Abstract
Amorpha fruticosa L. is a Chinese folk medicine and rich in polyphenols. Fifteen known compounds were isolated and identified from the leaves of A. fruticosa L. They are teph-rosin (1), 6a,12a-dehydrodeguelin (2), vitexin (3), afrormosin-7-O-β-d-glucopyranoside (4), 2″-O-α-l-rhamnopyranosyl isovitexin (5), rutin (6), chrysoeriol (7), 7-O-methylluteolin (8), trans-p-coumaric acid (9), 2-benzyl-4,6-dihydroxybenzoic acid-4-O-β-d-glucopyranoside (10), formononetin (11), quercetin (12), apigenin (13), β-sitosterol (14), and β-daucosterol (15). Compounds 3, 4, 5, and 7–9 were isolated from A. fruticosa L. for the first time. Cytotoxicity of individual compounds 3–10 and 90% ethanol extract against human cancer cell lines HCT116 and HepG2 were reported. The results suggested that compounds 7 and 8, and the crude extract exhibited inhibitory effects on human cancer cell line HCT116, at concentrations of 100 μg/mL, 5 μg/mL, and 25 μg/mL at <60% of cell viability rate, respectively. In addition, a valid high-performance liquid chromatography diode array detector method was established to quantitatively analyze compounds 1–12 in the leaves of A. fruticosa L., which was harvested at three different stages of maturity from May 20 to August 10, 2014. The results demonstrated that contents were greatly influenced by the maturity. Total amounts of the analytical constituents gradually increased from May 20 to August 10, with the values ranging from 10.86 mg/g to 18.84 mg/g, whereas bioactive compounds 7 and 8 presented the opposite variation trend. The results of this study may provide data for further study and comprehensive utilization of A. fruticosa L. resource.
Keywords: Amorpha fruticosa L., cytotoxicity, Leguminosae, phenolics, quantitative analysis
1. Introduction
Amorpha fruticosa L., a perennial deciduous shrub, belongs to the Leguminosae family and is native to North America [1]. It was introduced into China around the 1920s and widely planted in the Yellow River and Yangtze River basins, and northeast China for erosion control and afforestation [2]. A. fruticosa L. has been used as a Chinese folk medicine for the treatment of burn, ambustion, carbuncle, and eczema [3]. In recent years, increasing attention has been paid on this natural resource for its bioactivities.
Phytochemical studies have revealed that A. fruticosa L. is a polyphenol-rich plant containing bioactive constituents such as rotenoids [4–6], prenylated flavanones [7–9], isoflavones [10,11], and stilbenes [12,13]. Rotenoids are the most characteristic and functional constituents in this herbal plant, and their insecticidal activity has been known as the most important biological activity [14,15]. However, research has also demonstrated the antitumor activity [11,16–18] and bacterial neuraminidase inhibition effect of rotenoids [19,20]. In addition, potent anti-inflammatory, antidiabetic [21–23], antimicrobial [24], and other biological activities of amorfrutins have also been explored [25]. However, most phytochemical researches of A. fruticosa L. were focused on its fruits, roots, and flowers; less attention was devoted to the leaves of A. fruticosa L. To the best of our knowledge, quantitative analysis of this resource based on phenolic compounds remains virtually unknown except for one study on three amorfrutins [26].
In the present paper, we report the isolation and structural identification of the main compounds in the leaves of A. fruticosa L., as well as the cytotoxicity of some isolated compounds against human cancer cell lines (HepG2 and HCT116). In addition, the main compounds in A. fruticosa L. leaves of three different stages of maturity were analyzed by a high-performance liquid chromatography (HPLC) diode array detector (DAD) method.
2. Materials and methods
2.1. General experimental procedures
Isolation and purification were carried out by column chromatography. Agilent 1260 HPLC and thin-layer chromatography were used to monitor the separation, and thin-layer chromatography was performed on precoated silica gel 60 GF254 plates and visualized using UV illumination at 254 nm and 365 nm or by spraying with a 10% solution of sulfuric acid and 1% vanillin in ethanol. 1H and 13C nuclear magnetic resonance spectra were recorded on a Bruker Avance 400 MHz spectrometer (Bruker BioSpin GmbH, Beijing, China) with tetramethylsilane as the internal standard. Chemical shifts are expressed in δ values. HPLC quantitative analysis was performed on Agilent 1260 LC Series instrument (Agilent, Santa Clara, CA, USA) equipped with a G4212B DAD using a Luna C-18 column (5 μm, 4.6 mm i.d. × 250 mm; Phenomenex, Inc., Torrance, CA, USA). Flow rate was 1.0 mL/min. The mobile phase was a mixture of 0.2% (v/v) phosphoric acid–water solution (A) and methanol (B) with a gradient elution as follows: 0–6 minutes, 0–50% B; 6–13 minutes, 50–57% B; 13–25 minutes, 57–60% B; 25–40 minutes, 60–70% B; 40–50 minutes, 70–100% B; 50–57 minutes, 100% B; 57–60 minutes, 100–0% B. The injection volume was 10 μL, and the column oven was maintained at 25°C. DAD detection wavelength was set at 295 nm for all analytes.
2.2. Materials
The leaves of A. fruticosa L. used in this study were collected from Jiaxian, Shaanxi Province, China. Samples for isolation were harvested in June 2013. Leaves for quantitative analysis were collected from the same plants on May 20, June 30, and August 10, 2014, and samples at each sampling time were collected from three plants in a wild field. The sample collected on May 20 was named AL0520, and the remaining samples were also named the same way. Their botanical origins were identified by the corresponding author (Naisheng Bai), and a voucher specimen (AF-2013-01) has been deposited in Room 612, Department of Pharmaceutical Engineering, College of Chemical Engineering, Northwest University, Xi’an, China. Column chromatography was performed over silica gel (200–300 mesh; Qingdao Marine Chemical Factory, Qingdao, China), polyamide, MCI GEL CHP-20P, and Sephadex LH-20 (Sigma-Aldrich, St. Louis, MO, USA). Precoated silica gel 60 GF254 plates were supplied by Merck (Darmstadt, Germany).
2.3. Chemicals and reagents
CD3OD, CDCl3, and DMSO-d6 (HPLC grade) were obtained from Merck (Darmstadt, Germany). HPLC-grade methanol (Merck) and phosphoric acid (Hengxing Chemical Reagent Co., Ltd, Tianjin, China) were used for HPLC analysis. CCK-8 was obtained from Qihai Biological Technology Ltd (Shanghai, China). All other solvents used in this study, such as acetone, petroleum ether (PE), dichloromethane (CH2Cl2), ethyl acetate (EtOAc), ethanol, and methanol were of analytical grade and supplied by Hengxing Chemical Reagent Co., Ltd.
2.4. Cell culture
The human colorectal adenocarcinoma cell line HCT116 and human hepatoma cell line HepG2 were purchased from Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). These cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich) supplemented with 10% (v/v) fetal bovine serum (Sigma-Aldrich).
2.5. Extraction and isolation
Air-dried A. fruticosa L. leaves (10 kg) were crushed into powders before being extracted twice with 90% ethanol (48 hours each) to yield 1.29 kg of crude extract. The crude extract was then successively dissolved in water and partitioned with PE and EtOAc.
The PE-soluble portion (320 g) was subjected to normal-phase silica gel open column chromatography (550 g of silica gel). The sample was eluted with a stepwise gradient of PE:EtOAc (100 → 0) to obtain three major fractions (A, B, and C). Fraction B was rechromatographed on a silica gel column, and then crystallized by acetone to give compound 14 (14 mg). Fraction C was further purified by Sephadex LH-20 column to obtain compound 2 (5 mg) with the elution of 75% methanol, and compound 1 (4.9 mg) with the elution of 70% methanol. The fractions were monitored by Agilent 1260 HPLC and thin-layer chromatography.
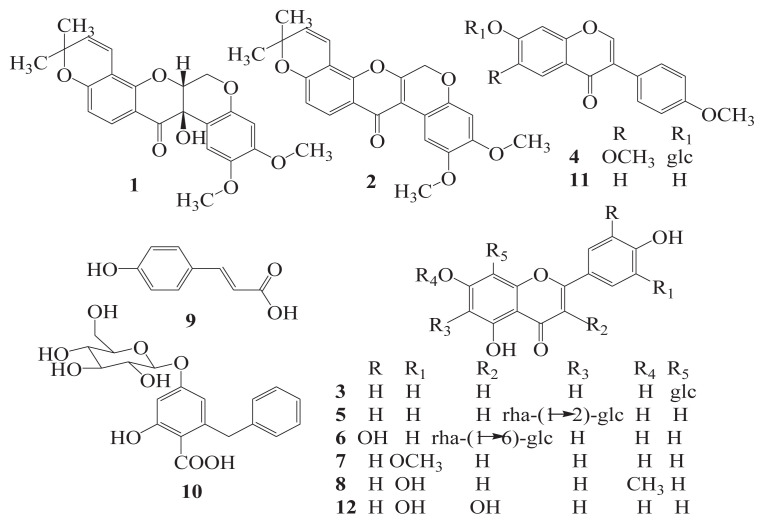
The EtOAc-soluble portion (170 g) was separated into five major fractions (B–F) by a normal-phase silica gel column (500 g of silica gel) using a stepwise gradient of PE:EtOAc (100 → 0) as eluent. Fraction C was further purified to yield compound 11 (42 mg) by a Sephadex LH-20 column eluting with 60% methanol, and compound 7 (26 mg) by an MCI-CHP20P column eluting with 90% methanol. Fraction D was further purified to give compound 13 (22 mg) by a normal-phase silica gel column, compound 12 (16 mg) by a Sephadex LH-20 column with the elution of 50% methanol, and compound 15 (15 mg) by an MCI-CHP20P column. Fractions E and F were subjected to polyamide column chromatography using water–ethanol mixtures (water: ethanol ratios of 75:1, 50:1, and 25:1) to afford five subfractions (E1 – E5) and (F1 – F5), respectively. Subfraction E3 was rechromatographed to give compounds 8 (115 mg), 9 (17 mg), and 10 (19 mg). Subfraction F2 was passed through a D101 column to afford three major fractions (F2–1, F2–2, and F2–3). Fraction F2–1 was separated by a Sephadex LH-20 column to yield compounds 4 (23 mg) and 5 (16 mg). Fraction F2–2 was successively purified by polyamide and an MCI-CHP20P column to yield compound 3 (75 mg). Compound 6 (26 mg) was obtained from fraction F2–3 by successively using polyamide, silica gel, and MCI-CHP20P column. The chemical structures of compounds 1–12 are shown in Figure 1.
Figure 1.
Chemical structures of compounds 1–12 isolated from the leaves of A. fruticosa L.
2.6. Cell viability assay
Cells were seeded into a 24-well plate (2 × 104 cells per well) overnight and then treated with various concentrations (5 μg/mL, 10 μg/mL, 25 μg/mL, 50 μg/mL, and 100 μg/mL) of individual compounds (3–10) and 90% ethanol extract for 48 hours at 37°C. After incubation, 10 μL CCK-8 was added to each well and incubated for further 3 hours. Results were measured by a spectrophotometer under 450 nm. Experiments were carried out in triplicate. Cell viability rate was calculated by the following formula: cell viability rate = (ODexperiment − ODblank)/(ODcontrol − ODblank) × 100%.
2.7. HPLC quantitative analysis
The HPLC method was carried out to quantitatively analyze compounds 1–12 isolated from the leaves of A. fruticosa L.
2.7.1. Preparation of sample solution
Prior to HPLC quantitative analysis, extraction conditions including extraction solvent (100%, 90%, 75%, 50%, and 25% ethanol and water), numbers (1, 2, 3, and 4 times), and time of sonication (30 minutes, 60 minutes, 90 minutes, and 120 minutes) were optimized on the sample AL0630. Under the optimized conditions, sun-dried samples were pulverized to homogeneous powders (40 mesh). Powder for each sample was accurately weighed (2.00 g) and ultrasonically extracted with 40 mL 90% (v/v) ethanol for 90 minutes. The residue was extracted once again, and the combined supernatants were evaporated and redissolved in 90% ethanol (10.0 mL). Extracting solutions were stored at 4°C and filtered through 0.45 μm membrane filters (Jiang Tian Unity, Tianjin, China) before HPLC analyses.
2.7.2. Preparation of standard solution
A mixed standard stock solution containing the reference compounds 1–12 was prepared in methanol. The working standard solutions for calibration curves were prepared by stepwise dilution of the mixed standard stock solution to a series of proper concentrations. All solutions were stored in a refrigerator at 4°C until use.
2.7.3. Method validation
To assess the validity of the developed method, linearity, limits of detection (LODs), limits of quantification (LOQs), precision, repeatability, stability, and recovery assays were performed on the sample AL0630. The linearity was assayed using external calibration curves with at least six concentration levels for each analyte, and each level was conducted in triplicate. The evaluation criterion for each regressive curve was a correlation coefficient (R2) greater than 0.999. LODs and LOQs were determined by diluting the mixed standard solution to the level when the signal-to-noise ratio was 3 and when it was 10, respectively. The intra- and interday precisions were determined by analyzing prepared sample solution six times on a single day and additionally on 3 consecutive days. Variations were expressed by the relative standard deviations (RSDs). Repeatability assay was performed by extracting six samples from one batch, and then each of the six extracts was analyzed (n = 3, each) and variations were expressed by RSD. Stability of the solution was assessed by analyzing one of the abovementioned solutions at 0 hours, 2 hours, 4 hours, 8 hours, 12 hours, and 24 hours. Recovery test was performed in triplicate by adding known quantities of standards into a certain amount (2.00 g) of the samples. The calculation formula was as follows: recovery (%) = (observed amount − original amount)/(spiked amount) × 100%.
2.7.4. Identification and quantification
Identification of compounds (1–12) was performed by comparing their HPLC retention times and UV spectra of target peaks with those of the standards isolated from the leaves of A. fruticosa L. In addition, standard substances were spiked in the sample solutions as a direct comparison. Quantitative determination was based on the external standard calibration curves of peak areas versus concentration. Amounts of the investigated compounds were calculated and expressed as mg/g of dried leaf weight.
3. Results and discussion
3.1. Extraction and isolation
Air-dried leaves of A. fruticosa L. (10 kg) were extracted twice with 90% ethanol (48 hours each) by maceration to yield 1.29 kg of crude extract. The crude extract was then dissolved in water and successively partitioned with PE and EtOAc. The PE and EtOAc partitions were subjected to fractionation with an initial separation by a normal-phase silica-gel column using a stepwise gradient of PE:EtOAc. Subsequent purification using a combination of column chromatography of Sephadex LH-20, MCI-CHP20P, D101, polyamide resin (PA), and silica gel to yield 15 compounds. Tephrosin (1) [27], 6a,12a-dehydrodeguelin (2) [28] and β-sitosterol (14) [29] were isolated from PE-soluble portion, and vitexin (3) [30], afrormosin-7-Oβ-d-glucopyranoside (4) [31], 2″-O-α-l-rhamnopyranosyl isovitexin (5) [32], rutin (6) [33], chrysoeriol (7) [34], 7-O-methyl-luteolin (8) [35], trans-p-coumaric acid (9) [36], 2-benzyl-4,6-dihydroxybenzoic acid-4-O-β-d-glucopyranoside (10) [37], formononetin (11) [38], quercetin (12) [39], apigenin (13) [40], and β-daucosterol (15) [29] were obtained from EtOAc-soluble portion. Structures of the 15 known compounds were characterized by chemical properties and spectroscopic methods (UV, and 1H 13C nuclear magnetic resonance), as well as by comparing nuclear magnetic resonance data with those reported in the literatures.
3.2. Cytotoxicity of individual compounds and crude extract
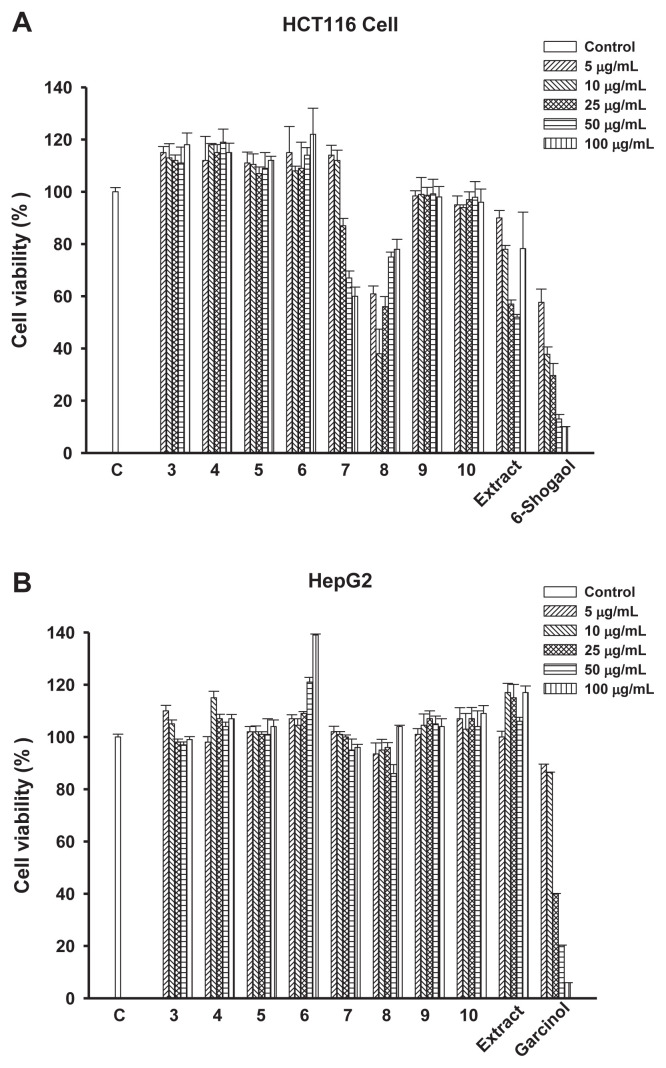
Anticancer activities of compounds 3–10 and 90% ethanol crude extract from A. fruticosa L. leaves were evaluated in two human cancer cell lines: HCT116 and HepG2. As shown in Figure 2, compounds 7 and 8, and 90% ethanol crude extract exhibited good inhibitory effect on human colorectal adenocarcinoma cell line HCT116, at concentrations of 100 μg/mL, 5 μg/mL, and 25 μg/mL at <60% of cell viability rate, respectively. However, no obvious effect on the inhibitory potency was observed for the test compounds and crude extract in human hepatoma cell line HepG2.
Figure 2.
Effect of compounds 3–10 and 90% ethanol crude extract on the viability of (A) HCT116 and (B) HepG2 cells. Cells were treated with 5 μg/mL, 10 μg/mL, 25 μg/mL, 50 μg/mL, or 100 μg/mL concentrations of the indicated compounds and 90% ethanol crude extract for 48 hours. Cell viability rate was then determined as described under the Materials and methods section.
3.3. HPLC quantitative analysis
3.3.1. Method validation
The quantitative analysis method was validated in terms of linearity, LOD, LOQ, precision, repeatability, stability, and accuracy. The results (Table 1) demonstrated that all calibration curves were good for the coefficients of linear regressions over 0.999. The values of LODs and LOQs were in the range of 0.10–0.40 μg/mL and 0.10–0.90 μg/mL, respectively. The results (Table 2) showed that the RSDs of intra- and interday variations, repeatability, and stability for the 12 analytes were all less than 1.98%. The overall recoveries were between 97.28% and 102.44%, with RSDs less than 1.69% (Table 2). Collectively, it indicated that the established analytical method was sensitive, precise, accurate, and repeatable for the determination of the 12 compounds in A. fruticosa L. leaves.
Table 1.
Calibration curves and LOD and LOQ data of the 12 compounds investigated by the HPLC-DAD method.
| Compound | Regression equationa | R 2 | Linear range (μg/mL) | LODb (μg/mL) | LOQb (μg/mL) |
|---|---|---|---|---|---|
| Tephrosin | Y = 6.35x − 7.25 | 0.9993 | 20.00–200.00 | 0.20 | 0.55 |
| 6a,12a-Dehydrodeguelin | Y = 2.21x + 23.01 | 0.9994 | 20.00–500.00 | 0.08 | 0.35 |
| Vitexin | y = 13.51x + 3.99 | 0.9997 | 20.00–200.00 | 0.40 | 0.90 |
| Afrormosin-7-O-β-d-glucopyranoside | y = 6.25x + 7.29 | 0.9995 | 20.00–200.00 | 0.15 | 0.50 |
| 2″-O-α-l-Rhamnopyranosyl isovitexin | y = 4.42x − 3.41 | 0.9996 | 20.00–500.00 | 0.35 | 0.85 |
| Rutin | y = 10.30x − 67.98 | 0.9993 | 20.00–500.00 | 0.05 | 0.22 |
| Chrysoeriol | y = 11.45x − 76.74 | 0.9993 | 1.00–200.00 | 0.25 | 0.60 |
| 7-O-Methylluteolin | y = 4.95x + 6.87 | 0.9996 | 20.00–500.00 | 0.10 | 0.42 |
| trans-p-Coumaric acid | y = 5.31x + 39.09 | 0.9998 | 1.00–200.00 | 0.17 | 0.48 |
| 2-Benzyl-4,6-dihydroxybenzoic acid-4-O-β-d-glucopyranoside | y = 5.59x − 33.77 | 0.9991 | 1.00–200.00 | 0.30 | 0.75 |
| Formononetin | y = 2.90x + 65.82 | 0.9998 | 1.00–200.00 | 0.13 | 0.45 |
| Quercetin | y = 82.37x + 18.64 | 0.9996 | 1.00–200.00 | 0.03 | 0.10 |
DAD = diode array detector; HPLC = high-performance liquid chromatography; LOD = limit of detection; LOQ = limit of quantification.
y is the value of peak area, and x is the value of the reference compound’s concentration (μg/mL).
LOD and LOQ were determined at S/N ratios of about 3 and 10, respectively.
Table 2.
Precision, repeatability, stability, and recovery of the 12 compounds.
| Compound | Precision (RSD, %) | Repeatability (RSD, %, n = 6) | Stability (RSD, %, n = 6) | Recovery (%, n = 3) | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Intraday (n = 6) | Interday (n = 6) | Mean | RSD, % | |||
| Tephrosin | 0.52 | 0.26 | 0.90 | 0.36 | 101.04 | 1.14 |
| 6a,12a-Dehydrodeguelin | 0.19 | 0.14 | 0.43 | 0.16 | 100.10 | 0.29 |
| Vitexin | 0.11 | 0.51 | 0.76 | 0.42 | 98.62 | 1.11 |
| Afrormosin-7-O-β-d-glucopyranoside | 0.32 | 0.37 | 0.54 | 0.36 | 98.55 | 0.96 |
| 2″-O-α-l-Rhamnopyranosyl isovitexin | 0.28 | 0.83 | 0.86 | 0.36 | 102.44 | 1.42 |
| Rutin | 0.29 | 0.21 | 0.24 | 0.26 | 99.00 | 1.05 |
| Chrysoeriol | 0.47 | 1.33 | 1.27 | 0.89 | 98.70 | 1.33 |
| 7-O-Methylluteolin | 0.26 | 0.32 | 0.23 | 0.33 | 100.07 | 0.20 |
| trans-p-Coumaric acid | 1.13 | 1.93 | 0.65 | 0.59 | 100.27 | 0.77 |
| 2-Benzyl-4,6-dihydroxybenzoic acid-4-O-β-d-glucopyranoside | 1.23 | 1.20 | 1.60 | 1.14 | 97.53 | 1.69 |
| Formononetin | 0.95 | 0.58 | 1.78 | 1.98 | 99.23 | 0.98 |
| Quercetin | 1.68 | 0.44 | 1.51 | 1.65 | 97.28 | 1.61 |
RSD = relative standard deviation.
3.3.2. Identification and quantification of the 12 compounds
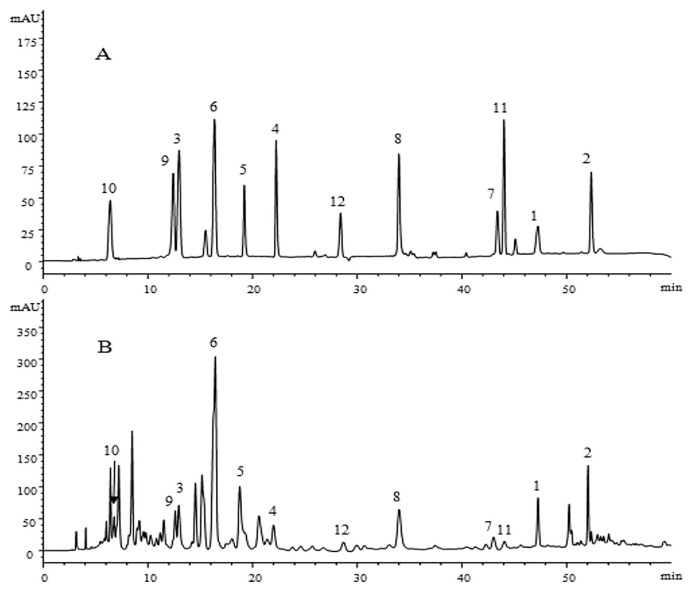
The established HPLC-DAD method was used for simultaneous determination of compounds 1–12 in the leaves of A. fruticosa L. Samples were collected at three different mature stages on May 20, June 30, and August 10, 2014 from Jiaxian, Shaanxi Province, China. Identification was carried out by comparing their HPLC retention times and UV spectral data with those of reference standards (Figure 3). Quantification was performed on the basis of an external standard method. The results of quantitative analysis are presented in Table 3.
Figure 3.
HPLC chromatograms of solution of (A) standards and (B) the sample A. fruticosa L. leaves at 295 nm. Peaks: 1, tephrosin (47.28 minutes); 2, 6a,12a-dehydrodeguelin (52.06 minutes); 3, vitexin (12.86 minutes); 4, afrormosin-7-O-β-d-glucopyranoside (22.03 minutes); 5, 2″-O-α-l-rhamnopyranosyl isovitexin (18.81 minutes); 6, rutin (16.42 minutes); 7, chrysoeriol (43.04 minutes); 8, 7-O-methylluteolin (34.01 minutes); 9, trans-p-coumaric acid (12.53 minutes); 10, 2-benzyl-4,6-dihydroxybenzoic acid-4-O-β-d-glucopyranoside (6.46 minutes); 11, formononetin (44.08 minutes); and 12, quercetin (28.70 minutes). HPLC =high-performance liquid chromatography.
Table 3.
Content of the 12 compounds in three different batches of A. fruticosa L. leaves.
| Compound | Content of compounds (mg/g, n = 3)a | ||
|---|---|---|---|
|
| |||
| AL0520 | AL0630 | AL0810 | |
| Tephrosin | 0.07 ± 0.001 | 0.76 ± 0.009 | 1.06 ± 0.002 |
| 6a,12a-Dehydrodeguelin | 0.45 ± 0.006 | 2.46 ± 0.001 | 3.14 ± 0.021 |
| Vitexin | 0.17 ± 0.002 | 0.76 ± 0.008 | 1.64 ± 0.014 |
| Afrormosin-7-O-β-d-glucopyranoside | 0.10 ± 0.002 | 0.65 ± 0.001 | 0.62 ± 0.002 |
| 2″-O-α-l-Rhamnopyranosyl isovitexin | 0.48 ± 0.005 | 2.00 ± 0.003 | 2.90 ± 0.026 |
| Rutin | 6.95 ± 0.013 | 4.78 ± 0.012 | 6.67 ± 0.016 |
| Chrysoeriol | 0.14 ± 0.001 | 0.11 ± 0.002 | 0.09 ± 0.001 |
| 7-O-Methylluteolin | 1.78 ± 0.002 | 1.63 ± 0.002 | 1.38 ± 0.003 |
| trans-p-Coumaric acid | 0.27 ± 0.004 | 0.46 ± 0.001 | 0.66 ± 0.001 |
| 2-Benzyl-4,6-dihydroxybenzoic acid-4-O-β-d-glucopyranoside | 0.16 ± 0.002 | 0.29 ± 0.003 | 0.52 ± 0.004 |
| Formononetin | 0.27 ± 0.001 | 0.18 ± 0.002 | 0.14 ± 0.003 |
| Quercetin | 0.02 ± 0.000 | 0.01 ± 0.000 | 0.02 ± 0.000 |
| Total | 10.86 | 14.09 | 18.84 |
SD = standard deviation.
Data are presented as the mean ± SD.
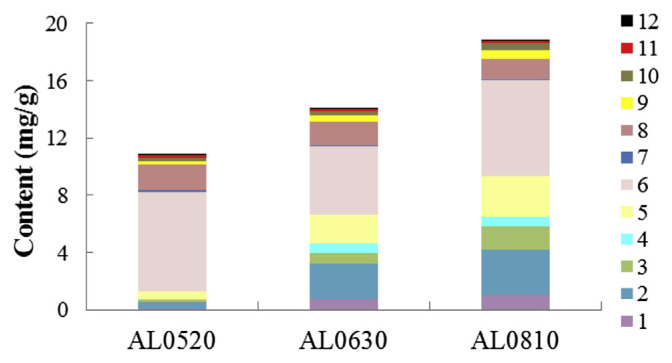
From Figure 4, we can clearly see that the total amount of the 12 analytes presented the increasing trend from May 20 to August 10, and it was highest in the sample AL0810 with a value of 18.84 mg/g, whereas it was 10.86 mg/g in the sample AL0520. Moreover, remarkable differences were also observed in individual compounds. For examples, the peak value of tephrosin (1), a potent antitumor agent [16,41], was registered on August 10, with a value of 1.06 mg/g. The compound 7-O-methylluteolin (8), which exerts a certain capacity against tumor cell lines HCT116, was highest in the sample AL0520, with a value of 1.78 mg/g. Despite the differences of individual compounds observed during the ripening of A. fruticosa L. leaves, the variation trend of the total amounts of the 12 compounds was also coincident with the trends of rotenoids (1 and 2), flavone glycosides (3–5), and phenolic acids (9 and 10). However, compounds 7, 8, 11, and 12 presented an overall decreasing trend in this period. Rutin (6), the most prevalent constituent in the leaves of A. fruticosa L., showed the highest value of 6.95 mg/g in the sample AL0520 and lowest value of 4.78 mg/g in AL0630. The differences observed for each compound probably correlated with physiological and environmental factors [42], such as tolerance to seasonal conditions and the need for defense against pathogenic agents to plants [43].
Figure 4.
Content of compounds 1–12 in three different batches of A. fruticosa L. leaves.
4. Conclusion
In conclusion, this study investigates and analyzes bioactive constituents of the leaves of A. fruticosa L. Six known phenols (3, 4, 5, and 7–9) were obtained from this plant for the first time, along with the other nine. Individual compounds chrysoeriol (7) and 7-O-methylluteolin (8), and 90% ethanol crude extract of A. fruticosa L. leaves exhibit inhibitory effects on human colorectal adenocarcinoma cell line HCT116, whereas there was no obvious effect on human hepatoma cell line HepG2. A validated HPLC-DAD method was used for quantitative analysis of compounds 1–12 isolated from this herb. The results indicated that their contents were greatly dependent on the stages of maturity. Total amounts of the isolated compounds presented an increasing trend from May 20 to August 10, with the value ranging from 10.86 mg/g to 18.84 mg/g. Tephrosin (1) presented an increasing trend from May 20 to August 10, and its highest level was registered in the sample AL0810 with a value of 1.06 mg/g. However, the highest contents of bioactive compounds 7 and 8 were 0.14 mg/g and 1.78 mg/g, respectively, in the sample AL0520. It will provide the optimal sampling time to use the rich resource as a source of bioactive compounds. The results of this study may provide data for further study and comprehensive utilization of A. fruticosa L. resource.
Footnotes
Conflict of interest statement
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Liu J, Wang ET, Chen WX. Diverse rhizobia associated with woody legumes Wisteria sinensis, Cercis racemosa and Amorpha fruticosa grown in the temperate zone of China. Syst Appl Microbiol. 2005;28:465–77. doi: 10.1016/j.syapm.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 2. Liang YP, Li XW, Gu ZM, Qin PW, Ji MS. Toxicity of amorphigenin from the seeds of Amorpha fruticosa against the larvae of Culex pipiens pallens (Diptera: Culicidae) Molecules. 2015;20:3238–54. doi: 10.3390/molecules20023238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinese PU. A dictionary of Chinese Materia Medica. 3rd ed. Beijing: Chinese Science and Technology Press of Medicine; 1997. [Google Scholar]
- 4. Claisse J, Crombie L, Peace R. Structure and stereochemistry of the vicianoside amorphin, the first rotenoid glycoside. J Chem Soc. 1964:6023–36. [Google Scholar]
- 5. Somleva T, Ognyanov I. New rotenoids in Amorpha fruticosa fruits. Planta Med. 1985;51:219–21. [Google Scholar]
- 6. Wu X, Liao HB, Li GQ, Liu Y, Cui L, Wu KF, Zhu XH, Zeng XB. Cytotoxic rotenoid glycosides from the seeds of Amorpha fruticosa. Fitoterapia. 2015;100:75–80. doi: 10.1016/j.fitote.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 7. Ohyama M, Tanaka T, Iinuma M. A prenylated flavanone from roots of Amorpha fruticosa. Phytochemistry. 1998;48:907–9. [Google Scholar]
- 8. Rózsa Z, Hohmann J, Szendrei K, Mester I, Reisch J. Amoradin, amoradicin and amoradinin, three prenylflavanones from Amorpha fruticosa. Phytochemistry. 1984;23:1818–9. [Google Scholar]
- 9. Cho JY, Kim PS, Park JB, Yoo ES, Baik KU, Kim YK, Park MH. Inhibitor of tumor necrosis factor-a production in lipopolysaccharide-stimulated RAW264.7 cells from Amorpha fruticosa. J Ethnopharmacol. 2000;70:127–33. doi: 10.1016/s0378-8741(99)00154-3. [DOI] [PubMed] [Google Scholar]
- 10. Ognyanov I, Somleva T. Rotenoids and 7,2′4′,5′-tetramethoxyisoflavone in Amorpha fruticosa L. fruits. Planta Med. 1980;38:279–80. [Google Scholar]
- 11. Konoshima T, Terada H, Kokumai M, Kozuka M, Tokuda H, Estes JR, Li LP, Wang HK, Lee KH. Studies on inhibitors of skin tumor promotion, XII. Rotenoids from Amorpha fruticosa. J Nat Prod. 1993;56:843–8. doi: 10.1021/np50096a006. [DOI] [PubMed] [Google Scholar]
- 12. Lee W, Ham J, Kwon HC, Yoon G, Bae GU, Kim YK, Kim SN. Amorphastilbol exerts beneficial effects on glucose and lipid metabolism in mice consuming a high-fat-diet. Int J Mol Med. 2015;36:527–33. doi: 10.3892/ijmm.2015.2227. [DOI] [PubMed] [Google Scholar]
- 13. Chen C, Wu Y, Chen Y, Du LL. Isolation and purification of prenylated phenolics from Amorpha fruticosa by high-speed counter-current chromatography. J Sep Sci. 2015;38:2924–9. doi: 10.1002/jssc.201500224. [DOI] [PubMed] [Google Scholar]
- 14. Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 15. Brett CH. Insecticidal properties of the indigobush (Amorpha fruticosa) J Agric Res. 1946;73:81–96. [PubMed] [Google Scholar]
- 16. Dat NT, Lee JH, Lee K, Hong YS, Kim YH, Lee JJ. Phenolic constituents of Amorpha fruticosa that inhibit NF-κB activation and related gene expression. J Nat Prod. 2008;71:1696–700. doi: 10.1021/np800383q. [DOI] [PubMed] [Google Scholar]
- 17. Choi Y, Lee JH. The combination of tephrosin with 2-deoxy-D glucose enhances the cytotoxicity via accelerating ATP depletion and blunting autophagy in human cancer cells. Cancer Biol Ther. 2011;12:989–96. doi: 10.4161/cbt.12.11.18364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee HJ, Kang HY, Kim CH, Kim HS, Kwon MC, Kim SM, Shin IS, Lee HY. Effect of new rotenoid glycoside from the fruits of Amorpha fruticosa LINNE on the growth of human immune cells. Cytotechnology. 2006;52:219–26. doi: 10.1007/s10616-006-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim YS, Ryu YB, Curtis-Long MJ, Yuk HJ, Cho JK, Kim JY, Kim KD, Lee WS, Park KH. Flavanones and rotenoids from the roots of Amorpha fruticosa L. that inhibit bacterial neuraminidase. Food Chem Toxicol. 2011;49:1849–56. doi: 10.1016/j.fct.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 20. Qu XL, Diao YP, Zhang Z, Wang SY, Jia YJ. Evaluation of antibacterial and wound healing activity of the fruits of Amorpha fruticosa L. AfrJ Tradit Complement AlternMed. 2013;10:458–68. doi: 10.4314/ajtcam.v10i3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuhr L, Rousseau M, Plauth A, Schroeder FC, Sauer S. Amorfrutins are natural PPARγ agonists with potent anti-inflammatory properties. J Nat Prod. 2015;78:1160–4. doi: 10.1021/np500747y. [DOI] [PubMed] [Google Scholar]
- 22. Shi H, Ma J, Mi CL, Li J, Wang F, Lee JJ, Jin XJ. Amorfrutin A inhibits TNF-α-induced NF-κB activation and NF-κB-regulated target gene products. Int Immunopharmacol. 2014;21:56–62. doi: 10.1016/j.intimp.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 23. Sauer S. Amorfrutins: a promising class of natural products that are beneficial to health. Chembiochem. 2014;15:1231–8. doi: 10.1002/cbic.201402124. [DOI] [PubMed] [Google Scholar]
- 24. Mitscher LA, Park YH, Alshamma A, Hudson PB, Haas T. Amorfrutin A and B, bibenzyl antimicrobial agents from Amorpha fruticosa. Phytochemistry. 1981;20:781–5. [Google Scholar]
- 25. Dzh ZD. Antioxidant and acetylcholinesterase inhibition properties of Amorpha fruticosa L. and Phytolacca americana L. Pharmacogn Mag. 2013;9:109–13. doi: 10.4103/0973-1296.111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C, Wu Y, Du L. Qualitative and quantitative analysis of amorfrutins, novel antidiabetic dietary natural products, by HPLC. Pharm Biol. 2015;54:488–93. doi: 10.3109/13880209.2015.1050115. [DOI] [PubMed] [Google Scholar]
- 27. Yea HY, Chen LJ, Li YF, Peng AH, Fu AF, Song H, Tang MH, Luo HD, Luo YF, Xu YB, Shi JY, Wei YQ. Preparative isolation and purification of three rotenoids and one isoflavone from the seeds of Millettia pachycarpa Benth by high-speed counter-current chromatography. J Chromatogr A. 2008;1178:101–7. doi: 10.1016/j.chroma.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 28. Ngandeu F, Bezabih M, Ngamga D, Tchinda AT, Ngadjui BT, Abegaz BM, Dufat H, Tillequin F. Rotenoid derivatives and other constituents of the twigs of Millettia duchesnei. Phytochemistry. 2008;69:258–63. doi: 10.1016/j.phytochem.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 29. Duan JA, Wang LY, Qian SH, Su SL, Tang YP. A new cytotoxic prenylated dihydrobenzofuran derivative and other chemical constituents from the rhizomes of Atractylodes lancea DC. Arch Pharm Res. 2008;31:965–9. doi: 10.1007/s12272-001-1252-z. [DOI] [PubMed] [Google Scholar]
- 30. Lin YL, Kuo YH, Shiao MS, Chen CC, Ou JC. Flavonoid glycosides from Terminalia catappa L. J Chin Chem Soc. 2000;47:253–6. [Google Scholar]
- 31. Tostes JBF, Silva AJR, Parente JP. Isoflavone glycosides from Centrosema pubescens. Phytochemistry. 1999;50:1087–90. [Google Scholar]
- 32. Otsuka H, Kijima K. An iridoid gentiobioside, a benzophenone glucoside and acylated flavone c-glycosides from Tripterospermum japonicum (SIEB. et ZUCC.) MAXIM. Chem Pharm Bull. 2001;49:699–702. doi: 10.1248/cpb.49.699. [DOI] [PubMed] [Google Scholar]
- 33. Li YL, Li J, Wang NL, Yao XS. Flavonoids and a new polyacetylene from Bidens parviflora Willd. Molecules. 2008;13:1931–41. doi: 10.3390/molecules13081931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim JH, Cho YH, Park SM, Lee KE, Lee JJ, Lee BC, Pyo HB, Song KS, Park HD, Yun YP. Antioxidants and inhibitor of matrix metalloproteinase-1 expression from leaves of Zostera marina L. Arch Pharm Res. 2004;27:177–83. doi: 10.1007/BF02980103. [DOI] [PubMed] [Google Scholar]
- 35. Noro R, Oda Y, Miyase T, Ueno A, Fukushima S. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem Pharm Bull. 1983;31:3984–7. doi: 10.1248/cpb.31.3984. [DOI] [PubMed] [Google Scholar]
- 36. KorP R, Vonk H, Xu X, Hoff WD, Crielaard W, Hellingwerf KJ. Evidence for trans–cis isomerization of the p-coumaric acid chromophore as the photochemical basis of the photocycle of photoactive yellow protein. FEBS Lett. 1996;382:73–8. doi: 10.1016/0014-5793(96)00149-4. [DOI] [PubMed] [Google Scholar]
- 37. Wu XH, Ruan JL, Cheng CR, Wu ZY, Guan SH, Tao SJ, Xu PP, Guo DA. Benzyl-β-resorcylates from Cassia obtusifolia. Fitoterapia. 2010;81:617–20. doi: 10.1016/j.fitote.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 38. Jeong CH, Shim KH. Tyrosinase inhibitor isolated from the leaves of Zanthoxylum piperitum. Biosci Biotech Biochem. 2004;68:1984–7. doi: 10.1271/bbb.68.1984. [DOI] [PubMed] [Google Scholar]
- 39. Jun M, Fu HY, Hong J, Wan X, Yang CS, Ho CT. Comparison of antioxidant activities of isoflavones from Kudzu root (Pueraria lobata Ohwi) J Food Sci. 2003;68:2117–22. [Google Scholar]
- 40. Stochmal A, Piacente S, Pizza C, Riccardis FD, Leitz R, Oleszek W. Alfalfa (Medicago sativa L.) flavonoids. 1. Apigenin and luteolin glycosides from aerial parts. J Agric Food Chem. 2001;49:753–8. doi: 10.1021/jf000876p. [DOI] [PubMed] [Google Scholar]
- 41. Choi SJ, Choi YJ, Dat NT, Hwangbo C, Lee JJ, Lee JH. Tephrosin induces internalization and degradation of EGFR and ErbB2 in HT-29 human colon cancer cells. Cancer Lett. 2010;293:23–30. doi: 10.1016/j.canlet.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 42. Lattanzio V. Bioactive polyphenols: their role in quality and storability of fruit and vegetables. J Appl Bot. 2003;77:128–46. [Google Scholar]
- 43. Kliebenstein DJ. Plant defense compounds: systems approaches to metabolic analysis. Annu Rev Phytopathol. 2012;50:155–73. doi: 10.1146/annurev-phyto-081211-172950. [DOI] [PubMed] [Google Scholar]