Abstract
In this study, 120 lactic acid bacterial strains from different fermented dairy products as well as 10 bacterial intestinal isolates were evaluated for in vitro and in vivo degradation of various food azo dyes. Of these isolates, lactic acid bacteria (LAB) strains 13 and 100 and the intestinal isolates Ent2 and Eco5 exhibited 96–98% degradation of the tested food azo dyes within 5–6 hours. High performance liquid chromatography mass spectra of sunset yellow (E110) and carmoisine (E122) anaerobic degradation products by the intestinal isolates showed that they were structurally related to toxic aromatic amines. For an in vivo study, eight groups of rats were treated for 90 days with either the food azo dyes or their degradation products. All groups were kept for a further 30 days as recovery period and then dissected at 120 days. Hematological, histopathological, and protein markers were assessed. Rats treated with either E110/E122 or their degradation products exhibited highly significant changes in red blood cell count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and white blood cell count. In addition, alanine and aspartate aminotransferases, amylase, total bilirubin, blood urea nitrogen, creatinine, glucose, total protein, and globulins were significantly increased. Furthermore, marked histopathological alterations in the liver, kidney, spleen, and small intestine were observed. Significant decreases in inflammation and a noticeable improvement in the liver, kidney, spleen, and small intestine of rats treated with LAB and food azo dyes simultaneously were observed. Finally, these results provide a reliable basis for not only a better understanding of the histological and biochemical effects of food additives, but also for early diagnostics. In addition, LAB strains 13 and 100 may play an important role as potential probiotics in food and dairy technology as a probiotic lactic acid starter.
Keywords: dairy products, food additives, food azo dyes, lactic acid bacteria, probiotic bacteria
1. Introduction
Synthetic colors are man-made compounds that are not found in nature; these are often azo dyes. Certain artificial colors that are used in foods have been linked to negative health issues [1]. In recent years, food containing synthetic colors have been increasingly avoided by consumers as studies of the effect of a combination of certain artificial food colors and sodium benzoate on childhood behavior concluded that there was a possible link between the consumption of these additives and increased hyperactivity in 3-year-old and 8–9-year-old children [2]. Food azo dyes are the largest group of synthetic chemicals containing one or more azo groups (–N=N–) that are widely used in foods to improve their appearance [3]; they are also used in drugs, cosmetics [4], textile dyeing, paper printing, color photography, and leather industries [5,6]. The annual global production of food azo dyes is estimated to be around 1 million tons [7], and more than 2000 structurally different dyes are currently in use [8].
The chronic effects of dyestuffs, especially food azo dyes, have been studied for several decades. The reduction of azo dyes, i.e., the cleavage of the azo linkage(s), leads to the formation of aromatic amines that are known mutagens and carcinogens. In mammals, metabolic reduction of food azo dyes is mainly attributable to bacterial activity in the anaerobic parts of the lower gastrointestinal tract [5]. Various other organs, especially the liver and the kidneys, can, however, also reduce food azo dyes. After degradation in the intestinal tract, the released aromatic amines are absorbed by the intestine and are then excreted via the kidneys [4].
Azo dyes are generally recalcitrant to biodegradation because of their complex structures, but some microbial consortia or combinations of anaerobic and aerobic systems achieve complete degradation [8–12]. Lactic acid bacteria (LAB) as promising probiotic isolates could completely metabolize some azo dyes under anaerobic/aerobic regimes [13]. Probiotics are health-promoting live microorganisms that improve the intestinal microbial balance and produce various compounds that inhibit the growth of various bacterial pathogens [14].
Tartrazine (E102), sunset yellow (E110), carmoisine (E122), and ponceau 4R (E124) are mostly used in ice cream, yoghurt, soft drinks, instant puddings, flavored chips, cake mixes, custard, candy, and fermented dairy products. They are also used in some pharmaceutical products, cosmetics, moisturizers, and crayons [15,16]. The European Parliament and Council of the European Union have made a political decision that food or drinks containing some artificial colorings must be labeled with the text “May have an adverse effect on activity and attention in children” [17]. So, this work aims to (1) study the potential degradation of food azo dyes by bacterial consortia, (2) identify the degradation products by high performance liquid chromatography (HPLC), and (3) evaluate the in vivo toxicity of both food azo dyes and their metabolites in rats as a mammalian model.
2. Materials and methods
2.1. Food azo dyes
The commercial food azo dyes tartrazine (E102), sunset yellow (E110), carmoisine (E122), and ponceau 4R (E124) were purchased from Sigma (Cairo, Egypt). These dyes were selected on the basis of their frequent use in the local food, dairy, drug, and cosmetics industries. For determination of the in vitro degradation, a stock solution of each dye (10 g/L) was prepared by dissolving in distilled water and filtering through a 0.45-μm filter.
2.2. Screening and identification of food azo dye degrading bacteria
A total of 120 LAB strains isolated from different fermented dairy products were screened for anaerobic degradation of different food azo dyes as described by Elbanna et al [13]. Furthermore, screening and isolation of intestinal food azo dye degrading bacteria were conducted using an enrichment technique. For this, Hungate tubes (16 mL) containing 14 mL of nutrient broth medium were prepared and sterilized at 121°C for 15 minutes before a final concentration of 100 mg/L of the stock solution of each dye was added. All tubes were inoculated with different fresh child feces, and then incubated at 37°C until complete decolorization was achieved within 48 hours. After that, a loop-full of each enrichment culture from each dye was streaked onto Eosin Methylene Blue agar plates and incubated at 37°C for 24 hours. After incubation, different colonies were picked and purified, then tested for degradation of the different food azo dyes. The most efficient intestinal strains capable of decolorizing all tested azo dyes were selected for further studies. Ten different intestinal isolates were characterized and identified by API 20 E kit (BioMérieux, Marcy l’Etoile, France) using the API database and the protocol given in Bergey’s Manual of Determinative Bacteriology [18].
2.3. Preparation of food azo dye degrading LAB
For preparation of the active fresh lactic acid bacteria used in this study, 100 μL of both LAB13 and LAB100 were propagated in sterilized and homogenized skim milk (3%) at 37°C for 48 hours, then subcultured in MRS broth several times. The final stock cultures were prepared and divided into two parts: the first was used fresh for determination of the potential degradation of food azo dyes; the other part was lyophilized, and then freshly prepared in sterile tap water (5 mg/L) for feeding the rats of Group 8 (G8).
2.4. Potential decolorization of food azo dyes
Decolorization of different azo dyes over time at different starting concentrations (50–600 mg/L) was followed using either a mixture of LAB or consortia isolates. For this, Hungate tubes containing MRS broth were inoculated with either selected lactic acid bacterial or intestinal isolates and incubated anaerobically at 37°C for 36 hours. To determine the decolorization by bacterial consortia, the selected strains were chosen based on their ability to degrade a wide variety of food azo dyes and the absence of an antagonism between each other. To develop a consortium, bacterial isolates Eco5 and Ent2 were grown individually overnight and mixed in equal proportions in a final mixture containing 3 × 109 cells/mL. A final concentration of 10 and 100 mg/L of each dye was added to each individual isolate as well as to the consortium. Cultures were then incubated anaerobically at 37°C for 10 hours or until discoloration was complete. Aliquots (1 mL) were withdrawn at different time intervals and centrifuged at 5000g for 15 minutes. The absorbance of the supernatant samples was measured at 428 nm, 480 nm, 515 nm, and 506 nm for tartrazine (E102), sunset yellow (E110), carmoisine (E122) and cochineal red (E124), respectively, using a JASCO V-530 UV–VIS spectrophotometer. All assays were performed in triplicate and compared with an uninoculated control. The decolorization efficiency (%) of different isolates was calculated as follows: Decolorization (%) = (Initial absorbance – Final absorbance)/Initial absorbance × 100.
2.5. Preparation of in vitro food azo dye degradation metabolites
To study the effect of the degradation products of E110 and E122 on rates, bacterial cultures of LAB strains, Eco5 and Ent2 isolates were grown individually in nutrient and MRS broth and incubated anaerobically at 37°C for 24 hours and 48 hours, respectively. To develop the intestinal consortium, bacterial isolates of Eco5 and Ent2 were mixed in equal proportion in final mixture contained 3 × 109/mL. A final concentration of 10 mg/L and 100 mg/L of each dye was added to each culture, respectively, and then incubated anaerobically at 37°C for 10 hours until discoloration was completed. Supernatant containing degradation metabolites were obtained by centrifugation, with bacterial culture at 5000g for 30 minutes, and subsequently extracted thrice with ethyl acetate. The extracts were pooled and dried. The dried extract was divided into two parts; the first was reconstituted in water (5 mg/L) and stored for the feeding experiment, and the other was analyzed by HPLC.
2.6. Analysis of food azo dye degrading metabolites by HPLC
To determine the degradation metabolites of E110 and E122 by high ultra performance liquid chromatography (UPLC), the dried residues were dissolved in methanol and analyzed according to the methods described by Supaka et al [19]. The UPLC system was coupled to a Quattro Premier triple quadrupole mass spectrometer (Micromass, Milford, MA, USA) using an electrospray ionization source (Z-spray) operating in negative ionization mode. The analysis was performed in multiple reactions monitoring mode. The parameters affecting the ion transmission and fragmentation conditions were optimized by infusing a standard solution of E110 or E122 at 5 μg/mL.
2.7. Experimental animals
Eighty male Wister rats (42 days old) were purchased from King Fahd Research Center, King Abdulaziz University (Jeddah, Saudi Arabia), and acclimatized for 2 weeks in plastic cages at 22 ± 2°C, with 50% humidity; they were given free access to chew and tap water prior to the start of the experimental period. Rats were divided randomly into eight groups (10 rats in each group). G1 was used as the control (untreated). G2 and G3 were administered with ½ LD50 of E110 (10 mg/mL) and E122 (100 mg/mL), for 90 days, respectively. G4 and G5 were administered for 90 days with 1 mL of the in vitro degradation products of E110 and E122 degraded by the intestinal consortium (Eco5 and Ent2), respectively. G6 and G7 were administered with 1 mL of in vitro degradation products of E110 and E122 degraded by the mixture of LAB strains (LAB13 and LAB100) for 90 days, respectively. G8 rats were administered with 0.5 mL solution containing a mixture of E110 and E122, followed by 0.5 mL solution containing a mixture (1:1) of 4 × 108 CFU/mL fresh LAB13 and LAB100 isolates (1:1) for 90 days (in vivo degradation). Rat groups (G2 to G8) were gavage administered daily. All groups were kept for a further 30 days as recovery period.
The experiments were performed in accordance with the guidelines for the care and use of laboratory animals of Umm Al-Qura University, Faculty of Applied Science, Institutional Animal Care Use Committee, Saudi Arabia.
Control and treated rats were fasted overnight prior to dissection. They were sacrificed under light ether anesthesia, and blood samples were drawn from the heart for complete blood count (CBC) and biochemical assays. Blood sera were separated from blood cells using a cooling centrifuge at 3000 rpm for 15 minutes at 4°C and kept frozen at −30°C until biochemical analysis. For histopathological investigations, small specimens were extracted from liver, kidney, spleen, stomach, and small intestine. These samples were immediately immersed in cold phosphate buffer and cut into small slices. Fixation was done as soon as possible in cold neutral buffered formalin (10%) for 24 hours. Fixed tissues were washed, dehydrated, and routinely processed for embedding in paraffin wax, sectioned to 5-μm thickness, deparaffinized, and stained with hematoxylin and eosin as described in standard techniques [20]. The selected sections were analyzed in triplicate for each rat to illustrate the histopathological alterations in the tested organs.
2.8. Blood samples
Two milliliters of venous blood was collected and pooled from rats of each group in plastic tubes, and the serum was obtained by centrifugation at 4°C, 4000g for 10 minutes, and then quantified and used fresh for electrophoresis.
2.9. Statistical analysis
Statistical analysis including mean, standard error, and analysis of variance (one-way analysis of variance) test were done using SPSS [21] version 17 for Windows.
3. Results and discussion
3.1. Screening and identification of food azo dye degrading bacteria
A total of 120 LAB isolates that were isolated and previously published [13] were screened for their ability to degrade different food azo dyes under anaerobic conditions. Of these, isolates LAB2, LAB11, LAB13, LAB100, and LAB107 were selected because of their ability to degrade more than one food azo dye (Table 1). The highest overall decolorization efficiency was recorded for strains LAB13 and LAB100, which were 95.70%, 96.20%, 94.20%, and 94.70%, respectively. Therefore, these strains were selected for in vivo degradation studies. Furthermore, 10 different human intestinal isolates were isolated and characterized. Based on the preliminary identification tests, as well as identification by API 20 E kit, the Gram-negative isolates Ent1, Ent4, and Ent5 were characterized as Enterobacter sp., isolates Ent2 and Ent3 were characterized as Enterobacter aerogenes, whereas isolates Eco1, Eco2, Eco3, Eco4, and Eco5 were characterized as Escherichia coli. Among the intestinal isolates, strains Eco5 and Ent2 were selected for in vitro food azo dye degradation studies.
Table 1.
Screening for food azo dye degrading bacteria.
| Bacterial isolates | Decolorization percentage (%) of different food azo dyes under anaerobic condition | ||||
|---|---|---|---|---|---|
|
| |||||
| E102 (D1) | E110 (D5) | E122 (D7) | E124 (D2) | ||
| Lactobacillus strains | LAB2 | 88.20 | 94.70 | 94.00 | 88.90 |
| LAB11 | 89.50 | 95.50 | 93.0 | 86.60 | |
| LAB13 | 92.00 | 95.70 | 96.20 | 92.40 | |
| LAB100 | 90.40 | 94.20 | 94.70 | 90.90 | |
| LAB107 | 87.60 | 91.10 | 95.00 | 88.50 | |
| Mean | 89.54 | 94.24 | 94.58 | 89.46 | |
| Escherichia coli strains | Eco1 | 68.20 | 70.80 | 73.40 | 64.40 |
| Eco2 | 69.70 | 72.40 | 75.00 | 61.50 | |
| Eco3 | 67.10 | 72.10 | 74.90 | 64.40 | |
| Eco4 | 69.80 | 70.50 | 73.70 | 65.80 | |
| Eco5 | 70.00 | 74.20 | 77.90 | 69.70 | |
| Mean | 68.96 | 72.00 | 74.98 | 65.16 | |
| Enterobacter strains | Ent1 | 57.00 | 62.70 | 66.80 | 54.60 |
| Ent2 | 58.50 | 68.90 | 67.20 | 60.50 | |
| Ent3 | 59.30 | 62.20 | 65.60 | 55.70 | |
| Ent4 | 56.80 | 64.80 | 64.70 | 57.20 | |
| Ent5 | 57.40 | 61.50 | 65.90 | 55.40 | |
| Mean | 57.80 | 64.02 | 66.04 | 56.68 | |
| Intestinal consortium* | 96.80 | 98.60 | 97.70 | 96.20 | |
Lactic acid bacterial strains (LAB 11), (LAB 2 and LAB 13), (LAB 100), and (LAB 107) were characterized as Lactobacillus rhamnosus (Accession No. HQ177094), Lactobacillus casei (Accession No. HQ177095), Lactobacillus paracasei (Accession No. HQ177096), respectively.
Note. From: “Safe biodegradation of textile azo dyes by newly isolated lactic acid bacteria and detection of plasmids associated with degradation,” by K. Elbanna, G. Hassan, M. Khider, R. Mandour, 2010, J Bioremed Biodegrad, 1, p. 110. Copyright year 2010, Journal of International Biodegradation and Bioremediation (ISSN: 2155-6199) is published by OMICS (https://www.omicsonline.org/bioremediation-biodegradation.php). With permission.
Bacterial consortium consists of isolates Eco5 and Ent2.
3.2. Potential decolorization of E110 and E122
Prior to the in vivo study of food azo dyes or their microbial metabolites, the degradation of food azo dyes E110 and E122 by the strains was assessed to estimate the decolorization rate and the maximum concentration of these dyes. The results (Table 1) showed the highest decolorization potential of tested food azo dyes were recorded by lactic acid bacterial strains, followed by E. coli and Enterobacter isolates, respectively. The decolorization efficiency of a consortium of the human intestinal Eco5 and Ent2 isolates was significantly increased to 97.32%, corresponding to a 5–27% increase compared to the average of the individual strains, suggesting a synergistic effect. Nigam et al [22] and Khadijah et al [23] also reported a higher degree of biodegradation and mineralization in bacterial consortia.
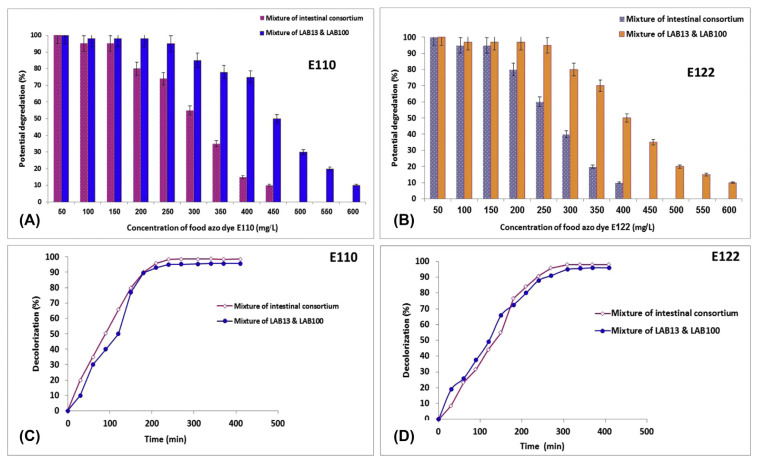
Figure 1 shows the potential degradation of both food azo dyes E110 and E122 at different concentrations by LAB and intestinal consortium at different intervals. It was noticed that the potential degradation of used dyes was decreased as their concentration increased (Figure 1A and 1B). LAB and the intestinal consortium achieved potential degradation of 98% and 96% for food azo dyes E110 and E122 within approximately 4–6 hours at 200 mg/L, respectively (Figure 1C and 1D). Furthermore, LAB strains were able to degrade 75% and 50% of E110 and E122 at 400 mg/L, respectively. Previous results indicate that LAB strains may possess an efficient enzymatic system for the cleavage of azo bonds. Similar results were reported by Elbanna et al [13], who found that some LAB could completely metabolize textile azo dyes under anaerobic/aerobic conditions.
Figure 1.
Degradation (%) of food azo dyes (A) E110 and (B) E122 at different concentrations by a mixture of lactic acid bacterial isolates LAB13 and LAB100 and by the intestinal consortium. Decolorization (%) at different intervals (min) of (C) E110 and (D) E122 by a mixture of LAB13 and LAB100 and by the intestinal consortium. LAB = lactic acid bacteria.
3.3. Analysis and mechanism of bacterial dye degradation
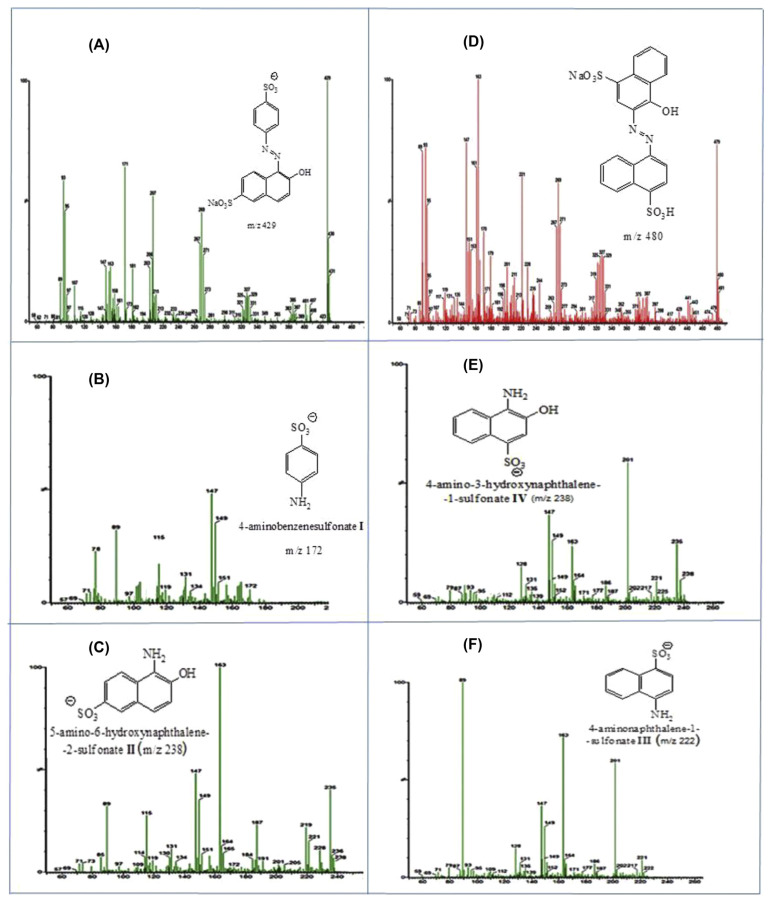
Azo dye reduction may involve different intracellular or extracellular mechanisms or combinations of mechanisms [19]. The exact mechanism of anaerobic azo dye reduction is not yet clearly understood. The incomplete degradation of food azo dyes E110 and E122 under anaerobic conditions by the intestinal consortium resulted in toxic aromatic amines (Figure 2). These compounds do not absorb light in the visible spectrum, and therefore azo dye reduction represents a decolorization process. The mass spectra of the E110 dye (Figure 2A) degradation products showed a signal at an m/z of 172, which was identified as 4-aminobenzenesulfonate (Figure 2B), and a signal at m/z = 238 that was ascribed to 5-amino-6-hydroxynaphthaline-2-sulfonate (Figure 2C). In the same manner, the E122 dye degradation products (Figure 2D) showed a signal at m/z = 238, which was identified as 4-amino-3-hydroxynaphthaline-1-sulfonate (Figure 2E), and a signal at m/z = 222, which corresponds to 4-aminonaphthaline-1-sulfonate (Figure 2F).
Figure 2.
Mass spectra of (A) E110 dye prior to degradation, (B, C) degradation products of E110 dye by the intestinal bacterial consortium, (D) E122 dye prior to degradation, (E, F) degradation products of E122 dye.
3.4. Effect of food azo dyes on rats
3.4.1. Body weight
The data in Table 2 reveal that the rats fed either E110 and E122 or their degradation products (G2–G7) exhibited a significant increase in body weight, with percentages ranging from 2.14% to 4.04% compared to the control group (G1), whereas rats in G8 fed with mixture of E110 and E122 and LAB showed 1.12% higher body weight. In contrast, other researchers found that food azo dyes did not affect the body weight gain [24–26], whereas Sharma et al [27] reported that tartrazine azo dye intake correlated with a highly significant decrease in body weight.
Table 2.
Effect of food azo dyes and their degradation products on body weight.
| Groups (n) | Rat groups (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | |
| Gained body weight | 148.52 ± 8.66e | 153.55 ± 7.47bc | 155.76 ± 7.92a | 151.71 ± 8.25d | 154.18 ± 6.97ab | 154.53 ± 8.56ab | 152.21 ± 7.67cd | 150.19 ± 7.74d |
| Body weight (%) | — | 3.38% | 4.87% | 2.14% | 3.81% | 4.04% | 2.48% | 1.12% |
Mean values ± standard error. Means of treatments having the same letter(s) within a column are not significantly different (p < 0.05).
3.4.2. Hematological studies
In Table 3, CBC and blood biochemistry showed significant changes in red blood cell (RBC) count, hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), blood platelets (PLT), and white blood cell (WBC) count of ratsreated with azo dyes compared to the control and recovery groups.
Table 3.
Effect of food azo dyes and their degradation products on complete blood count.
| Treatments | RBC (1012/L) | HGB (g/dL) | HCT (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PLT (109/L) | WBC (109/L) |
|---|---|---|---|---|---|---|---|---|
| G1 | 7.04 ± 0.14cd | 13.95 ± 0.38bc | 39.79 ± 0.28abcd | 56.50 ± 0.87a | 19.85 ± 0.95ab | 35.00 ± 1.21bcd | 772.67 ± 99 | 2.27 ± 0.35d |
| G2 | 7.94 ± 0.37abc | 13.85 ± 0.44bc | 42.53 ± 1.01abc | 54.00 ± 1.29abc | 17.50 ± 0.4cd | 32.60 ± 0.53cd | 612.50 ± 153 | 6.89 ± 1.26a |
| G3 | 7.17 ± 0.44bcd | 13.87 ± 0.48bc | 38.97 ± 2.91bcd | 54.33 ± 1.76abc | 19.40 ± 0.62abc | 35.87 ± 2.05bc | 718.50 ± 39 | 4.23 ± 1.13bcd |
| G4 | 8.45 ± 0.17a | 14.45 ± 0.32ab | 44.12 ± 0.16a | 53.00 ± 0.58bc | 21.35 ± 1.18a | 40.45 ± 2.86a | 865.50 ± 158 | 5.19 ± 0.46abc |
| G5 | 8.35 ± 0.47a | 15.05 ± 0.32a | 42.92 ± 1.37ab | 55.00 ± 1.15ab | 19.65 ± 0.72ab | 35.55 ± 0.49bcd | 707.50 ± 15 | 6.61 ± 1.32bc |
| G6 | 8.07 ± 0.04ab | 15.05 ± 0.03a | 41.38 ± 0.05abc | 54.90 ± 0.06ab | 18.40 ± 0.06bcd | 36.25 ± 0.09bc | 715.85 ± 0.09 | 6.60 ± 0.06ab |
| G7 | 8.37 ± 0.40a | 15.00 ± 0.33ab | 42.51 ± 1.24abc | 54.10 ± 1.10ab | 18.35 ± 0.15bcd | 35.51 ± 0.40bcd | 615.50 ± 150 | 6.92 ± 1.30a |
| G8 | 7.23 ± 0.13bcd | 14.10 ± 0.06abc | 38.24 ± 0.38bcd | 55.00 ± 0.58ab | 19.95 ± 0.14ab | 36.95 ± 0.03b | 713.50 ± 1.44 | 3.94 ± 0.03cd |
| ANOVA Sig | 0.005 | 0.009 | 0.021 | 0.013 | 0.001 | 0.001 | 0.445 | 0.000 |
Mean values ± standard error. Means of treatments having the same letter(s) within a column are not significantly different (p < 0.05).
ANOVA = analysis of variance; HCT = hematocrit; HGB = hemoglobin; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; PLT = blood platelets; RBC = red blood cells; WBC = white blood cells.
There were significant differences between rats administered with E110 (G4) and all the other groups, in terms of RBC, MCH, MCHC, and HCT values at p < 0.05, compared with the control group. Compared to control rats, significant increase in RBC counts were observed in G4–G7, whereas the MCV values were significantly decreased. These results indicate that RBCs with smaller diameter were induced; consequently, considerable increase in HGB, MCH, and MCHC values of G4–G7 was observed. The highest WBC numbers were recorded by G2 and G7, whereas the lowest count was recorded for the control group. These results confirm the damage observed in the tissues of the investigated organs including degeneration and inflammation, suggesting that the immune system has been accelerated to increase WBC production. These results might be attributed to the detected damage in the investigated tissues (Figs. 3–5). Regarding liver function, it was observed that alanine and aspartate aminotransferases (ALT and AST), amylase (AMY), and total bilirubin (TBIL) were significantly increased compared with the control group and G8 (Table 4). A similar increase in the WBC count of rats was reported for some synthetic azo dyes [26]. Sharma et al [27] reported that tartrazine and metanil yellow induced a decrease in RBC, HGB content, and HCT, but generated an increase in MCV and MCH. They also reported a significant decrease in glucose, triglycerides, alkaline phosphatase (ALP), and total cholesterol levels. The present study found decreased levels of albumin (ALB) and ALP. The kidney function parameters revealed a significant alteration in blood urea nitrogen (BUN), CRE, GLU, total phosphorus, and Glob (Table 5). The disruptive effects of different food azo dyes on these parameters were reported by numerous researchers [4,24,28,29]. Elevation of total protein indicates the stimulation of protein biosynthesis to produce the specific enzymes required for all processes. However, the increase of liver enzymes in blood sera indicated damage to the liver tissues. Serum globulin levels were increased in the groups fed with dyes or their degradation products, suggesting increasing immune-hyperactivity. These results are in agreement with those reported by Al-Shinnawy [24] and Amin et al [4], who stated that the elevation in globulin levels points toward increased immunoglobulin synthesis to protect the body from the toxic effects of this synthetic food dye. The results in Tables 2–5 suggest that daily intake for 90 days of E110 and E122 food azo dyes or their metabolites correlates with a significant increase in serum creatinine and urea levels when compared to control and G8. These results are in agreement with those of Helal et al [30], Varely [31], and Amin et al [4] in rats fed synthetic dye after 30 days.
Figure 3.
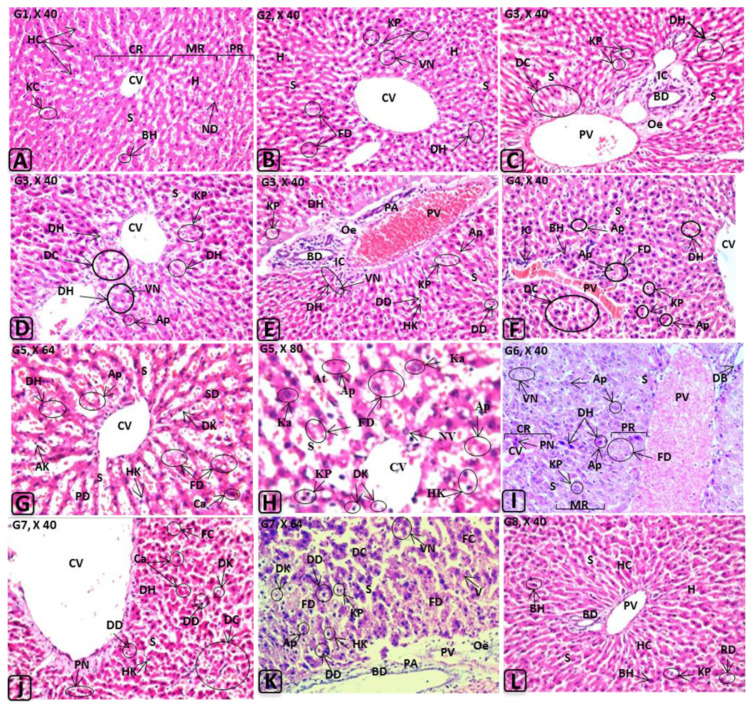
Histological changes in rat liver induced by E110, E122, and their in vitro or in vivo degradation products. Abbreviations (liver): AK = atrophied Kupffer cell(s); Ap = apoptotic nuclei; At = atrophied hepatocytes; BD = bile duct; BH = binucleated hepatocytes; Ca = canaliculus; CR = central region of the hepatic lobule; CV = central vein; DB = dilated bile duct; DC = destructed hepatic cords; DD = dilated Disse space; DH = degenerated hepatocytes; DK = degenerated Kupffer cells; FC = fatty changes; FD = fatty degeneration; H = hepatocytes; HC = hepatic cords; HK = hpertrophied Kupffer cells; IC = inflammatory cells infiltrations; Ka = karyomegaly; KC = Kupffer cells; KP = Kupffer cell proliferation; MR = medial region of the hepatic lobule; ND = normal Disse space; Oe = oedema; PA = portal area; PN = pyknotic nucleus; PR = peripheral region of the hepatic lobule; PV = portal vein; RD = remnants of degenerated hepatocytes; S = sinusoid(s); SD = sever degeneration in the hepatocytes; V = cytoplasmic vacuolations; VN = vacuolated nuclei.
Figure 4.
Histological changes in rat kidney induced by E110, E122, and their in vitro or in vivo degradation products. AG = atrophied glomerulus; BS = Bowman’s space; BV = dilated blood vessel; CB = congested blood vessel; CG = congested glomerular tufts; DE = degenerated epithelia of renal tubules; DG = degeneration in the glomerular tufts; DT = Degenerated tubules; DU = dilated urinary space; Gl = glomerulus; GT = glomerular tuft(s); Hc = hypercellularity; He = hemorrhage; Hy = hyalinosis; IC = inflammatory cells infiltrations; IF = interstitial fibrosis; Oe = oedema; PE = parietal epithelium; PF = polyps formation; PH = periglomerular hyalinosis; RA = renal arteriole; RS = renal space; RT = renal tubules; US = urinary space of Bowman’s capsule; VE = cytoplasmic vacuolization in the epithelium of renal tubules.
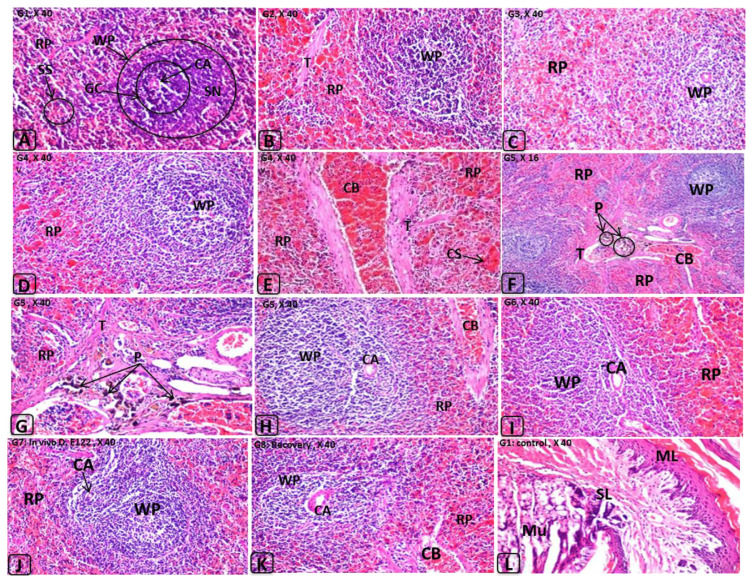
Figure 5.
Histological changes in rat spleen induced by E110 and E122 and their in vitro or in vivo degradation products. Abbreviations (spleen): AG = atrophied glomerulus; BS = Bowman’s space; BV = dilated blood vessel; CB = congested blood vessel; CG = congested glomerular tufts; DE = degenerated epithelia of renal tubules; DG = degeneration in the glomerular tufts; DT = degenerated tubules; DU = dilated urinary space; Gl = glomerulus; GT = glomerular tuft(s); Hc = hypercellularity; He = hemorrhage; Hy = hyalinosis; IC = inflammatory cells infiltrations; IF = interstitial fibrosis; Oe = oedema; PE = parietal epithelium; PF = polyps formation; PH = periglomerular hyalinosis; RA = renal arteriole; RS = renal space; RT = renal tubules; US = urinary space of Bowman’s capsule; VE = cytoplasmic vacuolization in the epithelium of renal tubules. Abbreviations (stomach): ML = muscle layer; MU = mucosal layer; SL = submucosal layer.
Table 4.
Effect of food azo dyes and their degradation products on liver function.
| Group | ALB (G/dL) | ALP (U/L) | ALT (U/L) | AST (U/L) | AMY (U/L) | TBIL (mg/dL) |
|---|---|---|---|---|---|---|
| G1 | 4.57 ± 0.10a | 5.05 ± 0.03a | 60.50 ± 0.29a | 132.33 ± 0.29f | 500.50 ± 0.29d | 0.21 ± 0.01c |
| G2 | 4.05 ± 0.03b | 5.00 ± 0.05ab | 122.00 ± 0.58f | 269.62 ± 0.58e | 618.00 ± 0.58cd | 0.31 ± 0.00ab |
| G3 | 4.10 ± 0.10b | 4.95 ± 0.03abc | 128.45 ± 0.32g | 285.88 ± 0.32d | 798.50 ± 0.29b | 0.32 ± 0.01a |
| G4 | 3.75 ± 0.05c | 5.00 ± 0.00ab | 156.50 ± 0.29d | 355.89 ± 0.29b | 737.50 ± 0.29b | 0.31 ± 0.01ab |
| G5 | 3.35 ± 0.05d | 4.95 ± 0.03abc | 185.50 ± 0.29b | 410.35 ± 0.29a | 684.50 ± 0.29c | 0.32 ± 0.00a |
| G6 | 4.05 ± 0.05b | 5.00 ± 0.00ab | 142.50 ± 0.29e | 315.93 ± 0.29c | 703.50 ± 0.29bc | 0.31 ± 0.00ab |
| G7 | 3.85 ± 0.05c | 4.85 ± 0.09bc | 158.50 ± 0.29c | 351.49 ± 0.29b | 937.50 ± 0.29a | 0.31 ± 0.00ab |
| G8 | 4.61 ± 0.04a | 5.15 ± 0.07a | 57.97 ± 0.34a | 128.13 ± 0.51f | 590.50 ± 0.29c | 0.25 ± 0.01c |
| ANOVA Sig. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Mean values ± standard error. Means of treatments having the same letter(s) within a column are not significantly different (p < 0.05).
ALB = albumin; ALP = alkaline phosphatase; ALT = alanine aminotransferase; AMY = amylase; ANOVA = analysis of variance; BUN = blood urea nitrogen; CRE = creatinine; Glob = globulins; TBIL = total bilirubin; TP = total phosphorus.
Table 5.
Effect of food azo dyes and their degradation products on kidney function.
| Treatments | CRE (mg/dL) | GLU (mg/dL) | TP (g/dL) | Glob (g/dL) | BUN (mg/dL) |
|---|---|---|---|---|---|
| G1 | 0.21 ± 0.01b | 82.52 ± 0.25c | 6.85 ± 0.03d | 2.70 ± 0.00c | 12.90 ± 0.58ef |
| G2 | 0.32 ± 0.01a | 100.50 ± 0.29b | 6.90 ± 0.06c | 3.65 ± 0.03b | 16.05 ± 0.03b |
| G3 | 0.32 ± 0.01a | 108.50 ± 0.29a | 6.80 ± 0.06d | 3.70 ± 0.06b | 17.90 ± 0.58a |
| G4 | 0.31 ± 0.00a | 69.50 ± 0.29e | 7.05 ± 0.03b | 3.75 ± 0.03ab | 15.40 ± 0.23c |
| G5 | 0.30 ± 0.00a | 71.03 ± 0.30d | 7.25 ± 0.03a | 3.85 ± 0.03a | 14.50 ± 0.29d |
| G6 | 0.31 ± 0.01a | 68.50 ± 0.29e | 7.19 ± 0.01a | 3.40 ± 0.06b | 14.50 ± 0.29d |
| G7 | 0.26 ± 0.00b | 75.10 ± 0.06d | 7.09 ± 0.06b | 3.60 ± 0.03b | 18.50 ± 0.29a |
| G8 | 0.21 ± 0.02b | 82.50 ± 0.29c | 6.82 ± 0.70d | 2.65 ± 0.03c | 13.00 ± 0.21ef |
| ANOVA Sig. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Mean values ± standard error. Means of treatments having the same letter(s) within a column are not significantly different (p < 0.05).
ANOVA = analysis of variance; BUN = blood urea nitrogen; CRE = creatinine; Glob = globulins; GLU, glucose; TP = total phosphorus.
3.5. Histological studies
The effects of the in vitro and in vivo degradation of food azo dye on liver, kidney, spleen, stomach, and small intestine are scantily studied. The hepatic parenchyma (Figure 3A) of rats treated with dyes or their degradation products (G2–G7) showed degenerated hepatocytes with pyknotic, vacuolated, or apoptotic nuclei, fatty degeneration, and dilation of the central vein and sinusoids compared with the control group and G8. In addition, hypertrophy and Kupffer cell proliferation were observed. Furthermore, edema, dilated portal veins, and bile ducts with aggregations of inflammatory cells were recorded (Figure 3B–3K). These results indicate that liver damage may be attributable to reactive oxygen species, indicating that oxidative stress may be inducted by the toxic metabolites of azo dyes. This perception was supported by Halliwell [32], who showed that oxidants induce serious pathological changes caused by loss of membrane fluidity and disruption of membrane integrity and function. Gil [33] and Cemek et al [34] reported that azo dyes are metabolized by azoreductase in the liver. Moreover, Himri et al [28] observed brown pigment deposition in Kupffer cells and fatty degeneration in the hepatocytes of rats treated with tartrazine. Compared to the treated groups (G2–G7), significant decreases in inflammation and a noticeable improvement in the liver, kidney, spleen, and small intestine of G8 rats were observed as a result of the beneficial effect of LAB (Figures 3L, 4Q, 4R, 5K, and 6I). These results highlight that the lactic acid bacterial strains (LAB13 and LAB100) used in this study could be used as potential probiotics to reduce the adverse effects of food azo dyes. These results are in agreement with those reported by Oyetayo et al [35], who confirmed that Lactobacillus sp. improves gastrointestinal tract and liver function. Our preliminary studies also showed that these strains exhibit a higher tolerance to acid and bile salt, and antimicrobial activity against a large number of pathogenic microorganisms (unpublished data).
Figure 6.
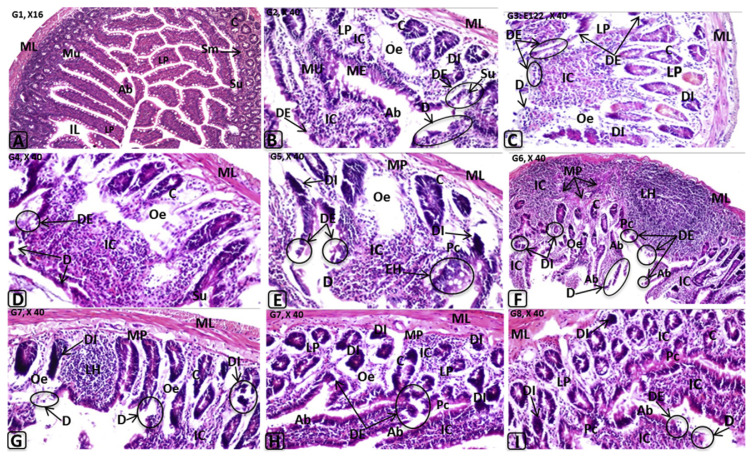
Histological changes in rat small intestine induced by E110, E122, and their in vitro or in vivo degradation products. C = cryptic region; D = desquamation of the surface epithelium; DI = degenerated intestinal crypts; EH = epithelial hyperplasia; Hp = hyperplasia in the mucosal epithelium; IC = inflammatory cells infiltrations; IL = intestinal lumen; LH = lymphoid hyperplasia; LP = lamina propria; ME = mucosal epithelium; ML = muscle layer; MM = muscularis mucosa; MU = mucosal layer; Oe = oedema; Pc = pericryptic region; SL = submucosal layer; Sm = submucosal; Su = surface epithelium; Vi = villi.
Histological studies of kidney showed atrophy in the renal glomeruli with hypercellularity and congestion in the glomerular tufts, dilation of the renal spaces, and renal tubule degeneration in treated rats (Figure 4A, 4Q, and 4R). Histological examination of the interstitial tissues showed edema, diffuse hemorrhage, dilatation and congestion in the intertubular blood vessels, and focal inflammatory cell infiltrations in the rats of G2–G7, but not in G8 and control group (Figure 4B–4P). The present investigation showed for the first time that the tested food azo dyes caused hyalinosis and interstitial fibrosis in the periglomerular portion with severe dilatation and congestion in the intertubular blood vessels. In addition, a thickening of the glomerular parietal epithelium was observed in G4–G7. Similarly, shrunken glomeruli with dilated periglomerular spaces glomerular capillaries, intercapillary sclerosis, atrophy of glomeruli, and degeneration of renal tubules with thickened basement membranes were reported in fish and rat kidneys exposed to some azo dyes [28,29,36]. Moreover, Rus et al [37] noted that guinea pigs treated with tartrazine and carmoisine for 3 weeks showed perivascular edema and vascular congestion. In addition, they observed atrophy of renal corpuscles, abnormal glomerular filtration, and protein material accumulating in the capsular space.
Spleens of rats treated with either E110 or E122 or their degradation products showed congestion and dilatation of the trabecular blood vessels and congestion in the red and white pulps with formation of lymphoid hyperplasia (Figure 5B–5J). No histopathological alterations were observed in the stomach (Figure 5L). In this context, it was reported that azo linkages are stable under the acidic conditions of the stomach [38]. The small intestine of rats treated with E110 and E122 or their degradation products (G2–G7) showed desquamation, degeneration in the intestinal crypts, absorptive epithelia, inflammatory cells infiltration, and lymphoid hyperplasia in the lamina propria. Although E110 and E122 were rapidly decolorized by either LAB strains or the intestinal consortium (Figure 1), significant adverse effects were observed in the investigated organs of the treated groups (Figure 6B–6H). These effects may be attributable to degradation products, including toxic metabolites characterized as aromatic amines. These results in accordance with the finding of Tsuda et al [39], who found DNA damage in the small intestine and colon of mice 3 hours after administration of amaranth azo dye. However, the same observations were noted in rats of G2–G7, whereas no perivascular infiltration was observed in G8, suggesting that the probiotic bacteria led to safe biotransformation and subsequent detoxification. In our previous studies, we reported that LAB exhibited safe degradation of azo dye under anaerobic/aerobic system [13].
4. Conclusion
We conclude that synthetic food colorants such as E110 and E122 as well as their degradation products may induce significant adverse hematological, biochemical, and histological effects in rats. Furthermore, hyperactivity was also observed in groups administered with such synthetic food colorants. Rats of G8 fed for 90 days with both azo dyes followed by LAB isolates, showed considerable improvements in liver, kidney, spleen, and small intestine. For the first time, we report that the tested food azo dyes caused hyalinosis and interstitial fibrosis in the renal periglomerular portion with severe dilatation and congestion in the intertubular blood vessels. Finally, the results provide a basis for not only a better understanding of the histological and biochemical markers of food additives, but also for development in the field of early diagnostics. Moreover, the lactic acid bacterial strains LAB13 and LAB100 may play an important role in food and dairy technology as a potential probiotic lactic acid starter in the future given that lactic acid bacterial species used in the production of fermented dairy products are generally regarded as safe microorganisms, and are cited for their ability to improve immune function and stimulate immunomodulatory cells, in vitro antimicrobial activity, and anticarcinogenic activity (reduction of carcinogens) in clinical trials [40,41]. To our knowledge, this is the first report to describe probiotic bacteria reducing the adverse effects of synthetic food colorants.
Acknowledgments
The authors thank Professor (Dr) Hamed Mutwally for allowing the use of his laboratory for blood analysis. The authors are also grateful to Dr Shady Elshehawy for his help in statistical analysis. The authors likewise thank Dr Martin Krehenbrink (Cysal Gmbh, Germany) for revising and correcting the (English) language of the entire manuscript.
Abbreviations of histopathological alterations
- Ab
Absorptive epithelium
- AG
Atrophied glomerulus
- AK
Atrophied Kupffer cell(s)
- Ap
Apoptotic nuclei
- At
Atrophied hepatocytes
- BD
Bile duct
- BH
Binucleated hsepatocytes
- BS
Bowman’s space
- BV
Blood vessel
- C
Cryptic region
- CA
Central arteriole
- Ca
Canaliculi
- CB
Congested blood vessel
- CG
Congested glomerular tufts
- CR
Centrolobular region
- CS
Congested Splenic nodule
- CV
Central vein
- D
Desquamation
- DB
Dilated bile duct
- DC
Destructed hepatic cords
- DD
Dilated Disse space
- DE
Degenerated epithelia of renal tubules
- DG
Degeneration in the glomerular tufts
- DH
Degenerated hepatocytes
- DI
Degenerated intestinal crypts
- DK
Degenerated Kupffer cell
- DT
Degenerated tubules
- DU
Dilated urinary space
- EH
Epithelial hyperplasia
- En
Endothelium or
- FC
Fatty changes
- FD
Fatty degeneration
- GC
Germinal center
- Gl
Glomerulus
- GT
Glomerular tuft(s)
- H
Hepatocytes
- Hc
Hypercellularity
- HC
Hepatic cords
- He
Hemorrhage
- HK
Hypertrophied Kupffer cells
- Hy
Hyalinosis
- Hp
Hyperplasia
- IC
Inflammatory cells infiltrations
- IF
Interstitial fibrosis
- IL
Intestinal lumen
- Ka
Karyomegaly
- KC
Kupffer cells
- KP
Kupffer cell Proliferation
- LH
Lymphoid hyperplasia
- LP
Lamina propria
- ME
Mucosal epithelium
- ML
Muscle layer
- MM
Muscularis mucosa
- MP
Muscularis propria
- MR
Medial-lobular region
- Mu
Mucosal layer
- ND
Normal Disse spaces
- Oe
Oedema
- P
Focal pigmentation
- PA
Portal area
- Pc
Pericryptic region
- PD
Pronounced degeneration in the hepatic parenchyma
- PE
Parietal epithelium
- PF
Polyp formation
- PH
Periglomerular hyalinosis
- PN
Pyknotic nucleus
- PR
Peripheral region of the hepatic lobule
- PV
Portal vein
- RA
Renal arteriole
- RD
Remnants of degenerated hepatocytes
- RP
Red pulp
- RS
Renal space
- RT
Renal tubules
- S
Sinusoid(s)
- SE
Swelling epithelium
- SN
Splenic nodule
- SL
Submucosal layer
- Sm
Submucosal
- SS
Splenic sinuses
- Su
Surface epithelium
- T
Splenic trabeculum
- TA
Trabecular artery
- US
Urinary space of Bowman’s capsule
- V
Cytoplasmic vacuolization
- VE
Cytoplasmic vacuolization in the epithelium of renal tubules
- Vi
Villi
- VN
Vacuolated nuclei
- WP
White pulp
Footnotes
Conflicts of interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
REFERENCES
- 1. Aberoumand A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J Dairy Food Sci. 2011;6:71–8. [Google Scholar]
- 2. Sarah C. Guidelines on approaches to the replacement of Tartrazine, Allura Red, Ponceau 4R, Quinoline Yellow, Sunset Yellow and Carmoisine in food and beverages. Food Standard Agency. 2011;4 [Google Scholar]
- 3. Węglarz-Tomczak E, Góreck T. Azodyes – biological activity and synthetic strategy. Chemik. 2012;66:1298–307. [Google Scholar]
- 4. Amin KA, Abdel Hameid H, Abd Elsttar AH. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem Toxicol. 2010;48:2994–9. doi: 10.1016/j.fct.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 5. Rafii F, Fraeankalin W, Cerniglia CE. Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl Environ Microbiol. 1990;56:2146–51. doi: 10.1128/aem.56.7.2146-2151.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuhad RC, Sood N, Tripathi KK, Singh A, Ward OP. Developments in microbial methods for the treatment of dye effluents. Adv Appl Microbiol. 2004;56:185–213. doi: 10.1016/S0065-2164(04)56006-9. [DOI] [PubMed] [Google Scholar]
- 7. Pandey A, Singh P, Lyengar L. Bacterial decolourisation and degradation of azo dyes. Int Biodeterior Biodegrad. 2007;59:73–84. [Google Scholar]
- 8. Vijaykumar MH, Vaishampayan PA, Shouche YS, Karegoudar TB. Decolourization of naphthalene-containing sulfonated azo dyes by Kerstesia sp. strain VKY1. Enzyme Microbiol Technol. 2007;40:204–11. [Google Scholar]
- 9. Stolz A. Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol. 2001;56:69–80. doi: 10.1007/s002530100686. [DOI] [PubMed] [Google Scholar]
- 10. Pearce CI, Lloyd JT, Guthrie JT. The removal of color from textile waste water using whole bacteria cells: a review. Dyes Pig. 2003;58:179–96. [Google Scholar]
- 11. Olukanni OD, Osuntoki AA, Gbenle GO. Textile effluent biodegradation potentials of textile effluent adapted and non adapted bacteria. Afr J Biotechnol. 2006;5:1980–4. [Google Scholar]
- 12. Lin YH, Leu JY. Kinetics of reactive azo-dye decolorization by Pseudomonas luteola in a biological activated carbon process. Biochem Eng J. 2008;39:457–67. [Google Scholar]
- 13. Elbanna K, Hassan G, Khider M, Mandour R. Safe biodegradation of textile azo dyes by newly isolated lactic acid bacteria and detection of plasmids associated with degradation. J Bioremed Biodegrad. 2010;1:110. [Google Scholar]
- 14. Tambekar DH, Bhutada SA. Studies on antimicrobial activity and characteristics of bacteriocins produced by Lactobacillus strains isolated from milk of domestic animals. Internet J Microbiol. 2010;8:1–6. [Google Scholar]
- 15. Tsai CF, Kuo CH, Shih DYC. Determination of 20 synthetic dyes in chili powders and syrup-preserved fruits by liquid chromatography/tandem mass spectrometry. J Food Drug Anal. 2015;23:453–62. doi: 10.1016/j.jfda.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu R, Chiu HM, Shiau CS, Yeh RL, Hung YT. Degradation and sludge production of textile dyes by Fenton and photo processes. Dyes Pig. 2007;73:1–6. [Google Scholar]
- 17. EU Council of the European. Union. L 354/16, (1333) 2008:16–33. [Google Scholar]
- 18.Holt JG, Krieg NR, Sneath PH, Staley JT, Williams ST. Bergey’s manual of determinative bacteriology. Baltimore, MD: Williams and Wilkins; 1994. [Google Scholar]
- 19. Supaka N, Juntongjin K, Damronglerd S, Delia ML, Strehaiano P. Microbial decolorization of reactive azo dyes in a sequential anaerobic–aerobic system. Chem Eng J. 2004;99:169–76. [Google Scholar]
- 20.Bancroft JD, Gamble M. Theory and practice of histological techniques. 5th ed. London, UK: Churchill Livingstone; 2002. [Google Scholar]
- 21.SPSS. Statistical package for social sciences program, Version 17 for Windows. Chicago, IL: SPSS Inc; 2008. [Google Scholar]
- 22. Nigam P, Banat IM, Marchant R. Microbial process for the decolourization of textile effluent containing azo, diazo and reactive dyes. Proceed Biochem. 1996;31:435–42. [Google Scholar]
- 23. Khadijah O, Lee KK, Mohd Faiz F, Abdullah Isolation, screening and development of local bacterial consortia with azo dyes decolorizing capability. Malay J Microbiol. 2009;5:25–32. [Google Scholar]
- 24. AL-Shinnawy SM. Physiological effect of a food additive on some haematological and biochemical parameters of male albino rats. Egypt Acad J Biol Sci. 2009;2:143–51. [Google Scholar]
- 25. Hesham MM, Atta AM, Arbid MS, Nada SA, Asaad GF. Immunological studies Amaranth sunset yellow and curcumin as food coloring agents in albino rats. Food Chem Toxicol. 2010;48:1581–6. doi: 10.1016/j.fct.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 26. Soltan SSA, Shehata MME. The effect of using color foods of children on immunity properties and liver, kidney on rats. Food Nutr Sci. 2012;3:897–904. [Google Scholar]
- 27. Sharma S, Kumar R, Nema RK, Sharma VK. Synthesis, anticonvulsant activity and in-silico study of some novel amino acids incorporated bicyclo compounds. Stamford J Pharm Sci. 2009;2:42–7. [Google Scholar]
- 28. Himri I, Bellaheen S, Souna F, Belmekki F, Aziz M, Bnouham M, Berkia Z, Mekhfi H, Saaluri EA. 90-day oral toxicity study of tartrazine, a synthetic food dye, in Wistar rats. Int J Pharm Pharmaceut Sci. 2011;3:159–69. [Google Scholar]
- 29. Barot J. Evaluation of azo dye toxicity using some haematological and histopathological alterations in fish Catla catla. Int J Biol Food Vet Agric Eng. 2015;9:401–4. [Google Scholar]
- 30. Helal GE, Zaahkouk SAM, Mekkaway AH. Effect of some food colourants (synthetic and natural products) of young albino rats. Egypt J Hosp Med. 2000;1:103–13. [Google Scholar]
- 31.Varely H. Practical clinical biochemistry. 6th ed. London, UK: Heinemann Medical Books; 1987. pp. 477–549. [Google Scholar]
- 32. Halliwell B. Oxidants and human disease: some view concepts. FASEB J. 1987;1:358–64. [PubMed] [Google Scholar]
- 33.Gil C. MS thesis. Uppsala Sweden: Swedish University of Agricultural Sciences; 2014. Toxicological effects of food additives azo dyes. [Google Scholar]
- 34. Cemek M, Büyükokuroğlu ME, Sertkaya F, Alpdağtas S, Hazini A, Önül A, Gönes S. Effects of food color additives on antioxidant functions and bioelement contents of liver, kidney and brain tissue in rats. J Food Nutr Res. 2014;2:686–91. [Google Scholar]
- 35. Oyetayo VO, Adetuyi FC, Akinyosoye FA. Safety and protective effect of Lactobacillus acidophilus and Lactobacillus casei used as probiotic agent in vivo. Afr J Biotechnol. 2003;2:448–52. [Google Scholar]
- 36. El-Neweshy MS, Srag MAA. Chronic malachite green toxicity in Nile tilapia: pathological and haematological studies with special reference to quantitative histopathological assessment. Researcher. 2011;3:55–64. [Google Scholar]
- 37. Rus V, Gherman C, Miclăuş‚ V, Mihalca AGC, Nadăş‚ GC. Comparative toxicity of food dyes on liver and kidney in guinea pigs: a histopathological study. Ann RSCB. 2008;15:161–5. [Google Scholar]
- 38. EFSA. Statement on Allura Red AC and other sulphonated mono azo dyes authorised as food and feed additives1 EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) EFSA J. 2013;11:3234. [Google Scholar]
- 39. Tsuda S, Murakami M, Matsusaka N, Kano K, Taniguchi K, Sasaki YF. DNA damage induced by red food dyes orally administered to pregnant and male mice. Toxicol Sci. 2001;61:92–9. doi: 10.1093/toxsci/61.1.92. [DOI] [PubMed] [Google Scholar]
- 40. Caselato de Sousa VR, Dos Santos EF, Sgarbieri VC. The importance of probiotics in functional foods and clinical practice. Food Nutr Sci. 2011;2:133–44. [Google Scholar]
- 41. Wu PW. A review on the analysis of ingredients with health care effects in health food in Taiwan. J Food Drug Anal. 2015;23:343–50. doi: 10.1016/j.jfda.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]