Abstract
In this study, polyphenols (LSP) were obtained from the fermentation broth of Lachnum singerianum. Two fractions were isolated by Sephadex LH-20 chromatographic column, and the primary fraction (LSP-1) was collected. The comprehensive physicochemical properties of phenolic acids and polyhydroxy phenolic compounds of LSP-1 were determined by UV-visible spectroscopy, Fourier transform infrared spectroscopy, and gas chromatography–mass spectrometry. Results of anticoagulant activity assay in vitro showed that LSP-1 could lengthen prothrombin time, activated partial thromboplastin time, and thrombin time of mouse plasma. In addition, anticoagulant activity results in vivo showed that high dose of LSP-1 could significantly prolong bleeding time, coagulation time, prothrombin time, activated partial thromboplastin time, and thrombin time of hypercoagulable mice induced by adrenaline, reduce the content of fibrinogen and enhance antithrombin III activity. All results indicated that the LSP-1 could serve well as an anticoagulant, and might be used as a potential natural drug candidate for thrombosis.
Keywords: anticoagulant activity, Lachnum singerianum, polyphenols
1. Introduction
Prevention and treatment of thrombotic diseases has become one of the demanding tasks in medicine as thrombosis becomes the main cause of acute myocardial infarction, cerebral infarction, and other cardiovascular diseases [1].
Formation of thrombus is mainly attributed to the damage of the cardiovascular wall, changes of blood state, and physicochemical properties [2,3]. Generally, the coagulation and anticoagulation systems are maintained in dynamic equilibrium in organisms. Inhibition of blood coagulation can be used as an important means to prevent formation of thrombosis [4,5]. Coagulation process is complex in the human body, and the key step for coagulation formation is to transform fibrinogen into fibrin, which is catalyzed by thrombin [6].
Polyphenols are widely distributed in fruits, vegetables, nuts, seeds, flowers, and saprophytic fungi [7,8]. Epidemiological studies have indicated that polyphenols could prevent the occurrence of cardiovascular diseases and maintain the integrality of blood vessels [9–12]. Curcumin, one of the polyphenolic compounds, can significantly prolong activated partial thromboplastin time (APTT) and prothrombin time (PT), and suppress activity of thrombin and the production of factor Xa. Therefore, curcumin can inhibit blood coagulation and formation of thrombus by endogenous and exogenous pathway [5]. Flavonoids in purple grape juice could inhibit platelet aggregation, increase endogenous platelet-derived nitric oxide, thus reducing endogenous peroxides [13]. Polyphenols of grape seed and Aronia melanocarpa can prolong the blood coagulation time [APTT, PT, thrombin time (TT)] and reduce the maximal velocity of fibrous protein aggregation [14].
Lachnum is a genus of saprophytic fungi in the family Hyaloscyphaceae which is distributed throughout the world [15]. So far, more than 250 species of Lachnum have been reported [16] and some can produce a variety of active metabolites such as polysaccharide [17], pigment [18], and lipid [19]. Previous studies have also found that Lachnum could produce polyphenols, which have antioxidation, antibacterial, and other biological activities and play an important role in food quality [20,21]. The aims of the study were to evaluate the physicochemical properties, anticoagulant effect and explore the mechanism of polyphenols.
2. Materials and methods
2.1. Materials
The fruiting bodies of Lachnum singerianum YM296 were collected from Huangshan, Anhui, China and then separated and preserved in the Microbial Resource and Application Laboratory of Hefei University of Technology.
Fibrinogen (FIB) kit, PT kit, TT kit, antithrombin III (ATIII) kit, and APTT kit were purchased from Nanjing Jiancheng Technology Co., Ltd (Nanjing, China). Aspirin enteric-coated tablets was purchased from Shenyang Aojina Pharmaceutical Co., Ltd (Shenyang, China). Sephadex LH-20 was purchased from Sigma Chemical Co., iMark ELIAS (Bio-Rad, Hercules, CA, USA).
2.2. Experimental animals
Forty male Kunming mice, weight 25 ± 1 g, were provided by the Experimental Animal Center of Anhui Medical University (Certificate number: No. 1 license of the Medical Laboratory Animal of Anhui). The mice were kept at 23 ± 2°C with humidity of 55 ± 5%, and cultured in a 12 h:12 h light–dark cycle. All procedures involving animals were conducted in accordance with the guidelines of Regulations for the Administration of Affairs Concerning Experimental Animals of Institutional Animal Care and Use Committee (IACUC).
2.3. Extraction and purification of L. singerianum polyphenols
L. singerianum was incubated at 26°C, 160 rpm for 8 days. The composition of cultivated broth is 20 g/L sucrose, 5 g/L peptone, 0.8 g/L MgCl2, 0.01 g/L tyrosine, and natural pH. After the completion of fermentation, the broth was concentrated by vacuum rotary evaporation. Three times the volume of 70% ethanol was added, and the solution was placed at 4°C for 24 hours. The supernatant was collected and evaporated to eliminate ethanol. The solution was extracted with iso-volumetric ethyl acetate and evaporated to remove organic phase. The crude polyphenols were lyophilized at −40°C for 24 h and isolated by Sephadex LH-20 chromatographic column. The loading concentration of the sample was 20 mg/mL. The eluent was methanol at a flow rate of 1 mL/min and was detected at 280 nm by spectrophotometer. The main fraction was gathered, concentrated, and lyophilized to obtain L. singerianum polyphenol (LSP)-1 [22].
2.4. Physicochemical characterization of LSP-1
2.4.1. UV and Fourier transform infrared spectroscopy characterization
LSP-1 was dissolved in methanol and recorded by a UV spectrophotometer with scanning range of 200–800 nm.
The IR absorption of LSP-1 was measured with KBr pellet (mass ratio 1:100) by Fourier transform infrared spectroscopy (FT-IR) spectrometer Nicolet 67 with the scanning range of 400–4000/cm.
2.4.2. Gas chromatography–mass spectrometry analysis
The method of Masek et al [23] was used with minor amendments: 50 mg LSP-1 was dissolved in 2 mL methanol to make a clear solution. After being dried with N2, 100 μL N,O-bis(-trimethylsilyl)trifluoroacetamide and 50 μL pyridine were added to the solution. Then the mixture was oscillated for 0.5 min and derived in the oven at 70°C for 45 min. Spectrogram was analyzed by the NIST 11 MS Database and MS Search Program 2.0.
Gas chromatography–mass spectrometry (GC-MS) analysis conditions are as follows: Quartz capillary column HP-5 (30 m × 0.25 mm × 0.25 μm), 1 μL of the derived solution was injected into injection port. Temperature programming was selected for column temperature which was increased from 50°C to 250°C at rate of 10°C/min. Temperature of the injection port was 280°C, while flow rate of He was 1 mL/min. The ion source was electrospray ionization and the electron bombing energy is 70 eV.
2.5. Anticoagulant activity assay in vitro
The mice were hocused with pentobarbital sodium solution before the eyeballs of mice were picked out to obtain the sample blood which had been exposed to the drug for 10 minutes. The sample blood was put into the centrifuge tube which contained 3.8% sodium citrate (whole blood: anticoagulant = 9:1, v/v), and was gently blended uniformly. After being centrifuged at 960 g for 10 minutes, the supernatant of the sample blood was collected and tested within 2 hours. Aliquots of 50 μL of plasma were added into 25 μL LSP-1 solutions with concentrations of 50 mg/L, 100 mg/L, 150 mg/ L, and 200 mg/L. To the negative and positive control groups, other than plasma, the same amount of saline and 50 mg/L aspirin were added, respectively. APTT, TT, and PT were determined according to the instructions of the enzyme-linked immunosorbent assay kit. All experiments were carried out in parallel three times.
2.6. Anticoagulant activity assay in vivo
After being kept for environmental adaptation for l week, the mice were randomly and equally divided into six groups, including normal control group, model control group, positive control group (20 mg/kg aspirin, mixed with carboxymethyl cellulose suspension), high-dose group (80 mg/kg LSP-1), medium-dose group (40 mg/kg LSP-1), and low-dose group (20 mg/kg LSP-1). The high coagulation models were built successfully by injecting adrenaline hydrochloride into the same place beneath the mice skin, after which the mice could be further divided into model group, positive group, high, medium, and low dose of LSP-1 groups. For the normal control group, the mice were just continuously injected with equivoluminal saline for 14 days [24]. Adrenaline could make the body in a hypercoagulable state, which was assessed by the experiment diagnosis index: AT-III ≤ 70%. In addition, TT, APTT or PT, and APTT were shortened significantly [25].
After 15 days, each group was gavaged with corresponding drug dose once a day. The mice in the normal and model groups were injected with equivoluminal saline. Then, the mice were successively administrated for 14 days. Drinking water was freely available for mice during administration. By the end of gavage, the mice fasted for 12 hours. Coagulation time (CT) and bleeding time (BT) were measured 1 hour after the last administration, and the mice were then hocused and the blood was collected by removing their eyeballs. Fresh whole blood was put into a centrifuge tube with sodium citrate as anticoagulant (whole blood: anticoagulant = 9:1, v/v) and was blended completely. After being centrifuged at 706 g for 5 minutes, the centrifugal supernatant was collected, which was used to measure PT, APTT, FIB, TT, and AT-III.
2.6.1. Determination of CT
One hour after the last gavage treatment, the mice were hocused. According to the method [26], coagulation time of whole blood was set. The tails of mice were sliced to obtain blood and then a drop of blood was placed on a slide with the timing starting. A syringe needle was used to stir the blood. The time of fibrin filament formation was recorded. Blood that was not provoked was used as control.
2.6.2. Determination of BT
The method of Cui et al [27] was used with minor amendments. One hour after the last gavage treatment, tails of the mice were wiped with alcohol to expand blood vessels. Afterwards, tails were cut at the point 0.5 cm away from the tip with scissors, and time was recorded immediately. The blood was sucked with filter paper every 20 s until the tails stopped bleeding (the time of no blood on filter paper as the criterion), and the BT was recorded.
2.6.3. Determination of FIB content
By the effect of excessive thrombin, soluble fibrinogen in plasma was transformed into insoluble aggregates and then formed into fibrin clots. Therefore, solidification time of plasma was inversely proportional to FIB content.
For preparation of standard curve, FIB standards were dissolved in deionized water to make a stock solution. The stock solution was diluted with imidazole buffer at the proportion of 1:5 (100 μL FIB: 500 μL imidazole buffer), 1:10, 1:15, 1:20, and 1:30 to make different concentrations. A 100-μL sample of thrombin was added into 200 μL FIB working solutions with different concentrations and the mixtures were pretreated at 37°C for 3 minutes. The solidification time was then recorded. A standard curve was drawn on log–log paper with different concentration of fibrinogen standard (mg/dL) as the abscissa and coagulation time as the ordinate.
Testing plasma was diluted with the imidazole buffer at 1:10 (100 μL plasma: 1000 μL imidazole buffer) to make a solution which was pretreated at 37°C for 3 minutes. After 100 μL thrombin was added into 200 μL solution, the solidification time was measured. The content of FIB was determined according to the standard curve.
2.6.4. Determination of AT-III activity
Chromogenic assays were used to determine the activity of AT-III. When concentration of the thrombin in blood was too high, thrombin could be combined with the AT-III in the plasma and formed into a complex that had no clotting activity. Unreacted thrombin would act on chromogenic peptide substrate Chromozym P to promote release of paranitroaniline, which has strong absorption at 405 nm. Thus, adsorption intensity of paranitroaniline in 405nm was inversely proportional to the activity of AT-III.
2.7. Statistical analysis
All data were processed by SPSS 18.0 statistical software and represented as mean ± standard deviation. The t test analysis and one-way analysis of variance were used to analyze the differences between groups with p < 0.05 indicating a significant difference and p < 0.01 a very significant difference.
3. Results and discussion
3.1. Isolation and purification of polyphenols from L. singerianum
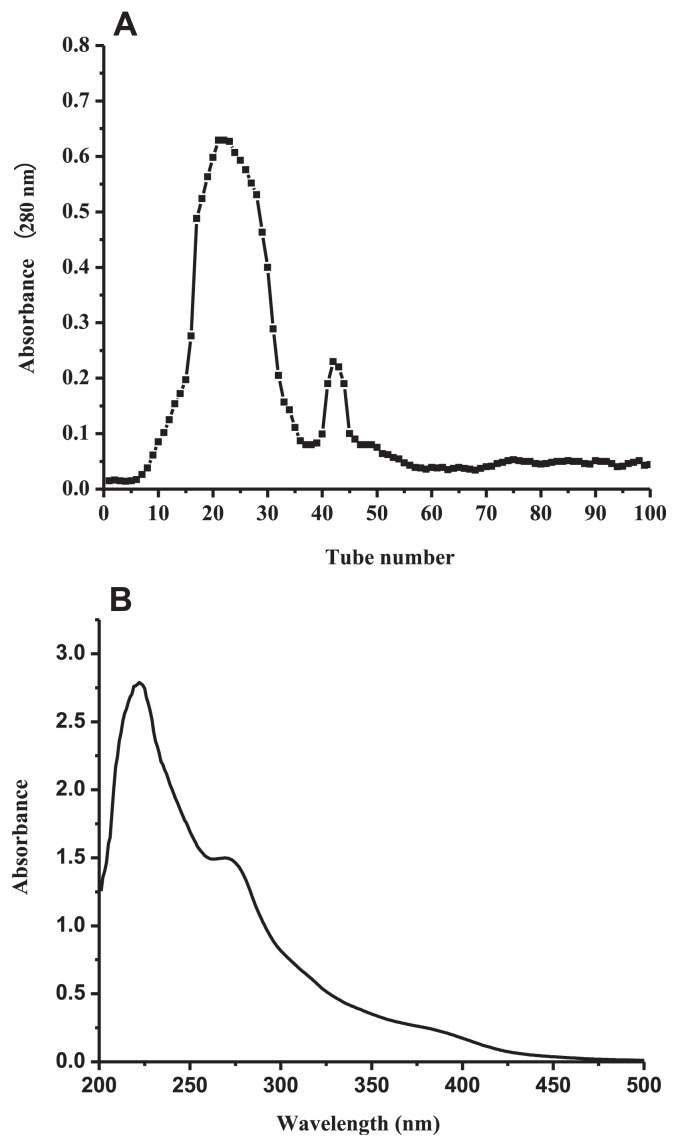
Polyphenols were isolated from L. singerianum extracted by Sephadex LH-20 chromatographic column, and the primary fraction (LSP-1) was collected (Figure 1A).
Figure 1.
(A) Chromatogram of Lachnum singerianum polyphenols on Sephadex LH-20 column and (B) UV-visible spectrum of Lachnum singerianum polyphenols.
3.2. Physicochemical characterization of LSP-1
3.2.1. UV-visible characterization
As shown in Figure 1B, there were two obvious ultraviolet absorption peaks at 222 nm and 269 nm (II belt) in the range of 200–300 nm. They were typical ultraviolet absorption peaks of phenolic components with benzoyl structure [23,28]. In addition, there was no ultraviolet absorption in the range of 300–400 nm which represented the characteristic absorption peaks of the flavonoids (I belt). Therefore, the primary content of extract was not flavonoids [29] but polyphenols.
3.2.2. FT-IR characterization
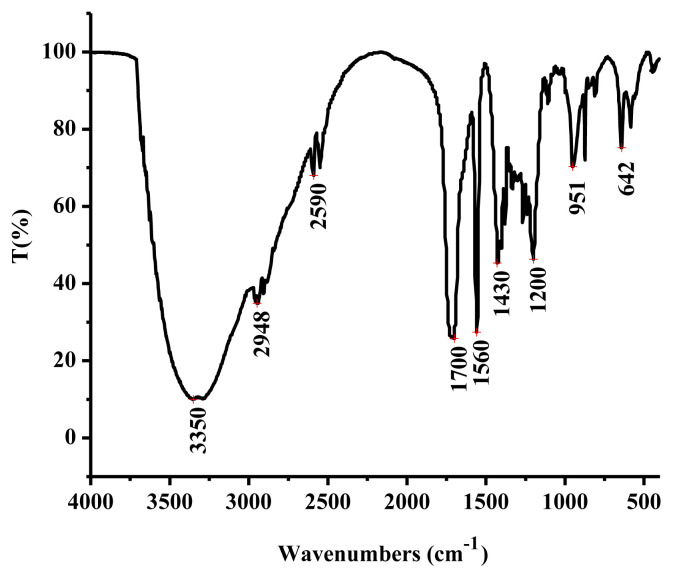
In the FT-IR spectrum (Figure 2), a broad peak at 3350/cm was assigned to the stretching vibration of −OH [30], while an adsorption peak at 2948/cm was caused by stretching vibration of = C–H in the benzene ring [31]. The peak at 1700/cm was resulted from stretching vibration of C‗O [30]. Two absorptions at 1560/cm and 1430/cm suggested that LSP-1 contained stretching vibration of aromatic ring [32], while the peak at 1200/cm was caused by the bending vibration of phenolic hydroxyl group. These results indicate that LSP-1 was composed of phenolic acids or polyhydric phenolics.
Figure 2.
Fourier transform infrared spectroscopy of Lachnum singerianum polyphenol-1.
3.2.3. GC-MS analysis
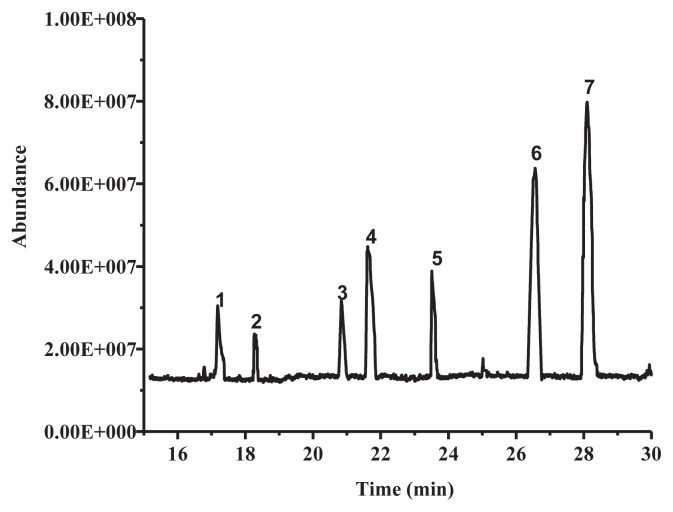
Phenolic compounds of LSP-1 were identified by GC-MS [23]. Seven kinds of phenolic compounds and their corresponding structures were determined by the NIST 11 MS Database and MS Search Program 2.0 (Figure 3 and Table 1). The total ion chromatogram of LSP-1 in Figure 3 indicated that the main ingredients of LSP-1 were phenolic acids, including 2,5-dihydroxybenzoic acid (Compound 4), 4-methylsalicylic acid (Compound 6), and 4-hydroxyphenyl lactic acid, ethyl ester (Compound 7). The other ingredients were polyhydric phenolics. The results of UV-vis and FT-IR also indicated that compounds of LSP-1 were phenolic acid derivatives with benzoyl structure.
Figure 3.
Total ion chromatogram of Lachnum singerianum polyphenols.
Table 1.
GC-MS analysis data of Lachnum singerianum polyphenol-1.
| Retention time (min) | Name | Structure | |
|---|---|---|---|
| 1 | 17.18 | Hydroquinone |
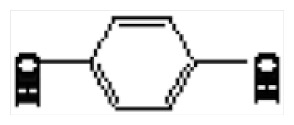
|
| 2 | 18.31 | 5-tert-Butylpyrogallol |
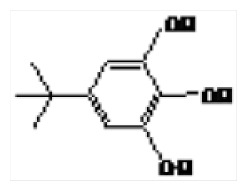
|
| 3 | 20.09 | 3,5-Dimethoxycinnamic acid |
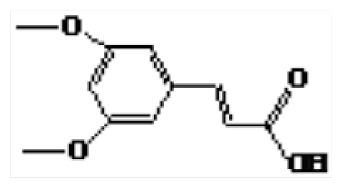
|
| 4 | 21.62 | 2,5-Dihydroxybenzoic acid |
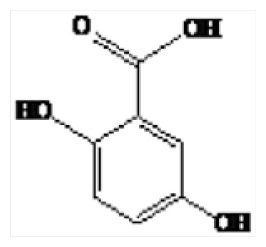
|
| 5 | 23.51 | 3-Methyl-hydroxybenzoic benzoic acid |
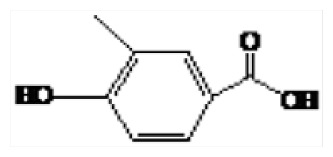
|
| 6 | 26.57 | 4-Methylsalicylic acid |
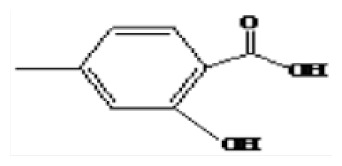
|
| 7 | 28.12 | 4-Hydroxyphenylactic acid, ethyl ester |
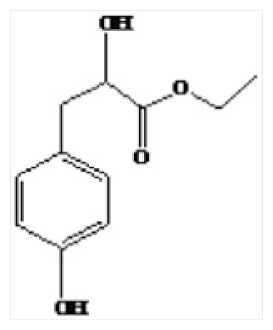
|
3.3. Anticoagulant activity assay in vitro
Coagulant and anticoagulant system in the body were in a dynamic equilibrium. Disorder of the body balance could cause the myocardial infarction, thrombosis, and other blood coagulation with thrombus being a common disease among the old. The activation of the anticoagulation system is an important mechanism for antithrombosis.
Resulting from a series of enzymatic reactions, coagulation process in the body could be divided into three stages: forming of thrombin activator; activation of prothrombin to thrombin; and transforming of fibrinogen into fibrin [33]. Initiated by different procedures, the coagulation process could be divided into exogenous and endogenous coagulation pathway. APTT extension reaction was caused by intrinsic coagulation and common coagulation pathways. PT extension reaction represented the exogenous coagulation pathway [34,35]. The TT extension reaction reflected both exogenous and endogenous coagulation pathways.
LSP-1 (100 mg/mL, 150 mg/mL, 200 mg/mL) could prolong APTT, PT, and TT significantly (p < 0.01). In addition, with the increased dose, APTT, PT, and TT were lengthened, exhibiting a dose-effect relationship (Table 2), which was similar to previous results [5]. The results showed that LSP-1 had inhibition effects on both endogenous and exogenous coagulation systems.
Table 2.
Effect of Lachnum singerianum polyphenol-1 (LSP-1) on activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT).
| Group | Dose (mg/L) | APTT (s) | PT (s) | TT(s) |
|---|---|---|---|---|
| Control | 25.67 ± 2.75 | 14.60 ± 1.05 | 19.4 ± 0.95 | |
| LSP | 50 | 32.57 ± 2.93a | 17.37 ± 0.85 | 26.7 ± 1.25b |
| 100 | 54.93 ± 1.88b | 23.27 ± 1.60b | 30.43 ± 1.86b | |
| 150 | 67.57 ± 3.89b | 27.50 ± 2.10b | 37.07 ± 1.90b | |
| 200 | 87.03 ± 2.53b | 32.17 ± 2.48b | 58.83 ± 2.25b | |
| Asp | 50 | 119.23 ± 3.55b | 39.73 ± 2.60b | 85.20 ± 2.72b |
p < 0.05,
p < 0.01, compared with the control group.
3.4. Anticoagulant activity assay in vivo
3.4.1. Determination of CT and BT
Adrenaline could facilitate the peripheral vascular to contract and increase the load on the heart. In addition, it increases the consumption of myocardial oxygen and decreases the cardiac function, causing blood circulation disorders. The mice were injected into adrenaline repeatedly which could simulate longer depression, causing higher viscosity and convert mouse blood to a hypercoagulable state [24]. CT and BT are basic indices to evaluate anticoagulation in vivo. BT related to the number and the function of platelet, the function of tissue factor, the function of the capillary, etc. CT can reflect the anticoagulation factor activity and the coagulation factor function [36].
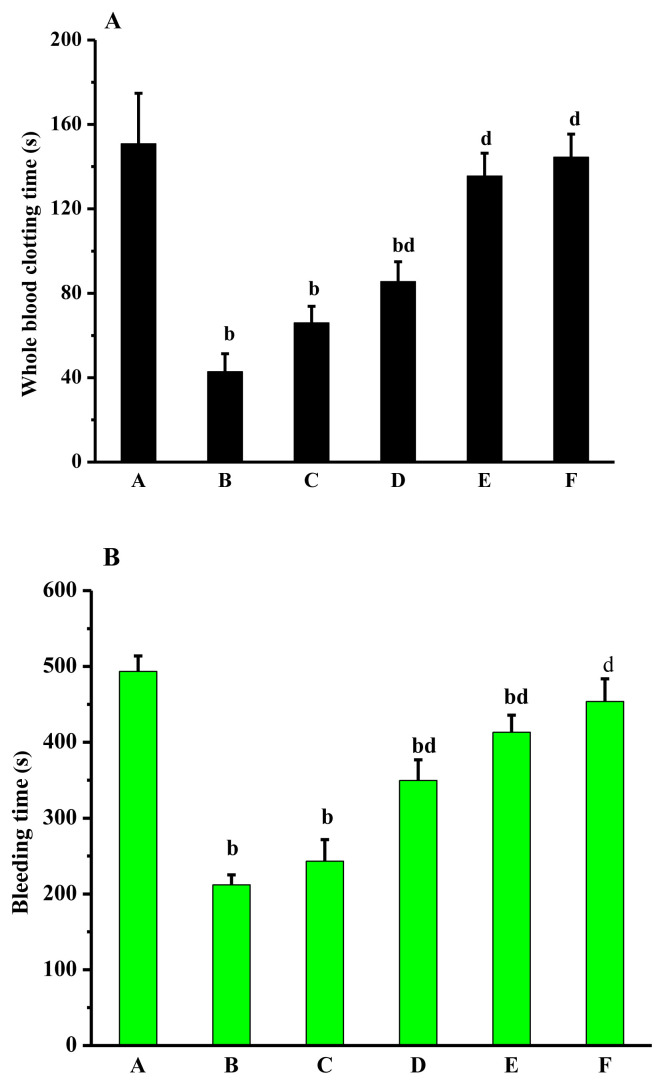
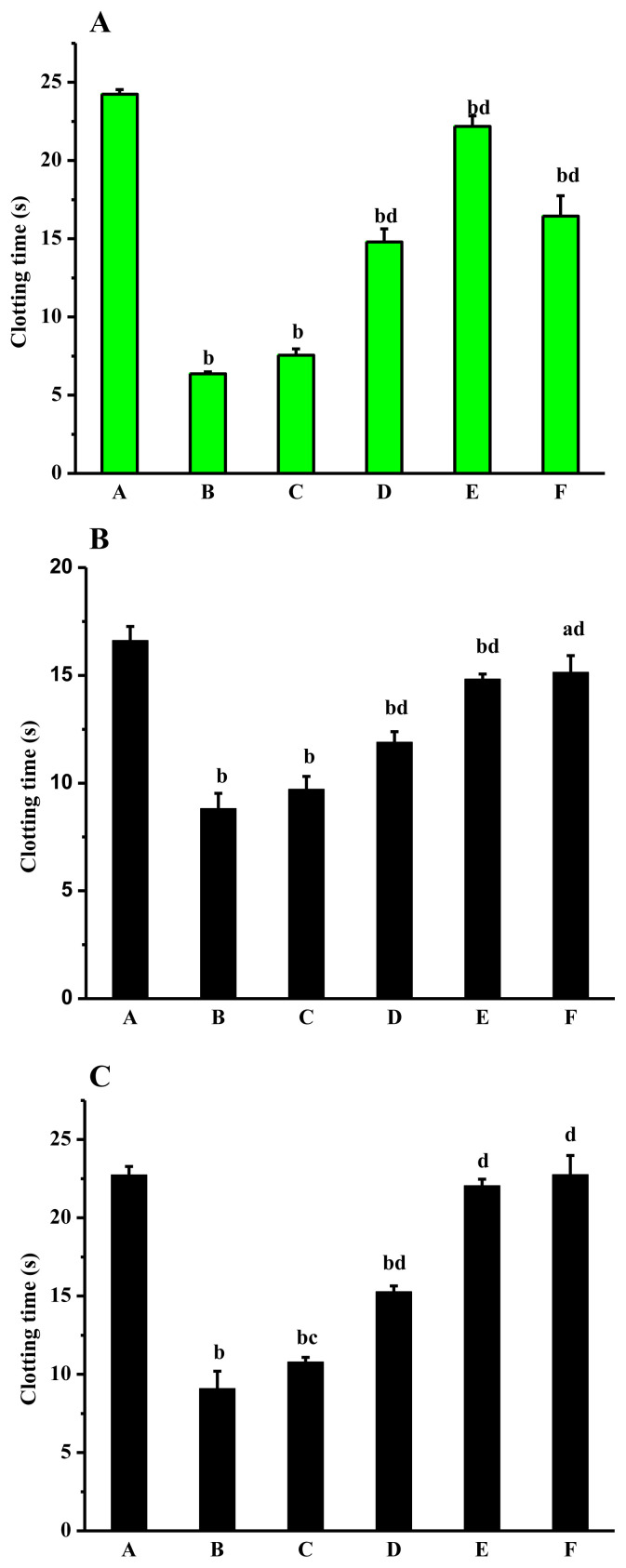
Compared with the normal group, CT and BT in the model group were reduced significantly (p < 0.01). Compared with the model group, CT and BT in the dose group and aspirin group were extended differently. With the increasing of dose, CT and BT also increased, showing a dose-effect relationship to a certain extent. CT and BT were significantly prolonged in middle- and high-dose groups (p < 0.01). CT and BT in normal group and high-dose group had no significant differences (Figure 4).
Figure 4.
Effects of Lachnum singerianum polyphenol-1 on (A) coagulation time and (B) bleeding time of the model mice. A = normal control group; B = model control group; C = low-dose group; D = middle-dose group; E = high-dose group; F = positive control group.
3.4.2. Determination of APTT, PT and TT
As shown in the Figure 5, compared with normal group, PT, APTT and TT in model group were decreased significantly (p < 0.01). The result showed that adrenaline could convert the blood of mice to a hypercoagulable state. Therefore, the model was established successfully. PT, APTT, and TT in the polyphenol dose groups were prolonged. The results between middle- and high-dose groups were compared and the effects of LSP-1 on PT, APTT, and TT were significant (p < 0.01). Overall, the results in vivo and in vitro indicated that LSP-1 had a synergic anticoagulant effect by endogenous and exogenous pathway [37].
Figure 5.
Effects of Lachnum singerianum polyphenol-1 on (A) activated partial thromboplastin time, (B) prothrombin time, and (C) thrombin time of the model mice. A = normal control group; B = model control group; C = low-dose group; D = middle-dose group; E = high-dose group; F = positive control group.
3.4.3. Determination of FIB content
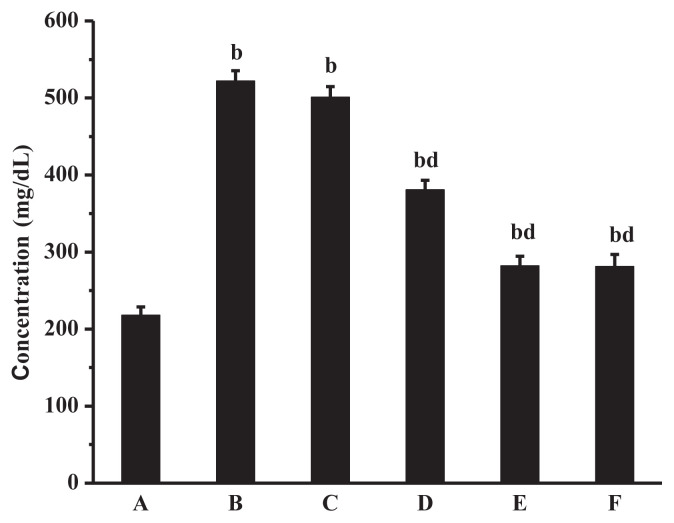
FIB is one of the highest concentration coagulation proteins in plasma. By the action of thrombin, FIB can form insoluble fibrin, which played an important role in the agglutination process of platelets [38]. Increasing fibrinogen levels can increase blood viscosity and retard blood flow, and is an independent risk factor for blood in a hypercoagulable state [39]. As can be seen from Figure 6, the fibrinogen content of the model group was significantly higher than that of the normal group (p < 0.01). Higher fibrin content could be found in medium- and high-dose groups. The dose groups had significant differences compared the model group. The result suggests that Lachnum polyphenols might accelerate the cleavage of FIB, inhibit the aggregation of platelet, and adjust the rheology of blood by activating the fibrinolytic system.
Figure 6.
Effect of Lachnum singerianum polyphenol-1 on fibrinogen of the model mice. A = normal control group; B = model control group; C = low-dose group; D = middle-dose group; E = high-dose group; F = positive control group.
3.4.4. Determination of AT-III activity
AT-III, synthesized by liver cells, macrophages, and endothelial cells, is a single-chain glycoprotein and a multifunctional serine protease inhibitor, playing an important role in the anticoagulation system. It can form a complex with heparin, which could accelerate inactivation of thrombin and thus inhibit the coagulation of blood [6].
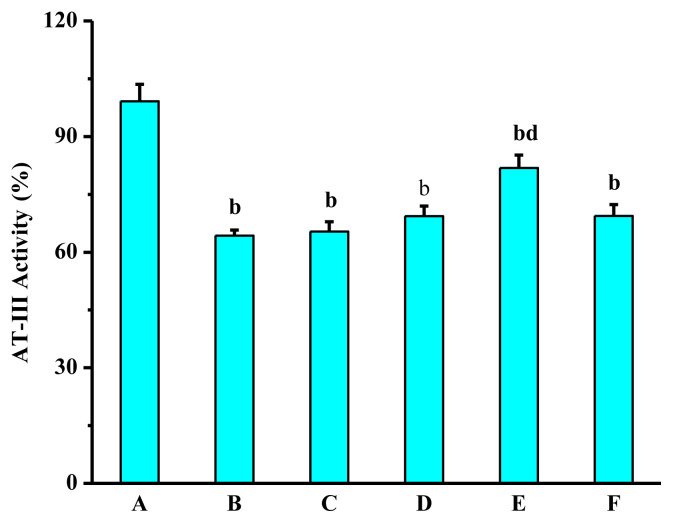
As shown in Figure 7, compared with the normal control group, the AT-III activity of the model group was significantly decreased. The underlying reason might be that adrenaline activated the coagulation factors in mice, which caused to produce large amounts of thrombin, and thus a lot of AT-III was consumed. Compared with the model group, AT-III activity of the positive control group was increased, but compared with the model group, there were no significant differences. The activity of AT-III in the high dose group was significantly enhanced. Possible reasons include that LSP-1 could significantly reduce the content of FIB, leading to decrease of the binding of fibrinogen and thrombin. In addition, after LSP-1 is absorbed into the blood stream, carboxyl of LSP-1 might reaction with metal ions to form a pendant group containing a carboxylate that could increase the anticoagulant III activity by modifying the affinity of AT-III proteins [40]. The exact mechanism underlying the enhancement of AT-III activity by LSP-1 needs further research.
Figure 7.
Effect of Lachnum singerianum polyphenol-1 on the antithrombin-III activity of the model mice. A = normal control group; B = model control group; C = low-dose group; D = middle-dose group; E = high-dose group; F = positive control group.
4. Conclusion
In this study, we evaluated the physicochemical properties and anticoagulant activity of the primary fraction (LSP-1) of polyphenols obtained from the fermentation broth of L. singerianum for the first time. Phenolic acids and polyhydroxy phenolic compounds of LSP-1 were determined. The results showed that LSP-1 had an inhibition effect on endogenous and exogenous coagulation system in vitro. In-vitro tests indicated that LSP-1 had significant anticoagulant activity compared to normal group. The mechanism of anticoagulation might be related to the improvement of AT-III activity induced by LSP-1. All these positive results suggest that LSP-1 is very promising for the future development of an anticoagulant drug candidate for thrombosis.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31270060).
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (No. 31270060).
Footnotes
Conflicts of interest
The authors declare no competing financial interest.
REFERENCES
- 1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anand M, Rajagopal K, Rajagopal KR. A model for the formation and lysis of blood clots. Pathophysiol Haemostat Thromb. 2005;34:109–20. doi: 10.1159/000089931. [DOI] [PubMed] [Google Scholar]
- 3. Biggs R, Douglas AS, Macfarlane RG. The formation of thromboplastin in human blood. J Physiol. 1953;119:89–101. doi: 10.1113/jphysiol.1953.sp004830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davie EW. Biochemical and molecular aspects of the coagulation cascade. Thromb Haemost. 1995;74:1–6. [PubMed] [Google Scholar]
- 5. Kim DC, Ku SK, Bae JS. Anticoagulant activities of curcumin and its derivative. Biochem Mol Biol Rep. 2012;45:221–6. doi: 10.5483/bmbrep.2012.45.4.221. [DOI] [PubMed] [Google Scholar]
- 6. Dahlbäck B. Blood coagulation. Lancet. 2000;355:1627–32. doi: 10.1016/S0140-6736(00)02225-X. [DOI] [PubMed] [Google Scholar]
- 7. Bai L, Guo S, Liu Q, Cui X, Zhang X, Zhang L, Yang X, Hou M, Ho CT, Bai N. Characterization of nine polyphenols in fruits of Malus pumila mill by high-performance liquid chromatography. J Food Drug Anal. 2016;43:1071–81. doi: 10.1016/j.jfda.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li W, He N, Tian L, Shi X, Yang X. Inhibitory effects of polyphenol-enriched extract from Ziyang tea against human breast cancer MCF-7 cells through reactive oxygen species-dependent mitochondria molecular mechanism. J Food Drug Anal. 2016:1–12. doi: 10.1016/j.jfda.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nardini M, Natella F, Scaccini C. Role of dietary polyphenols in platelet aggregation. A review of the supplementation studies. Platelets. 2007;18:224–43. doi: 10.1080/09537100601078083. [DOI] [PubMed] [Google Scholar]
- 10. Hussein L, Medina A, Barrionnevo A, Lammuela-Raventos RM, Andres-Lacueva C. Normal distribution of urinary polyphenol excretion among Egyptian males 7–14 years old and changes following nutritional intervention with tomato juice (Lycopersicon esculentum) Int J Food Sci Nutr. 2008;60:302–11. doi: 10.1080/09637480701780047. [DOI] [PubMed] [Google Scholar]
- 11. Jastrzebski Z, Medina OJ, Moreno LM, Gorinstein S. In vitro studies of polyphenol compounds, total antioxidant capacity and other dietary indices in a mixture of plants (Prolipid) Int J Food Sci Nutr. 2007;58:531–41. doi: 10.1080/09637480701335941. [DOI] [PubMed] [Google Scholar]
- 12. Rechner AR, Kroner C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb Res. 2005;116:327–34. doi: 10.1016/j.thromres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13. Freedman JE, Parker C, Li L, Perlman JA, Frei B, Ivanov V, Deak LR, Iafrati MD, Folts JD. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–8. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- 14. Bijak M, Bobrowski M, Borowiecka M, Podsędek A, Golański J, Nowak P. Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia. 2011;82:811–7. doi: 10.1016/j.fitote.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 15. Ye M, Chen X, Li GW, Guo GY, Yang L. Structural characteristics of pheomelanin-like pigment from Lachnum singerianum. Adv Mater Res. 2011;284–286:1742–5. [Google Scholar]
- 16. Ye M, Chen WX, Qiu T, Yuan RY, Ye YW, Cai JM. Structural characterisation and anti-ageing activity of extracellular polysaccharide from a strain of Lachnum sp. Food Chem. 2012;132:338–43. doi: 10.1016/j.foodchem.2011.10.087. [DOI] [PubMed] [Google Scholar]
- 17. He Y, Ming Y, Jing L, Du Z, Surahio M, Xu H, Li J. Preparation, characterization and bioactivities of derivatives of an exopolysaccharide from Lachnum. Carbohyd Polym. 2015;117:788–96. doi: 10.1016/j.carbpol.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 18. Ye M, Guo GY, Lu Y, Song S, Wang HY, Yang L. Purification, structure and anti-radiation activity of melanin from Lachnum YM404. Int J Biol Macromol. 2013;63:170–6. doi: 10.1016/j.ijbiomac.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 19. Lu Y, Ni L, Qian MS, Ye M. Purification of methyl linoleate and analysis of oils produced by Lachnum mutant strain. Food Sci Tech. 2013;38:15–20. [Google Scholar]
- 20. Qian MS, Ji J, Ye M, Shaikh F, Wang HY, Yang L. Effect of nitric oxide on Lachnum YMU50 extracellular polyphenol accumulation and antioxidant defense system. Appl Biochem Biotech. 2014;174:1761–70. doi: 10.1007/s12010-014-1154-1. [DOI] [PubMed] [Google Scholar]
- 21. Qian MS, Ji J, Chen TL, Yang L, Ye M. Physicochemical characteristics and biological activities of polyphenols from Lachnum. Adv Microbiol. 2013;2:102–8. [Google Scholar]
- 22. Pfundstein B, Desouky SKE, Hull WE, Haubner R, Erben G, Owen RW. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 2010;71:1132–48. doi: 10.1016/j.phytochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 23. Masek A, Chrzescijanska E, Kosmalska A, Zaborski M. Characteristics of compounds in hops using cyclic voltammetry, UV–VIS, FTIR and GC–MS analysis. Food Chem. 2014;156:353–61. doi: 10.1016/j.foodchem.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 24. Lan HE, Jiang WY, Mao TM. Preliminary exploration on establishing a simulated model of acute and chronic after-qi-stagnation blood stasis by adrenaline injection. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:244–6. [PubMed] [Google Scholar]
- 25. Wang GZ, Li XL, Ma SL. Study on the treatment of allergic purpura with high coagulation state and heparin anticoagulant therapy. Appl J Gen Pract. 2007;5:901–2. [Google Scholar]
- 26. Guo Q, Lianda LI, Hao W, Zhao L, Xianghuib QI. Effects of recombination of effective constituent of cortex moutan on platelet aggregation and blood coagulation in rabbits. Chin J Mod Appl Pharm. 2010;27:967–70. [Google Scholar]
- 27. Cui Y, Zhang J, Zhang R, Huang L. Study on structure analysis and anticoagulant function in vitro of Garlic polysaccharides. Food Fermen Ind. 2009;35:24–7. [Google Scholar]
- 28. Markham KR. Flavones, flavonols and their glycosides. Meth Plant Biochem. 1989;1:197–235. [Google Scholar]
- 29. He JB, Wang Y, Deng N, Lin XQ. Study of the adsorption and oxidation of antioxidant rutin by cyclic voltammetry–voltabsorptometry. Bioelectrochemistry. 2007;71:157–63. doi: 10.1016/j.bioelechem.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 30. Lan P, Gambier F, Pizzi A, Zhou DG, Brosse N. Wood adhesives from agricultural by-products: lignins and tannins for the elaboration of particleboards. Cell Chem Technol. 2012;46:457–62. [Google Scholar]
- 31. Baran NY, Demir HÖ, Kostekçi S, Sacak M. Poly-2-[(4-methylbenzylidene)amino]phenol: investigation of thermal degradation and antimicrobial properties. J Appl Polym Sci. 2014;132:357–67. [Google Scholar]
- 32. Saad H, Khoukh A, Ayed N, Charrier B, Bouhtoury CE. Characterization of Tunisian Aleppo pine tannins for a potential use in wood adhesive formulation. Ind Crop Prod. 2014;61:517–25. [Google Scholar]
- 33. Wang XB, Cheng H, Zhao Y, Huang HT. Study on the EGCG from tea on the blood anticoagulation effect of mice. J Tea Sci. 2011;31:532–6. [Google Scholar]
- 34. Bi Q, Han B, Feng Y, Jiang Z, Yang Y, Liu W. Antithrombotic effects of a newly purified fibrinolytic protease from Urechis unicinctus. Thromb Res. 2013;132:135–44. doi: 10.1016/j.thromres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 35. Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115:3355–62. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang QZ, Liu JT, Shi XL, Jing JL. Effect of non-heating leech extract on coagulation system and platelet aggregation in the animal model with blood hypercoagulable state. Chinese J Exp Tradit Med Form. 2006;12:47–9. [Google Scholar]
- 37. Wei YX, Jing LI, Wang JC, Qi HT. Screening of different fractions of phlorotannins from Sargassum thunbergii Kuntze for anticoagulant activity. Chinese J Biochem Pharm. 2007;28:227–9. [Google Scholar]
- 38. Påhlman LI, Mörgelin M, Kasetty G, Olin AI, Schmidtchen A, Herwald H. Antimicrobial activity of fibrinogen and fibrinogen-derived peptides–a novel link between coagulation and innate immunity. Thromb Haemost. 2013;109:930–9. doi: 10.1160/TH12-10-0739. [DOI] [PubMed] [Google Scholar]
- 39. Pop GA, Bisschops LL, Iliev B, Struijk PC, Hoeven JG, Hoedemaekers C. On-line blood viscosity monitoring in vivo with a central venous catheter, using electrical impedance technique. Biosens Bioelectron. 2013;41:595–601. doi: 10.1016/j.bios.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 40. da Silva E, Ficheux D, Coleman AW. Anti-thrombotic activity of water-soluble calix[n]arenes. J Incl Phenom Macro. 2005;52:201–6. [Google Scholar]