Abstract
The present study was designed to explore whether yam could protect the heart from doxorubicin (DOX)-induced oxidative stress leading to cardiotoxicity in vivo. In this study, the protective effects of water and ethanol extracts of three varieties of yam, including water extracts of Dioscorea japonica Thunb., ethanol extracts of D. japonica Thunb., water extracts of Dioscorea alata, ethanol extracts of D. alata, water extracts of Dioscorea purpurea, and ethanol extracts of D. purpurea, against DOX-induced cardiotoxicity in experimental mice were evaluated. DOX treatment led to significant decreases in the ratio of heart weight to body weight and heart rate, and increases in blood pressure and the serum level of lactate dehydrogenase, a marker of cardiotoxicity, were recovered by yam extracts, especially in water extracts of D. alata. Yam extracts also decreased the cardiac levels of thiobarbituric acid relative substances, reactive oxygen species, and inflammatory factors, as well as the expression of nuclear factor kappa B, while ethanol extracts of D. japonica Thunb. and D. purpurea were shown to be more potent. Moreover, yam extracts had a role in increasing the activities of glutathione peroxidase and superoxide dismutase, thus improving the DOX-induced alterations in oxidative status in the heart tissue of DOX-treated mice. All ethanol extracts of yam exhibited their antiapoptotic abilities on caspase-3 activation and mitochondrial dysfunction, and ethanol extracts of D. alata still exerted a superior effect. Based on these findings, it can be concluded that yam has significant cardioprotective properties against DOX-induced damage via its multiple effects on antioxidant, anti-inflammatory, or antiapoptotic activities.
Keywords: antiapoptosis, anti-inflammation, cardiotoxicity, doxorubicin, yam
1. Introduction
Doxorubicin (DOX), an anthracyclin antibiotic, has been used against cancer since 1960s. It is one of the most effective anticancer drugs. However, studies of cardiotoxic effects of DOX have been reported [1,2]. Therefore, chemotherapy with DOX is limited by its cardiotoxicity. The development of cumulative dose-dependent cardiomyopathy may occur many years after the cessation of DOX treatment. It has been calculated that approximately 10% of patients treated with DOX will develop cardiomyopathy and heart failure [3,4]. Owing to the importance of DOX in the chemotherapy treatment for many cancer types, strategies have been tried to prevent or attenuate the side effects of DOX administration, including the use of DOX analogs, alternative drug-delivery methods, and iron-chelating agents [1]. However, so far, the ability of treatments to prevent or attenuate DOX-induced damage has been limited, and discovery of novel agents for reducing its side effects is still needed.
Multiple mechanisms are involved in DOX-induced cardiotoxicity, such as the increase in cardiac oxidative stress and lipid peroxidation leading to inflammation- and apoptosis-related signaling pathway [5,6]. The heart is more susceptible to oxidative stress-induced damage, because it has relatively low levels of antioxidant enzymes including superoxide dismutase (SOD) and catalase [7]. Antioxidants have been reported to have beneficial effects against DOX-induced cardiotoxicity in mice and rats [8]. In previous studies, free radical scavengers, such as vitamin E and ellagic acid, have been demonstrated to protect from DOX-induced cardiotoxicity, indicating the involvement of reactive oxygen species (ROS) and nuclear factor kappa B (NF-κB) in the pathogenesis [9,10]. ROS have an important role in the progression of fibrosis and can influence the expression as well as activation of transforming growth factor-β (TGF-β) [11], a cytokine that has been associated with the evolution of tissue fibroinflammatory signaling. Acute DOX cardiotoxicity involves cardiomyocyte apoptosis. It is generally agreed that the elevated oxidative stress induced by DOX activates signaling pathways leading to cardiomyocyte apoptosis [12]. Caspase activity can be influenced by DOX, and mitochondrial dysfunction is associated with DOX administration [13]. Therefore, any agents with the ability to exhibit antioxidant, anti-inflammatory, or antiapoptotic activities might provide some protective effects against DOX.
Yam (Dioscorea spp.), a perennial herb, is distributed mainly in the tropical and subtropical regions of the world. It is estimated that there are more than 600 species in the world, 93 of which are found in China and 14 in Taiwan [14]. The tuber of yam serves as a good source of nutritional supply and staple food around the world. In addition, yam has been used for enhancement of health in oriental countries and traditionally considered as a superior Chinese herb to improve gastrointestinal function [15]. Nutritional assessment suggested that starch is the predominant fraction of yam, and the tuber contains choline, mucin, allantoin, crude fat, crude fiber, phytosterols, and steroidal saponins [16]. Yam is most noted for the abundance of diosgenin, a steroidal saponin, and the component has been found to ameliorate myocardial infarction by its antilipoperoxidant activity [17]. Recently, diosgenin has also been demonstrated to reduce DOX-induced cardiotoxicity in mice [18]. This implies that yam can be a potential cardioprotective agent. Furthermore, many studies have shown that an extract of yam possesses antioxidant [14,16,19], anti-inflammatory [16], lipid metabolism [20], and estrogenic [21] activities. A previous study indicated that a yam variety (called air potato) has been shown to exert a protective effect on myocardial ischemia/reperfusion injury in rats due to apoptosis and necrosis [22]. Hence, this study aimed to investigate whether the three varieties of yam, including Dioscorea japonica Thunb., Dioscorea alata (cultivars of Ta-Shan line 2), and Dioscorea purpurea (cultivar of Ming-Chien), have protective effects against DOX-induced cardiac damage in vivo.
2. Materials and methods
2.1. Chemicals
Doxorubicin hydrochloride, Tris-HCl, NaCl, ethylenediamin-etetraacetic acid (EDTA), Tween 80, phenylmethanesulfonyl fluoride (PMSF), KCl, MgCl2, and sodium dodecyl sulfate (SDS) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
2.2. Preparation of dried yam extracts
Three varieties of yam, including D. japonica Thunb., D. alata (cultivars of Ta-Shan line 2), and D. purpurea (cultivar of Ming-Chien), were used in the present study. All yam tubers were provided by the Taiwan Agricultural Research Institute (Taichung, Taiwan). The yam tubers were weighed, washed, peeled, cut into slices, and ground using an electric grinder. Then, the three parts were blended with water or 75% ethanol (1:1, w/w) overnight, and further freeze dried to obtain fine powder. The powders were sealed in polyester bags to prevent moisture absorption until used for further tests.
2.3. Animals and experimental protocol
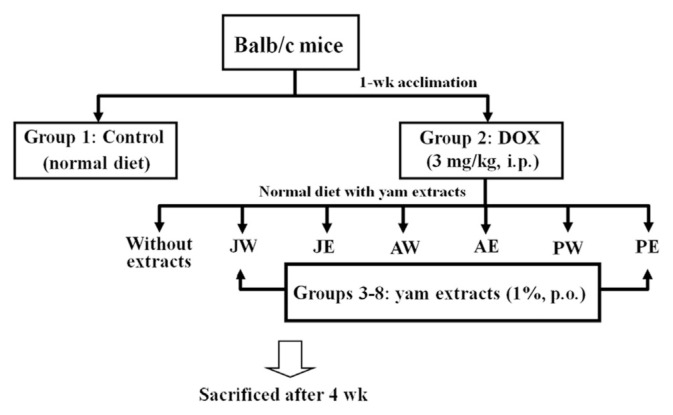
Five-week-old male Balb/c mice (15–21 g body weight) were obtained from National Laboratory Animal Center (National Science Council, Taipei City, Taiwan). Use of the mice was approved by the guidelines of the Instituted Animal Care and Use Committee of Chung Shan Medical University. Mice were housed on a 12-hour light/dark cycle and fed with mouse standard chow diet (MF-18; Oriental Yeast Co., Ltd. Tokyo, Japan); experiments were started after 1-week acclimation. The mice were randomly divided into eight groups (10 mice per group) and treated as follows:
Group 1 (control): vehicle
Group 2 (DOX): DOX at 3 mg/kg of body weight once a week, i.p.
Group 3 (DOX + JW): DOX with water extracts of D. japonica Thunb. (JW)
Group 4 (DOX + JE): DOX with ethanol extracts of D. japonica Thunb. (JE)
Group 5 (DOX + AW): DOX with water extracts of D. alata (AW)
Group 6 (DOX + AE): DOX with ethanol extracts of D. alata (AE)
Group 7 (DOX + PW): DOX with water extracts of D. purpurea (PW)
Group 8 (DOX + PE): DOX with ethanol extracts of D. purpurea (PE)
DOX was administered intraperitoneally to the mice of Groups 2–8 at a dose of 3 mg/kg once a week for 4 weeks (a total of 12 mg/kg). At the same time, six of these groups were receptively orally treated with different yam extracts at the same doses of 1% per mouse, equivalent to 3 g of daily basal diets containing 30 mg/mL (w/v) yam extracts, and this treatment was continued daily for 4 weeks (Figure 1). The doses and injection regiments for these treatments were based on the reports published previously [23], with some modification. At the end of 4 weeks, mice were euthanized by carbondioxide asphyxiation followed by exsanguination. Cardiac perfusion was performed as described previously [24]. The hearts were excised and weighed, and serum and cardiac samples were collected and used for analysis as described below.
Figure 1.
Schematic diagram of animal experiment. AE = ethanol extracts of D. alata; AW = water extracts of D. alata (Ta-Shan); DOX = doxorubicin; JE = ethanol extracts of D. japonica Thunb.; JW = water extracts of D. japonica Thunb.; PE = ethanol extracts of D. purpurea; PW = water extracts of D. purpurea.
2.4. Heart rate, blood pressure monitoring, and serum lactate dehydrogenase activity
Heart rate and blood pressure were estimated by the tail cuff method using a blood pressure monitor for rats and mice (Model MK 2000; Muromachi Kikai Co. Ltd, Tokyo, Japan). Serum lactate dehydrogenase (LDH) activity was assayed by commercial kits (Randox, Crumlin, UK).
2.5. Measurement of lipid peroxidation and antioxidant status in the heart
The level of thiobarbituric acid relative substances (TBARS, nmol/mg protein) in cardiac tissue was determined by a fluorescence spectrophotometer (excitation at 532 nm and emission at 600 nm) as described previously [25]. Quantification of TBARS was performed by comparison with a standard dosage of malondialdehyde, the lipid peroxidation product, which is generated by acid-catalyzed hydrolysis of 1,1,3,3-tetramethoxypropane. ROS in cardiac tissue were measured by using commercial kits (Calbiochem Inc., San Diego, CA, USA). Cardiac activities of glutathione peroxidase (GPx) and SOD were determined by commercial assay kits (Calbiochem Inc), and glutathione (GSH) was analyzed by commercial assay kits (OxisResearch, Portland, OR, USA).
2.6. Inflammatory cytokine analysis
Heart tissues were homogenized in 10mM Tris-HCl buffered solution (pH 7.4) containing 2M NaCl, 1mM EDTA, 0.01% Tween 80, and 1mM PMSF, and centrifuged at 9000g for 30 minutes at 4°C. The supernatant was used for cytokine determination. The levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were assayed by cytoscreen immunoassay kits (BioSource International, Camarillo, CA, USA).
2.7. Real-time polymerase chain reaction
Total RNA was isolated from cells with a guanidinium chloride procedure and the mRNA levels were analyzed by real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR) as described previously [26]. One microgram of total RNA was used to generate cDNA, which was amplified using Taq DNA polymerase. PCR was carried out in 50 μL reaction mixture containing Taq DNA polymerase buffer [20mM Tris-HCl at pH 8.4, 50mM KCl, 200mM deoxynucleotide triphosphates (dNTPs), 2.5mM MgCl2, and 0.5mM of each primer] and 2.5 U Taq DNA polymerase. The specific oligonucleotide primers for NF-κB, TGF-β, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, the housekeeping gene) are as follows: NF-κB: forward, 5′-CAG ACC GCA GTA TCC ATA GC-3′, and reverse, 5′-CGT GAA AGG GGT TAT TGT TGG-3′; TGF-β: forward, 5′-TGA CGT CAC TGG AGT TGT ACG G-3′, and reverse, 5′-GGT TCA TGT CAT GGA TGG TGC-3′; and GAPDH, forward, 5′-TGT GTC CGT CGT GGA TCT GA-3′, and reverse, 5′-TTG CTG TTG AAG TCG CAG GAG-3′. The cDNA was amplified under the following reaction conditions: 95°C for 20 seconds, 60°C for 1 minute, and 72°C for 1 minute. Twenty-eight cycles were performed for GAPDH, and 40 cycles for NF-κB and TGF-β. Fluorescence generated from each cycle was quantitatively analyzed by a Taqman system based on a real-time sequence detection system (ABI Prism 7700; Perkin-Elmer Inc., Foster City, CA, USA). Each cDNA sample was triplicated and the corresponding no-RT mRNA sample was included as a negative control. GAPDH primers were included in every plate to avoid sample variations. The mRNA level of each sample for each gene was normalized to that of the GAPDH mRNA.
2.8. Protein preparation and Western blot analysis
Western blotting was performed according to a method described previously [18]. The preparation of cytosolic and nuclear fractions of cells was performed according to the procedures described by Vasanthi et al [22]. Protein extracts were subjected to centrifugation at 10,000g for 10 minutes. Total protein (10–50 μg per lane) was electrophoresed and separated on 8–15% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. After blocking with 5% nonfat dry milk, the membranes were incubated with the indicated primary antibodies overnight at 4°C. The blots were incubated with the antibodies against NF-κB, C23, IκBα, caspase-3, caspase-8, caspase-9, poly (ADP-ribose) polymerase-1 (PARP-1), and β-actin, purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); β-actin served as an internal control. The blot was quantified by enhanced chemiluminescence detection (Amersham Pharmacia Biotech, Little Chalfont, Bucks, UK).
2.9. Isolation of mitochondria and cytochrome c assay
The preparation of cytosolic and mitochondrial fractions of cardiac tissue was performed as described previously [27]. The isolated hearts were washed in sterile phosphate-buffered saline, and the mitochondria were isolated according to the manufacturer’s instructions (Mitochondria Isolation Kit for Tissue; Pierce, Rockford, IL, USA). Briefly, tissues were minced after the addition of 800 mL Mitochondria Isolation Reagent A and carefully homogenized with 20 strokes on ice. The crude homogenates were then returned to the original tube, and 800 mL Mitochondria Isolation Reagent C was added. The tube was centrifuged at 700g for 10 minutes at 48°C. The supernatant was transferred and centrifuged at 3000g for 15 minutes at 48°C to obtain a more purified fraction of mitochondria. The resulting supernatant was transferred into a new tube and centrifuged at 12,000g to produce a more purified cytosolic fraction, which was saved for cytochrome c assay. The pellet contained the isolated mitochondria. Mitochondria Isolation Reagent C (500 mL) was added to the pellet, and the mixture was centrifuged at 12,000g for 5 minutes. The mitochondrial pellet was resuspended in 300 mL of mitochondria isolation buffer containing 0.1% Triton X-100 and protease inhibitors. Protein concentrations of both mitochondrial and cytosolic lysates were determined using BCA Protein Assay Reagents (Pierce). To detect cytochrome c release into the cytosol, Western blotting was performed with antibodies against cytochrome c and COX IV, a mitochondrial marker, purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
2.10. Statistical analysis
Results from 10 mice (n = 10) were analyzed and expressed as means ± standard deviation. Statistical analysis was done by one-way analysis of variance, and post hoc comparisons were carried out using Duncan’s multiple-range test. Differences with p < 0.05 were considered statistically significant.
3. Results
3.1. Effects of yam extracts on DOX-induced cardiotoxicity
Effect of DOX on cardiotoxic parameters are shown in Table 1. DOX significantly decreased the body weight, heart weight, and ratio of heart weight to body weight. Among the groups of DOX plus yam extracts, AW and AE significantly diminished the DOX-induced loss in the ratio of heart weight to body weight. Furthermore, heart rate and blood pressure were assessed after 4 weeks of DOX treatment. Table 1 also shows that DOX treatment resulted in significant cardiac functional deterioration, as characterized by a lower heart rate and an increase in blood pressure as compared with the control group (p < 0.05). The ethanol extracts of yam, including JE, AE, and PE, can increase heart rate and decrease blood pressure under DOX treatment. Serum level of LDH was also significantly elevated in the DOX-alone-treated group. All yam extracts significantly reduced LDH level as compared with DOX alone (p < 0.05), while AE was shown to be most potent.
Table 1.
Effects of yam extracts, including JW, JE, AW, AE, PW, and PE, on cardiac physical and functional parameters in DOX-treated mice.
| Normal | DOX | DOX + yam extracts | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| DOX + JW | DOX + JE | DOX + AW | DOX + AE | DOX + PW | DOX + PE | |||
| Body weight (g) | 30.40 ± 1.71b | 23.98 ± 1.52a | 24.60 ± 1.90a | 23.64 ± 1.72a | 23.28 ± 2.73a | 23.53 ± 2.44a | 23.04 ± 0.98a | 24.49 ± 2.70a |
| Heart weight (mg) | 168.77 ± 13.99c | 115.60 ± 16.45a | 124.35 ± 20.12ab | 127.16 ± 15.10ab | 127.21 ± 14.32ab | 139.26 ± 9.24b | 123.68 ± 9.73ab | 129.31 ± 17.81ab |
| Heart weight/body weight ratio | 5.75 ± 0.52b | 4.79 ± 0.87a | 5.05 ± 0.82ab | 5.38 ± 0.64ab | 5.46 ± 0.62b | 5.92 ± 0.39b | 5.37 ± 0.42 ab | 5.28 ± 0.73ab |
| Heart rate (bpm) | 616.10 ± 9.25d | 594.18 ± 18.02a | 596.30 ± 17.01ab | 610.60 ± 9.09cd | 601.75 ± 15.99ab | 603.90 ± 9.44bc | 599.50 ± 15.37ab | 603.70 ± 13.77bc |
| Blood pressure (mmHg) | 115.95 ± 11.59d | 124.09 ± 8.42e | 103.95 ± 9.71bc | 96.40 ± 12.17a | 103.70 ± 9.95bc | 93.35 ± 12.94a | 110.25 ± 11.32cd | 98.75 ± 11.82a |
| Serum LDH (U/L) | 167.77 ± 24.24a | 292.35 ± 66.96c | 229.69 ± 27.78ab | 191.91 ± 36.72a | 221.93 ± 55.92ab | 169.72 ± 27.71a | 208.29 ± 27.62ab | 204.74 ± 33.14ab |
Values are mean ± SD, n = 10.
Means in a row without a common letter differ, p < 0.05.
AE = ethanol extracts of D. alata; AW = water extracts of D. alata (Ta-Shan); bpm = beats per minute; DOX = doxorubicin; JE = ethanol extracts of D. japonica Thunb.; JW = water extracts of D. japonica Thunb.; LDH = lactate dehydrogenase; PE = ethanol extracts of D. purpurea; PW = water extracts of D. purpurea; SD = standard deviation.
3.2. Effects of yam extracts on DOX-induced alterations in cardiac oxidative status
To confirm that the induction of oxidative stress was mediated by DOX, tissue lipid peroxidation and antioxidant enzymes were also evaluated. As shown in Table 2, DOX treatment increased TBARS and ROS levels, and decreased GSH content as well as GPx and SOD activities in the heart. As compared with DOX alone, all yam extracts lowered ROS and TBARS levels (p < 0.05). Noticeably, PE significantly retained GSH content, and recovered cardiac GPx and SOD activities (p < 0.05, Table 2), indicating that PE could exert a better antioxidant activity than other extracts.
Table 2.
Effects of yam extracts, including JW, JE, AW, AE, PW, and PE, on DOX-induced alterations in cardiac oxidative status.
| Normal | DOX | DOX + yam extracts | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| DOX + JW | DOX + JE | DOX + AW | DOX + AE | DOX + PW | DOX + PE | |||
| TBARS (nmol/mg protein) | 0.42 ± 0.07a | 1.05 ± 0.39b | 0.57 ± 0.21a | 0.28 ± 0.24a | 0.37 ± 0.26a | 0.27 ± 0.18a | 0.47 ± 0.28a | 0.25 ± 0.10a |
| ROS (RFU/mg protein) | 0.99 ± 0.10a | 1.33 ± 0.35b | 0.92 ± 0.18a | 0.84 ± 0.08a | 0.90 ± 0.10a | 0.88 ± 0.02a | 0.88 ± 0.10a | 0.83 ± 0.16a |
| GSH (nmol/mg protein) | 8.53 ± 1.56cd | 4.87 ± 0.72a | 6.53 ± 1.49ab | 6.98 ± 1.44bc | 5.85 ± 0.65ab | 6.39 ± 0.88ab | 5.69 ± 0.82ab | 8.86 ± 1.25d |
| GPx (U/mg protein) | 37.04 ± 12.11c | 17.78 ± 4.54a | 21.80 ± 5.42a | 27.35 ± 1.29b | 23.44 ± 7.70ab | 23.57 ± 1.67abc | 32.73 ± 6.13bc | 33.07 ± 7.18bc |
| SOD (U/mg protein) | 7.12 ± 1.42c | 4.55 ± 1.61a | 5.70 ± 4.62a | 6.51 ± 2.23bc | 5.88 ± 1.65ab | 6.22 ± 3.23abc | 5.93 ± 2.15bc | 6.92 ± 1.74bc |
Values are mean ± SD, n = 10.
Means in a row without a common letter differ, p < 0.05.
AE = ethanol extracts of D. alata; AW = water extracts of D. alata (Ta-Shan); bpm = beats per minute; DOX = doxorubicin; GPx = glutathione peroxidase; GSH = glutathione; JE = ethanol extracts of D. japonica Thunb.; JW = water extracts of D. japonica Thunb.; LDH = lactate dehydro-genase; PE = ethanol extracts of D. purpurea; PW = water extracts of D. purpurea; RFU = relative fluorescence units; ROS = reactive oxygen species; SD = standard deviation; SOD = superoxide dismutase; TBARS = thiobarbituric acid relative substances.
3.3. Effects of yam extracts on DOX-induced cardiac inflammation
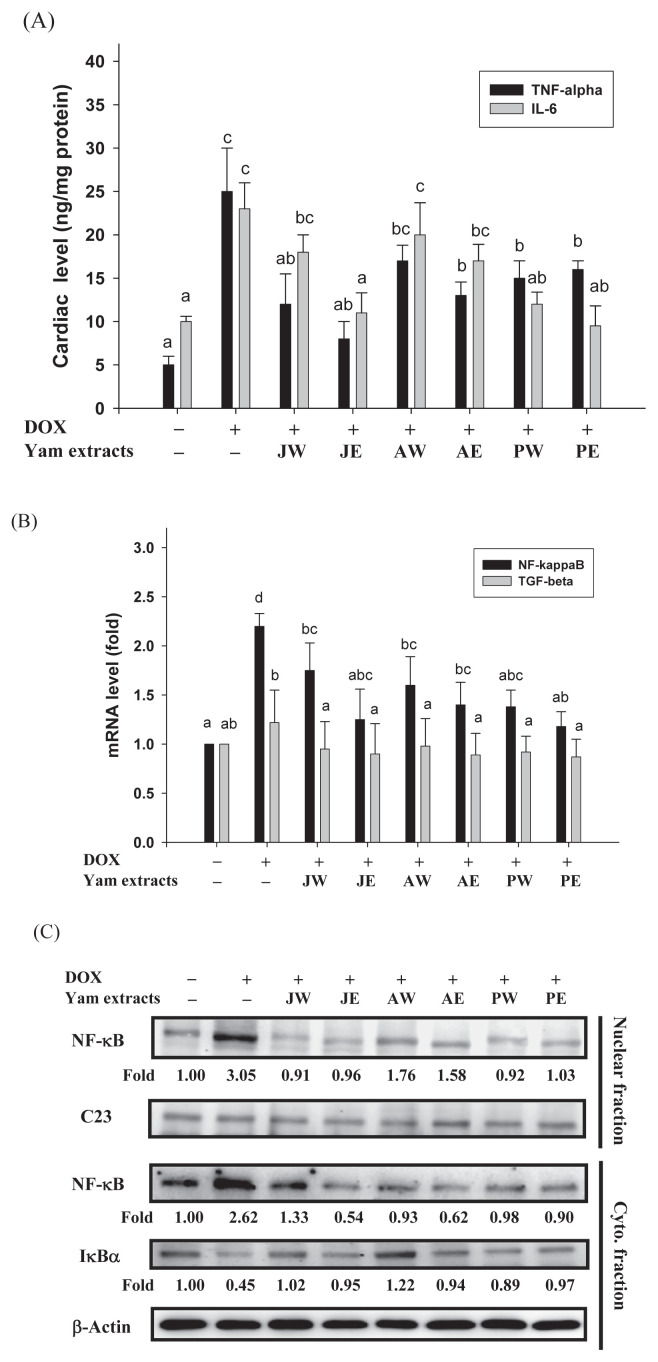
Previous studies have demonstrated that DOX can induce cardiotoxicity via activation of both apoptosis and inflammation-related signaling pathways in the heart [5,6]. Cytokines, particularly TNF-α and IL-6, are involved in the recruitment and activation of immune cells, both of which contribute to the development of cardiomyopathy. In the current study, the levels of both TNF-α and IL-6 in the heart were markedly elevated by DOX induction compared with those in the control group (p < 0.05, Figure 2A), indicating a severe inflammatory injury. In addition, mRNA expressions of NF-κB and TGF-β, two factors that are related to inflammation, were measured by real-time PCR. DOX treatment augmented the cardiac mRNA expression of NF-κB, but not of TGF-β (Figure 2B). Next, we tested whether yam extracts perturbed the translocation of NF-κB into the nucleus leading to NF-κB activation, by immunoblotting analysis of the nucleus fraction prepared from the heart tissues. The data in Figure 2C demonstrated that not only a marked increase of nuclear NF-κB level (Line 1), but also a coincided augment in the cytosolic level of NF-κB (Line 3) concomitantly with a decrease in the amount of cytosolic IκBα protein (Line 4) was observed upon the DOX injection. The nuclear and cytosolic levels of NF-κB were weaker in the yam extract groups than in the DOX-alone-treated group. Furthermore, the cytosolic level of IκB was augmented by DOX, and this, too, was significantly attenuated by treatment with yam extracts (Figure 2C), suggesting that yam extracts suppressed DOX-induced NF-κB activation. The yam extract-induced changes in the protein levels of nuclear and cytosolic NF-κB coincided well with its mRNA level, as evidenced by real-time PCR results (Figure 2B), indicating that yam extracts might regulate NF-κB expression at the transcriptional level. Significantly, when mice were cotreated with JE, PW, or PE, concomitant decreases in the levels of TNF-α and IL-6, as well as in mRNA and protein expressions of NF-κB, were observed, as compared with the DOX-alone-treated group (p < 0.05, Figure 2).
Figure 2.
Effects of yam extracts on the (A) levels of inflammatory cytokines TNF-α and IL-6, (B) mRNA expressions of NF-κB and TGF-β, and (C) nuclear translocation level of NF-κB in heart tissues of mice treated with DOX for 4 weeks. Values are mean ± SD, n = 10. The nuclear level of NF-κB in the nuclear fraction (Lines 1–2), and the cytosolic levels of NF-κB and IκBα in the Cyto. fraction (Lines 3–5) were determined by Western blotting. C23 and β-actin, respectively, served as internal controls of nuclear and Cyto. fractions. The protein levels above the figure represent relative density of the bands normalized to C23 or β-actin. The determined expression of the protein was subsequently quantified by densitometric analysis with that of control being 1.00-fold, as shown just below the gel data. Results are representative of at least three independent experiments. a–c Means in a row without a common letter differ, p < 0.05. AE = ethanol extracts of D. alata; AW = water extracts of D. alata (Ta-Shan); Cyto. = cytosolic; DOX = doxorubicin; IL = interleukin; JE = ethanol extracts of D. japonica Thunb.; JW = water extracts of D. japonica Thunb.; NF-κB = nuclear factor kappa B; PE = ethanol extracts of D. purpurea; PW = water extracts of D. purpurea; SD = standard deviation; TGF-β = transforming growth factor-β; TNF-α = tumor necrosis factor-alpha.
3.4. Effects of yam extracts on DOX-induced cardiac apoptosis
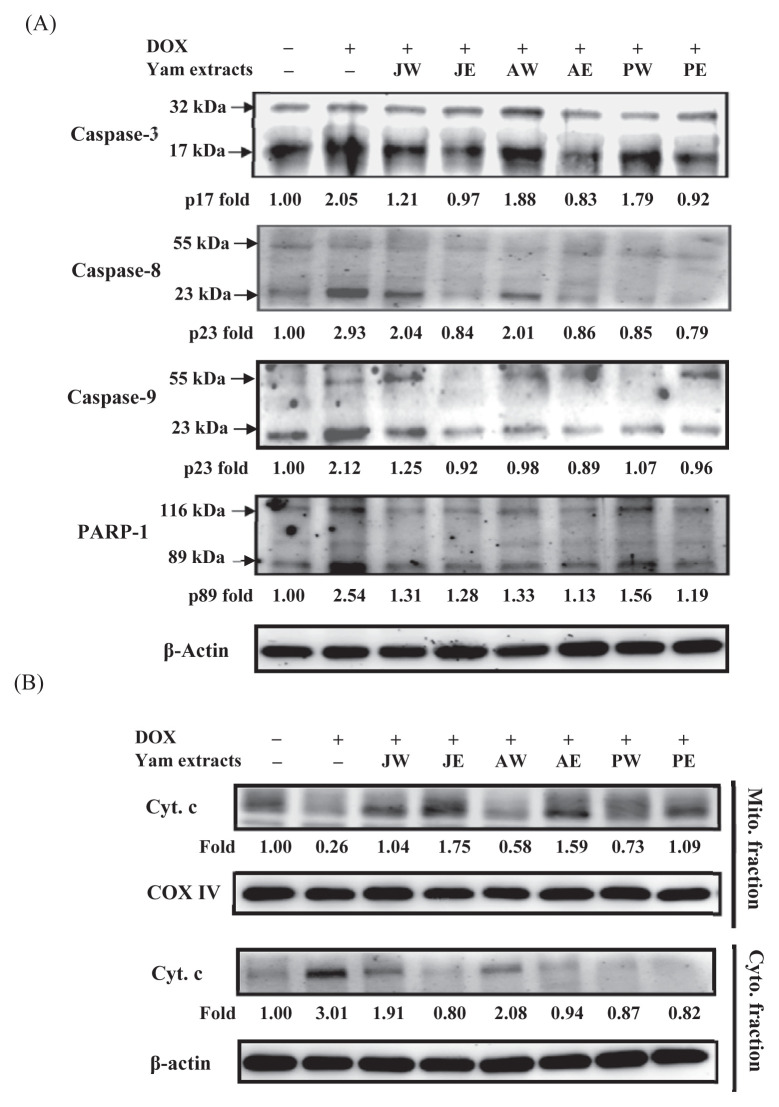
To investigate whether the protective effect of yam extracts against DOX occurred because they inhibited apoptotic pathways, we further studied the change in the expression of caspase-3, one marker of apoptosis, in the heart tissue (Figure 3A). Caspase-3 is a cytosolic protein that exists normally as an inactive precursor with a higher molecular weight (about 32 kDa). It is cleaved proteolytically into fragments of low molecular weights (11 kDa, 17 kDa, and 20 kDa) when a cell undergoes apoptosis [28]. In this study, treatment with DOX induced significantly the cleavages of caspase-3 (p20) and its upstream caspases, caspase-8 (p23) and caspase-9 (p23), as well as its downstream factorPARP-1 to 2.05-, 2.93-, 2.12-, and 2.54-fold of that of control, respectively. In the yam extract-cotreated groups, their active fragments were decreased (Lanes 3–8, Figure 3A). As shown in Figure 3B (Lane 2), DOX increased the cytosolic level of cytochrome c significantly, with a concomitant decrease in the mitochondria, implicating mitochondrial dysfunction due to DOX toxicity [29]. Quantitative data showed that the cytosolic level of cytochrome c was increased to 3.01-fold of that of control in the heart tissues of DOX-treated mice. This effect was significantly suppressed in the groups of DOX plus yam extracts (Lanes 3–8, Figure 3B). The DOX-induced release of cytochrome c from mitochondria was almost inhibited in the groups of DOX plus ethanol extracts of yam.
Figure 3.
Effects of yam extracts on (A) the protein expressions of caspase-3, caspase-8, caspase-9, and PARP-1, and (B) the release of Cyt. c in heart tissues of mice treated with DOX for 4 weeks. The protein levels of Cyt. c from Mito. (Lines 1–2) and Cyto. (Lines 3–4) fractions were determined by Western blotting. COX IV and β-actin, respectively, served as internal controls of Mito. and Cyto. fractions. The protein levels above the figure represent relative density of the bands normalized to COX IV or β-actin. The determined expression of the protein was subsequently quantified by densitometric analysis with that of control being 1.00-fold, as shown just below the gel data. Results are representative of at least three independent experiments. AE = ethanol extracts of D. alata; AW = water extracts of D. alata (Ta-Shan); Cyt. c = cytochrome c; Cyto. = cytosolic; DOX = doxorubicin; IL = interleukin; JE = ethanol extracts of D. japonica Thunb.; JW = water extracts of D. japonica Thunb.; Mito. = mitochondrial; PARP-1 = poly (ADP-ribose) polymerase-1; PE = ethanol extracts of D. purpurea; PW = water extracts of D. purpurea.
4. Discussion
DOX continues to be an effective and widely used broad-spectrum chemotherapeutic agent in the treatment of many types of cancer. However, many side effects limit its benefits [1]. Cardiotoxicity, a major side effect of DOX, can be observed in clinical patients and animal studies. DOX-induced cardiomyopathy is an important public health concern because this may limit its therapeutic use for clinical patients [3]. The search for cardioprotective agents will continue to rely on increasing our understanding of the mechanisms of DOX-induced cardiotoxicity and how to counteract and overcome it. The present study examined the possible protective effects of different species of yam currently available and commonly consumed in Taiwan on the cardiac function in a mouse model of DOX-induced cardiotoxicity. Our data indicated that water or ethanol extracts of three varieties of yams (D. japonica Thunb., D. alata, and D. purpurea), including JW, JE, AW, AE, PW, and PE, cotreated with DOX for 4 weeks improved cardiac physical and functional parameters during the DOX-induced cardiotoxicity, as demonstrated by improvements in the body weight, heart weight, and ratio of heart weight to body weight, as well as in cardiac function, as indicated by increased heart rates, lower blood pressure, and lower serum LDH levels (Table 1). The preservation of heart function was associated with a decrease in the level of oxidative stress (Table 2) and inflammation status in cardiomyocytes (Figure 2), as well as a significant reduction in apoptotic response (Figure 3).
To date, a lot of strategies using pharmaceutical agents to reduce DOX cardiotoxicity have been tested in many experimental models and clinical studies. Use of antioxidants is one promising pharmaceutical approach to prevent DOX cardiotoxicity [10,30]. DOX generates ROS, which have been suggested to play an important role in the development of cardiotoxicity. Myocardial tissue is more susceptible to ROS damage due to a fewer number of antioxidant enzymes present in the heart [31]. Production of ROS damages the myocardium and results in an increase in cell membrane permeability leading to leakage of LDH, as reported earlier [32]. Therefore, free radical scavengers have been proposed to protect cardiac tissues from oxidative stress and relieve cardiotoxicity. In this study, generation of lipid peroxidation is confirmed by elevated TBARS in the DOX treatment group. The inhibitory effects of yam extracts were observed on the levels of not only TBARS but also ROS (Table 2), with a concomitant decrease in the serum level of LDH (Table 1), suggesting that yam extracts play important roles in protection against oxidative stress. Moreover, yam extracts restored the GSH level, and GPx and SOD activities in the heart tissue (Table 2), supporting the antioxidant effect of yam extracts against DOX-induced oxidative damage. Previous studies have demonstrated that yam possesses antioxidant property in various oxidative conditions [14,16,19]. The antioxidant supplements have been suggested to patients with cancer to enhance the benefits of treatment [33]. Consistent with previous reports, this study confirmed that dietary supplements of yam, especially in three ethanol extracts, may provide a helpful effect on the heart against DOX. Compared with the same ethanol extraction of yam, AE was shown to be most potent on elevating the GSH level, and GPx and SOD activities.
A previous study has demonstrated oxidative stress-induced NF-κB activation in neonatal rat cardiomyocytes, which leads to cardiomyocyte loss [34]. The cardioprotective activity of yam extracts was further supported by the decreasing extent of inflammation status. Our present study showed that yam extracts declined the mRNA and protein levels of NF-κB (Figures 2B and 2C) and cardiac levels of proinflammation cytokines, including TNF-α and IL-6 (Figure 2A). Although all ethanol extracts of yam improved the cardiac inflammation status, JE and PE exerted superior effects to AE.
Several studies have shown that the DOX-induced apoptosis plays an important role in the cardiotoxicity caused by ROS production or oxidative stress [5,27]. Caspase-3 activity can be influenced by DOX [12]. As shown in Figure 3, the results confirmed that DOX significantly increased caspase-3, caspase-9, caspase-8, and PARP-1 activation, as well as mitochondrial dysfunction. In contrast, yam extract cotreatments dramatically reduced the DOX-dependent the expressions of cleavage caspases and PARP-1 (Figure 3A), and the cytosolic release of cytochrome c (Figure 3B). However, the detailed mechanism(s) of the inhibitory effect of yam extracts on caspase activation is not well understood. The data further indicated that AE could have a better antiapoptotic effect against DOX than other yam extracts. Similar results were found for DOX-induced cardiotoxic parameters, such as LDH, as shown in Table 1. By contrast, the anti-inflammatory capacity of ethanol extracts of yam directly correlated with their antioxidant activity (PE ≥ JE > AE). As a whole, these results revealed that the ethanol extracts of yam possess higher cardioprotective activities than the water extracts in each tested, including cardiac physical and functional parameters, measurement of lipid peroxidation, ROS and antioxidant enzymes, inflammatory cytokines analysis, real-time PCR, Western blotting, and cytochrome c assay. Looking back on our and other previous studies, Qin et al [35] reported that ethanol extracts of yam contains 28.34% diosgenin. Diosgenin is known to significantly ameliorate the cardiac damage induced by DOX [18]. According to these findings, diosgenin may be a candidate mediator, which is responsible for the antioxidant, anti-inflammatory, or antiapoptotic activities in the ethanol extracts of yam. However, further work is needed to clarify this issue.
In conclusion, these results demonstrate that yam extracts were able to reduce the DOX-induced cardiotoxicity in multiple ways. Our results suggest that yam can be a promising agent to prevent DOX-induced cardiotoxicity via its antioxidant, anti-inflammatory, and antiapoptotic effects.
Acknowledgments
This study was supported by the grant from the National Science Council (NSC95-2313-B-040-007), Taiwan.
Funding Statement
This study was supported by the grant from the National Science Council (NSC95-2313-B-040-007), Taiwan.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 2. Jain D. Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol. 2000;7:53–62. doi: 10.1067/mnc.2000.103324. [DOI] [PubMed] [Google Scholar]
- 3. Fu LX, Waagstein F, Hjalmarson A. A new insight into adriamycin-induced cardiotoxicity. Int J Cardiol. 1990;29:15–20. doi: 10.1016/0167-5273(90)90267-9. [DOI] [PubMed] [Google Scholar]
- 4. Singal PK, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J. 1997;11:931–6. doi: 10.1096/fasebj.11.12.9337145. [DOI] [PubMed] [Google Scholar]
- 5. Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem. 2002;234–235:119–24. [PubMed] [Google Scholar]
- 6. Tong J, Ganguly PK, Singal PK. Myocardial adrenergic changes at two stages of heart failure due to adriamycin treatment in rats. Am J Physiol. 1991;260:H909–16. doi: 10.1152/ajpheart.1991.260.3.H909. [DOI] [PubMed] [Google Scholar]
- 7. Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980;65:128–35. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X, Chen Z, Chua CC, Ma YS, Youngberg GA, Hamdy R, Chua BH. Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2002;283:H254–63. doi: 10.1152/ajpheart.01023.2001. [DOI] [PubMed] [Google Scholar]
- 9. Hadi N, Yousif NG, Al-amran FG, Huntei NK, Mohammad BI, Ali SJ. Vitamin E and telmisartan attenuates doxorubicin induced cardiac injury in rat through down regulation of inflammatory response. BMC Cardiovasc Disord. 2012;12:63. doi: 10.1186/1471-2261-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin MC, Yin MC. Preventive effects of ellagic acid against doxorubicin-induced cardiotoxicity in mice. Cardiovasc Toxicol. 2013;13:185–93. doi: 10.1007/s12012-013-9197-z. [DOI] [PubMed] [Google Scholar]
- 11. Nitobe J, Yamaguchi S, Okuyama M, Nozaki N, Sata M, Miyamoto T, Takeishi Y, Kubota I, Tomoike H. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas mediated apoptosis in cardiac myocytes. Cardiovasc Res. 2003;57:119–28. doi: 10.1016/s0008-6363(02)00646-6. [DOI] [PubMed] [Google Scholar]
- 12. Krstić J, Trivanović D, Mojsilović S, Santibanez JF. Transforming growth factor-beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev. 2015;2015:654594. doi: 10.1155/2015/654594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ueno M, Kakinuma Y, Yuhki K, Murakoshi N, Iemitsu M, Miyauchi T, Yamaguchi I. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci. 2006;101:151–8. doi: 10.1254/jphs.fp0050980. [DOI] [PubMed] [Google Scholar]
- 14. Liu SY, Chang TW, Lin YK, Chen SF, Wang JY, Zu GL, Wang SC. Studies on the varietal characters, production potential, phytochemical properties, and antioxidant effect of Dioscorea spp. J Agric Res China. 1999;48:1–22. [Google Scholar]
- 15. Hsu CC, Huang YC, Yin MC, Lin SJ. Effect of yam (Dioscorea alata vs. Dioscorea japonica) on gastrointestinal function and antioxidant activity in mice. J Food Sci. 2006;71:S513–6. [Google Scholar]
- 16. Chiu CS, Deng JS, Chang HY, Chen YC, Lee MM, Hou WC, Lee CY, Huang SS, Huang GJ. Antioxidant and anti-inflammatory properties of Taiwanese yam (Dioscorea japonica Thunb. var. pseudojaponica (Hayata) Yamam.) and its reference compounds. Food Chem. 2013;141:1087–96. doi: 10.1016/j.foodchem.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 17. Jayachandran KS, Vasanthi HR, Rajamanickam GV. Antilipoperoxidative and membrane stabilizing effect of diosgenin, in experimentally induced myocardial infarction. Mol Cell Biochem. 2009;327:203–10. doi: 10.1007/s11010-009-0058-9. [DOI] [PubMed] [Google Scholar]
- 18. Chen CT, Wang ZH, Hsu CC, Lin HH, Chen JH. In vivo protective effects of diosgenin against doxorubicin-induced cardiotoxicity. Nutrients. 2015;7:4938–54. doi: 10.3390/nu7064938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farombi EO, Britton G, Emerole GO. Evaluation of the antioxidant activity and partial characterization of extracts from browned yam flour diet. Food Res Int. 2000;33:493–9. [Google Scholar]
- 20. Chen H, Wang C, Chang CT, Wang T. Effects of Taiwanese yam (Dioscorea japonica Thunb var. pseudojaponica Yamamoto) on upper gut function and lipid metabolism in Balb/c mice. Nutrition. 2003;19:646–51. doi: 10.1016/s0899-9007(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 21. Wu WH, Liu LY, Chung CJ, Jou HJ, Wang TA. Estrogenic effect of yam ingestion in healthy postmenopausal women. J Am Coll Nutr. 2005;24:235–43. doi: 10.1080/07315724.2005.10719470. [DOI] [PubMed] [Google Scholar]
- 22. Vasanthi HR, Mukherjee S, Ray D, Pandian Jayachandran KS, Lekli I, Das DK. Protective role of air potato (Dioscorea bulbifera) of yam family in myocardial ischemic reperfusion injury. Food Funct. 2010;1:278–83. doi: 10.1039/c0fo00048e. [DOI] [PubMed] [Google Scholar]
- 23. Patil L, Balaramanb R. Effect of green tea extract on doxorubicin induced cardiovascular abnormalities: antioxidant action. Iran J Pharm Res. 2011;10:89–96. [PMC free article] [PubMed] [Google Scholar]
- 24. Amsen D, de Visser KE, Town T. Approaches to determine expression of inflammatory cytokines. Methods Mol Biol. 2009;511:107–42. doi: 10.1007/978-1-59745-447-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Remya S, Chikku AM, Renjith RS, Arunima S, Rajamohan T. Coconut kernel protein in diet protects the heart by beneficially modulating endothelial nitric oxide synthase, tumor necrosis factor-alpha, and nuclear factor-kappaB expressions in experimental myocardial infarction. J Food Drug Anal. 2013;21:325–31. [Google Scholar]
- 26. Wang ZH, Hsu CC, Lin HH, Chen JH. Antidiabetic effects of Carassius auratus complex formula in high fat diet combined streptozotocin-induced diabetic mice. Evid Based Complement Alternat Med. 2014;2014:628473. doi: 10.1155/2014/628473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, Liu M, Chen G, Xiao X. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res. 2011;90:538–45. doi: 10.1093/cvr/cvr022. [DOI] [PubMed] [Google Scholar]
- 28. Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F, Reed JC, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Green PS, Leeuwenburgh C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta. 2002;1588:94–101. doi: 10.1016/s0925-4439(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 30. Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti A, 3rd, von Hoff D, Schuchter LM. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127–45. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 31. Khan M, Shobha JC, Mohan IK, Naidu MU, Sundaram C, Singh S, Kuppusamy P, Kutala VK. Protective effect of Spirulina against doxorubicin-induced cardiotoxicity. Phytother Res. 2005;19:1030–7. doi: 10.1002/ptr.1783. [DOI] [PubMed] [Google Scholar]
- 32. Viswanatha Swamy AH, Wangikar U, Koti BC, Thippeswamy AH, Ronad PM, Manjula DV. Cardioprotective effect of ascorbic acid on doxorubicin-induced myocardial toxicity in rats. Indian J Pharmacol. 2011;43:507–11. doi: 10.4103/0253-7613.84952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, Kelly KM. Antioxidants and cancer therapy: a systematic review. J Clin Oncol. 2004;22:517–28. doi: 10.1200/JCO.2004.03.086. [DOI] [PubMed] [Google Scholar]
- 34. Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–40. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qin Y, Wu X, Huang W, Gong G, Li D, He Y, Zhao Y. Acute toxicity and sub-chronic toxicity of steroidal saponins from Dioscorea zingiberensis C.H.Wright in rodents. J Ethnopharmacol. 2009;126:543–50. doi: 10.1016/j.jep.2009.08.047. [DOI] [PubMed] [Google Scholar]