Abstract
The purpose of this study was to investigate the effects of Gelidium amansii (GA) hot-water extracts (GHE) on lipid metabolism in hamsters. Six-week-old male Syrian hamsters were used as the experimental animals. Hamsters were divided into four groups: (1) control diet group (CON); (2) high-fat diet group (HF); (3) HF with GHE diet group (HF + GHE); (4) HF with probucol diet group (HF + PO). All groups were fed the experimental diets and drinking water ad libitum for 6 weeks. The results showed that GHE significantly decreased body weight, liver weight, and adipose tissue (perirenal and paraepididymal) weight. The HF diet induced an increase in plasma triacylglycerol (TG), total cholesterol (TC), low-density lipoprotein cholesterol and very-low-density lipoprotein cholesterol levels. However, GHE supplementation reversed the increase of plasma lipids caused by the HF diet. In addition, GHE increased fecal cholesterol, TG and bile acid excretion. Lower hepatic TC and TG levels were found with GHE treatment. GHE reduced hepatic sterol regulatory element-binding proteins (SREBP) including SREBP 1 and SREBP 2 protein expressions. The phosphorylation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) protein expression in hamsters was decreased by the HF diet; however, GHE supplementation increased the phosphorylation of AMPK protein expression. Our results suggest that GHE may ameliorate lipid metabolism in hamsters fed a HF diet.
Keywords: AMP-activated protein kinase, Gelidium amansii hot-water extract, hamsters, lipid metabolism, sterol regulatory element-binding, proteins
1. Introduction
A diet high in fat may cause hyperlipidemia, which is characterized by elevated plasma levels of total cholesterol (TC), triacylglycerol (TG), and low-density lipoprotein cholesterol (LDL-C) [1,2]. All of these symptoms are known to be major risk factors for developing cardiovascular disease [3] and nonalcoholic fatty liver disease [4,5].
Scientists recently demonstrated that algae can play some beneficial effects on the improvement of hyperlipidemia, nonalcoholic fatty liver disease, and obesity. Chlorella pyrenoidosa, a type of green algae, has been shown to lower serum TC, TG, and LDL-C in rats and hamsters [6]. In addition, Ecklonia cava, an edible brown algae, is effective in reducing adipose tissue, plasma TC concentration, and liver TG content in mice [7]. Moreover, brown seaweed extracts can decrease serum TG levels and liver fat contents in obese nondiabetic women [8]. These results indicate that algae may play a critical role in regulating lipid metabolism in animals and humans.
Gelidium amansii (GA) is an edible red algae that is widely distributed in Japan, Korea, China, and northeast Taiwan. Agar jelly made from hot-water extracts of GA is a traditional desert in Taiwan and Japan. Our previous study demonstrated that supplementation with GA powder in a high-fat (HF) and high-cholesterol diet can reduce plasma and liver lipids in diabetic rats [9]. It was suggested that the abundance of water-soluble fiber in GA was an active component in reducing lipid accumulation in liver and adipose tissue. Other studies have shown that ethanol extracts of GA can inhibit lipid accumulation in 3T3-L1 cells [10], and decrease body weight, epididymal fat weight, and serum TC and TG levels in obese mice [11]. These results indicate that GA, including both water-soluble components (e.g., water-soluble polysaccharides) and water-insoluble components (e.g., flavonoids), may have beneficial effects on the improvement of lipid metabolism. However, the active component and mechanism of action of the lipid-lowering effect of GA is still unclear.
In Taiwan, GA is traditionally processed by exposure to sunlight followed by extraction with hot water. However, this process may result in a loss of phytochemicals and, therefore, the water-soluble fiber in GA is suggested to play a role in regulating lipid metabolism [9]. Cholesterol and bile acid metabolism of hamsters have been reported to closely resemble that of humans, and thus are considered as a suitable animal model for studying lipid metabolism [12,13]. In the present study we further investigated the effect of the hot-water extract of GA on lipid metabolism in hamsters fed a HF diet, and evaluated its possible mechanism of action.
2. Materials and methods
2.1. GA hot-water extract
GA (Lamouroux) (dry material) was purchased in the market at Keelung, the northeast corner of Taiwan. It was stored at 4°C until used. A 20-g quantity of GA was added to 400 mL of deionized water and autoclaved at 121°C for 20 min. After cooling, the GA hot-water extract (GHE) was filtered through filter paper No. 1 (Advantec. Toyo Roshi Kaisha, Ltd., Tokyo, Japan) and then lyophilized. The harvest weight of hot-water extracts obtained from 20 g GA was 5.71 g (recovery about 28.5%). General compositions of GHE were determined using the methods of the Association of Official Agricultural Chemists (AOAC) [14], including moisture, 6.5%; ash, 4.6%; crude fat, 0.25%; and crude protein, 6.7%. In addition, according to analysis by the Food Industry Research and Development Institute, Hsinchu, Taiwan, GHE contains 68.6% water-soluble dietary fiber and 0% water-insoluble dietary fiber [14]. Moreover, GHE contains 4.1% sulfate (in dry weight). Sulfate content in GHE was determined by the rhodizonate method using sodium sulfate as standard [15].
2.2. Animals and treatments
Six-week-old male Syrian hamsters were purchased from The National Laboratory Animal Center (Taipei, Taiwan). Hamsters were housed in individual stainless steel cages in a room kept at 23°C ± 1°C and 40% to 60% relative humidity, with a 12-h light–dark cycle. Hamsters were fed a standard laboratory diet (5001 rodent diet, Lab Diet, PMI Nutrition International Inc., Brenwood, MO, USA) for 1 week and were then divided into four groups of eight hamsters each. The four groups were as follows: (1) normal diet group [control (CON)]; (2) HF diet group (HF group); (3) HF diet with 1.5% GHE group (HF + GHE group); and (4) HF diet with 1% probucol (Sigma St. Louis, MO, USA) group (HF + PO group). The daily dose of probucol in the HF + PO group was approximately equivalent to 640 mg/kg body weight. Probucol is an antihyperlipidemic drug developed in the treatment of coronary artery disease, which can decrease blood and liver cholesterol in animals and humans [16–18]. The composition of the normal control diet was 5001 rodent diet. Our previous study showed that hamsters fed a diet containing 3% Ching-Shan oil and 0.2% cholesterol can have increased plasma and liver lipid levels [19]. In the present study, the HF diet contained 94.9% (w/w) normal diet, 5% (w/ w) Ching-Shan oil, and 0.1% (w/w) cholesterol. The composition of the four diets is shown in Table 1.
Table 1.
Composition of the experimental diet (%).
| Ingredient (%) | CON | HF | HF + GHE | HF + PO |
|---|---|---|---|---|
| Chow diet | 100 | 94.9 | 93.4 | 94.9 |
| Ching-Shan oila | 0 | 5 | 5 | 5 |
| Cholesterol | 0 | 0.1 | 0.1 | 0.1 |
| Gelidium hot extract | 0 | 0 | 1.5 | 0 |
| Total | 100 | 100 | 100 | 100 |
| Probucol | — | — | — | 1 |
| Total energy Kcal/100 g | 336.0 | 363.9 | 362.1 | 363.9 |
CON, normal diet group; HF, high-fat diet group; HF + GHE, Gelidium amansii hot-water extract diet group; HF + PO, probucol diet group.
Ching-Shan oil: a mixture oil of palm oil, lard, and canola oil. In addition, Ching-Shan oil contains saturated fat (32.7%) and cholesterol (162 mg/100 g). The fatty acid composition of Ching-Shan oil is: 14:0 (1.9%); 16:0 (29.3%); 16:1 (2.8%); 18:0 (8.3%); 18:1 (42.5%); 18:2 (14.8%); 18:3 (0.4%).
The hamsters were fed the experimental diets for 6 weeks. Food and drinking water were available ad libitum. Body weight was measured every week and feces were collected during the final 3 days of the experiment. The feces samples were then dried and weighed. This study was approved by the Animal House Management Committee of the National Taiwan Ocean University. The animals were maintained in accordance with the guidelines for the care and use of laboratory animals as issued by the Animal Center of the National Science Council.
2.3. Collection of blood and tissue samples
At the end of the experiment, hamsters were fasted overnight and then sacrificed by exsanguination via the abdominal aorta while under CO2 anesthesia. Heparin was used as the anti-coagulant. Plasma was collected by centrifugation at 1750g for 20 min (4°C) from whole blood. Liver and adipose tissues (perirenal and paraepididymal) were excised from each hamster and weighed. All tissue samples were immediately frozen and were stored at −80°C until further analysis.
2.4. Determinations of plasma lipids, aspartate aminotransferase, and alanine aminotransferase
Plasma TC and TG concentrations were determined by use of a cholesterol enzymatic kit (Audit Diagnostics, Cork, Ireland) and TG enzymatic kit (Audit Diagnostics), respectively. The concentration of lipoprotein in plasma was determined by ultracentrifugation (194,000g for 3 h at 10°C) [20]. The cholesterol levels of the lipoprotein fraction were measured by use of a cholesterol enzymatic kit (Audit Diagnostics). Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were determined using an enzymatic kit (Randox Ltd., Antrim, UK) and ALT enzymatic kit (Randox Ltd.), respectively.
2.5. Determinations of liver lipids, fecal lipids, and bile acid concentrations
Liver and fecal lipids were extracted with a chloroform/ methanol solution (v/v, 2:1) according to the method of Folch et al [21]. TG and TC were determined using the method of Carlson and Goldfarb [22]. Fecal bile acids were extracted using the method of Cheng and Lai [23], and quantified enzymatically according to the procedures provided in the assay kit (Randox Ltd.)
2.6. Liver fatty acid synthase and acetyl-CoA carboxylase enzymes activity
Preparation of liver enzymes was according to the method of Nepokroeff et al [24]. Fatty acid synthase (FAS) activity was determined using a modified version of Goodridge’s method [25]. The reaction mixture contained 2M potassium phosphate buffer, pH 7.1, 20mM dithiothreitol, 0.25mM acetyl-CoA, 60mM EDTA, 0.39mM malonyl-CoA, and 6mM NADPH. FAS activity was determined at 37°C by following the decrease of NADPH at 340 nm. Acetyl-CoA carboxylase (ACC) activity was determined using a modified version of the method of Mohan and Kekwick [26]. The liver enzyme incubation mixture consisted of 50mM Tris–HCl buffer, pH 7.4, 3mM magnesium chloride, 10mM potassium citrate, 3.75mM glutathione, 12.5mM potassium bicarbonate, 0.675mM bovine serum albumin, 0.125mM acetyl-CoA, 3.75mM ATP, and 10mM NADPH. ACC activity was determined at 37°C by following the decrease of NADPH at 340 nm.
2.7. Western blot analysis
Liver proteins were extracted using radioimmunoprecipitation buffer (Cell Signaling Technology, Beverly, MA, USA). Total protein containing 40 μg was separated on 10% sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Bio-Rad, CA, USA). After blocking, the primary antibodies employed were: β-actin (Biovision, Mountain View, USA), adenosine monophosphate (AMP)-activated protein kinase α [(AMPK) Cell Signaling Technology], phosphor-AMPK (Thr172) [(p-AMPK) Cell Signaling Technology], sterol regulatory element binding protein 1 [(SREBP1) Novus Biologicals, Littleton, CO, USA] and sterol regulatory element binding protein 2 [(SREBP2) Proteintech, Manchester, UK]. The membranes were incubated overnight at 4°C with primary antibodies, followed by incubation with horseradish peroxidase-linked secondary antibodies for 1 h at room temperature. The blots were incubated with appropriate horseradish peroxidase-linked secondary antibodies and then detected by enhanced chemiluminescence (Western Lightning ECL Pro; PerkinElmer, Waltham, MA, USA). Signal intensities were quantitated using the Quantity One Software (BioRad, Hercules, CA, USA).
2.8. Statistical analysis
Results are given as mean ± standard deviation values. The significant difference from the respective controls for each experimental test condition was assessed by one-way analysis of variance and two-tailed Student t test. The statistical software SPSS for Windows version 12.0 (SPSS, Chicago, IL, USA) was used.
3. Results
3.1. Effects of GHE on body and tissue weight in HF diet fed hamster
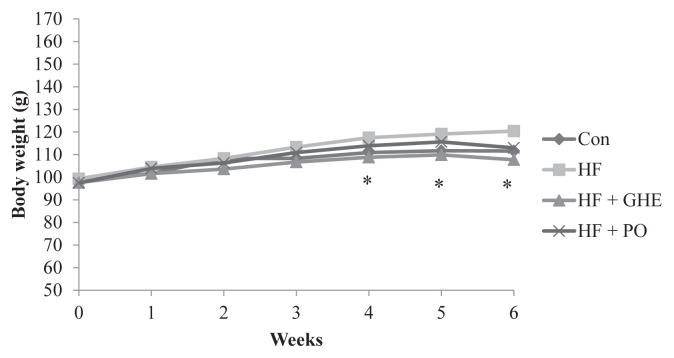
Lower body weight was found in hamsters fed a diet with GHE supplementation when compared with the HF group (p < 0.05) (Figure 1). The hamsters fed a HF diet for 6 weeks had increased liver weight (p < 0.05) (Table 2). However, GHE supplementation reversed the increase of adipose tissue weight and liver weights caused by HF diet feeding. GHE had no effect on food intake in hamsters fed with a HF diet (Table 2) (p > 0.05).
Figure 1.
The change of body weight in Syrian hamsters fed a different diet for 6 weeks. Results are expressed as mean for eight Syrian hamsters. * Significant difference from the HF group at p < 0.05. CON, control; HF, high fat; HF + GHE: high fat + Gelidium amansii hot-water extract; HF + PO: high fat + probucol.
Table 2.
The change of body weights and tissue weights in Syrian hamsters fed different diets for 6 weeks.
| CON | HF | HF + GHE | HF + PO | |
|---|---|---|---|---|
| Fasting body weight (g) | 109.2 ± 9.1 | 115.8 ± 7.2 | 103.5 ± 6.2* | 110.0 ± 7.7 |
| Food intake (g/days) | 7.1 ± 0.7 | 7.1 ± 1.0 | 6.5 ± 1.1 | 6.5 ± 0.5 |
| Liver weight (g) | 3.2 ± 0.3 | 4.1 ± 0.3** | 3.5 ± 0.2* | 3.8 ± 0.3 |
| Relative liver weight (g/100 g BW) | 2.9 ± 0.1 | 3.5 ± 0.2** | 3.4 ± 0.2 | 3.4 ± 0.2 |
| Perirenal fat (g) | 1.4 ± 0.3 | 1.6 ± 0.3 | 1.1 ± 0.2* | 1.5 ± 0.4 |
| Paraepididymal fat (g) | 1.3 ± 0.2 | 1.5 ± 0.2 | 1.1 ± 0.2* | 1.4 ± 0.4 |
Results are expressed as mean ± SD for eight Syrian hamsters.
Significant difference from the HF group at p < 0.05;
significant difference from the CON group at p < 0.05.
CON: normal; HF: high fat; HF+GHE: high fat +Gelidium amansii hot-water extracts; HF+PO: high fat +probucol.
3.2. Effects of GHE on plasma, liver, and fecal lipid metabolism in HF diet fed hamster
Hamsters fed the HF diet had elevated plasma TC, TG, low-density lipoprotein cholesterol (LDL-C), very-low-density lipoprotein cholesterol (VLDL-C), and TC/high-density-lipoprotein cholesterol (HDL-C) ratio (p < 0.05); however, GHE showed decreased plasma levels of AST, ALT, TC, TG, LDL-C, LDL-C + VLDL-C, and TC/HDL-C ratio (p < 0.05) (Table 3). In this study, animals fed the HF diet also had enhanced TC and TG contents in the liver (p < 0.05). However, GHE supplementation decreased the TC and TG content in liver (p < 0.05) (Table 4). These results indicate that GHE feeding reduced hepatic lipid accumulation in hamsters fed the HF diet. However, GHE supplementation increased fecal TC, TG, and bile acids in hamsters (p < 0.05) (Table 4).
Table 3.
Effect of different diet on plasma lipids and glucose in Syrian hamsters for 6 weeks.
| CON | HF | HF + GHE | HF + PO | |
|---|---|---|---|---|
| Triacylglycerol (mg/dL) | 56.4 ± 27.0 | 88.4 ± 9.6** | 70.6 ± 19.9* | 95.5 ± 31.6 |
| Total cholesterol (mg/dL) | 90.6 ± 8.2 | 181.3 ± 19.7** 157.6 ± 23.9* 146.9 ± 10.6* | ||
| HDL-C (mg/dL) | 62.9 ± 5.9 | 103.5 ± 8.0** | 102.4 ± 12.1 | 93.9 ± 14.5 |
| LDL-C (mg/dL) | 15.1 ± 6.7 | 54.8 ± 19.6** | 36.6 ± 12.8* | 25.5 ± 12.3* |
| VLDL-C (mg/dL) | 12.6 ± 4.6 | 23.0 ± 5.7** | 18.6 ± 5.6 | 27.5 ± 6.2 |
| LDL-C +VLDL-C (mg/dL) | 27.7 ± 4.1 | 77.8 ± 17.5** | 55.2 ± 16.0* | 53.0 ± 11.5* |
| TC/HDL-C ratio | 1.4 ± 0.1 | 1.8 ± 0.2** | 1.5 ± 0.1* | 1.6 ± 0.2 |
| AST (U/L) | 12.4 ± 6.1 | 20.2 ± 7.8** | 12.9 ± 3.2* | 11.8 ± 2.1* |
| ALT (U/L) | 15.1 ± 3.0 | 21.1 ± 7.2** | 15.0 ± 3.1* | 14.4 ± 3.7* |
Results are expressed as mean ± SD for eight Syrian hamsters.
Significant difference from the HF group at p < 0.05;
significant difference from the CON group at p < 0.05.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CON, control; HDL-C, high-density-lipoprotein cholesterol; HF, high fat; HF + GHE, high fat + Gelidium amansii hot-water extracts; HF + PO, high fat + probucol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; VLDL-C, very-low-density lipoprotein cholesterol.
Table 4.
Effect of different diets on liver and fecal lipid concentrations in Syrian hamsters for 6 weeks.
| CON | HF | HF + GHE | HF + PO | |
|---|---|---|---|---|
| Liver | ||||
| Total cholesterol (mg/Liver) | 20.5 ± 6.0 | 159.3 ± 27.4** | 122.0 ± 24.1* | 104.4 ± 19.5* |
| Triacylglycerol (mg/Liver) | 23.7 ± 7.0 | 51.0 ± 7.0** | 40.3 ± 5.5* | 40.9 ± 4.3* |
| Feces | ||||
| Total cholesterol (mg/day) | 4.3 ± 1.8 | 5.1 ± 1.5 | 7.5 ± 2.4* | 8.1 ± 2.2* |
| Triacylglycerol (mg/day) | 7.1 ± 2.9 | 6.7 ± 2.4 | 9.0 ± 1.5* | 7.5 ± 2.5 |
| Bile acid (μmol/ day) | 2.6 ± 0.7 | 2.7 ± 0.7 | 4.0 ± 0.7* | 4.6 ± 0.6* |
Results are expressed as mean ± SD for eight Syrian hamsters.
Significant difference from the HF group at p < 0.05;
significant difference from the CON group at p < 0.05.
CON, control; HF, high fat; HF + GHE, high fat + Gelidium amansii hot-water extracts; HF + PO, high fat + probucol.
3.3. Effects of GHE on lipogenic enzyme activities in liver
Hamsters fed the HF diet showed increased liver FAS and ACC activities (Figures 2A and 2B, p < 0.05), but GHE reduced the hepatic FAS and ACC activities (p < 0.05). Lower FAS activity was observed in animals after probucol treatment (p < 0.05).
Figure 2.
Changes in liver lipid enzyme activity. (A) Fatty acid synthase; (B) acetyl-CoA carboxylase in Syrian hamsters for 6 weeks. Results are expressed as mean ± SD for eight Syrian hamsters. * Significant difference from the HF group at p < 0.05; ** significant difference from the CON group at p < 0.05. CON, control; HF, high fat; HF + GHE, high fat + Gelidium amansii hot-water extract; HF + PO, high fat + probucol.
3.4. Expression of lipid metabolism-related proteins
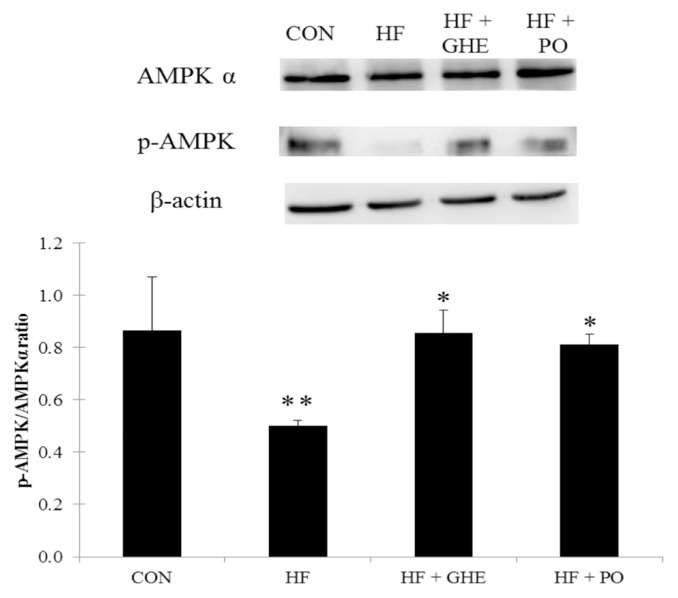
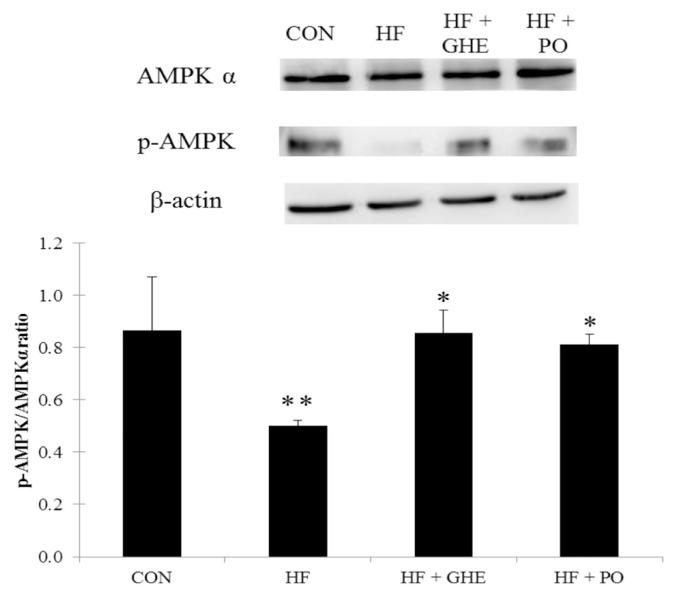
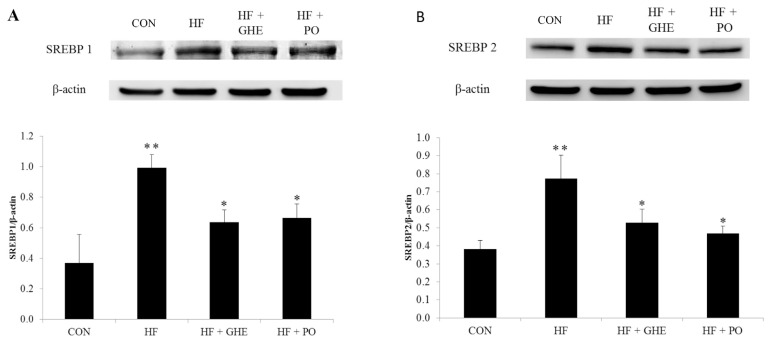
After 6 weeks of the feeding study, the HF diet significantly decreased the phosphorylation of AMPK protein expression in the liver of hamsters (Figure 3, p < 0.05). However, GHE and probucol treatments induced an increase in hepatic phosphorylation of AMPK protein expression (p < 0.05). Figure 4 shows the hepatic SREBP1 and SREBP2 protein expressions. Hamsters fed the HF diet had elevated protein expressions of SREBP1 and SREBP2 (p < 0.05). GHE or probucol supplementation reduced the SREBP1 and SREBP2 protein expressions in hamsters fed a HF diet (p < 0.05).
Figure 3.
Expression analysis of adenosine monophosphate-activated protein kinase phosphorylation levels in the liver from hamsters fed the different experimental diets for 6 weeks. Results are expressed as mean ± SD for four Syrian hamsters. * Significant difference from the HF group at p < 0.05; ** significant difference from the CON group at p < 0.05. CON, normal; HF, high fat; HF + GHE, high fat + Gelidium amansii hot-water extract; HF + PO, high fat + probucol.
Figure 4.
Expression analysis of (A) SREBP1 (B) SREBP2 levels in the liver from hamsters fed the different experimental diets for 6 weeks. Results are expressed as mean ± SD for four Syrian hamsters. * Significant difference from the HF group at p < 0.05; ** significant difference from the CON group at p < 0.05. CON, control; HF, high fat; HF + GHE, high fat + Gelidium amansii hot-water extract; HF + PO, high fat + probucol.
4. Discussion
In this study, we found that GHE reduced plasma and liver lipids in hamsters fed with the HF diet. GHE reduced not only plasma TC, TG, LDL-C, and LDL-C + VLDL-C concentrations but also lowered hepatic TC and TG levels. These results might be due to the ability of GHE to increase fecal excretions of cholesterol and bile acids.
Scientists have reported that algae possess hypolipidemic effects in animal models [6,27]. Algae are rich in dietary fiber and the total dietary fiber contents of the main edible algae ranged from 25% to 75% (on a dry weight basis). About 51% to 85% of total dietary fiber was found as water-soluble fiber [28]. In this study, GHE contained abundant water-soluble fiber (about 68.6%). It has been demonstrated that consuming water-soluble dietary fiber can reduce plasma and liver lipids [29,30]. The cholesterol-lowering effects of water-soluble fibers are believed to be a result of the interference with the enterohepatic circulation of bile acids [31]. It has been shown that animals fed a high-cholesterol diet had an increased hepatic cholesterol content, resulting in increased TG biosynthesis and reduced fatty acid oxidation [32,33]. Indeed, our data showed that increased hepatic ACC and FAS activities as a result of the HF diet supported the hypothesis of these previous studies. In this study, fecal bile acid excretion, the major degradation of endogenous cholesterol, was enhanced after GHE feeding in hamsters and this might stimulate the biosynthesis of bile acid using cholesterol as the precursor. Thus, these results suggest that the hepatic lipid-lowering effect of GHE in cholesterol-fed hamsters may be primarily related to lower absorption of bile acids, resulting in an increase in hepatic cholesterol catabolism and thus leading to a reduction in hepatic cholesterol and thereafter TG accumulation. Furthermore, due to lowering the cholesterol pool by GHE supplementation, the hepatic LDL receptor might be induced to enhance the uptake of LDL-C from the circulation, thus lowering blood cholesterol concentration [34]. Numerous studies have demonstrated that the lipid-lowering effects of dietary water-soluble fibers are primarily related to the increased fecal excretions of cholesterol and bile acids. Therefore, it is suggested that the reduction of plasma and liver lipids after GHE treatment is probably due to its abundance of dietary water-soluble fiber (68.6%), which may increase fecal lipid excretion. Moreover, the sulfated polysaccharides from green algae showed a high anti-hyperlipidemia activity in mice [35]. Ginzberg et al [36] reported that chickens fed sulfated polysaccharides from red algae had reduced blood cholesterol. Some water extracts of red algae have sulfated polysaccharides [37], which contain around 3% to 10% of sulfate in their structures [38]. Similar to the results of previous studies, our data also showed that GHE contained sulfated polysaccharides (4.1% sulfate). Therefore, we suggest that sulfated polysaccharides in GHE, at least in part, may play an important role in improving lipid metabolism in hamsters.
AMPK and SREBP played critical roles in the regulation of lipid metabolism [39]. They were considered as the important factors of hyperlipidemia [40] and liver lipid accumulation [41]. AMPK activation has been proposed as a major therapeutic target for obesity [42], fatty liver [41], and hyperlipidemia [43]. AMPK phosphorylation suppresses the activity of the key proteins involved in lipogenesis, such as SREBP 1 and SREBP 2 [44]. SREBP1 primarily regulates the lipogenic process by activating the FAS and ACC involved in TG synthesis [45], and SREBP 2 mainly controls cholesterol homeostasis by activating the genes required for cholesterol synthesis [44,46]. In the present study, hamsters fed the HF diet showed a decrease in the expression of the phosphorylation of AMPK, and an increase in both SREBP 1 and SREBP 2 protein expressions, indicating that lipogenesis is increased in liver. In this study, GHE supplementation increased hepatic AMPK phosphorylation and reduced SREBP 1 and SREBP 2 protein expressions. These alternations by GHE treatment might explain a lower lipogenesis in liver. Galisteo et al [47] reported that water-soluble fiber can increase the phosphorylation of AMPK, consequently inhibiting ACC protein expression. In addition, water-soluble fiber displayed a lower hepatic SREBP2 mRNA expression, thereby reducing the serum TC [48]. Moreover, water-soluble fiber has been shown to down-regulate SREBP1 and FAS mRNA expression [49] and thus reduced TG synthesis in liver [50]. Because GHE is rich in water-soluble fiber, it is possible that the reduction of liver lipids by GHE may be related to the activation of AMPK phosphorylation and inhibition of SREBP pathways in hamsters fed the HF diet. Studies have shown that water-soluble fiber can enhance the amount of AMPK phosphorylation in HepG2 cells [51] and mouse liver [52]. However, the reason why water-soluble fiber can activate AMPK is still unknown. Yamauchi et al [53] showed that adiponectin may be an important factor in the activation of AMPK. We have reported that feeding a dry powder of GA can increase plasma adiponectin levels in rats with diabetes [9]. Therefore, it is proposed that the increased AMPK activation by GHE may be due to the increased plasma adiponectin.
Our previous study demonstrated that GA feeding increased adipose tissue hormone-sensitive lipase activity, thereby reducing the adipose tissue weight [9]. In the present study, GHE reduced body weight, liver weight, and adipose tissue weight in hamsters fed a HF diet. The reduced body weight might be due to the decreased liver weight, adipose tissue weight, and their lipid accumulation in these tissues. Therefore, GHE might have a beneficial effect on reducing lipid accumulation in liver and adipose tissue in hamsters fed a HF diet. Again, the lipid-lowering effects of GHE may have been due to the increase in fecal lipid excretion. Similar actions were shown in other dietary fiber materials, such as GA powder [9] or chitosan [54].
In conclusion, the present study demonstrated the lipid-lowering potential of GHE in hamsters, and this effect may be possibly be a result of enhanced fecal excretions of cholesterol, TG, and bile acids. Our results also show that GHE is able to activate AMPK phosphorylation and reduce SREBP 1 and SREBP 2 protein expressions in liver, thereby decreasing the hepatic lipogenesis which may result in lipid accumulation in liver. Further studies are needed to elucidate the different Molecular Weight (MW) of water-soluble fiber or sulfated polysaccharides content in GA, underlying the lipid-lowering effects.
Footnotes
Conflicts of interest
There is no potential conflict of interest.
References
- 1. Talayero BG, Sacks FM. The role of triglyceride in atherosclerosis. Curr Cardiol Rep. 2011;13:544–52. doi: 10.1007/s11886-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durrington P, Soran H. Hyperlipidemia. In: Lammert E, Zeeb M, editors. Metabolism of Human Diseases. Vienna: Springer; 2014. pp. 295–302. [Google Scholar]
- 3. Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40:195–211. doi: 10.1016/j.pop.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ni HC, Li J, Jin Y, Zang HM, Peng L. The experimental animal model of hyperlipidemia and hyperlipidemic fatty liver in rats. Chin Pharmacol Bull. 2004;20:703–6. [Google Scholar]
- 5. Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14:6395–400. doi: 10.3748/wjg.14.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cherng JY, Shih MF. Preventing dyslipidemia by Chlorella pyrenoidosa in rats and hamsters after chronic high fat diet treatment. Life Sci. 2005;76:3001–13. doi: 10.1016/j.lfs.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 7. Park EY, Kim EH, Kim MH, Seo YW, Lee JI, Jun HS. Polyphenol-rich fraction of brown alga Ecklonia cava collected from Gijang, Korea, reduces obesity and glucose levels in high-fat diet-induced obese mice. Evid Based Complement Alternat Med. 2012;2012:1–11. doi: 10.1155/2012/418912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abidov M, Ramazanov Z, Seifulla R, Grachev S. The effects of Xanthigen™ in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes Metab. 2010;12:72–81. doi: 10.1111/j.1463-1326.2009.01132.x. [DOI] [PubMed] [Google Scholar]
- 9. Yang TH, Yao HT, Chiang MT. Red algae (Gelidium amansii) reduces adiposity via activation of lipolysis in rats with diabetes induced by streptozotocin-nicotinamide. J Food Drug Anal. 2015;23:758–65. doi: 10.1016/j.jfda.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seo MJ, Lee OH, Choi HS, Lee BY. Extract from edible red seaweed (Gelidium amansii) inhibits lipid accumulation and ros production during differentiation in 3T3-L1 cells. Prev Nutr Food Sci. 2012;17:129–35. doi: 10.3746/pnf.2012.17.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang MC, Kang N, Kim SY, Lima IS, Ko SC, Kim YT, Kim YB, Jeung HD, Choi KS, Jeon YJ. Popular edible seaweed, Gelidium amansii prevents against diet-induced obesity. Food Chem Toxicol. 2016;90:181–7. doi: 10.1016/j.fct.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Z, Wang H, Jiao R, Peng C, Wong YM, Yeung VS, Chen ZY. Choosing hamsters but not rats as a model for studying plasma cholesterol-lowering activity of functional foods. Mol Nutr Food Res. 2009;53:921–30. doi: 10.1002/mnfr.200800517. [DOI] [PubMed] [Google Scholar]
- 13. Guo F, Huang C, Liao X, Wang Y, He Y, Feng R, Li Y, Sun C. Beneficial effects of mangiferin on hyperlipidemia in high-fat-fed hamsters. Mol Nutr Food Res. 2011;55:1809–18. doi: 10.1002/mnfr.201100392. [DOI] [PubMed] [Google Scholar]
- 14.Association of Official Agricultural Chemists (AOAC) Official Methods of Analysis of AOAC International. 16th ed. Gaithersburg, MD: AOAC; 1996. [Google Scholar]
- 15. Terho TT, Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971;41:471–6. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- 16. Ferns GA, Forster L, Stewart-Lee A, Nourooz-Zadeh J, Änggård EE. Probucol inhibits mononuclear cell adhesion to vascular endothelium in the cholesterol-fed rabbit. Atherosclerosis. 1993;100:171–81. doi: 10.1016/0021-9150(93)90203-7. [DOI] [PubMed] [Google Scholar]
- 17. Dujovne CA, Harris WS, Gerrond LLC, Fan J, Muzio F. Comparison of effects of probucol versus vitamin E on ex vivo oxidation susceptibility of lipoproteins in hyperlipoproteinemia. Am J Cardiol. 1994;74:38–42. doi: 10.1016/0002-9149(94)90488-x. [DOI] [PubMed] [Google Scholar]
- 18. Lee CL, Tsai TY, Wang JJ, Pan TM. In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl Microbiol Biotechnol. 2006;70:533–40. doi: 10.1007/s00253-005-0137-0. [DOI] [PubMed] [Google Scholar]
- 19. Yao HT, Chiang MT. Effect of chitosan on plasma lipids, hepatic lipids, and fecal bile acid in hamsters. J Food Drug Anal. 2006;14:183–6. [Google Scholar]
- 20. Takehisa F, Suzuki Y. Effect of guar gum and cholestyramine on plasma lipoprotein cholesterol in rats. J Jpn Soc Nutr Food Sci. 1990;43:269–74. [Google Scholar]
- 21. Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22. Carlson SE, Goldfarb S. A sensitive enzymatic method for the determination of free and esterified tissue cholesterol. Clin Chim Acta. 1977;79:575–82. doi: 10.1016/0009-8981(77)90178-4. [DOI] [PubMed] [Google Scholar]
- 23. Cheng HH, Lai MH. Fermentation of resistant rice starch produces propionate reducing serum and hepatic cholesterol in rats. J Nutr. 2000;130:1991–5. doi: 10.1093/jn/130.8.1991. [DOI] [PubMed] [Google Scholar]
- 24. Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Methods Enzymol. 1975;35:37. doi: 10.1016/0076-6879(75)35136-7. [DOI] [PubMed] [Google Scholar]
- 25. Goodridge AG. Regulation of the activity of acetyl coenzyme A carboxylase by palmitoyl coenzyme A and citrate. J Biol Chem. 1972;247:6946–52. [PubMed] [Google Scholar]
- 26. Mohan SB, Kekwick RG. Acetyl-coenzyme A carboxylase from avocado (Persea americana) plastids and spinach (Spinacia oleracea) chloroplasts. Biochem J. 1980;187:667–76. doi: 10.1042/bj1870667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee SJ, Kim CW, Jang HJ, Cho SY, Choi JW. Anti-hyperlipidemia and anti-arteriosclerosis effects of Laminaria japonica in sprague-dawley rats. Fish Aquat Sci. 2011;14:235–41. [Google Scholar]
- 28. Jiménez-Escrig A, Sánchez-Muniz FJ. Dietary fibre from edible seaweeds: chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr Res. 2000;20:585–98. [Google Scholar]
- 29. Jenkins DJ, Kendall CW, Vuksan V, Vidgen E, Parker T, Faulkner D, Mehling CC, Garsetti M, Testolin G, Cunnane SC, Ryan MA, Corey PN. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am J Clin Nutr. 2002;75:834–9. doi: 10.1093/ajcn/75.5.834. [DOI] [PubMed] [Google Scholar]
- 30. Martinez-Flores HE, Chang YK, Martinez-Bustos F, Sgarbieri V. Effect of high fiber products on blood lipids and lipoproteins in hamsters. Nutr Res. 2004;24:85–93. [Google Scholar]
- 31. Fernandez ML. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr Opin Lipidol. 2001;12:35–40. doi: 10.1097/00041433-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 32. Fungwe TV, Cagen LM, Cook GA, Wilcox HG, Heimberg M. Dietary cholesterol stimulates hepatic biosynthesis of triglyceride and reduces oxidation of fatty acids in the rat. J Lipid Res. 1993;34:933–41. [PubMed] [Google Scholar]
- 33. Liu CH, Huang MT, Huang PC. Sources of triacylglycerol accumulation in livers of rats fed a cholesterol-supplemented diet. Lipids. 1995;30:527–31. doi: 10.1007/BF02537027. [DOI] [PubMed] [Google Scholar]
- 34. Gunness P, Gidley MJ. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010;1:149–55. doi: 10.1039/c0fo00080a. [DOI] [PubMed] [Google Scholar]
- 35. Pengzhan Y, Quanbin Z, Ning L, Zuhong X, Yanmei W, Zhi’en L. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J Appl Psychol. 2003;15:21–7. [Google Scholar]
- 36. Ginzberg A, Cohen M, Sod-Moriah UA, Shany S, Rosenshtrauch A, Arad SM. Chickens fed with biomass of the red microalga Porphyridium sp. have reduced blood cholesterol level and modified fatty acid composition in egg yolk. J Appl Psychol. 2000;12:325–30. [Google Scholar]
- 37. Stoloff L, Silva P. An attempt to determine possible taxonomic significance of the properties of water extractable polysaccharides in red algae. Econ Bot. 1957;11:327–30. [Google Scholar]
- 38.Nussinovitch A. Agar. In: Vainstein C, editor. Hydrocolloid Applications. US: Springer; 1997. pp. 1–18. [Google Scholar]
- 39. Li W, Li Y, Wang Q, Yang Y. Crude extracts from Lycium barbarum suppress SREBP-1c expression and prevent diet-induced fatty liver through AMPK activation. Biomed Res Int. 2014;2014:1–10. doi: 10.1155/2014/196198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liao CC, Ou TT, Wu CH, Wang CJ. Prevention of diet-induced hyperlipidemia and obesity by caffeic acid in C57BL/6 mice through regulation of hepatic lipogenesis gene expression. J Agric Food Chem. 2013;61:11082–8. doi: 10.1021/jf4026647. [DOI] [PubMed] [Google Scholar]
- 41. Shen L, Xiong Y, Wang DQ, Howles P, Basford JE, Wang J, Xiong YQ, Hui DY, Woods SC, Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54:1430–8. doi: 10.1194/jlr.M035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee JW, Choe SS, Jang H, Kim J, Jeong HW, Jo H, Tadi S, Park MG, Kwak TH, Kim JM, Hyum DH, Kim JB. AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J Lipid Res. 2012;53:1277–86. doi: 10.1194/jlr.M022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo P, Kai Q, Gao J, Lian ZQ, Wu CM, Wu CA, Zhu HB. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J Pharmacol Sci. 2010;113:395–403. doi: 10.1254/jphs.10041fp. [DOI] [PubMed] [Google Scholar]
- 44. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Gao B, Park O, Luo Z, Lefai E, Shyy JYJ, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–88. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vázquez-Vela MEF, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39:715–28. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 46. Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297:E28–37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Galisteo M, Morón R, Rivera L, Romero R, Anguera A, Zarzuelo A. Plantago ovata husks-supplemented diet ameliorates metabolic alterations in obese Zucker rats through activation of AMP-activated protein kinase. Comparative study with other dietary fibers. Clin Nutr. 2010;29:261–7. doi: 10.1016/j.clnu.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 48. Nakamura Y, Kanazawa M, Liyanage R, Iijima S, Han KH, Shimada KI, Sekikawa M, Yamauchi A, Hashimoto N, Ohba K, Fukushima M. Effect of white wheat bread containing sugar beet fiber on serum lipids and hepatic mRNA in rats fed on a cholesterol-free diet. Biosci Biotechnol Biochem. 2009;73:1280–5. doi: 10.1271/bbb.80787. [DOI] [PubMed] [Google Scholar]
- 49. Brockman DA, Chen X, Gallahe DD. High-viscosity dietary fibers reduce adiposity and decrease hepatic steatosis in rats fed a high-fat diet. J Nutr. 2014;144:1415–22. doi: 10.3945/jn.114.191577. [DOI] [PubMed] [Google Scholar]
- 50. Bartley GE, Yokoyama W, Young SA, Anderson WH, Hung SC, Albers DR, Langhorst ML, Kim H. Hypocholesterolemic effects of hydroxypropyl methylcellulose are mediated by altered gene expression in hepatic bile and cholesterol pathways of male hamsters. J Nutr. 2010;140:1255–60. doi: 10.3945/jn.109.118349. [DOI] [PubMed] [Google Scholar]
- 51. Yun H, Lee JH, Park CE, Kim MJ, Min BI, Bae H, Choe W, Kang I, Kim SS, Ha J. Inulin increases glucose transport in C2C12 myotubes and HepG2 cells via activation of AMP-activated protein kinase and phosphatidylinositol 3-kinase pathways. J Med Food. 2009;12:1023–8. doi: 10.1089/jmf.2009.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang ZQ, Yu Y, Zhang XH, Floyd ZE, Boudreau A, Lian K, Cefalu WT. Comparing the effects of nano-sized sugarcane fiber with cellulose and psyllium on hepatic cellular signaling in mice. Int J Nanomedicine. 2012;7:2999–3012. doi: 10.2147/IJN.S30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamauchi T, Kamon J, Minokoshi YA, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre O, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 54. Chiu CY, Chan IL, Yang TH, Liu SH, Chiang MT. Supplementation of chitosan alleviates high-fat diet-enhanced lipogenesis in rats via adenosine monophosphate (AMP)-activated protein kinase activation and inhibition of lipogenesis-associated genes. J Agric Food Chem. 2015;63:2979–88. doi: 10.1021/acs.jafc.5b00198. [DOI] [PubMed] [Google Scholar]