Abstract
The objective of this research was to access the determination of metabolite profiles and antioxidant properties in the leaves of green perilla (Perilla frutescens), where these are considered functional and nutraceutical substances in Korea. A total of 25 compositions were confirmed as six phenolic acids, two triterpenoids, eight flavonoids, seven fatty acids, and two glucosides using an ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS/MS) technique from the methanol extract of this species. The individual and total compositions exhibited significant differences, especially rosmarinic acid (10), and linolenic acids (22 and 23) were detected as the predominant metabolites. Interestingly, rosmarinic acid (10) was observed to have considerable differences with various concentrations in three samples (Doryong, 6.38 μg/g; Sinseong, 317.60 μg/g; Bongmyeong, 903.53 μg/g) by UPLC analysis at 330 nm. The scavenging properties against 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radicals also showed potent effects with remarkable differences at a concentration of 100 μg/mL, and their abilities were as follows: Sinseong (DPPH, 86%; ABTS, 90%) > Bongmyeong (71% and 84%, respectively) > Doryong (63% and 73%, respectively). Our results suggest that the antioxidant activities of green perilla leaves are correlated with metabolite contents, especially the five major compositions 10 and 22–25. Moreover, this study may be useful in evaluating the relationship between metabolite composition and antioxidant activity.
Keywords: antioxidant activity, green perilla leaves, metabolite, rosmarinic acid, UPLC-ESI-Q-TOF-MS/MS
1. Introduction
In recent years, metabolites including phenolic compounds and triterpenoids have been of great interest in food and medical industries because of their beneficial effects on human health [1–5]. Phenolic compounds are distributed in crops, fruits, vegetables, and edible natural plants [6–8] and are associated with a wide range of health beneficial effects including antioxidant, antidiabetic, anti-inflammatory, and anticancer agents [1,3,7,9]. Triterpenoids have also been reported to play essential roles in preventing human diseases because of their anticancer, antioxidant, antibacterial, and antiatherosclerotic properties [2,4,10]. Moreover, many studies have reported that fatty acids have beneficial properties on lipoprotein profile, blood cholesterol level, and basal metabolism of humans [11,12]. For these reasons, several researchers have focused on natural sources with high phytochemical contents for the manufacture of supplements with preventive and therapeutic capacities. In our continuing survey of bioactive substances, an investigation of the metabolite profile in the leaves of green perilla was carried out.
Perilla [Perilla frutescens (L.) Britt], which belongs to the Labiatae family, is a widely cultivated edible and medicinal plant in Asian countries such as China, Japan, India, and South Korea [13]. Moreover, this plant has long been used as an important traditional medicine for treating diseases such as tumor, cough, allergy, and intoxication [14,15]. Numerous researches have described that the health beneficial capacities of perilla are related to its metabolite contents (phenolic acids, monoterpenes, flavonoids, and triterpenoids) [2,6,16,17]. In particular, the leaves, seeds, and stems of this species have shown to have antipyretic and antibiotic effects for treating intestinal disorders [18]. Perilla leaves are used as food and medicinal materials for their antimicrobial, antioxidant, anticancer, antidiabetic, antitumor, and antiallergic effects owing to the presence of phenolic compounds, monoterpenes, and triterpenoids [18–21]. Perilla seeds are an important source of fatty acids (α-linolenic acid, linoleic acid, oleic acid, and palmitic acid) and possess health benefits, such as lowering risk of colon cancer as well as plasma lipid levels, reducing the cholesterol level, and reducing the triglyceride level [22–24]. Commonly, human health benefits are associated with many antioxidant metabolites. Among these various biological benefits, perilla plant has demonstrated potent antioxidant properties by in vitro method. For example, studies on purple (red) perilla have revealed that the high antioxidant activities are attributable to several anthocyanins [25]. Also, green perilla is known to be associated with phenolic compounds except anthocyanins [26]. We recently reported the optimal harvest time for the phytochemical contents in the leaves of purple perilla [27]. Moreover, we evaluated information concerning the antioxidant effects and inhibitory activities against α-glucosidase as well as aldose reductase of perilla seeds [28]. Several studies have also established the metabolite contents and their biological activities in perilla plant [18–24]. Even though many reports have evaluated the beneficial health effects of perilla regarding metabolites, the exact chemical components in the leaves of green perilla have still not been fully characterized. In addition, the antioxidant capacities of natural plants are considered to have higher synergistic activities in metabolite extracts compared to the effects of a single phytochemical in recent years [1,3,6]. For these reasons, in order to evaluate the antioxidant abilities of perilla leaves, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzthiazo-line-6-sulphonic acid) (ABTS) radical methods, based on an electron transfer and involving the reduction of a colored oxidant (DPPH: purple; ABTS: blue/green), have been measured by the spectrophotometric assay. Therefore, our work was designed to investigate the metabolite profiles and antioxidant capacities from the methanol extract of a widely used green perilla leaves.
The purpose of the present research was to characterize the metabolite profiles in the leaves of Korean green perilla using ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS/MS) system. Furthermore, this study is the first to investigate the changes in metabolite compositions from three methanol extracts of this species. We also determined the antioxidant properties of the scavenging abilities on DPPH and ABTS radicals in view of simplicity, easy control, and cost-effectiveness aspects.
2. Materials and methods
2.1. Plant material and chemicals
The leaves of green perilla were obtained from local markets (Daejeon) in Korea. Three samples (Doryong, Sinseong, Bongmyeong) of perilla leaves were air-dried for 3 days at room temperature to remove the moisture. The collected perilla leaves were immediately freeze-dried at −40°C until analysis. Caffeic acid and rosmarinic acid standards were isolated by chromatographic techniques using silica gel column chromatography (230–400 mesh silica gel, kieselgal 60; Merck, Darmstadt, Germany) based on the data reported in a previous study [6]. Silica gel column chromatography was carried out using 230–400 mesh silica gel (kieselgal 60; Merck) and DPPH and ABTS were purchased from Sigma (St. Louis, MO, USA). Analytical-grade methanol, formic acid, and water were purchased from J.T. Baker (Phillipsburg, NJ, USA). Moreover, other chemicals, reagents, and solvents used in the present research were of analytical grade.
2.2. Instruments
The isolated phenolic compounds, caffeic acid and rosmarinic acid, were elucidated with a Bruker AM 500 [1H nuclear magnetic resonance (NMR) at 500 MHz, 13C NMR at 125 MHz] spectrometer (Bruker, Karlsruhe, Germany) using DMSO-d6 with tetramethylsilane. Two phenolic contents were performed using an Agilent 1200 UPLC series (Agilent Technologies, Inc., Wilmington, DE, USA) including a quaternary gradient pump and an Agilent 1200 series photodiode array detector. For analysis of multiple compositions in perilla leaves, the UPLC system (Agilent 1200 series) was coupled with triple quadrupole time-of-flight tandem mass spectrometry (LC/MS-Triple TOF 5600+; AB SCIEX, Foster City, CA, USA) equipped with an electrospray ionization (ESI) interface. To evaluate the antioxidant capacities concerning the scavenging effects against DPPH and ABTS radicals, UV–Vis absorption spectra were measured on a Beckman DU650 spectrophotometer (Beckman Coulter, Fullerton, CA, USA).
2.3. Sample preparation and calibration curves
The dried leaves of green perilla were pulverized (60 mesh) using an HR 2860 coffee grinder (Philips, Drachten, Netherlands) for 3 minutes. The powdered leaves (1.0 g) were extracted with methanol (10 mL) in a shaking incubator for 8 hours at room temperature. The supernatant was centrifuged at 3000g for 3 minutes using VS-6000CFN (VISION, Seoul, Korea), and then filtered through a 0.45-μm syringe filter (Whatman Inc., Maidstone, UK) prior to UPLC analysis. For the calibration curve, the peak area of the identified phenolic compounds (rosmarinic acid and caffeic acid) was integrated with the UPLC chromatogram at 330 nm and plotted against the concentration to create a linear curve. The stock solutions of standard material were prepared with methanol to obtain a 1 mg/mL concentration, and calibration curves were made by dilution of each stock solution in methanol to six concentrations (1 μg/mL, 5 μg/mL, 10 μg/mL, 20 μg/mL, 50 μg/mL, and 100 μg/mL). The correlation coefficients (r2) of two phenolic standards were detected, higher than 0.999.
2.4. UPLC conditions
The UPLC analysis was carried out using an Agilent Technologies 1290 Infinity series instrument coupled to a binary pump, a photodiode array detector, an autosampler, and a column compartment (kept at 25°C). A 5-μL sample of the methanol extract in perilla leaves was injected into an analytical reverse phase column (Poroshell 120 EC-C18, 150 × 2.1 mm, I.D., 4 μm). Separation was performed with 0.1% formic acid in water (eluent A) and methanol (eluent B). The gradient elution program was follows: 0–5 minutes, 20% B; 20 minutes, 35% B; 40 minutes, 75% B; and then held for 10 minutes prior to returning to the initial conditions. The total running time was 20 minutes at a flow rate of 0.4 mL/min, and the wavelength of detection was recorded at 330 nm.
2.5. UPLC-ESI-Q-TOF-MS/MS conditions
The metabolite profile in the methanol extract of perilla leaves was analyzed on a triple TOF 5600+ system coupled to the UPLC system. The mass spectrometer was operated in the negative ESI mode with Duo-Spray source, and the mass scan range was set at m/z 100–1000 for both TOF-MS and TOF-MS/ MS scan using a resolution of 2700. The following parameter conditions were used: ion spray voltage, −4500 V; ion source heater, 500°C; curtain gas, 25 psi; nebulizer gas 50°C (GS1 50 and GS2 50); collision energy, −10 eV; declustering potential −100. The analyst TF software (version 1.7) combined with the information-dependent acquisition packing was used to acquire the MS/MS data. The mobile phase was composed of 0.1% formic acid in water (elution A) and methanol (elution B) using a gradient elution of 30% elution B (0–5 minutes), from 30% to 50% of elution B (5–20 minutes), from 50% to 90% elution B (20–40 minutes), and from 90% to 100% of elution B (40–45 minutes).
2.6. Antioxidant activity
In order to measure antioxidant abilities, the DPPH and ABTS radical scavenging methods were used in this research. The DPPH radical scavenging effect in the methanol extract of perilla leaves was determined as described by Lee et al [6]. The grounded leaves (1.0 g, 60 mesh) were extracted with methanol (10 mL) for 6 hours at room temperature. The crude extract was filtered through Whatman No. 42 filter paper, and the supernatants were measured. BHT (positive control) or sample (0.1 mL) of various concentrations (300 μg/mL, 200 μg/ mL, 100 μg/mL, 50 μg/mL, 20 μg/mL, 10 μg/mL, and 5 μg/mL) were mixed with 0.49 mL of methanol and 0.39 mL methanolic solution of 1mM DPPH. The reaction mixture was vortexed and left to stand for 30 minutes at room temperature in darkness. The scavenging activity was reported as a percentage using the following formula at 517 nm: DPPH radical scavenging effect (%) = (1 − absorbance of sample/absorbance control) × 100.
The scavenging capacity of the ABTS radical was defined as the ability of different substances to scavenge the ABTS•+ radical cation, in comparison with a positive control (Trolox) [6]. This radical cation was generated by reacting the 7mM ABTS•+ stock solution with 2.45mM potassium persulfate, and the mixture was maintained for 10 hours in the dark. The ABTS•+ stock solution was melted with ethanol to an absorbance of 0.70 at 734 nm. The ABTS•+ solution (0.9 mL) was mixed with 0.1 mL sample (300 μg/mL, 200 μg/mL, 100 μg/mL, 50 μg/mL, 20 μg/mL, 10 μg/mL, and 5 μg/mL) and then measured using a spectrophotometer. This effect was determined as a percentage using the following formula: ABTS•+ scavenging capacity (%) = [(absorbance of control − absorbance of sample)/absorbance of control] × 100.
2.7. Statistical analysis
All determinations were based on three replicate samples, and the results for the caffeic acid and rosmarinic acid contents are shown as mean values. The results were determined using Microsoft Excel (Microsoft 2013; Microsoft Corporation, Roselle, IL, USA).
3. Results and discussion
3.1. Determination of two phenolic acid contents in the leaves of green perilla
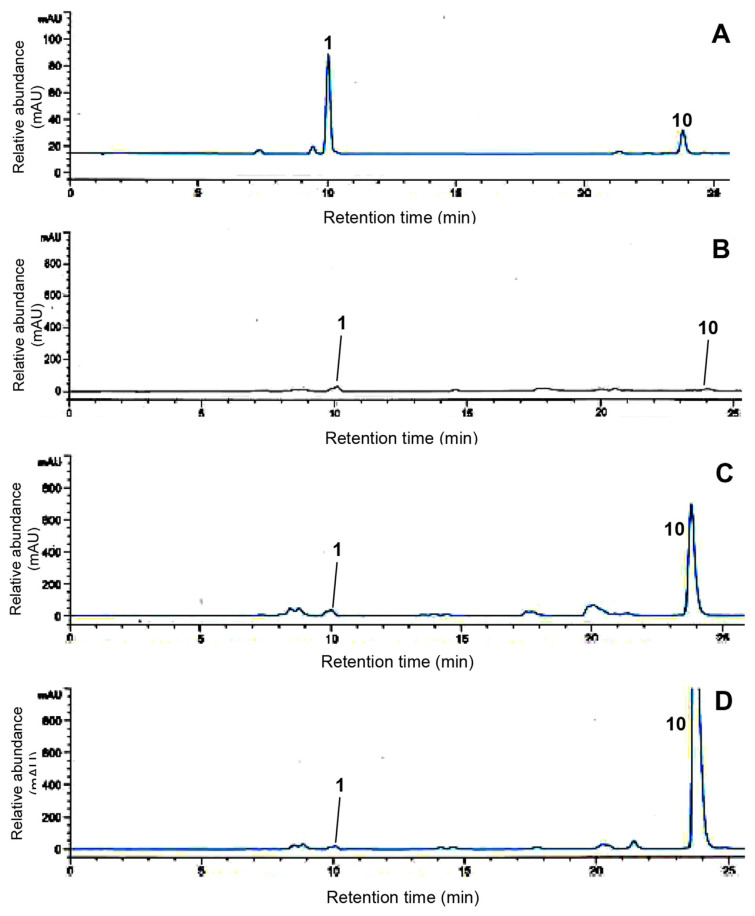
Perilla contains remarkably high amounts of phytochemical contents for the manufacture of supplements with therapeutic and preventive properties for human beneficial health effects, such as antioxidant, anticancer, and antidiabetic properties [18–21,29]. Among the various phytochemicals, caffeic acid and rosmarinic acid are of great interest in food and crop industries because of their attractive biological activities [6,19,28]. We evaluated the main phenolic acids in the methanol extract of perilla leaves using UPLC analysis. A representative UPLC chromatogram of two phenolic acids was produced within 25 minutes at a wavelength of 330 nm (Figure 1A). The identification of each peak in the sample was confirmed using the retention time in the chromatogram, and their retention times are as follows: peak 1 (caffeic acid), tR = 9.8 minutes and peak 2 (rosmarinic acid), tR = 23.7 minutes. To quantitatively analyze phenolic acids, calibration curves were constructed in the range of 100–1 μg/ mL, and their equations were calculated as y = 1352.35x + 105.13 (caffeic acid, r2 = 0.9998) and y = 760.68x + 25.27 (rosmarinic acid, r2 = 0.9997). We have chosen methanol for extraction of two phenolic acids in perilla leaves because it is an appropriate solvent for the maximum extraction of phenolic compounds [30,31]. The typical UPLC chromatograms of three perilla leaves are shown in Figures 1B–1D. As can be seen, the metabolite profiles exhibited similar patterns except one peak. Briefly, target phenolic acids, caffeic acid (1) and rosmarinic acid (10), presented in all samples and peak 10 exhibited significant differences. The highest rosmarinic acid content was 903.53 μg/g in Bongmyeong (Figure 1D), whereas the lowest was 6.38 μg/g in the Doryong sample (Figure 1B). Moreover, Sinseong showed the second highest content with 317.60 μg/g (Figure 1C). Caffeic acid (1) exhibited little differences with 6.69 μg/g (Doryong), 8.54 μg/g (Sinseong), and 2.54 μg/g (Bongmyeong), respectively. Although numerous studies have revealed that the major metabolites of perilla leaves are caffeic acid and rosmarinic acid, this study observed one major phenolic acid (rosmarinic acid) with various contents. These results suggest that the distribution of phenolic acids in natural plants may be influenced by various factors including environmental stress (light, temperature, region, and moisture) and agronomic conditions [1,7,32]. Their contents may also be affected by cultivars, sowing periods, years, and genetics, as previously reported data [6,7]. Our work agreed with earlier studies reporting the distribution of phenolics in many foods and crops [27,32,33].
Figure 1.
UPLC chromatograms concerning caffeic acid (1) and rosmarinic acid (10) in the methanol extracts of green perilla leaves. (A) The isolated phenolic acid standard mixture. (B) Doryong sample. (C) Sinseong sample. (D) Bongmyeong sample.
3.2. Characterization of metabolites using UPLC-ESI-Q-TOF-MS/MS analysis
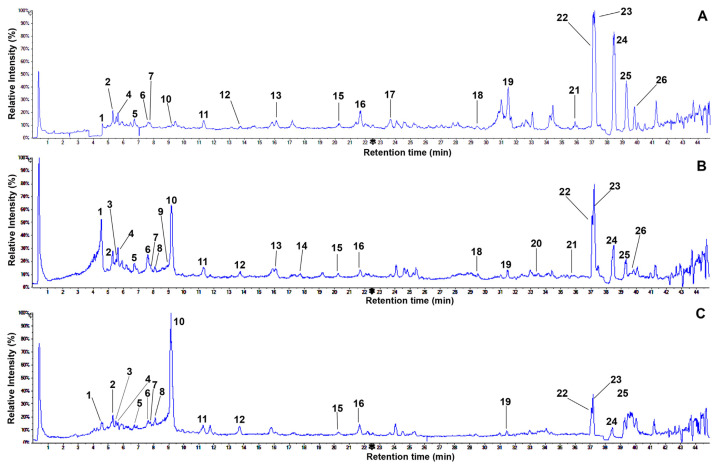
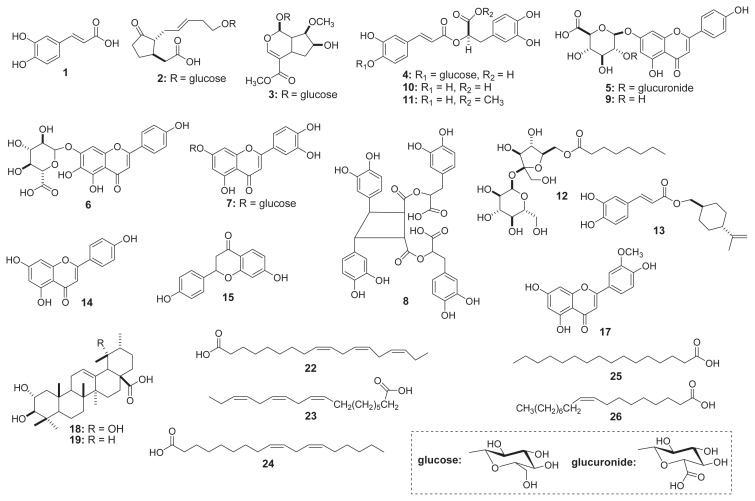
It is commonly demonstrated that the UPLC system coupled with MS is an excellent analytical method for simultaneously identifying complicated metabolites owing to the product ions produced from the fragmentation of a selected precursor ion [32,34]. Therefore, metabolite profiles in the leaves of three perilla samples were tentatively investigated by UPLC-ESI-Q-TOF-MS/MS through the full scan negative ESI mode. In the current research, a complete chromatographic separation of various metabolites, including major and minor peaks, was reached within 45 minutes (Figure 2). Their compositions were observed to have significant differences in samples. Table 1 shows the retention times, elementary compositions, and mass spectral data (molecular and fragment ions) of the identified metabolites by comparison with those of previous studies. First, we identified 21 peaks from the Doryong sample. Among the various peaks, four major compositions (peak 22, tR = 37.1 minutes; peak 23, tR = 37.2 minutes; peak 24, tR = 38.5 minutes; and peak 25, tR = 39.3 minutes) were detected with identical molecular ions [M–H]− at m/z 277.2165, m/z 277.2174, m/z 279.2332, and m/z 255.2336, representing approximately 80% of the total peak area (Figure 2A).
Figure 2.
Representative total ion chromatograms of the methanol extracts of green perilla leaves obtained by UPLC-ESI-Q-TOF/MS in negative ion mode: (A) Doryong sample. (B) Sinseong sample. (C) Bongmyeong sample. UPLC-ESI-Q-TOF/ MS = ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry.
Table 1.
Metabolite profile in methanol extract of green perilla leaves by UPLC-ESI-Q-TOF-MS/MS analysis.
| Peak | tR (min) | Formula | Calculated mass [M] (m/z) | Calculated mass [M-H]− (m/z) | Observed molecular ion in MS [M-H]− (m/z) | Observed fragment ions in MS and MS/MS (m/z) | Identification | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.5 | C9H8O4 | 180.0423 | 179.0344 | 179.0346 | 179.0336, 161.0265, 135.0446 | Caffeic acid | Lee et al [6], Zhou et al[35], Chen et al [41] |
| 2 | 5.3 | C18H28O9 | 388.1733 | 387.1655 | 387.1647 | 387.1649, 207.1022 | 5′-Gluco-pyranosyoxyjasmanic acid | Fujita et al [36] |
| 3 | 5.9 | C17H26O10 | 390.1526 | 389.1448 | 389.1451 | 167.0358 | Loganin | Song et al [51], Ye et al [52] |
| 4 | 6.2 | C24H26O13 | 522.1373 | 521.1295 | 521.1847 | 521.1847, 359.0757, 285.0391 | Rosmarinic acid-3-O- glucoside | Lee et al [6], Zhou et al [35] |
| 5 | 6.7 | C27H26O17 | 622.1170 | 621.1092 | 621.1109 | 621.1109, 351.0582, 269.0451, 113.0225 | Apigenin-7-O-diglucuronide | Kaufmann et al [38] |
| 6 | 7.6 | C21H18O12 | 462.0798 | 461.0720 | 461.0723 | 461.0723, 285.0408 | Scutellarein-7-O-glucuronide | Yamazaki et al [39], Kaufmann et al [38] |
| 7 | 7.8 | C21H20O11 | 448.1006 | 447.0927 | 447.0907 | 447.0926, 285.0394 | Luteolin-7-O-glucoside | Lee et al [6], Chen et al [41] |
| 8 | 8.1 | C36H32O16 | 720.1690 | 719.1612 | 719.1601 | 719.1601, 359.0763, 197.0417, 161.0224 | Sagerinic acid | Lu and Foo [53] |
| 9 | 9.0 | C21H18O11 | 446.0849 | 445.0771 | 445.0779 | 445.0779, 269.0460, 176.0344, 161.0253 | Apigenin-7-O-glucuronide | Kaufmann et al [38] |
| 10 | 9.2 | C18H16O8 | 360.0845 | 359.0773 | 359.0772 | 359.0772, 197.0455, 179.0360, 161.0243 | Rosmarinic acid | Lee et al [6], Zhou et al [35] |
| 11 | 11.3 | C19H18O8 | 374.1002 | 373.0923 | 373.1871 | 373.1880, 161.0758, 148.0529 | Rosmarinic acid methyl ester | Zhou et al [35] |
| 12 | 13.8 | C20H36O12 | 468.2207 | 467.2129 | 467.2119 | 467.2119, 289.1660, 161.0462 | N-Octanoylsucrose | Tirupati et al [43] |
| 13 | 16.1 | C19H24O4 | 316.1675 | 315.1596 | 315.0875 | 164.9840, 119.0514 | trans-p-Menth-8-en-yl caffeate | Tada et al [44] |
| 14 | 17.2 | C15H10O5 | 270.0528 | 269.0450 | 269.0459 | 269.0445, 117.0359 | Apigenin | Lee et al [6], Zhou et al [35] |
| 15 | 20.2 | C15H12O4 | 256.0736 | 255.0657 | 255.0658 | 255.0674, 213.0565, 145.0652 | Liquiritigenin | Liu et al [45] |
| 16 | 21.8 | C18H32O5 | 328.2250 | 327.2171 | 327.2174 | 233.1152, 229.1437 | Not identified | — |
| 17 | 23.7 | C16H12O6 | 300.0634 | 299.0556 | 299.0921 | 299.0921, 284.0715, 269.0450, 241.0508 | Chrysoeriol | Lee et al [6] |
| 18 | 29.5 | C30H48O5 | 488.3502 | 487.3423 | 487.3422 | 487.3432, 469.3319, 249.1881 | Tomentic acid | Chaturvedula and Prakash [48] |
| 19 | 31.5 | C30H48O4 | 472.3553 | 471.3474 | 471.3477 | 471.3397, 361.1995, 315.1947, | Corosolic acid | Chaturvedula and Prakash [48] |
| 20 | 33.0 | C34H58O16 | 722.3725 | 721.3647 | 721.3660 | 721.3660, 470.3346, 451.3192, 277.2169, 235.0860 | Palmitoleic–linolenic glucoside | Pierson et al [50] |
| 21 | 35.4 | C32H60O16 | 700.3881 | 699.3803 | 699.3812 | 397.2595 | Palmitic–oleic glucoside | Pierson et al [50] |
| 22 | 37.1 | C18H30O2 | 278.2246 | 277.2168 | 277.2165 | 277.2164, 127.0762 | α-Linolenic acid | Pierson et al [50] |
| 23 | 37.2 | C18H30O2 | 278.2246 | 277.2168 | 277.2174 | 277.2170 | Linolenic acid | Pierson et al [50] |
| 24 | 38.5 | C18H32O2 | 280.2402 | 279.2324 | 279.2332 | 279.2334 | Linoleic acid | Pierson et al [50] |
| 25 | 39.3 | C16H32O2 | 256.2402 | 255.2324 | 255.2329 | 255.2336 | Palmitic acid | Pierson et al [50] |
| 26 | 39.8 | C18H34O2 | 282.2559 | 281.2481 | 281.2480 | 281.2490 | Oleic acid | Pierson et al [50] |
tR = retention time; UPLC-ESI-Q-TOF-MS/MS = ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry.
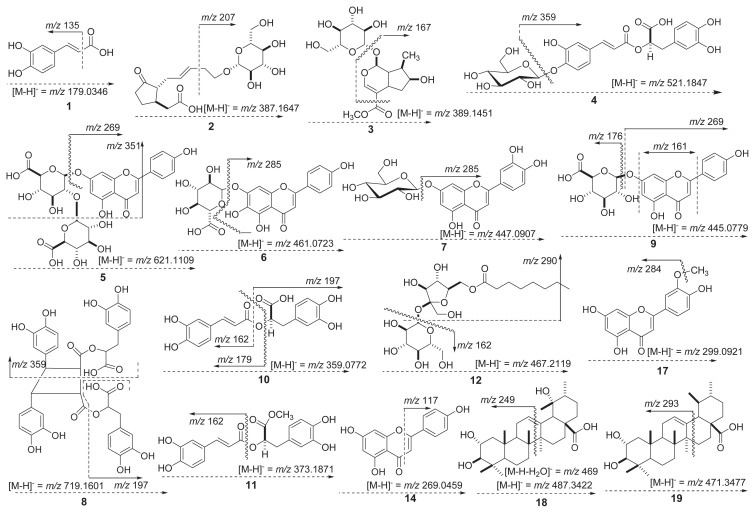
The product ion mass spectrum of peak 1 (tR = 4.5 minutes) showed as deprotonated ion [M–H]− at m/z 179.0346. In the MS/MS spectrum, the distinctive product ion at m/z 135.0446 ([M–H]−–44 amu) corresponded to the loss of a carboxylic acid (−COOH) moiety (Figures 3 and 4A) . According to the fragment interpretation and the published data, peak 1 was confirmed to be caffeic acid (1) with the elemental formula C9H8O4 (Figures 4A and 5) [19,35]. The TOF-MS data of peak 2 (tR = 5.3 minutes) was detected with the precursor ions at m/z 387.1647 ([M–H]−), and the fragmentation patterns of the MS/ MS spectrum were detected with signals at m/z 387.1649 and m/z 207.1022 ([M–H]−–180 amu, loss of glucopyranosy moiety) (Figures 3 and 4B). This peak was identified as 5′-glucopyr-anosyoxyjasmanic acid (2) (C18H28O9) by comparison with jasmonoid glucoside of the earlier data [36]. Subsequently, peak 4 (tR = 6.2 minutes) presented a deprotonated molecular ion [M–H]− at m/z 521.1847 and one fragment ion at m/z 359.0757 in negative ESI-TOF-MS analysis (Figure 4D). The fragmentation at m/z 359.0757 was determined to be characteristic of rosmarinic acid by the loss of a glucose residue (162 amu) (Figure 3) [35]. Therefore, peak 4 was assumed to be rosmarinic acid-3-O-glucoside (4) with the formula C24H26O13 (Figure 5) [6,35]. Full TOF-MS scan analysis of peak 5 (tR = 6.7 minutes) showed an identical molecular ion [M–H]− ion at m/z 621.1109 (Figure 4E). In addition, the MS/MS spectrum possessed two peaks including one major fragment ion at m/z 269.0451 and one minor ion at m/z 351.0582 (Figure 4E). The fragment ion (m/z 269.0451) was characteristic of apigenin aglycone through the loss of the deprotonated glucuronide resides (C-7 site, 2 × 176 amu) (Figure 3) from the molecular ion [M–H]− at m/z 621.1109 [35]. The remaining MS/MS fragment ion (m/z 351.0582) was observed as the loss of the glucuronide moiety (176 amu) and 2-phenyl group (93 amu) in flavone skeleton (Figure 3) [37]. Based on these observations, peak 5 was identified as apigenin-7-O-diglucuronide (5) (C27H26O17) (Figure 5) [38]. The ESI-TOF-MS in the negative ion mode of peak 6 (tR = 7.6 minutes) exhibited the deprotonated precursor [M–H]− ion at m/z 461.0723 (Figure 4A). The fragment ion in the MS/MS spectrum exhibited one major peak at m/z 285.0408 (Figure 4F). This fragment ion (m/z 285.0408) was formed by the loss of m/z 176 fragment (m/z 461 → m/z 285; [M–H]−–176 amu) (Figure 3) [37], indicating the characteristic of a glucuronide moiety [37,38]. Furthermore, the major fragmentation at m/z 285.0428 (−176 amu) was characterized as luteolinidin moiety (Figure 3), in agreement with a previous study [39]. On the basis of these observations, this compound was tentatively identified as scutellarein-7-O-glucuronide (6) with the elemental formula of C21H18O12 [39,40]. In the TOF/MS and MS/MS spectra, peak 7 (tR = 7.8 minutes) was detected as a deprotonated ion [M–H]− at m/z 447.0907 and one major fragment ion at m/z 285.0394 ([M–H]−–162 amu) (Figures 3 and 4G). The main fragmentation at m/z 285.0394 resulted from the loss of a glucose moiety, which corresponding to luteolin aglycone (m/z 285) [6]. On the basis of the fragment interpretation, peak 7 has already been identified as luteolin-7-O-glucoside (7) with the formula C21H20O11 (Figure 3). Also, this peak was confirmed by comparison of data reported in previous studies [6,41]. The TOF mass scan analysis of peak 10 (tR = 9.2 minutes) exhibited an deprotonated molecular ion at m/z 359.0772. The MS/MS fragmentation patterns possessed three fragment ions at m/z 197.0455, m/z 179.0360, and m/z 161.0243 (Figure 4J). A fragment ion at m/z 197.0455, which was formed by the loss of m/z 162 fragment (m/z 359 → m/z 197; [M–H]−–162 amu) (Figure 3). This ion peak was determined to be the characteristic of a caffeoyl moiety [42]. In addition, the fragment ion at m/z 161.0243 corresponded to the deprotonated caffeoyl residue, and the fragmentation at m/z 179.0360 was in agreement with the pattern of peak 1 (caffeic acid). As a result, peak 10 was tentatively assigned as rosmarinic acid (10) (C18H16O8) (Figure 3) [6,19,35]. The ESI-TOF-MS and MS/MS in the negative ion mode of peak 11 (tR = 11.3 minutes) presented the molecular ion at m/z 373.1871 and two fragment ions at m/ z 161.0758 and m/z 148.0529 (Figure 4K). The fragmentation at m/z 161.0758 resulted from the deprotonated caffeoyl residue [42] as the ion pattern of peak 10 (Figure 3). Moreover, the pseudomolecular ion at m/z 373.1871 was in agreement with the rosmarinic acid derivative of published data [35]. As discussed previously, this peak was elucidated as rosmarinic acid methyl ester (11) with the elemental composition of C19H18O8 (Figure 3) [6,35]. The product ion mass spectrum of peak 12 (tR = 13.8 minutes) appeared as deprotonated ion [M–H]−at m/ z 467.2119. In addition, three fragment ions at m/z 289.1660 and m/z 161.0462 were observed in the MS/MS spectrum (Figure 4L). A fragment ion was observed at m/z 289.1660, which was formed by the loss of m/z 178 fragment (m/z 467 → m/z 289; {[M–H]−–(glucose + methyl)}) (Figure 3). In brief, this ion peak (m/z 289.1660) may correspond to the deprotonated fructose lipid residue, and the fragmentation ion at m/z 161.0462 was characterized as the deprotonated glucose moiety [6,27]. Based on the described fragmentation ions, peak 12 was assumed to be N-octanoylsucrose (12) (C20H36O12). Although this composition was previously identified by Synechocystis sp. PCC [43], we have documented for the first time its presence in perilla plant. The negative ion mode of peak 13 possessed the molecular ion at m/z 315.0875, and its chemical structure was identified as trans-p-menth-8-en-yl caffeate (13) (C19H24O4) based on the earlier literature [44]. Peak 15 was observed with a retention time at 20.2 minutes in UPLC-ESI-Q-TOF-MS chromatogram, and its mass measurement was observed at m/z 255.0658 ([M–H]−). The chemical structure was tentatively assigned as liquiritigenin (C15H12O4) in reference to a previously reported study [45]. As far as we know, it is reported for the first time in perilla plant. Peak 16 was detected as a molecular ion [M–H]− at m/z 327.2174 with the formula C18H32O5 and two fragment ions (m/z 233.1152 and m/z 229.1437). The above fragmentation ions may be formed by the losses of m/z 94 and m/z 98. However, with this peak, it was not possible to completely characterize the chemical structure. The TOF-MS and MS/MS analyses of peak 17 (tR = 23.7 minutes) presented the molecular ion [M–H]− at m/z 299.0921 with three fragmentations at m/z 284.0715, m/z 269.0450, and m/z 241.0508 (Figure 4N). In particular, a fragment ion was found at m/z 284.0715, which was formed by the loss of the m/z 15 fragment (methyl moiety) (Figure 3) [6,46]. According to the molecular weight in published data [6], the structure of peak 17 was determined as chrysoeriol (17) (C16H12O6). The deprotonated molecular ion [M–H]− at m/z 487.3422 was found in the UPLC ESI-TOF-MS spectrum of peak 18 (Figure 4O). Furthermore, two fragment ions at m/z 469.3319 and m/z 249.1881 were produced from the parent ion at m/z 487.3422 in MS/MS data (Figure 4O). The precursor ion at m/z 469.3319 resulted from the loss of a H2O (−18 amu), and the fragmentation pattern of the ion at m/z 249.1881 would correspond to the molecular ion of diterpenoid moiety regarding B/C ring retro-Diels-Alder cleavage (Figure 3) [47]. According to the above summarized fragmentation, peak 18 was tentatively characterized as tomentic acid (18) (C30H48O5) [48]. The full mass scan analysis of peak 19 (tR = 31.5 minutes) was observed with [M–H]−at m/z 471.3477 and three fragment ions at m/z 361.1995, m/z 293.2123, and m/z 275.2013 (Figure 4P). The major fragmentation at m/z 293.2123 ([M–H]−–178 amu) resulted from the cleavage of the D-ring, which is in accordance with an earlier study [49]. Therefore, the fragmentation characteristics were attributed to the corosolic acid (19) (C30H48O4) (Figure 3) [48]. The ESI-TOF-MS spectrum of peak 21 (tR = 35.4 minutes) displayed a [M–H]− ion at m/z 699.3812, and its characteristic fragment ion was observed at m/z 397.2595 ([M–H]−–302 amu) in MS/MS analysis. Based on these lines of evidence, this composition was identified as palmitic–oleic glucoside (21), with the formula C32H60O16 [50]. The ESI-TOF-MS in negative ion mode of peaks 22–26 exhibited the molecular ions at m/z 277.2165 (Figure 4R), m/z 277.2174, m/z 279.2332, m/z 255.2329, and m/z 281.2480. Their molecular weights and retention times were coincident with those of mango fatty acids in a previously reported study [50]. Therefore, five peaks were confirmed as α-linolenic acid (22), linolenic acid (23), linoleic acid (24), palmitic acid (25), and oleic acid (26) (Figure 5).
Figure 3.
Mass fragmentation patterns of metabolite compositions in the leaves of green perilla.
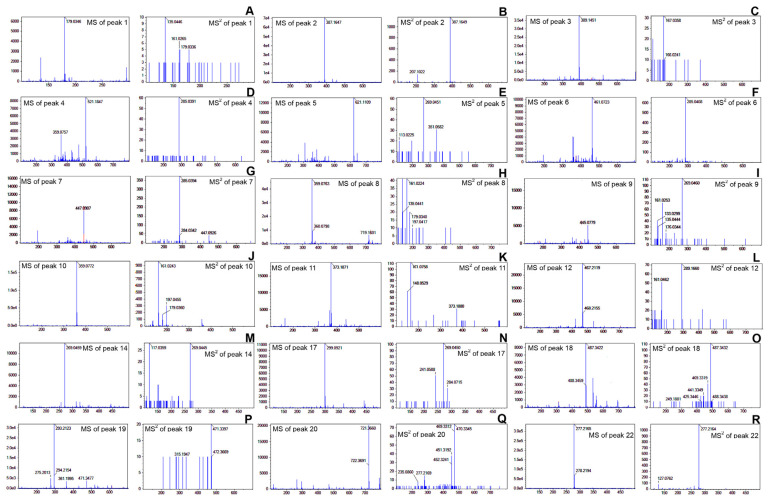
Figure 4.
The negative ESI-TOF/MS and MS/MS spectra of metabolites in the leaves of green perilla. (A) caffeic acid (1), (B) 5′-gluco-pyranosyoxyjasmanic acid (2), (C) loganin (3), (D) rosmarinic acid-3-O-glucoside (4), (E) apigenin-7-O-diglucuronide (5), (F) scutellarein-7-O-glucuronide (6), (G) luteolin-7-O-glucoside (7), (H) sagerinic acid (8), (I) apigenin-7-O-glucuronide (9), (J) rosmarinic acid (10), (K) rosmarinic acid methyl ester (11), (L) N-octanoylsucrose (12), (M) apigenin (14), (N) chrysoeriol (17), (O) tomentic acid (18), (P) corosolic acid (19), (Q) palmitoleic–linolenic glucoside (20), and (R) α-linolenic acid (22).
ESI = electrospray ionization; MS = mass spectrometry; TOF = time of flight.
Figure 5.
Chemical structures of metabolites found in the methanol extract of green perilla leaves.
Second, we examined the metabolite profile from the methanol extract of Sinseong sample to gather more information in the leaves of Korean green perilla (Figure 2B). Twenty-five compositions were characterized by the molecular weights of mass spectral data in previously reported studies (Figure 5). As illustrated in Figure 2B, metabolite peaks exhibited important differences by comparison with those of Doryong. In particular, caffeic acid (1), rosmarinic acid (10), α-linolenic acid (22), and linolenic acid (23) were the predominant metabolites, representing approximately 80% of the total peak area (Figure 2B). Moreover, five compositions were newly observed—loganin (3), sagerinic acid (8), apigenin-7-O-glucuronide (9), apigenin (14), and palmitoleic–linolenic glucoside (20)—with identical molecular ions [M–H]− at m/z 389.1451, m/ z 719.1601, m/z 445.0779, m/z 269.0459, and m/z 721.3660 (Figure 4). In this study, we report the fragment patterns of UPLC-ESI-TOF-MS/MS data, as detailed below.
Peak 3 (tR = 5.9 minutes) possessed an identical molecular ion [M–H]− at m/z 389.1451 and the fragmentation pattern at m/z 167.0358 (Figure 4C). The fragment ion (m/z 167.0358, −222 amu) resulted from the losses of glucose (−162 amu) and ester (−60 amu, −COOCH3) groups (Figure 3). By comparing its mass values with data from earlier reports [51,52], this peak was tentatively evaluated as loganin (3) (C17H26O10). To the best of our knowledge, this composition is the first reported in perilla plant. The product ion mass spectrum of peak 8 (tR = 8.1 minutes) presented the molecular ion [M–H]− at m/z 719.1601 and a fragment ion at m/z 359.0763, which was formed by the loss of the m/z 360 fragment (Figure 4H). The fragment ion resulted from the loss of a rosmarinic acid moiety in dimer skeleton (Figure 3) by comparison with an earlier report [53]. A fragment ion at m/z 197.0417 in the MS/MS spectrum, which was formed by the loss of m/z 161.0224 fragment (m/z 359 → m/z 197; [M–H]−–162 amu) (Figures 3 and 4H), was related with the characteristic of a caffeoyl group [6]. Briefly, peak 8 has already been identified as rosmarinic acid dimer, where dimerization had occurred by olefinic moieties. According to these observations, peak 8 was tentatively assigned as sagerinic acid (8) (C36H32O16). We identified for the first time that peak 8 was present in perilla plant. Using UPLC-ESI-TOF/MS, peak 9 was detected with the precursor ion of m/z 445.0779 ([M–H]−) at the retention time of 9.0 minutes (Figure 4I). Moreover, two major fragment ions at m/z 269.0460 and m/z 161.0253 as well as one minor peak at m/z 176.0344 were observed in the TOP/MS/MS spectrum (Figure 4I). The fragmentation patterns of this peak were similar to those of peak 5; in particular, the fragmentation at m/z 269.0460 (−176 amu) was characterized as apigenin aglycone. A minor ion at m/z 176.0344 ([M–H]−–269), which would correspond to the loss of an apigenin moiety, and a major ion at m/z 161.0253 resulted from the losses of a glucuronide (m/z 176) group as well as 2-phenyl (m/z 93) and hydroxyl (m/z 17) moieties of apigenin aglycone. Thus, peak 9 was assumed to be apigenin-7-O-glucuronide (9) with the formula C21H18O11 (Figure 5) [38]. Peak 14 (tR = 17.2 minutes) possessed an identical molecular ion [M–H]− at m/z 269.0459 as the fragmentation patterns of peaks 5 and 9 (Figure 4M). This ion was characteristic of apigenin aglycone by comparison with published data [6]. In addition, a fragment ion at m/z 117.0359 of the MS/MS spectrum may be attributed to the result from the cleavage of Cring bonds in deprotonated flavonoids (Figures 3 and 4M) [54]. This structure was tentatively assigned as apigenin (14) (Figure 5) [6,35]. The ESI TOF/MS profile of peak 20 (tR = 33.0 minutes) that exhibited an identical molecular ion [M–H]− at m/z 721.3660 was characterized by an ion signal at m/z 721.3660, and the MS/MS fragment ions were observed with four values, including m/z 470.3346, m/z 451.3192, m/z 277.2169, and m/z 235.0860 (Figure 4Q). On the basis of the lines of evidence presented, this structure was coincident with those of palmitoleic–linolenic glucoside (20) in mango fatty acids [50]. The crude methanol extract of the Bongmyeong sample was also screened for metabolite profile on Korean green perilla. This material was observed to have various minor peaks in total ion chromatogram (Figure 2C), and the individual component was not fully characterized. We revealed 18 metabolites, including the identified compositions of Doryong and Sinseong samples (Figure 5). Interestingly, only one main phenolic acid (rosmarinic acid 10) was found in the chromatography, representing approximately 55% of the total peak area (Figure 2C). Consequently, metabolite compositions and their contents in the leaves of Korean green perilla showed remarkable differences in each sample. This phenomenon suggests that metabolites may be affected by multiple factors, such as environmental stresses, cultivars, and genetics [5–7,27,39]. In addition, our result may be an important factor for the determination of the quality of green perilla.
3.3. Antioxidant effects against DPPH and ABTS radicals from the leaves of green perilla
The evaluation of antioxidant capacity is commonly used as a screening method for crops, foods, fruits, vegetables, and edible plants [6,19,45]. Specifically, the scavenging activities against radical sources have been used in the screening of antioxidants with regard to phenolic compounds, flavonoids, carotenoids, and anthocyanins for beneficial human health properties [3,6,9,12,27]. Among various radical sources, DPPH and ABTS radical assays are of great interest in food and crop industries owing to their simple quality control and reproducibility [6,9,35]. For these reasons, we investigated antioxidant properties including DPPH and ABTS radicals in the present research. The scavenging effects of green perilla leaves were determined by comparing the percentage inhibition through the formation of DPPH and ABTS radicals by the methanol extract of each sample, BHT (positive control), and Trolox (positive control). The scavenging abilities against DPPH and ABTS radicals of the samples and positive controls increased with increasing concentrations (5 μg/mL, 10 μg/mL, 20 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, and 300 μg/mL). Although 100% scavenging capacity was observed at 200 μg/mL and 300 μg/mL, the antioxidant properties on the radical scavengers were carried out at 100 μg/mL because of the dose-dependent changes and comparison of the inhibition rates in samples. In the current study, the rank order of the scavenging effects against DPPH radical was consistent with the results of the ABTS assay. The highest scavenging effects were observed in the Sinseong sample with 86% (DPPH) and 90% (ABTS) at a concentration of 100 μg/mL. These values were slightly higher than BHT (82%) and Trolox (86%) of positive controls (Table 2).
Table 2.
Antioxidant effects against DPPH and ABTS radicals from the leaves of three green perilla samples.
| Sample | Radical scavenging activity (%)a | |
|---|---|---|
|
| ||
| DPPH radical | ABTS radical | |
| Doryong | 63b | 73 |
| Sinseong | 86 | 90 |
| Bongmyeong | 71 | 84 |
| Positive control (DPPH: BHT) (ABTS: Trolox) | 82 | 86 |
ABTS = 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid); BHT = butylated hydroxytoluene; DPPH = 2,2-diphenyl-1-picrylhydrazyl.
All values of samples and positive controls are expressed as the mean of triplicate determinations.
Radical scavenging effects of the methanol extracts of samples and positive controls were carried out at 100 μg/mL.
In more detail, the DPPH and ABTS radical scavenging abilities of different perilla leaves were, in increasing order, as follows: Sinseong (86 and 90%) > Bongmyeong (71 and 84%) > Doryong (63 and 73%) (Table 2). These results reveal that the identified metabolite contents in the methanol extract of perilla leaves may be the major portion of antioxidant properties through DPPH and ABTS radical scavenging capacities, as described in previous studies [6,19,44]. The unidentified peaks may also be potential factors in determining the two radical scavenging properties [55]. Interestingly, three samples showed higher ABTS radical capacities than the results on DPPH radical. This phenomenon suggests that DPPH radical may be attributed to the scavenging activities regarding hydrogen donating compositions of various metabolites, whereas ABTS radical was related to the scavenging abilities of chain breaking and hydrogen donating metabolites, which was shown in previous studies [6,7]. Additionally, it is well documented that perilla contains many metabolites, namely, phenolic acids, flavonoids, triterpenoids, and fatty acids [2,6,17,19,27]. Even though all of the identified metabolites do not indicate the antioxidant effects, phenolic acid, flavonoids, and triterpenoid derivatives are responsible for the beneficial health properties such as antioxidant effects against radicals in previously published data [35,56–59]. Therefore, the metabolite contents may also be attributed to the scavenging activities on DPPH and ABTS radicals in the methanol extract of perilla leaves. As a result, the leaves of green perilla were observed to have potential antioxidant properties against DPPH and ABTS radicals because of their high metabolite contents. Moreover, this species may be considered a radical scavenger for food and nutraceutical uses.
4. Conclusion
We have characterized metabolite profiles from the leaves of green perilla using UPLC-ESI-Q-TOF-MS/MS analysis. Twenty-five metabolites were demonstrated as six phenolic acids, two triterpenoids, eight flavonoids, seven fatty acids, and two glucosides. Among them, logainin (3), sagerinic acid (8), N-octanoylsucrose (12), and liquiritigenin (15) were reported for the first time in this species. The metabolite compositions exhibited remarkable differences in three samples, especially Doryong, which exhibited the highest fatty acid contents. Moreover, α-linolenic acid (22), linolenic acid (23), and linoleic acid (24) showed high contents in Doryong and Sinseong samples. The well-known phenolic acid, rosmarinic acid (10), was also observed to have significant differences in samples as follows: Doryong, 6.38 μg/g; Sinseong, 317.60 μg/g; and Bongmyeong, 903.53 μg/g. In addition, the antioxidant properties such as DPPH and ABTS radical scavenging effects differed significantly according to the sample (100 μg/mL): Doryong, 63% and 73%; Sinseong, 86% and 90%; and Bongmyeong, 71% and 84%. We believe that metabolites may be an important factor for the quality and antioxidant property of green perilla leaves. To the development of functional food and nutraceutical sources, future researches are needed to evaluate the human health beneficial properties such as anticancer, antidiabetic, antioxidant, anti-inflammatory, and antiartherogenic effects of various metabolites in green perilla.
REFERENCES
- 1. Zhang B, Deng Z, Dan Ramdath D, Tang Y, Chen PX, Liu R, Liu Q, Tsao R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015;172:862–72. doi: 10.1016/j.foodchem.2014.09.144. [DOI] [PubMed] [Google Scholar]
- 2. Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa J, Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem. 2004;68:85–90. doi: 10.1271/bbb.68.85. [DOI] [PubMed] [Google Scholar]
- 3. Galasso S, Pacifico S, Kretschmer N, Pan SP, Marciano S, Piccolella S, Monaco P, Bauer R. Influence of seasonal variation on Thymus longicaulis C. Presl chemical composition and its antioxidant and anti-inflammatory properties. Phytochemistry. 2014;107:80–90. doi: 10.1016/j.phytochem.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 4. Wang J, Ren H, Xu QL, Zhou ZY, Wu P, Wei XY, Cao Y, Chen XX, Tan JW. Antibacterial oleanane-type triterpenoids form pericarps of Akebia trifoliate. Food Chem. 2015;168:623–9. doi: 10.1016/j.foodchem.2014.07.105. [DOI] [PubMed] [Google Scholar]
- 5. Stefanović OD, Tešić JD, Čomić LR. Melilotus albus and Dorycnium herbaceum extracts as source of phenolic compounds and their antimicrobial, antibiofilm, and antioxidant potentials. J Food Drug Anal. 2015;23:417–24. doi: 10.1016/j.jfda.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee JH, Park KH, Lee MH, Kim HT, Seo WD, Kim JY, Baek IY, Jang DS, Ha TJ. Identification, characterization, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013;136:843–52. doi: 10.1016/j.foodchem.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 7. Lee JH, Cho KM. Changes occurring in compositional components of black soybeans maintained at room temperature for different storage periods. Food Chem. 2012;131:161–9. [Google Scholar]
- 8. Seo WD, Kim JY, Ryu HW, Kim JH, Han SI, Ra JE, Seo KH, Jang KC, Lee JH. Identification and characterisation of coumarins from the roots of Angelica dahurica and their inhibitory effects against cholinesterase. J Funct Foods. 2013;5:1421–31. [Google Scholar]
- 9. Denardin CC, Hirsch GE, da Rocha RF, Vizzotto MV, Henriques AT, Moreira JCF, Guma FTCR, Emanuelli T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J Food Drug Anal. 2015;23:387–98. doi: 10.1016/j.jfda.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Somova LI, Shode FO, Ramnanan P, Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J Ethnopharmacol. 2003;84:299–305. doi: 10.1016/s0378-8741(02)00332-x. [DOI] [PubMed] [Google Scholar]
- 11. Hyson D, Schneeman BO, Davis PA. Almonds and almond oil have similar effects on plasma lipids and LDL oxidation in healthy men and women. J Nutr. 2002;132:703–7. doi: 10.1093/jn/132.4.703. [DOI] [PubMed] [Google Scholar]
- 12. Doğan HH. Evaluation of phenolic compounds, antioxidant activities and fatty acid composition of Amanita ovoidea (Bull.) Link. in Turkey. J Food Compos Anal. 2013;31:87–93. [Google Scholar]
- 13. Igarashi M, Miyazaki Y. A review on bioactivities of perilla: progress in research on the functions of perilla as medicine and food. Evid Based Complement Alternat Med. 2013;2013:1–7. doi: 10.1155/2013/925342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee KN, Shin HH, Han DS, Kim YO, Choi KE, Kwag JS, Back SH. Development of anticancer agents from Korean medicinal plants: part 5. Cytotoxic activity of the butanol soluble fraction of Perilla frutescens against human skin melanoma cells. Saengyak Hakhoechi. 1997;28:264–70. [Google Scholar]
- 15. Liu JH, Steigel A, Reininger E, Bauer R. Two new prenylated 3-benzoxepin derivatives as cyclooxygenase inhibitors from Perilla frutescens var. acuta. J Nat Prod. 2000;63:403–5. doi: 10.1021/np990362o. [DOI] [PubMed] [Google Scholar]
- 16. Asif M. Phytochemical study of polyphenols in Perilla frutescens as an antioxidant. Avicenna J Phytomed. 2012;2:169–78. [PMC free article] [PubMed] [Google Scholar]
- 17. You C, Wang Y, Zhang W, Yang K, Wu Y, Geng Z, Chen H, Jiang H, Du S, Deng Z, Liu Z. Chemical constituents and biological activities of the purple perilla essential oil against Lasioderma serricorne. Ind Crop Prod. 2014;61:331–7. [Google Scholar]
- 18. Nakazawa T, Ohsawa K. Metabolites of orally administered Perilla frutescens extract in rats and humans. Biol Pharm Bull. 2000;23:122–7. doi: 10.1248/bpb.23.122. [DOI] [PubMed] [Google Scholar]
- 19. Jun HI, Kim BT, Song GS, Kim YS. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens var. acuta) leaves. Food Chem. 2014;148:367–72. doi: 10.1016/j.foodchem.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 20. Feng LJ, Yu CH, Ying KJ, Hua J, Dai XY. Hypolipidemic and antioxidant effects of total flavonoids of Perilla frutescens leaves in hyperlipidemia rat induced by high-fat diet. Food Res Int. 2011;44:404–9. [Google Scholar]
- 21. Makino T, Furuta Y, Wakushima H, Fujii H, Saito KI, Kano Y. Antiallergic effect of Perilla frutescens its active constituent. Phytother Res. 2003;17:240–3. doi: 10.1002/ptr.1115. [DOI] [PubMed] [Google Scholar]
- 22. Chang HH, Chen CS, Lin JT. Dietary perilla oil lowers serum lipids and ovalbumin-specific IgG1, but increase total IgE levels ovalbumin-challenged mice. Food Chem Toxicol. 2009;47:848–54. doi: 10.1016/j.fct.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 23. Peiretti PG, Gasco L, Brugiapaglia A, Gai F. Effects of perilla (Perilla frutescens L.) seeds supplementation on performance, carcass characteristics, meat quality and fatty acid composition of rabbits. Livest Sci. 2004;138:118–24. [Google Scholar]
- 24. Kim HK, Choi H. Dietary α-linolenic acid lowers postprandial lipid levels with increase of eicosapentaenoic acid and docosahexaenoic acid contents in rat hepatic membrane. Lipids. 2001;36:1331–6. doi: 10.1007/s11745-001-0849-7. [DOI] [PubMed] [Google Scholar]
- 25. Yoshida K, Kondo T, Kameda K, Goto T. Structure of anthocyanins isolated from purple leaves of Perilla ocimoides L. Var. crispa Benth and their isomerization by irradiation of light. Agric Biol Chem. 1990;54:1745–51. [Google Scholar]
- 26.Kosuna K, Haga M. The development and application of perilla extract as an anti-allergic substance. In: Yu HC, editor. Perilla: the genus Perilla. Amsterdam: Harwood Academic Publishers; 1997. pp. 143–8. [Google Scholar]
- 27. Kang NS, Lee JH. Characterisation of phenolic phytochemicals and quality changes related to the harvest times from the leaves of Korean purple perilla (Perilla frutescens) Food Chem. 2011;124:556–62. [Google Scholar]
- 28. Ha TJ, Lee JH, Lee MH, Lee BW, Kwon HS, Park CH, Shim KB, Kim HT, Baek IY, Jang DS. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012;135:1397–403. doi: 10.1016/j.foodchem.2012.05.104. [DOI] [PubMed] [Google Scholar]
- 29. Chen JH, Xia ZH, Tan RX. High-performance liquid chromatographic analysis of bioactive triterpenes in Perilla frutescens. J Pharm Biomed Anal. 2003;32:1175–9. doi: 10.1016/s0731-7085(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 30. Naczk M, Shahidi F. Extraction and analysis of phenolic in food. J Chromatogr A. 2004;1054:95–111. [PubMed] [Google Scholar]
- 31. Umphress ST, Murphy SP, Franke AA, Custer LJ, Blitz CL. Isoflavone content of foods with soy additives. J Food Compost Anal. 2005;18:533–50. [Google Scholar]
- 32. Dartora N, de Souza LM, Santana-Filho AP, Iacomini M, Valduga AT, Gorin PAJ, Sassaki GL. UPLC-PDA-MS evaluation of bioactive compounds from leaves of Ilex paraguariensis with different growth conditions, treatments, and ageing. Food Chem. 2011;129:1453–61. [Google Scholar]
- 33. Lee SJ, Ahn JK, Kim SH, Kim JT, Han SJ, Jung MY, Chung IM. Variations in isoflavone of soybean cultivars with location and storage duration. J Agric Food Chem. 2003;51:3382–9. doi: 10.1021/jf0261405. [DOI] [PubMed] [Google Scholar]
- 34. Zhang L, Jiang Z, Yang J, Li Y, Wang Y, Chai X. Chemical material basis study of Xuefu Zhuyu decoction by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Food Drug Anal. 2015;23:811–20. doi: 10.1016/j.jfda.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou XJ, Yan LL, Yin PP, Shi LL, Zhang JH, Liu YJ, Ma C. Structural characterization and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014;164:150–7. doi: 10.1016/j.foodchem.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 36. Fujita T, Terato K, Nakayama Two jasmonoid glucosides and a phenylvaleric acid glucoside from perilla frutescens. Biosci Biotechnol Biochem. 1999;60:732–5. [Google Scholar]
- 37. Rydevik A, Bondesson U, Thevis M, Hedeland M. Mass spectrometric characterization of glucuronides formed by a new concept, combining Cunninghamella elegans with TEMPO. J Pharm Biomed Anal. 2013;84:278–84. doi: 10.1016/j.jpba.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 38. Kaufmann CM, Grassmann J, Letzel T. HPLC method development for the online-coupling of chromatographic Perilla frutescens extract separation with xanthine oxidase enzymatic assay. J Pharm Biomed Anal. 2016;124:347–57. doi: 10.1016/j.jpba.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 39. Yamazaki M, Nakajima J, Yamanashi M, Sugiyama M, Makita Y, Springob K, Awazuhara M, Saito K. Metabolomics and differential gene expression in anthocyanin chemo-varietal forms of Perilla frutescens. Phytochemistry. 2003;62:987–95. doi: 10.1016/s0031-9422(02)00721-5. [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Xia H, Liu Y, Qiu F, Di X. Simultaneous determination of three glucuronide conjugates of scutellarein in rat plasma by LC-MS/MS for pharmacokinetic study of breviscapine. J Chromatogr B. 2014;965:79–84. doi: 10.1016/j.jchromb.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 41. Chen HJ, Inbaraj BS, Chen BH. Determination of phenolic acids and flavonoids in Taraxacum formosanum kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int J Mol Sci. 2012;13:260–85. doi: 10.3390/ijms13010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olivier DK, van Wyk BE, van Heerden FR. The chemotaxonomic and medicinal significance of phenolic acids in Arctopus and Alepidea (Apiaceae subfamily Saniculoideae) Biochem Syst Ecol. 2008;36:724–9. [Google Scholar]
- 43. Tirupati B, Vey JL, Drennan CL, Bollinger JM., Jr Kinetic and structural characterization of Slr0077/SufS, the essential cysteine desulfurase from Synechocystis sp. PCC 6803. J Biochem. 2004;43:12210–9. doi: 10.1021/bi0491447. [DOI] [PubMed] [Google Scholar]
- 44. Tada M, Matsumoto R, Yamaguchi H, Chiba K. Novel antioxidants isolated from Perilla frutescens Britton var. crispa (Thumb.) Biosci Biotechnol Biochem. 1996;60:1093–5. doi: 10.1271/bbb.60.1093. [DOI] [PubMed] [Google Scholar]
- 45. Liu J, Luo L, Zhang H, Jia B, Lu J, Li P, Chen J. Rapid screening for novel antioxidants in Glycyrrhiza inflata using high-resolution peak fractionation. J Funct Foods. 2015;16:40–9. [Google Scholar]
- 46. Marin PD, Veitch NC, Grayer RJ, Kite GC, Soković M, Janaćković P. Flavonoids from Phlomis fruticosa (Lamiaceae) growing in Montenegro. Biochem Syst Ecol. 2007;35:462–6. [Google Scholar]
- 47. Liu Y, Liu Z, Lu C, Li J, Ning Z, Song Z, Wang C, Du Z, Lu X, Zhao S, Lu A. Comprehensive identification of active triterpenoid metabolites in frankincense using a coupling strategy. J Chromatogr B. 2014;963:90–8. doi: 10.1016/j.jchromb.2014.05.054. [DOI] [PubMed] [Google Scholar]
- 48. Chaturvedula VSP, Prakash I. Isolation and structure elucidation of two triterpene acids from the leaves of Perilla frutescens. J Pharmacogn Phytochem. 2013;1:49–53. [Google Scholar]
- 49. Guo XY, Han J, Ye M, Ma XC, Shen X, Xue BB, Che QM. Identification of major compouds in rat bile after oral administration of total triterpenoids of Ganoderma lucidum by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2012;63:29–39. doi: 10.1016/j.jpba.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 50. Pierson JT, Monteith GR, Roberts-Thomson SJ, Dietzgen RG, Gidley MJ, Shaw PN. Phytochemical extraction, characterization and comparative distribution across four mango (Mangifera indica L.) fruit varieties. Food Chem. 2014;149:253–63. doi: 10.1016/j.foodchem.2013.10.108. [DOI] [PubMed] [Google Scholar]
- 51. Song Y, Li SL, Wu MH, Li HJ, Li P. Qualitative and quantitative analysis of iridoid glycosides in the flower buds of Lonicera species by capillary high performance liquid chromatography coupled with mass spectrometric detector. Anal Chim Acta. 2006;564:211–8. [Google Scholar]
- 52. Ye J, Zhang X, Dai W, Yan S, Huang H, Liang X, Li Y, Zhang W. Chemical fingerprinting of Liuwei Dihuang Pill and simultaneous determination of its major bioactive constituents by HPLC coupled with multiple detections of DAD, ELSD, and ESI-MS. J Pharm Biomed Anal. 2009;49:638–45. doi: 10.1016/j.jpba.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 53. Lu Y, Foo LY. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry. 1999;51:91–4. [Google Scholar]
- 54. Li J, Wang YH, Smillie TJ, Khan IA. Identification of phenolic compounds from Scutellaria lateriflora by liquid chromatography with ultraviolet photodiode array and electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2012;63:120–7. doi: 10.1016/j.jpba.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 55. Alu’datt MH, Rababah T, Ereifej K, Alli I. Distribution, antioxidant and characterisation of phenolic compounds in soybeans, flaxseed and olives. Food Chem. 2013;139:93–9. doi: 10.1016/j.foodchem.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 56. Lee JH, Kang NS, Ha TJ, Ko JM, Han WY, Suh DY, Park KH, Baek IY. Antioxidant activities and determination of phenolic acids from leaves of Perilla frutescens. Agric Chem Biotechnol. 2006;49:11–5. [Google Scholar]
- 57. Falé PL, Borges C, Amorim Madeira PJ, Ascensão L, Araújo MEM, Floréncio MH, Serralheiro MLM. Rosmarinic acid, scutellarein 4′-methyl ether 7-O-glucuronide and (16S)-coleon E are the main compounds responsible for the antiacetylcholinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (“falso boldo”) Food Chem. 2009;114:798–805. [Google Scholar]
- 58. Mamadalieva NZ, Herrmann F, El-Readi MZ, Tahrani A, Hamoud R, Egamberdieva DR, Azimova SS, Wink M. Flanonoids in Scutellaria immaculate and S. ramosissima (Lamiaceae) and their biological activity. J Pharm Pharmacol. 2011;63:1346–57. doi: 10.1111/j.2042-7158.2011.01336.x. [DOI] [PubMed] [Google Scholar]
- 59. Aladedunye FA, Okorie DA, Ighodaro OM. Anti-inflammatory and antioxidant activities and constituents of Platostoma africanum P. Beauv. Nat Prod Res. 2008;22:1067–73. doi: 10.1080/14786410802264004. [DOI] [PubMed] [Google Scholar]