Abstract
Uric acid (UA) is an end product of purine metabolism by the enzyme xanthine oxidase (XOD). Hyperuricemia is characterized by the accumulation of serum UA and is an important risk factor for gout and many chronic disorders. XOD inhibitors or uricase (catalyzes UA to the more soluble end product) can prevent these chronic diseases. However, currently available hypouricemic agents induce severe side effects. Therefore, we developed new microbial fermented extracts (MFEs) with substantial XOD inhibition activity from Lactobacillus (MFE-21) and Acetobacter (MFE-25), and MFE-120 with high uricase activity from Aspergillus. The urate-lowering effects and safety of these MFEs were evaluated. Our results showed that MFE-25 exerts superior urate-lowering effects in the therapeutic model. In the preventive model, both MFE-120 and MFE-25 significantly reduced UA. The results of the safety study showed that no organ toxicity and no treatment-related adverse effects were observed in mice treated with high doses of MFEs. Taken together, the results showed the effectiveness of MFEs in reducing hyperuricemia without systemic toxicity in mice at high doses, suggesting that they are safe for use in the treatment and prevention of hyperuricemia.
Keywords: hyperuricemia, microbial fermented extracts, uricase, uric acid, xanthine oxidase
1. Introduction
Uric acid (UA) is an end product of purine metabolism produced mainly by the liver and intestines, as well as other peripheral tissues such as muscles, the endothelium, and the kidney [1]. Unlike other mammals, human beings have a higher serum UA level because they lack UA oxidase (uricase), an enzyme that catalyzes UA to the more soluble end product allantoin [2]. Under normal conditions, two-thirds of UA is eliminated in the urine and one-third is removed by the biliary tree [1]. Hyperuricemia is characterized by the accumulation of serum UA beyond its solubility point in water (6.8 mg/dL), and it develops because of UA overproduction, UA under-secretion, or a combination of both [3]. Under normal conditions, UA provides an antioxidant defense in humans; therefore, it might be protective against oxidative stress in cardiac, vascular, and neuronal cells [4]. However, elevated serum UA can decompose and generate free radicals in various conditions, leading to increased oxidative stress, inflammation, cell proliferation, and angiotensin II production; therefore, hyperuricemia is an important risk factor for gout and is associated with the development of many disorders such as cardiovascular disease, diabetes, renal failure, obesity, dyslipidemia, cancer, and increased mortality [5,6]. Accordingly, it is necessary to treat or prevent hyperuricemia by physiological regulation in order to reduce UA-related disorders.
In humans, UA forms in the final step of the purine catabolic pathway, as a product of the oxidation xanthine by the enzyme xanthine oxidoreductase. Xanthine oxidoreductase is a molybdoflavoprotein enzyme that exists in two functionally distinct forms—xanthine dehydrogenase and xanthine oxidase (XOD)—which are present in significant concentrations in the liver and intestines. XOD catalyzes the two terminal steps of purine degradation by the oxidation of hypoxanthine to xanthine and xanthine to UA, with the concomitant production of hydrogen peroxide and superoxide anions in humans [4]. Xanthine dehydrogenase and XOD inhibitors such as allopurinol (AP) or febuxostat are available to block the final step in UA synthesis and to reduce the production of UA. At present, AP is the most common clinically applied XOD inhibitor prescribed for the treatment of hyperuricemia. However, these existing hypouricemic agents possess a number of adverse side effects such as allergic and hypersensitivity problems, nephropathy, liver toxicity, and the enhancement of 6-mercaptopurine toxicity [7].
Taking into account the adverse side effects of existing hypouricemic agents, developing novel urate-lowering agents is necessary for the management of hyperuricemia. It has been reported that many flavonoids ubiquitously distributed in various foods and beverages have antioxidant and XOD inhibitory activities [8,9]. The consumption of flavonoids has been shown to be associated with the protective effects of certain diets and herbs against hyperuricemia and gout [10,11]. Many microorganisms, such as pink oyster mushrooms, also have antioxidant and XOD inhibitory activities, suggesting that microorganisms possess XOD inhibitory activities that could be a potential resource for developing hypouricemic agents [12]. In addition to XOD inhibitors, the treatment of hyperuricemia entails other approaches including using recombinant uricase or inhibitors of renal urate reabsorption such as probenecid and benzbromarone [2]. Uricase has been found in mammals, plants, and also in microorganisms such as bacteria, yeast, and filamentous fungi [13]. Previous studies indicated that uricase is an important medical enzyme that has advantages for gout treatment compared with AP [14]. Thus, the development of new hypouricemic agents from microorganisms, including XOD inhibitors, or agents with uricase activity, with a more favorable toxicological profile is highly warranted.
Accordingly, we studied the XOD inhibitory activities and uricase activity of fermented food microorganisms from two strains, Lactobacillus microbial fermented extract (MFE)-21 and Acetobacter MFE-25, which expressed a high XOD inhibitory effect, and one strain, Aspergillus MFE-120, which expressed high uricase activity. The aim of this study was to evaluate the therapeutic and preventive effects of hyperuricemia using these MFEs. Furthermore, we provide evidence of safety for the use of high doses of MFEs for 14 days via toxicological experiments in mice. The results showed the effectiveness of MFEs in reducing hyperuricemia without systemic toxicity in mice at high doses, suggesting they are safe for use in the treatment and prevention of hyperuricemia.
2. Methods
2.1. Chemicals and reagents
The XOD inhibitor AP and uricase inhibitor potassium oxonate (PO; or oxonic acid potassium salt) were purchased from Sigma (St. Louis, MO, USA). The UA assay kit and Xanthine Oxidase Fluorometric Assay Kit was purchased from Cayman Chemical Company (Ann Arbor, MI, USA).
2.2. MFE preparation (MFE-21, MFE-25, and MFE-120)
Lactobacillus MFE-21 (1 μL) was inoculated into 10-mL de Man–Rogosa–Sharpe broth (purchased from DIFCO, Becton, Dickinson and Company, NJ, USA) and incubated for 1 day at 37°C under anaerobic conditions. Then, 500 μL of Acetobacter MFE-25 was inoculated onto a 250-mL flask containing 50 mL of M1 medium (2.5% mannitol, 0.5% yeast extract, and 0.3% peptone) and incubated for 2 days at 30°C. Aspergillus oryzae MFE-120 (0.5 mL) was inoculated onto a 250-mL flask containing 50 mL of SYP medium (soy peptone 1%, yeast extract 1%, peptone 1%, malt extract 1%, glucose 1%) and incubated for 4 days at 30°C and 150 rpm. The fermented broths of the three strains were homogenized (20,000 rpm, 30 seconds at 4°C), centrifuged (10,000 rpm, 10 minutes at 4°C), and concentrated by lyophilization. The concentrates were assayed for their XOD inhibitory activity or uricase activity.
2.3. In vitro XOD inhibition (MFE-21 and MFE-25)
The in vitro XOD inhibitory activity of various samples from strains MFE-21 and MFE-25 was assayed spectrophotometrically under aerobic conditions using xanthine as the substrate [12]. In a reaction tube, 880 μL of xanthine (50 μg/mL in 100 mM phosphate-buffered saline) and 40 μL of 50 mM phosphate-buffered saline or 40 μL of the culture supernatants of MFE-21 or MFE-25 were premixed, and 80 μL XOD (0.1 U) was added to initiate the reaction. The reaction was incubated at 30°C for 30 minutes, after which an equal volume of absolute ethanol was added to terminate the reaction. The terminated reaction was filtered through a 0.25-μm membrane filter, and the content of xanthine was analyzed by high-performance liquid chromatography. The XOD inhibitory activity of the samples was calculated as follows:
| (1) |
2.4. Animals
Four-week-old ICR mice were purchased from BioLASCO Taiwan Co., Ltd, Taipei, Taiwan. They were housed (five mice per cage) in a pathogen-free environment, maintained on Lab Diet 5010 chow (PMI Feeds, Inc., St. Louis, MO, USA) at 24 ± 2°C and 50 ± 10% relative humidity, and subjected to a 12-hour light/12-hour dark cycle in the Laboratory Animal Center of the National Cheng Kung University Medical College. All animal studies were approved by the Laboratory Animal Center of the National Cheng Kung University Medical College and performed according to the local guidelines for animal care and protection.
2.5. Hyperuricemia therapeutic animal model
At 5 weeks of age, male ICR mice were randomly divided into different groups. Food, but not water, was withdrawn from the animals 1 hour prior to drug treatment. The vehicle control groups were given 0.9% normal saline 200 μL orally. To produce hyperuricemia, mice received PO at 400 mg/kg for 1 hour prior to testing the drug administration by gastric gavage. PO is usually used to develop a rodent model of hyperuricemia by inhibiting uricase activity [15]. AP (10 mg/kg) or tested MFEs (MFE-21 or MFE-25) with various concentrations dissolved in 0.9% normal saline were given orally immediately after PO for 1 hour. All mice were then euthanized under ether anesthesia, and whole blood samples were collected from cardiac puncture. The blood was allowed to clot for approximately 30 minutes at room temperature and then centrifuged at 2000g for 15 minutes to collect the serum. The serum was stored at −80°C until assayed.
2.6. Hyperuricemia preventive animal model
The preventive animal model was adapted from a previous report [16]. As shown in Figure 2A, control mice were orally administered with 200 μL of 0.9% normal saline. Hyperuricemia mice were treated with 400 mg/kg PO at Days 1, 3, 5, and 7 by gastric gavage. The preventive drugs (AP and MFEs) were dissolved in 0.9% normal saline and orally administration once daily from Day 1 to Day 7. At the end of the experiments, all mice were euthanized under ether anesthesia 1 hour after final administration on Day 7. Whole blood samples were collected from the heart and centrifuged at 2000g for 15 minutes to measure the serum UA levels. Livers were removed, cut into pieces, and stored at −80°C for XOD analysis.
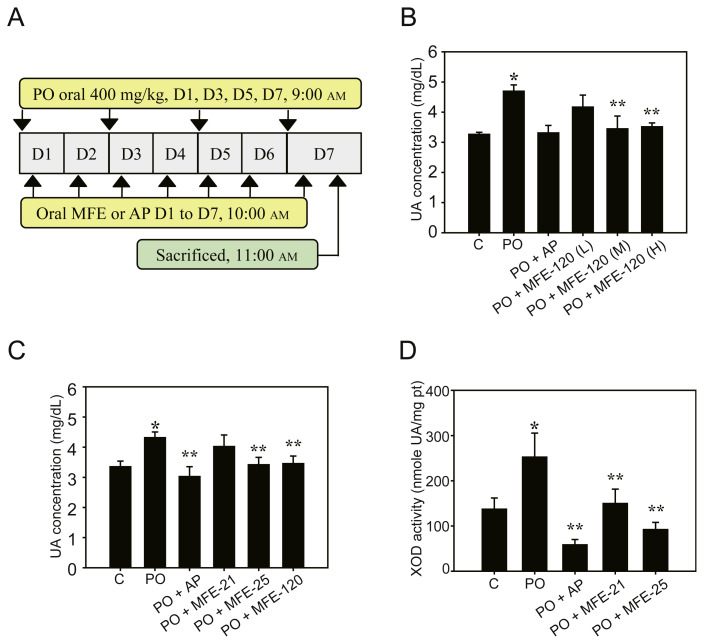
Figure 2.
Preventive effects of microbial fermented extracts (MFEs) in a hyperuricemia preventive animal model. (A) Scheme of hyperuricemia preventive animal model as described in the Methods section; (B) concentration-dependent inhibitory effects of serum uric acid (UA) levels in mice treated with MFE-120. Control mice received 0.9% saline (control group), and the potassium oxonate (PO) group was given PO at 400 mg/kg on Days 1, 3, 5, and 7. Different doses of MFE-120 were given continuously for 7 days. MFE-120 (L): 5 mg/kg; MFE-120 (M): 10 mg/kg; and MFE-120 (H): 20 mg/kg. Data represent the mean ± standard deviation (SD) of three mice per group; (C) hyperuricemia prevention effects of MFEs in mice treated with 0.9% saline, PO, PO + MFE-21 (150 mg/kg), PO + MFE-25 (150 mg/kg), or PO + MFE-120 (10 mg/kg) for 7 days. Data represent the mean ± SD of 10 mice per group; (D) effects of MFEs on xanthine oxidase (XOD) activities in the livers of a hyperuricemia preventive animal model. Mice were treated with 0.9% saline (control group), PO, PO + MFE-21 (150 mg/kg), or PO + MFE-25 (150 mg/kg). Data represent the mean ± SD of five mice per group. * p < 0.05 compared with the control groups. ** p < 0.05 compared with PO groups. C = control; H = high dose; L = low dose; XOI = xanthine oxidase inhibitory activity.
2.7. UA analysis
The serum UA level was determined using the UA Assay Kit according to the manufacturer’s instructions. This kit provides a fluorescence-based method for detecting UA. In the assay, uricase catalyzed the conversion of UA (in the serum) to allantoin, hydrogen peroxide (H2O2), and carbon oxide. In the presence of horseradish peroxidase, H2O2 reacts with 10-acetyl-3,7,-dihydroxyphenoxazine to produce the highly fluorescent compound resorufin. Resorufin fluorescence was analyzed with an excitation wavelength at 530 nm and an emission wavelength at 595 nm. The concentration of UA in the serum sample was calculated using the equation determined from different doses of standards.
2.8. Assays of mouse liver XOD activities
Mouse livers collected from the hyperuricemia preventive animal model were homogenized by lysis buffer [137 mM NaCl, 20 mM Tris, pH 7.9, 10 mM NaF, 5 mM EDTA, pH 8.0, 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 10 % (volume/volume) glycerol, 1 % Triton X-00, 5 mM sodium orthovanadate, 1 mM pyrophosphate, 100 μM β-glycerophosphate, 2 mM phenylmethanesulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 2 μg/mL pepstatin, and 5 mM 1,4-dithiothreitol]. The homogenates were centrifuged at 14,000 rpm for 30 minutes at 4°C. The final supernatant was used for XOD analysis with the Xanthine Oxidase Fluorometric Assay Kit according to the manufacturer’s instructions. Briefly, XOD oxidized hypoxanthine then produced H2O2 during the multiple enzymatic reactions. H2O2 reacts with 10-acetyl-3,7,-dihydroxyphenoxazine to produce the highly fluorescent compound resorufin, which can be detected with an excitation wavelength at 530 nm and an emission wavelength at 595 nm.
2.9. Clinical biochemical analysis
Mouse serum samples collected from the hyperuricemia preventive animal model were monitored by the following biochemical indices: glutamic pyruvate transferase (GPT), blood urea nitrogen, creatinine, total protein, and albumin. Serum samples were analyzed by the Taiwan Mouse Clinic (TMC, Taipei, Taiwan).
2.10. Safety studies conducted on high dose of MFEs in mice
ICR mice were divided into three groups: control groups, which received 0.9% saline; MFE-25 groups, which received MFE-25 450 mg/kg; and MFE-120 groups, which received MFE-120 100 mg/kg (five male and five female mice in each group). All treatments were given once daily by gavage for 14 days. All mice were observed daily for clinical signs of toxicity including survival rates, and physical examination of skin, eyes, respiratory system, somatomotor patterns, and behavior. After the 14-day treatment, mice were sacrificed, blood samples were collected by cardiac puncture, then centrifuged at 2000g for 15 minutes, and the serum was immediately removed. The clinical biochemistry of GPT was measured using a Fuji Dri-chem slide GPT/ALT-PIII by Fuji Dri-chem 4000i (FUJIFILM Corporation, Tokyo, Japan). The necropsy procedure was a thorough and systemic examination and dissection of the viscera and carcass, collection, and weighing. Detailed gross necropsy, including careful examination of the body external surface, orifices, abdominal cavities and their contents, were performed in all groups. The liver, kidney, spleen, stomach, and intestine were excised, trimmed of any adherent tissues, and their wet weights were immediately recorded. The excised liver and kidney were fixed in 10% buffered formalin for histopathological study. The tissues were then embedded in paraffin, sectioned in 4-μm slices, and stained with hematoxylin and eosin for examination under a light microscope.
2.11. Statistical analysis
Results are expressed as the mean ± standard deviation. Experimental data were analyzed using Student t test. All statistical tests were two-tailed, and a p value less than 0.05 was considered statistically significant.
3. Results
3.1. Urate-lowering effects of MFEs in the hyperuricemia therapeutic animal model
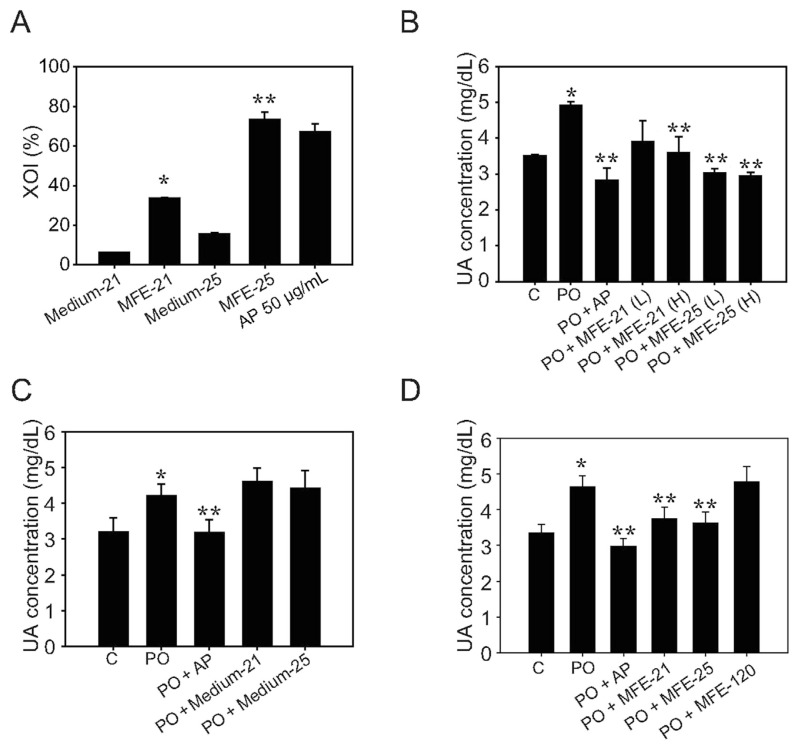
Hyperuricemia is a main risk factor for gout, kidney dysfunction, and cardiovascular diseases [17], but recent therapeutic agents for lowering UA are limited because of the undesirable adverse effects. Therefore, it is necessary to develop novel antihyperuricemic agents for clinical usage. Previous studies indicated that XOD is a key enzyme in purine metabolism that catalyzes the oxidation of hypoxanthine to xanthine and finally to UA. We examined the XOD inhibitory activity by in-vitro experimentation using positive control AP (50 μg/mL), which showed inhibition of XOD by 67.1% (Figure 1A). The negative control, using cultured medium Medium-21 and Medium-25, showed that XOD inhibitory activity was 6.2% and 15.6%, respectively. The concentrates of MFEs (40 μL of MFE-21 and MFE-25) showed that the XOD inhibitory activity of MFE-21 and MFE-25 was 44.5% and 73.6%, respectively (Figure 1A). We then established a hyperuricemia therapeutic animal model by oral administration of PO at 400 mg/kg for 2 hours. The UA concentration in the PO groups (4.91 ± 0.10 mg/dL) was significantly higher than those in control groups (3.51 ± 0.03 mg/dL), which indicated that the model was successful at inducing hyperuricemia in mice (Figure 1B). Then, MFE-21 and MFE 25 were given to hyperuricemic mice dose-dependently after PO administration for 1 hour. The results showed that MFE-21 (200 mg/kg), and MFE-25 at 150 mg/kg and 200 mg/kg significantly lowered serum UA to 3.60 ± 0.44 mg/dL, 3.03 ± 0.13 mg/dL, and 2.94 ± 0.12 mg/dL, respectively, compared with the PO groups (Figure 1B). To test whether cultured medium for MFE-21 and MFE 25 would influence UA concentration, mice were treated with PO followed by Medium-21 or Medium-25 at 200 mg/kg. The results indicated that the UA concentration did not show a significant difference from the medium treatment and PO groups (Figure 1C). These results further suggested that the cultured medium did not influence the urate-lowering effects of MFE-21 and MFE-25. We then investigated the urate-lowering effects of MFE-21 and MFE-25 (both processes XOD inhibitory activity) at a dosage of 150 mg/kg and MFE-120 (processes uricase activity) in the therapeutic model by using 10 mice in each group. As shown in Figure 1D, MFE-21 and MFE-25, there was a significant reduction in UA concentrations (3.74 ± 0.33 mg/dL and 3.63 ± 0.30 mg/dL) compared with PO groups (4.63 ± 0.32 mg/dL) and MFE-120 groups (5.04 ± 0.66 mg/ dL). The reference drug AP also significantly reduced the UA concentration (3.00 ± 0.23 mg/dL) to normal values in mice (Figure 1D). These results indicated that MFE-21 and MFE-25 with XOD inhibitory activity were able to induce an immediate decrease in serum UA levels in a hyperuricemia therapeutic animal model, and the urate-lowering effect of MFE-25 was more efficient than that of MFE-21.
Figure 1.
Antihyperuricemic effects of microbial fermented extracts (MFEs) in a hyperuricemia therapeutic animal model. Experiments were performed as described in the Methods section. (A) The in vitro xanthine oxidase (XOD) inhibitory activity (%) of samples from strains MFE-21 and MFE-25 was assayed. Medium-21 and Medium-25 were used as negative controls, and allopurinol (AP; 50 μg/mL) was used as positive control. Data represent the mean ± standard deviation (SD) of three independent experiments; (B) serum uric acid (UA) levels of control mice orally receiving 200 μL of 0.9% saline or hyperuricemic mice receiving 400 mg/kg potassium oxonate (PO) by gastric gavage (PO groups). The PO + AP groups received PO at 400 mg/kg for 1 hour followed by AP at 10 mg/kg. The MFE treatment groups received PO followed by MFE-21 at 150 mg/kg [PO + MFE-21(L) group], MFE-21 at 200 mg/kg [PO + MFE-21(H) group], MFE-25 at 150 mg/kg [PO + MFE-25(L) group], and MFE-25 at 200 mg/kg [PO + MFE-25(H) group], respectively. Data represent the mean ± SD of three mice per group; (C) serum UA concentrations of mice receiving 0.9% saline (control groups), PO, and PO-induced hyperuricemic mice receiving AP (PO + AP groups), Medium-21 200 mg/kg (PO + Medium-21 groups), and Medium-25 200 mg/kg (PO + Medium-25 groups), respectively. Data represent the mean ± SD of three mice per group; (D) hypouricemic effects of MFEs in mice pretreated with PO. C = control; PO = mice treated with potassium oxonate at 400 mg/kg; PO + AP = mice pretreated with PO followed by AP at 10 mg/kg; PO + MFE-21 = mice pretreated with PO followed by MFE-21 at 150 mg/kg; PO + MFE-25 = mice pretreated with PO followed by MFE-25 at 150 mg/kg; PO + MFE-120 = mice pretreated with PO followed by MFE-120 at 10 mg/kg. Data represent the mean ± SD of 10 mice per group. * p < 0.05 compared with the control groups. ** p < 0.05 compared with the PO groups.
3.2. Preventive effects of MFE-21, MFE-25, and MFE-120 in a hyperuricemia preventive animal model
Hyperuricemia is a chronic disease that often lacks symptoms in patients bearing genes that alter purine metabolism, which results in various chronic diseases [4]. Therefore, prevention of UA elevation by modulate dietary food intake or use of some preventive agent may reduce hyperuricemic-related chronic diseases [4]. To this end, we used a preventive hyperuricemic mouse model (see Methods section) to test whether MFEs could act as preventive agents to prevent hyperuricemia using the preventive animal model (Figure 2A).
Although MFE-120 could not reduce the urate level immediately, as shown in the therapeutic model (Figure 1D), recent clinical trials have indicated that recombinant uricase is effective in reducing the urate level for chronic treatment [18]. Therefore, the effective urate-lowering dose of MFE-120 with uricase activity was first tested using the preventive model (Figure 2A). After PO administration for 1 day, 3 days, 5 days, and 7 days, the UA concentration (4.70 ± 0.21 mg/dL) was significantly increased compared with that of the control group (3.27 ± 0.07 mg/dL; Figure 2B). MFE-120 administered for 7 days at different doses (5 mg/kg, 10 mg/kg, and 20 mg/kg) effectively decreased UA levels compared with those in the PO group (4.17 ± 0.40 mg/dL, 3.45 ± 0.42 mg/dL, and 3.52 ± 0.13 mg/ dL, respectively). The urate-lowering effects of 10-mg/kg and 20-mg/kg MFE-120 were similar; therefore, 10-mg/kg MFE-120 was used for further study.
We then compared the urate-lowering effects of MFE-21, MFE-25, and MFE-120 in the hyperuricemia preventive model. The results indicated that MFE-21, MFE-25, and MFE-120 significantly reduced UA concentrations (4.02 ± 0.38 mg/kg, 3.42 ± 0.24 mg/kg, and 3.46 ± 0.25 mg/kg, respectively) compared with the PO group (4.32 ± 0.19 mg/dL; Figure 2C), and the urate-lowering effects of MFE-25 and MFE-120 were more potent than those of MFE-21. AP also significantly lowered serum UA levels in mice (3.03 ± 0.33 mg/dL). MFE-120 showed a more potent effect than MFE-25 in preventing hyperuricemia because of the lower doses (only 10 mg/kg) applied in this model. Hence, MFE-120, a novel MFE with uricase activity, showed promise as an agent for handling hyperuricemia.
To perform detailed investigations of the urate-lowering properties of MFE-21 and MFE-25, we further assayed XOD activity using mouse livers. As shown in Figure 2D, the liver XOD activity in control and PO mice was 137.08 ± 24.81 nmol UA/mg protein, and 252.42 ± 53.00 nmol UA/mg protein, respectively. The XOD activities in the MFE-21 and MFE-25 treatment groups were 149.59 ± 31.00 nmol UA/mg protein and 92.03 ± 15.00 nmol UA/mg protein, respectively. Thus, MFE-21 and MFE-25 inhibited XOD activity by 40.8% and 63.5%, respectively, compared with the PO groups (Figure 2D). AP also significantly inhibited XOD activities (76.9% inhibition) at a dose of 10 mg/kg (Figure 2D). The results confirmed that the XOD inhibitory activity of MFE-21 and MFE-25 might be one of the antihyperuricemia mechanisms, and further indicated that the urate-lowering effects of MFE-25 were superior to those of MFE-21.
3.3. Daily oral administration of high dose MFE-25 and MFE-120 for 14 days is not harmful to mice
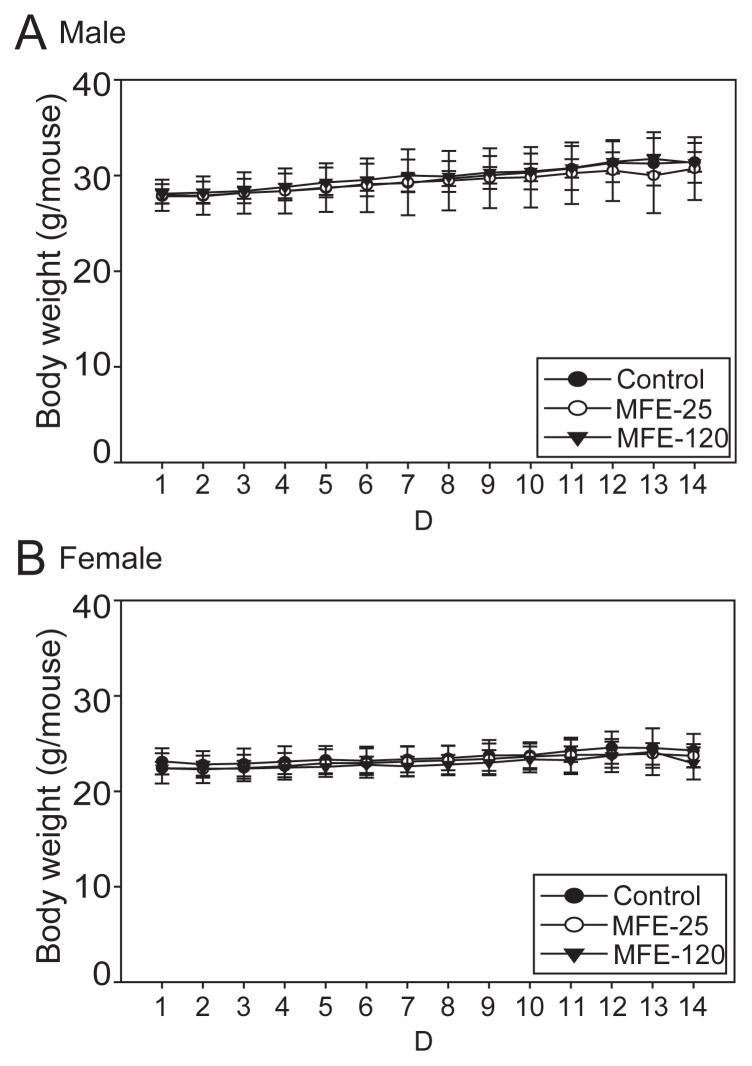
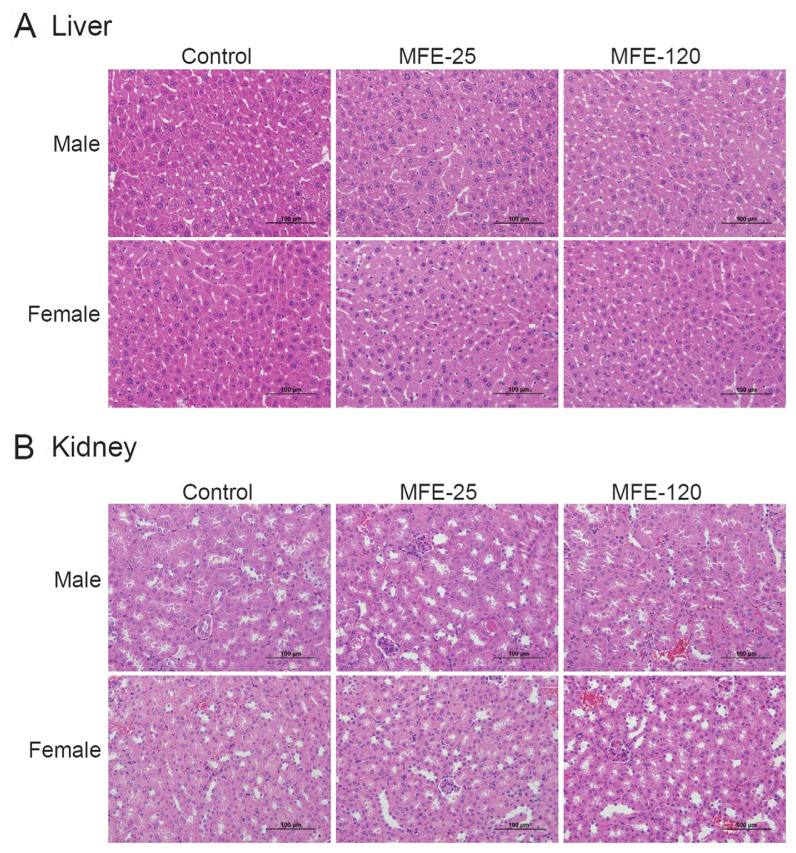
Based on the hyperuricemia preventive model, in which MFE-21, MFE-25, and MFE-120 were administered to 10 mice in each group, the results of clinical chemistry measurements that included GPT, blood urea nitrogen, creatinine, total protein, and albumin did not differ between the groups (Table 1), and no systemic toxicological effects were observed. However, the GPT levels in the PO + MFE-25 groups were elevated without significant differences (Table 1). Therefore, we further confirmed that it is safe to use MFEs for longer periods and at a higher dosage via a 14-day oral toxicological study in mice. After daily oral administration of high dose MFE-25 (450 mg/ kg) and MFE-120 (100 mg/kg) for the 14-day period, there were no deaths (Table 2), suggesting that MFE-25 and MFE-120 lack acute toxicity in mice. Moreover, MFE-25 and MFE-120 did not cause any treatment-related adverse effects in mice. The body weight and organ weights were comparable in all groups during the period of the experiment. These results suggest that there was no significant alteration in body weight (Figure 3) and organ weights (Table 3) of mice treated with high dose treatment of MFE-25 and MFE-120 during the experimental period. A postmortem examination of internal organs did not show macroscopic differences in size, color, or texture between the control and MFE-treated groups. Neither pathological signs nor gross lesions were observed in vital organs examined by microscopy. The histological examination showed that no toxicity-related alteration in either liver or kidney examined in all groups (Figure 4). A clinical chemistry analysis for liver function by measuring the GPT levels showed no significant toxicity to liver function (Table 4). Thus, our results suggest that there were no significant toxicological effects observed in body weights, organ weights, food consumption, clinical chemistry, and gross and histologic pathology in control and high dose MFE-treated mice.
Table 1.
Clinical chemistry measurements in mice treated with 0.9% saline, potassium oxonate (PO; 400 mg/kg), PO combined with allopurinol (AP; 10 mg/kg), microbial fermented extract (MFE)-21 (150 mg/kg), MFE-25 (150 mg/kg), or MFE-120 (10 mg/kg) in a hyperuricemia preventive model.
| Parameters | Control | PO | PO + AP | PO + MFE-21 | PO + MFE-25 | PO + MFE-120 |
|---|---|---|---|---|---|---|
| GPT (U/L) | 23.4 ± 1.82 | 24.4 ± 7.57 | 30.2 ± 7.73 | 25.4 ± 7.27 | 36.4 ± 13.12 | 20.6 ± 3.29 |
| BUN (mg/dL) | 22.02 ± 3.56 | 21.34 ± 0.41 | 23.22 ± 6.78 | 17.18 ± 1.57 | 17.9 ± 1.47 | 23.42 ± 1.11 |
| CRE (mg/dL) | 0.14 ± 0.05 | 0.22 ± 0.04 | 0.14 ± 0.05 | 0.22 ± 0.04 | 0.1 ± 0.00*,# | 0.15 ± 0.58 |
| TP (g/dL) | 4.34 ± 0.39 | 4.26 ± 0.26 | 4.38 ± 0.15 | 4.22 ± 0.28 | 4.84 ± 0.16 | 4.32 ± 0.19 |
| ALB (g/dL) | 2.4 ± 0.24 | 2.02 ± 0.08 | 2.06 ± 0.15 | 2.16 ± 0.25 | 2.3 ± 0.82 | 2.06 ± 0.11 |
Mice were treated with 0.9% saline, PO, PO + MFE-21, PO + MFE-25, or PO + MFE-120 for 7 days. Data represent the mean ± standard deviation of five mice per group.
ALB = albumin; BUN = blood urea nitrogen; CRE = creatinine; GPT = glutamic pyruvate transferase; TP = total protein.
p.
p<0.05 compared with PO groups.
Table 2.
Survival rate of male and female mice in the oral toxicity test.
| Group | Treatment | Survival mice/total mice, male survival rate (%) | Survival mice/total mice, female survival rate (%) |
|---|---|---|---|
| Control | Saline, 200 μL | 5/5 (100) | 5/5 (100) |
| MFE-25 | 450 mg/kg | 5/5 (100) | 5/5 (100) |
| MFE-120 | 100 mg/kg | 5/5 (100) | 5/5 (100) |
Male and female mice in the oral toxicity model were treated with 0.9% saline (control), MFE-25 (450 mg/kg), or MFE-120 (100 mg/kg) for 14 days. Each group consisted of five mice.
MFE = microbial fermented extract.
Figure 3.
Body weight changes in the toxicity model in (A) male mice and (B) female mice treated with 0.9% saline (control), microbial fermented extract (MFE)-25 (450 mg/kg), or MFE-120 (100 mg/kg) for 14 days. Data represent the mean ± standard deviation of each mice per group.
Table 3.
Organ weights of male and female mice in the oral toxicity test.
| Organ | Male | ||
|---|---|---|---|
|
| |||
| Control | MFE-25 | MFE-120 | |
| Spleen | 0.108 ± 0.009 | 0.121 ± 0.018 | 0.118 ± 0.011 |
| Left kidney | 0.266 ± 0.026 | 0.262 ± 0.021 | 0.301 ± 0.038 |
| Right kidney | 0.270 ± 0.038 | 0.265 ± 0.029 | 0.297 ± 0.035 |
| Liver | 1.812 ± 0.154 | 1.634 ± 0.293 | 1.829 ± 0.113 |
| Stomach | 0.557 ± 0.100 | 0.611 ± 0.119 | 0.531 ± 0.093 |
| Intestine | 3.313 ± 0.328 | 3.350 ± 0.322 | 3.208 ± 0.297 |
|
| |||
| Female | |||
|
| |||
| Control | MFE-25 | MFE-120 | |
|
| |||
| Spleen | 0.111 ± 0.010 | 0.098 ± 0.016 | 0.114 ± 0.035 |
| Left kidney | 0.189 ± 0.020 | 0.174 ± 0.010 | 0.171 ± 0.020 |
| Right kidney | 0.192 ± 0.018 | 0.173 ± 0.013 | 0.168 ± 0.020 |
| Liver | 1.267 ± 0.162 | 1.234 ± 0.058 | 1.227 ± 0.068 |
| Stomach | 0.404 ± 0.104 | 0.416 ± 0.068 | 0.436 ± 0.047 |
| Intestine | 2.523 ± 0.268 | 2.851 ± 0.356 | 2.541 ± 0.345 |
The weights of the spleen, left kidney, right kidney, liver, stomach, and intestine are shown in male and female mice treated with 0.9% saline (control), MFE-25 (450 mg/kg), or MFE-120 (100 mg/kg) for 14 days. Each group consists of five mice in the oral toxicity test. MFE = microbial fermented extract.
Figure 4.
Representative hematoxylin and eosin staining micrographs of (A) liver and (B) kidney morphologies in male and female mice in the toxicity model. Mice were treated with 0.9% saline (control), microbial fermented extract (MEF)-25 (450 mg/kg), or microbial fermented extract-120 (100 mg/kg) for 14 days.
Table 4.
Biochemistry glutamic pyruvate transferase (GPT) analysis of mice treated with 0.9% saline (control), microbial fermented extract (MFE)-25 (450 mg/kg), and MFE-120 (100 mg/kg) in an oral toxicity test.
| Sex | Male | Female | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Parameters | Control | MFE-25 | MFE-120 | Control | MFE-25 | MFE-120 |
| GPT (U/L) | 21.2 ± 3.34 | 26.6 ± 6.02 | 20.6 ± 6.5 | 30.2 ± 7.25 | 21.6 ± 4.39 | 24.6 ± 3.78 |
The results of serum GPT (n = 5 in each group) collected from control, MFE-25 (450 mg/kg), and MFE-120 (100 mg/kg) treated groups are shown.
4. Discussion
Hyperuricemia refers to a supersaturation status of extracellular urate caused by purine metabolic disorders or the reduction of UA excretion. The deposition of urate may damage the tissues and internal organs resulting in gout, chronic nephritis, renal dysfunction, and cardiovascular disease [1,4]. Therefore, hypouricemic agents are used for the treatment or prevention of hyperuricemia and relative chronic diseases. Current hypouricemic agents in use include XOD inhibitors such as AP, uricosuric agents including benzbromarone and probenecid, and the enzyme urate oxidase (uricase) [2]. However, the utilization of these agents is sometimes limited by the association of undesirable side effects. For instance, 5% of patients are unable to tolerate the adverse side effects induced by AP, which include gastrointestinal irritation, bone marrow suppression, hypersensitivity, fever, hepatitis, and worsened renal function [2]. Therefore, the search for better therapeutic and preventive agents for hyperuricemia is highly warranted.
In recent years, there has been an increasing interest in the search for effective or novel bioactive compounds for anti-hyperuricemia from a wide variety of traditional herbal plants, natural products, or MFEs such as lactic acid bacteria, acetic acid bacteria, and koji [19]. People have been eating these foods for more than 100 years in Taiwan and China, and the bioactivities of these microorganisms for human health have also been shown over the past 30 years. In this study, we identified the novel XOD inhibitors, MFE-21 and MFE-25, from traditional fermented food microorganisms. The therapeutic hyperuricemia model was established by using ICR mice treated with high doses of PO, and the therapeutic effects of MFE-21 and MFE-25 were confirmed (Figure 1). It was found that, compared with MFE-21, MFE-25 exerts superior urate-reducing effects in the therapeutic model (Figure 1D). Consistent with our findings, recent studies also indicated that novel synthesized or natural products derivatives from pink oyster mushroom extracts had potential inhibitory effects on XOD and tyrosine inhibitory activity, and antioxidant activity in vivo [4]. Moreover, some strains of lactic acid bacteria isolated from Chinese sauerkraut were reported to efficiently degrade purine compounds. These results, in accordance with our findings, suggest that MFEs may be promising candidates as an adjunctive treatment in patients with hyperuricemia [20].
Maintaining UA at normal levels is important for the prevention of gout and other disorders. Therefore, we also evaluated the urate-lowering effects of the XOD inhibitors MFE-21, MFE-25, and uricase MFE-120 using a preventive model. MFE-25 seemed to be more potent in reducing UA than MFE-21 after administration for 7 days, which was consistent with the XOD inhibition results that suggested that the magnitude of the hypouricemic action paralleled the reduction in the liver XOD activities (Figure 2). However, a previous report indicated that XOD inhibitors are widely used but are not satisfactory to handle hyperuricemia. Indeed, direct comparison of MFE-120, MFE-25, and AP in this model indicated that MFE-120 with uricase activity had similar urate-lowering effects as AP and was more effective than MFE-25 (the effective dose of MFE-120 is far less than that of MFE-25). Therefore, we first identified a novel extract from A. ory-zae (MFE-120) with good uricase activity that has urate-lowering effects. Uricase is an enzyme found in the liver peroxisomes that catalyzes the oxidation of UA to a more soluble and easily excreted compound, allantoin, but it is absent in humans [14]. Therefore, recent studies indicated that recombinant fungal uricase is a promising drug for treating hyperuricemia in patients for short-term treatment [21]. However, when administered in high doses, uricase may result in an increase of hydrogen peroxide in plasma and cause deleterious effects [2]. Previous studies also indicated that uricase may induce immune responses to clear the unmodified uricase by macrophages and hydrolyzed by proteases [17]. Accordingly, we suggested that continuous administration of low concentrations of uricase could achieve a higher activity and longer half-life in vivo instead of being given once to mice in the therapeutic model. Therefore, we first confirmed that MFE-120 with uricase activity reduced UA levels in the prevention for hyperuricemia at low concentrations after being administered for 7 days, but not in the therapeutic model (Figure 2B).
To determine whether there was any toxicity of MFEs, we then examined the safety of two effective urate-lowering MFEs (MFE-25 and MFE-120) for 2 weeks of continuous intake at high doses. This is necessary to confirm the in vivo health-promoting effects of MFEs. If any adverse effects are found, additional complete toxicological studies should be performed. For this reason, we assessed the oral administration of MFE-25 at 450 mg/kg or MFE-120 at 100 mg/kg for 14 days and found that MFEs did not affect the final body weight or the mean growth rate. Our results indicated that MFE-25 and MFE-120 were well tolerated by animals at high doses and with the routes of administration that were tested. The biochemical tests indicated that there were no changes in GPT values, which indicated that the liver integrity did not differ between the control and treated groups. We did not test glutamic oxaloacetic transaminase (GOT) levels because of previous reports indicating that GPT is a more reliable variable for the evaluation of liver toxicity; GOT is not useful because of its wide distribution [22]. Our results also indicated that the appearance of vital organs including liver, kidney, spleen, stomach, and intestine was normal in the macroscopic examinations after necropsy (data not shown). The examination of histopathology indicated there were no treatment-related adverse effects in mice. Taken together, the lack of harmful effects indicated that the MFE-25 and MFE-120 treatments have a large safety margin and might be used in the future as novel hypouricemic agents.
Although the urate-lowering effect of MFE-25 appeared to be less than that of AP, the onset of hypouricemic action of MFE-25 appeared to be as fast as that of AP with the onset time being less than 1 hour after the administration. In addition, MFE-120 at the same dose as AP (10 mg/kg) possessed similar urate-lowering effects as AP after 7 days of administration. More importantly, both MFE-25 and MFE-120 at high doses did not show any toxic reactions in mice, indicating that they might be much safer than AP. The present study indicated that MFEs could serve as possible alternatives for AP, or at least in combination therapy to minimize the side effect of AP, particularly in long-term application.
In conclusion, this is the first study to test the urate-lowering effects of MFEs, which were extracted from fermented food microorganisms. Extracts of MFE-25 possessed in vivo potent urate-lowering effects in both therapeutic and preventive models and were found to inhibit liver XOD activities. The other extraction, MFE-120, possessed uricase activity and urate-lowering effects and was assessed using the preventive animal model. Therefore, further purification of MFE-25 and MFE-120 for their effective compounds is a worthwhile direction for the future development of potential urate-lowering agents. The stability and detailed mechanism of the molecular action of MFE-25 and MFE-120 on lowering UA, in addition to their XOD inhibitory or uricase activity, warrant further study. Relevant experiments are in progress.
Acknowledgments
This study was supported by the Ministry of Economic Affairs, Taiwan (102-EC-17-D-02-11-1236) and the Ministry of Science and Technology, Taiwan (MOST 103-2314-B-006-060-MY2 and MOST 103-2321-B-006 -019 -MY3).
Funding Statement
This study was supported by the Ministry of Economic Affairs, Taiwan (102-EC-17-D-02-11-1236) and the Ministry of Science and Technology, Taiwan (MOST 103-2314-B-006-060-MY2 and MOST 103-2321-B-006 -019 -MY3).
Footnotes
Conflicts of interest
All authors declared that there is no conflicts of interest.
REFERENCES
- 1. Jalal DI, Chonchol M, Chen W, Targher G. Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61:134–46. doi: 10.1053/j.ajkd.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu Z, Fong WP, Cheng CH. The dual actions of morin (3,5,7,2′,4′-pentahydroxyflavone) as a hypouricemic agent: uricosuric effect and xanthine oxidase inhibitory activity. J Pharmacol Exp Ther. 2006;316:169–75. doi: 10.1124/jpet.105.092684. [DOI] [PubMed] [Google Scholar]
- 3. Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Clev Clin J Med. 2008;75:S13–6. doi: 10.3949/ccjm.75.suppl_5.s13. [DOI] [PubMed] [Google Scholar]
- 4. Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol. 2012;59:235–42. doi: 10.1016/j.jjcc.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 5. Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27:967–78. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, Dai C, Yang J. Uric acid induces renal inflammation via activating tubular NF-kappaB signaling pathway. PloS One. 2012;7:e39738. doi: 10.1371/journal.pone.0039738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kong LD, Yang C, Ge F, Wang HD, Guo YS. A Chinese herbal medicine Ermiao wan reduces serum uric acid level and inhibits liver xanthine dehydrogenase and xanthine oxidase in mice. J Ethnopharmacol. 2004;93:325–30. doi: 10.1016/j.jep.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 8. Haidari F, Rashidi MR, Keshavarz SA, Mahboob SA, Eshraghian MR, Shahi MM. Effects of onion on serum uric acid levels and hepatic xanthine dehydrogenase/xanthine oxidase activities in hyperuricemic rats. Pak J Biol Sci. 2008;11:1779–84. doi: 10.3923/pjbs.2008.1779.1784. [DOI] [PubMed] [Google Scholar]
- 9. Niu Y, Zhu H, Liu J, Fan H, Sun L, Lu W, Liu X, Li L. 3,5,2′,4′-Tetrahydroxychalcone, a new non-purine xanthine oxidase inhibitor. Cell Biol Int. 2011;189:161–6. doi: 10.1016/j.cbi.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 10. Kimira M, Arai Y, Shimoi K, Watanabe S. Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol. 1998;8:168–75. doi: 10.2188/jea.8.168. [DOI] [PubMed] [Google Scholar]
- 11. Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc. 2002;102:1414–20. doi: 10.1016/s0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- 12. Alam N, Yoon KN, Lee KR, Kim HY, Shin PG, Cheong JC, Yoo YB, Shim MJ, Lee MW, Lee TS. Assessment of antioxidant and phenolic compound concentrations as well as xanthine oxidase and tyrosinase inhibitory properties of different extracts of Pleurotus citrinopileatus fruiting bodies. Mycobiology. 2011;39:12–9. doi: 10.4489/MYCO.2011.39.1.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Chen Z, Hou L, Fan H, Weng S, Xu C, Ren J, Li B, Chen W. High-level expression, purification, and characterization of non-tagged Aspergillus flavus urate oxidase in Escherichia coli. Protein Express Purif. 2006;49:55–9. doi: 10.1016/j.pep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14. Chen Z, Wang Z, He X, Guo X, Li W, Zhang B. Uricase production by a recombinant Hansenula polymorpha strain harboring Candida utilis uricase gene. Appl Microbiol Biotechnol. 2008;79:545–54. doi: 10.1007/s00253-008-1472-8. [DOI] [PubMed] [Google Scholar]
- 15. Li JM, Zhang X, Wang X, Xie YC, Kong LD. Protective effects of cortex fraxini coumarines against oxonate-induced hyperuricemia and renal dysfunction in mice. Eur J Pharmacol. 2011;666:196–204. doi: 10.1016/j.ejphar.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 16. Hua J, Huang P, Zhu CM, Yuan X, Yu CH. Antihyperuricemic and nephroprotective effects of modified simiao decoction in hyperuricemic mice. J Ethnopharmacol. 2012;142:248–52. doi: 10.1016/j.jep.2012.04.052. [DOI] [PubMed] [Google Scholar]
- 17. Feng J, Li X, Yang X, Zhang C, Yuan Y, Pu J, Zhao Y, Xie Y, Yuan H, Bu Y, Liao F. A new practical system for evaluating the pharmacological properties of uricase as a potential drug for hyperuricemia. Arch Pharm Res. 2010;33:1761–9. doi: 10.1007/s12272-010-1108-2. [DOI] [PubMed] [Google Scholar]
- 18. Reinders MK, Jansen TL. New advances in the treatment of gout: review of pegloticase. Ther Clin Risk Manag. 2010;6:543–50. doi: 10.2147/TCRM.S6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tung YT, Hsu CA, Chen CS, Yang SC, Huang CC, Chang ST. Phytochemicals from Acacia confusa heartwood extracts reduce serum uric acid levels in oxonate-induced mice: their potential use as xanthine oxidase inhibitors. J Agric Food Chem. 2010;58:9936–41. doi: 10.1021/jf102689k. [DOI] [PubMed] [Google Scholar]
- 20. Li M, Yang D, Mei L, Yuan L, Xie A, Yuan J. Screening and characterization of purine nucleoside degrading lactic acid bacteria isolated from Chinese sauerkraut and evaluation of the serum uric acid lowering effect in hyperuricemic rats. PloS One. 2014;9:e105577. doi: 10.1371/journal.pone.0105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang X, Yuan Y, Zhan CG, Liao F. Uricases as therapeutic agents to treat refractory gout: xurrent states and future directions. Drug Dev Res. 2012;73:66–72. doi: 10.1002/ddr.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J Nutr. 2002;132:257–60. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]