Abstract
Dimer sesquiterpene lactones (SLs), uvedafolin and enhydrofolin, against four monomer SLs isolated from yacon, Smallanthus sonchifolius, leaf were the most cytotoxic substances on HeLa cells (IC50 values 2.96–3.17 μM at 24 hours). However, the cytotoxic mechanism of dimer SL has not been elucidated yet. Therefore, in this study, we clarified the in vitro cytotoxic mechanism of uvedafolin on the HeLa cells, and evaluated the cytotoxicity against NIH/3T3 cells which were used as normal cells. In consequence, the dimer SLs had low toxicity for the NIH/3T3 cells (IC50 4.81–4.98 μM at 24 hours) and then the uvedafolin mediated cell cycle arrest at the G2/M phase and induced apoptosis on the HeLa cells evidenced by appearance of a subG1 peak. Uvedafolin induced apoptosis was attributed to caspase-9 and caspase-3/7 activities. An effectively induced apoptosis pathway was demonstrated from mitochondria membrane potential change and cytochrome c release to cytosol. These results reveal that uvedafolin induced apoptosis via the mitochondria pathway. The present results indicate the potential of uvedafolin as a leading compound of new anticancer agents.
Keywords: apoptosis, dimer sesquiterpene lactone, G2/M arrest, HeLa cells, Smallanthus sonchifolius
1. Introduction
Cancer is the leading cause of death worldwide. In 2012, there were 8.2 million deaths, 14.6% of the total global deaths, attributed to cancer [1] and the number of new cancer cases has been estimated to rise by about 23 million over the next two decades.
Uncontrolled cell proliferation is a characteristic of cancer, and then cancer cells become a solid tumor [2]. Cancer treatments, typically chemotherapy and radiation therapy, are conducted to inhibit cancer cell proliferation. Most chemotherapeutic agents contribute to DNA damage in cancer cells and consequently induce apoptosis [3].
Natural products have been discovered as sources of therapeutic agents for cancer. There are 206 anticancer agents used in chemotherapy of which 63% are derived from natural products or their derivatives [4]. In spite of many anticancer agents being discovered, some cancer cells have resistance to these new agents. Furthermore, the side-effects of cancer drugs are severe and can lead to secondary problems, as well as discomfort for patients during treatment. To reduce cancer cell resistance and the side-effects of drugs, and to get more active substances, drug designs, modifications, and isolations of effective novel substances from natural resources are needed. Investigation of effective novel substances may lead to new anticancer compounds with increased diversity of chemical and biological activities. Natural substances, such as polyphenolic compounds (e.g., flavonoids and phenylpropanoids), sesquiterpenes, diterpenes, polyketides, macrolides and alkaloids are being investigated as target resources, focusing on new types of chemicals and biological functions of anticancer agents [5,6].
Sesquiterpene lactones (SLs) have been identified in more than 5000 compounds and have demonstrated a wide range of biological activities such as antimicrobial, antiinflammatory, antifungal, antimalarial, and anticancer activities [7]. Parthenolide is well-known as an antiinflammatory and antitumor active substance in vitro and in vivo, and its derivative, dimethylamino-parthenolide, has been studied in a Phase 1 cancer clinical trial [8]. An α, β-unsaturated lactone structure on the SLs is important for the biological properties which can form a Michael-type addition reaction [9]. Currently, a novel dimer SL has been isolated and identified. More than 60 dimer SLs have been cited in recent literature. Biological activities, such as antiprotozoal [10] and cytotoxicity activities [11–13] have been reported for dimer SLs. Interestingly, the dimer SLs are assumed to have more valuable biologically active and drug-like characteristics when compared with the monomers, due to their unique structures [14].
In our previous study, two dimer SLs, uvedafolin and enhydrofolin, isolated from Smallanthus sonchifolius leaf (Figure 1), especially certain varieties of the plant, were found to be the most cytotoxic substance on HeLa cervical cancer cells due to the ester linkage of two sesquiterpene monomers [12]. However, the cytotoxic mechanism of the dimer SLs is not understood yet. Therefore, the aim of this study is to clarify the in vitro cytotoxic mechanism of dimer SL, uvedafolin from the S. sonchifolius leaf, on the HeLa cells, and the influence of cytotoxicity on the NIH/3T3 cell line used as a normal cell line.
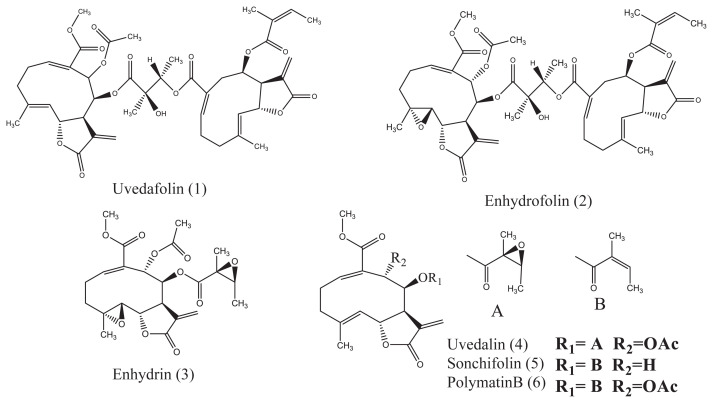
Figure 1.
Structures of sesquiterpene lactones (1–6) isolated from Smallanthus sonchifolius leaf.
2. Material and methods
2.1. Chemicals
Compounds 1–6 (purity > 95%, Figure 1) were isolated from S. sonchifolius leaf as described in a previous report by Kitai et al [12]. Etoposide, taxol, and parthenolide were purchased from Sigma-Aldrich Co. Ltd., (Tokyo, Japan). Propidium iodide (PI), RNase A, and 4′, 6-diamidino-2-phenylindole (DAPI) were obtained from Sigma-Aldrich Co. Ltd., (Tokyo, Japan). A JC-1 assay kit was purchased from Cayman Chemical Co. Ltd., (Ann Arbor, MI, USA). Anti-cytochrome c and immunoglobulin G horseradish peroxidase (HRP) antibody were obtained from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA, USA). A β-actin antibody was purchased from Gene Tex, Inc., (Irvine, CA, USA).
2.2. Cell lines and cultures
The HeLa and NIH/3T3 cell lines were purchased from RIKEN BRC (Tsukuba, Japan) and cells were cultured in an E-MEM (Wako, Ltd., Tokyo, Japan) medium supplemented with 4.2 mM HEPES (Dojindo Molecular Inc., Kumamoto, Japan), 10% (v/v) fetal bovine serum (Biosera, Boussens, France), 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere containing 95% air and 5% CO2.
2.3. Cytotoxicity assay
The cytotoxicities for the HeLa and NIH/3T3 cells were evaluated using a WST-8 assay. Cells (2 × 103 cell/well) were exposed to the test samples at different concentrations among 0.5–10 μM for 24 hours and 48 hours at 37°C. Control and blank (no cells) were exposed to 0.1% dimethyl sulfoxide (DMSO). After exposure was completed, 10 μL WST-8 reagent (Dojindo Molecular Inc., Kumamoto, Japan) was added to each well and then incubated for 4 hours. The optical density (OD) values at 450 nm were measured using a Multiskan FC Microplate Photometer (Thermo Scientific Inc., Waltham, MA, USA). The cell viability in treated populations was determined and expressed (n = 3) as percentages against that of the control treatment. The cytotoxicity inhibition concentration 50% (IC50) value was calculated from the dose-response curve using the Hillslope.
2.4. Cell cycle analysis
The HeLa cells (1 × 106 cells) were treated with Compound 1 at 3 μM for 24 hours and 48 hours. The cells were collected and fixed with 70% EtOH at −20°C. The fixed cells were treated with RNase A (250 μg/mL) for 1 hour at 37°C. The cells were stained with PI (50 μg/mL) for 30 minutes at room temperature in the dark. Cell cycle analysis was carried out using a FACS Cabibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Data was analyzed by using “Multi cycle AV for windows version 4.0” software. For each experiment, at least 20,000 cells were measured. Results are expressed as means ± standard deviation (SD) from triplicate assays.
2.4.1. DAPI staining
Compound 1 treated cells (2 × 103 cell/well) were fixed with 10% formaldehyde overnight at 4°C. The cells were stained with DAPI (1 μg/mL) for 30 minutes at room temperature in the dark. After washing, the cells were coated with a SlowFade-Antifade reagent and then covered by a cover glass. The stained cells were observed under a florescence microscope (372 nm excitation and 456 nm emission) at ×200 magnification (Olympus BX70, Tokyo, Japan).
2.4.2. Measurement of caspase activities
Caspase activities were measured by using FAM-FILICA caspase inhibitor fluorescence probes (Immunochemistry Technologies, LLC, Bloomington, MN, USA) as descried in a previous report [15]. FAM-DEVD-FMK, FAM-LETD-FMK, and FAM-LEHD-FMK were used for labeling caspase-3/7, caspase-8, and caspase-9, respectively. The cells (3 × 106 cells) were exposed to Compound 1 at 3 μM for 24 hours. The treated cells were collected and then incubated with 10 μL of each caspase probe for 1 hour at 37°C. After washing, the labeled cells were measured at 485 nm excitation and 530 nm emission using a CytoFluor Series 4000 fluorescence plate reader (Applied Biosystems, Waltham, MA, USA). The results were expressed as an n-fold increase in caspase activities against control.
2.5. JC-1 assay
Mitochondria membrane potential (MMP) was determined using a lipophilic cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) assay. The cells (5 × 105 cells) were exposed to Compound 1 at 3 μM for 24 hours and 48 hours. After exposure, the cells were collected and stained with 10 μL of JC-1 dye for 30 minutes at 37°C. The stained cells, corresponding to apoptotic cells, were washed and measured at 485 nm excitation and 530 nm emission using the fluorescence plate reader. Subsequently, the red fluorescence intensity, at 520 nm excitation and 595 nm emission, was saved as digital photos under a florescence microscope at ×400 magnification and the intensity was calculated by using the software, ‘Image J’, Wayne Rasband (NIH).
2.5.1. Determination of cytochrome c by western blot
The cells (3 × 106 cells) were exposed to Compound 1 at 3 μM for 24 hours and 48 hours. After exposure, the cells were washed and then collected by a cell scraper. The cytosolic fraction was extracted by using an Ezsubcell fractionation kit (ATTO, Tokyo, Japan) according to the manufacturer’s protocol. The lysed cells were centrifuged at 12,000g for 10 minutes at 4°C, and the supernatants were collected and kept at −20°C until analysis. The protein concentration was measured by the bicinchoninic acid (BCA) method (Thermo Scientific Inc.). Proteins (20 μg) were separated on a 12% acryl-amide gel by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then the separated proteins on the gel were transferred to a polyvinylidene difluoride membrane. The polyvinylidene difluoride membrane was blocked with 5% nonfat milk in phosphate buffered saline with Tween 20 (PBST) for 1 hour at room temperature. The membrane was probed with an anti-cytochrome c (1:100) antibody and a β-actin (1:1000) antibody. The membrane was washed with PBST and then incubated with an HRP-conjugated secondary antibody (1:10,000). Bound antibodies were detected by the enhanced chemiluminescence (ECL) method (PerkinElmer, Inc., Waltham, MA, USA).
3. Results
3.1. Cytotoxicity of uvedafolin on HeLa cells and NIH/3T3 cells
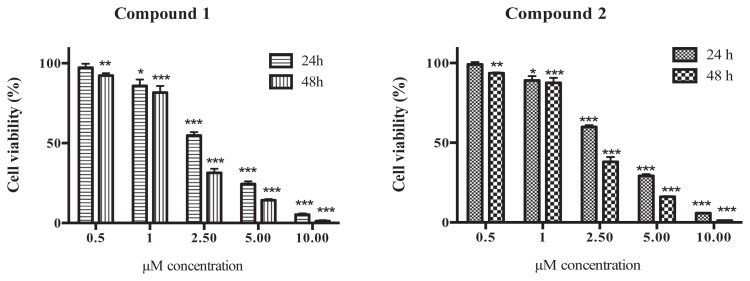
Effective concentration of dimer SL, Compound 1 against the cytotoxicity of HeLa cell line was tested at 24 hours and 48 hours incubation. The IC50s at 24 hours and 48 hours on HeLa cells were 2.96 μM and 1.69 μM, respectively (Table 1). Dimer sesquiterpene lactones (SLs), Compound 1 and Compound 2, decreased cell viability in a time- and dose-dependent manner on the HeLa cells (Figure 2). The dimer SL, Compound 1, showed three times greater activity than the 5 monomer SLs tested, including parthenolide and etoposide, against the HeLa cells at 24 hours. The IC50 of Compound 1 on NIH/3T3 cell line at 24 hours was 4.98 μM, which was a rate 1.68-times safer than the HeLa cells. The IC50 of enhydrin on the NIH/3T3 cells was 2.70 μM for 24 hours and 1.53 μM for 48 hours, showing the highest toxicity among seven kinds of SLs. Moreover, the IC50 of dimer SLs, Compound 1 and Compound 2 at 48 hours had a similar concentration between the HeLa cells and the NIH/3T3 cells, but the other five monomer SLs and etoposide showed high toxicity for the NIH/3T3 cells rather than for the HeLa cells.
Table 1.
Cytotoxic IC50 value (μM) of sesquiterpene lactones and anticancer drugs.
| HeLa a | NIH/3T3 b | |||
|---|---|---|---|---|
| Compounds | 24 h | 48 h | 24 h | 48 h |
| Uvedafolin (1) | 2.96 | 1.69 | 4.98 | 1.66 |
| Enhydrofolin (2) | 3.17 | 1.93 | 4.81 | 1.67 |
| Enhydrin (3) | 13.56 | 6.55 | 2.70 | 1.53 |
| Uvedalin (4) | 12.74 | 5.99 | 5.29 | 2.66 |
| Sonchifolin (5) | 17.55 | 9.92 | 13.93 | 11.12 |
| Polymatin B (6) | 9.38 | 5.51 | 9.09 | 5.24 |
| Parthenolide | 24.33 | 16.55 | 12.26 | 8.95 |
| Etoposide | 12.52 | 3.20 | 7.43 | 0.82 |
| Taxol | 0.055 | 0.0067 | 0.39 | 0.12 |
| Mitomycin C | 2.41 | 1.44 | 4.43 | 1.26 |
HeLa: cervical cancer cells.
NIH/3T3: mouse embryonic fibroblast cells.
Figure 2.
Effect of dimer sesquiterpene lactones (SLs), uvedafolin and enhydrofolin, on HeLa cell viability for 24 hours and 48 hours. Chemical structures of Compound 1 and Compound 2 are described in Figure 1. Results are expressed as means ± standard deviation (SD) from triplicates experiment. The data was analyzed by Dunnett’s test using SPSS. * p < 0.05, ** p < 0.01, and *** p < 0.001.
As the IC50 of Compound 1 at 24 hours was 2.96 μM, the concentration for further studies was set at 3 μM, meaning that the NIH/3T3 cell line had a high viability for survival.
3.2. Effect of uvedafolin on cell cycle in HeLa cells
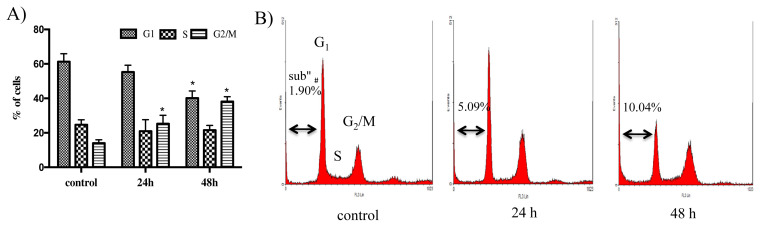
The HeLa cells treated by Compound 1 at 3 μM for 24 hours and 48 hours were compared with untreated cells (control). Cell cycle distribution (Figure 3) showed a significant increase in the percentage of G2/M phase from 14.0% to 22.9% for 24 hours and 38.1% for 48 hours time-dependently when compared with control. Furthermore, the percentage of G1 phase decreased significantly from 61.2% to 40.1% at 48 hours. Moreover, an increase in a sub-G1 peak, a parameter of fragmented DNA caused by apoptosis, was observed from 1.9% to 5.1% for 24 hours and 10.0% for 48 hours after treatment with Compound 1.
Figure 3.
Effect of uvedafolin on HeLa cell cycle distribution. Cells were exposed to uvedafolin at 3 μM for the indicated time and stained with propidium iodide (PI). DNA content was analyzed by flow cytometory. (A) Percentage of cell cycle distribution after treatment with compound 1. (B) DNA content of histogram (FL-3 Lin). Results are expressed as means ± standard deviation (SD) from triplicates experiment. The data was analyzed by Student t test using SPSS. * p < 0.05.
3.3. Effect of uvedafolin on nuclear morphology of HeLa cells
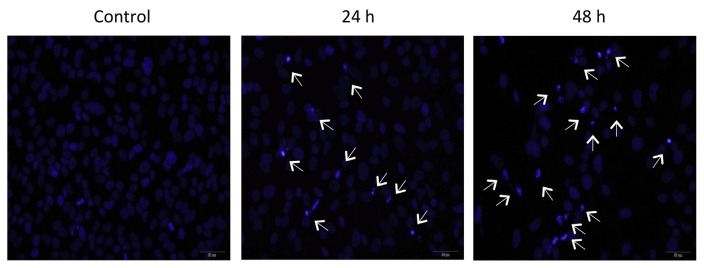
To observe the morphological changes of the nuclei, the cells were stained with DAPI fluorescence dye that specifically binds to A-T bond in chromatin. Untreated cells (control) displayed a typical HeLa cell nuclear pattern, while Compound 1 treated cells at 3 μM exhibited an increase of the condensed and fragmented nuclei time-dependently, detaching the apoptotic cells from the plate (Figure 4).
Figure 4.
Effect of uvedafolin on nuclear morphology of HeLa cells. Cells were exposed to uvedafolin at 3 μM for the indicated time and stained with 4′,6-diamidino-2-phenylindole (DAPI). The apoptotic cells with light blue color were visualized by the fluorescence microscope (excitation: 372 nm and emission: 456 nm). Arrows displayed the typical apoptotic cells.
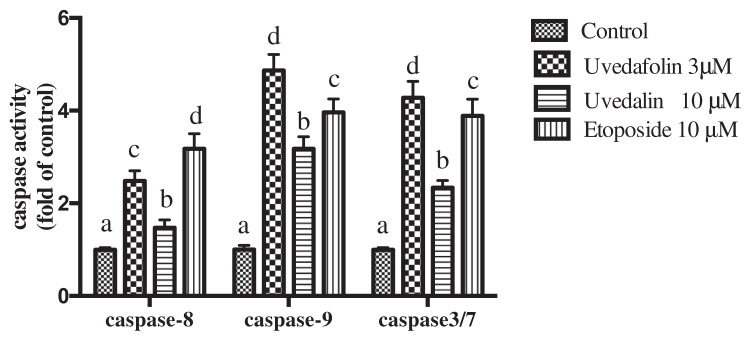
3.4. Effect of uvedafolin on caspase-3/7, caspase-8 and caspase-9 activities
When Compound 1 was added in the growth media of HeLa cells, all of the caspase activities were increased (Figure 5). Caspases are substrates that show specific enzyme activity, and originally present as inactive proenzyme form in the cells. The increases of caspase activities with Compound 1, uvedafolin, were 2.42-fold for caspase-8, 4.86-fold for caspase-9, and 4.27-fold for caspase-3/7 against those of control. The increases by Compound 4, uvedalin, were 1.47-fold increase for caspase-8, 3.17-fold for caspase-9, and 2.34-fold for caspase-3/7 against that of the untreated cells. Etoposide, an anticancer drug, treated cells exhibited 3.18-fold for caspase-8, 3.96-fold for caspase-9, and 3.88-fold for caspase-3/7. Compound 1 was the most effect to caspase-9, followed by caspase-3/7.
Figure 5.
Effect of uvedafolin on caspase-8, caspase-9, and caspase-3/-7 activities for 24 hours. The cells were labeled with fluorochrome inhibitor of caspase (FILCA) and measured by the fluorescence microplate reader (excitation: 485 nm and emission: 530 nm). Results are expressed as means ± standard deviation (SD). The data was analyzed by Duncan test using SPSS (p < 0.05).
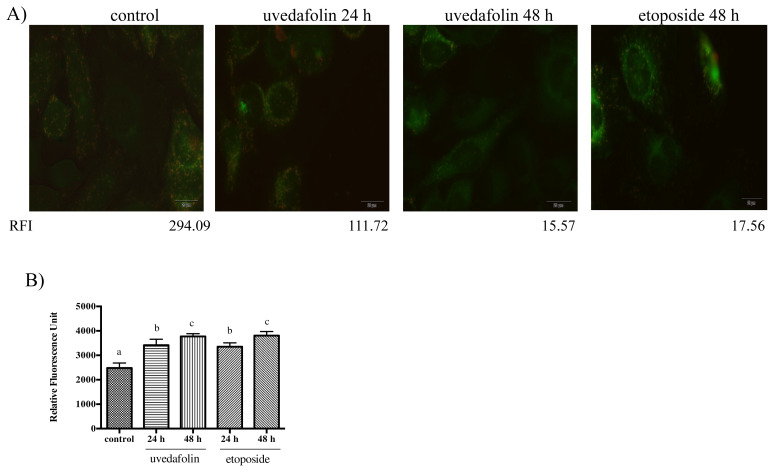
3.5. Effect of uvedafolin on MMP change
MMP was determined by using the JC-1 dye. The highly negative charge in healthy cells indicated that JC-1 shifts into mitochondria and then J-aggregates formed results in an increase of red fluorescence. By contrast, depolarization mitochondria in apoptotic cells led to an increase of green fluorescence, indicating that monomer JC-1 is kept in the cytosol without entering into the mitochondria.
The photo (Figure 6A) of untreated cells (control) bearing red and green fluorescence indicates healthy cells. Red fluorescence of untreated cells showed as 294 in intensity, meaning healthy cells, whereas the etoposide treated cells (48 hours) had decreased red fluorescence (red fluorescence intensity: 17.56). Treated cells with Compound 1 (24 hours and 48 hours) had decreased red fluorescence (red fluorescence intensity: 111.72 for 24 hours and 15.57 for 48 hours), indicating time-dependent apoptosis for the majority of cells. The green fluorescence unit of Compound 1 increased time-dependently and showed a similar effect with etoposide on the location of monomer JC-1 at cytosol, without entering into the mitochondria (Figure 6B).
Figure 6.
Effect of uvedafolin on mitochondrial membrane potential of HeLa cells. Cells were treated with 3 μM uvedafolin and 10 μM etoposide. (A) Fluorescent microscopy images (excitation: 520 nm and emission: 595 nm). The red fluorescence intensity (RFI) was described as the values at the bottom of images. (B) The relative fluorescence unit, apoptotic cells, of control and uvedafolin treatment cells. The cells stained with JC-1 and then measured at excitation: 485 nm and emission: 530 nm by fluorescence reader. Results are expressed as means ± standard deviation (SD). The data was analyzed by Duncan test using SPSS (p < 0.05).
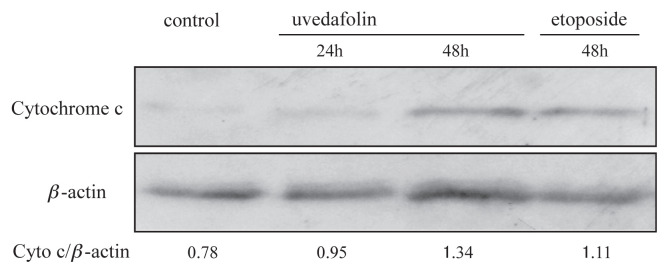
3.6. Effect of uvedafolin on cytochrome c releasing from mitochondria
Since cytochrome c plays an important role as a trigger of apoptosis signal, the existence of cytochrome c in cytosol was monitored by western blot analysis. Thus, after treatment with uvedafolin, release of cytochrome c in cytosol was enhanced time-dependently from 0.95 intensity to 1.34 (Figure 7). This result indicated that translocation of cytochrome c into cytosol sufficiently activated downstream caspases, such as caspase-9 and caspase-3/7.
Figure 7.
Effect of uvedafolin on cytochrome c releasing of HeLa cells. Cells were treated with 3 μM uvedafolin and 10 μM etoposide. The cytosolic fractions which contained 20 μg of proteins were subjected to western blot. β-actin was used as the internal standard. Specific antibodies were used for detection of cytochrome c and β-actin.
4. Discussion
The dimeric SL, uvedafolin, found in S. sonchifolius, is a unique chemical that has great cytotoxicity against the HeLa cells. The dimer SL is composed of two monomer SLs. Mixtures of monomer SLs on a mole basis did not show high cytotoxic activity, which differed from dimer SLs, Compound 1 and Compound 2 [12]. The dimer SLs have the two α-methylene-γ-lactone rings, alkylating elements, which are responsible for the activity. Biosynthesis of the dimer SL, Compound 1, is proposed as an acid form of Compound 5, derived by hydrolysis of the methyl ester moiety, and can couple with the epoxyangeloyloxy moiety of Compound 4 (Figure 1). Dimer SLs, including Compound 1, are chemically classified into more than six groups as follows: (1) esterification type; (2) Diels-Alder addition type; (3) [2+2] photosynthetic addition; (4) acetal type; (5) Michael addition type; and (6) others [16]. Compound 1 can be classified as one of the esterification type.
The cytotoxicities of Compound 1 on the HeLa cells were determined to be 2.96 μM at 24 hours and 1.69 μM at 48 hours as the IC50 (Table 1). Great cytotoxicity of dimeric SL against the HeLa cell line has been reported only in guaianolide dimer SL (esterification type), artemisinin dimer SL (acetal type), and melampolide dimer SL (esterification type, Compound 1). Among these substances, the greatest cytotoxicity was observed in melampolide dimer SL, Compound 1, when guaianolide dimer SL (IC50, 2.0 μM at 72 hours) [17] and artemisinin dimer SL (IC50, 67.4 μM at 96 hours) [18] were compared. For other cancer cell lines, cytotoxic activity was demonstrated for dimer SLs, biguaiascorzolide A on K562/ADM cell line (IC50, 39.8 μM at 72 hours) [11], neojaponicone B and six similar compounds on 6T-CEM and Jurkat cell line (IC50, 2.2–5.9 μM at 72 hours) [13], rufescenolide C on HT-29 cell line (IC50, 0.15 μM at 72 hours) [19], and three dimers of monomeric melampomagnolide B on eight kinds of cancer cell lines (IC50, 0.16–0.99 μM at 48 hours) [20]. All of these dimers showed greater activities than monomer SLs. Compound 1 (IC50, 1.69 μM at 48 hours) shows the greatest potent compound activity of these compounds.
The IC50 values of SLs and anticancer drugs against the HeLa cells should be lower than those against the NIH/3T3 cells, as normal cells (Table 1). However, the IC50 values of monomer SLs such as enhydrin, uvedalin, and parthenolide, and etoposide against the HeLa cells were higher in cytotoxicity than those against the NIH/3T3 cells. Only dimer SLs taxol and mitomycin C showed lower concentration or similar levels of concentration in toxicity for the HeLa cells rather than those of NIH/3T3 cells. Among the dimer SLs, taxol and mitomycin C, the dimer SLs had a similar trend in cytotoxicity with mitomycin C on the HeLa cells. For anticancer substances, there are some molecular mechanisms in which etoposide inhibits DNA replication, taxol builds block of the microtubules, and mitomycin C alkylates and cross-links between DNA. These three different mechanisms commonly induce cell cycle arrest at the G2/M phase. As the dimer SLs induce cell cycle arrest at the G2/M phase, the dimer SLs could be categorized in these mechanisms, especially because the mechanism is closely related to the mitomycin C type, as suggested by the similarity of the cytotoxicity trend.
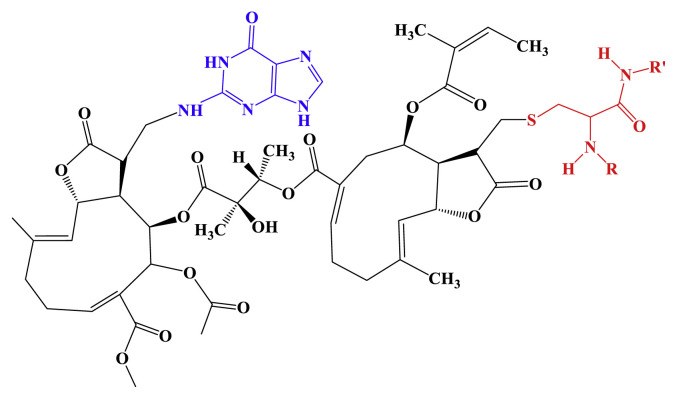
Furthermore, it has been reported that monomer SLs act to form monoadducts to DNA [21] and α-methylene-γ-lactone covalently linked to guanine and adenine [22]. The dimer SLs bearing two sets of α-methylene-γ-lactone in the molecule have the possibility to associate with cross-linkage between DNA, and/or DNA and protein. A possible representation of the mechanism of SLs on cytotoxicity is shown in Figure 8. Further study is necessary to clarify a direct target of uvedafolin and enhydrofolin in HeLa cells.
Figure 8.
Representation of the cytotoxicity mechanism of sesquiterpene lactones (SLs) for the uvedafolin that interacts with guanine and cysteine residue of protein. Guanine base is shown in blue and cysteine residue of protein with SL is shown in red.
In cell cycle analysis, treatment of Compound 1 on the HeLa cells exhibited an increase of G2/M phase of 2.7 times at 48 hours, when compared to the control (no treatment) (Figure 3). It has been known that DNA damages and deformation of the microtubules are associated with G2/M phase arrest, which results in mitotic catastrophe [23]. Robles et al [24] reported that germacranolides SLs involve inhibition of mitotic spindle function as inhibition tubulin polymerization. It is possible that Compound 1 could cause mitotic catastrophe by DNA damage and/or deformation of the microtubules reacting with the α-methylene-γ-lactone on SL.
While it has been established that cytotoxicity of the WST-8 assay against HeLa cells is due to apoptotic and necrotic cells [25], from our results (Table 1), the cytotoxic pathway of the dimer SLs could not be concluded to be apoptosis. Further study of the induction pathway of apoptosis of Compound 1 is needed. In subsequent analysis in apoptosis, it well known that two factors are related to apoptosis induction after treatment with Compound 1 against HeLa cells: the 10% appearance of the sub G1 peak, which is a parameter of DNA fragmentation based on cell cycle analysis (Figure 3); and the typical apoptotic bodies, which can be observed by DAPI stain occurring in time-dependent manner (Figure 4).
It is known that DNA fragmentation as an apoptotic feature is mediated by caspases, cysteine aspartate-specific proteases. There are two caspase cascades in apoptotic pathways: extrinsic and intrinsic pathways. Initiator caspase-8 is essential for the extrinsic pathway and caspase-9 is essential for the intrinsic pathway [26]. Dimer SL, Compound 1, activated caspase-9, rather than caspase-8, on the HeLa cells (Figure 5). Among several SLs, the apoptotic pathways are not consistent. Parthenolide induces a decline of mitochondrial potential and the release of cytochrome c resulted in the activation of caspase-9 and then induction of apoptosis via the intrinsic pathway [27], whereas it sensitizes cancer cells to tumor necrosis factor-related apoptosis-inducing ligand-induction apoptosis via the extrinsic pathway by enhancing the activities of caspase-3, caspase-8, and caspase-9 [28].
It can be concluded that dimer SL, Compound 1, clearly activated caspase-9 in the intrinsic pathway rather than caspase-8, and in the extrinsic pathway when caspase activities of monomer SLs and dimer SL, Compound 1, were compared with that of etoposide (Figure 5). Activation of caspase-8, caspase-9, and caspase-3/7 by Compound 1 was around 1.5 times greater than that of uvedalin. Siriwan et al [25] also found that the monomer SLs, uvedalin and sonchifolin, are representative SLs for great caspase-3/7 activity. So, dimer SL may be a particularly effective chemical for activation of caspases among SLs in S. sonchifolius. Furthermore, dimer SL increased the caspase-9 and caspase -3/7 activities more than etoposide, but etoposide showed caspase-8 activity greater than dimer SL. Therefore, it can be surmised that dimer SL is a good candidate for drugs that specifically enhance caspase-9.
Mitochondria play an important role in the triggering of apoptosis via the intrinsic pathways. Mitochondria release cytochrome c and other pro-apoptotic proteins with a loss of MMP by opening the mitochondria permeability transition pore [29]. The loss of MMP was observed by the JC-1 assay. Figure 6A shows MMP change after treatment with Compound 1 as the red color of mitochondria decreased from 294 to 15.6 in intensity. The large decrease of red color indicates the decrease of JC-1 aggregates and living cells, and the loss of MMP, time-dependently.
Figure 7 shows a clear band of cytochrome c that was detected at 24 hours and 48 hours in cytosol from mitochondria by western blot analysis after addition of dimer SL, Compound 1. The dimer SL induced cytochrome c release had similar intensity to the band to etoposide. Once in the cytosol, the cytochrome c forms apoptosomes which consist of cytochrome c, Apaf-1, and caspase-9, and then trigger caspase-3/7 activation [29]. This is more evidence for apoptosis induction by Compound 1 through the mitochondrial pathway by detection of cytochrome c in cytosol. It is reported that anti-cancer drugs targeted for apoptosis induction are mediated by the mitochondrial pathway [30]. Therefore, release of cytochrome c from mitochondria will support a great relationship of dimer SL, Compound 1, to a potent anticancer drug.
In conclusion, this study demonstrated the cytotoxic mechanism of dimer SL, Compound 1, against the HeLa cells with low toxicity to the NIH/3T3 cells. One of the cytotoxic mechanisms of Compound 1 is to mediate the cell cycle arrest at the G2/M phase and then to induce apoptosis on the HeLa cells. The other function of cytotoxicity by Compound 1 is to mediate apoptosis through an intrinsic apoptotic pathway, demonstrated from caspase-9 activity, MMP change, and cytochrome c detection at cytosol. The molecular targets of dimer SL, effective cytotoxicity against cancer cell lines, should be clarified to develop new anticancer agents in the future.
Acknowledgments
We would like to thank Professor Osamu Kawamura and Assistant Professor Yasunori Sugiyama of Kagawa University for providing access to western blot analysis in this study. We also are grateful to Associate Professor Peter Lutes of Kagawa University for language editing.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal DA. Global cancer statistics. Ca Cancer J Clin. 2012;2015(65):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2. Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 3. Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 4. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Millimouno FM, Dong J, Yang L, Li J, Li X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev Res. 2014;7:1081–107. doi: 10.1158/1940-6207.CAPR-14-0136. [DOI] [PubMed] [Google Scholar]
- 6. Murtaza G, Sajjad A, Mehmod Z, Shah S, Siddiqi A. Possible molecular targets for therapeutic applications of caffeic acid phenethyl ester in inflammation and cancer. J Food Drug Anal. 2015;23:11–8. doi: 10.1016/j.jfda.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci. 2013;14:12780–805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woods JR, Mo H, Bieberich AA, Alavanja T, Coldy DA. Amino-derivatives of the sesquiterpene lactone class of natural products as prodrugs. Med Chem Commun. 2012;4:27–33. [Google Scholar]
- 9. Ghantous A, Sinjab A, Herceg Z, Darwiche N. Parthenolide: from plant shoots to cancer roots. Drug Discov Today. 2013;18:894–905. doi: 10.1016/j.drudis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 10. Maas M, Hensel A, Costa FB, Brun R, Kaiser M, Schmidt TJ. An unusual dimeric guaianolide with antiprotozoal activity and further sesquiterpene lactones from Eupatorium perfoliatum. Phytochemistry. 2011;72:635–44. doi: 10.1016/j.phytochem.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 11. Zhu Y, Wu QX, Hu PZ, Wu WS. Biguaiascorzolides A and B: Two novel dimeric guaianolides with a rare skeleton, from Scorzonera austriaca. Food Chem. 2009;114:1316–20. [Google Scholar]
- 12. Kitai Y, Hayashi K, Otsuka M, Nishiwaki H, Senoo T, Ishii T, Sakane G, Sugiura M, Tamura H. New sesquiterpene lactone dimer, uvedafolin, extracted from eight yacon leaf varieties (Smallanthus sonchifolius): cytotoxicity in HeLa, HL-60, and Murine B16-F10 melanoma cell lines. J Agric Food Chem. 2015;63:10856–61. doi: 10.1021/acs.jafc.5b05229. [DOI] [PubMed] [Google Scholar]
- 13. Xu XY, Sun P, Guo DA, Liu X, Liu JH, Hu LH. Cytotoxic sesquiterpene dimers isolated from Inula japonica. Fitoterapia. 2015;101:218–23. doi: 10.1016/j.fitote.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 14. Ye J, Qin JJ, Su J, Lin S, Huang Y, Jin HZ, Zhang WD. Identification and structural characterization of dimeric sesquiterpene lactones in Inula japonica Thunb. by high-performance liquid chromatography/electrospray ionization with multi-stage mass spectrometry. Rapid Commun Mass Spectrom. 2013;27:2159–69. doi: 10.1002/rcm.6677. [DOI] [PubMed] [Google Scholar]
- 15. Trisonthi P, Sato A, Nishiwaki H, Tamura H. A new diterpene from Litsea cubeba fruits: structure elucidation and capability to induce apoptosis in HeLa cells. Molecules. 2014;19:6838–50. doi: 10.3390/molecules19056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lian G, Yu B. Naturally occurring dimers from chemical perspective. Chem Biodiversity. 2010;7:2660–91. doi: 10.1002/cbdv.201000038. [DOI] [PubMed] [Google Scholar]
- 17. Hilmi F, Gertsch J, Bremner P, Valovic S, Heinrich M, Stichera O, Heilmanna J. Cytotoxic versus anti-inflammatory effects in HeLa, Jurkat T and human peripheral blood cells caused by guaianolide-type sesquiterpene lactones. Bioorg Med Chem. 2003;11:3659–63. doi: 10.1016/s0968-0896(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 18. Beekman AC, Woerdenbag HJ, Kampinga HH, Konings AWT. Cytotoxicity of artemisinin, a dimer of dihydroartemisinin, artemisitene and eupatoriopicrin as evaluated by the MTT and clonogenic assay. Phytother Res. 1996;10:140–4. [Google Scholar]
- 19. Ren Y, Jiménez F, García R, Mejía M, Chai H, Farnsworth NR, Soejarto DD, Kinghorna AD. A cytotoxic dimeric furanoheliangolide from Piptocoma rufescens. Tetrahedron Lett. 2013;54:5457–60. doi: 10.1016/j.tetlet.2013.07.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janganati V, Ponder J, Jordan CT, Borrelli MJ, Penthala NR, Crooks PA. Dimers of melampomagnolide B exhibit potent anticancer activity against hematological and solid tumor cell. J Med Chem. 2015;58:8896–906. doi: 10.1021/acs.jmedchem.5b01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woynarowski JM, Napier C, Koester SK, Chen SF, Troyer D, Chapman W, MacDonald JR. Effects on DNA integrity and apoptosis induction by a novel antitumor sesquiterpene drug, 6-hydroxymethylacylfulvene (HMAF, MGI 114) Biochem Pharmacol. 1997;54:1181–93. doi: 10.1016/s0006-2952(97)00321-3. [DOI] [PubMed] [Google Scholar]
- 22. Lee KH, Rice GK, Hall IH, Amarnath V. Antitumor agents. 86. Synthesis and cytotoxicity of α-methylene-γ-lactone-bearing purines. J Med Chem. 1987;30:586–8. doi: 10.1021/jm00386a025. [DOI] [PubMed] [Google Scholar]
- 23. Spies L, Koekemoer TC, Sowemimo AA, Goosen ED, Van de Vender M. Caspase-dependent apoptosis is induced by Artemisia afra Jacq. ex Willd in a mitochondria-dependent manner after G2/M arrest. S Afr J Bot. 2013;84:104–9. [Google Scholar]
- 24. Robles AJ, Peng J, Hartley RM, Lee B, Mooberry SL. Melampodium leucanthum, a source of cytotoxic sesquiterpenes with antimitotic activities. J Nat Prod. 2015;78:388–95. doi: 10.1021/np500768s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siriwan D, Naruse T, Tamura H. Effect of epoxides and α-methylene-γ-lactone skeleton of sesquiterpenes from yacon (Smallanthus sonchifolius) leaves on caspase-dependent apoptosis and NF-κB inhibition in human cervical cancer cells. Fitoterapia. 2011;82:1093–101. doi: 10.1016/j.fitote.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 26. Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–31. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 27. Zhang S, Ong CN, Shen HM. Involvement of proapoptotic Bcl-2 family members in parthenolide-induced mitochondrial dysfunction and apoptosis. Cancer Lett. 2004;211:175–88. doi: 10.1016/j.canlet.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 28. Kim SL, Liu YC, Park YR, Seo SY, Kim SH, Kim IH, Lee SO, Lee ST, Kim DG, Kim SW. Parthenolide enhances sensitivity of colorectal cancer cells to TRAIL by inducing death receptor 5 and promotes TRAIL-induced apoptosis. Int J Oncol. 2015;46:1121–30. doi: 10.3892/ijo.2014.2795. [DOI] [PubMed] [Google Scholar]
- 29. Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 30. Kim R, Tanabe K, Uchida Y, Emi M, Inoue H, Toge T. Current status of the molecular mechanisms of anticancer drug-induced apoptosis: The contribution of molecular-level analysis to cancer chemotherapy. Cancer Chemother Pharmacol. 2002;50:343–52. doi: 10.1007/s00280-002-0522-7. [DOI] [PubMed] [Google Scholar]