Abstract
Prolonged use of an antineoplastic agent methotrexate (MTX), can cause numerous side effects such as nephrotoxicity. The aim of this study was to examine the effects of MTX on kidneys and demonstrate the protective effects of gallic acid (GA). Twenty-four, male, rats distributed into three groups. Each groups consisted eight rats and only saline was administered to the control group. The MTX group received a single dose (20 mg/kg) MTX intraperitoneally. The MTX + GA group received same dose MTX and 100 mg/kg GA orally during the 7 days. Renal functions, oxidative stress markers, histopathological and immunohistochemical changes were evaluated at the end of the experiment. Blood urea nitrogen, creatinine, uric acid levels and tissue oxidative stress markers, total oxidant status and oxidative stress index levels significantly increased and total antioxidant status levels significantly decreased in MTX group compared with the control group. At the histopathological examination hemorrhages, tubular cell necrosis, glomerulosclerosis, inflammatory cell infiltrations and proteinous materials in tubules were noticed in MTX group. Immunohistochemical examination revealed that increased expressions of serum amyloid A (SAA), tumor necrosis factor alpha (TNF-α), prostaglandin E2 (PGE-2) and C-reactive protein (CRP) in tubular epithelial cells of kidneys in this group. There were no immunoreaction with SAA and CRP, only small number of PGE-2 and TNF-α positive tubular epithelial cells were observed in MTX + GA group. In conclusion, all evidence suggested that oxidative stress caused MTX-induced nephrotoxicity and GA prevent the kidney from the nephrotoxicity due to its antioxidant and anti-inflammatory activities.
Keywords: , Gallic acid, Methotrexate, Nephrotoxicity, Oxidative stress, Pathology
1. Introduction
Methotrexate (MTX) is an antineoplastic agent and it may be used in the treatment of several maladies, such as cancer and inflammatory diseases. Prolonged use of this agent led to causes many side effects on different organs including kidney, liver, lung, testis, bone marrow and brain. Because of drug excretion from the kidneys by glomerular filtration and active transport, nephrotoxicity occurs more than other side effects. Mainly, this side effect restricts the use of MTX for treatment.
The most common mechanism of MTX induced damages is oxidative stress which triggered by inflammation due to producing reactive oxygen species (ROS) [1–3]. The levels of the various classical inflammatory mediators, such as an acute phase protein C-reactive protein (CRP), proinflammatory cytokine tumor necrosis factor-α (TNF-α) and Prostaglandin E2 (PGE-2) are important for monitoring the severity of inflammation during the damage [4]. Additionally, serum amyloid A (SAA) is the major acute-phase indicator of inflammation, which is secreted in inflammation, trauma or infection [5]. Besides, it expresses by vitamin D-binding protein isoform-1 precursor, plasma kallikrein, and apolipoprotein A-I in a malignant tumor, multiple myeloma (MM), in which methotrexate is used for treatment [6].
There are several agents that used to ameliorate the potential side effects of MTX due to their antioxidant and anti-inflammatory activities [2,7]. Gallic acid (3,4,5-trihydrox-ybenzoic acid, GA), a natural endogenous product, presented in red wine, green tea, strawberries, pineapples, bananas, lemons, gallnuts, sumac, witch hazel, tea leaves, oak bark, and apple peels [8]. GA, strong chelating agent, protects human cells or tissues against oxidative stress, by its biological activities, including anti-oxidant and anti-inflammatory effects [9–12]. It does not only protect the integrity of plasma membrane, but at the same time increases the regenerative and reparative capacity of the liver and kidney [13]. Additionally, GA and its derivations have anticancer activities due to several mechanisms. For example, in one study, lauryl gallate induced acute myeloid leukemia cell apoptosis, resulted in down-regulation of anti-apoptotic proteins (Bcl-2, Mcl-1, and Bcl-xL); and in another study, matrix metalloproteinases-2 and matrix metalloproteinases-9 downregulation in GA treated human leukemia K562 cells are mediated through the suppression of Jun N-terminal kinases-1(JNK-1) mediated c-Jun/Activating transcription factor 2 (Akt-2) and Akt/ERK-mediated c-Jun/c-Fos pathways [14,15].
The aim of this study was to focus on the knowledge of the effects of MTX on the kidneys, and demonstrate the protective effects of GA through the CRP, TNF-α, PGE-2 and SAA pathways.
2. Materials and methods
2.1. Experimental conditions
All experiments were performed in accordance with the guidelines for animal research from the National Institutes of Health, and were approved by the Committee on Animal Research of Suleyman Demirel University, Isparta.
Twenty-four, male, Wistar Albino rats, weighing 300–350 g, were placed in a temperature (21–22 °C) and humidity (60 ± 5%) controlled room in which a 12:12 h light/dark cycle was maintained. All the rats were fed with standard commercial chow diet (Korkuteli yem, Antalya, Turkey). The rats were distributed into three groups that consisted eight rats:
Control group; 0,1 ml saline by oral gavage for 7 days, and only intraperitoneally (i.p.) on the second day;
MTX group; 20 mg/kg MTX (i.p., Methotrexate 50 mg/ml flk, Kocak, Turkey) in a single dose on the second day and 0,1 ml saline by oral gavage for 7 days [16];
MTX + GA group; 20 mg/kg MTX (i.p.) in a single dose on the second day and 100 mg/kg GA by oral gavage for 7 days [17].
Twenty-four hours after the last GA administration, all rats were anesthetized by intraperitoneal injection of 90 mg/kg ketamine (Alfamin, Alfasan IBV, Turkey) and 10 mg/kg xylazine (Alfazin, Alfasan IBV, Turkey). Blood samples were collected for blood urea nitrogen (BUN), uric acid and creatinine analyses. Both kidneys were quickly removed and cut in two parts, one half of the kidneys was fixed in 10% neutral formalin solution for histopathological and immunohistochemical examinations. The other half of the kidneys was placed into the liquid nitrogen and stored at −20 °C until for biochemical analyses of Total Antioxidant Status (TAS), Total Oxidant Status (TOS) and Oxidative Stress Index (OSI).
2.2. Biochemical analyses
Kidneys were homogenized in ice-cold phosphate buffer (pH 7.4) to produce 10% homogenate. Tissues were homogenized in a motor-driven tissue homogenizer (IKA Ultra-Turrax T25 Basic; Labortechnic, Staufen, Germany) and sonicator (UW–2070 Bandelin Electronic, Germany) with phosphate buffer (pH 7.4). Unbroken cells, nuclei and cell debris were sedimented by centrifugation at 10000g for 10 min at 4 °C. Protein levels in the homogenate were determined according to the method of Bradford et al. [18]. This tissue homogenate was used for to determination of TAS and TOS levels [19,20]. The TAS levels of samples were measured spectrophotometrically at the 660 nm absorbance. The results were expressed as mmol Trolox Eq/mg protein. The color intensity is related to the total amount of oxidant (TOS) molecules in the samples. The results are expressed in terms of mM hydrogen peroxide equivalent per g liter (mmol H2O2 Equiv/L, mmol H2O2 Equiv/ mg protein). Determination of OSI, which is an indicative parameter of oxidative stress level and the ratio of TOS to TAS was calculated using the following formula [21]:
TAS and TOS were measured by the automated chemistry analyzer Beckman Coulter AU5800 (Japan). Serum BUN, uric acid and creatinine levels were determined using the Olympus AutoAnalyzer (Olympus Instruments, Tokyo, Japan) and results are expressed as mg/dl.
2.3. Histopathological analysis
Kidney samples were fixed in 10% buffered formalin, routinely processed, embedded in paraffin and then stained with hematoxylin and eosin (HE) to be examined by light microscopy. Histopathological changes were graded in a blinded manner by a specialized pathologist from another university who unawareness the study groups.
Hyperemia, edema, inflammatory reaction, degeneration, necrosis at tubular epithelium and proteinous material in tubular lumen were evaluated according to the severity of lesions using a 0–3 scoring system, where 0, normal; 1, slight hyperemia and slight degeneration in tubular epithelial cells; 2, mild to severe degeneration and inflammatory reaction; 3, necrosis of tubular epithelium, proteinous material in lumen, and severe inflammatory reactions.
2.4. Immunohistochemical examination
All antibodies were purchased (Abcam, Cambridge, UK) and used in 1/100 dilution. Kidney samples were immunostained with primary antibodies, by PGE-2 [Anti-PGE-2 antibody (ab2318)]; C-reactive protein [Anti-C Reactive Protein antibody-Amino terminal end (ab65842)]; Anti-TNF-α antibody (ab6671)]; and Serum amyloid A [Anti-Serum Amyloid A antibody [mc1] (ab655)], according to the manufacturer’s instructions. EXPOSE Mouse and Rabbit Specific HRP/DAB Detection IHC kit (ab80436) used as seconder kit. All the slides were analyzed for immunopositivity and a semiquantitative analysis was carried out. Samples were analyzed by examining five different sections in each sample, which were then scored from 0 to 3, according to the intensity of staining (0, absence of staining; 1, slight, 2, medium and 3, marked). Morphometric evaluation was made using the Database Manual Cell Sens Life Science Imaging Software System (Olympus Corporation, Tokyo, Japan).
2.5. Statistical analysis
Variables were presented as mean ± standard deviations. ANOVA, and Bonferroni Dunn tests were used to compare histopathological and immunohistochemical scores between the groups. Biochemical parameters demonstrated normal distribution. ANOVA and post hoc LSD test were used to compare groups. Calculations were made using the SPSS 15.0 program pack. p < 0.05 was set as the value for significance.
3. Results
3.1. Biochemical analyses
All biochemical blood parameters BUN, creatinine and uric acid levels increased in MTX group compared with the control group, but only creatinine levels were statistically significant (p = 0.028). GA administration ameliorated all these biochemical parameters significantly in MTX + GA group (p < 0.05) (Table 1).
Table 1.
Biochemical markers of kidney.
| Groups | BUN (mg/dL) | Creatinin (mg/dL) | UricAcid (mg/dL) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | |
| Control (n = 8) | 22.4 ± 1.51 | 0.48 ± 0.01 | 1.06 ± 0.2 | |||
| MTX (n = 8) | 25.4 ± 3.36 | 0.51 ± 0.02* | *:0.028 | 1.15 ± 0.41 | ||
| MTX + GA (n = 8) | 17.8 ± 2.22*,** | *: 0.009 | 0.49 ± 0.01** | **:0.005 | 0.54 ± 0.2*,** | *:0.013 |
| **: 0.001 | **:0.008 | |||||
- Values are presented as means ± SD. The relationships between groups and results of biochemical markers are assessed by one-way ANOVA.
- MTX: Methotrexate, GA: Gallic acid, BUN: Blood Urea Nitrogen.
- p values statistically significant compared with *: Control and **: MTX groups.
In MTX group, oxidative stress markers, TOS and OSI levels significantly increased (p = 0.001 and p = 0.021; respectively), and antioxidant activity marker, TAS levels significantly decreased compared with the control group (p = 0.002). In MTX + GA group, GA treatment reversed these parameters significantly compared with the MTX group (p < 0.05) (Table 2).
Table 2.
Oxidative stress markers of kidney.
| Groups (n = 8) | TAS (mmolTroloxequivalents/L) | TOS (mmol H2O2 Equiv./L) | OSI (Index) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | |
| Control | 1.37 ± 0.1 | 16.64 ± 1.93 | 1.48 ± 0.22 | |||
| MTX | 1,08 ± 0.09* | *: 0.002 | 22.69 ± 2.28* | *: 0.001 | 1.90 ± 0.25* | *: 0.021 |
| MTX + GA | 1.23 ± 0.08** | **: 0.044 | 16.65 ± 3.17** | **: 0.002 | 1.35 ± 0.29** | **: 0.007 |
- Values are presented as means ± SD. The relationships between groups and results of biochemical markers are assessed by one-way ANOVA.
- MTX: Methotrexate, GA: Gallic acid, TAS: Total antioxidant capacity, TOS: Total oxidant status, OSI: Oxidative stress index.
- p values statistically significant compared with *: Control and **: MTX groups.
3.2. Histopathological analyses
In MTX group, some kidneys were slightly swollen and pale at gross examination. Normal kidney architecture was observed in the control and MTX + GA groups. The histopathological examination of the kidneys of rats in the MTX group showed hemorrhages, inflammatory cell infiltrations, tubular cell necrosis, glomerulosclerosis and proteinous materials in tubules (Figs. 1–2). Histopathological lesions were scored between 0 and 3 according to lesions and group lesion scores calculated. Because of no pathologies were found in control group, lesions in MTX treated groups were attributed to the MTX toxicity.
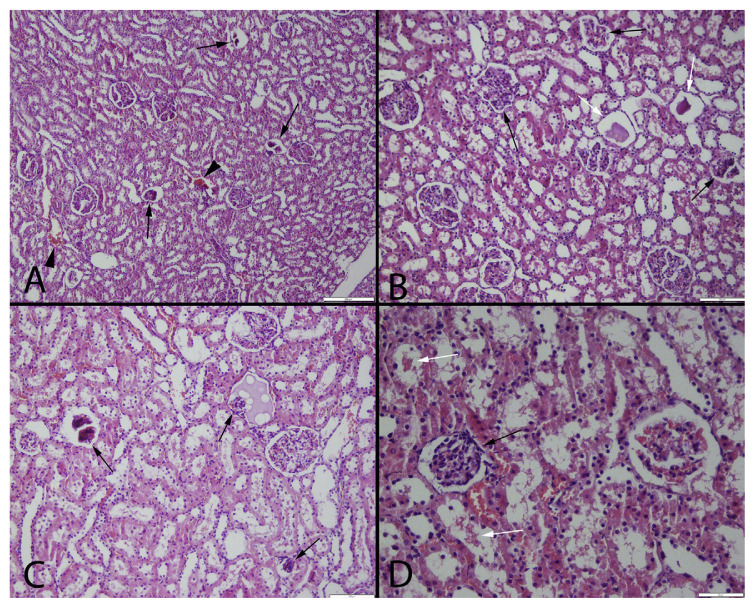
Fig. 1.
Microscopical findings of the kidneys in MTX group by different magnification. Marked glomerulosclerosis in glomeruli (black arrows), hemorrhages (arrow heads) and proteinous materials in tubules (white arrows), HE, (A) Bar = 200 μm, (B)and (C) Bar = 100 μm, (D) Bar = 50 μm.
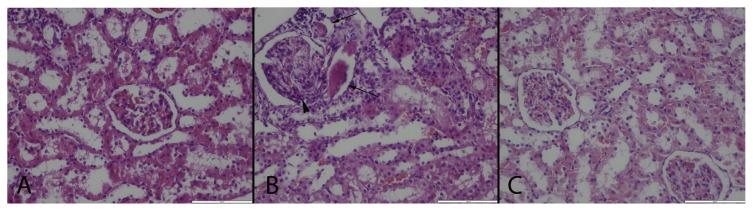
Fig. 2.
Histopathological examination results of the kidneys. (A): Normal histological appearance of the kidney in a rat belonging the control group, (B): Marked glomerulosclerosis (arrow head) and proteinous material in the lumen of the tubules (arrows), (C): Relatively normal histology of a kidney in a rat from MTX + GA group, HE, Bars = 100 μm.
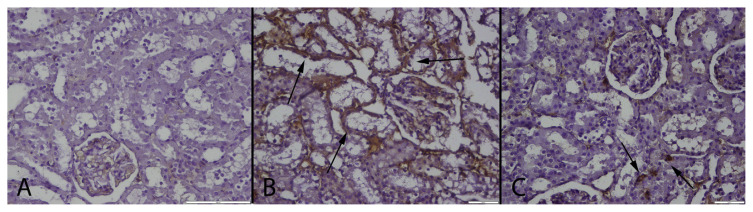
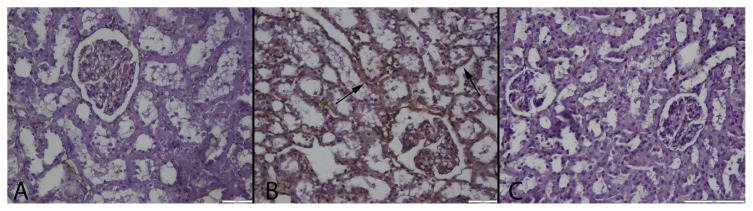
At the immunohistochemical examination, there was no immunoreaction in control group. Numerous strong SAA, TNF-α, PGE-2 and CRP expressions were observed in tubular epithelial cells of MTX group (Figs. 3–6); the strongest positive reaction was seen in proximal tubular epithelial cells (Table 3). Small number of PGE-2 and TNF-α positive tubular epithelial cell were observed in MTX + GA group.
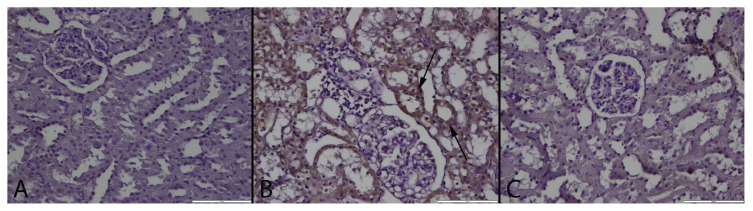
Fig. 3.
CRP immunoreaction in the groups. (A): Negative immunoreaction in a kidney of a rat belonging control group, (B): Marked CRP expression in tubular epithelial cells (arrows) in a rat from MTX treated group, (C): Negative immunoreaction of CRP in a kidney from a rat belonging MTX + GA group, Streptavidin biotin peroxidase method, Bars = 100 μm.
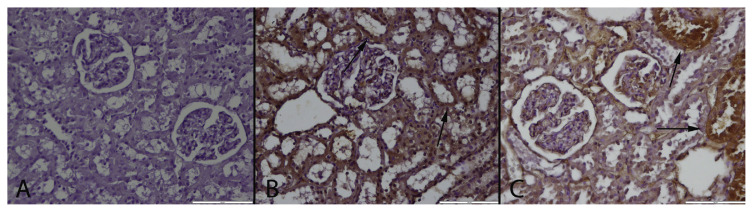
Fig. 4.
PGE-2 immunoreaction in the groups. (A): Negative immunoreaction in a kidney of a rat belonging control group, (B): Marked PGE2 expression in numerous proximal tubular epithelium (arrows) in a rat from MTX treated group, (C): PGE2 expression in some tubules (arrows) in a kidney from MTX + GA group, Streptavidin biotin peroxidase method, Bars = 100 μm.
Fig. 5.
TNF-α immunoreaction in the groups. (A): Negative immunoreaction in control group, (B): Marked TNF-α expression in proximal tubular epithelium (arrows) in a rat from MTX treated group, (C): Slight immunoreaction in some tubular epithelial cells (arrows) in a kidney from MTX + GA group, Streptavidin biotin peroxidase method, Bars = 100 μm.
Fig. 6.
SAA immunoreaction in the groups. (A): Negative immunoreaction in a kidney of a rat belonging control group, (B): Marked SAA expression in proximal tubular epithelium (arrows) in a rat from MTX treated group, (C): Negative immunoreaction in a kidney from MTX + GA group, Streptavidin biotin peroxidase method, Bars = 100 μm.
Table 3.
Statistical analysis results of histopathological and immunohistochemical scoring results of kidney.
| Control | MTX | MTX + GA | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| Mean ± SD (max-min) | Mean ± SD (max-min) | P values | Mean ± SD (max-min) | P values | |
| Histopathology | 0.00 ± 0.00 (0–0) | 2.40 ± 0.69* (1–3) | 0.001 | 0.50 ± 0.16** (0–1) | 0.001 |
| CRP | 0.00 ± 0.00 (0–0) | 1.90 ± 0.73* (1–3) | 0.001 | 0.00 ± 0.00** (0–0) | 0.001 |
| TNF alpha | 0.00 ± 0.00 (0–0) | 1.60 ± 0.69* (1–2) | 0.001 | 0.40 ± 0.51** (0–1) | 0.001 |
| PGE-2 | 0.00 ± 0.00 (0–0) | 2.20 ± 0.78* (1–3) | 0.001 | 0.80 ± 0.91** (0–2) | 0.001 |
| SAA | 0.00 ± 0.00 (0–0) | 1.90 ± 0.73* (1–3) | 0.001 | 0.00 ± 0.00** (0–0) | 0.001 |
- Values are presented as means ± SD. The relationships between groups and results of histochemical markers area assessed by one-way ANOVA and Bonferroni Dunn tests.
- MTX: Methotrexate, GA: Gallic Acid, CRP: C-Reactive Protein, TNF α: Tumor Necrosis Factor Alpha, PGE-2: Prostaglandin E2.
- p values statistically significant compared with *: Control and **: MTX groups.
4. Discussion
Kidney toxicity can occur with MTX treatment in both low and high doses. High doses of MTX make kidney damage in two ways: tubular injury with the precipitation of MTX in kidney tubules, and decrease in the glomerular filtration rate [22]. It can be ameliorated mostly by hydration and making the urine alkaline. In patients receiving MTX treatment, the risk of kidney toxicity was 2% [23]. To induce nephrotoxicity, we used a single dose MTX which was described in the literature. According to the biochemical markers of kidney, MTX elevated BUN, creatinine and uric acid levels, which is an indicator of the renal function. Armagan et al. use the same single MTX dose, and found similar biochemical findings about impairment of renal function induced by MTX [7]. Besides, the oxidative stress markers of the renal tissue, TOS and OSI levels, were also similar to the blood markers. Ahmed et al. investigated that MTX-induced nephrotoxicity and role of protective effect of garlic aqueous extract in rats. Their results shown blood urea and creatinine levels and MDA, adenosine deaminase and nitric oxide higher and catalase and GSH levels lower in the MTX administration group [24]. Similarly, Asvadi et al., showed that MTX administration caused increase blood urea and creatinine levels [25]. In this study, nephrotoxicity was induced by single dose of MTX, as shown in the literature and GA treatment ameliorated all biochemical and oxidative stress markers.
GA treatment improve the kidney damage via antioxidant property but there are no enough studies about oxidative stress and antioxidant parameters TAS, TOS and OSI in MTX induced kidney damage. Baradaran et al., found that Aloe Vera, containing GA, has protective effects on gentamicin-induced nephrotoxicity in male rats due to its antioxidant activity, and Nabavi et al. found that GA isolated from Peltiphyllum peltatum protects the rat kidneys from the sodium fluoride-induced oxidative stress [26,27]. According to this study, it indicates that more time is needed to fit the damage that shows the more significant rise in oxidative stress parameters than biochemical parameters. Also, according to both findings, GA has huge antioxidant capacity that normalized the abnormal outcomes.
Based on gross examination and histopathological findings such as hemorrhages, inflammatory cell infiltrations, tubular cell necrosis, glomerulosclerosis and proteinous materials in tubules in MTX group, showed that oxidative stress induced renal damage mainly occurred at histopathological level in this study. In a previous study where the equivalent dose was used, MTX caused marked degenerative changes, such as tubular degeneration, tubular dilatation, tubular cell swelling, and tubular damage [28]. MTX-induced kidney toxicity was associated with the activation of the systemic inflammatory response and proinflammatory cytokines. Ibrahim et al. studied peroxisome proliferator-activated receptor alpha and γ ligands against methotrexate-induced nephrotoxicity and found upregulation of TNF-α and apoptotic markers [29]. In another study, montelukast was used for the same indications and researchers found that increased of BUN and serum creatinine levels, and TNF-α expression in renal tissue, which was similar to this study [30]. El-Boghdady used antioxidant agents, such as ellagic acid and pumpkin seed oil, against MTX-induced small intestine damage and found that pumpkin seed oil decreased the intestinal damage by inhibition of increased PGE-2, malondialdehyde, nitric oxide, myeloperoxidase and xanthine oxidase levels [31]. In this study, the acute inflammation markers TNF-α, PGE-2 and CRP expressions also significantly increased in MTX administrated group immunohistochemically. Strong antioxidants, such as lycopene, combined with melatonin, provided significant reduction in TNF-α, interleukin 1β and ceruloplasmin levels, which protected the kidney against MTX induced nephrotoxicity [32]. In this study, GA treatment decreased expressions of all these elevated inflammation parameters: TNF-α, CRP, and PGE-2 immunohistochemically. These findings reflected the anti-inflammatory effects of GA on MTX induced nephrotoxicity. In accordance with these parameters, SAA levels increased in MTX induced nephrotoxicity, because it was secreted by the liver in response to the inflammation [5]. Obayashi et al. found that plasma TNF-α, interleukin-6, and SAA concentrations showed obvious 24 h rhythms with higher levels during the light phase, and lower levels during the dark phase after the rheumatoid arthritis crisis, and in Jamnitski et al. study on 100 patients which used TNF-α blockers for rheumatoid arthritis, SAA levels decreased 4 months after the beginning of the drug therapy [33,34]. GA treatment decreased SAA expression significantly in tubules of the kidney in this study. Olayinka et al. reported that oxidative stress has been identified as a toxicological mechanism of MTX nephrotoxicity. Free radicals release and ROS plays a significant role in this toxicity [35]. They reported MTX related nephrotoxicity in rats and amelioration with GA treatment in biochemical parameters. GA has ameliorative effect of cells even some drugs [36] Similar findings were observed and mechanisms of the MTX damage and ameliorative effect of GA evaluated in this study.
In conclusion, all evidence suggested that oxidative stress, caused by the abnormal production of ROS, has been accused in the etiology of MTX-induced nephrotoxicity. GA prevents the kidneys from the nephrotoxicity due to its antioxidant and anti-inflammatory activities especially through the novel biomarker of SAA. Due to the decrease of nephrotoxic side effects, GA administration can be used for long term therapy. Consequently, it could be combined with the pharmaceutical formulation of MTX and may be used to treat cancer or autoimmune diseases safely and effectively. Cancer patients should consume more food sources which contain of GA during the MTX therapy.
Footnotes
Conflicts of interest
There is no conflict interest.
REFERENCES
- 1. Daggulli M, Dede O, Utangac MM, Bodakci MN, Hatipoglu NK, Penbegul N, et al. Protective effects of carvacrol against methotrexate-induced testicular toxicity in rats. Int J Clin Exp Med. 2014;7(12):5511–6. [PMC free article] [PubMed] [Google Scholar]
- 2. Celik F, Gocmez C, Bozkurt M, Kaplan I, Kamasak K, Akil E, et al. Neuroprotective effects of carvacrol and pomegranate against methotrexate –induced toxicity in rats. Eur Rev Med Pharmacol Sci. 2013;17(22):2988–93. [PubMed] [Google Scholar]
- 3. Selimoglu Sen H, Sen V, Bozkurt M, Turkçu G, Guzel A, Sezgi C, et al. Carvacrol and pomegranate extract in treating methotrexate-induced lung oxidative injury in rats. Med Sci Monit. 2014;20:1983–90. doi: 10.12659/MSM.890972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sugitharini V, Prema A, Berla Thangam E. Inflammatory mediators of systemic inflammation in neonatal sepsis. Inflamm Res. 2013;62(12):1025–34. doi: 10.1007/s00011-013-0661-9. [DOI] [PubMed] [Google Scholar]
- 5. Kovacevic A, Hammer A, Stadelmeyer E, Windischhofer W, Sundl M, Ray A, et al. Expression of serum amyloid A transcripts in human bone tissues differentiated osteoblast-like stem cells and human osteosarcoma cell lines. J Cell Biochem. 2008;103(3):994–1004. doi: 10.1002/jcb.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang HT, Tian EB, Chen YL, Deng HT, Wang QT. Proteomic analysis for finding serum pathogenic factors and potential biomarkers in multiple myeloma. Chin Med J Engl. 2015;128(8):1108–13. doi: 10.4103/0366-6999.155112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armagan I, Bayram D, Candan IA, Yigit A, Celik E, Armagan HH, et al. Effects of pentoxifylline and alpha lipoic acid on methotrexate-induced damage in liver and kidney of rats. Environ Toxicol Pharmacol. 2015;39(3):1122–31. doi: 10.1016/j.etap.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 8. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. Am J Clin Nutr. 2005;81(1):230–42. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 9. Farbood Y, Sarkaki A, Hashemi S, Mansouri MT, Dianat M. The effects of gallic acid on pain and memory following transient global ischemia/reperfusion in wistar rats. Avicenna J Phytomed. 2013;3(4):329–40. [PMC free article] [PubMed] [Google Scholar]
- 10. Rangkadilok N, Sitthimonchai S, Worasuttayangkurn L, Mahidol C, Ruchirawat M, Satayavivad J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem Toxicol. 2007;45:328–36. doi: 10.1016/j.fct.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 11. Badavi M, Sadeghi N, Dianat M, Samarbafzadeh A. Effects of gallic acid and cyclosporine on antioxidant capacity and cardiac markers of rat isolated heart after ischemia/ reperfusion. Iran Red Crescent Med J. 2014;16(6):e16424. doi: 10.5812/ircmj.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haute GV, Caberlon E, Squizani E, de Mesquita FC, Pedrazza L, Martha BA, et al. Gallic acid reduces the effect of LPS on apoptosis and inhibits the formation of neutrophil extracellular traps. Toxicol Vitro. 2015;30(1 Pt B):309–17. doi: 10.1016/j.tiv.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 13. Vijaya Padma V, Sowmya P, Arun Felix T, Baskaran R, Poornima P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem Toxicol. 2011;49(4):991–8. doi: 10.1016/j.fct.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 14. Teng CL, Han SM, Wu WC, Hsueh CM, Tsai JR, Hwang WL, et al. Mechanistic aspects of lauryl gallate-induced differentiation and apoptosis in human acute myeloid leukemia cells. Food Chem Toxicol. 2014;71:197–206. doi: 10.1016/j.fct.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 15. Chen YJ, Chang LS. Gallic acid downregulates matrix metalloproteinase-2 (MMP-2) and MMP-9 in human leukemia cells with expressed Bcr/Abl. Mol Nutr Food Res. 2012;56(9):1398–412. doi: 10.1002/mnfr.201200167. [DOI] [PubMed] [Google Scholar]
- 16. Hemeida RA, Mohafez OM. Curcumin attenuates methotraxate-induced hepatic oxidative damage in rats. J Egypt Natl Canc Inst. 2008;20(2):141–8. [PubMed] [Google Scholar]
- 17. Sarkaki A, Farbood Y, Gharib-Naseri MK, Badavi M, Mansouri MT, Haghparast A, et al. Gallic acid improved behavior, brain electrophysiology, and inflammation in a rat model of traumatic brain injury. Can J Physiol Pharmacol. 2015;93(8):687–94. doi: 10.1139/cjpp-2014-0546. [DOI] [PubMed] [Google Scholar]
- 18. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 19. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–85. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 20. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 21. Demirbag R, Gur M, Yilmaz R, Kunt AS, Erel O, Andac MH. Influence of oxidative stress on the development of collateral circulation in total coronary occlusions. Int J Cardiol. 2007;116(1):14–9. doi: 10.1016/j.ijcard.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 22. Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65:168–73. [PubMed] [Google Scholar]
- 23. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 24. Ahmed W, Zaki A, Nabil T. Prevention of methotrexate-induced nephrotoxicity by concomitant administration of garlic aqueous extract in rat. Turk J Med Sci. 2015;45:507–16. doi: 10.3906/sag-1408-121. [DOI] [PubMed] [Google Scholar]
- 25. Asvadi I, Hajipour B, Asvadi A, Asl NA, Roshangar L, Khodadadi A. Protective effect of pentoxyfilline in renal toxicity after methotrexate administration. Eur Rev Med Pharmacol Sci. 2011;15:1003–9. [PubMed] [Google Scholar]
- 26. Baradaran A, Nasri H, Nematbakhsh M, Rafieian-Kopaei M. Antioxidant activity and preventive effect of aqueous leaf extract of Aloe Vera on gentamicin-induced nephrotoxicity in male Wistar rats. Clin Ter. 2014;165(1):7–11. doi: 10.7471/CT.2014.1653. [DOI] [PubMed] [Google Scholar]
- 27. Nabavi SM, Habtemariam S, Nabavi SF, Sureda A, Daglia M, Moghaddam AH, et al. Protective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress in rat’s kidney. Mol Cell Biochem. 2013;372(1–2):233–9. doi: 10.1007/s11010-012-1464-y. [DOI] [PubMed] [Google Scholar]
- 28. Uzar E, Koyuncuoglu HR, Uz E, Yilmaz HR, Kutluhan S, Kilbas S, et al. The activities of antioxidant enzymes and the level of malondialdehyde in cerebellum of rats subjected to methotrexate: protective effect of caffeic acid phenethyl ester. Mol Cell Biochem. 2006;291:63–8. doi: 10.1007/s11010-006-9196-5. [DOI] [PubMed] [Google Scholar]
- 29. Ibrahim MA, El-Sheikh AA, Khalaf HM, Abdelrahman AM. Protective effect of peroxisome proliferator activator receptor (PPAR)-α and -γ ligands against methotrexate-induced nephrotoxicity. Immunopharmacol Immunotoxicol. 2014;36(2):130–7. doi: 10.3109/08923973.2014.884135. [DOI] [PubMed] [Google Scholar]
- 30. Abdel-Raheem IT, Khedr NF. Renoprotective effects of montelukast, a cysteinyl leukotriene receptor antagonist, against methotrexate-induced kidney damage in rats. Naunyn Schmiedeb Arch Pharmacol. 2014;387(4):341–53. doi: 10.1007/s00210-013-0949-x. [DOI] [PubMed] [Google Scholar]
- 31. El-Boghdady NA. Protective effect of ellagic acid and pumpkin seed oil against methotrexate-induced small intestine damage in rats. Indian J Biochem Biophys. 2011;48(6):380–7. [PubMed] [Google Scholar]
- 32. Oguz E, Kocarslan S, Tabur S, Sezen H, Yilmaz Z, Aksoy N. Effects of lycopene alone or combined with melatonin on methotrexate-induced nephrotoxicity in rats. Asian Pac J Cancer Prev. 2015;16(14):6061–6. doi: 10.7314/apjcp.2015.16.14.6061. [DOI] [PubMed] [Google Scholar]
- 33. Obayashi K, Tomonari M, Yoshimatsu H, Fukuyama R, Ieiri I, Higuchi S, et al. Dosing time-dependency of the arthritis-inhibiting effect of tacrolimus in mice. J Pharmacol Sci. 2011;116(3):264–73. doi: 10.1254/jphs.11029fp. [DOI] [PubMed] [Google Scholar]
- 34. Jamnitski A, Levels JH, van den Oever IA, Nurmohamed MT. High-density lipoprotein profiling changes in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a cohort study. J Rheumatol. 2013;40(6):825–30. doi: 10.3899/jrheum.121358. [DOI] [PubMed] [Google Scholar]
- 35. Olayinka ET, Ore A, Adeyemo OA, Ola OS. Ameliorative effect of gallic acid on methotrexate-induced hepatotoxicity and nephrotoxicity in rat. J Xenobiotics. 2016;6:6092. doi: 10.4081/xeno.2016.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oboh G, Ogunsuyi OB, Ogunbadejo MD, Adefegha SA. Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. J Food Drug Anal. 2016;24(3):627–34. doi: 10.1016/j.jfda.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]