Abstract
The purpose of this study is to evaluate the efficiency of using propidium monoazide (PMA) real-time quantitative polymerase chain reaction (qPCR) to count the viable cells of Lactobacillus gasseri and Lactobacillus salivarius in probiotic products. Based on the internal transcription spacer and 23S rRNA genes, two primer sets specific for these two Lactobacillus species were designed. For a probiotic product, the total deMan Rogosa Sharpe plate count was 8.65 ± 0.69 log CFU/g, while for qPCR, the cell counts of L. gasseri and L. salivarius were 8.39 ± 0.14 log CFU/g and 8.57 ± 0.24 log CFU/g, respectively. Under the same conditions, for its heat-killed product, qPCR counts for L. gasseri and L. salivarius were 6.70 ± 0.16 log cells/g and 7.67 ± 0.20 log cells/g, while PMA-qPCR counts were 5.33 ± 0.18 log cells/g and 5.05 ± 0.23 log cells/g, respectively. For cell dilutions with a viable cell count of 8.5 log CFU/mL for L. gasseri and L. salivarius, after heat killing, the PMA-qPCR count for both Lactobacillus species was near 5.5 log cells/mL. When the PMA-qPCR counts of these cell dilutions were compared before and after heat killing, although some DNA might be lost during the heat killing, significant qPCR signals from dead cells, i.e., about 4–5 log cells/mL, could not be reduced by PMA treatment. Increasing PMA concentrations from 100 μM to 200 μM or light exposure time from 5 minutes to 15 minutes had no or, if any, only minor effect on the reduction of qPCR signals from their dead cells. Thus, to differentiate viable lactic acid bacterial cells from dead cells using the PMA-qPCR method, the efficiency of PMA to reduce the qPCR signals from dead cells should be notable.
Keywords: Lactobacillus gasseri, Lactobacillus salivarius, propidium monoazide, quantitative polymerase chain reaction, viable and heat-killed cells
1. Introduction
Probiotics including lactic acid bacteria (LAB), such as Lactobacillus spp. and Bifidobacterium spp., are living microorganisms that, upon ingestion, exert health benefits on human and animals. Owing to the increasing use of lactobacilli in probiotic products and feed supplements, the manufacturers should declare the right LAB species and viable cell counts for total or each of the LAB strains in the product so that the consumers’ rights and interests could be protected. Thus, correct identification and quantification of viable cells for each LAB strains in probiotic products are important. According to Coeuret et al [1], for human nutritional supplements, in general, viable cell counts of specific LAB strains are 8–9 log CFU/g. Since most of the probiotic products may contain two or more LAB species, rapid methods that allow simultaneous identification and quantification of viable cells of different LAB species are required.
Lactobacillus gasseri is one of the common species of the human gut flora [2]. Strains of this species have been found to have wide inhibitory activity against pathogenic and food-spoilage bacteria [3,4]. Reports regarding its anti-inflammatory properties and expression of superoxide dismutase using a mouse model [5], regulatory effect on gut environment and intestinal functionality [6], reduction of blood glucose levels and body weight in a mouse model of type 2 diabetes [7], as well as the protective effect against gastric ulcers [8] have been revealed. As for Lactobacillus salivarius, its influence on the incidences of dental health in children [9], and production of bacteriocins to inhibit the pathogens and influence the host immune system [10] have been reported. Recently a probiotic product, containing L. salivarius and L. gasseri and claiming to have antiallergic function for humans, has been commercialized in the market.
For the quantification of viable cells, recently, the use of selective nucleic acid intercalating dyes, such as propidium monoazide (PMA), has been suggested as a method to reduce polymerase chain reaction (PCR) signals from DNA in dead cells. The approach is based on the difference of membrane integrity between viable and nonviable cells [11]. Ideally, PMA should only penetrate into membrane-compromised dead cells and intercalate with double-strand DNA in the cells. This method has been used to differentiate viable cells from dead cells for different bacterial species, such as Escherichia coli and Campylobacter [12,13]. For LAB, it has been used for the quantification of viable cells (>105 CFU/g) in spray-dried probiotic lactobacilli [14], and differentiation of viable as well as heat-killed cells of specific strains of Bifidobacterium breve and Bifidobacterium bifidum cells (1010 cells/mL) added in human feces [15,16]. In this study, based on the 16S and internal transcription spacer (ITS)–23S rRNA sequences, we designed PCR primers specific for L. gasseri and L. salivarius, and attempted to use these primers for simultaneous identification and quantification of viable LAB cells in the probiotic product. Meanwhile, the possible loss of DNA during heat killing of these two LAB species cells and the efficiency of PMA treatment in the reduction of quantitative polymerase chain reaction (qPCR) signals from heat-killed cells of these two Lactobacillus species were evaluated.
2. Materials and methods
2.1. Bacterial strains and heat-killed cells
Strains used in this study include strains of Lactobacillus spp., Bifidobacterium spp., Enterococcus spp., Bacillus spp., and other bacterial species, such as those of the family of Enterobacteriaceae (Table 1). These strains were obtained from Bioresources Collection and Research Center (BCRC; Hsin-Chu, Taiwan) and American Type Culture Collection (Manassas, VA, USA). All LAB strains were maintained at −80°C as 25% glycerol stocks. Strains of Lactobacillus spp. were grown in deMan Rogosa Sharpe (MRS) broth (Merck, Darmstadt, Germany), while strains of Bifidobacterium spp. were grown in MRS broth plus 0.05% L-cystine under anaerobic conditions at 37°C. By contrast, strains of Enterococcus spp. were grown in Brain Heart Infusion broth (Difco, Detroit, MI, USA) at 37°C. For all these LAB strains, the culture time was 16–20 hours. For bacteria other than the LAB strains, Luria broth (yeast extract 5 g, tryptone 10 g, NaCl 5 g, and sterile H2O to 1000 mL) was used and the culture time was 20–24 hours at 37°C.
Table 1.
Bacterial strains used in this study and the PCR results using LaITS-1F/LGA-1R and LaITS-1F/LSA-1R as primers.
| Species | No. of isolate tested | Strain numbera and source | No. of PCR-positive or PCR-negative strains | |
|---|---|---|---|---|
|
| ||||
| LaITS-1F/LGA-1R | LaITS-1F/LSA-1R | |||
| Lactobacillus acidophilus | 3 | BCRC 10695, 14064, 14065 | −3 | −3 |
| Lactobacillus brevis | 2 | BCRC 12945, 12187 | −2 | −2 |
| Lactobacillus casei | 5 | BCRC 10697, 14080, 14082, 14084, 17002 | −5 | −5 |
| Lactobacillus crispatus | 1 | BCRC 14618 | −1 | −1 |
| Lactobacillus delbrueckii subsp. delbrueckii | 1 | BCRC 12195 | −1 | −1 |
| L. delbrueckii subsp. lactis | 1 | BCRC 12256 | −1 | −1 |
| L. delbrueckii subsp. bulgaricus | 1 | BCRC 10696 | −1 | −1 |
| Lactobacillus fermentum | 3 | BCRC 10360, 14625, 14691 | −3 | −3 |
| L. gasseri | 4 | BCRC 14619, 17614, 17615, 17616 | +4 | −4 |
| Lactobacillus heleveticus | 2 | BCRC 12936, 14092 | −2 | −2 |
| Lactobacillus jensenii | 1 | BCRC 12939 | −1 | −1 |
| Lactobacillus johnsonii | 1 | BCRC 17010 | −1 | −1 |
| Lactobacillus paracasei | 4 | BCRC 12248, 14001, 14023, 16100 | −4 | −4 |
| Lactobacillus plantarum | 4 | BCRC 10069, 10357, 12250, 12251 | −4 | −4 |
| Lactobacillus pentosus | 2 | BCRC 11503, 15317 | −2 | −2 |
| Lactobacillus reuteri | 3 | BCRC 14625, 16090, 16091 | −3 | −3 |
| Lactobacillus ruminis | 1 | BCRC 14620 | −1 | −1 |
| Lactobacillus rhamnosus | 4 | BCRC 10940, 11672, 12321, 16000 | −4 | −4 |
| Lactobacillus saliverius | 4 | BCRC 12574, 14759, LS01, 02 | −4 | +4 |
| Bifidobacteria spp.b | 44 | −44 | −44 | |
| Othersc | 42 | −42 | −42 | |
ATCC = American Type Culture Collection; BCRC = Bioresources Collection and Research Center; PCR = polymerase chain reaction.
BCRC, Hsin-Chu, Taiwan.
Bifidobacteria species including Bifidobacterium adolescentis BCRC 14606, 14607 14609, 14658; Bifidobacterium angulatum BCRC 14665, 15971; Bifidobacterium animalis BCRC 14668; Bifidobacterium asteroids BCRC 14659; B. bifidum BCRC 11844, 12584, 14146, 14611, 14613, 14615; Bifidobacterium boum BCRC 14677; B. breve BCRC 11846, 14601, 14632; Bifidobacterium catenulatum 14667; Bifidobacterium coryneforme BCRC 14675; Bifidobacterium cuniculi BCRC 14672; Bifidobacterium dentium BCRC 14662; Bifidobacterium gallinarum BCRC 14679, 16012; Bifidobacterium pseudolongum subsp. globosum BCRC 14663; Bifidobacterium indicum BCRC 14674; Bifidobacterium infantis BCRC 14602, 14603, 14604, 14633, 14661; Bifidobacterium longum BCRC 11847, 14605, 14634, 14664; Bifidobacterium magnum BCRC 14676; Bifidobacterium minimum BCRC 14666; B. pseudolongum BCRC 16013, 14673, 15476; Bifidobacterium pullorum BCRC 14678; Bifidobacterium subtile BCRC 14660; Bifidobacterium suis BCRC 14671; Bifidobacterium thermophilum BCRC 14669.
Enterococcus avium BCRC 10801, 14728; Enterococcus casseliflavus BCRC 14926; Enterococcus durans BCRC 10790; Enterococcus faecalis BCRC 10066, 12298, 12301; Enterococcus faecium BCRC 10067, 910248; Enterococcus gallinarum BCRC 15477; Enterococcus hirae BCRC 12496; Lactococcus lactis subsp. lactis BCRC 14016; Lactococcus pseudomesenteroides BCRC 11651; Lactococcus mesenteroides subsp. mesenteroides BCRC 11652; L. mesenteroides subsp. dextranicum BCRC 12660; Lactococcus lactis BCRC 12261; L. mesenteroides subsp. cremoris BCRC 14047; Streptococcus salivarius subsp. thermophilus BCRC 14086; Streptococcus faecium BCRC 14089, 14070; Bacillus cereus BCRC 10446; Brevibacterium linens ATCC 19391; Citrobacter freundii BCRC 10041, FR 12291; Enterobacter cloacae ATCC 23355; Erwinia carotovora BCRC 11298; Hafnia alvei BCRC 10906; Kluyvera ascorbata BCRC 11645; Micrococcus roseus BCRC 11577; Morganella morganii BCRC 10706; Proteus vulgaris ATCC 8427; Pseudomonas cepacia BCRC 10735; Salmonella arizonae BCRC 10742; Salmonella typhimurium ATCC 13311; Serratia marcescens ATCC 13880; Shigella flexneri BCRC 10772, 13894; Shigella sonnei BCRC 10773, 10774; Shigella boydii BCRC 15959; Shigella dysenteria BCRC 13893; Staphylococcus aureus BCRC 12653; Vibrio parahaemolyticus ATCC 17803; Yersinia enterocolitica BCRC 10807.
For probiotic samples, amarket-available probiotic product with antiallergic activity (Kan-Min 2) manufactured by Promed Biotech (Tainan, Taiwan) was used. This product was labeled with only two LAB species, i.e., L. gasseri and L. salivarius, but not with their viable cell counts. It was in the form of capsule (0.6 g/capsule). To prepare the heat-killed product, 1 g/mL of the sample in phosphate-buffered saline (PBS) was mixed with 9 mL PBS, while for reference strains, L. gasseri (BCRC 14619) and L. salivarius (BCRC 12574) in PBS, with different cell counts, were heated at 100°C for 30 minutes and then cooled at 4°C for 10 minutes, followed by centrifugation at 6000 g (Eppendorf Cat. No. 5424) for 10 minutes to collect the cells and supernatant.
2.2. Preparation of genomic DNA from bacterial strains
For LAB cells, total chromosomal DNA was prepared from the overnight culture as described earlier using the Blood & Tissue Genomic DNA Extraction Miniprep System according to the bacterial protocol in the instruction sheet from the manufacturer (Viogene Laboratories, Taipei, Taiwan). Briefly, cells collected from 500 μL of the overnight culture were washed with 1× PBS. After spinning down the cells, the cell pellet was mixed with 200 μL lysozyme (20 mM Tris–HCl, pH 8.0, 2 mM EDTA, 20 mg/mL lysozyme). After incubation at 37°C for 60 minutes, 20 μL proteinase K (10 μg/mL) and 200 μL EX buffer were mixed, followed by incubation at 60°C for 60 minutes until the solution became clear. After incubation at 37°C for 30 minutes, total DNA was prepared by mixing the solution with 400 μL absolute ethanol followed by separation of DNA with B/T Genomic DNA minicolumn according to the manufacturer’s manual. These DNA samples were then stored at −20°C.
For bacterial species other than LAB, total DNA was prepared from 100 μL of the overnight cell culture after 10-fold dilution with sterile water. The bacterial suspension was boiled (100°C for 30 minutes) to decompose the cells, followed by cooling (−20°C for 10 minutes). After centrifugation at 6000 g for 5minutes, 1 μL of the supernatant was used for PCR amplification.
2.3. PCR primers and probes
Methods for designing of PCR primers and probes, and PCR amplification were modified from those of Sheu et al [17,18]. DNA sequences coding for 23S rRNA and ITS region of LAB were selected for designing L. gasseri- and L. salivarius-specific oligonucleotides. Sequences available in the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) were retrieved and aligned using software, such as cluster X, GCG, Seqweb, and Array designer. Sequences of 23S rRNA and ITS regions of L. gasseri (Accession No. AF182721) and L. salivarius (Accession No. AF182725) were aligned and selected for designing primers specific to L. gasseri and L. salivarius (Table 2). These primers were then tested for their specificity by PCR assay with the strains shown in Table 2.
Table 2.
Primers and conditions for the PCR assay of L. gasseri and L. salivarius.
| Species | Primer or probe | Oligonucleotide sequence (5′–3′) | Location within gene and (target gene) | Product size (bp) | Accession no.a |
|---|---|---|---|---|---|
| L. gasseri | LaITS-1F | AAGGGCGCACGGTGAATGCCT | 222–242 (ITS) | 329 | AF182726 |
| LGA-1R | TGCTATCGCTTCAAGTGCTT 530–549 (23S rDNA) AF182721 | ||||
| L. salivarius | LaITS-1F | AAGGGCGCACGGTGAATGCCT | 222–242 (ITS) | 396 | AF182726 |
| LSA-1R | GAACTGAGGAAACGAAGTTTCGCTT 598–617 (23S rDNA) AF182725 |
ITS = internal transcription spacer; PCR = polymerase chain reaction.
Accession numbers were obtained from GenBank database.
2.4. PCR amplification
Each PCR primer set was used in a single PCR reaction consisting of 1× PCR buffer (1× PCR buffer: 10mMTris-HCl, pH 8.8; 1.5 mM MgCl2; 50 mM KCl; and 0.1% Trition X-100), 200 μM concentration of each deoxynucleotide triphosphate (PROtech Technology Enterprise Co., Ltd, Taipei, Taiwan), 0.2 μMof each of the PCR primers, 0.6 units of Prozyme (PROtech Technology Enterprise Co., Ltd), 150–200 ng of bacterial DNA, and double-deionized water to a final volume of 25 μL. The PCR mixture was heated at 94°C for 5 minutes using a thermal cycler (Gene Amp PCR system 2720; Applied Biosystems, Carlsbad, CA, USA). Afterward, 35 PCR cycles were followed. For each cycle, denaturation, annealing, and extension were carried out at 94°C for 30 seconds, 59°C for 30 seconds (for LaITS-1F/LGA-1R primer set) or 66°C for 10 seconds (for LaITS-1F/LSA-1R primer set), and 72°C for 40 seconds, respectively. Final extension was carried out at 72°C for 5 minutes. To detect the amplified product, 10 μL of the PCR product was examined by electrophoresis through a 2% agarose gel in 1× TAE buffer (4mMTris acetate, pH 7.6; 1mMNa2-EDTA) using a 100-bp DNA ladder as a marker.
2.5. Total plate counts and real-time qPCR of L. gasseri and L. salivarius
Viable cell counts of L. gasseri and L. salivarius in the cell dilutions or probiotic product were determined according to the methods modified from Sheu et al [18]. For the probiotic product, 2 g of the sample was suspended in 2 mL of the sterilized water. One milliliter of the serial cell dilutions was then smeared on an MRS agar plate (5.3 cm diameter), followed by incubation at 37°C for 20 hours for viable cell counting. Meanwhile, L. gasseri and L. salivarius cells were counted by real-time qPCR using the SYBR Green PCR Master kit (nr. 4369155; Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instruction. Conditions for DNA extraction and PCR were as those described earlier for PCR amplification. A dissociation stage was followed for the melting curve analysis after the final extension step. The dissociation analysis determines the melting temperature (Tm) of the amplified products generated during PCRs. Melting curves were obtained for samples indentified as positive for L. gasseri and L. salivarius. For the standard curve, serial dilutions of L. gasseri and L. salivarius (1–9 log cells/mL) in 0.1 mL sterilized water, respectively, were subjected to DNA extraction and qPCR. For plate counting and qPCR assay, each assay was performed in triplicate.
2.6. PMA qPCR
Conditions for PMA treatment were according to the instruction from the PMA manufacturer (Biotium, Inc., Hayward, CA, USA) and the reports of Nocker et al [19] and Fujimoto and Watanabe [15]. PMA was dissolved in 20% dimethyl sulfoxide to a final concentration of 20 mM. Then, 2.5 μL PMA (20 mM) was added to 500 μL of cell suspension (final concentration of PMA, 100 μM), held in dark for 5 minutes. Then triplicate samples were placed in an ice bath and photoactivated using a 500 W halogen light source for 5minutes. After washing twice with PBS by suspension of the cells and centrifugation at 13,000 g (Eppendorf 5424) for 5 minutes, the PMA-treated cells were preserved at −20°C until DNA extraction and qPCR assay. To study the effect of PMA concentration and photoactivation time on the PMA-qPCR results, similar conditions were used except that 100–200 μM PMA concentrations and 5–15 minutes of photoactivation time were used.
2.7. Statistical analysis
Values were compared using a paired t test for parametric data and considered significant if p values were <0.05. Data were expressed as the mean ± standard error of mean (SEM) unless otherwise stated.
3. Results
3.1. Specificity of PCR primers
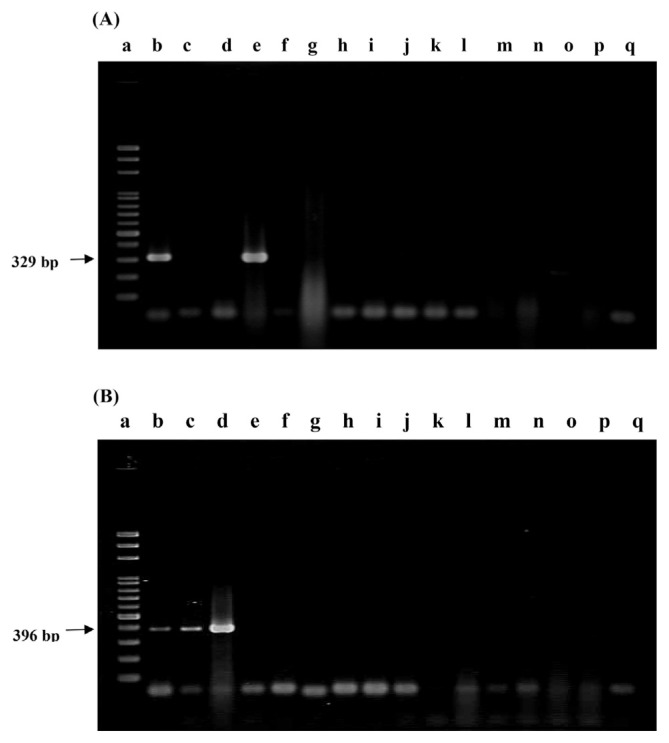
Two sets of PCR primers were designed for the specific detection of L. gasseri and L. salivarius. These oligonucleotide primers were LaITS-1F/LGA-1R and LaITS-1F/LSA-1R, respectively. Under the conditions described in the Methods section, all the four strains of L. gasseri and four strains of L. salivarius obtained from BCRC generated the expected PCR products, with molecular weights being equal to 329 bp and 396 bp, respectively (Figure 1). The specificity of these primers was further confirmed by a lack of any cross reaction with other strains including 39 Lactobacillus strains of 17 other Lactobacillus spp. and 44 strains of 23 Bifidobacterium spp., as well as 42 strains other than Lactobacillus and Bifidobacterium spp., such as those of the Enterococcus spp. and Bacillus species, and the family of Enterobacteriaceae (Table 1 and Figure 1). In addition, when strains in a commercial probiotic product, i.e., Kan-Min 2, labeled with L. gasseri and L. salivarius were assayed with primers LaITS-1F/LGA-1R (Figure 1A), and LaITS-1F/LSA-1R (Figure 1B), respectively, only two PCR products with molecular weights of 329 bp and 396 bp, respectively, were observed. Thus, specificity of these PCR primers was assured. These PCR products represented the presence of L. gasseri and L. salivarius in the product.
Figure 1.
PCR results using (A) L. gasseri-specific and (B) L. salivarius-specific primers. Lane a, 100-bp ladder; lane b, PCR products amplified from probiotic product; lanes c–q, PCR products amplified from strains of L. salivarius BCRC 12574 (c), L. salivarius BCRC 14759 (d), L. gasseri BCRC14619 (e), L. plantarum BCRC10069 (f), L. bulgaricus BCRC10696 (g), B. longum BCRC11847 (h), L. lactis BCRC12256 (i), L. acidophilus BCRC14065 (j), L. jensenii BCRC12939 (k), L. fermentum BCRC14691 (l), L. rhamnosus BCRC16000 (m), L. johnsonii BCRC17010 (n), Enterococcus faecium TM39 BCRC 910248 (o), other probiotic product (p), and negative control (q). PCR = polymerase chain reaction.
3.2. Total viable and culturable cell counts of the probiotic product
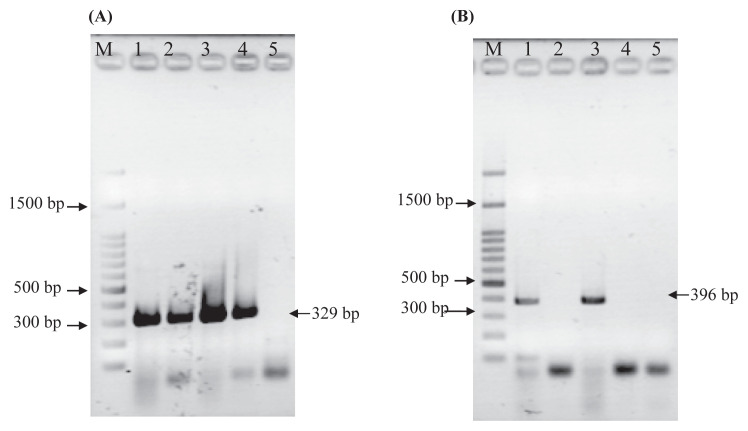
Based on the conditions described in the Methods section, LAB cells in the probiotic product were serially diluted to 107-fold and spread on an MRS agar plate for viable cell counting. A total cell count of 8.65 ± 0.69 log CFU/g of the sample was obtained. After heat treatment at 100°C for 30 minutes, none of the colonies was observed on the MRS agar plate. Such results indicate that all the cells were heat killed (Table 3). Regarding the heat-killing process, it should be mentioned that after heat treatment, the cell-free supernatant fraction from L. gasseri, but not from L. salivarius, generated detectable PCR products (Figure 2).
Table 3.
Detection and quantification of the Lactobacillus cells in probiotic products using plate count, and methods of realtime qPCR and PMA-qPCR.
| Unheated product | Heat-killed (100°C, 30 min) product | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Total plate counts | qPCR (log CFU/g)* | PMA-qPCR (log CFU/g)* | Total plate count | qPCR (log CFU/g)* | PMA-qPCR (log CFU/g)* | ||||
|
|
|
|
|
||||||
| LSA-1 | LGA-1 | LSA-1 | LGA-1 | LSA-1 | LGA-1 | LSA-1 | LGA-1 | ||
| 8.65 ± 0.69b | 8.57 ± 0.24 | 8.39 ± 0.14 | 8.49 ± 0.12 | 8.17 ± 0.44 | 0 | 7.67 ± 0.20 | 6.70 ± 0.16 | 5.33 ± 0.18 | 5.05 ± 0.23 |
| 8.79 ± 0.131,a | 8.67 ± 0.061,a | 7.72 ± 0.171,b | 5.59 ± 0.091,c | ||||||
PMA = propidium monoazide; qPCR = quantitative polymerase chain reaction.
Each value in the table represents the mean value ± standard deviation from three trials; different superscript letters in the row indicate significant differences (p < 0.05).
Sum of L. salivarius and L. gasseri.
Figure 2.
PCR assay of the cell pellets and supernatants from heat-killed (100°C, 10 minutes and 30 minutes) cells (8.5 log cells/mL) of (A) L. gasseri and (B) L. salivarius. Experimental conditions were as described in the Methods section. Lane M represents a 100-bp λ ladder. Lanes 1–5 represent the PCR results from pelleted cells (1 and 3) and cell-free supernatant (2 and 4), of cells heated for 10 minutes and 30 minutes, respectively. Lane 5 represents a negative control with sterilized H2O as the sample. PCR = polymerase chain reaction.
3.3. Quantification of L. gasseri and L. salivarius by qPCR
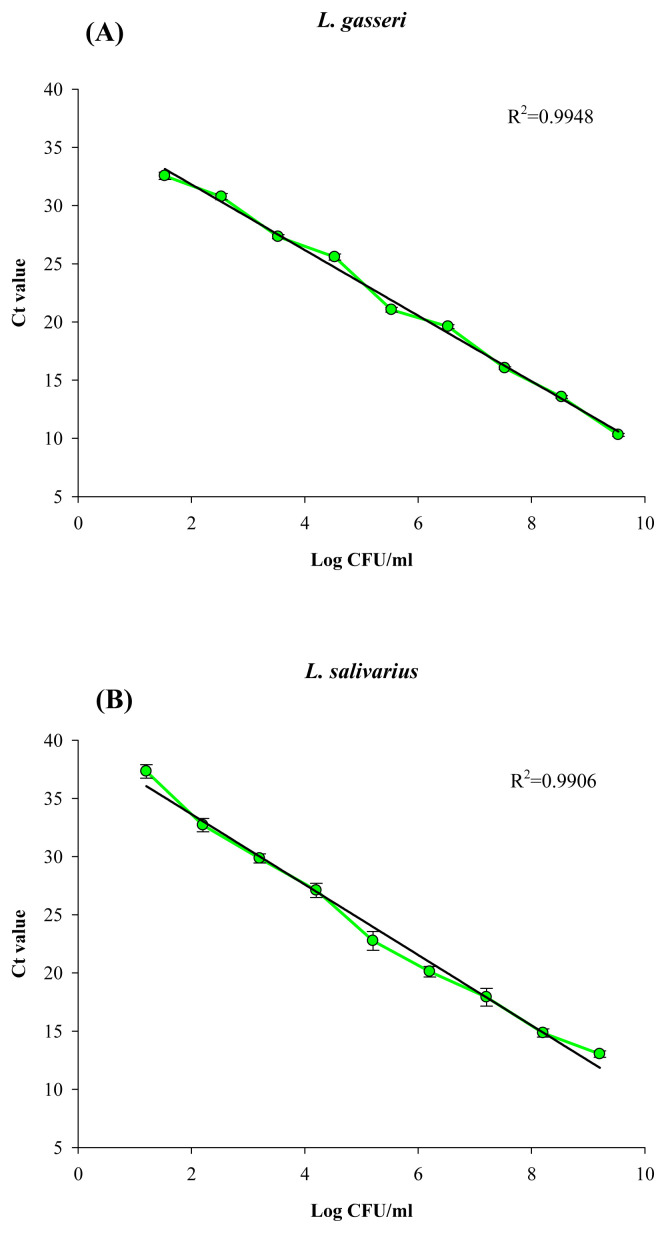
The standard curve of qPCR for each primer set was determined using 10-fold serial dilutions of the genomic DNA extracted from a known viable cell count of each target organism. Within the range of 2.0–8.5 log CFU/mL of L. gasseri and L. salivarius, the R2 of Ct values versus cell counts, using primers LaITS-1F/LGA-1R and LaITS-1F/LSA-1R, respectively, were 0.9948 and 0.9906, respectively (Figure 3A and B).
Figure 3.
Standard curve for quantification of (A) L. gasseri and (B) L. salivarius cells by real-time qPCR using specific primers of LaITS-1F/LGA-1 and LaITS-1F/LSA-1, respectively. Experimental conditions were as described in the Methods section. Error bars represent the standard deviation from the mean for three individual assays. qPCR = quantitative polymerase chain reaction.
The qPCR results of L. gasseri and L. salivarius in the product are shown in Table 3. For qPCR, viable cell counts of L. gasseri and L. salivarius in the product were determined as 8.39 ± 0.14 log CFU/g and 8.57 ± 0.24 log CFU/g, respectively. For heat-killed (100°C, 30 minutes) samples, qPCR counts of 6.70 ± 0.16 log cells/g and 7.67 ± 0.20 log cells/g for L. gasseri and L. salivarius, respectively, were observed. Such results were due to the persistence of residual DNA in the heat-killed cells (Table 3). Some DNA may leak or be damaged during the heat-killing process (Figure 2).
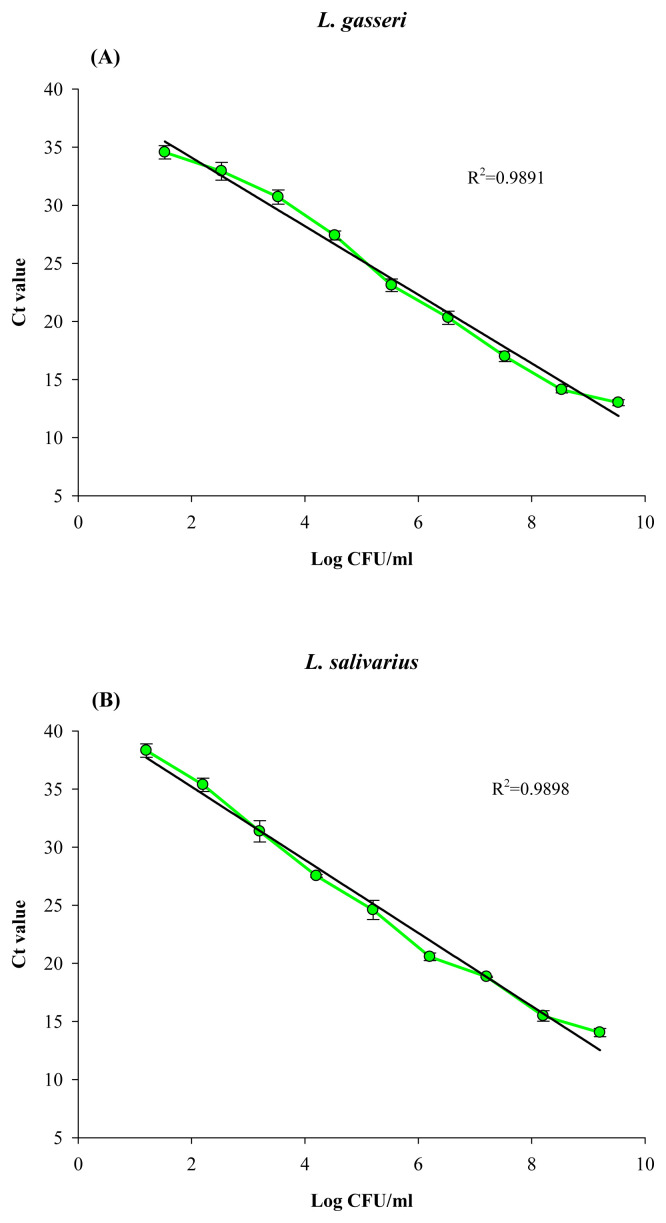
Based on the standard curve of Ct values versus viable cell counts for PMA-qPCR assay (Figure 4), the cell counts determined by PMA-qPCR for L. gasseri and L. salivarius in the probiotic product were 8.17 ± 0.44 and 8.49 ± 0.12, respectively. Comparing with the qPCR results without PMA treatment, i.e., 8.39 ± 0.14 CFU/g and 8.59 ± 0.24 CFU/g for L. gasseri and L. salivarius, respectively, most of the cells of both Lactobacillus strains in the original probiotic product could be viable cells (Table 3). By contrast, the PMA-qPCR counts determined for the heat-killed product were 5.05 ± 0.23 log cells/g sample and 5.33 ± 0.18 log cells/g sample for L. gasseri and L. salivarius, respectively. As such cell counts were compared with the qPCR counts without PMA treatment for both LAB strains in the heat-killed product (Table 3), such results indicated that despite the loss of some DNA during heat treatment, PMA did not fully inhibit the PCR signal from heat-killed cells of both LAB strains. Since the heat-killed probiotic product was prepared by heating at 100°C for 30 minutes, and its MRS agar plate counting showed none of the bacterial colonies; thus, it was impossible that the remaining PMA-qPCR counts were due to the residual viable cells in the heat-killed product.
Figure 4.
Standard curve for quantification of (A) L. gasseri and (B) L. salivarius by PMA-qPCR. Experimental conditions were as those described in Figure 2, except that cells were treated with PMA prior to qPCR. PMA = propidium monoazide; qPCR= quantitative polymerase chain reaction.
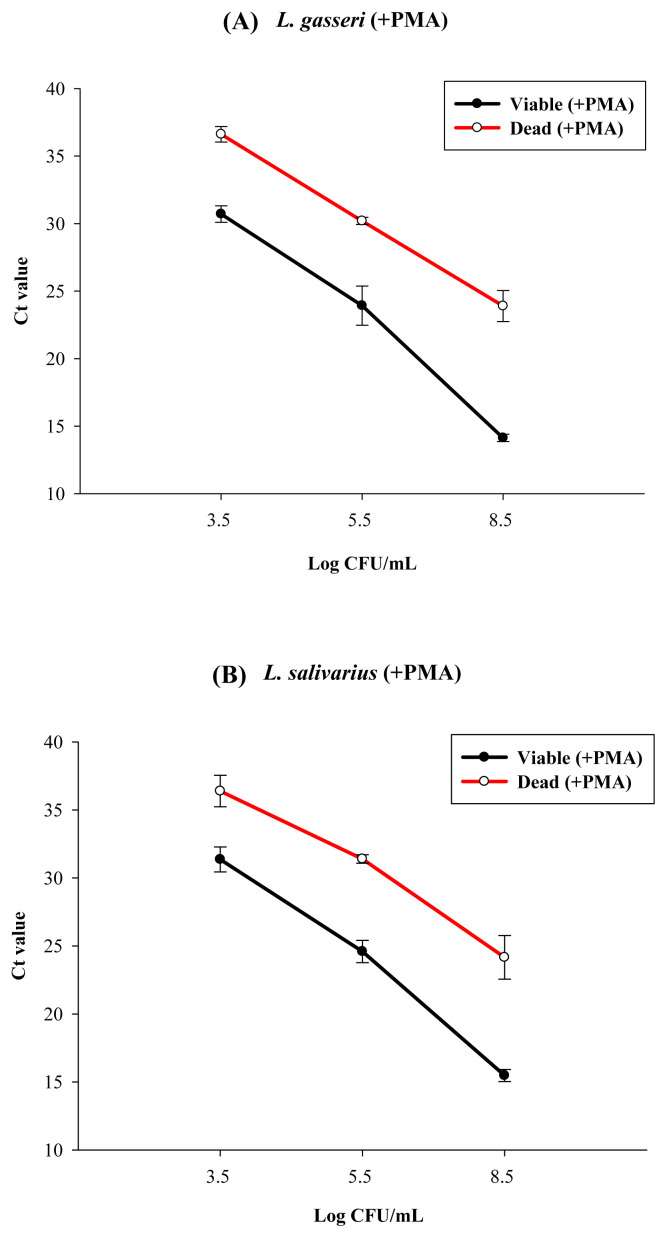
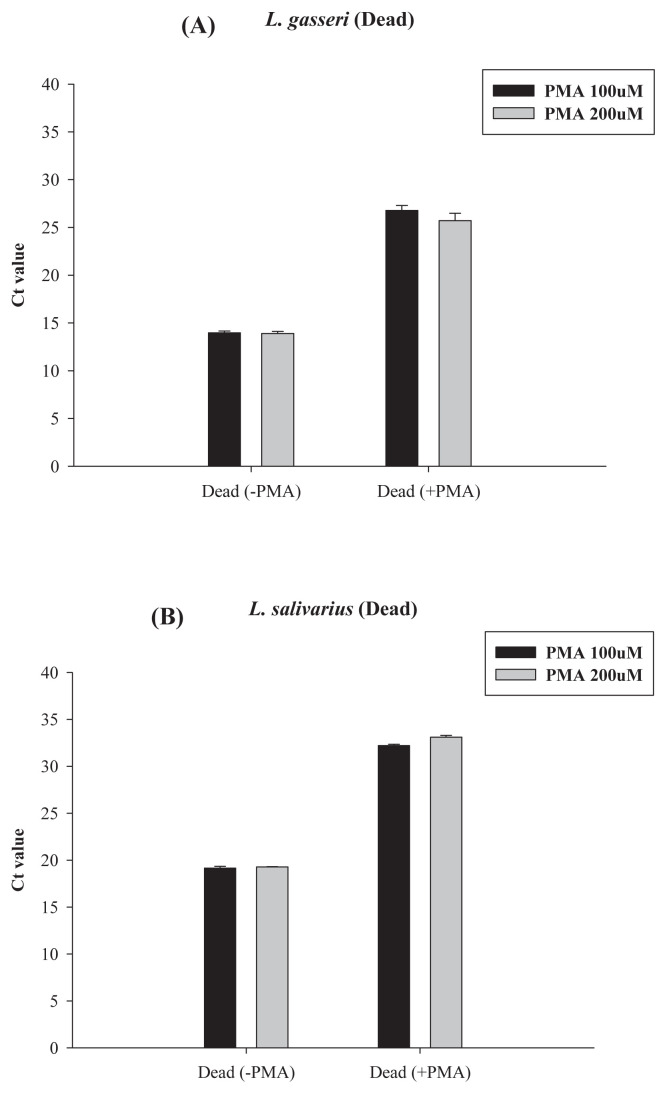
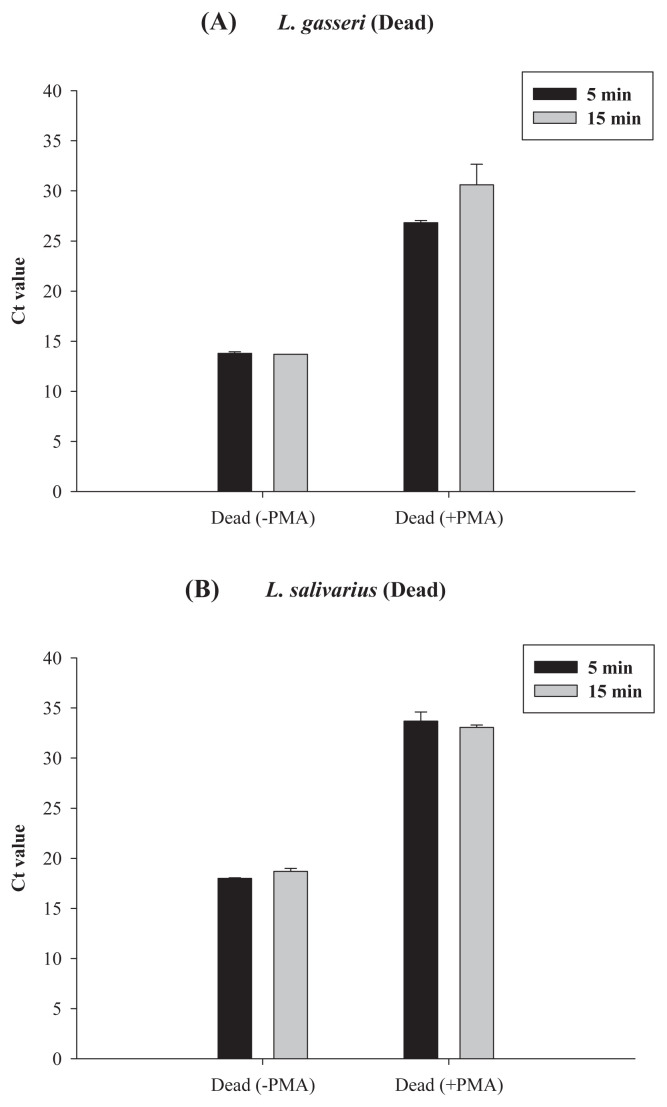
To further confirm the PMA-qPCR results for the heat-killed probiotic product, in a separate study, we used both the qPCR and the PMA-qPCR method to count the cells in the heat-killed (100°C, 30 minutes) solutions of L. gasseri and L. salivarius strains, with original viable cell concentrations being near to 3.5 log CFU/mL, 5.5 log CFU/mL, and 8.5 log CFU/mL for L. gasseri and L. salivarius (Figure 5). Results showed that for L. gasseri with an original viable cell count of 8.5 log CFU/mL, its Ct value from PMA-qPCR was 14 (Figure 5A); after heat killing, its Ct value from PMA-qPCR was near to 25, i.e., equal to the cell count near 5.5 log cells/mL. However, without PMA, its Ct value from qPCR was 15, i.e., with cell counts near to 7.4 log cells/mL (Figure 3A). Such data also imply that during heat killing, some DNA might be damaged or lost. Results in Figure 5A also meant that a significant ratio of qPCR signal, i.e., about 5.5 log cells/mL from dead cells, was not reduced by PMA treatment. Similar results were found for L. salivarius. About 5.5 log cells/mL qPCR signal from heat-killed cells could not be reduced by PMA treatment (Figure 5B). Only when their original viable cell concentrations were ≤3.5 log CFU/mL, after heat killing, the Ct values for both Lactobacillus strains could reach to 37–38, which meant that the residual cell counts were less than 1–2 log cells/mL, which was close to the detection limit of qPCR. Thus, for both L. gasseri and L. salivarius, when viable cells in cell dilutions were heat killed, the cell count determined by PMA-qPCR was about 1/1000–1/10,000 of that detected by qPCR depending on the original viable cell concentration. To improve the efficiency of PMA treatment, we have tried to increase the final PMA concentration from 100 μM to 200 μM, and the light exposure time from 5 minutes to 15 minutes (Figures 6 and 7). However, the increase in PMA concentration from 100 μM to 200 μM could not further reduce the qPCR signal from the heat-killed cells of those two LAB strains (Figure 6). Conversely, at 100 μM PMA concentration, the increase in the light exposure time from 5 minutes to 15 minutes might have minor effects, if any, on the qPCR signal from the heat-killed cells of L. gasseri, but not that from L. salivarius (Figure 7).
Figure 5.
qPCR and PMA-qPCR quantitative detection of heat-killed cells (100°C, 30 minutes) of (A) L. gasseri and (B) L. salivarius, with original viable cell concentrations of 3.5 log cells/mL, 5.5 log cells/mL, and 8.5 log cells/mL for L. gasseri and L. salivarius. Experimental conditions were as those described in the Methods section. Error bars represent the standard deviation from the mean for three independent replicates. PMA = propidium monoazide; qPCR = quantitative polymerase chain reaction.
Figure 6.
Effect of PMA concentrations on the PMA-qPCR assay of heat-killed cells of (A) L. gasseri and (B) L. salivarius. The qPCR results for heat-killed cells without PMA treatment were used for comparison. For all PMA-qPCR assays, the light exposure time was 5 minutes. PMA = propidium monoazide; qPCR = quantitative polymerase chain reaction.
Figure 7.
Effect of light exposure time on the PMA-qPCR assay of heat-killed cells of (A) L. gasseri and (B) L. salivarius. Real-time PCR results for heat-killed cells without PMA treatment were used for comparison. For all PMA-qPCR assays, the PMA concentration was 100 μM. PMA = propidium monoazide; qPCR = quantitative polymerase chain reaction.
4. Discussion
As described earlier, to assure the health function of probiotic products, manufacturers and public health administrators should be concerned about the right labeling of LAB species and viable cell counts of probiotic products. Thus, correct identification of each of the LAB species and their viable cell counts in the products, especially those containing different species of strains, is important. Since L. gasseri and/or L. salivarius have recently been used in different probiotic products, in this report, we developed PCR primers and assured their specificity to detect these two LAB species (Table 1). Using these primers, we were able to evaluate the efficiency of PMA-qPCR counting of the viable cells of the two LAB species, i.e., L. gasseri or L. salivarius, in the probiotic product. However, we found that PMA treatment could not effectively reduce the qPCR signal from heat-killed cells.
Although qPCR is regarded as a rapid and useful method to identify and quantify specific bacterial species in probiotics containing different bacterial species, its use as an accurate method for viable cell counting is limited since DNA can be detected hours or even days after cell death [20]. In this study, the primers we designed for the detection of L. gasseri and L. salivarius were based on the rRNA gene, which was one of the most common genes used for primer designing. However, because rRNA is not as liable as mRNA in dead cells, reverse transcription qPCR using rRNA gene-based primers may not be useful for the quantification of viable cells [21]. Thus, to count viable LAB cells, the PMA-qPCR method was used. Using the PMA-qPCR method to quantify the viable cells in a probiotic product without heat treatment, we found that statistically, the sum of the cell counts determined for L. gasseri and L. salivarius by PMA-qPCR was not significantly different from that obtained from either the total viable counts determined with an MRS agar plate or the sum of qPCR counts of these two LAB strains (Table 3). Since only two Lactobacillus species, i.e., L. gasseri and L. salivarius, were labeled on the probiotic product, most of the viable cells in this probiotic product could be viable cells of these two Lactobacillus species.
Recently, qPCR in combination with the use of PMA has become a recognized method to discriminate viable bacterial cells from dead cells, such as E. coli, Campylobacter, and LAB strains [11–13,15,16]. However, as qPCR in combination with PMA being widely used for counting viable bacterial cells, much confusion exists in the correct interpretation of results. For example, studies have found that the size of amplicon in a target gene, concentration of PMA, intensity of light and light exposure time, different causes of cell death, and even bacterial species as well as their growth phase may all affect the efficiency of reduction of qPCR signal from dead cells [12–14,22–24]. In this regard, to evaluate the effect of PMA on dead cells, it should be assured that all the LAB cells were killed and dead. In this study, heat treatment at 100°C for 30 minutes was thus used to assure the killing of all LAB cells (Table 1). Other heating conditions such as 60°C and 30 minutes, or 80°C and 30 minutes were not tried. Under our heating conditions, we also found that during the heating process, trace DNA was PCR detectable in the cell-free fraction of L. gasseri, but not of L. salivarius (Figure 2). Such results may be due to the difference in cell surface architecture of different Lactobacillus species or even strains [25,26].
In this study, conditions for PMA treatment, such as PMA concentration and light intensity as well as light exposure time for LAB treatment, were according to the protocol of the instruction sheet from the PMA manufacturer (Biotium, Inc.). Such conditions were similar to those used for many different microorganisms including LAB [14,27–29]. However, we found that under such conditions, when heat-killed cells with an original viable cell count of 8.5 log CFU/mL were treated with PMA followed by qPCR, PMA treatment could only reduce the qPCR signal to near to 5.5 log cells/mL for both L. salivarius and L. gasseri (Figure 5A and B). Increasing the PMA concentration from 100 μM to 200 μM could not affect the qPCR signal for both LAB strains. However, increasing the light exposure time from 5 minutes to 15 minutes seems to have a minor effect on the reduction of more qPCR signal from the heat-killed cells of L. gasseri (Figures 6 and 7). Such results might be due to the difference in the membrane properties of these two LAB species (Figure 2). In this regard, reports have shown that, in general, 2–10 minutes of light exposure has typically been used to detect viable bacteria or viruses [30–32]. As for the effect of PMA concentration, most of the studies used PMA concentrations ranging from 50–100 μM (25–50 μg/mL). Moreover, it should be reminded that optimized PMA concentration may be affected by many parameters, such as bacterial species, cell concentration, ratio between live and dead cells, and dye incubation time [33]. Using strain-specific primers, Fujimoto and Watanabe [15] found that for the viable cells of a specific Bifidobacterium bifidum strain, i.e., strain BF1, added to the fecal samples, the results from PMA-qPCR assay gave a viable cell count of 10.4 (±0.1) log cells/mL. However, when these B. bifidum BF1 cells were heat killed and then introduced into fecal samples, the number of BFI cells detected in the feces by PMA-qPCR was 1/10,000 of that detected without PMA treatment. In another report, Fujimoto et al [16] used strain-specific primers for the PMA-qPCR assay of viable and heat-killed cells of a B. breve strain Yakult (BbrY) strain added in human fecal samples. In the case of heat-killed BbrY cells, the use of qPCR without PMA treatment resulted in a reduced count of BbrY (from 9.6 to 8.6 log cells/mL). However, using qPCR with PMA treatment of the cells, only qPCR signal equivalent to 4.7 (±0.3) log cells/mL was reduced as compared with that of the cells without PMA treatment, i.e., 8.6 (±0.1) log cells/mL. Changes in light exposure time and PMA concentration from 50 to 150 μM also did not influence the enumeration of their heat killed BbrY cells. Thus the accuracy of the PMA-qPCR for enumeration of the viable BbrY cells in the feces was highly and significantly correlated with the number within the range of 5–9 log cells/g added to the fecal samples. Such results were similar to those of our study, as shown in Figures 5–7. Similar observations were found by Deng et al [34]. They tried to differentiate between the viable and dead cells of beer spoilage LABA, i.e., Lactobacillus acidotolerans, by the PMA-qPCR method based on horA-specific primers and found that the detection limit of PMA-qPCR was 100 cells/PCR assay, i.e., 5.0 log cells/mL bacterial culture. These results also seem to be similar to the cell counts obtained from our PMA-qPCR assay of the heat-killed cells of L. gasseri and L. salivarius with an original viable cell count of 8.5 log CFU/mL (Figure 5). Thus, for LAB strains, PMA may not be able to fully reduce the qPCR signal from dead cells.
5. Conclusions
In this study, two sets of species-specific primers were designed for the detection of L. gasseri and L. salivarius. Using these primers and PMA for the differentiation of viable and heat-killed cells of these two LAB species, we found that the efficiency of PMA in reducing qPCR signal from dead cells of different LAB species was notable. In addition, except for the facts that many factors may influence the PMA-qPCR results, for different LAB species, different responses of their cell membranes to the heat-killing conditions and thus PMA-qPCR results may also be considered. To improve the efficiency of PMA-qPCR detection of the viable cells of different LAB species, more studies are needed.
Acknowledgments
The authors would like to thank the Ministry of Science and Technology, Taipei, Taiwan, for the support of this project. The project numbers are MOST 103-2313 B-241-001 and NSC 102-2632-B-241-001-MY3-3.
Funding Statement
The authors would like to thank the Ministry of Science and Technology, Taipei, Taiwan, for the support of this project. The project numbers are MOST 103-2313 B-241-001 and NSC 102-2632-B-241-001-MY3-3.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Coeuret V, Gueguen M, Vernoux JP. Numbers and strains of lactobacilli in some probiotic products. Int J Food Microbiol. 2004;97:147–56. doi: 10.1016/j.ijfoodmicro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 2. Walter J. Ecological role of lactobacilli in the gastrointestinal track: implications for fundamental and biomedical research. Appl Environ Microbiol. 2008;74:4985–96. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Itoh T, Fujimoto Y, Kawai Y, Toba T, Saito T. Inhibition of food-borne pathogenic bacteria by bacteriocins from Lactobacillus gasseri. Lett Appl Microbiol. 1995;21:137–41. doi: 10.1111/j.1472-765x.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 4. Nishiyama K, Seto Y, Yoshioka K, Kakuda T, Takai S, Yamamoto Y, Mukai T. Lactobacillus gasseri SBT2055 reduces infection by and colonization of Campylobacter jejuni. PLoS One. 2014;9:e108827. doi: 10.1371/journal.pone.0108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carroll IM, Andrus JM, Bruno-Barcena JM, Klaenhammer TR, Hassan HM, Threadgill DS. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G729–38. doi: 10.1152/ajpgi.00132.2007. [DOI] [PubMed] [Google Scholar]
- 6. Sugawara T, Sawada D, Ishida Y, Aihara K, Aoki Y, Takehara I, Takano K, Fujiwara S. Regulatory effect of paraprobiotic Lactobacillus Gasseri cp2305 on gut environment and function. Microb Ecol Health Dis. 2016;27(30259):1–11. doi: 10.3402/mehd.v27.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107:1681–6. doi: 10.1111/j.1365-2672.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- 8. Uchida M, Shimizu K, Kurakazu K. Yogurt containing Lactobacillus gasseri OLL 2716 (LG21 yogurt) accelerated the healing of acetic acid-induced gastric ulcer in rats. Biosci Biotechnol Biochem. 2010;74:1891–4. doi: 10.1271/bbb.100287. [DOI] [PubMed] [Google Scholar]
- 9. Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM, Hale JDF, Wescombe PA. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J Med Microbiol. 2013;62:875–84. doi: 10.1099/jmm.0.056663-0. [DOI] [PubMed] [Google Scholar]
- 10. Messaoudi S, Manai M, Kergourlay G, Prevost H, Connil N, Chobert JM, Dousset X. Lactobacillus salivarius: bacteriocin and probiotic activity. Food Microbiol. 2013;36:296–304. doi: 10.1016/j.fm.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 11. Bae S, Wuertz S. Discrimination of viable and dead fecal Bacteroidales bacteria by quantitative PCR with propidium monoazide. Appl Environ Microbiol. 2009;75:2940–4. doi: 10.1128/AEM.01333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang X, Badoni M, Gill CO. Use of propidium monoazide and quantitative PCR for differentiation of viable Escherichia coli from E. coli killed by mild or pasteurizing heat treatments. Food Microbiol. 2011;28:1478–82. doi: 10.1016/j.fm.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 13. Pacholewicz E, Swart A, Lipman LJ, Wagenaar JA, Havelaar AH, Duim B. Propidium monoazide does not fully inhibit the detection of dead Campylobacter on broiler chicken carcasses by qPCR. J Microbiol Methods. 2013;95:32–8. doi: 10.1016/j.mimet.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 14. Radulovic Z, Mirkovic N, Bogovic-Matijasic B, Petrusic M, Petrovic T, Manojlovic V, Nedovic V. Quantification of viable spray-dried potential probiotic lactobacilli using real-time PCR. Arch Biol Sci. 2012;64:1465–72. [Google Scholar]
- 15. Fujimoto J, Watanabe K. Quantitative detection of viable Bifidobacterium bifidum BF-1 cells in human feces by using propidium monoazide and strain-specific primers. Appl Environ Microbiol. 2013;79:2182–8. doi: 10.1128/AEM.03294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujimoto J, Tanigawa K, Kudo Y, Makino H, Watanabe K. Identification and quantification of viable Bifidobacterium breve strain Yakult in human faeces by using strain-specific primers and propidium monoazide. J Appl Microbiol. 2011;110:209–17. doi: 10.1111/j.1365-2672.2010.04873.x. [DOI] [PubMed] [Google Scholar]
- 17. Sheu SJ, Hwang WZ, Chen HC, Chiang YC, Tsen HY. Development and use of tuf gene–based primers for the multiplex PCR detection of Lactobacillus acidophilus, Lactobacillus casei group, Lactobacillus delbrueckii, and Bifidobacterium longum in commercial dairy products. J Food Sci. 2009;72:93–100. doi: 10.4315/0362-028x-72.1.93. [DOI] [PubMed] [Google Scholar]
- 18. Sheu SJ, Hwang WZ, Chiang YC, Lin WH, Chen HC, Tsen HY. Use of tuf gene-based primers for the PCR detection of probiotic Bifidobacterium species and enumeration of bifidobacteria in fermented milk by cultural and quantitative real-time PCR methods. J Food Sci. 2010;75:M521–7. doi: 10.1111/j.1750-3841.2010.01816.x. [DOI] [PubMed] [Google Scholar]
- 19. Nocker A, Cheung CY, Camper AK. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J Microbiol Methods. 2006;67:310–20. doi: 10.1016/j.mimet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 20. Reimann S, Grattepanche F, Rezzonico E, Lacroix C. Development of a real-time RT-PCR method for enumeration of viable Bifidobacterium longum cells in different morphologies. Food Microbiol. 2010;27:236–42. doi: 10.1016/j.fm.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 21. McKillip JL, Jaykus LA, Drake M. Nucleic acid persistence in heat-killed Escherichia coli O157:H7 from contaminated skim milk. J Food Prot. 1999;62:839–44. doi: 10.4315/0362-028x-62.8.839. [DOI] [PubMed] [Google Scholar]
- 22. Kruger NJ, Buhler C, Iwobi AN, Huber I, Ellerbroek L, Appel B, Stingl K. “Limits of control”—crucial parameters for a reliable quantification of viable Campylobacter by real-time PCR”. PloS One. 2014;9:e88108. doi: 10.1371/journal.pone.0088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor MJ, Bentham RH, Ross KE. Limitations of using propidium monoazide with qPCR to discriminate between live and dead Legionella in biofilm samples. Microbiol Insights. 2014;7:15–24. doi: 10.4137/MBI.S17723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li B, Chen JQ. Development of a sensitive and specific qPCR assay in conjunction with propidium monoazide for enhanced detection of live Salmonella spp. in food. BMC Microbiol. 2013;13:273. doi: 10.1186/1471-2180-13-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sengupta R, Altermann E, Anderson RC, McNabb WC, Moughan PJ, Roy NC. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediators Inflamm. 2013;2013:237921. doi: 10.1155/2013/237921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chapot-Chartier MP, Kulakauskas S. Cell wall structure and function in lactic acid bacteria. Microb Cell Fact. 2014;13(Suppl 1):S9. doi: 10.1186/1475-2859-13-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yanez MA, Nocker A, Soria-Soria E, Murtula R, Martinez L, Catalan V. Quantification of viable Legionella pneumophila cells using propidium monoazide combined with quantitative PCR. J Microbiol Methods. 2011;85:124–30. doi: 10.1016/j.mimet.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 28. Desfosses-Foucault E, Dussault-Lepage V, Le Boucher C, Savard P, Lapointe G, Roy D. Assessment of probiotic viability during cheddar cheese manufacture and ripening using propidium monoazide-PCR quantification. Front Microbiol. 2012;3:350. doi: 10.3389/fmicb.2012.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Frankenhuyzen JK, Trevors JT, Flemming CA, Lee H, Habash MB. Optimization, validation, and application of a real-time PCR protocol for quantification of viable bacterial cells in municipal sewage sludge and biosolids using reporter genes and Escherichia coli. J Ind Microbiol Biotechnol. 2013;40:1251–61. doi: 10.1007/s10295-013-1319-x. [DOI] [PubMed] [Google Scholar]
- 30. Tseng CC, Hsiao PK, Chang KC, Chen WT, Yiin LM, Hsieh CJ. Optimization of propidium monoazide quantitative PCR for evaluating performances of bioaerosol samplers for sampling airborne Staphylococcus aureus. Aerosol Sci Technol. 2014;48:1308–19. [Google Scholar]
- 31. Nocker A, Camper AK. Novel approaches toward preferential detection of viable cells using nucleic acid amplification techniques. FEMS Microbiol Lett. 2009;291:137–42. doi: 10.1111/j.1574-6968.2008.01429.x. [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Mustapha A. Detection of viable Escherichia coli O157:H7 in ground beef by propidium monoazide real-time PCR. Int J Food Microbiol. 2014;170:48–54. doi: 10.1016/j.ijfoodmicro.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 33. Fittipaldi M, Nocker A, Codony F. Progress in understanding preferential detection of live cells using viability dyes in combination with DNA amplification. J Microbiol Methods. 2012;91:276–89. doi: 10.1016/j.mimet.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 34. Deng Y, Zhao J, Li H, Xu Z, Liu J, Tu J, Xiong T. Detection of culturable and viable but non-culturable cells of beer spoilage lactic acid bacteria by combined use of propidium monoazide and horA-specific polymerase chain reaction. J Inst Brew. 2016;122:29–33. [Google Scholar]