Abstract
Herein, we present a convenient method for quantitative spectrophotometric determination of citrate ions in aqueous solutions in the middle-UV range. It involves measuring the absorbance of citric acid at 209 nm under suppressed dissociation at pH < 1.0 in the presence of hydrochloric acid. Validation of the method was performed according to the guidelines of the International Conference on Harmonization. A very good linear dependence of the absorbance on concentration (r2 = 0.9999) was obtained in a citrate concentration range of 0.5–5.0 mmol/L. This method is characterized by excellent precision and accuracy; the coefficient of variation in each case is below the maximal permissible value (%RSD < 2). The proposed analytical procedure has been successfully applied to the determination of citrates in oral electrolyte formulations.
Keywords: Citrates, Oral rehydration, Ultraviolet spectrophotometry, Validation studies
1. Introduction
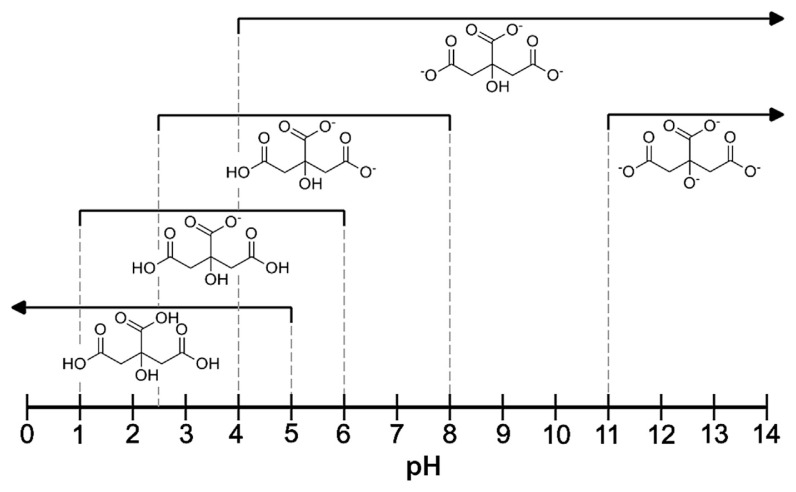
Citric acid (C6H8O7) and its salts are widely used in food and pharmaceutical industries. One of the common pharmaceutical applications of citrates is their use in oral rehydration treatment [1]. For this purpose, electrolytes are normally supplied in the form of effervescent tablets or powders for the preparation of oral solutions. In addition to the citrate salts (most often trisodium citrate) [1], they usually contain glucose, inorganic sodium, and potassium salts (chlorides or bicarbonates). In these formulations, citric acid is typically an acidity regulator. As a rule, citric acid is regarded as a triprotic acid, having three dissociation constants (pKa = 3.13, 4.76 and 6.40) [2] and forming mono-, di- or tribasic salts. However, there are some reports of a fourth dissociation constant related to the deprotonation of the hydroxyl group at the central carbon atom in strongly basic solutions (pKa = 13.0) [2,3]. The unionized acid and related citrates exist in aqueous solutions in specific pH ranges (Figure 1) [2–7].
Figure 1.
Specific pH ranges of the occurrence of citric acid and various citrates in aqueous solutions (25°C, I = 0.1 M) [2–7].
Aqueous solutions of citric acid and its salts are colorless, since they absorb in the middle-UV range. This absorption is attributed to the carbonyl groups (C=O) of unionized or ionized carboxyl groups (electronic n→π* transitions). The absorption maximum of citrates in aqueous solutions is observed at about 200 nm [8,9]. However, because citrates produce broad UV absorption bands close to the lower detectable wavelength limit accessible for standard spectrometers, the direct spectrophotometric method is rarely used. Usually, citrates are analyzed using an indirect procedure necessitating their complexation with transition metal ions [10–12] or enzymatic derivation to achieve clear absorption bands, preferably in the visible range [13,14]. Rather than the spectrophotometric method, chromatographic analysis [6,15–18], capillary electrophoresis [19,20], or classical titration [18] are typically used.
In aqueous solutions of citric acid and citrate salts, the processes of dissociation and hydrolysis lead to complex equilibria which are dependent on nominal concentrations of solutes, pH, and temperature. Therefore, at a constant temperature, an analyst is concerned with a mixture of various forms of citrates (H3Cit, H2Cit−, HCit2−, Cit3−) present in the solution at a given pH. The spectrophotometric determination of citrates proposed in this paper requires neither complicated reactions nor the use of specialized reagents. According to the Le Chatelier–Braun principle, the addition of strong acid to a solution of citrates shifts the dissociation backwards towards a less dissociated species. In view of the pKa values of citric acid, only undissociated H3Cit molecules are present in aqueous solutions at pH < 1.0 in practice. Thus, the spectrophotometric analytical conditions are significantly improved, as shown in this paper: the absorption maximum moves to a higher wavelength and becomes more discernible, while the absorption bands become narrower.
This study is aimed at the quantitative analysis of citrates in highly acidic aqueous solutions using UV spectrophotometry. Citric acid and trisodium citrate solutions have been studied with the admixture of hydrochloric acid to lower the pH below 1.0. The spectrophotometric analytical procedure is carefully examined and validated on standards. Following this, it is verified against five different commercial medications, which are distributed in sachets as powders containing trisodium citrate (oral electrolyte medications for rehydration therapy). Our method can be applied in pharmaceutical, clinical, and chemical analyses provided that there is no spectral or chemical interference from accompanying compounds.
2. Materials and methods
The commercial oral electrolyte formulations used in this study were procured from the local drugstore; they are listed as follows: Acidolit powder – code E1-AC (Z.F. Polpharma S.A., Starogard Gdanski, Poland), composition: trisodium citrate, glucose, sodium chloride, potassium chloride; Dicodral 60 powder – code E2-DI (Dicofarm S.p.A., Rome, Italy), composition: trisodium citrate, glucose, sodium chloride, potassium chloride, banana flavor; Floractin electrolytes – code E3-FL (Novascon Pharmaceuticals Sp. z o.o., Warsaw, Poland), composition: trisodium citrate, tripotassium citrate, glucose, sodium chloride, orange flavor; Hydronea Baby – code E4-HY (Aflofarm Farmacja Polska Sp. z o.o., Pabianice, Poland), composition: trisodium citrate, glucose, sodium chloride, potassium chloride, lemon flavor; and Orsalit – code E5-OR (Biomed S.A., Cracow, Poland), composition: trisodium citrate, glucose, sodium chloride, potassium chloride.
Validation of the analytical method was performed according to the guidelines of the International Conference on Harmonization [21]. The statistical analysis of the results was done using STATISTICA software (StatSoft, Inc.; version 12; 2014).
2.1. Preparation of standard solutions for the method validation
Two series of standard solutions (A and B), each containing 11 solutions, were prepared using anhydrous citric acid (ACS reagent, ≥99.5%, Sigma-Aldrich) and trisodium citrate dihydrate (ACS reagent, ≥99.0%, Sigma-Aldrich) respectively (Table 1). This was done using class A volumetric flasks (25 mL). A stock HCl solution (0.25 mol/L; Chempur, analytical grade) was used to prepare the series of solutions with added hydrochloric acid. The examined citrate solutions were mixed with this HCl solution in the ratio 1:1 v/v (2 mL + 2 mL). The resultant acidic solutions all had a pH < 1.0. In the H3Cit/HCl series (A2), all solutions had pH = 0.90. In the Na3Cit/HCl series (B2), the pH value varied from 0.91 to 0.96 with increasing citrate concentration.
Table 1.
Description of the examined solutions. The mixing ratios are expressed as v/v. The primary and measured (actual) citrate concentrations correspond to solutions before and after the addition of HCl, respectively.
| Series A, citric acid | Series B, trisodium citrate | Primary citrate concentration (mmol/L) | Measured citrate concentration (mmol/L) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Series A1 | Series A2 | Series B1 | Series B2 | ||
| Reference: H2O | Reference: H2O + HCl (1:1) | Reference: H2O | Reference: H2O + HCl (1:1) | 0 | 0 |
| Studied solution + H2O (1:1) | Studied solution + HCl (1:1) | Studied solution + H2O (1:1) | Studied solution + HCl (1:1) | 0.5 | 0.25 |
| 1.0 | 0.50 | ||||
| 2.0 | 1.0 | ||||
| 3.0 | 1.5 | ||||
| 4.0 | 2.0 | ||||
| 5.0 | 2.5 | ||||
| 6.0 | 3.0 | ||||
| 7.0 | 3.5 | ||||
| 8.0 | 4.0 | ||||
| 9.0 | 4.5 | ||||
| 10.0 | 5.0 | ||||
2.2. Preparation of drug solutions
For each sample analyzed, 500 mg of powder was transferred into a class A 100-mL volumetric flask. The powder was diluted with 50 mL of deionized water and was ultrasonicated for 5 min. Then the flask was filled up to 100 mL with deionized water and the solution was mixed. All the solutions of the examined powders were colorless and clear. Finally, they were mixed with 0.25-mol/L HCl solution in the ratio 1:1 v/v (2 mL + 2 mL). The resultant acidic solutions all had a pH < 1.0.
2.3. Spectrophotometric measurements
The measurements were done using a Shimadzu UV-1800 spectrophotometer in quartz cells (transmission in the 190–2700 nm range) with a standard optical path length l = 1 cm. The absorbance (A) of the solutions was measured in the middle-UV range (190–280 nm) at a temperature of 25° C. The concentrations of the solutes in the A2 and B2 series (Table 1) were suitable for maintaining peak absorbance in the range 0.1–1.0 (except for the least concentrated solutions), wherein the relative error of the measured concentrations resulting from the apparatus noise was the lowest [9].
3. Results and discussion
3.1. Optimization of analytical conditions
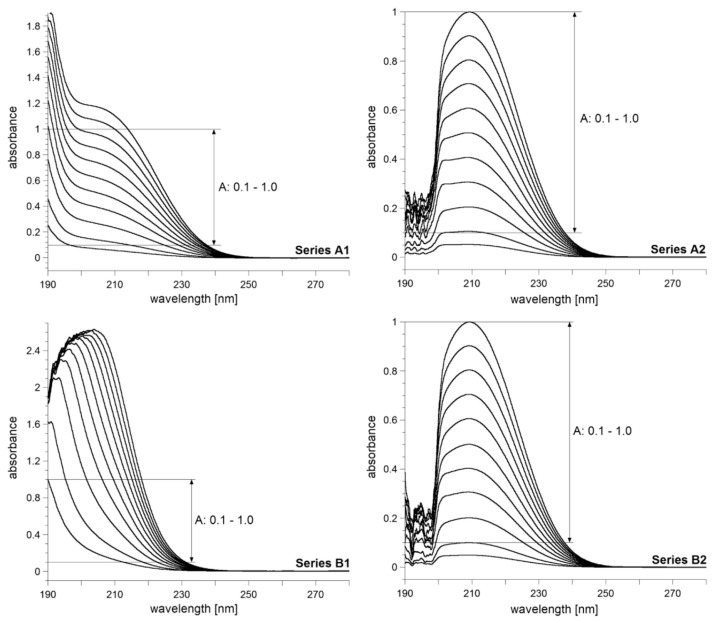
The UV spectra of the citric acid and trisodium citrate solutions are presented in Figure 2. The results in both cases demonstrate the spectacular effect of the addition of strong acid on the position, intensity and width of the absorption bands.
Figure 2.
The UV spectra of citric acid (Series A) and trisodium citrate (Series B) solutions in the concentration range 0.25–5.0 mmol/L: series A1 and B1—without HCl added; series A2 and B2—with HCl added (pH < 1.0).
First, we comment on the spectra of solutions without the added HCl. The peak absorption for citric acid in the series A1 (Figure 2) is difficult to determine for all the solutions studied. With increasing solute content, the top of the band at about 205 nm becomes more visible; however, the measurement accuracy decreases when absorbance exceeds 1.0 for measured concentrations over 4 mmol/L. Conversely, for most spectra from the series B1 (Figure 2), the absorption maxima are clearly visible. However, in this series, the band position varies with concentration (190–205 nm). Then, the band maxima are measurable only for absorbances higher than 1.0, and this excludes quantitative work with high accuracy. For the B1 solutions with A < 1, the absorption maxima are out of the spectrometer range, and spectrophotometric analysis is not feasible.
The A2 and B2 series (with HCl added) both exhibit distinct absorption maxima in the concentration range 0.5–5.0 mmol/ L (Figure 2). The bandwidths are narrower than for A1 and B1. Only the least concentrated solutions in the A2 and B2 series do not give clear-cut absorption peaks. Therefore, they have been excluded from the further analysis. The spectra of series A2 and B2 indicate that the band position is stable and easy to determine (λmax = 209 nm in both series). Furthermore, one can readily measure peak absorbance, construct a calibration curve, and find the linear part that fulfils the Beer–Lambert law. It follows that the analysis of citrates should be done in highly acidic conditions of pH < 1.0. Hereafter, the solutions of the A2 and B2 series will be treated as standard solutions in the validation procedure of the proposed citrate analysis.
3.2. Linearity, limit of detection, and limit of quantification
Absorbance measurements for standard solutions were performed to determine the linearity of calibration. For each concentration, the measurements were repeated three times. The rectilinear dependence of absorbance on concentration, confirmed by a very good linear fit, was obtained for both the analyzed series. Thus, the described method satisfies the Beer–Lambert law and can be successfully used for the quantitative analysis of citrates in the examined concentration range of 0.5–5.0 mmol/L (actual concentrations obtained after mixing analyzed samples with the HCl solution).
The limit of detection (LOD) was calculated using the equation: LOD = 3.3 × (s / a), where s is the average standard deviation determined from all the measurements (10 concentrations for particular solute, each measured three times), and a is the slope of the regression equation. The limit of quantification (LOQ) was calculated from LOD using the following equation: LOQ = 3 × LOD. The results are presented in Table 2.
Table 2.
Optical parameters, linearity and measurement range.
| Parameter | Citric acid | Trisodium citrate |
|---|---|---|
| λmax (nm) | 209 | 209 |
| Molar absorption coefficient ɛ (L/mol·cm, mean ± SD) | 203.3 ± 1.9 | 201.1 ± 1.4 |
| Regression equation (y = ax + b) | y = 0.1992x + 0.0078 | y = 0.2001x + 0.0026 |
| a (slope; mean ± SD) | 0.1992 ± 0.0005 | 0.2001 ± 0.0006 |
| b (intercept; mean ± SD) | 0.0078 ± 0.0035 | 0.0026 ± 0.0018 |
| Regression coefficient (r2) | 0.9999 | 0.9999 |
| Determination range in accordance with Beer–Lambert law (mmol/L) | 0.5–5.0 | 0.5–5.0 |
| LOD (mmol/L) | 0.0098 | 0.0099 |
| LOQ (mmol/L) | 0.029 | 0.030 |
3.3. Precision
The measures of precision, i.e. repeatability (intra-day precision) and intermediate precision (inter-day precision), were determined for three standard solutions (Table 3). For each concentration, the absorbance measurement was repeated six times on the same day (repeatability examination). This procedure was continued over three consecutive days (intermediate precision examination).
Table 3.
Repeatability and intermediate precision of absorbance measurements for selected concentrations of citric acid and trisodium citrate: mean values from six repetitions (n = 6).
| Measured concentration (mmol/L) | Repeatability (intra-day precision) | Intermediate precision (inter-day precision) | ||
|---|---|---|---|---|
|
|
|
|||
| Absorbance (mean ± SD) | RSD (%) | Absorbance (mean ± SD) | RSD (%) | |
| Citric acid | ||||
| 1.0 | 0.2047 ± 0.0008 | 0.399 | 0.2046 ± 0.0006 | 0.297 |
| 2.5 | 0.5073 ± 0.0008 | 0.161 | 0.5073 ± 0.0008 | 0.148 |
| 4.5 | 0.9035 ± 0.0005 | 0.061 | 0.9037 ± 0.0007 | 0.074 |
| Trisodium citrate | ||||
| 1.0 | 0.2007 ± 0.0005 | 0.257 | 0.2005 ± 0.0005 | 0.257 |
| 2.5 | 0.5008 ± 0.0008 | 0.150 | 0.5006 ± 0.0006 | 0.121 |
| 4.5 | 0.9033 ± 0.0005 | 0.057 | 0.9036 ± 0.0005 | 0.057 |
The proposed analytical method is characterized by its high precision; the repeatability and intermediate precision both had very low coefficients of variation for each examined concentration (%RSD < 2).
3.4. Accuracy
The accuracy was determined by an examination of the recovery (%) of citric acid and trisodium citrate from the standard solution samples enriched at three levels with respect to their primary concentration (Table 4). For each solution, its concentration was measured in five replications.
Table 4.
Accuracy of the method: mean values from five repetitions (n = 5).
| Primary concentration [mmol/L] | Enrichment [%] | Citric acid | Trisodium citrate | ||
|---|---|---|---|---|---|
|
|
|
||||
| Recovery (%, mean ± SD) | RSD (%) | Recovery (%, mean ± SD) | RSD (%) | ||
| 2.0 | 80 | 99.73 ± 0.75 | 0.76 | 100.23 ± 0.64 | 0.64 |
| 100 | 100.10 ± 0.81 | 0.80 | 99.80 ± 0.68 | 0.69 | |
| 120 | 100.08 ± 0.55 | 0.55 | 100.13 ± 0.63 | 0.63 | |
The proposed analytical method has high accuracy, and the results are characterized by a very low coefficient of variation at each level of enrichment (%RSD < 2).
3.5. Determination of citrates in the commercial medications
The developed method was applied to determine the citrate content of five commercial electrolyte products (λ = 209 nm, regression equation for trisodium citrate). Each product was analyzed using five replications, with each solution being prepared from a new batch of powder. The citrate content in grams of Cit3− is reported per 100 g of the initial powder (Table 5). The determined concentrations of the citrates are in good agreement with the claims made on the labels of the medications.
Table 5.
Content of citrates in analyzed pharmaceutical products (mean values; n = 5).
| Product code | Label claim of citrates (g/100 g) | Amount found (g/100 g, mean ± SD) | Potency (%) | RSD (%) |
|---|---|---|---|---|
| E1-AC | 8.74 | 8.738 ± 0.005 | 99.97 | 0.062 |
| E2-DI | 11.25 | 11.248 ± 0.003 | 99.98 | 0.030 |
| E3-FL | 7.60 | 7.595 ± 0.013 | 99.94 | 0.166 |
| E4-HY | 7.21 | 7.204 ± 0.007 | 99.92 | 0.095 |
| E5-OR | 7.84 | 7.839 ± 0.011 | 99.98 | 0.138 |
4. Summary and conclusions
Herein, we have proposed a convenient method for determining the concentrations of citrates in aqueous solutions. It requires a pH < 1.0 to suppress the dissociation of citric acid present in an acidic solution and perform spectrophotometric determination at 209 nm. The method is characterized by high precision and accuracy, and the analytical procedure is fast and simple. It was verified using citric acid and trisodium citrate. The method works well for a concentration range of 0.5–5.0 mmol/L (actual concentrations after mixing the analyzed solutions with the HCl solution) using 1 cm spectrophotometric cells. This concentration range can be extended if cells with other path lengths are to be used. It is possible that other citrate salts with alkali and alkaline earth cations can be determined in the same way. Since the addition of HCl is required in this method, this technique can be probably applied to determine citrate salts which are insoluble in water but soluble in HCl. However, these above-mentioned developments will require validation.
Regardless of which citric/citrate species are present in the original solution to be analyzed, after the addition of hydrochloric acid to lower the pH to below 1.0, only undissociated citric acid will be present (pH < 1 in Figure 1). This was proved by performing two parallel analyses of solutions containing either citric acid or trisodium citrate. As expected, in both cases, the slope and intercept of the calibration curves, and the band positions (λmax) and molar absorption coefficients (ɛ), were the same within experimental errors (Table 2). Furthermore, the validation of the proposed method performed for both the acid and salt demonstrated that the linearity, precision, and accuracy are not dependent on the citrate form in the initial solution (Tables 2–4). It follows that no competitive reactions and/or equilibria have an influence on the quantitative results obtained under the described analytical conditions.
Beyond the scope of this work, we have already done preliminary studies to assess the performance of our analytical method against other water-soluble, simple but common organic acids and their sodium salts. Thus, we have performed tests on acetic acid, sodium acetate, oxalic acid, and sodium oxalate (Figure S1 in Supplemental Materials); tartaric acid and sodium tartrate (Figure S2 in Supplemental Materials); and glycine, L-alanine, L-proline, and L-lysine. We have found that our analytical method can be applied to this wide group of bioorganic compounds. It is easy to implement and does not require any additional techniques and/or reagents. There is only one issue requiring caution: the compounds amenable to our analytical method should not be in a mixture because in this case, only their total concentration can be determined. This method cannot be applied in the presence of substances which give interfering absorption maxima in the 200–210 nm range. Fortunately, this rarely concerns pharmaceutical formulations (although it occurs often in food).
Overall, the analytical method presented here can be readily applied to the determination of citrates in pharmaceutical preparations in the way it has been demonstrated in this work using the example of oral electrolyte formulations, and its application can be easily extended to other similar systems.
Acknowledgments
The authors are grateful to the Medical University of Warsaw for financial support (grants: FW23/PM31D/14, FW23/PM31D/ 15/15, FW23/NM4/16 and FW23/N/2016).
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2017.01.009.
Funding Statement
The authors are grateful to the Medical University of Warsaw for financial support (grants: FW23/PM31D/14, FW23/PM31D/ 15/15, FW23/NM4/16 and FW23/N/2016).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Santosham M, Greenough WB., III Oral rehydration therapy: a global perspective. J Pediatr. 1991;118:S44–51. doi: 10.1016/s0022-3476(05)81425-8. [DOI] [PubMed] [Google Scholar]
- 2. Gacsi A, Kutus B, Bucko A, Csendes Z, Peintler G, Palinko I, Sipos P. Some aspects of the aqueous solution chemistry of the Na+/Ca2+/OH−/Cit3− system: the structure of a new calcium citrate complex forming under hyperalkaline conditions. J Mol Struct. 2016;1118:110–6. [Google Scholar]
- 3. Heller A, Barkleit A, Foerstendorf H, Tsushima S, Heim K, Bernhard G. Curium(III) citrate speciation in biological systems: a europium(III) assisted spectroscopic and quantum chemical study. Dalton Trans. 2012;41:13969–83. doi: 10.1039/c2dt31480k. [DOI] [PubMed] [Google Scholar]
- 4. Silva AMN, Kong X, Parkin MC, Cammack R, Hider RC. Iron(III) citrate speciation in aqueous solution. Dalton Trans. 2009;40:8616–25. doi: 10.1039/b910970f. [DOI] [PubMed] [Google Scholar]
- 5. Ohyoshi E, Ohyoshi A. A study of complexes with a polybasic acid Am(III) citrate complexes. J Inorg Nucl Chem. 1971;33:4265–73. [Google Scholar]
- 6. Lopez-Macipe A, Gomez-Morales J, Rodriguez-Clemente R. The role of pH in the adsorption of citrate ions on hydroxyapatite. J Colloid Interface Sci. 1998;200:114–20. [Google Scholar]
- 7. Lito MJGHM, Camoes MFGFC, Covington AK. Effect of citrate impurities on the reference pH value of potassium dihydrogen buffer solution. Anal Chim Acta. 2003;482:137–46. [Google Scholar]
- 8.Mistry BD. A handbook of spectroscopic data chemistry. Jaipur, Oxford: Book Company; 2009. [Google Scholar]
- 9.Owen T. Fundamentals of UV-visible spectroscopy: a primer. Palo Alto: Hewlett Packard; 1996. [Google Scholar]
- 10. Sun Y, Xiao F, Zhong Ch, Xue P, Fu E. Colorimetric sensing ensemble for citrate in water. Sens Actuators B. 2014;194:269–75. [Google Scholar]
- 11. Krug A, Kellner R. Determination of citric acid by means of competitive complex formation in a flow injection system. Mikrochim Acta. 1994;113:203–10. [Google Scholar]
- 12. Itabashi H, Umetsu K, Teshima N, Satoh K, Kawashima T. Indirect spectrophotometric determination of complexing agents by flow-injection analysis based on redox reaction of copper(II) with iron(II) Anal Chim Acta. 1992;261:213–8. [Google Scholar]
- 13. Moreno-Cid A, Yebra MC, Santos X. Flow injection determinations of citric acid: a review. Talanta. 2004;63:509–14. doi: 10.1016/j.talanta.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 14. Planta M, Lazaro F, Puchades R, Maquieira A. Determination of citric acid and oxalacetic acid in foods by enzymic flow injection. Analyst. 1993;118:1193–7. [Google Scholar]
- 15. Destandau E, Vial J, Jardy A, Hennion MC, Bonnet D, Lancelin P. Development and validation of a reversed-phase liquid chromatography method for the quantitative determination of carboxylic acids in industrial reaction mixtures. J Chromatogr A. 2005;1088:49–56. doi: 10.1016/j.chroma.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16. Chalgeri A, Tan HSI. Indirect photometric detection for determination of citrate in pharmaceutical matrices by ion chromatography. J Pharm Biomed Anal. 1996;14:835–44. doi: 10.1016/0731-7085(96)01731-1. [DOI] [PubMed] [Google Scholar]
- 17. Shapiro F, Silanikove N. Rapid and accurate determination of malate, citrate, pyruvate and oxaloacetate by enzymatic reactions coupled to formation of a fluorochromophore: application in colorful juices and fermentable food (yogurt, wine) analysis. Food Chem. 2011;129:608–13. doi: 10.1016/j.foodchem.2011.04.074. [DOI] [PubMed] [Google Scholar]
- 18. Hotha KK, Patel T, Roychowdhury S, Subramanian V. Development of better-quality assay method for the citric acid and sodium citrate in ophthalmic/oral solutions and their application to deformulation studies. Am J Anal Chem. 2014;5:1249–60. [Google Scholar]
- 19. Jaworska M, Cygan P, Wilk M, Anuszewska E. Capillary electrophoresis with indirect UV detection for the determination of stabilizers and citrates present in human albumin solutions. J Pharm Biomed Anal. 2009;50:90–5. doi: 10.1016/j.jpba.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 20. Munoz JA, Lopez-Mesas M, Valiente M. Development and validation of a simple determination of urine metabolites (oxalate, citrate, uric acid and creatinine) by capillary zone electrophoresis. Talanta. 2010;81:392–7. doi: 10.1016/j.talanta.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 21.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Validation of Analytical Procedures: Text and Methodology Q2(R1); Geneva, Switzerland. 2005; [Google Scholar]