, AMP, PNG or 0.75 μg/mL for CXA and DCX were prepared daily by diluting the intermediate standard solution with water/acetonitrile (50:50, v/v). These solutions were used for spiking negative milk samples and for constructing calibration curves.
2.3.2. Sample preparation and LC-MS/MS conditions
All milk samples determined to be positive for drug residues were stored at −80°C until analysis. The sample preparation was a modification of the procedure described by Holstage et al [28]. Briefly, residues from 5-g milk samples were extracted with 10 mL of acetonitrile by mechanical shaking for 10 minutes. The organic phase was separated from solid residue by centrifugation at 4000g for 5 minutes at 4°C. The supernatant was transferred in a second 15-mL centrifuge tube, and the extraction was repeated a second time by adding 10 mL of acetonitrile in the centrifuge tube with the precipitate. After shaking and centrifugation at 4000g for 5 minutes, the acetonitrile fractions were combined and evaporated to 0.5 mL volume under an air stream at 50°C using a TurboVap evaporator (Zymarck, Hopkinton, MA, USA). A volume of 4mL phosphate buffer (0.05M at pH 7.5) was added to each sample, and the extract was defatted with 5 mL n-hexane. After centrifugation at 4000g for 5 minutes, the upper organic layer was eliminated and the aqueous phase was purified through STRATA-X SPE cartridges (60 mg, 3 mL) obtained from Phenomenex (Torrance, CA, USA). Each cartridge was previously activated with 2 mL methanol, 2 mL distilled water, and 2 mL phosphate buffer (0.05M at pH 7.5).
The loaded cartridges were washed with 3 mL phosphate buffer (0.05M at pH 7.5) and 1 mL distilled water, then eluted with 5 mL acetonitrile. The eluate was dried under an air stream at 50°C, and the residue was redissolved with 500 μL ammonium acetate (0.05M at pH 7.5) in acetonitrile (90:10, v/v). The samples were sonicated for 10 minutes, centrifuged at 14,000g for 15 minutes and subsequently transferred into LC vials for LC-MS/MS analysis.
All analyses were performed on a liquid chromatographic system (LC) Accela 600 (Thermo Fischer Scientific, San Jose, CA, USA) provided with a quaternary solvent delivery system, a column heater module, and a sampling cooling device, coupled to an LTQ ion trap from Thermo Fischer Scientific. The chromatographic separation was achieved with a Kinetex C18 column (inner diameter, 2.1 mm; length, 100 mm; particle size, 2.6 μm; Phenomenex Ltd.). The mass analyzer was set in the full scan monitoring mode. The analytical conditions are summarized in Table 1.
Table 1.
Chromatographic conditions (timing and percentages of linear gradients used) and MS/MS acquisition conditions for β-lactams.
| Time (min) | Percentage acetic acid 0.005% | Percentage ACN with 0.005% acetic acid | |
|---|---|---|---|
| 0.0 | 100 | 0 | |
| 8.00 | 10 | 90 | |
| 9.00 | 10 | 90 | |
| 10.00 | 100 | 0 | |
| 14.00 | 100 | 0 | |
|
| |||
| Flow rate (mL/min) | 0.2 | ||
| Injection volume (μL) | 10 | ||
| Autosampler temperature (°C) | 4 | ||
| Column temperature (°C) | 30 | ||
|
| |||
| Acquisition conditions | |||
|
| |||
| Analyte | Precursor ion (m/z) | Collision energy (%) | Product ion (m/z) |
|
| |||
| AMOXY (ESI +) | 366 | 15 | 305, 234, 211, |
| 349 | 18 | 208,a 160, 114 | |
| AMPI (ESI +) | 350 | 15 | 333, 305, 191, 174, 160,a 106 |
| PEN G (ESI −) | 333 | 20 | 289, 192a |
| CLOXA (ESI −) | 434 | 15 | 390,a 293 |
| DICLOXA (ESI −) | 468 | 15 | 424,a 327 |
|
| |||
| Ionization conditions for positive and negative mode | |||
|
| |||
| Sheath gas flow (arbitrary unit) | 30 | ||
| Auxiliary gas flow (arbitrary unit) | 15 | ||
| Capillary temperature (°C) | 275 | ||
ACN= acetonitrile; AMOXY= amoxicillin; AMPI= ampicillin; CLOXA = cloxacillin; DICLOXA= dicloxacillin; ESI= electrospray ionization; MS/MS= tandem mass spectrometry; PEN G= penicillin G.
Product ion used for quantification.
2.3.3. Method validation
The confirmatory method for β-lactams was validated to be in compliance with the Commission Decision 657/2002/EC [29]. To avoid possible variability in the instrument response owing to matrix effects, all analytes were quantified by calibration curves prepared daily by processing blank milk samples and spiking the final evaporated extract with amixture of drugs at four concentration levels including zero (blank). An external standard calibration was used for the quantification of all analytes.
The linearity of the methods was tested in milk over the range of 0.5–10 μg/kg for AMX, AMP, and PNG, and over the range of 2.5–90 μg/kg for CXA and DCX. All the calibration curves tested were characterized by excellent linearity—verified by lack-of-fit tests—and by satisfactory correlation coefficients (r2) greater than 0.98. Specificity was verified by the lack of chromatographic interference at the retention time of the analytes of interest on a minimum number of 20 blank milk samples.
Because no certified reference materials were available for β-lactams, to determine trueness and precision, recovery and repeatability were evaluated by means of spiked blank samples around the MRL of each analyte. Blank milk samples were spiked prior to the beginning of the extraction procedure with the analytes under investigation: three concentrations levels were chosen (0.5 × MRL, 1 × MRL, 1.5 × MRL), and six replicates were carried out for each spiking level and repeated on 3 different days for a total of 72 samples. The precision of the method was evaluated by calculating the relative standard deviation (RSD %) in intraday repeatability conditions (r %, RSD calculated from the six replicates for each spiking level) and in intralaboratory reproducibility conditions (R %, RSD calculated from the 18 replicates for each spiking level over 3 days). Trueness was calculated by dividing the mean measured value by the fortification level and multiplying by 100 to express the result as a percentage.
The results, which are shown in Table 2, reveal that all RSD % values, for intraday repeatability (r %) and intralaboratory reproducibility (R %) ranging from 3.5% to 14.8% and from 6.8% to 13.8%, respectively, meet the requirement of the Commission Decision 2002/657/EC at all fortification levels. The trueness, expressed as the relative recovery, ranged from 88.3% to 108.6% for AMX, AMP, and PNG, and from 76.5% to 109.7% for CXA and DCX, which are in agreement with the limits (from ≥1.0 μg/kg to 10 μg/kg, 70–110%, and ≥10 μg/kg, and 80–110%) established by Commission Decision 2002/657/EC.
Table 2.
Validation results for the confirmation of β-lactam residues in milk: results of trueness (recovery in %), intraday repeatability (r %), and intralaboratory reproducibility (R %) in spiked milk samples.
| LC-MS/MS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Intra- & interday repeatability for the determination of β-lactams, in fortified milk samples | |||||||||||||
|
| |||||||||||||
| Compound | Spike level (μg/kg) | Day 1 (n = 6) | Day 2 (n = 6) | Day 3 (n = 6) | Interday (n = 18) | ||||||||
|
|
|
|
|
||||||||||
| Mean found (μg/kg) | RSD (%) | Recovery (%) | Mean found (μg/kg) | RSD (%) | Recovery (%) | Mean found (μg/kg) | RSD (%) | Recovery (%) | Mean found (μg/kg) | RSD (%) | Recovery (%) | ||
| AMOXY | 2.0 | 1.8 | 13.6 | 88.3 | 2.0 | 6.6 | 101.5 | 2.0 | 11.8 | 100.1 | 1.9 | 11.9 | 96.6 |
| 4.0 | 3.9 | 12.5 | 97.3 | 3.9 | 6.6 | 97.8 | 4.3 | 11.5 | 107.9 | 4.0 | 11.1 | 101.0 | |
| 6.0 | 5.8 | 7.9 | 96.1 | 5.9 | 7.6 | 98.6 | 6.0 | 10.8 | 100.0 | 5.9 | 8.6 | 98.2 | |
| CCα | 4.9 μg/kg | ||||||||||||
| CCβ | 5.7 μg/kg | ||||||||||||
| AMPI | 2.0 | 1.8 | 13.8 | 90.5 | 1.9 | 5.8 | 96.1 | 2.1 | 7.0 | 103.5 | 1.9 | 10.4 | 96.7 |
| 4.0 | 3.8 | 11.9 | 95.6 | 4.3 | 8.6 | 106.5 | 3.9 | 10.9 | 96.5 | 4.0 | 11.1 | 99.5 | |
| 6.0 | 5.8 | 8.5 | 96.8 | 6.1 | 11.9 | 101.4 | 6.1 | 10.7 | 101.2 | 6.0 | 10.1 | 99.8 | |
| CCα | 4.7 μg/kg | ||||||||||||
| CCβ | 5.5 μg/kg | ||||||||||||
| PEN G | 2.0 | 1.9 | 10.2 | 93.0 | 2.2 | 11.1 | 108.6 | 2.0 | 15.1 | 99.4 | 2.0 | 13.3 | 100.3 |
| 4.0 | 3.8 | 14.8 | 95.5 | 4.1 | 11.4 | 103.1 | 4.0 | 10.1 | 100.6 | 4.0 | 11.9 | 99.8 | |
| 6.0 | 5.4 | 13.3 | 89.6 | 6.1 | 9.7 | 100.9 | 6.0 | 7.1 | 99.8 | 5.8 | 11.0 | 96.8 | |
| CCα | 5.5 μg/kg | ||||||||||||
| CCβ | 7.0 μg/kg | ||||||||||||
| CLOXA | 15 | 11.5 | 9.1 | 76.5 | 15.5 | 5.5 | 103.1 | 15.1 | 9.3 | 100.7 | 14.2 | 13.8 | 94.9 |
| 30 | 24.2 | 14.4 | 80.6 | 28.8 | 3.5 | 96.2 | 29.8 | 10.9 | 99.2 | 27.6 | 13.2 | 92.0 | |
| 45 | 41.5 | 8.7 | 92.2 | 45.7 | 6.3 | 101.5 | 45.1 | 6.3 | 100.2 | 44.1 | 7.9 | 97.9 | |
| CCα | 35.8 μg/kg | ||||||||||||
| CCβ | 41.6 μg/kg | ||||||||||||
| DICLOXA | 15 | 16.5 | 11.8 | 109.7 | 14.8 | 11.6 | 98.9 | 15.1 | 5.8 | 100.4 | 15.4 | 10.7 | 103.0 |
| 30 | 31.8 | 12.2 | 105.9 | 30.3 | 8.5 | 100.9 | 29.9 | 7.1 | 99.6 | 30.6 | 9.5 | 102.1 | |
| 45 | 45.1 | 7.3 | 100.3 | 43.0 | 8.6 | 96.6 | 45.1 | 4.1 | 100.1 | 44.4 | 6.8 | 98.7 | |
| CCα | 35.3 μg/kg | ||||||||||||
| CCβ | 40.6 μg/kg | ||||||||||||
AMOXY = amoxicillin; AMPI = ampicillin; CLOXA = cloxacillin; DICLOXA = dicloxacillin; LC-MS/MS = liquid chromatography-tandem mass spectrometry; PEN G = penicillin G; RSD = relative standard deviation.
CCα and CCβ were determined by analyzing 20 blank samples fortified at their corresponding permitted limit. CCα was calculated as the mean measured concentration at the MRL of each compound plus 1.64 times the standard deviation (SD) of intraday precision at this concentration; CCβ was calculated as CCα plus 1.64 times the SD of intraday repeatability at CCα [29,30].
3. Results
3.1. Results by screening methods
The qualitative detection of antibiotic residues by the Delvotest SP screening test applied to 1734 raw milk samples collected over 2 years (2009–2010) led to the identification of 106 positive samples (6.11%), and 1628 negative samples (93.9%). In 2009, 52 out of 1015 samples were drug-positive (5.12%), and in 2010, 54 out of 719 samples were positive (7.51%). The total number of drug-positive samples found in the study is shown in Table 3. Enzyme-linked receptor-binding assay (SNAP) test was used to check the positive samples, thus using the new β-lactam test for the determination of antibiotic residues in positive samples—40/52 in 2009 and 54/54 in 2010 samples were found to be positive; no positive samples were detected by SNAP tetracycline test and SNAP sulfamethazine test (see Table 4).
Table 3.
Raw milk samples screened by the Delvotest SP test to detect the presence of antibiotic residues in 2009 and 2010.
| Raw milk | No. of samples | No. of positive samples, n (%) |
|---|---|---|
| 2009 | 1015 | 52 (5.12) |
| 2010 | 718 | 54 (7.51) |
| Total | 1734 | 106 (6.11) |
Table 4.
Raw milk samples screened by SNAP test in 2009 and 2010.
| Analytical test used | 2009 | 2010 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. of samples | No. of negative samples | No. of positive samples | No. of samples | No. of negative samples | No. of positive samples | |
| New SNAP beta lactam test | 52 | 0 | 40 | 54 | 0 | 54 |
| SNAP tetracycline test | 52 | 52 | 0 | 54 | 54 | 0 |
| SNAP sulfamethazine test | 52 | 52 | 0 | 54 | 54 | 0 |
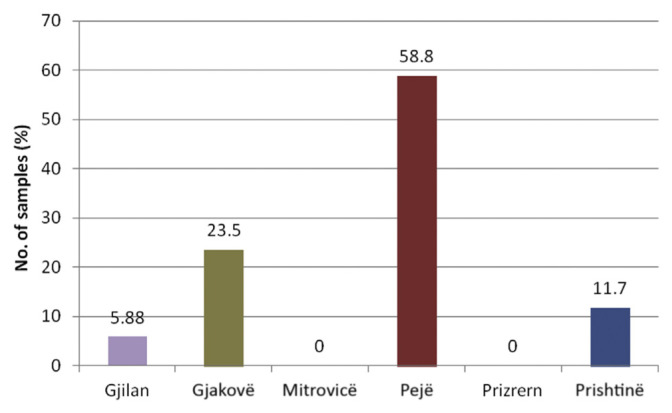
In terms of the regional distribution of milk samples containing drug residues, the highest numbers of positive milk samples were obtained from the Pejë (58.8%) and Gjakovë (23.5%) regions of Kosovo, whereas relatively low numbers of positive milk samples were from Gjilan (5.88%), and no drug-positive samples were detected in Mitrovicë or Prizren (Figure 1).
Figure 1.
Distribution of β-lactam positive milk samples in different regions of Kosovo (2009 and 2010).
3.2. Confirmation of qualitative results
Fifty-five out of 106 drug-positive samples were analyzed by LC-MS/MS, and β-lactam antimicrobial drug residues were detected in 32 samples. The concentrations of β-lactams (AMX, AMP, PNG, CXA) in these samples ranged from 2.1 μg/kg to 1973 μg/kg, as shown in Table 5. Eighteen samples out of 55 would be considered noncompliant because the concentrations of one or more β-lactam residues exceeded the CCα of 5 for AMX, 7 for PNG, 2 for CXA, 1 for AMP, 1 for both AMP and CXA, 1 for both AMP and PNG, and 1 for the combination of AMP, CXA, and PNG. Seven samples were deemed compliant with respect to the established CCα, and six additional milk samples contained only trace amounts of β-lactam residues that were not quantified because they were below the limit of quantification.
Table 5.
Confirmatory results for suspect samples analyzed by LC-MS/MS.
| Sample | AMPI (μg/kg) | AMOXY (μg/kg) | PEN G (μg/kg) | CLOXA (μg/kg) | DICLOXA (μg/kg) |
|---|---|---|---|---|---|
| 2009 | |||||
| DP021 | nd | nd | C (2.2) | nd | nd |
| BF037 | nd | nd | nd | nd | nd |
| DP071 | nd | nd | nd | nd | nd |
| BB110 | nd | nd | NC (98) | nd | nd |
| DE115 | nd | nd | <LoQ | nd | nd |
| BB120 | nd | nd | nd | nd | nd |
| EI121 | nd | nd | NC (1973) | nd | nd |
| DO125 | nd | nd | nd | nd | nd |
| GO128 | nd | nd | nd | nd | nd |
| DE138 | nd | nd | NC (5.5) | nd | nd |
| DP146 | nd | nd | nd | nd | nd |
| DE168 | nd | NC (7.2) | nd | nd | nd |
| DP175 | nd | nd | C (20) | nd | nd |
| DE185 | nd | C (2.1) | C (4.1) | nd | nd |
| DE191 | nd | NC (14) | nd | nd | nd |
| DP195 | nd | NC (7.6) | nd | nd | nd |
| DY196 | nd | nd | nd | nd | nd |
| IK196 | NC (8.9) | nd | nd | C (20) | nd |
| FV200 | nd | nd | nd | nd | nd |
| FV201 | NC (171) | nd | <LoQ | NC (439) | nd |
| PM203 | nd | nd | nd | nd | nd |
| BD206 | nd | nd | <LoQ | nd | nd |
| BL209 | <LoQ | nd | nd | nd | nd |
| DO213 | nd | nd | nd | nd | nd |
| GO214 | nd | nd | nd | nd | nd |
| 2010 | |||||
| KA216 | <LoQ | nd | nd | <LoQ | nd |
| GO236 | nd | nd | NC (24) | nd | nd |
| GO244 | nd | nd | NC (24) | nd | nd |
| KA270 | nd | nd | nd | nd | nd |
| KP291 | nd | nd | NC (6.4) | nd | nd |
| DE292 | nd | nd | nd | <LoQ | nd |
| DK293 | nd | nd | nd | nd | nd |
| IK294 | <LoQ | nd | C (5.1) | <LoQ | nd |
| EI294 | <LoQ | nd | nd | NC (42) | nd |
| DY296 | nd | nd | nd | nd | nd |
| DP297 | nd | NC (15) | nd | nd | nd |
| BD298 | nd | nd | nd | nd | nd |
| DO299 | nd | nd | nd | NC (49) | nd |
| BL317 | nd | nd | nd | nd | nd |
| DE318 | nd | C (4.6) | nd | nd | nd |
| DE319 | nd | nd | nd | nd | nd |
| FR320 | NC (784) | nd | NC (156) | NC (542) | nd |
| KP379 | nd | nd | NC (6.9) | nd | nd |
| GA390 | nd | nd | nd | <LoQ | nd |
| DO391 | nd | nd | nd | nd | nd |
| DP392 | nd | nd | nd | nd | nd |
| PM393 | nd | nd | nd | <LoQ | nd |
| FV394 | nd | nd | nd | nd | nd |
| GO426 | nd | nd | nd | nd | nd |
| DP461 | C (2.5) | nd | nd | C (32.4) | nd |
| DP514 | nd | NC (43) | nd | nd | nd |
| GO554 | nd | nd | nd | nd | nd |
| DE587 | NC (7.0) | nd | C (15) | nd | nd |
| KP661 | nd | nd | C (5.1) | ND | nd |
| DK671 | nd | nd | nd | nd | nd |
AMOXY = amoxicillin; AMPI = ampicillin; C = compliant (sample containing a concentration of β-lactam residues lower or equal to CCα); CLOXA = cloxacillin; DICLOXA = dicloxacillin; LC-MS/MS = liquid chromatography-tandem mass spectrometry; NC = not compliant (sample containing a concentration of β-lactam residues higher than CCα); LoQ = limit of quantification (corresponding to the 1st spiking level used in validation of the LC-MS/MS method: 2 μg/kg for AMP, AMX, PNG; 15 μg/kg for CXA and DCX); nd = not detected; PEN G = penicillin G; RSD = relative standard deviation.
4. Discussion
This study confirms that penicillins are the main group of antibiotics detected in milk samples, and these findings are in agreement with the observations reported by several other investigative teams [28,31–38]. This is likely to be a reflection of the frequent use of chemotherapeutic drugs in the therapy and prevention of specific diseases in dairy cattle and the use of intra-mammary infusions containing β-lactams for the treatment of mastitis. A report by Chung et al [23] identified 21 antibiotic contaminated samples out of 269 analyzed milk samples, representing 7.8% of the total. Khaskheli et al [39] analyzed 137 milk samples and, using the qualitative microbial method with Bacillus subtilis in plates for detection of βlactam residues, identified 87 samples (63.5%) as negative and 50 samples (36.5%) as positive. In Romania, a survey was conducted [40], in which 124 milk samples (4.45%) out of a total of 2785 were found to be contaminated with antibiotic residues, with 2531 samples (90.88%) free of antibiotic residues. Nikolić et al [41] analyzed 6161 raw milk samples in Montenegro, of which 7.84% of the samples were drug-positive, and in Croatia [42], a very low percentage (0.69% [42]) or no milk samples [43,44] containing antibiotics were detected above the maximum residue levels (MRLs) established by European Union and Croatian legislation. Similar results were obtained in raw milk samples from Slovenia [45]. By contrast, tetracyclines (48.9%), sulfonamides (18.4%), and quinolones (6.8%) were found in milk samples from Macedonia, although drug residues were below the maximum residue limits [46].
5. Conclusion
The present investigation is the first performed in Kosovo to evaluate the presence of antibiotic residues in foodstuffs, and in particular, milk and dairy products. Our results indicate that β-lactams are the main class of antimicrobial drugs detected in milk intended for human consumption in Kosovo. The considerable levels of residues detected in raw milk, although regionally limited, are a human health concern that prompts a number of recommendations addressed to public authorities, veterinarians, livestock producers, and consumers. In addition to implementing appropriate regulatory legislation and providing an adequately controlled sampling network, we should be able to provide effective means for food control with appropriate risk assessments that will instill confidence in consumers. Competent authorities should establish and maintain continuous dairy monitoring programs to ensure risk-free milk products to Kosovo consumers. In addition, there is a pressing need for additional research to accurately assess other aspects of this problem and identify effective corrective actions that are designed to reduce milk contaminants.
Acknowledgments
The project was partially funded with grants from Padova University (Fund PVS 2008 and 2009). The authors thank also the Kosovo Food and Veterinary Agency for their local support.
Funding Statement
The project was partially funded with grants from Padova University (Fund PVS 2008 and 2009).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Tollefson L, Miller MA. Antibiotic use in food animals: controlling the human health impact. J AOAC Int. 2000;83:245–56. [PubMed] [Google Scholar]
- 2. Lee HJ, Lee MH, Ruy PD. Public health risks: chemical and antibiotic residues. Asian-Aust J Anim Sci. 2001;14:402–13. [Google Scholar]
- 3. Paige JC, Kent R. Tissue residue briefs. FDA Vet. 1987;11:10–1. [Google Scholar]
- 4. Van Dresser WR, Wilcke JR. Drug residues in food animals. J Am Vet Med Assoc. 1989;194:1700–10. [PubMed] [Google Scholar]
- 5. Guest GB, Paige JC. The magnitude of the tissue residue problem with regard to consumer needs. J Am Vet Med Assoc. 1991;198:805–8. [PubMed] [Google Scholar]
- 6. Paige JC. Analysis of tissue residues. FDA Vet. 1994;9:4–6. [Google Scholar]
- 7. Wicher K, Reisman RE, Arbesman CE. Allergic reaction to penicillin present in milk. J Am Med Assoc. 1969;208:143–5. [PubMed] [Google Scholar]
- 8. Lindemayr H, Knobler R, Kraft D. Challenge of penicillin-allergic volunteers with penicillin-contaminated meat. Allergy. 1981;36:471–8. doi: 10.1111/j.1398-9995.1981.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Joint FAO/WHO Expert Committee on Food Additives (JECFA) Toxicological evaluation of certain veterinary drug residues in food: Monograph prepared by 93 the Thirty-sixth meeting of the Joint FAO/WHO Expert Committee on Food Additives. 1991. (WHO Technical Report Series 1991). No 815. [Google Scholar]
- 10. Kanny G, Puygrenier J, Beaudoin E, Moneret-Vautrin DA. Alimentary anaphylactic shock: implication of penicillin residues. Allerg Immunol (Paris) 1994;26:181–3. [PubMed] [Google Scholar]
- 11. Sundlof SF. Human risks associated with drug residues in animal derived foods. J Agromed. 1994;1:5–22. [Google Scholar]
- 12.Riviere JE, Spoo JW. Tetracycline antibiotics. In: Adams RH, editor. Veterinary pharmacology and therapeutic. 7th ed. Ames, IA: Iowa State University Press; 1995. pp. 784–96. [Google Scholar]
- 13. Molina A, Molina MP, Althaus RL, Gallego L. Residue persistence in sheep milk following antibiotic therapy. Vet J. 2003;165:84–9. doi: 10.1016/s1090-0233(02)00173-9. [DOI] [PubMed] [Google Scholar]
- 14. Payne MA, Craigmill A, Riviere JE, Webb AI. Extralabel use of penicillin in food animals. J Am Vet Med Assoc. 2006;229:1401–3. doi: 10.2460/javma.229.9.1401. [DOI] [PubMed] [Google Scholar]
- 15. Choma I, Grenda D, Malinowska I, Suprynowics Z. Determination of flumequine and doxycycline in milk by a simple thin-layer chromatographic method. J Chromatogr B. 1999;734:7–14. doi: 10.1016/s0378-4347(99)00328-x. [DOI] [PubMed] [Google Scholar]
- 16. Schenck FJ, Callery PS. Chromatographic methods of analysis of antibiotics in milk. J Chromatogr A. 1998;812:99–109. doi: 10.1016/s0021-9673(97)01291-0. [DOI] [PubMed] [Google Scholar]
- 17. Ramirez A, Gutiérrez R, González C, Escobar I, Castro G, Díaz G, Noa M. High-performance thin-layer chromatography bioautography for multiple antibiotic residues in cow’s milk. J Chromatogr B. 2003;784:315–22. doi: 10.1016/s1570-0232(02)00819-x. [DOI] [PubMed] [Google Scholar]
- 18. Council Directive 96/23/EC of the European Parliament and of the Council of 29 April 1996. Off J Eur Commun OJ L. 1996;125:10. [Google Scholar]
- 19.Commission Decision of 27 October 1997 fixing the levels and frequencies of sampling provided for by Council Directive 96/23/EC for the monitoring of certain substances and residues thereof in certain animal products (Text with EEA relevance) 1997;(97/747/EC).
- 20. Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82 /EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. J Eur Commun. 2009;152:11. [Google Scholar]
- 21.Commission Regulation (EU) no 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. J Eur Commun 2010;no L151
- 22. De Zayas-Blanco F, Garcia-Falcon MS, Simal-Gandara J. Determination of sulfamethazine in milk by solid phase extraction and liquid chromatographic separation with ultraviolet detection. Food Control. 2004;15:375–8. [Google Scholar]
- 23. Lee KG, Chung HH, Lee JB, Chung YH. Analysis of sulfonamide and quinolone antibiotic residues in Korean milk using microbial assays and high performance liquid chromatography. Food Chem. 2009;113:297–301. [Google Scholar]
- 24. Zhang HY, Wang SO. Review on enzyme-linked immunosorbent assays for sulfonamide residues in edible animal products. J Immunol Methods. 2009;350:1–13. doi: 10.1016/j.jim.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Petrova M. Final Report. Kosovo: USAID; 2006. Improving dairy plant operations in Kosovo. [Google Scholar]
- 26. Ministry of Agriculture Forestry, and Rural Development(MAFRD), Republic of Kosovo, DEAAS-MAFRD. Annual Report. 2011 [Google Scholar]
- 27. Suhren G, Beukers R. Delvotest SP for detection of cloxacillin and sulfamethoxazole in milk: IDF interlaboratory study. International Federation Dairy. J AOAC Int. 1998;81:978–90. [PubMed] [Google Scholar]
- 28. Holstage DM, Punchner B, Whitehead G, Galey FD. Screening and mass spectral confirmation of β-lactam antibiotic residues in milk using LC-MS/MS. J Agric Food Chem. 2002;16:406–11. doi: 10.1021/jf010994s. [DOI] [PubMed] [Google Scholar]
- 29. Commission Decision (EC) No. 657/02. Off J Eur Communities Legis. 2002;L221:8. [Google Scholar]
- 30. Verdon E, Hurtaud-Pessel D, Sanders P. Evaluation of the limit of performance of an analytical method based on a statistical calculation of its critical concentrations according to ISO Standard 11843 – Application to Routine Control of Banned Veterinary Drug residues in food According to European Decision. Accred Qual Assur. 2006;11:58–62. [Google Scholar]
- 31. Allison JRD. Antibiotic residues in milk. Br Vet J. 1985;141:9–16. doi: 10.1016/0007-1935(85)90121-6. [DOI] [PubMed] [Google Scholar]
- 32. Booth JM, Harding F. Testing for antibiotic residues in milk. Vet Rec. 1986;119:565–9. [PubMed] [Google Scholar]
- 33. McEwen SA, Meek AH, Black WD. A dairy farm survey of antibiotic treatment practices, residue control methods and associations with inhibitors in milk. J Food Prot. 1991;54:454–9. doi: 10.4315/0362-028X-54.6.454. [DOI] [PubMed] [Google Scholar]
- 34. Adesiyun AA, Webb LA. Prevalence of antimicrobial residues in preprocessed and processed cows milk in Trinidad. J Food Saf. 1997;16:301–10. [Google Scholar]
- 35. Mitchell JM, Griffiths MW, McEwen SA, McNab WB, Yee AJ. Antimicrobial drug residues in milk and meat: causes, concerns, prevalence, regulations, tests, and test performance. J Food Prot. 1998;61:742–56. doi: 10.4315/0362-028x-61.6.742. [DOI] [PubMed] [Google Scholar]
- 36. Ghidini SM, Zanardi E, Varisco G, Chizzolini R. Prevalence of molecules of β-lactam antibiotics in bovine milk in Lombardia and Emilia Romagna (Italy) Ann Fac Medi Vet Parma. 2002;22:245–52. [Google Scholar]
- 37. Ramírez A, Gutiérrez R, González C, Escobar I, Castro G, Díaz G, Noa M. Detección de antibióticos en leche comercializada en la ciudad de México. Rev Salud Anim. 2001;23:37–41. [in Spanish] [Google Scholar]
- 38. Allara M, Izquierdo P, Torres G, Rodríguez BB. Penicillin G in pasteurized milk produced in Zulia State-Venezuela. Rev Cient Fac Cienc Vet. 2002;12:683–7. [Google Scholar]
- 39. Khaskheli M, Malik RS, Arain MA, Soomroand AH, Arain HH. Detection of beta-lactam antibiotic residues in market milk. Pak J Nutr. 2008;7:682–5. [Google Scholar]
- 40. Grădinaru AC, Popescu O, Solcan G. Antibiotic residues in milk from Moldavia, Romania. Int J Bioflux Soc. 2011;3 [Google Scholar]
- 41. Nikolić N, Mirecki S, Blagojević M. Presence of inhibitory substances in raw milk in the area of Montenegro. Mljekarstvo. 2011;61:182–7. [Google Scholar]
- 42. Bilandzic N, Kolanovic BS, Varenina I, Scortichini G, Annunziata L, Brstilo M, Rudan N. Veterinary drug residues determination in raw milk in Croatia. Food Control. 2011;22:1941–8. [Google Scholar]
- 43. Bilandzic N, Kolanovic BS, Varenina I, Jurkovic Z. Concentrations of veterinary drug residues in milk from individual farms in Croatia. Mljekarstvo. 2011;61:260–7. [Google Scholar]
- 44. Vragovic N, Bažulic DN, Zdolec N. Dietary exposure assessment of β-lactam antibiotic residues in milk on Croatian market. Croat J Food Sci Technol. 2012;4:81–4. [Google Scholar]
- 45. Gacnik SK, Kirbis A, Cerkvenik V. Residues of veterinary drugs in Slovenia milk 1995–1998. Mljekarstvo. 2000;50:191–8. [Google Scholar]
- 46. Dimitrieska-Stojkovic E, Hajrulai-Musliu Z, Stojanovska-Dimzoska B, Sekulovski P, Uzunov R. Screening of veterinary drug residues in milk from individual farms in Macedonia. Mac Vet Rev. 2001;34:5–13. [Google Scholar]