Abstract
Pearl powder, a well-known traditional mineral medicine, is reported to be used for well-being and to treat several diseases from centuries in Taiwan and China. We investigated the in vitro antihemolytic and antioxidant properties of pearl powder that could protect erythrocytes against 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidative damage to membrane proteins/lipids. Human erythrocytes were incubated with different concentrations of pearl powder (50–200 μg/mL) for 30 minutes and then exposed to AAPH for 2–6 hours. We found that AAPH alone time dependently increased the oxidative hemolysis of erythrocytes, while pearl powder pretreatment substantially inhibited the hemolysis in a concentration-/time-dependent manner. AAPH-induced oxidative damage to erythrocyte membrane lipids was evidenced by the elevated malondialdehyde (MDA) levels. However, pearl powder remarkably inhibited the malondialdehyde formation, and the 200 μg/mL concentration showed almost similar malondialdehyde values to the control. Furthermore, pearl powder suppressed the AAPH-induced high-molecular-weight protein formation and concomitantly increased the low-molecular-weight proteins in erythrocytes. Antioxidant potential that was measured as superoxide dismutase activity and glutathione content was significantly dropped by AAPH incubation, which suggests the vulnerability of erythrocytes to AAPH-induced oxidative stress. Noteworthy, erythrocytes pretreated with pearl powder showed restored superoxide dismutase activity and glutathione levels against AAPH-induced loss. Our findings conclude that pearl powder attenuate free radical-induced hemolysis and oxidative damage to erythrocyte membrane lipids/proteins. The potent antioxidant property of pearl powder may offer protection from free radical-related diseases.
Keywords: antioxidants, glutathione, hemolysis, pearl powder, superoxide dismutase
1. Introduction
From ancient times, pearl powder has been used as a traditional Chinese mineral medicine to treat ulcers, epilepsy, convulsions, palpitations, and insomnia. Pearl powder has also been used as a cosmetic, antiaging substance and to promote the wound healing process [1,2]. Traditionally, medicinal pearls are used in the form of powder obtained through fine smashing and pulverizing of natural pearls to make the ingredients more bioavailable. Several studies have indicated that pearls are a rich source of calcium (~90% of weight) and amino acids, followed by conchiolin proteins and a small amount of trace elements [3,4]. Pearl powder has widely been used by Taiwanese women as a health-boosting supplement during pregnancy and postpartum period [5]. Shao and colleagues [2] demonstrated the antioxidant and antiradiation properties of different kinds of pearl powder, and indicated their use in cosmetics, aging resistance, and clinical medical treatment. A recent study reported the anti-inflammatory effect of pearl extract against UVB irradiation in skin keratinocytes [6]. However, it is still unclear whether pearl powder can attenuate the oxidative hemolysis and membrane damage, and improve the antioxidant capacity in human erythrocytes.
Erythrocytes are the most common type of blood cells that deliver oxygen to body tissues via blood. Owing to their principal role, erythrocytes (red blood cells) are under continuous stress by oxygenation and deoxygenation cycles, and strong shearing forces in narrow blood vessels and endogenously generated reactive oxygen species (ROS) during their 120-day lifespan [7]. In addition, erythrocytes are extremely vulnerable to oxidative damage due to the high cellular concentration of oxygen and hemoglobin, and high content of membrane polyunsaturated fatty acid (PUFA) [8,9]. Oxidative damage to the lipid membrane of erythrocytes is implicated in hemolysis that is associated with certain hemoglobinopathies, radiation, exposure to transition metals, and dearth in the antioxidant defense system [10]. ROS-induced erythrocyte oxidative stress is characterized by increased membrane lipid peroxidation, protein oxidation, and decreased glutathione (GSH) levels. These events eventually contribute to the architectural damage of erythrocytes. Such morphological changes and oxidative damage are usually observed in erythrocyte disorders and other diseases, including Alzheimer’s disease, sickle cell anemia, renal failure, and β-thalassemia, as well as during cellular aging [7,11,12].
Malondialdehyde (MDA) is a principal byproduct of PUFA degradation induced by ROS and is widely considered a marker of lipid peroxidation. Interaction of this highly reactive and toxic aldehyde with biomolecules, such as DNA and proteins, has often been referred to as potentially mutagenesis and atherogenic [13]. MDA content has shown to cross link erythrocyte phospholipids and proteins, which then impair most of the membrane-related functions and eventually decrease erythrocyte survival (hemolysis) [14,15]. The increased erythrocyte lipid peroxidation is further involved in hemolysis and normal cell aging, and correlates with several diseases such as cancer, diabetes, and liver and cardiovascular diseases [13,16]. ROS-induced detrimental effects on erythrocyte membrane are manifested by the decreased cytoskeletal protein content (low molecular weight, LMW) and formation of high-molecular-weight (HMW) proteins, which lead to abnormalities in erythrocyte morphology and disturbances in microcirculation [15,17]. Thus, inhibition of oxidative damage by supplementation of antioxidants can be a valuable strategy to protect erythrocytes and reduce the risk of ROS-associated diseases.
The cellular antioxidant system is a sophisticated defensive mechanism to protect cells and tissues from oxidative damage caused by highly reactive free radicals or ROS. Among several antioxidant enzymes, superoxide dismutase (SOD) acts as a frontline defense against free radical toxicity and scavenges toxic superoxide radicals into less toxic hydrogen peroxide. GSH is a thiol-based antioxidant that plays a pivotal role in preventing membrane lipid peroxidation and maintaining cellular redox homeostasis [18–21]. Literature revealed that antioxidant treatment not only attenuates ROS-mediated oxidative insults, but also improves cellular anti-oxidant capacity to cope with oxidative damages [15,22,23]. Therefore, we hypothesized that treatment of pearl powder may protect human erythrocytes from ROS-induced oxidative stress. This study aimed to evaluate the protective effects of pearl powder on 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced hemolysis, lipid peroxidation, and architectural damage of erythrocyte membrane proteins. In addition, GSH levels and SOD activity were also measured in human erythrocytes to strengthen our findings.
2. Materials and methods
2.1. Chemicals
Reagents and chemical used in this study, including bovine serum albumin, 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), EDTA, 2-thiobarbituric acid (TBA), 1,1,3,3-tetramethoxypropane, sodium citrate, sodium chloride (NaCl), and sodium phosphate dibasic (Na2HSO4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). AAPH, phosphoric acid (H3PO4), and trichloro-acetic acid were purchased from Wako Pure Chemical Industries Ltd (Osaka, Japan). Coomassie Brilliant Blue and sodium dodecyl sulfate (SDS) were obtained from Bio-Rad Co. (Hercules, CA, USA). All other chemicals were of the highest grade commercially available and were supplied either by Merck (Darmstadt, Germany) or by Sigma-Aldrich.
2.2. Sample preparation
Pearl powder (35 nm) was kindly provided by a local commercial company. The pearl solution was prepared by dissolving pearl powder (25 mg) in 1 mL of 0.5N HCl. Then the pH of the pearl solution was adjusted to 7.2. The calcium content in pearl powder was about 34.5–35.0%, as reported in the chemical analysis data provided by the company.
2.3. Preparation of erythrocyte suspensions
Blood samples (about 10 mL each) were obtained by venipuncture from healthy volunteers, who were aware of the study design, and informed consent was obtained from the volunteers. Erythrocytes from the citrated blood samples were isolated by centrifugation at 3000g for 10 minutes. Then the samples were washed four times with phosphate buffer saline (PBS) and resuspended to the desired hematocrit level in the same buffer. The cells were stored at 4°C and used within 6 hours of sample preparation. To induce free radical chain oxidation in erythrocytes, aqueous peroxyl radicals were generated by thermal decomposition of AAPH (an azo compound) in oxygen [24].
2.4. Hemolysis assay
Erythrocyte suspension (5% hematocrit) was incubated with PBS (control) and preincubated with pearl powder (50–200 μg/ mL) for 30 minutes, followed by incubation with or without 25mM AAPH in PBS at pH 7.4 for 2 hours, 4 hours, or 6 hours. The reaction mixture was shaken gently during incubation for a fixed interval at 37°C. A 200 μL aliquot of the reaction mixture was removed and centrifuged at 3000g for 2 minutes. The absorbance of the supernatant was determined at 540 nm. Reference values were determined using the same volume of erythrocytes in a hypotonic buffer (5mM phosphate buffer at pH 7.4; 100% hemolysis). The hemolysis percentage was calculated using the following formula: absorbance of sample supernatant/reference value × 100.
2.5. Determination of lipid peroxidation in erythrocytes
Lipid peroxidation in erythrocytes was measured indirectly through estimation of the TBA reaction. Hundred microliters of H3PO4 (0.44M) and 250 μL (0.67%) TBA were added to 1 mL reaction mixture, and incubated at 95°C for 1 hour. Then these contents were cooled in an ice bath for 10 minutes before 150 μL trichloroacetic acid (20%) was added. After centrifugation at 13,000 rpm for 10 minutes, peroxide content of the supernatant obtained was assayed using a TBA reaction with the molar extinction coefficient (O.D532) of MDA. MDA values in the samples were expressed as p mole/g Hb. Tetraethoxypropane was used as standard. An aliquot of lysate was also used for determination of hemoglobin content using colorimetry. Briefly, 8 μL of lysate was added into a final volume of 2 mL in Drabkin’s solution (Randox Laboratories, San Diego, CA, USA), and the absorbance of samples was read against a reagent blank at 540 nm. Hemoglobin was expressed as g/dL.
2.6. Preparation of erythrocyte ghosts and analysis of membrane proteins using SDS polyacrylamide gel electrophoresis
Erythrocyte ghosts were prepared from the reaction mixture using hypotonic lysis buffer in 30 volumes of 5mM NaH2PO4 (pH 7.4). Hemolysate preparations were washed six times with the lysis buffer and centrifuged at 12,000g for 30 minutes. Next, erythrocyte ghosts were treated with or without desired concentrations of pearl powder (50–200 μg/mL) for 4 hours or 6 hours. Protein concentrations of the erythrocyteghost pellets were determined using bovine serum albumin as the standard. Erythrocyteghost pellets were dissolved to a concentration of 2 mg protein/mL (12.5 μL) in SDS sample buffer and brought to a total volume of 17 μL. The ghost was incubated at 95°C for 5 minutes and kept on ice for 5 minutes, then centrifuged at 3000 rpm for 3 minutes. SDS polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a 1.5-mm-thick slab gel using 3% and 8% gels for condensation and separation, and then stained with Coomassie Brilliant Blue [25]. Molecular weight determination of the gel system was calibrated by measuring migration of the standard proteins. Densitometry analyses were performed using commercially available quantitative software (AlphaEase; Genetic Technology Inc. Miami, FL, USA).
2.7. Determination of erythrocyte GSH levels
The reduced form of intracellular GSH levels was determined by DTNB titration as described previously [26]. After centrifugation of the reaction mixture (2 mL), 0.6 mL distilled water was added to the erythrocyte pellets to lyse the cells. Then, 0.6 mL of cell lysate was precipitated by the addition of 0.6 mL metaphosphoric acid solution (1.67 g metaphosphoric acid, 0.2 g EDTA, and 30 g NaCl in 100 mL water). After 5 minutes, the protein precipitate was isolated from the remaining solution by centrifugation at 18,000g for 10 minutes. Then 0.45 mL of solution was mixed with 0.45 mL of 300mM Na2HPO4, and absorbance was read at 412 nm against a blank consisting of 0.45 mL solution plus 0.45 mL water. Continuously, 100 μL DTNB solution (20 mg DTNB in 100 mL of 1% citrate solution) was added to the blank and the sample. Absorbance of the sample was read against the blank at 412 nm. The GSH values were expressed as μmole/g Hb.
2.9. Measurement of SOD activity in erythrocytes
SOD activity in erythrocytes was determined by the nitro blue tetrazolium method as described by Sptiz and Oberley [27]. The reaction mixture (100 μL) was centrifuged at 3000 rpm for 2 minutes and the supernatant was removed. Then, 35 μL of ice water was added for breaking the erythrocytes. The SOD kit from Randox Laboratories was used to measure the SOD activity. This method uses xanthine and xanthine oxidase to generate superoxide radicals that react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazoliun chloride to form a red formazan dye. SOD activity was measured by the inhibition degree of this reaction. The calculation was performed according to the instructions and SOD activity was expressed as U/mg Hb.
2.10. Statistical analyses
Data are presented as mean ± standard deviation). All study data were analyzed using analysis of variance followed by Dunnett’s test for pairwise comparison. Values are expressed as mean ± standard deviation (n = 3). Results are significant at p < 0.05 compared with the untreated control and significant at p < 0.05 compared with AAPH-treated sample.
3. Results
3.1. Pearl powder attenuates AAPH-induced hemolysis in human erythrocytes
In our study, pearl powder has been investigated for its in vitro antihemolytic property against AAPH-induced hemolysis in human erythrocytes. We found that incubation of an aqueous suspension of erythrocytes with AAPH (25mM, 2–6 hours), a water-soluble free radical generator, drastically increased the hemolysis in a time-dependent manner. The increased oxidative hemolysis at 4 hours and 6 hours after AAPH treatment reached about 96.5% and 100.3%, respectively, whereas it was only 13.2% at 2 hours following AAPH incubation. The lagged hemolysis at 2 hours explaining that endogenous antioxidants exist in erythrocytes may take part in trapping the AAPH-induced free radicals as part of a defensive mechanism. However, continuous exposure of erythrocytes to AAPH caused maximum utilization of endogenous antioxidants, leading to an enormous increase of hemolysis [10,28]. However, preincubation of erythrocytes with different concentrations of pearl powder (50–200 μg/mL) significantly attenuated AAPH-induced erythrocyte hemolysis in a concentration- and time-dependent manner (Table 1). The inhibition of hemolysis by pearl powder indicates that pearl powder probably improved erythrocytes’ antioxidant capacity to quench the free radicals and thereby attenuate oxidative hemolysis. Since oxidative hemolysis was prominent at 4 hours and 6 hours after AAPH incubation, changes in oxidative stress and antioxidant biomarkers were assayed at 4 hours and 6 hours following AAPH treatment.
Table 1.
Antihemolytic property of pearl powder against AAPH-induced oxidative hemolysis of human erythrocytes.
| Experimental condition | Time (h) | Hemolysis (%) |
|---|---|---|
| Control | 2 | 2.9 ± 0.1# |
| AAPH | 13.2 ± 0.1* | |
| +50 μg/mL pearl powder | 8.7 ± 0.4*,# | |
| +100 μg/mL pearl power | 3.5 ± 0.5*,# | |
| +150 μg/mL pearl power | 2.8 ± 0.3*,# | |
| +200 μg/mL pearl power | 3.0 ± 0.4*,# | |
| Control | 4 | 5.3 ± 0.3# |
| AAPH | 96.5 ± 0.5* | |
| +50 μg/mL pearl powder | 96.7 ± 0.1* | |
| +100 μg/mL pearl power | 41.0 ± 9.6*,# | |
| +150 μg/mL pearl power | 26.9 ± 3.5*,# | |
| +200 μg/mL pearl power | 20.7 ± 0.6*,# | |
| Control | 6 | 7.1 ± 0.9# |
| AAPH | 100.3 ± 0.1* | |
| +50 μg/mL pearl powder | 98.5 ± 1.8* | |
| +100 μg/mL pearl power | 71.0 ± 3.9*,# | |
| +150 μg/mL pearl power | 66.1 ± 0.9*,# | |
| +200 μg/mL pearl power | 46.7 ± 0.5*,# |
Erythrocyte suspension at 5% hematocrit was incubated with PBS (control) or preincubated with different concentrations of pearl powder (50–200 μg/mL) for 30 minutes. The suspension was then incubated with and without AAPH (25mM) for 2 hours, 4 hours, or 6 hours at 37°C. Values are expressed as mean ± SD (n = 3).
AAPH = 2,2′-azobis(2-amidinopropane) dihydrochloride; PBS = phosphate buffer saline; SD = standard deviation.
Results are significant at p < 0.05 compared with untreated control cells.
Results are significant at p < 0.05 compared with AAPH-treated cells.
3.2. Pearl powder inhibits AAPH-induced lipid peroxidation in erythrocytes
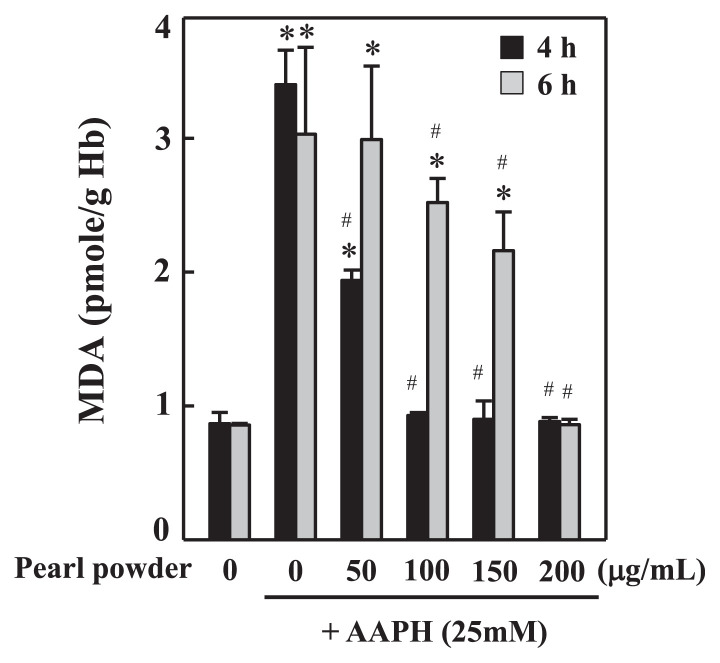
MDA is a widely studied marker of membrane peroxidation in various biological samples [13,22]. In our study, AAPH-induced erythrocyte membrane lipid peroxidation was reflected by a large increase in MDA content, a byproduct of lipid peroxidation. The elevated MDA content in AAPH-treated (25mM, 4 hours or 6 hours) erythrocytes was approximately three-fold greater than the control samples. To address whether pearl powder is able to attenuate AAPH-induced membrane peroxidation and protect erythrocytes, we treated erythrocyte suspension with different concentrations of pearl powder (50–200 μg/mL) for 30 minutes prior to AAPH incubation. We found that preincubation of erythrocytes with pearl powder concentration dependently inhibited MDA levels against AAPH-induced elevation. Among the tested concentrations, pearl powder at 200 μg/mL showed substantial suppression of MDA production, which is almost similar to the control samples (Figure 1).
Figure 1.
Pearl powder suppresses AAPH-induced lipid peroxidation (MDA) in erythrocytes. Erythrocyte suspension at 5% hematocrit was incubated with PBS (control) or preincubated with different concentrations of pearl powder (50–200 μg/mL) for 30 minutes. Then erythrocytes were incubated with AAPH (25mM) for 4 hours and 6 hours. MDA levels were expressed as pmole/g Hb. Values are expressed as mean ± SD (n = 3). * Results are significant at p < 0.05 compared with untreated control cells. # Results are significant at p < 0.05 compared with cells treated with AAPH alone. AAPH = 2,2’-azobis(2-amidinopropane) dihydrochloride; MDA = malondialdehyde; PBS = phosphate buffer saline; SD = standard deviation.
3.3. Pearl powder ameliorates AAPH-induced changes in erythrocyte membrane proteins
Erythrocyte membrane proteins are basically composed of Bands 1 and 2 (spectrins), 2.1 (ankyrin), 3, 4.1, 4.2, and 5, and other accessory proteins. Free radicals or oxidants are known to cause architectural damage to erythrocyte membranes, as manifested by decreased cytoskeletal proteins (LMW proteins) and increased formation of HMW proteins [15,17]. To address whether pearl powder treatment can revert the architectural damage to erythrocyte membrane proteins, we performed SDS-PAGE to determine the changes in various membrane proteins in the presence or absence of AAPH. We demonstrated that incubation of erythrocytes with AAPH for 6 hours increased HMW proteins, while LMW proteins from Band 2.1 to other bands were decreased compared with the control. Nevertheless, preincubation of erythrocytes with pearl powder (50–200 μg/mL) for 30 minutes attenuated the AAPH-induced morphological changes of erythrocyte membrane proteins (Figure 2).
Figure 2.
Pearl powder pretreatment attenuates AAPH-induced changes in erythrocyte membrane proteins analyzed by SDS-PAGE. Erythrocyte suspension at 5% hematocrit was incubated with PBS (control) or preincubated with different concentrations of pearl powder (50–200 μg/mL) for 30 minutes. Then erythrocytes were incubated with AAPH (25mM) for 4 hours. Lane 1: marker; Lane 2: intact erythrocyte membrane proteins; Lane 3: erythrocyte oxidized with AAPH; Lane 4, 5, 6 or 7: erythrocyte preincubated with pearl powder at 50 μg/mL, 100 μg/mL, 150 μg/mL, and 200 μg/mL, respectively, and then oxidized with AAPH. The amount of layered protein was 25 μg in each case. This experiment was repeated three times with similar results. AAPH = 2,2’-azobis(2-amidinopropane) dihydrochloride; HMWP = high-molecular-weight proteins; PBS = phosphate buffer saline; SDS-PAGE = sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Densitometric analyses of HMW and LMW proteins with response to AAPH and pearl powder are presented in Table 2. The higher levels of HMW proteins (117 ± 4%) with AAPH alone were significantly decreased in the presence of pearl powder treatment. LMW proteins, particularly, Bands 2.1, 5.1/5.2, 6, and 7 were significantly higher with pearl powder compared with erythrocytes exposed to AAPH alone (Table 2). We noticed that the attenuation of AAPH-induced hemolysis and MDA levels by pearl powder was concentration dependent, but amelioration of erythrocyte membrane proteins did not occur in a similar fashion. These results suggest that a longer period of pearl powder treatment may be necessary to protect of erythrocyte membrane proteins from the free radical-induced damage.
Table 2.
Effects of pearl powder on AAPH-induced relative changes in erythrocyte membrane proteins by densitometric analysis.
| Experimental condition | HMWP | Band 1/2 | Band 2.1 | Band 3 | Band 4.1/4.2 | Band 5.1/5.2 | Band 6 | Band 7 |
|---|---|---|---|---|---|---|---|---|
| AAPH (25mM) | 117 ± 4* | 102 ± 3 | 71 ± 7* | 81 ± 14 | 104 ± 5 | 65 ± 22* | 82 ± 5* | 65 ± 3* |
| +50 μg/mL pearl powder | 102 ± 7# | 98 ± 2 | 73 ± 10# | 87 ± 17 | 91 ± 3*,# | 91 ± 25# | 86 ± 6 | 76 ± 7*,# |
| +100 μg/mL pearl powder | 102 ± 6# | 100 ± 4 | 99 ± 11# | 97 ± 27 | 108 ± 1* | 102 ± 10# | 94 ± 8# | 81 ± 4*,# |
| +150 μg/mL pearl powder | 106 ± 4# | 102 ± 7 | 101 ± 13# | 104 ± 16 | 108 ± 2* | 100 ± 3# | 100 ± 2# | 85 ± 4*,# |
| +200 μg/mL pearl powder | 107 ± 3# | 101 ± 3 | 101 ± 10# | 105 ± 16 | 110 ± 7* | 100 ± 2# | 107 ± 9# | 93 ± 2# |
Erythrocyte suspension at 5% hematocrit was incubated with PBS (control), or preincubated with different concentrations of pearl powder (50–200 μg/mL) for 30 minutes. Then it was incubated with AAPH (25mM) for 6 hours at 37°C. Relative changes in protein bands were measured using densitometric analysis with the control being 100%. Values are expressed as mean ± SD (n = 3).
AAPH = 2,2′-azobis(2-amidinopropane) dihydrochloride; HMWP = high-molecular-weight proteins; PBS = phosphate buffer saline; SD = standard deviation.
Significant at p < 0.05 compared with untreated control cells.
Significant at p < 0.05 compared with AAPH-treated cells.
3.4. Pearl powder prevents AAPH-induced depletion of erythrocyte GSH levels
Intracellular GSH levels are crucial in maintaining cellular redox homeostasis and attenuation of oxidative damage. In this study, erythrocyte GSH levels were determined at 4 hours and 6 hours after PBS (control) or AAPH treatment. Incubation of erythrocytes with AAPH (25mM) for 4 hours and 6 hours caused a greater time-dependent depletion of GSH levels compared with the control. The drastic decrease in GSH levels indicates that erythrocytes are under severe oxidative stress upon exposure to AAPH alone. As shown in Figure 3, incubation of erythrocytes with different concentrations of pearl powder (50–200 μg/mL, 30 minutes) prior to AAPH treatment attenuated the GSH depletion. Pearl powder at concentrations of 100 μg/mL, 150 μg/mL, and 200 μg/mL showed significant restoration of GSH compared with AAPH-alone-induced loss (Figure 3). The restoration of GSH levels by pearl powder may explain the antioxidant property of pearl powder.
Figure 3.
Pearl powder treatment restores AAPH-induced depleted erythrocyte GSH levels. Erythrocyte suspension at 5% hematocrit was incubated with PBS (control) or preincubated with different concentrations of pearl powder (50–200 μg/mL) for 30 minutes. Then erythrocytes were incubated with AAPH (25mM) for 4 hours and 6 hours. Erythrocyte GSH content was expressed as μmole/g Hb. Values are expressed as mean ± SD (n = 3). * Results are significant at p < 0.05 compared with untreated control cells. # Results are significant at p < 0.05 compared with cells treated with AAPH alone. AAPH = 2,2’-azobis(2-amidinopropane) dihydrochloride; GSH = glutathione; PBS = phosphate buffer saline; SD = standard deviation.
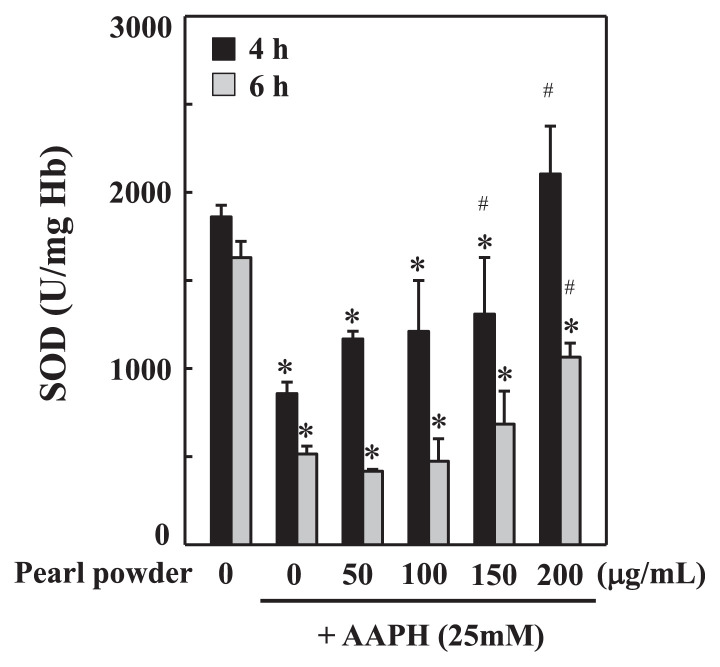
3.5. AAPH-induced loss of erythrocyte SOD activity was restored by pearl powder
SOD is a primary antioxidant enzyme that typically scavenges superoxide anion radicals into less toxic hydrogen peroxide. The decrease in SOD activity reflects the oxidative state of cells, and cells are prone to ROS-mediated attacks. To address the antioxidant property of pearl powder, we measured erythrocyte SOD activity in the presence and absence of AAPH. We found that exposure of erythrocytes to AAPH (25mM) alone for 4 hours and 6 hours drastically decreased the SOD activity in a time-dependent fashion compared with the control. Nevertheless, preincubation of erythrocytes with pearl powder (50–200 μg/mL) for 30 minutes prevented the AAPH-induced loss of SOD activity. Moreover, high concentrations of pearl powder (150 μg/mL and 200 μg/mL) appear to be more effective in the restoration of SOD activity compared with treatment with AAPH alone (Figure 4).
Figure 4.
Pearl powder pretreatment prevents AAPH-induced loss of erythrocyte SOD activity. Erythrocyte suspension at 5% hematocrit was incubated with PBS (control) or preincubated with different concentrations of pearl powder (50–200 μg/mL) for 30 minutes. Then erythrocytes were incubated with AAPH (25mM) for 4 hours and 6 hours. SOD activity was expressed as U/mg Hb. Values are expressed as mean ± SD (n = 3). * Results are significant at p < 0.05 compared with untreated control cells. # Results are significant at p < 0.05 compared with cells treated with AAPH alone. AAPH = 2,2’-azobis(2-amidinopropane) dihydrochloride; PBS = phosphate buffer saline; SD = standard deviation; SOD = superoxide dismutase.
4. Discussion
Pearl powder has widely been used in traditional Chinese medicine as a complementary medicine for general health improvement and to treat various diseases, including convulsions, epilepsy, ulcers, and impaired wound healing [2,3,29]. In the present study, for the first time we have demonstrated that pearl powder is capable of inhibiting AAPH-induced hemolysis, lipid peroxidation, and architectural damage of erythrocyte membrane proteins. The AAPH-induced oxidative hemolysis of human erythrocytes was used as a model to study the antihemolytic and antioxidant properties of pearl powder. Preincubation of erythrocytes with different concentrations of pearl powder substantially inhibited AAPH-induced hemolysis. This was accompanied by decreased MDA production and attenuated derangement of erythrocyte membrane proteins. The antioxidant property of pearl powder was evidenced by significantly increased erythrocyte SOD activity and GSH levels against AAPH-induced loss. Taken together, our findings conclude that the potential antioxidant property of pearl powder may help diminish AAPH-induced oxidative hemolysis, lipid peroxidation, and protein oxidation in human erythrocytes.
The antihemolytic property of pearl powder against AAPH-induced oxidative hemolysis was studied via preincubation of erythrocytes with different concentrations of pearl powder. AAPH, a water-soluble azo compound, has been reported to increase hemolysis through increasing intracellular free radicals. On their excessive production, ROS or free radicals that are highly reactive in nature eventually attack erythrocyte membrane lipids and proteins to cause irreversible damage. This phenomenon is usually terminated by erythrocyte hemolysis or death of healthy erythrocytes [7,8]. At physiological temperature, AAPH decomposes in an erythrocyte aqueous suspension to produce alkyl radicals, which then convert to peroxyl radicals in the presence of oxygen. These peroxyl radicals cause oxidative damage to erythrocyte membrane lipids/PUFA, resulting in the loss of membrane integrity, which leads to the release of hemoglobin (hemolysis) and intracellular K+ ions [30]. In our study, pearl powder treatment prior to AAPH exposure inhibited erythrocyte hemolysis in a concentration- and time-dependent fashion. Similar to our studies, Dai and colleagues [31] reported that flavonols and their glycosides, considered as effective antioxidants, effectively protect human erythrocytes against free radical-induced oxidative hemolysis. Another study from our laboratory demonstrated that fermented culture broth of Antrodia salmonea significantly inhibited the AAPH-induced oxidative hemolysis of human erythrocytes along with suppressed lipid peroxidation and increased antioxidant status [10]. By contrast, oral administration of carbonyl iron powder has been shown to restore the hemoglobin concentration in rats with iron deficiency anemia [32]. Since pearls are rich in amino acids, trace elements, calcium, and proteins [3,4], we assume that these components in pearl powder possibly contributed to the inhibition of AAPH-induced free radical production and prevention of free radical-induced oxidative hemolysis.
Evidence has shown that increased erythrocyte hemolysis is associated with increased oxidative modification of membrane lipids/proteins and ruined antioxidant capacity [8,31]. Therefore, we studied the changes in lipid peroxidation byproduct along with antioxidant status in pearl powder-treated erythrocytes. To demonstrate the potential antioxidant property of various compounds, AAPH has been used extensively in several in vitro studies, which triggers the lipid peroxidation process followed by an increased production of free radicals [10,31]. Consistent to previous reports, we found that incubation of erythrocytes with AAPH is represented by a greater increase of MDA content, an index of erythrocyte membrane peroxidation. At physiological temperature, AAPH can decompose to generate alkyl radicals to initiate lipid peroxidation and hemolysis. Since erythrocyte membranes are rich in PUFA, AAPH-induced radicals certainly oxidize membrane lipids, which cause quick damage to the membrane and a loss of its integrity leading to lipid peroxidation [30]. Despite AAPH-induced MDA elevation, preincubation of erythrocytes with pearl powder considerably suppressed MDA production. This result explains that pearl powder is able to alleviate membrane peroxidation and protect erythrocytes from ROS-mediated damage. The decreased MDA levels in pearl powder-treated erythrocytes may be due to either increased erythrocyte antioxidant status or suppressed ROS production, and/or both. Laboratory studies indicated that pearl powder is effective in scavenging of 2,2-diphenyl-1-pic-rylhydrazyl (DPPH)-induced free radicals, which is almost similar to the well-established antioxidants [2]. Besides, a large body of evidence demonstrated that increased antioxidant status in cells or tissues refers to decreased lipid peroxidation [10,15,22]. In our study, elevation of erythrocyte GSH and SOD with pearl powder supports the antioxidant property of pearl powder, which may contribute to decreased MDA levels. Moreover, pearls are reported to contain various nutrients, including calcium, amino acids, proteins, and trace elements [3,4], which possibly participate in the termination of lipid peroxidation chain reaction and enrichment of endogenous antioxidant potential.
It has been well documented that free radicals could damage erythrocyte membrane proteins mostly by aggregation of Band 3, spectrin hemoglobin, increased formation of HMW proteins, or decreased LMW proteins [15,33]. Previously, we have shown that AAPH-treated erythrocytes subjected to SDS-PAGE represented by a greater loss in Bands 3, 6, and 7, while no apparent change in Bands 1 and 2 (spectrins) [15]. Another study emphasized a preferential decrease of Band 3 and no change in spectrin when erythrocytes were exposed to AAPH [34]. The cross linking and interaction between LMW proteins and oxidized lipids result in the formation of HMW proteins, which leads to abnormalities in erythrocyte morphology [15,33]. Compared with membrane lipids, erythrocyte inner surface proteins are more vulnerable to oxidative attack by ROS. Previous studies reported that hydrogen peroxide treatment to erythrocytes altered the membrane spectrin complex, but the same was inhibited by pretreatment of cells with carbon monoxide, which interferes with hemoglobin peroxidative reactions [33]. Moreover, it has been described that continuous damage of erythrocyte membrane proteins could also cause hemolysis [8]. Our findings showed that AAPH-induced damage to erythrocyte membrane proteins, particularly Bands 2.1, 5.1/ 5.2, 6, and 7, was accompanied by increased hemolysis. However, pretreatment of erythrocytes with pearl powder attenuated the AAPH-induced oxidative modification of membrane proteins. Some studies claimed that peal extracts possess anti-inflammatory, antiapoptotic, and wound-healing properties, and protect skin keratinocytes against radiation or oxidative insults [15,29]. Other studies demonstrated that dermatoprotective or antiapoptotic properties of natural compounds are mostly due to their potent antioxidant activity [35,36]. In this context, we speculate that the antioxidant property of pearl powder may attenuate the AAPH-induced derangement of erythrocyte membrane proteins. Owing to its cytoprotective effects, pearl powder may also play a key role in maintaining cell architecture by preventing the damage of membrane proteins/lipids. These pharmacological effects could be considerable points to include pearl powder in the preparation of cosmetics and antiaging substances.
GSH, a reduced form of glutathione that comprises three amino acids (γ-glutamyl–cysteinyl–glycine), plays a crucial role in the regulation of cellular redox homeostasis by scavenging a variety of free radicals/ROS through its electron-donating property. As a result, the reduced GSH becomes oxidized, and two such molecules are linked by a disulfide bridge to form oxidized GSH [18,19]. Depletion of cellular GSH levels is considered as a foot marker of oxidative stress, and it is correlated with increased lipid peroxidation and protein oxidation [19,30,37]. In agreement with these, greater loss of erythrocyte GSH levels with AAPH was accompanied by elevated lipid peroxidation and oxidative modification of membrane proteins. Such oxidative damage to lipids and proteins may destabilize the membrane skeleton, thereby compromising erythrocyte survival. It is worth noting that depleted GSH levels were concentration dependently restored by pearl powder treatment along with suppressed lipid peroxidation. The restored GSH levels imply that pearl powder can prevent erythrocyte hemolysis and oxidative modification of membrane proteins and lipids, at least in part, due to the improved antioxidant status. In redox reactions, the thiol group of cysteine is able to donate a reducing equivalent or electron to other unstable radicals to stabilize their reactivity. Among other amino acids, cysteine residues are reported to be important for the antioxidant activity of a protein [38]. In this regard, amino acids present in pearl powder may take part in GSH biosynthesis and thereby promote erythrocyte GSH levels. A most recent study by Zhang and colleagues [3] reported that different kinds of pearl proteins contained similar amino acids, mainly glycine, glutamic acid, arginine, cysteine, and lysine. Most of these amino acids contribute to boosting the antioxidant activity under oxidative stress conditions. It is also reported that different kinds of pearl powder are effective in scavenging DPPH-induced free radicals in vitro, which explains the antioxidant property of pearl powder [2].
SOD is a primary antioxidant enzyme that effectively scavenges superoxide anion radicals into less toxic hydrogen peroxide [18]. Trace elements, such as Cu and Zn, are part of the catalytic center of the enzyme, where Cu is involved in the redox mechanism and Zn acts as a structural element [39]. In our study, we found that erythrocyte SOD activity was remarkably decreased when cells were exposed to AAPH alone. The decreased SOD activity indicates that AAPH increased free radical production, and endogenous SOD was fully utilized to neutralize the toxic radicals. In vitro incubation of cells with AAPH was reported to trigger free radicals, including superoxide anions, leading to decreased radical scavenging capacity of cells. The continuous increase of radical production and a subsequent decrease of defensive antioxidant enzymes are expected to damage membrane proteins and lipids [28,30,40]. This scenario was evidenced in our study as we found increased MDA levels and deranged erythrocyte membrane proteins. However, the important finding in our study is that the AAPH-induced greater loss in SOD activity was remarkably restored with pearl powder preincubation. Increased SOD activity reveals that pearl powder possesses potent antioxidant activity, which contributes to the eradication of AAPH-induced superoxide anion radicals in erythrocytes. The increased GSH and decreased MDA levels further support the protective effects of pearl powder against AAPH-induced oxidative insults. It has been documented that pearl powder contain different proteins, including water-soluble, acid-soluble, and conchiolin proteins, with almost similar amino acid profile [3]. By contrast, amino acids are vital molecules to boost the cellular antioxidant potential when cells are exposed to highly reactive radicals [41]. A study by Rükgauer and colleagues [39] demonstrated that higher concentrations of trace elements, Cu and Se, in erythrocytes contributed to an increase in SOD (Cu-dependent) and GSH peroxidase (Se-dependent) activities, and a decrease in plasma MDA levels. Therefore, amino acids and trace elements present in pearl powder are expected to promote erythrocyte SOD activity in order to quench toxic free radicals.
5. Conclusions
For the first time, we demonstrated that pearl powder treatment attenuated AAPH-induced hemolysis and oxidative modification of lipids/proteins in erythrocytes possibly through the increased antioxidant status. The antihemolytic activity of peal powder was evidenced by a significant diminution of AAPH-induced hemolysis. This was accompanied by a substantial suppression of membrane lipid peroxidation and protein oxidation, and greater restoration of SOD activity and GSH levels with pearl powder treatment. The increased erythrocyte antioxidant status reveals that pearl powder potentially inhibited AAPH-induced oxidative damage and hemolysis. This study concludes that pearl powder as a complementary medicine could be a valid substance to include in the preparation of novel therapeutic drugs to treat diseases with an impaired antioxidant system and hemolysis. Our results further suggest that in-depth molecular studies should be carried out to reveal the molecular mechanisms underlying the therapeutic effects of pearl powder.
Acknowledgments
This work was supported by the grants MOST-104-2320-B-039-040-MY3, MOST-103-2320-B-039-038-MY3, NSC-103-2622-B-039-001-CC2, CMU103-ASIA-12, and CMU 103-ASIA-09 from the Ministry of Science and Technology, Asia University, and China Medical University, Taiwan.
Funding Statement
This work was supported by the grants MOST-104-2320-B-039-040-MY3, MOST-103-2320-B-039-038-MY3, NSC-103-2622-B-039-001-CC2, CMU103-ASIA-12, and CMU 103-ASIA-09 from the Ministry of Science and Technology, Asia University, and China Medical University, Taiwan.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Jian-Ping D, Jun C, Yu-Fei B, Bang-Xing H, Shang-Bin G, Li-Li J. Effects of pearl powder extract and its fractions on fibroblast function relevant to wound repair. Pharm Biol. 2010;48:122–7. doi: 10.3109/13880200903046211. [DOI] [PubMed] [Google Scholar]
- 2. Shao DZ, Wang CK, Hwang HJ, Hung CH, Chen YW. Comparison of hydration, tyrosinase resistance, and antioxidant activation in three kinds of pearl powders. J Cosm Sci. 2010;61:133–45. [PubMed] [Google Scholar]
- 3. Zhang JX, Li SR, Yao S, Bi QR, Hou JJ, Cai LY, Han SM, Wu WY, Guo DA. Anticonvulsant and sedative–hypnotic activity screening of pearl and nacre (mother of pearl) J Ethnopharmacol. 2016;181:229–35. doi: 10.1016/j.jep.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 4. Chen H, Chang J, Wu J. Calcium bioavailability of nanonized pearl powder for adults. J Food Sci. 2008;73:H246–51. doi: 10.1111/j.1750-3841.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 5. Chuang CH, Chang PJ, Hsieh WS, Tsai YJ, Lin SJ, Chen PC. Chinese herbal medicine use in Taiwan during pregnancy and the postpartum period: a population-based cohort study. Int J Nurs Stud. 2009;46:787–95. doi: 10.1016/j.ijnurstu.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 6. Yang YL, Chang CH, Huang CC, Liu HW. Anti-inflammation and anti-apoptosis effects of pearl extract gel on UVB irradiation HaCaT cells. Biomed Mater Eng. 2015;26(s1):139–45. doi: 10.3233/BME-151299. [DOI] [PubMed] [Google Scholar]
- 7. Barodka VM, Nagababu E, Mohanty JG, Nyhan D, Berkowitz DE, Rifkind JM, Strouse JJ. New insights provided by a comparison of impaired deformability with erythrocyte oxidative stress for sickle cell disease. Blood Cells Mol Dis. 2014;52:230–5. doi: 10.1016/j.bcmd.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 8. Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010;3:2–12. doi: 10.4161/oxim.3.1.10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott M, Van Den Berg J, Repka T, Rouyer-Fessard P, Hebbel R, Beuzard Y, Lubin B. Effect of excess alpha-hemoglobin chains on cellular and membrane oxidation in model beta-thalassemic erythrocytes. J Clin Invest. 1993;91:1706–12. doi: 10.1172/JCI116380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hseu Y-C, Lee CC, Chen YC, Kumar KS, Chen CS, Tsai CT, Huang HC, Wang HM, Yang HL. Antrodia salmonea in submerged culture exhibits antioxidant activities in vitro and protects human erythrocytes and low-density lipoproteins from oxidative modification. Food Chem Toxicol. 2014;66:150–7. doi: 10.1016/j.fct.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 11. Vellosa JCR, Regasini LO, Khalil NM, Bolzani VdS, Khalil OA, Manente FA, Pasquini Netto H, Oliveira OM. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclética Quimica. 2011;36:7–20. [Google Scholar]
- 12. Piccinini G, Minetti G, Balduini C, Brovelli A. Oxidation state of glutathione and membrane proteins in human red cells of different age. Mech Ageing Dev. 1995;78:15–26. doi: 10.1016/0047-6374(94)01511-j. [DOI] [PubMed] [Google Scholar]
- 13. Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14. Sugihara T, Rawicz W, Evans E, Hebbel R. Lipid hydroperoxides permit deformation-dependent leak of monovalent cation from erythrocytes. Blood. 1991;77:2757–63. [PubMed] [Google Scholar]
- 15. Yang HL, Chen SC, Chang NW, Chang JM, Lee ML, Tsai PC, Fu HH, Kao WW, Chiang HC, Wang HH, Hseu YC. Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food Chem Toxicol. 2006;44:1513–21. doi: 10.1016/j.fct.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 16. Yang S, Jensen MK, Rimm EB, Willett W, Wu T. Erythrocyte superoxide dismutase, glutathione peroxidase, and catalase activities and risk of coronary heart disease in generally healthy women: a prospective study. Am J Epidemiol. 2014;180:901–8. doi: 10.1093/aje/kwu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Somer T, Meiselman HJ. Disorders of blood viscosity. Annals Med. 1993;25:31–9. doi: 10.3109/07853899309147854. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. New York, USA: Oxford University Press; 2015. [Google Scholar]
- 19. Pompella A, Visvikis A, Paolicchi A, Tata VD, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 20. Chang HC, Yang HL, Pan JH, Korivi M, Pan JY, Hsieh MC, Chao PM, Huang PJ, Tsai CT, Hseu YC. Hericium erinaceus inhibits TNF-α-induced angiogenesis and ROS generation through suppression of MMP-9/NF-κB signaling and activation of Nrf2-mediated antioxidant genes in human EA.hy926 endothelial cells. Oxid Med Cell Longev. 2016;2016:15. doi: 10.1155/2016/8257238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaur G, Kathariya R, Bansal S, Singh A, Shahakar D. Dietary antioxidants and their indispensable role in periodontal health. J Food Drug Anal. 2016;24:239–46. doi: 10.1016/j.jfda.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen KN, Peng WH, Hou CW, Chen CY, Chen HH, Kuo CH, Korivi M. Codonopsis javanica root extracts attenuate hyperinsulinemia and lipid peroxidation in fructose-fed insulin resistant rats. J Food Drug Anal. 2013;21:347–55. [Google Scholar]
- 23. Mallikarjuna K, Shanmugam KR, Nishanth K, Wu MC, Hou CW, Kuo CH, Reddy KS. Alcohol-induced deterioration in primary antioxidant and glutathione family enzymes reversed by exercise training in the liver of old rats. Alcohol. 2010;44:523–9. doi: 10.1016/j.alcohol.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 24. Tsuchiya M, Asada A, Maeda K, Ueda Y, Sato EF, Shindo M, Inoue M. Propofol versus midazolam regarding their antioxidant activities. Am J Respir Crit Care Med. 2001;163:26–31. doi: 10.1164/ajrccm.163.1.9911120. [DOI] [PubMed] [Google Scholar]
- 25. Arduini A, Stern A. Spectrin degradation in intact red blood cells by phenylhydrazine. Biochem Pharmacol. 1985;34:4283–9. doi: 10.1016/0006-2952(85)90286-2. [DOI] [PubMed] [Google Scholar]
- 26. van den Berg JJ, den Kamp JAO, Lubin BH, Roelofsen B, Kuypers FA. Kinetics and site specificity of hydroperoxide-induced oxidative damage in red blood cells. Free Radic Biol Med. 1992;12:487–98. doi: 10.1016/0891-5849(92)90102-m. [DOI] [PubMed] [Google Scholar]
- 27. Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Analyt Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 28. Zou CG, Agar NS, Jones GL. Oxidative insult to human red blood cells induced by free radical initiator AAPH and its inhibition by a commercial antioxidant mixture. Life Sci. 2001;69:75–86. doi: 10.1016/s0024-3205(01)01112-2. [DOI] [PubMed] [Google Scholar]
- 29. Li YC, Chen CR, Young TH. Pearl extract enhances the migratory ability of fibroblasts in a wound healing model. Pharmaceut Biol. 2013;51:289–97. doi: 10.3109/13880209.2012.721130. [DOI] [PubMed] [Google Scholar]
- 30. Banerjee A, Kunwar A, Mishra B, Priyadarsini K. Concentration dependent antioxidant/pro-oxidant activity of curcumin: studies from AAPH induced hemolysis of RBCs. Chem Biol Interact. 2008;174:134–9. doi: 10.1016/j.cbi.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 31. Dai F, Miao Q, Zhou B, Yang L, Liu ZL. Protective effects of flavonols and their glycosides against free radical-induced oxidative hemolysis of red blood cells. Life Sci. 2006;78:2488–93. doi: 10.1016/j.lfs.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 32. Zhu Q, Qian Y, Yang Y, Wu W, Xie J, Wei D. Effects of carbonyl iron powder on iron deficiency anemia and its subchronic toxicity. J Food Drug Anal. 2016;24:746–53. doi: 10.1016/j.jfda.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Snyder L, Fortier N, Trainor J, Jacobs J, Leb L, Lubin B, Chiu D, Shohet S, Mohandas N. Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics, and spectrin-hemoglobin cross-linking. J Clin Invest. 1985;76:1971–7. doi: 10.1172/JCI112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Celedón G, González G, Lissi EA, Hidalgo G. Free radical-induced protein degradation of erythrocyte membrane is influenced by the localization of radical generation. IUBMB Life. 2001;51:377–80. doi: 10.1080/152165401753366140. [DOI] [PubMed] [Google Scholar]
- 35. Hseu YC, Tsai YC, Huang PJ, Ou TT, Korivi M, Hsu LS, Chang SH, Wu CR, Yang HL. The dermato-protective effects of lucidone from Lindera erythrocarpa through the induction of Nrf2-mediated antioxidant genes in UVA-irradiated human skin keratinocytes. J Funct Foods. 2015;12:303–18. [Google Scholar]
- 36. Hseu YC, Lo HW, Korivi M, Tsai YC, Tang MJ, Yang HL. Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated human keratinocytes. Free Radic Biol Med. 2015;86:102–17. doi: 10.1016/j.freeradbiomed.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 37. Korivi M, Hou CW, Huang CY, Lee SD, Hsu MF, Yu SH, Chen CY, Liu YY, Kuo CH. Ginsenoside-Rg1 protects the liver against exhaustive exercise-induced oxidative stress in rats. Evid Based Complement Alternat Med. 2011;2012:932165. doi: 10.1155/2012/932165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iwao Y, Ishima Y, Yamada J, Noguchi T, Kragh Hansen U, Mera K, Honda D, Suenaga A, Maruyama T, Otagiri M. Quantitative evaluation of the role of cysteine and methionine residues in the antioxidant activity of human serum albumin using recombinant mutants. IUBMB Life. 2012;64:450–4. doi: 10.1002/iub.567. [DOI] [PubMed] [Google Scholar]
- 39. Rükgauer M, Neugebauer RJ, Plecko T. The relation between selenium, zinc and copper concentration and the trace element dependent antioxidative status. J Trace Elem Med Biol. 2001;15:73–8. doi: 10.1016/S0946-672X(01)80046-8. [DOI] [PubMed] [Google Scholar]
- 40. Antosiewicz J, Spodnik J, Teranishi M, Herman-Antosiewicz A, Kurono C, Soji T, Woźniak M, Borkowska A, Wakabayashi T. NADH-generating substrates reduce peroxyl radical toxicity in RL-34 cells. Folia Morphol. 2009;68:247–55. [PubMed] [Google Scholar]
- 41. Anraku M, Shintomo R, Taguchi K, Kragh-Hansen U, Kai T, Maruyama T, Otagiri M. Amino acids of importance for the antioxidant activity of human serum albumin as revealed by recombinant mutants and genetic variants. Life Sci. 2015;134:36–41. doi: 10.1016/j.lfs.2015.05.010. [DOI] [PubMed] [Google Scholar]