Abstract
Bai-Hu-Tang (BHT), a classic traditional Chinese medicine (TCM) formula used for clearing heat and promoting body fluid, consists of four traditional Chinese medicines, i.e., Gypsum Fibrosum (Shigao), Anemarrhenae Rhizoma (Zhimu), Glycyrrhizae Radix et Rhizoma Praeparata cum Melle (Zhigancao), and nonglutinous rice (Jingmi). The chemical composition of BHT still remains largely elusive thus far. To qualitatively and quantitatively characterize secondary metabolites and carbohydrates in BHT, here a combination of analytical approaches using ultraperformance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry and ultraperformance liquid chromatography coupled with photodiode array detector was developed and validated. A total of 42 secondary metabolites in BHT were tentatively or definitely identified, of which 10 major chemicals were quantified by the extracting ion mode of quadrupole time-of-flight mass spectrometry. Meanwhile, polysaccharides, oligosaccharides, and monosaccharides in BHT were also characterized via sample pretreatment followed by sugar composition analysis. The quantitative results indicated that the determined chemicals accounted for 35.76% of the total extract of BHT, which demonstrated that the study could be instrumental in chemical dissection and quality control of BHT. The research deliverables not only laid the root for further chemical and biological evaluation of BHT, but also provided a comprehensive analytical strategy for chemical characterization of secondary metabolites and carbohydrates in traditional Chinese medicine formulas.
Keywords: Bai-Hu-Tang, carbohydrate, secondary metabolite, ultraperformance liquid chromatography coupled with photodiode array detector, ultraperformance liquid, chromatography coupled with quadrupole time-of-flight mass spectrometry
1. Introduction
Bai-Hu-Tang (BHT, also known as white tiger decoction), originally documented in Treatise on Exogenous Febrile Diseases (Shang-Han-Lun, a renowned medicinal classic at the Eastern Han Dynasty), is a classic traditional Chinese medicine (TCM) formula used for clearing heat and promoting body fluid [1]. The potential of BHT for treating septicemia and hyperglycemia is also demonstrated by pharmacological experiments [2,3]. BHT is composed of one mineral drug, i.e., Gypsum Fibrosum (GF, Shigao), and three herbal drugs including Anemarrhenae Rhizoma (AR, Zhimu), Glycyrrhizae Radix et Rhizoma Praeparata cum Melle (GR, Zhigancao), and nonglutinous rice (NR, Jingmi) [1]. Bioactive ingredients of the four drugs have been individually explored. Specifically, GF is constituently explicit with no less than 95% hydrated calcium sulfate (CaSO4·2H2O) [4]; secondary metabolites, mainly involving saponins (steroidal and triterpene) and flavonoids (xanthones, flavanonols, and chalcones), are believed to be largely responsible for pharmacological effects of AR [5] and GR [6]; NR mainly contains multiple primary metabolites (e.g., carbohydrates, amino acids, and vitamins) and microelements, of which polysaccharides, especially amylose and amylopectin, were much abundant occupying up to around 80% [7]. Nevertheless, the constituents of BHT still remain qualitatively and quantitatively unknown, especially those contributed by the three herbal drugs due to their complex chemical compositions. Whether and how the bioactive chemicals in the individual drugs occur in BHT are crucial to both control the quality and ascertain the effective substances of BHT, and therefore deserve further investigation.
Secondary metabolites and carbohydrates are commonly deemed as the two major kinds of ingredients in most TCMs, in particular herbal TCMs [8,9]. However, nowadays chemical characterization of TCMs extensively focuses on secondary metabolites since they have been understood adequately [10], just as in the cases of AR and GR. By contrast, carbohydrates (polymeric and monomeric carbohydrates) are largely overlooked due to scientifically restricted definition. However, the situation is becoming increasingly debatable given the recently revealed chemical and bioactive roles of TCM carbohydrates. To be specific, first, natural carbohydrates are abundant in many TCMs and are easily extracted as the taken chemicals since TCMs are usually prepared by water extraction [11]. Second, accumulated in vivo and in vitro studies have demonstrated that TCM carbohydrates have various pharmacological actions—anticancer [12], immune regulation [13,14], hyperglycemic [15], and prebiotic-like effects [16], to name but a few. In addition to their direct bioactivity, we recently verified that carbohydrates could also exert indirect effects in vivo by synergy with co-occurring secondary metabolites in herbal medicines [17]. Therefore, carbohydrates should be taken into account for overall chemical characterization of TCMs as well as TCM formulas.
Thus, we seek to further explore chemical compositions of BHT in this study by characterizing both carbohydrates and secondary metabolites. First, BHT was accordingly prepared. Then, an ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS/MS) method was developed for qualitative and quantitative characterization of the secondary metabolites in BHT, for the latter of which the extracting ion mode of QTOF-MS was adopted. Meanwhile, carbohydrates in BHT, including polysaccharides, oligosaccharides, and monosaccharides, were also qualitatively and quantitatively determined by sample pretreatment and then sugar composition analysis using ultra-performance liquid chromatography coupled with photodiode array detector (UPLC-PDA).
2. Methods
2.1. Reagents, chemicals, and materials
Acetonitrile [high-performance liquid chromatography (HPLC) and MS grade], ammonium acetate (HPLC grade), ethanol (absolute), and formic acid (MS grade) were purchased from Merck (Darmstadt, Germany). Trifluoroacetic acid used for acid hydrolysis of polymeric carbohydrates was from Riedelde Haën (Honeywell, Seelze, Germany). For monosaccharide derivatization, 3-methyl-1-phenyl-5-pyrazolone (PMP) was bought from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was produced by a Milli-Q water purification system (Merck Millipore, Milford, MA, USA).
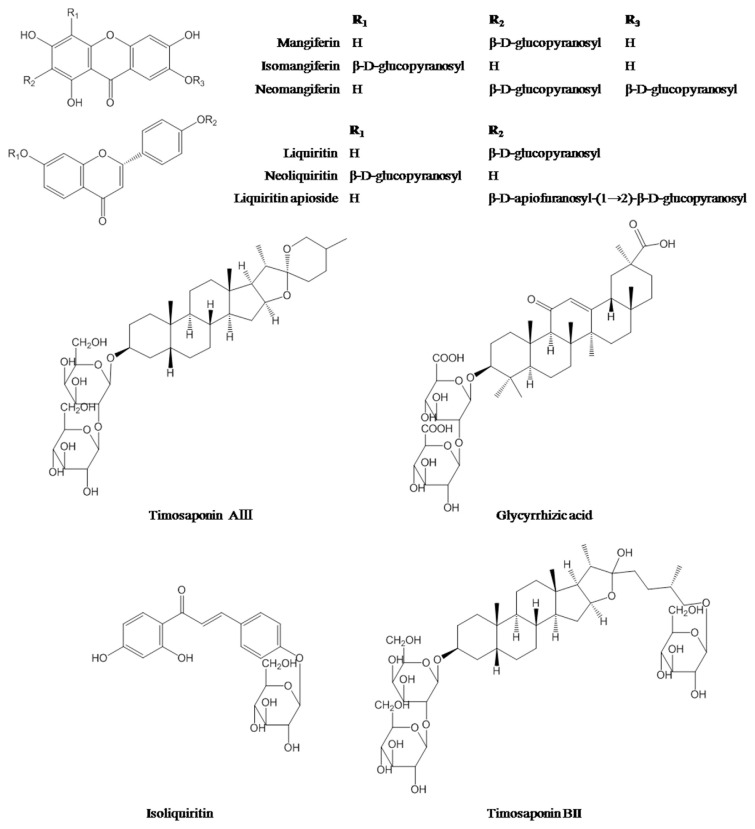
Reference substances including neomangiferin, isomangiferin, mangiferin, isoliquiritin, liquiritin, neoliquiritin, liquiritin apioside, timosaponin BII, glycyrrhizic acid, and timosaponin AIII (Figure 1) were supplied from Chengdu Pufei De Biotech Co. Ltd (Szechwan, China). The purity of these reference standards was determined to be higher than 95% by HPLC–MS analysis (the same analytical condition as described in Section 2.3). Sugar reference substances, namely, D-mannose (Man), L-rhamnose monohydrate (Rha), D-glucuronic acid (GlcA), D-galacturonic acid monohydrate (GalA), D-glucose (Glc), D-galactose (Gal), L-arabinose (Ara), D-fucose (Fuc), sucrose, and maltotriose, were obtained from Sigma.
Figure 1.
Chemical structures of 10 investigated secondary metabolites.
GF and decoction pieces of AR and GR were purchased from Hong Kong herbal market, and were authenticated by Professor H.B. Chen based on the morphological and histological features according to the standards of Chinese Pharmacopoeia (2015 version). NR was bought from Hong Kong supermarket. Voucher specimens were deposited in School of Chinese Medicine, Hong Kong Baptist University.
2.2. Sample preparation
2.2.1. Extraction and pretreatment
GF (crushed, 10.00 g), AR (3.75 g), GR (1.25 g), and NR (3.76 g) were accurately weighed, immersed together in 40 mL water for 30 minutes, and then refluxed twice at 100°C for 30 minutes each to generate BHT. Next, the two extract solutions were centrifuged (4000 ×g for 10 minutes), and the supernatants were combined for freeze drying. The lyophilized powder was then weighed and stored at 4°C before analysis. Component drugs were also individually treated as mentioned above.
For the analysis of secondary metabolites in BHT, the lyophilized powder (20 mg) was accurately weighed and ultrasonic extracted by 20.0 mL of 80% methanol at room temperature for 40 minutes. The extract was then centrifuged (4000 ×g for 10 minutes), and the supernatant was filtered through a 0.22 μm syringe filter (Agilent Technologies, Santa Clara, CA, USA) for UPLC-QTOF-MS/MS analysis. The lyophilized powder of AR and GR extracts was also treated as mentioned above.
For the analysis of carbohydrates in BHT, the lyophilized powder (30 mg) was accurately weighed and redissolved in 1 mL of water by ultrasonication for 10 minutes. The extract was then centrifuged (4000 ×g for 10 minutes) to obtain the supernatant. Subsequently, the supernatant (0.5 mL) was successively extracted with water-saturated n-butanol (0.5 mL) and ethyl acetate (0.5 mL) (3 times each). After the extraction, the aqueous layer was subjected to acid hydrolysis followed by PMP derivatization or direct PMP derivatization without acid hydrolysis; meanwhile, the supernatant (5 mL) was precipitated by adding ethanol to make a final concentration of 95% and left overnight (12 hours) at 4°C. After centrifugation (4000 ×g for 10 minutes), the precipitate was collected, washed with ethanol, and dried (water bath, 70°C) to remove the residual ethanol; then it was completely redissolved in 5 mL hot water (60°C) by drastic mechanical vibration for 2 hours to yield the crude polysaccharide solution, and the polysaccharide content was determined by acid hydrolysis and PMP derivatization.
2.2.2. Acid hydrolysis of water extracts and polysaccharides
The prepared water extract or polysaccharide solution (0.50 mL) was mixed with 2.50 mL of 2.4 M trifluoroacetic acid (final concentration 2 M) solution in a screw-cap vial and hydrolyzed for 2 hours at 120°C. After cooling, the hydrolysate was evaporated at 55°C on a rotary evaporator until dry. Then 1 mL of water was added to dissolve the hydrolysate, and the precipitate was removed after centrifugation (15,700 ×g for 5 minutes); the supernatant was then subjected to PMP derivatization.
2.2.3. PMP derivatization of monosaccharides
Sugar derivatization was performed according to our previous report [18]. Briefly, the acid hydrolysate (100 μL) was mixed with the same volume of ammonia water and 0.5 M PMP methanolic solution (200 μL). The mixture was allowed to react at 70°C for 30 minutes and then cooled to room temperature. Afterward, 100 μL glacial acetic acid and 500 μL chloroform were successively added to neutralize the reaction solution and remove the excess PMP reagents, respectively. After vigorous shaking followed by centrifugation (15,700 ×g for 5 minutes), the organic phase was discarded. The operation was performed five times, and finally the aqueous layer was filtered through a 0.22 μm syringe filter (Agilent Technologies) before UPLC-PDA analysis. A standard solution, containing six monosaccharides (Man, Rha, Glc, Gal, Ara, and Fuc) and two uronic acids (GlcA and GalA), was also treated as mentioned above.
2.3. UPLC-QTOF-MS/MS analysis
2.3.1. Liquid chromatography
Analysis of secondary metabolites was performed on an Agilent UPLC system equipped with a binary pump, a thermostatted column compartment, an autosampler, and a degasser. A sample (2 μL) was injected onto an ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm; Waters, Milford, MA, USA) operated at 40°C. The separation was achieved using gradient elution with 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) at a flow rate of 0.35 mL/min: 0–10 minutes, 2–15% B; 10–18 minutes, 15–45% B; 18–23 minutes, 45–75% B; 23–25 minutes, 75–100% B; 25–28 minutes, 100% B; 28–28.1 minutes, 100–2% B; 28.1–31 minutes, 2% B.
2.3.2. Mass spectrometry
MS data were recorded using an Agilent 6540 QTOF mass spectrometer (Agilent Technologies) equipped with a QTOF mass spectrometer and a jet stream electrospray ion source. Data acquisition were controlled by Mass Hunter Qualitative Analysis B.06 and Quantitative Analysis B.04 (Agilent Technologies). The optimized operating parameters in the negative ion modes were as follows: nebulizing gas (N2) flow rate, 8.0 L/min; nebulizing gas temperature, 300°C; jet stream gas flow, 8 L/min; sheath gas temperature, 350°C; nebulizer pressure, 45 psi; capillary voltage, 3000 V; skimmer, 65 V; Octopole RFV, 600 V; and collision energy, 15 eV. Mass spectra were recorded across the range m/z 100–1300 with accurate mass measurement of all mass peaks. Deprotonated molecular ions of the 10 analytes were selected as their respective extraction ions in quantitative analysis using the extracting ion mode.
2.3.3. Establishment of in-house library and peak assignment
Previous reports on secondary metabolites from the constituted drugs of BHT were collected and summarized in a Microsoft Office Excel table (Microsoft Corporation, Redmond, WA, USA) to establish an in-house database, which includes compound name, molecular formula, accurate molecular weight, UV maximum wavelength, chemical structure, and related references. The empirical molecular formula was deduced by a comparison of the accurate mass value and the theoretical exact mass value of putative deprotonated molecular ions and/or further confirmed by elucidating the fragment ions based on previous reports, and then matched with that of known compounds in the database using the “Find” function of Microsoft Office Excel (Microsoft Corporation).
2.4. UPLC-PDA analysis
Analysis of PMP derivatives of monosaccharides was performed on a Waters UPLC system (Waters), which was equipped with a vacuum degasser, a binary pump, an auto-sampler, and a PDA detector. Samples (2 μL) were injected onto an ACQUITY UPLC HSS C18 column (2.1 mm × 100 mm, 1.8 μm; Waters) operated at 40°C. Separation was achieved using gradient elution with 100 mM ammonium acetate (A) (pH 5.58) and acetonitrile (B) at a flow rate of 0.3 mL/min: 0–9 minutes, 19% B; 9–14 minutes, 19–30% B; and 14–15 minutes, 30–19% B. UV detection wavelength was set at 245 nm.
2.5. Quantitative method validation
The UPLC-PDA and UPLC-QTOF-MS methods for quantitative analysis of carbohydrates and secondary metabolites in BHT were validated in terms of linearity, sensitivity, precision, accuracy, and stability.
Stock solutions of reference compounds were diluted to appropriate concentrations for the construction of calibration curves. Six concentrations of the solution were analyzed, and the calibration curves were constructed by plotting the peak areas versus the concentrations of analytes. Limits of detection and lower limits of quantification under the present conditions were determined at a signal to noise ratio of about 3 and the lowest point calibration standard, respectively.
Intra- and interday variations were chosen to determine the precision of the developed assay. For intraday variability test, the BHT sample was extracted and analyzed for six replicates within 1 day, while for interday variability test, the BHT sample was examined in duplicates for 3 consecutive days. Variations were expressed by the relative standard deviations (RSDs) of the data.
The spike recovery test was used to evaluate the accuracy of the method. The BHT sample with known contents of the target analytes was weighed, and different amounts (low, middle, and high levels) of reference standards (neomangiferin: 60.00 μg, 90.00 μg, and 120.00 μg; isomangiferin: 6.00 μg, 9.00 μg, and 12.00 μg; mangiferin: 48.00 μg, 56.00 μg, and 80.00 μg; isoliquiritin: 0.40 μg, 0.50 μg, and 1.00 μg; liquiritin: 5.00 μg, 10.00 μg, and 15.00 μg; neoliquiritin: 1.00 μg, 2.00 μg, and 4.00 μg; liquiritin apioside: 7.46 μg, 9.32 μg, and 11.18 μg; timosaponin BII: 192.00 μg, 240.00 μg, and 288.00 μg; glycyrrhizic acid: 2.50 μg, 4.00 μg, and 10.00 μg; timosaponin AIII: 1.00 μg, 2.00 μg, and 5.00 μg; mannose: 121.11 μg, 100.92 μg, and 80.74 μg; glucuronic acid: 86.2 μg, 72.18 μg, and 57.75 μg; glucose: 5.76 mg, 4.79 mg, and 3.83 mg; galactose: 153.06 μg, 127.55 μg, and 102.04 μg; arabinose: 49.98 μg, 41.65 μg, and 33.32 μg; sucrose: 3.00 mg, 6.00 mg, and 9.00 mg; and maltotriose: 3.00 mg, 6.00 mg, and 9.00 mg) were spiked, and then extracted and analyzed in triplicates. Spike recoveries were calculated by the following equation:
| (1) |
The stability test was performed by analyzing the sample extract over periods of 2 hours, 4 hours, 6 hours, 8 hours, 10 hours, 12 hours, and 24 hours; RSDs of the peak areas of each analyte were taken as the measures of stability.
3. Results and discussion
3.1. Methodology optimization
According to the original description in Treatise on Exogenous Febrile Disease, BHT is produced by boiling GF 1 Jin, AR 6 Liang, GR 2 Liang, and NR 6 Ge in 1 Dou (also called “dipper”) of water and then removing the dregs. These traditional units of measurement at the Eastern Han Dynasty are officially converted into the modern ones by equating 1 Jin to 250 g, 1 Liang to 15.625 g, 1 Ge to 20 mL, and 1 Dou to 2 L [1]. The BHT sample was, therefore, prepared accordingly but scaled down by 25 times in this study, in which the NR amount was measured using a measuring cylinder and then weighed.
The QTOF-MS is very adept at qualitative analysis of complicated composition systems by providing accurate mass measurement, high resolution, and selectivity. Thus, it is widely employed for qualitative identification of secondary metabolites in TCMs [19,20]. In recent years, it is also attempted for quantitative determination of TCM constituents [21]. Moreover, using the extracting ion mode, the quantitative selectivity and sensitivity of QTOF-MS could be improved, and sometimes even comparative with that of triple quadrupole mass spectrometer [22]. These favorable features make QTOF-MS more attractive for simultaneous quantitative and qualitative analysis of TCMs than triple quadrupole mass spectrometer. It was therefore used here for the quantitative and qualitative determination of secondary metabolites in BHT. Both the positive and negative ion mode were tried in MS analysis, and the data collected in the negative ion mode were used for the qualitative identification and quantitative analysis as it provided higher sensitivity.
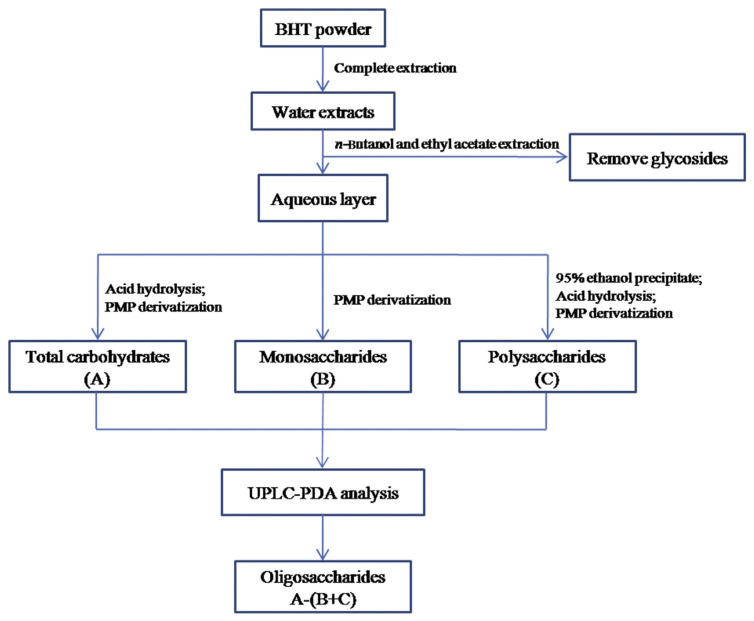
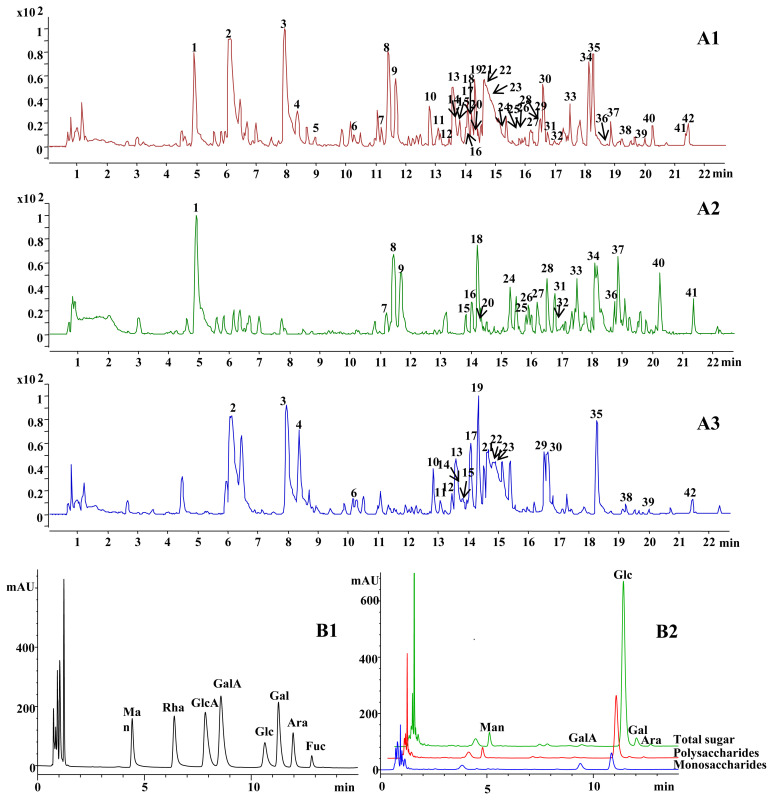
To examine the carbohydrates in BHT, an analytical strategy was newly adopted (Figure 2), by which carbohydrates with different polymeric degrees, including polysaccharides, oligosaccharides, and monosaccharides, could be determined. First, the extraction efficiency of saccharides was examined by the sulfuric acid–phenol method, and the result showed that the saccharides in the materials could be completely extracted because no sugar was detected in the subsequent third extraction. Since the BHT sample was rich in glycosides (saponins and flavonoids being the constituents, see Section 3.3) that would release saccharides by acidic hydrolysis and thereby result in overestimated contents of free carbohydrates, the water extracts of BHT were successively extracted by water-saturated n-butanol and ethyl acetate to remove the glycosides [23]. Meanwhile, we examined monosaccharides in the organic extracts using the developed UPLC-PDA method, and no peaks were detected (data not shown), thereby suggesting that free carbohydrates in the BHT were not removed by this step. Subsequently, the extracted aqueous layer was hydrolyzed, and the monosaccharides in the hydrolysate were then determined by PMP derivatization and UPLC-PDA analysis as the total free carbohydrate content of BHT. Besides, the extracted water layer was directly subjected to derivatization and chromatographic analysis, and the determined monosaccharides on this occasion were regarded as the free monosaccharides in BHT, since polymeric carbohydrates did not release monosaccharides without acidic hydrolysis. Meanwhile, the polysaccharides of BHT were produced by ethanol precipitation and then characterized by the same method. Afterward, the difference between the value of the total carbohydrate content and the sum of polysaccharides and monosaccharides was treated as the content of oligosaccharides in BHT (Figure 2). We previously demonstrated that the commonly used 70–80% ethanol (final concentration) for natural polysaccharide precipitation could easily result in the loss of polysaccharides [11]. Here, ethanol at a higher concentration (95%) was thus used for precipitating the polysaccharides in BHT as much as possible. In addition, the hydrolysis conditions for polymeric carbohydrates were investigated by spiking two oligosaccharides, sucrose and maltotriose, with known amounts into the samples that were then subjected to hydrolysis, derivatization, and chromatographic analysis (see Section 2.5). Recoveries of the glucose released from the hydrolyzed sucrose and maltotriose were between 94.45% and 113.73% (Table 1), indicating a satisfactory hydrolysis condition. Meanwhile, the original procedure of PMP derivatization was modified using ammonia water instead of sodium hydroxide as the alkaline medium, since it does not produce salt end products and thereby facilitates the subsequent analysis [24]. In this case, the amino group and the reducing end (aldehyde group) of reducing carbohydrates could be reacted to generate Schiff base (glycamine derivatives) [25]. However, the good method validation data (see Section 3.2), in particular the linearity, sensitivity, and accuracy, suggested that the side reaction was slight, stable, and therefore acceptable. Besides, the chromatographic specificity in the analysis of the released and free monosaccharides in BHT was observed by analyzing the untreated water extract in the same concentration and analytical conditions, and the result showed no obvious interference peaks. However, some minor peaks except for the PMP-labeled monosaccharides were detected in the water extract that was not subjected to acid hydrolysis before derivatization (Figure 3). We deduced that they might be the PMP-labeled oligosaccharides in BHT since PMP derivatization followed by reversed-phase chromatography analysis was also occasionally employed for natural oligosaccharide determination [26].
Figure 2.
Strategy for quantitative analysis of carbohydrate including polysaccharides, oligosaccharides, and monosaccharides in the BHT sample. BHT =Bai-Hu-Tang; PMP =3-methyl-1-phenyl-5-pyrazolone; UPLC-PDA =ultraperformance liquid chromatography coupled with photodiode array detector.
Table 1.
Calibration curves, linear ranges, sensitivity, precision, stability, and accuracy for quantitative assay of 20 analytes.
| Analyte | Linearity | LLOQ (μg/mL) | LODa (ng/mL) | Repeatability (RSD, %, n = 6) | Spike recovery % (RSD, %, n = 3) | Stability (RSD, %, n = 6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Range (μg/mL) | Equation | R 2 | Intraday | Interday | High | Middle | Low | ||||
| UPLC-QTOF-MS/MS assay | |||||||||||
| Neomangiferin | 0.05–10 | y = 522.37x – 26,433.83 | 0.9998 | 0.05 | 0.71 | 1.53 | 4.26 | 96.51 (2.62) | 101.76 (1.48) | 105.87 (5.47) | 4.21 |
| Mangiferin | 0.125–50 | y = 701.07x + 49,783 | 0.9993 | 0.125 | 0.33 | 4.25 | 4.62 | 106.72 (0.51) | 102.98 (5.51) | 105.54 (4.30) | 2.72 |
| Isomangiferin | 0.05–25 | y = 430.23x – 8113 | 0.9999 | 0.05 | 0.96 | 4.11 | 5.08 | 107.54 (4.46) | 106.64 (1.60) | 101.15 (0.61) | 3.93 |
| Neoliquiritin | 0.02–25 | y = 341.5x – 7107.5 | 0.9996 | 0.02 | 6.16 | 1.86 | 3.53 | 101.28 (2.46) | 100.07 (5.09) | 99.07 (4.81) | 3.38 |
| Liquiritin | 0.02–50 | y = 612.15x – 3713.8 | 0.9999 | 0.02 | 3.95 | 2.18 | 4.09 | 104.56 (0.50) | 101.05 (3.27) | 102.82 (4.25) | 3.07 |
| Liquiritin apioside | 0.125–100 | y = 431.08x – 46,525 | 0.9997 | 0.125 | 3.76 | 1.94 | 3.82 | 105.23 (0.56) | 105.62 (0.51) | 109.65 (2.11) | 3.63 |
| Isoliquiritin | 0.01–25 | y = 914.32x – 3529.1 | 0.9998 | 0.01 | 1.88 | 2.02 | 3.01 | 102.65 (3.40) | 100.65 (2.25) | 110.38 (0.41) | 1.76 |
| Timosaponin BII | 0.25–100 | y = 203.08x – 33,875 | 0.9995 | 0.25 | 1.25 | 1.73 | 3.73 | 105.67 (5.40) | 101.96 (0.58) | 106.94 (4.28) | 4.77 |
| Glycyrrhizic acid | 0.01–1.25 | y = 343.74x – 3113.7 | 0.9994 | 0.01 | 0.39 | 2.14 | 5.00 | 109.36 (0.90) | 97.30 (4.64) | 100.94 (8.68) | 2.91 |
| Timosaponin AIII | 0.02–25 | y = 144.56x – 489.78 | 0.9993 | 0.02 | 1.25 | 1.69 | 4.01 | 98.08 (2.74) | 97.12 (3.88) | 102.53 (3.24) | 3.34 |
| UPLC-PDA assay | |||||||||||
| Mannose | 0.39–200 | y = 8406.6x – 3645.8 | 0.9997 | 0.39 | 0.10 | 3.52 | 2.92 | 99.50 (4.12) | 96.57 (1.59) | 100.11 (4.42) | 4.39 |
| Rhamnose | 3.91–2000 | y = 1179.3x + 9760.6 | 0.9992 | 3.91 | 0.49 | 3.29 | 2.83 | — | 5.12 | ||
| Glucuronic acid | 0.78–400 | y = 8626.7x – 9639.6 | 0.9997 | 0.78 | 0.39 | 1.22 | 4.00 | — | 3.58 | ||
| Galacturonic acid | 0.78–400 | y = 4388.4x – 24,087 | 0.9993 | 0.78 | 0.33 | 2.22 | 4.01 | 91.97 (1.97) | 103.15 (2.88) | 95.58 (0.73) | 4.18 |
| Glucose | 3.91–2000 | y = 3229.6x + 37,825 | 0.9995 | 3.91 | 0.49 | 4.39 | 2.84 | 107.16 (1.59) | 96.52 (3.70) | 108.35 (1.00) | 2.70 |
| Galactose | 0.78–400 | y = 6392.9x + 6833.8 | 0.9997 | 0.78 | 0.20 | 4.74 | 3.58 | 105.55 (3.23) | 105.43 (4.27) | 93.71 (2.62) | 3.48 |
| Arabinose | 0.39–200 | y = 3040.3x + 6130.3 | 0.9996 | 0.39 | 0.17 | 2.97 | 4.92 | 93.61 (3.09) | 102.16 (4.76) | 107.97 (2.06) | 2.30 |
| Fucose | 3.91–2000 | y = 19,907x + 21,304 | 0.9991 | 3.91 | 0.98 | 3.56 | 4.12 | – | 3.56 | ||
| Sucrose | 105.22 (1.38) | 112.13 (3.21) | 101.19 (1.32) | ||||||||
| Maltotriose | 94.45 (2.99) | 108.85 (5.08) | 113.73 (3.98) | ||||||||
LLOQ = lower limit of quantification; LOD = limit of detection; RSD = relative standard deviation; UPLC-PDA = ultraperformance liquid chromatography coupled with photodiode array detector; UPLC-QTOF-MS = ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry.
The LOD unit of UPLC-PDA assay is μg/mL.
Figure 3.
(A) Typical base peak chromatograms of the BHT sample (A1), GR (A2), and AR (A3), and (B) PDA chromatograms of standards (B1) and the BHT sample (B2). The peak numbers in Figure 3A are the same as those in Table 2. AR =Anemarrhenae Rhizoma; BHT =Bai-Hu-Tang; GR =Glycyrrhizae Radix et Rhizoma Praeparata cum Melle; PDA =photodiode array detector.
The HPLC method previously developed for monosaccharide analysis was transplanted into UPLC with minor modifications in this study, by which the analytical time was greatly shortened and the sensitivity was improved as well [27]. Different types of reversed-phase columns, namely, BEH C18, HSS T3, and HSS C18, were tried for secondary metabolite and monosaccharide analysis. The result indicated that the separation of polar monosaccharide derivatives was better achieved on HSS C18, while BEH C18 could give consideration of the total secondary metabolites with a wide range of polarity.
3.2. Quantitative method validation
The linearity, sensitivity, precision, accuracy, and stability of total 18 analytes (10 secondary metabolites, 6 neutral monosaccharides, and 2 uronic acids), analyzed by UPLC-QTOF-MS and UPLC-PDA, are summarized in Table 1. The data indicated a good relationship between concentrations and peak areas of the analytes within the test ranges (R2 ≥ 0.9991). The limits of detection of all analytes, as assessed by UPLC-QTOF-MS and UPLC-PDA, were < 6.16 ng/mL and < 0.98 μg/mL on the columns, respectively. The overall RSDs of intra- and interday variations for the analytes were not more than 4.74% and 5.08%, respectively. The established methods also had acceptable accuracy with a spike recovery of 91.97–110.38% for all analytes. As to the stability test, the RSDs of the peak areas for all analytes detected within 24 hours were < 5.12%. All these results indicated that the established methods were linear, sensitive, precise, accurate, and stable enough for quantification of the selected secondary metabolites as well as the released and free monosaccharides in the BHT sample.
3.3. Analysis of secondary metabolites
By the optimized gradient elution, the main secondary me-tabolites in BHT were separately eluted within 22 minutes on the column (Figure 3). A total of 42 major secondary metabolites were tentatively or definitely identified from the BHT sample, 10 of which were confirmed by comparing the mass spectra and retention times with those of reference compounds, while the others were temporarily assigned by the elemental composition data determined from accurate molecular weight measurements and/or fragment ions, and a comparison with those of the published known chemicals in the established in-house library. Details of the secondary metabolites are summarized in Table 2. Mass accuracy for all quasimolecular ions were < 5 ppm, indicating that the empirical molecular formula match well with the putative deprotonated ions. As shown in Table 2, the major secondary metabolites in the BHT sample belonged to steroidal saponins, triterpene saponins, and flavonoids. For example (Figure S1), Peak 21 showed [M–H]− and [M–H+HCOOH]− at m/z 919.4903 and m/z 965.4942, respectively, which indicated that its molecular formula was C45H76O19. In the MS2 spectrum of Peak 21, fragment ions at m/z 757.4371, m/z 595.3841, and m/z 433.3301 formed by sequential losses of glucosyls and galactosyl were observed. Therefore, Peak 21 was identified as timosaponin BII [31], which was further confirmed by a reference substance. Similarly, the molecular formula of Peak 34 was determined as C42H62O16 based on its mass-to-charge ratios of m/z 821.3968 ([M–H]−) and m/z 1643.7956 ([2M–H]−). In addition, deglucuronosylated product ions at m/z 645.3623 ([M–H–GlcA]−) and m/z 469.3304 ([M–H–2GlcA]−) and glucuronosyl ions at m/z 351.0566 ([2GlcA–2H2O–H]−) and m/z 193.0353 ([GlcA–H]−) were found. Glycyrrhizic acid was thus assigned to Peak 34 [36,40], and it was also confirmed by reference substance. Peak 8 showed [M–H]− and [2M–H]− at m/z 417.1188 and m/z 835.2425, respectively, indicating a molecular formula of C21H22O9. Fragment ions at m/z 255.0659 ([M–H–Glc]−) and m/z 135.0085 ([M–H–Glc–C8H8O]−) suggested that the compound was liquiritin, and it could be degraded by O-glycoside cleavage and then RDA cleavage [6,29]. Peak 5 showed mass-to-charge ratios of m/z 593.1506 ([M–H]−) and m/z 639.1584 ([M–H+HCOOH]−). By comparing with chemicals in the library, Peak 5 was tentatively identified as vicenin-2 (C27H30O15). The speculation was supported by the daughter ion at m/z 337.0384 formed by C-glycoside cleavage and B-ring cleavage [29]. These results revealed that different types of secondary metabolites in BHT showed different mass fragmentation patterns.
Table 2.
Chromatographic and mass spectral data of the 42 compounds analyzed by UPLC-QTOF-MS/MS.
| Peak no. | Identification | tR (min) | Molecular formula | [M–H]− | Assigned adduct and fragment ions | Occurrence | Classification | |
|---|---|---|---|---|---|---|---|---|
| Measured mass (Da) | Mass accuracy (ppm) | |||||||
| 1 | p-Hydroxybenzyl malonic acid [28] | 4.92 | C10H10O5 | 209.0451 | 1.97 | 419.0975 [2M–H]− 165.0562 [M–H–CO2]− 121.0654 [M–H–2CO2]− |
GR | Organic acid |
| 2 | Neomangiferin [5] | 6.11 | C25H28O16 | 583.1299 | 1.32 | 1167.2665 [2M–H]− 421.0770 [M–H–Glc]− |
AR | Xanthone |
| 3 | Mangiferin [5] | 7.94 | C19H18O11 | 421.0769 | 1.85 | 843.1591 [2M–H]− | AR | Xanthone |
| 4 | Isomangiferin [5] | 8.38 | C19H18O11 | 421.0762 | 3.35 | 843.1595 [2M–H]− | AR | Xanthone |
| 5 | Vicenin-2 [29] | 8.91 | C27H30O15 | 593.1506 | 4.12 | 639.1584 [M–H+HCOOH]− 337.0384 [M–H–Glc–C6H6O]− |
GR | Flavone |
| 6 | Iriflophenone [5] | 10.27 | C13H10O5 | 245.0450 | 2.06 | 491.0976 [2M–H]− | AR | Benzophenone |
| 7 | Neoliquiritin [29] | 11.19 | C21H22O9 | 417.1178 | 3.10 | 463.1210 [M–H+HCOOH]− 835.2416 [2M–H]− |
GR | Flavanone |
| 8 | Liquiritin [6,29] | 11.42 | C21H22O9 | 417.1188 | 2.03 | 835.2425 [2M–H]− 255.0659 [M–H–Glc]− 135.0085 [M–H–Glc–C8H8O]− |
GR | Flavanone |
| 9 | Liquiritin apioside [6,29] | 11.67 | C26H30O13 | 549.1598 | 2.92 | 1099.3267 [2M–H]− 417.1181 [M–H–Api]− 255.0661 [M–H–Api–Glc]− 135.0086 [M–H–Api–Glc–C8H8O]− |
GR | Flavanone |
| 10 | Baohuoside I [30] | 12.80 | C27H30O10 | — | 0.29a | 559.1821 [M–H+HCOOH]− | AR | Flavonol |
| 11 | 15OH-timosaponin N (or isomer)/ 15OH-macrostemonoside J [31,32] | 13.08 | C45H76O21 | 951.4780 | 2.73 | 997.4830 [M–H+HCOOH]− 789.4241 [M–H–Glc]− 627.3734 [M–H–2Glc]− 465.3139 [M–H–2Glc–Gal]− |
AR | Furostanol saponins |
| 12 | Timosaponin N + Rha or isomer [33] | 13.41 | C51H86O24 | 1081.5403 | 3.10 | 1127.5462 [M–H+HCOOH]− 935.4829 [M–H–Rha]− 773.4316 [M–H–Rha–Glc]− 611.3790 [M–H–Rha–2Glc]− |
AR | Furostanol saponin |
| 13 | Timosaponin E1/timosaponin N (or isomer)/ hydroxyl-timosaponin BII/macrostemonoside J [31,32] | 13.59 | C45H76O20 | 935.4827 | 3.24 | 981.4878 [M–H+HCOOH]− 773.4315 [M–H–Glc]− 611.3791 [M–H–2Glc]− 449.3270 [M–H–2Glc–Gal]− |
AR | Furostanol saponin |
| 14 | Karatavioside C [34] | 13.81 | C56H92O29 | 1227.5586 | 4.79 | 1095.5214 [M–H–Xyl]− 933.4705 [M–H–Xyl–Glc]− 771.4175 [M–H–Xyl–2Glc]− |
AR | Furostanol saponin |
| 15 | Isoliquiritin apioside (licuraside) [29] | 13.83 | C26H30O13 | 549.1603 | 1.95 | 417.1164 [M–H–Api]− 255.0660 [M–H–Api–Glc]− 135.0084 [M–H–Api–Glc–C8H8O]− |
GR | Chalcone |
| 16 | Isoliquiritin [29] | 14.01 | C21H22O9 | 417.1180 | 2.75 | 835.2420 [2M–H]− 255.0663 [M–H–Glc]− 135.0086 [M–H–Glc–C8H8O]− |
GR | Chalcone |
| 17 | Timosaponin E1/27OH-timosaponin BII + Rha [33] | 14.06 | C45H76O20 | 935.4831 | 2.82 | 981.4881 [M–H+HCOOH]− 773.4316 [M–H–Glc]− 611.3791 [M–H–2Glc]− |
AR | Furostanol saponin |
| 18 | Ononin [35,36] | 14.22 | C22H22O9 | 429.1187 | 0.88 | 475.1233 [M–H+HCOOH]− 267.0657 [M–H–Glc]− |
GR | Isoflavone |
| 19 | 2,4′,6-Trihydroxy-4-methoxybenzophenone (or isomer) [37] | 14.32 | C14H12O5 | 259.0607 | 1.76 | 519.1254 [2M–H]− 243.0290 [M–H–CH4]− |
AR | Benzophenone |
| 20 | Licorice glycoside B [29] | 14.42 | C35H36O15 | 695.1963 | 2.68 | 1391.3971 [2M–H]− 417.1190 [M–H–C14H14O6]− 255.0661 [M–H–C14H14O6–Glc]− |
GR | Flavonoid glycoside |
| 21 | Timosaponin BII [31] | 14.66 | C45H76O19 | 919.4903 | 1.70 | 965.4942 [M–H+HCOOH]− 757.4371 [M–H–Glc]− 595.3841 [M–H–2Glc]− 433.3301 [M–H–2Glc–Gal]− |
AR | Furostanol saponin |
| 22 | Asparagoside G/petunioside N/terrestrosin H [33,38,39] | 14.75 | C51H86O24 | 1081.5411 | 2.32 | 1127.5484 [M–H+HCOOH]− 919.4909 [M–H–Glc]− 757.4372 [M–H–2Glc]− 595.3778 [M–H–2Glc–Gal]− |
AR | Furostanol saponin |
| 23 | Timosaponin H1 [33] | 14.83 | C56H92O28 | 1211.5673 | 2.39 | 1257.5679 [M–H+HCOOH]− 1079.5268 [M–H–Xyl]− |
AR | Furostanol saponins |
| 24 | Formononetin [35,36] | 15.29 | C16H12O4 | 267.0657 | 2.33 | 252.0425 [M–H–CH3]− | GR | Isoflavone |
| 25 | 22-Acetoxyl licorice-saponin G2 [40] | 15.80 | C44H64O19 | 895.3943 | 2.87 | 837.3899 [M–H–C2H2O2]− | GR | Triterpene saponin |
| 26 | Licochalcone B [6] | 15.90 | C16H14O5 | 285.0760 | 3.07 | 571.164 [2M–H]− | GR | Chalcone |
| 27 | Licorice-saponin A3 (or isomer) [40] | 16.18 | C48H72O21 | 983.4465 | 2.89 | 821.3958 [M–H–Glc]− | GR | Triterpene saponin |
| 28 | 22-Acetoxyl-glycyrrhizin [29] | 16.49 | C44H64O18 | 879.3994 | 2.93 | 351.0568 [2GlcA–2H2O–H]− 193.0351 [GlcA–H]− |
GR | Triterpene saponin |
| 29 | Timosaponin C/timosaponon B/ macrostemonoside F/anemarsaponin C [33] | 16.52 | C45H74O18 | 901.4771 | 3.45 | 947.4827 [M–H+HCOOH]− 739.4267 [M–H–Glc]− 577.3723 [M–H–2Glc]− |
AR | Furostanol saponin |
| 30 | Timosaponin C/timosaponon B/ macrostemonoside F/anemarsaponin C [33] | 16.59 | C45H74O18 | 901.4775 | 3.06 | 947.4824 [M–H+HCOOH]− 739.4269 [M–H–Glc]− 577.3743 [M–H–2Glc]− |
AR | Furostanol saponin |
| 31 | Licorice-saponin G2 or isomer [40] | 16.75 | C42H62O17 | 837.3889 | 3.02 | 1675.7769 [2M–H]− 351.0572 [2GlcA–2H2O–H]− 193.0350 [GlcA–H]− |
GR | Triterpene saponin |
| 32 | Liquiritigenin [36,40] | 16.98 | C15H12O4 | 255.0656 | 2.72 | 301.0706 [M–H+HCOOH]− 135.0085 [M–H–C8H8O]− 119.0500 [M–H–C7H4O3]− |
GR | Flavanone |
| 33 | Licorice-saponin G2 or isomer [40] | 17.49 | C42H62O17 | 837.3885 | 3.46 | 1675.7826 [2M–H]− 351.0567 [2GlcA–2H2O−H]− 193.0349 [GlcA–H]− |
GR | Triterpene saponin |
| 34 | Glycyrrhizic acid [36,40] | 18.13 | C42H62O16 | 821.3968 | 2.52 | 1643.7956 [2M–H]− 645.3623 [M–H–GlcA]− 469.3304 [M–H–2GlcA]− 351.0566 [2GlcA–2H2O–H]− 193.0353 [GlcA–H]− |
GR | Triterpene saponin |
| 35 | Anemarrhena saponin I/II [31] | 18.26 | C39H66O14 | 757.4354 | 3.35 | 803.4418 [M–H+HCOOH]− 1515.8785 [2M–H]− 595.3840 [M–H–Glc]− |
AR | Furostanol saponin |
| 36 | Licorice-saponin B2/dehydroxyl-uralsaponin C [40] | 18.74 | C42H64O15 | 807.4150 | 2.80 | 631.3819 [M–H–C6H8O6]− 351.0566 [2GlcA–2H2O–H]− 193.0354 [GlcA–H]− |
GR | Triterpene saponin |
| 37 | Licorice-saponin H2/K2 [40] | 18.87 | C42H62O16 | 821.3939 | 3.16 | 351.0568 [2GlcA–2H2O−H]− 193.0351 [GlcA–H]− |
GR | Triterpene saponin |
| 38 | Nyasol [5] | 19.25 | C17H16O2 | 251.1071 | 2.61 | 297.1122 [M–H+HCOOH]− | AR | Lignan |
| 39 | Timosaponin AII/anemarrhenasaponin III/ anemarrhenasaponin A2/timosaponin A2/ timosaponin G | 19.99 | C39H64O14 | 755.4200 | 3.05 | 801.4256 [M–H+HCOOH]− 593.3693 [M–H–Glc]− |
AR | Spirostanol saponin |
| 40 | Gancaonin C [6] | 20.25 | C20H18O6 | 353.1021 | 2.73 | 323.0923 [M–H–CH2O]− | GR | Isoflavone |
| 41 | Licoisoflavone B/semilicoisoflavone B [6] | 21.35 | C20H16O6 | 351.0869 | 1.49 | 703.1801 [2M–H]− | GR | Flavone |
| 42 | Timosaponin AIII [31] | 21.45 | C39H64O13 | 739.4245 | 4.00 | 785.4298 [M–H+HCOOH]− 577.3737 [M–H–Glc]− |
AR | Spirostanol saponin |
UPLC-QTOF-MS = ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry.
Calculated by [M–H+HCOOH]−.
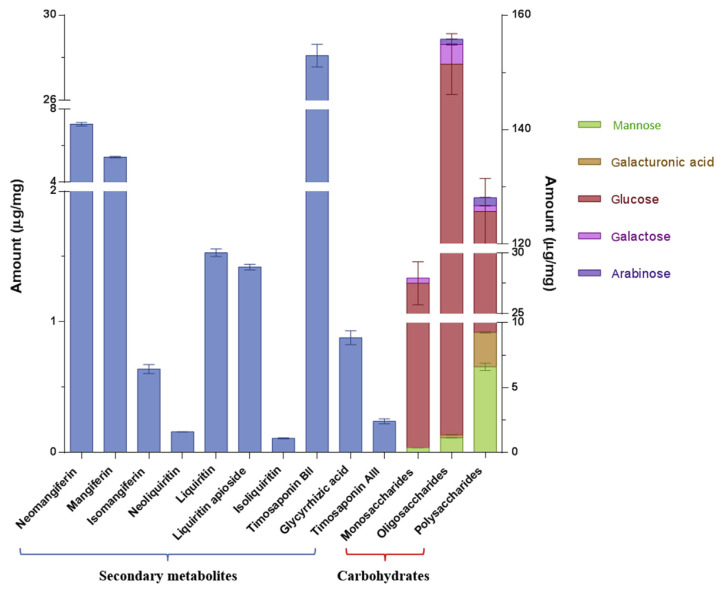
Moreover, it was manifested that the secondary metabolites in BHT were from AR and GR rather than from GF and NR, by comparing the respective chemical profiles of BHT, AR, and GR (Figure 3 and Table 2), which is in agreement with the fact that no secondary metabolites from GF and NR have been reported so far. Steroidal saponins and flavonoids from AR together with triterpene saponins and flavonoids from GR constituted the majority of secondary metabolites in BHT, while some other types of constituents occurred as well, such as lignans and organic acids, which is also in conformity with the reported chemical compositions of AR and GR [5,6]. As mentioned earlier, saponins and flavonoids are regarded as the main bioactive chemicals of AR and GR. Thus, 10 major (and/or characteristic) saponins and flavonoids, including three xanthones (mangiferin, isomangiferin, and neomangiferin), three flavanonols (liquiritin, neoliquiritin, and liquiritin apioside), one chalcone (isoliquiritin), one spirostanol saponin (timosaponin AIII), one furostanol saponin (timosaponin BII), and one triterpene saponin (glycyrrhizic acid), were selected as the quantitative markers and then were determined by the extracting ion mode analysis, in which deprotonated ions served as the selected ions for each analyte due to their high mass spectrum response. The extracted ion chromatograms shown in Figure S2 demonstrated that the extracting ion mode provided satisfactory chromatographic resolution for the 10 analytes compared with the base peak ion chromatogram, which is indispensable for quantitative analysis. The results indicated that the 10 analytes in the BHT samples varied quantitatively, accounting for 4.56% of the BHT extract in total (Figure 4). Among them, liquiritin and glycyrrhizic acid in GR as well as mangiferin and timosaponin BII in AR are regarded as their respective quality control markers in the Chinese Pharmacopoeia (2015 edition) [4]. We verified that they were also abundant in the BHT sample, in particular timosaponin BII (2.81%).
Figure 4.
Amount of chemicals determined in the BHT sample. BHT =Bai-Hu-Tang.
3.4. Analysis of carbohydrates
Quantification of carbohydrates in the BHT sample is demonstrated in Figure 4. Based on the results, it could be concluded that the polysaccharides and oligosaccharides of BHT consisted of five monosaccharide units, namely Glc, Man, Gal, Ara, and GalA, while Gal, Glc, and Ara were the free monosaccharides in the BHT sample; the total content of polysaccharides, oligosaccharides, and monosaccharides calculated by the sugar composition analysis were 12.81%, 15.59%, and 2.80%, respectively, in which Glc was the major compositional sugar of all three kinds of carbohydrates with a percentage of 90.87%, 94.88%, and 96.97%, respectively. These carbohydrates should be derived from three constituted herbal drugs of BHT, i.e., AR, GR, and NR. Several neutral and acidic polysaccharides in arabino-galacto-glucan or arabino-galactan types have been extracted from GR before, and the structural elucidation indicated that they were mainly composed of Glc, Ara, Gal, and/or GalA [41,42]. Reportedly, a similar composition mainly including Glc, Ara, Gal, and Man structured the AR polysaccharides [43]. Moreover, Glc was the predominant monosaccharide unit of the investigated polysaccharides in both AR and GR. These previous study results were highly consistent with the findings here. Besides, the high contents of amylose and amylopectin in NR should also significantly contribute to the high content of released Glc in the BHT sample [7]. Additionally, oligosaccharides in BHT were unexpectedly found with a higher content than polysaccharides and monosaccharides therein. However, unfortunately, oligosaccharides in AR, GR, and NR have hardly been concerned so far. Natural oligosaccharides have been proved to be existent in a wide range of sources, including herbal medicines and foods [44], and thus also potentially occur in the constituted drugs of BHT. Moreover, it is well defined that starch could release various kinds of oligosaccharides by hydrolysis, such as malto-oligosaccharides and isomalto-oligosaccharides [45]. We therefore surmised that the starch in NR could be incompletely hydrolyzed to oligosaccharides during the BHT preparation under the GF-induced weak acidic condition, which thereby, at least partially, resulted in the high content of oligosaccharides.
Taking secondary metabolites and carbohydrates together, the established method was able to quantify 35.76% of the BHT extract. The undetermined part should include CaSO4·2H2O, unquantified metabolites, amino acids, vitamins, and microelements. The quantitative level was improved compared with the analysis of TCM formulas focusing on secondary metabolites, and should, therefore, be instrumental in chemical dissection and quality control of BHT. More significantly, this study provided an overall chemical basis for further biological evaluation, e.g., elucidation of bioactive substances. Given the considerable content and experimentally demonstrated bioactivities of the determined secondary metabolites and carbohydrates [5,41–43,46], we have reason to believe that these chemicals in BHT should be therapeutically contributive, which however still warrants further investigation.
4. Concluding remarks
In this study, an efficient combination of analytical approaches using UPLC-QTOF-MS/MS and UPLC-PDA was developed and validated to qualitatively and quantitatively characterize secondary metabolites and carbohydrates, respectively, in BHT. The results showed that the determined chemicals accounted for 35.76% of the total extract of BHT. This research not only set a basis for further chemical and biological investigation of BHT, but also provided a comprehensive analytical strategy for chemical characterization of secondary metabolites and carbohydrates in TCM formulas.
Acknowledgments
The study was financially supported by the Program of Study Abroad for Young Teachers of Agricultural University of Hebei and FRG2/14-15/101 of Hong Kong Baptist University.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2016.12.007.
Funding Statement
The study was financially supported by the Program of Study Abroad for Young Teachers of Agricultural University of Hebei and FRG2/14-15/101 of Hong Kong Baptist University.
Footnotes
Conflicts of interest
All authors declare no competing financial interest.
REFERENCES
- 1.Jiang JG. Treatise on exogenous febrile disease. 1st ed. Beijing: China Press of Traditional Chinese Medicine; 2004. [Google Scholar]
- 2. Lin CJ, Su YC, Lee CH, Li TC, Chen YA, Lin SJS. Bai-Hu-Tang, ancient Chinese medicine formula, may provide a new complementary treatment option for sepsis. Evid Based Complement Alternat Med. 2013;2013:193084. doi: 10.1155/2013/193084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen CC, Hsiang CY, Chiang AN, Lo HY, Li CI. Peroxisome proliferator-activated receptor gamma transactivation-mediated potentiation of glucose uptake by Bai-Hu-Tang. J Ethnopharmacol. 2008;118:46–50. doi: 10.1016/j.jep.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Pharmacopoeia Commission of P.R. China. Pharmacopoeia of P.R China. Vol. 1. Beijing: China: Medical Science Press; 2015. [Google Scholar]
- 5. Wang YL, Dan Y, Yang DW, Hu YL, Zhang L, Zhang CH, Zhu H, Cui ZH, Li MH, Liu YZ. The genus Anemarrhena Bunge: a review on ethnopharmacology, phytochemistry and pharmacology. J Ethnopharmacol. 2014;153:42–60. doi: 10.1016/j.jep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 6. Zhang QY, Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice) J Chromatogr A. 2009;1216:1954–69. doi: 10.1016/j.chroma.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 7. Hu PS, Zhao HJ, Duan ZY, Zhang LL, Wu DX. Starch digestibility and the estimated glycemic score of different types of rice differing in amylose contents. J Cereal Sci. 2004;40:231–7. [Google Scholar]
- 8. Ngo LT, Okogun JI, Folk WR. 21st Century natural product research and drug development and traditional medicines. Nat Prod Rep. 2013;30:584–92. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li SP, Wu DT, Lv GP, Zhao J. Carbohydrates analysis in herbal glycomics. Trends Anal Chem. 2013;52:155–69. [Google Scholar]
- 10. Li P, Qi LW, Liu EH, Zhou JL, Wen XD. Analysis of Chinese herbal medicines with holistic approaches and integrated evaluation models. Trends Anal Chem. 2008;27:66–77. [Google Scholar]
- 11. Xu J, Yue RQ, Liu J, Ho HM, Yi T, Chen HB, Han QB. Structural diversity requires individual optimization of ethanol concentration in polysaccharide precipitation. Int J Biol Macromol. 2014;67:205–9. doi: 10.1016/j.ijbiomac.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 12. Zong AZ, Cao HZ, Wang FS. Anticancer polysaccharides from natural resources: a review of recent research. Carbohyd Polym. 2012;90:1395–410. doi: 10.1016/j.carbpol.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 13. Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA. Structure function relationships of immunostimulatory polysaccharides: a review. Carbohyd Polym. 2015;132:378–96. doi: 10.1016/j.carbpol.2015.05.079. [DOI] [PubMed] [Google Scholar]
- 14. Tsai CH, Yen YH, Yang JPW. Finding of polysaccharide-peptide complexes in Cordyceps militaris and evaluation of its acetylcholinesterase inhibition activity. J Food Drug Anal. 2015;23:63–70. doi: 10.1016/j.jfda.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen C, You LJ, Abbasi AM, Fu X, Liu RH. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohyd Polym. 2015;130:122–32. doi: 10.1016/j.carbpol.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 16. Shi LL, Li Y, Wang Y, Feng Y. MDG-1, an Ophiopogon polysaccharide, regulate gut microbiota in high-fat diet-induced obese C57BL/6 mice. Int J Biol Macromol. 2015;81:576–83. doi: 10.1016/j.ijbiomac.2015.08.057. [DOI] [PubMed] [Google Scholar]
- 17. Zhou SS, Xu J, Zhu H, Wu J, Xu JD, Yan R, Li XY, Liu HH, Duan SM, Wang Z, Chen HB, Shen H, Li SL. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci Rep. 2016;6:1–13. doi: 10.1038/srep22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu J, Li SL, Yue RQ, Ko CH, Hu JM, Liu J, Ho HM, Yi T, Zhao ZZ, Zhou J, Leung PC, Chen HB, Han QB. A novel and rapid HPGPC-based strategy for quality control of saccharide-dominant herbal materials: Dendrobium officinale, a case study. Anal Bioanal Chem. 2014;406:6409–17. doi: 10.1007/s00216-014-8060-9. [DOI] [PubMed] [Google Scholar]
- 19. Zhou JL, Qi LW, Li P. Herbal medicine analysis by liquid chromatography/time-of-flight mass spectrometry. J Chromatogr A. 2009;1216:7582–94. doi: 10.1016/j.chroma.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Jiang Z, Yang J, Li Y, Wang Y, Chai X. Chemical material basis study of Xuefu Zhuyu decoction by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Food Drug Anal. 2015;23:811–20. doi: 10.1016/j.jfda.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou SS, Xu JD, Zhu H, Shen H, Xu J, Mao Q, Li SL, Yan R. Simultaneous determination of original, degraded ginsenosides and aglycones by ultra high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry for quantitative evaluation of Du-Shen-Tang, the decoction of ginseng. Molecules. 2014;19:4083–104. doi: 10.3390/molecules19044083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li L, Luo GA, Liang QL, Hu P, Wang YM. Rapid qualitative and quantitative analyses of Asian ginseng in adulterated American ginseng preparations by UPLC/Q-TOF-MS. J Pharm Biomed Anal. 2010;52:66–72. doi: 10.1016/j.jpba.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Song XM. Extraction, isolation and purification of chemical ingredients in Chinese medicine. 1st ed. Beijing: People’s Medical Publishing House Co., Ltd; 2004. [Google Scholar]
- 24. Rozaklis T, Ramsay SL, Whitfield PD, Ranieri E, Hopwood JJ, Meikle PJ. Determination of oligosaccharides in Pompe disease by electrospray ionization tandem mass spectrometry. Clin Chem. 2002;48:131–9. [PubMed] [Google Scholar]
- 25. Honda S, Suzuki S, Taga A. Analysis of carbohydrates as 1-phenyl-3-methyl-5-pyrazolone derivatives by capillary/ microchip electrophoresis and capillary electrochromatography. J Pharm Biomed Anal. 2003;30:1689–714. doi: 10.1016/s0731-7085(02)00512-5. [DOI] [PubMed] [Google Scholar]
- 26. Pitt JJ, Gorman JJ. Oligosaccharide characterization and quantitation using 1-phenyl-3-methyl-5-pyrazolone derivatization and matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry. Anal Biochem. 1997;248:63–75. doi: 10.1006/abio.1997.2080. [DOI] [PubMed] [Google Scholar]
- 27. Xu J, Chen HB, Liu J, Kwok KY, Yue RQ, Yi T, Ho HM, Zhao ZZ, Han QB. Why are Angelicae Sinensis radix and Chuanxiong Rhizoma different? An explanation from a chemical perspective. Food Res Int. 2013;54:439–47. [Google Scholar]
- 28. Simmier C, Nikolic D, Lankin DC, Yu Y, Friesen JB, van Breemen RB, Lecomte A, Le Quemener C, Audo G, Pauli GF. Orthogonal analysis underscores the relevance of primary and secondary metabolites in licorice. J Nat Prod. 2014;77:1806–16. doi: 10.1021/np5001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu W, Huang M, Li H, Chen X, Zhang Y, Liu J, Xu W, Chu K, Chen L. Chemical profiling and quantification of Gua-Lou-Gui-Zhi decoction by high performance liquid chromatography/quadrupole-time-of-flight mass spectrometry and ultra-performance liquid chromatography/triple quadrupole mass spectrometry. J Chromatogr B. 2015;986–87:69–84. doi: 10.1016/j.jchromb.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 30. Qin L, Han T, Zhang Q, Cao D, Nian H, Rahman K, Zheng H. Antiosteoporotic chemical constituents from Er-Xian decoction, a traditional Chinese herbal formula. J Ethnopharmacol. 2008;118:271–9. doi: 10.1016/j.jep.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 31. Kang LP, Zhang J, Cong Y, Li B, Xiong CQ, Zhao Y, Tan DW, Yu HS, Yu ZY, Cong YW, Liu C, Ma BP. Steroidal glycosides from the rhizomes of Anemarrhena asphodeloides and their antiplatelet aggregation activity. Planta Med. 2012;78:611–6. doi: 10.1055/s-0031-1298223. [DOI] [PubMed] [Google Scholar]
- 32. Peng J, Yao X, Okada Y, Okuyama T. Further studies on new furostanol saponins from the bulbs of Allium macrostemon. Chem Pharm Bull. 1994;42:2180–2. doi: 10.1248/cpb.42.2180. [DOI] [PubMed] [Google Scholar]
- 33. Zhao Y, Kang L, Yu H, Zhang J, Xiong C, Pang X, Gao Y, Liu C, Ma B. Structure characterization and identification of steroidal saponins from the rhizomes of Anemarrhena asphodeloides by ultra performance liquid chromatography and hybrid quadrupole time-of-flight mass spectrometry. Int J Mass Spectrom. 2013;341–2:7–17. [Google Scholar]
- 34. Vollerner YS, Gorovits MB, Gorovits TT, Abubakirov NK. Steroid saponins and sapogenins of Allium. XVII. The structure of karatavioside C. Chem Nat Comp. 1980;16:264–8. [Google Scholar]
- 35. Dong J, Zhu Y, Gao X, Chang Y, Wang M, Zhang P. Qualitative and quantitative analysis of the major constituents in Chinese medicinal preparation Dan-Lou tablet by ultra high performance liquid chromatography/diode-array detector/ quadrupole time-of-flight tandem mass spectrometry. J Pharm Biomed Anal. 2013;80:50–62. doi: 10.1016/j.jpba.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 36. Tao S, Chen G, Yang M, Deng S, Zhang J, Guo DA. Identification of the major constituents in Shi-Quan-Da-Bu decoction by HPLC-ESI-MS/MS. Nat Prod Commun. 2008;3:689–96. [Google Scholar]
- 37. Youn UJ, Lee YS, Jeong H, Lee J, Nam JW, Lee YJ, Hwang ES, Lee JH, Lee D, Kang SS, Seo EK. Identification of antiadipogenic constituents of the rhizomes of Anemarrhena asphodeloides. J Nat Prod. 2009;72:1895–8. doi: 10.1021/np900397f. [DOI] [PubMed] [Google Scholar]
- 38. Yan W, Ohtani K, Kasai R, Yamasaki K. Steroidal saponins from fruits of Tribulus terrestris. Phytochemistry. 1996;42:1417–22. doi: 10.1016/0031-9422(96)00131-8. [DOI] [PubMed] [Google Scholar]
- 39. Shvets SA, Naibi AM, Kintya PK. Steroid glycosides of the seeds of Petunia hybrida II. The structures of petuniosides I, L, and N. Chem Nat Comp. 1995;31:203–6. [Google Scholar]
- 40. Qi Y, Li S, Pi Z, Song F, Lin N, Liu S, Liu Z. Chemical profiling of Wu-tou decoction by UPLC-Q-TOF-MS. Talanta. 2014;118:21–9. doi: 10.1016/j.talanta.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 41. Shimizu N, Tomoda M, Kanari M, Gonda R, Satoh A, Satoh N. A novel neutral polysaccharide having activity on the reticuloendothelial system from the root of Glycyrrhiza uralensis. Chem Pharm Bull. 1990;38:3069–71. doi: 10.1248/cpb.38.3069. [DOI] [PubMed] [Google Scholar]
- 42. Tomoda M, Shimizu N, Kanari M, Gonda R, Arai S, Okuda Y. Characterization of 2 polysaccharides having activity on the reticuloendothelial system from the root of Glycyrrhiza uralensis. Chem Pharm Bull. 1990;38:1667–71. doi: 10.1248/cpb.38.1667. [DOI] [PubMed] [Google Scholar]
- 43. Takahashi M, Konno C, Hikino H. Isolation and hypoglycemic activity of Anemaran-a, Anemaran-B, Anemaran-C and Anemaran-D, glycans of Anemarrhena asphodeloides rhizomes. Planta Med. 1985;51:100–2. doi: 10.1055/s-2007-969417. [DOI] [PubMed] [Google Scholar]
- 44. Mussatto SI, Mancilha IM. Non-digestible oligosaccharides: a review. Carbohyd Polym. 2007;68:587–97. [Google Scholar]
- 45. Sako T, Matsumoto K, Tanaka R. Recent progress on research and applications of non-digestible galacto-oligosaccharides. Int Dairy J. 1999;9:69–80. [Google Scholar]
- 46. Wang XY, Zhang H, Chen LL, Shan LH, Fan GW, Gao XM. Liquorice, a unique “guide drug” of traditional Chinese medicine: a review of its role in drug interactions. J Ethnopharmacol. 2013;150:781–90. doi: 10.1016/j.jep.2013.09.055. [DOI] [PubMed] [Google Scholar]