Abstract
An investigation was carried out to extract polyphenols from the peel of kinnow (Citrus reticulate L.) by maceration and ultrasound-assisted extraction (UAE) techniques. The antioxidant potential of these polyphenols was evaluated using ferric reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and superoxide radical scavenging assays; and their antimicrobial activity was assessed against bacterial strains Staphyloccoccus aureus, Bacillus cereus, and Salmonella typhimurium. The highest extraction yield was obtained through the solvent ethanol at 80% concentration level, whereas UAE was a more efficient technique and yielded comparatively higher polyphenol contents than maceration. Maximum polyphenols were extracted with 80% methanol [32.48 mg gallic acid equivalent (GAE)/g extract] using UAE, whereas minimum phenolics (8.64 mg GAE/g extract) were obtained with 80% ethyl acetate through the maceration technique. Elevated antioxidant activity of kinnow peel extracts was exhibited in three antioxidant assays, where 80% methanolic extracts showed the highest antioxidant activity (27.67 ± 1.11mM/100 g for FRAP) and the highest scavenging activity, 72.83 ± 0.65% and 64.80 ± 0.91% for DPPH and superoxide anion radical assays, respectively. Strong correlations between total polyphenols and antioxidant activity were recorded. Eleven phenolic compounds—including five phenolic acids and six flavonoids—were identified and quantified by high performance liquid chromatography. Ferulic acid and hesperidin were the most abundant compounds whereas caffeic acid was the least abundant phenolic compound in kinnow peel extracts. Maximum inhibition zone was recorded against S. aureus (16.00 ± 0.58 mm) whereas minimum inhibition zone was noted against S. typhimurium (9.00 ± 1.16 mm). It was concluded that kinnow mandarin peels, being a potential source of phenolic compounds with antioxidant and antimicrobial properties, may be used as an ingredient for the preparation of functional foods.
Keywords: antimicrobial activity, antioxidant capacity, kinnow peel, maceration, phenolic compounds, ultrasound
1. Introduction
Polyphenols are natural antioxidants in plants, especially in fruits and vegetables, which have a vital role in human health because of their free radical scavenging activity, antioxidant enzyme cofactors, as well as chelation of pro-oxidant metal ions in the body [1,2]. Epidemiological studies have reported a positive correlation between fruit and vegetable intake and a decrease in the rate of cardiovascular disease, aging, certain cancers, and other degenerative diseases related to oxidative stress, which is attributed to the antioxidant activity of phenolic compounds in fruits and vegetables [3,4].
During the industrial processing of fruits, large quantities of agroindustrial wastes such as peels, seeds, stones, and other residues are produced. The fruit processing wastes contain valuable nutrients and biomass, which may be converted into value-added by-product fruit wastes. In particular, peels have a comparatively higher concentration of phenolic compounds and thus have more antioxidant potential than fruit pulps [5–7].
Citrus is one of the major fruit crops of Pakistan, and comprises kinnow, orange, grapefruit, lemon, lime, sweet orange, etc. The annual production of citrus is 2.33 million tons, of which about 90% is kinnow mandarin [8]. Kinnow mandarin (Citrus reticulate L.) peel is about 35–40% of the fruit weight and is the major waste component after processing. High disposal costs of waste have prompted researchers to explore the potential benefits of wastes as well as minimize their environmental hazards [9]. Currently, only a fraction of total peel residue mass is being utilized as beverage bases, marmalades, and candied peel. However, citrus peel is the richest source of bioactive phenolic compounds, especially flavonoids, with comparatively higher polyphenol content compared with the edible parts. The flavonoids present in citrusconsist of flavones, isoflavones, flavonones, flavonols, and anthocyanidins [10]. The beneficial effects of citrus peel against certain degenerative diseases (e.g., coronary heart disease) as an antiinflammatory and anticarcinogenic agent have been observed [11].
Solvent extraction is generally used for the preparation of plant material extracts because of its wide applicability, efficiency, and ease of use. Most common organic solvents used for the extraction of phenolic compounds include methanol, ethanol, acetone, and ethyl acetate [12]. Conventional solid–liquid extraction techniques such as maceration are mostly used for obtaining bioactive compound extracts from plant material [13]. However, conventional solvent extraction processes have certain limitations such as high extraction temperature, lower efficiency, low extraction yield, use of large quantity of solvents, mass transfer resistance, and health hazards [14,15]. Ultrasound-assisted extraction (UAE) of polyphenols is a nonconventional technique that involves mixing the sample with organic solvent in a flask or beaker and placing it in an ultrasonic bath with preset time and temperature. Sound waves, which are produced during the process, generate cavitation and rupture sample cell walls, leading to extraction of phenolic compounds from the sample to the solvent medium [16]. Generally, the UAE process duration is less than 1 hour, but the extraction yield is 6% to 35% higher than that obtained using traditional extraction techniques with longer extraction time of 12 or more hours [7,17]. During a study on orange peel polyphenols, Khan et al [18] compared the conventional solvent extraction process with UAE using the 80% ethanol solvent. Significantly high extraction yield and polyphenols flavanone concentration at an ultrasound frequency 25 of kHz and 15 minutes of treatment time was observed as compared to conventional extraction (40°C for 60 minutes). Similarly, Pan et al [19] studied pomegranate peel polyphenols and reported that for the extraction of bioactive compounds, 20 to 100 kHz ultrasonic radiations was effective and could be efficiently used because of high reproducibility, low energy and solvent consumption, and the low temperature used, and thus lower the loss of phenolic compounds.
Antioxidant activity determination of polyphenols in vitro is generally carried out using various assays such as ferric reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH), superoxide radical scavenging assay, trolox equivalent antioxidant capacity, and oxygen radical absorbance capacity. However, DPPH radical scavenging assay is the most popular and widely used technique to evaluate antioxidant capacity [20,21].
Plants generate a variety of secondary metabolites as part of their defense system during growth. These secondary metabolites or phytochemicals have strong inhibitory activity against microorganisms such as bacteria and fungi [22]. Bacteria as well as fungal infections pose a big threat to mankind, and indiscriminate use of antimicrobial drugs has caused resistance in microbes. Because they have the least antibiotic-related side effects and better activity against drug-resistant strains, researchers have focused their attention toward phytochemicals [23]. Phytochemicals abundant in plants include phenolic acids, flavonoids, tannins, and alkaloids. The antimicrobial characteristics of certain polyphenol classes have been investigated to develop novel therapies for the treatment of different microbial infections [24,25]. Agro-industrial wastes were studied for their potential antimicrobial activity by different researchers such as lemon peels [26], pomegranate peels [27], grape marcs [28], and grape seeds [29].
Keeping in view the abovementioned facts, a research study was designed to optimize extraction conditions for polyphenols from kinnow peels, determination of antioxidant and antimicrobial activity of phenolic compounds in kinnow peels.
2. Materials and methods
2.1. Plant material
Kinnow mandarin (C. reticulate L.) were procured from a fruit market in Islamabad and taken to the Food Science and Product Development Institute research laboratory, National Agricultural Research Center. Fruits were thoroughly washed under tap water to remove dirt, dust, microflora, and pesticide residue on the surface. Peeling of kinnow mandarin was carried out; the, the peels were further cut into small pieces using a stainless steel knife and oven-dried at 50°C for 48 hours in hot air oven until moisture content fell below 10%. Dried peels were ground to fine powder through the cyclotec sample mill with sieve size 0.5 mm. Kinnow mandarin peel powder was packed in airtight polyethylene zip bags and stored at refrigeration temperature.
2.2. Proximate analysis of peel powder
Kinnow peel powder was analyzed for moisture, ash, crude protein, crude protein, crude fat, and crude fiber according to the standard methods of the Association of Analytical Communities [30]. Available carbohydrate in peel powder was estimated by difference [100 − (% moisture + % ash + % crude protein + % crude fat + % crude fiber)].
2.3. Extraction of polyphenols
Maceration and UAE procedures were used for polyphenol extraction from kinnow mandarin peel powders.
2.4. Maceration
Kinnow mandarin peel powders were subjected to extraction through the maceration technique as described by Elfalleh et al [31] with slight modifications. Preliminary studies were performed to evaluate an optimal sample/solvent ratio (1:10, 1:15, 1:20) and extraction temperature (30°C and 40°C). After the preliminary studies, extraction was carried out using different solvents—ethanol, methanol, acetone, and ethyl acetate—at three solvent concentrations (50%, 80%, 100%) with a 1:15 sample/solvent ratio and extraction temperature of 40°C. Briefly, 5-g kinnow peel powder samples were extracted by specific solvent, concentration level, extraction temperature, and sample/solvent ratio in a shaking water bath (Tecator 1024; Tecator AB, Höganäs, Sweden) for 20 hours. The extracts were filtered through Whatman filter paper 1 and centrifuged (Beckman J2-21; Beckman Coulter, Fullerton, CA, USA) at 5000 rpm for 10 minutes. The supernatant was collected, and the solvent was evaporated with a rotary evaporator (BUCHI Rotavapor, Flawil, Switzerland) under vacuum at 45°C to obtain the extract, which was further filtered through 0.45-μm membrane filter, then collected in amber glass bottles and stored at refrigeration temperature.
2.5. Ultrasound-assisted extraction
The extraction of polyphenols from kinnow mandarin peel powder was conducted using the UAE technique as described by Bimakr et al [32] with slight modifications. Preliminary studies were carried out to determine the optimal sample/solvent ratio (1:10, 1:15, 1:20), extraction temperature (35°C, 45°C, 55°C), and extraction time (40 minutes, 50 minutes, 60 minutes, and 70 minutes). After the preliminary studies, 5-g kinnow peel powder samples were extracted by solvents ethanol and methanol at 50%, 80%, 100% concentration levels under optimal extraction conditions: sample/solvent ratio, 1:20; extraction temperature, 45°C; extraction time, 60 minutes in a sonicator (Transsonic 700; Elma, Wetzikon, Switzerland) at 35 kHz frequency. The extracts were subjected to filtration, centrifugation, solvent vacuum evaporation, microfiltration, collection in amber glass bottles, and refrigeration (storage) in a similar manner with maceration extracts.
2.6. Yield (%) of peel extracts
The percent yield of kinnow peel extracts through maceration and UAE was assessed by dividing the weight of the extract with the sample weight and multiplying by 100.
2.7. Total polyphenols determination
The total polyphenol content of kinnow mandarin peel extracts was measured using the Folin–Ciocalteau method as described by Singleton et al [33]. Methanolic solutions of kinnow peel extracts (10 mg/mL) were prepared for the analysis. Briefly, 0.5 mL methanolic extract solution was mixed with 2.5 mL of 10% Folin–Ciocalteu reagent dissolved in distilled water and 2.5 mL 7.5% sodium carbonate. The blank contained 0.5 mL methanol, 2.5 mL Folin–Ciocalteu reagent (10 times diluted), and 2.5 mL of 7.5% sodium carbonate. Then the samples were incubated at 25°C for 30 minutes for the development of a blue color. The absorbance was measured at 765 nm with a UV–VIS Spectrophotometer (Agilent 8453; Santa Clara, California, USA). A similar procedure was carried out for gallic acid standard solution, and the calibration curve was prepared from various concentrations of gallic acid. The total polyphenol content was expressed as mg gallic acid equivalent (GAE)/g extract.
2.8. Antioxidant activity evaluation
2.8.1. FRAP assay
The FRAP assay was carried using the procedure described by Benzie and Strains [34] with several modifications. The FRAP reagent was prepared by mixing 25 mL of 0.3M acetate buffer (pH 3.6) with 2.5 mL 2,4,6-Tripyridyl-s-Triazine (TPTZ) solution (0.01M) and 2.5 mL of FeCl3·6H2O (0.02M). A 200-μL diluted sample was added to 1.5 mL FRAP reagent and warmed at 37°C for 10 minutes. The absorbance was measured at 593 nm, and the antioxidant activity of the sample was expressed as millimoles per 100 g of extract.
2.8.2. DPPH radical scavenging assay
The antioxidant activity of kinnow mandarin peel extracts was measured using the DPPH (1,1-diphenyl-2-picryl-hydra-zyl) assay according to the method described by Brand-William et al [35] with slight modifications. Briefly, 24 mg DPPH was dissolved in 100 mL methanol to prepare a stock solution. The working standards were prepared by diluting DPPH stock solution with methanol to obtain about 0.98 (±0.02) absorbance at 517 nm. Then, 3 mL of the solution was mixed with 100 μL of samples at different concentrations (25–400 μg/mL), shaken well, incubated in the dark at room temperature for 15 minutes, and absorbance was measured at 517 nm. A parallel control (without extract) and standard ascorbic acid were also analyzed in a similar manner. The scavenging activity was calculated based on the DPPH radical percentage scavenged.
where Ac is the absorbance of control and As is the absorbance of the sample.
2.8.3. Superoxide radical scavenging power assay
The antioxidant activity of kinnow peel extracts was determined by superoxide radical scavenging assay in accordance with the procedure described by Vaidya et al [36]. Initially, 1 mL kinnow peel extract at different concentrations (25–400 μg/mL) was added to 1 mL sodium carbonate (5%), 0.3 mL EDTA (0.5%), and 0.4 mL nitroblue tetrazolium (150 μm). After mixing all the reagents, absorbance was measured immediately at 560 nm. The reaction was initiated by the addition of 0.4 mL hydroxlylamine hydrochloride and incubated at 25°C for 5 minutes. The nitroblue tetrazolium (NBT) reduction was determined with a spectrophotometer at 560 nm. A parallel control (without extract) and standard ascorbic acid were also analyzed in a similar manner. The % scavenging activity was calculated as follows:
where A1 is the absorbance of extract sample and A0 is the absorbance of control.
2.9. High performance liquid chromatography analysis of phenolic compounds
Identification and quantification of phenolic acids and flavonoids in the extracts were determined with high performance liquid chromatography (HPLC) according to the method described by Salvador et al [37] with slight modifications. Kinnow mandarin peel extract samples filtered through a 0.45-μm membrane filter were injected into the HPLC system, which consisted of a Perkin Elmer HPLC equipped with Binary LC pump 250, an LC 295 UV/VIS detector, and a reversed phase C18-WP.100 column (CNW Technologies, Dusseldorf, Germany) with internal dimensions of 4.6 mm × 250 mm, 5 μm. The mobile phase consisted of a linear gradient with a combination of solvent A (acetonitrile) and solvent B (distilled water/acetic acid, 99:1, v/v, pH 2.30 ± 0.1). The following gradient program was used for the separation of flavonoids and phenolic acids: 20% A (5 min), 80% A (10 min), 20% A (5 min). The analyses were conducted at a flow rate of 1 mL/min with the UV detector set at 280 nm for phenolic acids and 370 nm for flavonoids and a sample injection volume of 20 μL. The analytes were identified by comparing the retention times and spike samples with polyphenol standards (gallic acid ≥99%, coumaric acid ≥98%, chlorogenic acid ≥95%, caffeic ≥98%, ferulic acid ≥99%, catechin ≥98%, epicatechin ≥98%, hesperdin ≥97%, naringenin ≥95%, quercetin ≥95%, kaempferol ≥90%; Sigma Aldrich, Saint-Quentin-Fallavier, France) and subsequent quantification of phenolic compounds were determined.
2.10. Antimicrobial activity determination
The antimicrobial activity of kinnow mandarin peel extracts was determined using the disk diffusion method [38,39]. Two Gram-positive bacterial strains (Staphylococcus aureus ATCC 25923 and Bacillus cereus ATCC 10876) and one Gram-negative strain (Salmonella typhimurium ATCC 14028) were selected for the study of the antimicrobial activity of kinnow peel extracts.
For the preparation of media, nutrient agar was dissolved in distilled water and the pH was adjusted to 7. Sterilization of media was carried out in the autoclave at 121°C for 15 minutes, and the media were cooled at room temperature after sterilization. The petri plates without any contamination were selected for further investigations. Sterile paper disks (6 mm diameter) were placed on the agar medium. Then, 10 μL extract sample of various concentrations (25 mg/mL, 50 mg/mL, 100 mg/mL, 200 mg/mL extract) was applied on sterile disks and allowed to dry. For comparison, the antibiotic chloramphenicol was used as a standard. Bacterial cultures were injected to sterilize petri plates. The petri plates were incubated at 37°C for 24 hours, after which the zones of growth inhibition (mm) around the disks were measured.
2.11. Statistical analysis
Data were statistically analyzed using analysis of variance to determine the significance level. The least square design (LSD) test was used to calculate the least significant difference among means. Minitab software was used for conducting statistical analysis of data.
3. Results and discussion
3.1. Extraction yield
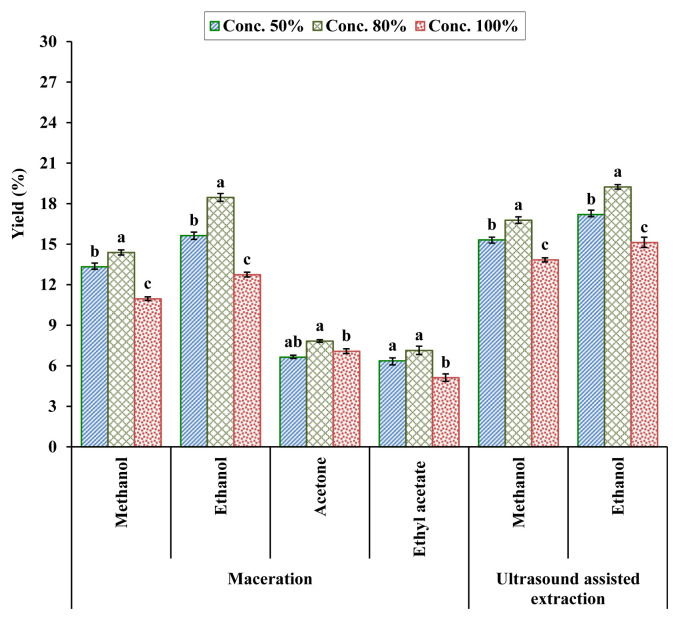
The percent yield of kinnow mandarin peel extracts through UAE and maceration techniques at different solvent concentration levels reveals that the highest extraction yield was obtained with solvent ethanol either through maceration or UAE (Figure 1). In the case of the maceration technique, extraction with 80% ethanol resulted in the highest yield (18.46%) followed by 50% ethanol extraction (15.64%), whereas the lowest extraction yield (5.12%) was recorded in samples extracted with 100% ethyl acetate. As regards UAE, extraction with 80% ethanol led to a comparatively higher yield (19.24%) than samples extracted with solvent methanol. Extraction with 100% methanol solvent concentration resulted in the lowest extraction yield (13.84%). Statistically, solvent concentration levels were significantly different from each other for solvent methanol and ethanol used in the UAE method, whereas there were nonsignificant difference between concentration levels 50% and 80% for solvent ethyl acetate as well as 80% and 100% for acetone in the maceration technique. However, the solvent concentration level of 80% was more effective than 50% or 100% solvent concentration of all solvents used during maceration and UAE. Overall, UAE had a comparatively higher extraction yield at all solvent concentration levels compared with the maceration technique. Variations in extraction yield among the various solvents used may be attributable to the different polarities of solvents. Sultana et al [40] investigated various agro wastes and observed a polyphenol extraction yield of 21.5% from citrus peels with 80% methanol solvent. Similarly, Hegazy and Ibrahim [41] reported the orange peel extract yield within the range of 8.27% from solvent hexane to 28.32% from methanol. The yield of phenolic compounds from plants is associated with the polarity, solubility, as well as certain extraction parameters such as nature of solvent, solvent concentration, extraction temperature, and time [42,43].
Figure 1.
Yield (%) of kinnow mandarin peel extracts by maceration and ultrasound-assisted extraction. Values are presented as mean ± standard error of triplicate analyses. Same alphabetical letters denote nonsignificant difference at p < 0.05.
3.2. Total polyphenols content
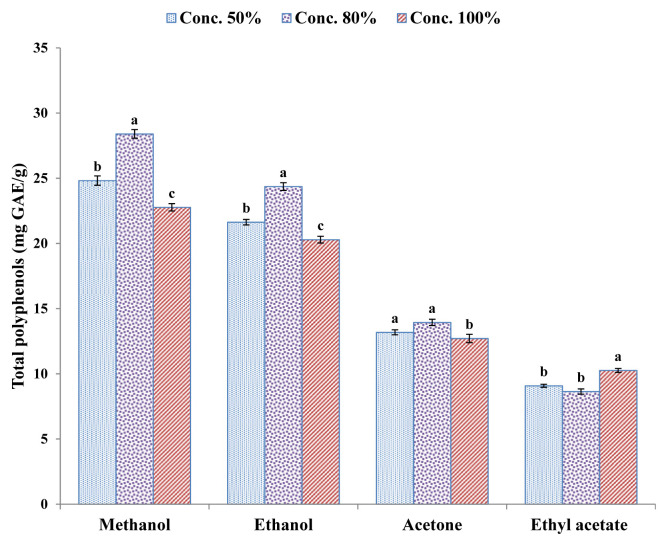
Maceration and UAE methods were applied for the extraction of polyphenols, which were then determined using the Folin–Ciocalteau reagent assay. The total polyphenol content of kinnow mandarin peel extracts shows that maceration (Figure 2) was comparatively less efficient than the UAE (Figure 3) technique, which yielded higher polyphenol content. As regards maceration extraction, methanol was the most effective solvent followed by ethanol, whereas ethyl acetate had the lowest polyphenol extraction rate. The highest total polyphenol contents (28.40 ± 0.33 mg GAE/g extract) were extracted with solvent methanol at 80% concentration level, whereas lowest polyphenol contents (8.64 ± 0.20 mg GAE/g extract) were obtained with 80% ethyl acetate. Results of the LSD test reveal that solvent concentration levels were significantly different from each other for methanol and ethanol used, whereas there were nonsignificant differences between concentration levels 50% and 80% for solvent acetone and ethyl acetate when the maceration technique was used. Al-Juhaimi [44] extracted polyphenols through the maceration technique from the peel and pulp of mandarin, lemon, and orange using 80% ethanol as solvent at 70°C for 3 hours, and phenolic compounds of extracts were evaluated with the Folin–Ciocalteau reagent assay. They observed that mandarin, orange, and lemon peels contained 169.54 mg GAE/100 g, 178.90 mg GAE/100 g, and 61.22 mg GAE/100 g total phenolics, which were higher than the amount of phenolic compounds extracted from pulp.
Figure 2.
Total polyphenol content (mg GAE/g extract) of kinnow peel extracts by maceration technique. Values are presented as mean ± standard error of triplicate analyses. Data with different letters denote significant difference at p < 0.05. GAE =gallic acid equivalent.
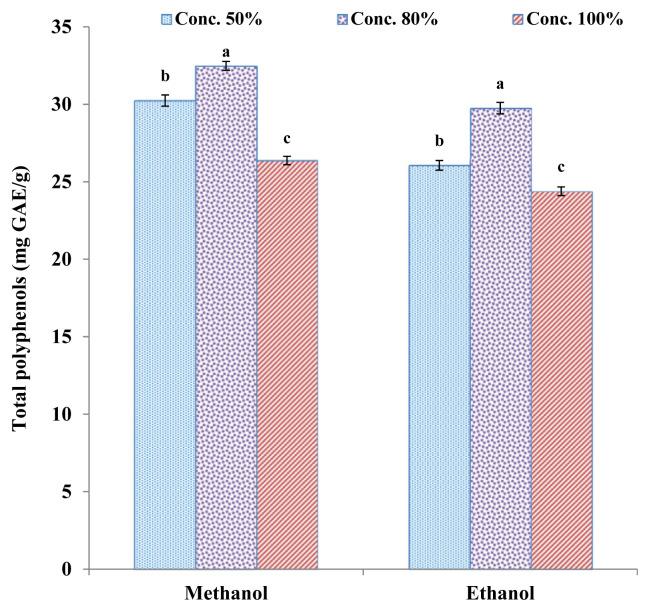
Figure 3.
Total polyphenol content (mg GAE/g extract) of kinnow peel extracts by ultrasound assisted extraction. Values are presented as mean ± standard error of triplicate analyses. Different alphabetical letters denote significant difference at p < 0.05. GAE = gallic acid equivalent.
In the case of UAE, maximum polyphenols were extracted with 80% methanol (32.48 ± 0.36 mg GAE/g extract) whereas 100% ethanolic extracts had minimum phenolics (24.39 ± 0.28 mg GAE/g extract). The LSD test result reveals that solvent concentration levels had a significant effect on phenolic extraction and were significantly different from each other at all concentration levels of both solvents. Because of the ultrasonic cavitation phenomenon of UAE, the cavitation generates currents in the solvent, which in turn increases the mass transfer rate between the sample material and the solvent medium [16], causing mechanical effects on samples cell walls that result in cell disruption and particle breakdown [45]. The advantage of UAE over the maceration technique is the comparatively higher extraction of polyphenols in a shorter time, thus saving energy inputs [46]. Furthermore, phenolic compounds being thermosensitive remained stable during UAE as compared to soxhlet and other conventional techniques where elevated temperature are used [47]. The total polyphenol content of kinnow peel extracts obtained by UAE was 14.37% higher than the polyphenols extracted through the maceration technique at 80% methanol solvent concentration. These results are in agreement with the findings of Petigny et al [48], who used maceration and UAE methods for the extraction of polyphenols from boldo leaves and observed 20% more polyphenolic content extracted through UAE compared with maceration. Likewise, UAE and maceration techniques were compared by Quiroz-Reyes et al [49] for the extraction of phenolic compounds from cocoa beans and reported 50% higher polyphenol content extracted by UAE compared with maceration.
Extraction of polyphenols also depends on the type of solvent used. Chan et al [50] compared various solvents such as ethanol, acetone, and methanol for extraction of polyphenols from limau purut (Citrus hystrix) peel and concluded that aqueous acetone was slightly were more efficient than aqueous ethanol and aqueous methanol water with the following extraction conditions: 60% solvent; temperature, 25°C; extraction time, 3 hours. Similarly, the efficiency of various solvents such as ethanol, methanol, acetone, dichloromethane, ethyl acetate, and hexane were assessed for the extraction of polyphenols from orange peel [41]. It was observed that there was variation in total polyphenol content among different solvent extracts with ethanolic extract having the highest total polyphenols (169.56 mg/g) whereas hexane extract contained the lowest total polyphenol content (63.20 mg/g). The total polyphenols of each solvent at absolute concentration level were lowest for both extraction methods, which established the findings of Chan et al [50] that absolute solvent could not ensure fair extraction of polyphenols than aqueous solvents. Selection of the appropriate extraction solvent is vital for complex food matrices as it will estimate the type and quantity of polyphenols being extracted. Variations in extracted polyphenol content depend on the polarities of the solvents used as well as their concentration level, either aqueous or absolute. In general, aqueous alcohols such as methanol and ethanol are used in extraction of phenolic compounds from plant materials [51]. Solvent ethanol categorized under GRAS (Generally Recognized as Safe) is preferred because of its application in the food system. Ethanol enhances the solubility of a solute, whereas water accelerates its desorption from the sample matrix [52]. The low solubility of phenolic compounds in absolute solvents may be attributable to the strong hydrogen bonding between protein and polyphenols. However, the solubility increases upon addition of water to organic solvents that weakens the hydrogen bonds [53]. In a related study, Nepote et al [54] investigated the phenolic content of peanut skins with different concentrations of ethanol and reported that 50% ethanol led to the highest polyphenol content, which decreased with the increase in ethanol concentration above 70%.
3.3. Antioxidant activity
3.3.1. FRAP assay
The antioxidant power of a sample extract corresponds to its reducing capacity to transfer electrons to a FRAP reagent. The FRAP data (Table 1) indicate that kinnow mandarin peel extracts exhibited high antioxidant activity extracted with methanol as well as ethanol solvents. However, peel samples extracted with methanol had significantly higher antioxidant activity than samples extracted with ethanol. As regards the solvent concentration level, 80% methanolic extracts exhibited highest antioxidant activity (27.67 ± 1.11mM/100 g) followed by 80% ethanolic extracts (25.82 ± 0.67mM/100 g), whereas polyphenols extracted with 50% ethanol had the least antioxidant activity (21.29 ± 0.70mM/100 g). The LSD test results show that there were nonsignificant differences between 50% and 100% ethanolic extracts, but significant difference from 80% ethanolic extracts. However, methanolic extracts at different concentration levels were significantly different from each other. While investigating different fruit wastes for antioxidant activity, Farha et al [55] observed that 50% methanolic extracts of sweet lime(Citrus limetta) had a FRAP value of 7.48 mmol Fe2+/mL, which was considered a medium antioxidant activity. During a related study on various fruit peels' antioxidant activity, Zulkifli et al [56] reported a FRAP value of 20.03 ± 1.46mM/100 g for Navel orange (Citrus sinensis) peel using water extraction. Similarly, Oikeh et al [57] evaluated the in vitro antioxidant activity of sweet orange (C. sinensis) wastes and found that 70% ethanolic extract of flavedo had maximum FRAP value (800.30 ± 1.53 μmol Fe2+/g extract) whereas the absolute ethanolic sweet orange seed extracts had minimum FRAP value (329 ± 1.53 μmol Fe2+/g extract). Variations in the FRAP activity of citrus peel among different studies may be influenced by type of citrus variety, solvent used, as well as solvent concentration.
Table 1.
Antioxidant activity of kinnow mandarin peel extracts.
| Antioxidant assays | Methanol | Ethanol | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 100% | 80% | 50% | 100% | 80% | 50% | |
| FRAP | 21.95 ± 1.44c | 27.67 ± 1.91a | 24.08 ± 1.59b | 22.53 ± 1.32b,c | 25.82 ± 1.15a,b | 21.29 ± 1.66c |
| DPPH | 55.61 ± 1.69d | 72.83 ± 1.12a | 60.67 ± 1.24c | 57.18 ± 1.49d | 69.74 ± 1.97a,b | 56.52 ± 0.92d |
| Super oxide | 56.86 ± 1.37c | 64.80 ± 1.57a | 59.19 ± 0.83b | 55.28 ± 0.99c | 61.37 ± 1.63b | 54.06 ± 1.11d |
Data sharing similar letters in a row are statistically nonsignificant (p > 0.05).
All values represent the mean of three replications ± standard error (n = 3).
DPPH = 2,2-diphenyl-1-picrylhydrazyl; FRAP = ferric reducing antioxidant power.
3.3.2. DPPH radical scavenging activity
DPPH assay has been used widely and is a popular technique to assess the free radical scavenging activity of different plant extracts. It is a stable free radical that dissolves in either ethanol or methanol, and DPPH free radical reduction is determined by the decrease in its absorption at 517 nm when the color of the DPPH assay solution changes from purple to light yellow. The scavenging potential of plant extract anti-oxidants corresponds to the degree of the discoloration [58].
The effect of different solvents and their concentration levels on DPPH radical scavenging activity of kinnow mandarin peel extracts (Table 1) reveals high antioxidant activity of all sample extracts. However, the highest scavenging activity (72.83 ± 0.65%) was exhibited by samples extracted with solvent methanol at 80% concentration level followed by 80% ethanolic extract (69.74 ± 1.14%), whereas samples extracted with 100% methanol had the lowest scavenging activity (55.61 ± 0.98%). Aqueous solvent extracts had higher inhibitory activity against the DPPH radical as compared to corresponding absolute solvents, which may be attributed to the higher polyphenol content in these extracts. Among solvents, methanol extracted samples exhibited more scavenging activity than samples extracted with ethanol. When compared to standard ascorbic acid, the DPPH radical scavenging activity of kinnow peel extracts was lower (95.83 ± 0.75%). The extracting solvent effect on DPPH radical scavenging activity was earlier reported by Turkmen et al [59]. During a study on natural antioxidants from citrus mandarin peels, Karsheva et al [60] observed that 50% ethanolic extracts had the highest DPPH radical scavenging activity compared with 20% and 70% ethanolic extracts. Similarly, Do et al [61] investigated the effect of extraction solvent on the antioxidant activity of Limnophila aromatica and observed that 100% ethanolic exatract exhibited the maximum DPPH radical scavenging activity. Likewise, the peel and pulp of kinnow mandarin, orange, and lemon were assessed for total polyphenols and free radical scavenging activities. It was reported that orange pulp exhibited the highest radical scavenging activity (69.31%) followed by kinnow mandarin peel (68.57%), and lemon peel had the lowest radical-scavenging activity (46.98%). Park et al [62] found that the DPPH radical scavenging activity of orange flesh was higher than that of orange peel and reported that the acetone extract of orange flesh had the highest DPPH radical scavenging activity (compared with ethanolic and methanolic extracts). The IC50 value (i.e., the sample concentration required to scavenge 50% free radicals) was lowest in orange flesh acetone extracts (3333.7 μg/mL). The IC50 value is negatively correlated to antioxidant activity, and the lower the IC50 value, the higher the sample antioxidant activity [63]. Similarly, Oikeh et al [57] observed that the IC50 value of 70% ethanolic extract of sweet orange (C. sinensis) seeds was lowest (0.18 mg/mL) and hence had more radical scavenging activity than albedo and flavedo extracts.
3.3.3. Superoxide radical scavenging power assay
Although considered a weak oxidant, superoxide anion radical may lead to the generation of dangerous and powerful hydroxyl radicals and singlet oxygen, which are responsible for oxidative stress-related disorders. The antioxidants scavenge the superoxide anion and the percentage scavenging of superoxide anion radical increases with the increase in concentration of antioxidants [64].
The effect of different solvents and their concentration levels on superoxide anion radical scavenging activity of kinnow mandarin peel extracts (Table 1) shows the fairly high antioxidant activity of sample extracts. However, kinnow peel methanolic extracts at 80% concentration level exhibited the highest activity to scavenge superoxide anion radical (64.80 ± 0.91%) followed by 80% ethanolic extract (61.37 ± 0.91%), whereas the 50% ethanolic extract had the lowest scavenging activity (54.06 ± 0.64%). Overall, the superoxide anion radical scavenging activity of kinnow mandarin peels was comparatively lower than that of the standard ascorbic acid (87.83 ± 0.92%). In general, aqueous solvent extracts demonstrate lower inhibitory activity against superoxide anion radical compared with absolute solvent concentration extracts. The antioxidant activities of the pulp and peel of citrus fruits kinnow, orange, and shaddock were assessed by Mathur et al [65]. It was noted that the ethanolic extract of the peel and pulp of citrus fruits had higher superoxide radical scavenging activity than aqueous and chloroform extracts. Kinnow peel (87%) and shaddock pulp (90%) exhibited the highest scavenging activity. Similarly, Kalpna et al [58] evaluated the antioxidant potential of different fruit and vegetable peels using methanol, acetone, chloroform, and hexane. It was observed that the acetone extract of mango peels had the highest superoxide radical scavenging activity compared with methanolic, chloroform, and hexane extracts as well as other fruit and vegetable extracts. Jahan [66] investigated the superoxide anion radical scavenging activity of different medicinal plants and reported that methanolic extracts had a stronger antioxidant activity compared with water extracts, which might be attributable to the presence of high concentrations of hydrophilic and hydrophobic phenolic compounds.
3.4. HPLC analysis of phenolic compounds
Identification and quantification of phenolic acids and flavonoids in kinnow mandarin peel extracts were determined with HPLC. A total of 11 phenolic compounds—including five phenolic acids and six flavonoids at wavelength 280 nm and 370 nm, respectively—were identified and quantified according to retention time and their peaks' spectral characteristics against those of standards (Table 2). HPLC chromatograms of peel extracts and phenolic standards are presented in Figures 4 and 5. It is evident from the data that kinnow mandarin peel extracts phenolic compounds varied considerably as a function of solvent composition and concentration level. Maximum phenolic compounds were quantified in 80% ethanolic extracts (371.16 ± 6.79 μg/g) followed by 50% methanolic extracts (350.17 ± 4.47 μg/g) whereas minimum phenolic compounds were quantified in 100% ethanolic extracts (178.75 ± 2.12 μg/g) of kinnow mandarin peels. Among the phenolic compounds, ferulic acid and hesperidin were the most abundant in kinnow mandarin peel extracts. Maximum ferulic acid (102.13 ± 1.51 μg/g) and hesperidin (92.94 ± 1.23μg/g) were determined in 50% methanolic and 80% ethanolic extracts, respectively. Gallic acid and catechin were the other phenolic compounds present in high concentration, whereas caffeic acid and naringenin were the least quantified phenolic compounds and were present in traces only. To assess the effect of solvents and their concentration level, means of each phenolic compound quantified were compared statistically using the LSD test. There were significant variations observed in phenolic acids and flavonoids content with respect to solvent concentrations. Gallic acid content of 50% and 80% methanolic extracts were nonsignificant to each other, but were significantly different to other concentration levels. Ferulic acid and hesperidin at all concentration levels as well as solvent types differed significantly, whereas quercetin compound 100% methanolic and ethanolic extracts differed nonsignificantly but were significantly different from other concentration levels. Overall, 50% and 80% methanolic and ethanolic extracts lead to more phenolic compounds quantified as compared to absolute concentration levels. The antioxidant activity of mango peel extracts might be attributable to the phenolic acids and flavonoids contribution. Earlier, Chun et al [67] reported that flavonoids were responsible for the antioxidant activities of plants. Hesperidin and naringenin are the major flavanones present in orange, with the former being higher in concentration compared with the latter [42]. During a study on orange peel phenolic compounds, Khan et al [18] quantified flavanones hesperidin and naringin in orange peel extracts through HPLC and reported them as 205.20 mg/100 g and 70.30 mg/100 g fresh weight, respectively. Peels of Magnifera indica, C. sinensis, Malus sylvestris, and Psidium guajava were quantified for phenolic compounds through HPLC and compared by Zulkifli et al [56]. It was noted that all fruit peels had significantly higher concentrations of phenolic acids, especially gallic and chlorogenic acid along with flavonoids myricetin, quercetin, and kaempferol. They concluded that peels of M. indica and C. sinensis had the highest phenolic compounds and antioxidant activity.
Table 2.
Effect of solvent type and concentration on the phenolic compounds profile in kinnow peel.
| Phenolic compounds (μg/g) | Methanol | Ethanol | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 100% | 80% | 50% | 100% | 80% | 50% | |
| Gallic acid | 37.86 ± 1.03c | 39.54 ± 1.29c | 48.05 ± 0.71b | 12.02 ± 0.44e | 54.13 ± 1.12a | 25.60 ± 0.70d |
| Chlorogenic acid | 18.48 ± 0.41b | 12.91 ± 0.47d | 22.48 ± 0.85a | 17.25 ± 0.64b,c | 20.52 ± 0.82a,b | 15.86 ± 0.42c |
| Ferulic acid | 50.16 ± 0.75d | 88.41 ± 0.86b | 102.13 ± 1.51a | 22.37 ± 0.94f | 65.21 ± 1.16c | 42.56 ± 1.05e |
| Coumaric acid | 17.12 ± 0.34b,c | 11.23 ± 0.50d | 22.51 ± 0.61a | 15.93 ± 1.04c | 27.29 ± 0.44f | 20.18 ± 0.35a,b |
| Caffeic acid | 1.28 ± 0.38b | N.D. | N.D. | N.D. | 2.43 ± 0.30a | N.D. |
| Catechins | 26.24 ± 0.93d | 32.06 ± 0.44c | 37.89 ± 0.54b | 18.54 ± 0.49e | 49.46 ± 1.03a | 36.42 ± 0.88b |
| Epicatechins | 20.54 ± 0.53a | 17.25 ± 0.63a,b | 14.46 ± 0.33b | N.D. | 18.62 ± 0.54a | 7.73 ± 0.60c |
| Hesperidin | 44.38 ± 1.08f | 52.14 ± 1.22e | 61.02 ± 1.17d | 75.66 ± 1.67c | 92.94 ± 1.23a | 84.41 ± 1.01b |
| Naringenin | 1.97 ± 0.37b | N.D. | 3.74 ± 0.45a | N.D. | N.D. | 2.52 ± 0.28b |
| Quercetin | 18.44 ± 0.65d | 29.78 ± 0.86a | 25.71 ± 0.80b,c | 16.98 ± 0.39d | 23.71 ± 0.50c | 26.98 ± 0.65b |
| Kaempferol | 12.52 ± 0.32b | 13.87 ± 0.54a,b | 12.18 ± 0.39b | N.D. | 16.85 ± 0.41a | 14.26 ± 0.66a,b |
| Total | 248.99 ± 5.04c | 297.19 ± 2.64b | 350.17 ± 4.47a | 178.75 ± 2.12d | 371.16 ± 6.79a | 276.52 ± 5.26b,c |
All values are the mean of three replications.
Means followed by same letters do not differ significantly (p < 0.05).
Different superscript letters within same row denote significant difference (p < 0.05).
N.D. = not detected.
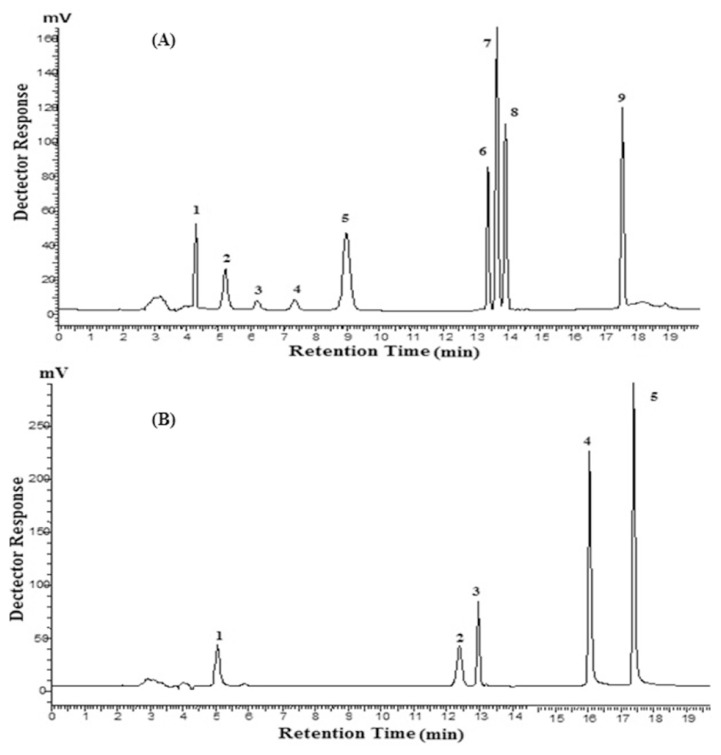
Figure 4.
(A) Typical chromatogram of polyphenols standards (200 μg/mL) at 280 nm. 1 = gallic acid, 2 = chlorogenic acid, 3 = catechin; 4 = epicatechin; 5 = caffeic acid; 6 = hesperidin; 7 = transferulic acid; 8 = coumaric acid; 9 = naringenin. (B) Typical chromatogram of polyphenol standards (200 μg/mL) at 370 nm. 1 = magniferin; 2 = myricetin; 3 = rutin; 4 = quercetin; 5 = kaempferol.
Figure 5.
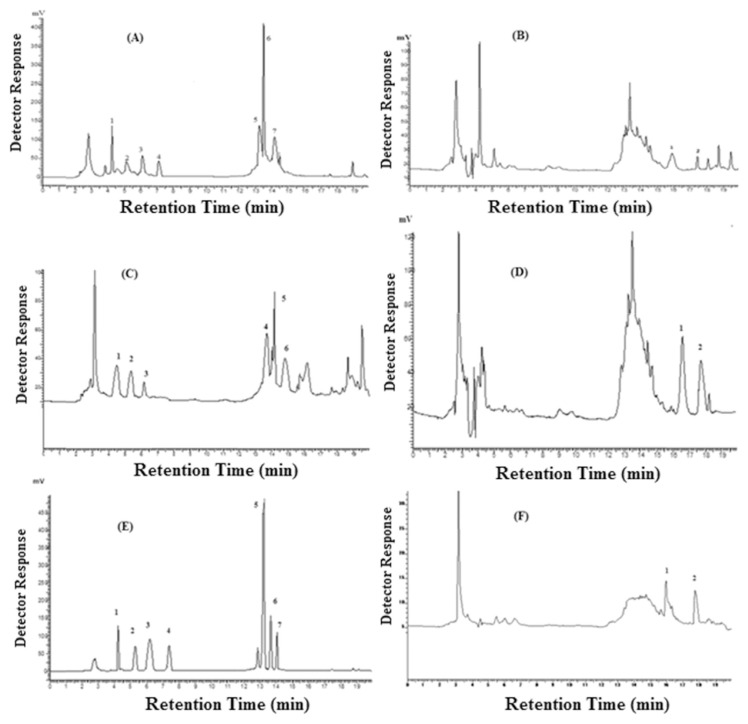
(A) Typical chromatogram of polyphenols of kinnow mandarin peel 50% methanolic extract at 280 nm. 1 = gallic acid, 2 = chlorogenic acid, 3 = catechin, 4 = epicatechin, 5 = hesperidin, 6 = ferulic acid, 7 = coumaric acid. (B) Typical chromatogram of polyphenols of kinnowmandarin peel 50% methanolic extract at 370 nm, 1 = quercetin, 2 = kaempferol. (C) Typical chromatogram of polyphenols of kinnow mandarin peel 100% methanolic extract at 280 nm, 1 = gallic acid, 2 = chlorogenic acid, 3 = catechin, 4 = hesperidin, 5 = ferulic acid, 6 = coumaric acid. (D) Typical chromatogram of polyphenols of kinnow mandarin peel 100% methanolic extract at 370 nm, 1 = quercetin, 2 = kaempferol. (E) Typical chromatogram of polyphenols of kinnow mandarin peel 80% ethanolic extract at 280 nm, 1 = gallic acid, 2 = chlorogenic acid, 3 = catechin, 4 = epicatechin, 5 = hesperidin, 6 = ferulic acid, 7 = coumaric acid. (F) Typical chromatogram of polyphenols of kinnow mandarin peel 80% ethanolic extract at 370 nm, 1 = quercetin, 2 = kaempferol.
3.5. Antimicrobial activity
Ethanolic extracts of kinnow mandarin peels were assessed for their antimicrobial activity against three foodborne bacteria (Table 3). Statistical analysis reveals that peel extracts exhibited significantly different antimicrobial potential against bacterial strains. As evident from the table, the bacterial growth inhibition activity was increased with higher concentrations of kinnow peel extracts, which implies that microbial growth inhibition is dose dependent. Kinnow mandarin peel extracts at a concentration level of 250 μg/disk exhibited no antimicrobial activity, whereas at 500 μg/disk concentration, slight inhibitory activity (8.67 ± 0.33) was observed against S. aureus. However, in the case of a kinnow peel extract at a concentration level of 1000 μg/disk, maximum inhibition zone (16.00 ± 0.58 mm) was recorded against S. aureus whereas minimum inhibition zone (9.00 ± 1.16 mm) was noted against S. typhimurium. The LSD test results reveal that there were significant differences between extract concentration against three bacterial strains. It was observed that Gram-positive strains (S. aureus and B. cereus) were more sensitive to kinnow peel extracts as compared to Gram-negative strain (S. typhimurium). The variation in sensitivity among bacterial strains is ascribed to cell wall structure differences of strains. The cell wall of Gram-negative bacteria are bestowed with outer membrane as well as peri-plasmic space, which hinders the penetration of antimicrobial substances, thus providing more resistance to Gram-negative bacteria [68,69].
Table 3.
Antimicrobial activity of kinnow mandarin peel extracts.
| Zone of inhibition (mm) | ||||
|---|---|---|---|---|
|
| ||||
| Extract conc. (μg/disk) | Staphylococcus aureus | Bacillus cereus | Salmonella typhimurium | Mean |
| 250 | N.D. | N.D. | N.D. | N.D. |
| 500 | 8.67 ± 0.33d,e | N.D. | N.D. | 2.89 ± 0.56C |
| 750 | 14.00 ± 1.16b,c | 12.67 ± 1.02c | 7.33 ± 0.96e | 11.33 ± 1.05B |
| 1000 | 16.00 ± 0.58a | 14.33 ± 0.88b | 9.00 ± 1.16d | 13.11 ± 0.87A |
Data sharing similar letters in a row or in a column are statistically nonsignificant (p > 0.05). Small letters represent comparison among interaction means and capital letters are used for overall mean.
All values represent the mean of inhibition zone (mm) ± standard error (n = 3).
N.D. = not detected.
The antimicrobial activity of plant extracts may be attributed to the presence of polyphenols in extracts as high antimicrobial activity is exhibited by plant extracts with elevated polyphenol content [70]. The effects of plant extracts as antimicrobial agents depend on the polyphenol type such as phenolic acids, flavonoids, and tannins. Flavonols such as quercetin are considered potent antimicrobial agents [71]. Antimicrobial activity is substantially influenced by the position and number of hydroxyl groups because these groups may interact with the bacterial cell membrane to disrupt its structure, which leads to cellular components leakage [72]. Results are in accordance with the findings of Mathur et al [65], who reported that kinnow peel ethanolic extracts possessed maximum antimicrobial activity against S. aureus than other tested microorganisms. Similarly, the higher antimicrobial activity of orange, lemon, and banana peels against S. aureus compared with other studied bacterial, yeast, and fungal strains was observed by El Zawawy [73].
4. Conclusion
Kinnow mandarin peels are a rich source of phenolic compounds with strong antioxidant activity. UAE, which led to higher polyphenol extraction, is a more efficient technique than maceration. Absolute solvents could not ensure fair extraction of polyphenols than aqueous solvents as well as lower antioxidant activity in comparison with absolute solvents. Although methanol and ethanol are efficient solvents for extraction of polyphenols, ethanol categorized under GRAS is preferred because of its application in the food system. Strong correlations between total polyphenols and antioxidant activity were observed. Eleven phenolic compounds, including five phenolic acids and six flavonoids, were identified and quantified by HPLC. Ferulic acid and hesperidin were the most abundant whereas caffeic acid was the least quantified phenolic compounds in kinnow peel extracts. As regards the antimicrobial activity of kinnow mandarin peels against three foodborne bacterial strains, maximum inhibition zone was recorded against S. aureus at a concentration level of 1000 μg/disk, whereas minimum inhibition zone was noted against S. typhimurium. It was concluded that kinnow mandarin peels is a potential source of phenolic compounds with antioxidant and antimicrobial properties and can be utilized as an ingredient for the preparation of functional foods.
Acknowledgments
This research work was supported by the Research For Agricultural Development Program (RADP), No. CS-72/2013/RADP, PARC, Islamabad, Pakistan.
Funding Statement
This research work was supported by the Research For Agricultural Development Program (RADP), No. CS-72/2013/RADP, PARC, Islamabad, Pakistan.
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
REFERENCES
- 1. Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–9. [Google Scholar]
- 2. Osman H, Rahim AA, Isa NM, Bakhir NM. Antioxidant activity and phenolic content of Paederia foetida and Syzygium aqueum. Molecule. 2009;14:970–8. doi: 10.3390/molecules14030970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang CS, Yin MC, Chiu LC. Antihyperglycemic and antioxidative potential of Psidium guajava fruit in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49:2189–95. doi: 10.1016/j.fct.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 4. Sesso HD, Wang L, Ridker PM, Buring JE. Tomato-based food products are related to clinically modest improvements in selected coronary biomarkers in women. J Nutr. 2012;142:326–33. doi: 10.3945/jn.111.150631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deng GF, Shen C, Xu XR, Kuang RD, Guo YJ, Zeng LS, Gao LL, Lin X, Xie JF, Xia EQ. Potential of fruit wastes as natural resources of bioactive compounds. Int J Mol Sci. 2012;13:8308–23. doi: 10.3390/ijms13078308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goulas V, Manganaris G. Exploring the phytochemical content and the antioxidant potential of Citrus fruits grown in Cyprus. Food Chem. 2012;131:39–47. [Google Scholar]
- 7. Jabbar S, Abid M, Wu T, Hashim MM, Saeeduddin M, Hu B, Lei S, Zeng X. Ultrasound-assisted extraction of bioactive compounds and antioxidants from carrot pomace: a response surface approach. J Food Proc Preserv. 2015;39:1878–88. [Google Scholar]
- 8.GOP. 50-Years of Pakistan in statistics volume. Islamabad: Federal Bureau of Statistics; 2012. [Google Scholar]
- 9. Magda R, Awad A, Selim K. Evaluation of mandarin and navel orange. Peels as natural sources of antioxidant in biscuits. Alexandria J Food Sci Technol. 2008:75–82. [Google Scholar]
- 10. Senevirathne M, Jeon YJ, Ha JH, Kim SH. Effective drying of citrus by-product by high speed drying: a novel drying technique and their antioxidant activity. J Food Eng. 2009;92:157–63. [Google Scholar]
- 11. Tripoli E, La Guardia M, Giammanco S, Di Majo D, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007;104:466–79. [Google Scholar]
- 12. Ross K, Beta T, Arntfield S. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 2009;113:336–44. [Google Scholar]
- 13. Belova V, Voshkin A, Kholkin A, Payrtman A. Solvent extraction of some lanthanides from chloride and nitrate solutions by binary extractants. Hydrometal. 2009;97:198–203. [Google Scholar]
- 14. Jadhav D, Rekha B, Gogate PR, Rathod VK. Extraction of vanillin from vanilla pods: a comparison study of conventional soxhlet and ultrasound assisted extraction. J Food Eng. 2009;93:421–6. [Google Scholar]
- 15. Adjé F, Lozano YF, Lozano P, Adima A, Chemat F, Gaydou EM. Optimization of anthocyanin, flavonol and phenolic acid extractions from Delonix regia tree flowers using ultrasound-assisted water extraction. Ind Crop Prod. 2010;32:439–44. [Google Scholar]
- 16. Da Porto C, Decorti D. Ultrasound-assisted extraction coupled with under vacuum distillation of flavour compounds from spearmint (carvone-rich) plants: comparison with conventional hydrodistillation. Ultrason Sonochem. 2009;16:795–9. doi: 10.1016/j.ultsonch.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 17. Vilkhu K, Mawson R, Simons L, Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry—a review. Innov Food Sci Emerg Technol. 2008;9:161–9. [Google Scholar]
- 18. Khan MK, Abert-Vian M, Fabiano-Tixier AS, Dangles O, Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119:851–8. [Google Scholar]
- 19. Pan Z, Qu W, Ma H, Atungulu GG, McHugh TH. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason Sonochem. 2011;18:1249–57. doi: 10.1016/j.ultsonch.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 20. Bendini A, Cerretani L, Pizzolante L, Toschi TG, Guzzo F, Ceoldo S, Marconi AM, Andreetta F, Levi M. Phenol content related to antioxidant and antimicrobial activities of Passiflora spp. extracts. Eur Food Res Technol. 2006;223:102–9. [Google Scholar]
- 21. Pérez-Jiménez J, Serrano J, Tabernero M, Arranz S, Díaz-Rubio ME, García-Diz L, Goñi I, Saura-Calixto F. Bioavailability of phenolic antioxidants associated with dietary fiber: plasma antioxidant capacity after acute and long-term intake in humans. Plant Food Hum Nutr. 2009;64:102–7. doi: 10.1007/s11130-009-0110-7. [DOI] [PubMed] [Google Scholar]
- 22. Anwar N, Salik S, Ahmad D. Antibacterial activity of Otostegia limbata. Int J Agric Biol. 2009;11:647–50. [Google Scholar]
- 23. Khan R, Islam B, Akram M, Shakil S, Ahmad AA, Ali SM, SiddiquiM Khan AU. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecule. 2009;14:586–97. doi: 10.3390/molecules14020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jayaraman P, Sakharkar MK, Lim CS, Tang TH, Sakharkar KR. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int J Biol Sci. 2010;6:556–68. doi: 10.7150/ijbs.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saavedra MJ, Borges A, Dias C, Aires A, Bennett RN, Rosa ES, Simões M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med Chem. 2010;6:174–83. doi: 10.2174/1573406411006030174. [DOI] [PubMed] [Google Scholar]
- 26. Mahmud S, Saleem M, Siddique S, Ahmed R, Khanum R, Perveen Z. Volatile components, antioxidant and antimicrobial activity of Citrus acida var. sour lime peel oil. J Saudi Chem Soc. 2009;13:195–8. [Google Scholar]
- 27. Al-Zoreky N. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int J Food Microbiol. 2009;134:244–8. doi: 10.1016/j.ijfoodmicro.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 28. Katalinić V, Možina SS, Skroza D, Generalić I, Abramović H, Miloš M, Ljubenkov I, Piskernik S, Pezo I, Terpinc P. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia) Food Chem. 2010;119:715–23. [Google Scholar]
- 29. Adámez JD, Samino EG, Sánchez EV, González-Gómez D. In vitro estimation of the antibacterial activity and antioxidant capacity of aqueous extracts from grape-seeds (Vitis vinifera L.) Food Control. 2012;24:136–41. [Google Scholar]
- 30.AOAC. Official methods of analysis. Gaithersburg, MD: 2010. [Google Scholar]
- 31. Elfalleh W, Hannachi H, Tlili N, Yahia Y, Nasri N, Ferchichi A. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J Med Plant Res. 2012;6:4724–30. [Google Scholar]
- 32. Bimakr M, Abdul Rahman R, Taip FS, Mohd Adzahan N, Sarker ZI, Ganjloo A. Ultrasound-assisted extraction of valuable compounds from winter melon (Benincasa hispida) seeds. Int Food Res J. 2013;20:331–8. [Google Scholar]
- 33. Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–78. [Google Scholar]
- 34. Benzie IF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 35. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 36. Vaidya SK, Viswanatha G, Ramesh C, Nandakumar K, Srinath R. Antimutagenic (anticlastogenic) and antioxidant activities of bark extract of Terminalia arjuna. J Genet Toxicol. 2008;1:1–7. [Google Scholar]
- 37. Salvador MJ, Ferreira EO, Mertens-Talcott SU, De Castro WV, Butterweck V, Derendorf H, Dias DA. Isolation and HPLC quantitative analysis of antioxidant flavonoids from Alternanthera tenella Colla. Zeitschr Naturforsch C. 2006;61:19–25. doi: 10.1515/znc-2006-1-204. [DOI] [PubMed] [Google Scholar]
- 38. Rios J, Recio M, Villar A. Screening methods for natural products with antimicrobial activity: a review of the literature. J Ethnopharmacol. 1988;23:127–49. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 39. Ripa FA, Haque M, Imran-Ul-Haque M. In vitro antimicrobial, cytotoxic and antioxidant activity of flower extract of Saccharum spontaneum Linn. European J Sci Res. 2009;30:478–83. [Google Scholar]
- 40. Sultana B, Anwar F, Rafique Asi M, Ali Shahid Chatha S. Antioxidant potential of extracts from different agro wastes: stabilization of corn oil. Gras Aceites. 2008;59:205–17. [Google Scholar]
- 41. Hegazy A, Ibrahim M. Antioxidant activities of orange peel extracts. World Applied Sci J. 2012;18:684–8. [Google Scholar]
- 42. Wang YC, Chuang YC, Hsu HW. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008;106:277–84. [Google Scholar]
- 43. Naseer R, Sultana B, Anwar F, Mehmood Z, Mushtaq M. Variations in phenolics, antioxidant and antifungal activities among different parts of selected medicinal plants. Pak J Bot. 2014;42:705–12. [Google Scholar]
- 44. Al-Juhaimi FY. Citrus fruits by-products as sources of bioactive compounds with antioxidant potential. Pak J Bot. 2014;46:1459–62. [Google Scholar]
- 45. Paniwnyk L, Cai H, Albu S, Mason T, Cole R. The enhancement and scale up of the extraction of antioxidants from Rosmarinus officinalis using ultrasound. Ultrason Sonochem. 2009;16:287–92. doi: 10.1016/j.ultsonch.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 46. Veggi PC, Santos DT, Fabiano-Tixier A-S, Le Bourvellec C, Meireles MAA, Chemat F. Ultrasound-assisted extraction of polyphenols from Jatoba (Hymenaea courbaril L. var stilbocarpa) Bark. Food Pub Health. 2013;3:119–29. [Google Scholar]
- 47. Zhang ZS, Wang LJ, Li D, Jiao SS, Chen XD, Mao ZH. Ultrasound-assisted extraction of oil from flaxseed. Sep Purif Technol. 2008;62:192–8. [Google Scholar]
- 48. Petigny L, Périno-Issartier S, Wajsman J, Chemat F. Batch and continuous ultrasound assisted extraction of boldo leaves (Peumus boldus Mol.) Int J Mol Sci. 2013;14:5750–64. doi: 10.3390/ijms14035750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quiroz-Reyes CN, Aguilar-Mendez MA, Ramirez-Ortiz ME, Ronquillo-De Jesus YE. Comparative study of ultrasound and maceration techniques for the extraction of polyphenols from cocoa beans (Theobroma cacao L.) Rev Mexicana Ingen Quim. 2013;12:11–8. [Google Scholar]
- 50. Chan S, Lee C, Yap C, Mustapha WAW, Ho C. Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Int Food Res J. 2009;16:203–13. [Google Scholar]
- 51. Hayouni EA, Abedrabba M, Bouix M, Hamdi M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007;105:1126–34. [Google Scholar]
- 52. Mustafa A, Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal Chim Acta. 2011;703:8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 53. Wissam Z, Ghada B, Wassim A, Warid K. Effective extraction of polyphenols and proanthocyanidins from pomegranate's peel. Int J Pharm Pharm Sci. 2012;4:675–82. [Google Scholar]
- 54. Nepote V, Grosso NR, Guzmán CA. Optimization of extraction of phenolic antioxidants from peanut skins. J Sci Food Agric. 2005;85:33–8. [Google Scholar]
- 55. Farha S, Chatterjee E, Manuel SG, Reddy SA, Kale RD. Isolation and characterization of bioactive compounds from fruit wastes. Dynamic Biochem Proc Biotechnol Mol Biol. 2012;6:92–4. [Google Scholar]
- 56. Zulkifli KS, Abdullah N, Abdullah A, Aziman N, Kamarudin WSSW. Bioactive phenolic compounds and antioxidant activity of selected fruit peels. Int Conf Environ Chem Biol. 2012;49:66–70. [Google Scholar]
- 57. Oikeh EI, Oriakhi K, Omoregie ES. Phenolic content and in vitro antioxidant activities of sweet orange (Citrus sinensis L.) fruit wastes. Arch Basic Appl Med. 2015;2:119–26. [Google Scholar]
- 58. Kalpna R, Mital K, Sumitra C. Vegetable and fruit peels as a novel source of antioxidants. J Med Plants Res. 2011;5:63–71. [Google Scholar]
- 59. Turkmen N, Velioglu YS, Sari F, Polat G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12:484–96. doi: 10.3390/12030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Karsheva M, Kirova E, Alexandrova S. Natural antioxidants from citrus mandarin peels. Extraction of polyphenols; effect of operational conditions on total polyphenols contents and antioxidant activity. J Chem Technol Metal. 2013;48:35–41. [Google Scholar]
- 61. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Park JH, Lee M, Park E. Antioxidant activity of orange flesh and peel extracted with various solvents. Prev Nutr Food Sci. 2014;19:291. doi: 10.3746/pnf.2014.19.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chanda S, Dave R, Kaneria M. In vitro antioxidant property of some Indian medicinal plants. Res J Med Plant. 2011;5:169–79. [Google Scholar]
- 64. Jayakumar T, Thomas P, Geraldine P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innov Food Sci Emerg Technol. 2009;10:228–34. [Google Scholar]
- 65. Mathur A, Verma SK, Purohit R, Gupta V, Dua V, Prasad G, Mathur D, Singh SK, Singh S. Evaluation of in vitro antimicrobial and antioxidant activities of peel and pulp of some citrus fruits. IJPI's J Biotechnol Biother. 2011;1:1–17. [Google Scholar]
- 66.Jahan N. Correlation of polyphenolic contents of indigenous medicinal plants with their bioactivities. Faisalabad, Pakistan: University of Agriculture Faisalabad; 2011. [Google Scholar]
- 67. Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of US adults. J Nutr. 2007;137:1244–52. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 68. Von Staszewski M, Pilosof AM, Jagus RJ. Antioxidant and antimicrobial performance of different Argentinean green tea varieties as affected by whey proteins. Food Chem. 2011;125:186–92. [Google Scholar]
- 69. Oliveira DA, Salvador AA, Smânia A, Smânia EF, Maraschin M, Ferreira SR. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J Biotechnol. 2013;164:423–32. doi: 10.1016/j.jbiotec.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 70. Sanhueza L, Tello M, Vivanco M, Mendoza L, Wilkens M. Relation between antibacterial activity against food transmitted pathogens and total phenolic compounds in grape pomace extracts from Cabernet Sauvignon and Syrah varieties. Adv Microbiol. 2014;4:225–32. [Google Scholar]
- 71. Li M, Xu Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch Pharm Res. 2008;31:640–4. doi: 10.1007/s12272-001-1206-5. [DOI] [PubMed] [Google Scholar]
- 72. Xue J, Davidson PM, Zhong Q. Thymol nanoemulsified by whey protein–maltodextrin conjugates: the enhanced emulsifying capacity and antilisterial properties in milk by propylene glycol. J Agric Food Chem. 2013;61:12720–6. doi: 10.1021/jf4043437. [DOI] [PubMed] [Google Scholar]
- 73. El Zawawy NA. Antioxidant, antitumor, antimicrobial studies and quantitative phytochemical estimation of ethanolic extracts of selected fruit peels. Int J Curr Microbiol Appl Sci. 2015;4:298–309. [Google Scholar]