Abstract
In recent years, the use of fermented plant products to protect against various metabolic syndromes has been increasing enormously. The objective of this study was to check the regulatory efficacy of fermented plant extract (FPE) on intestinal microflora, lipid profile, and antioxidant status in mildly hypercholesterolemic volunteers. Forty-four mildly hypercholesterolemic individuals (cholesterol 180–220 mg/dL) were recruited and assigned to two groups: experimental or placebo. Volunteers were requested to drink either 60 mL of FPE or placebo for 8 weeks. Anthropometric measurements were done in the initial, 4th, 8th, and 10th weeks. The anthropometric parameters such as body weight, body fat, and body mass index were markedly lowered (p < 0.05) on FPE intervention participants. Moreover, the total antioxidant capacity and total phenolics in plasma were considerably increased along with a reduction (p < 0.05) in total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c) after FPE supplementation. Participants who drank FPE showed a pronounced increase (p < 0.05) in the number of beneficial bacteria such as Bifidobacterium spp. and Lactobacillus spp., whereas the number of harmful bacteria such as Escherichia coli and Clostridium perfringens (p < 0.05) were concomitantly reduced. Furthermore, the lag time of LDL oxidation was substantially ameliorated in FPE-administered group, thus indicating its antioxidative and cardioprotective properties. Treatment with FPE substantially improved the intestinal microflora and thereby positively regulated various physiological functions by lowering the anthropometric parameters, TC, and LDL-c, and remarkably elevated the antioxidant capacity and lag time of LDL oxidation. Therefore, we recommended FPE beverage for combating hypercholesterolemia.
Keywords: anthropometric parameters, antioxidant capacity, fermented plant extract, intestinal microflora, lipid profile
1. Introduction
Hypercholesterolemia (elevated blood cholesterol level) has been recognized as a crucial risk factor for cardiovascular disease (CVD) and coronary heart disease (CHD). CVD and CHD are the main reasons for the increased morbidity and mortality globally [1,2]. Hypercholesterolemia is highly implicated in the overproduction of free radicals and subsequently leads to endothelial dysfunction and other detrimental effects [3]. It is widely accepted that maintaining a normal level of blood cholesterol (lipid) would be the best strategy for preventing or lowering the incidence of both CVD and CHD. Likewise, the Lipid Research Clinics Coronary Primary Prevention Trial pointed out that 1% decline in total cholesterol (TC) results in a 2% decrease in the risk of CVD/CHD [4]. Although many cholesterol/lipid-lowering synthetic drugs are currently available on the market, they are not without adverse events. Therefore, researchers have turned their focus on developing a natural hypocholesterolemic or hypolipidemic drug with minimal or no side effects.
Plant-based food products (vegetables, fruits, and herbs) might be a better option to fight against various chronic diseases such as CVD and diabetes mellitus without any adverse events. Several studies have hinted that an inverse correlation exists between the intake of plant-based products with the risk rate of CVD [3,5]. Ample amount of data suggest that fermented plant products (fruits, vegetables, leaves, and roots) and milk-based products might act as good food matrices (substrate) and thereby ameliorate the indigenous microflora (probiotics) and thus optimize the health status by positively regulating various physiological or metabolic functions [6–8]. Intestinal microflora plays a crucial role in maintaining human health status by improving nutrient supply (absorption), suppressing pathogen colonization, maintaining normal mucosal immunity, effectively removing toxins, and regulating fat metabolism [9,10]. However, to date, no studies have been conducted with a different mixture of fruits, vegetables, herbs, and oligosaccharides as beverage products (food matrices) to improve intestinal microflora and their subsequent health-promoting effects.
Moreover, numerous studies indicated that phytocomponents such as polyphenols, tannins, saponins, phytosterols, and dietary fibers can effectively lower the cholesterol level by various mechanisms [2,3]. Fermented plant extract (FPE) is a fermented beverage made from several fruits, vegetables, and herbs (plant-based products) and also contains oligosaccharides, yeast, starch, and dietary fibers (well-known prebiotic), which make it as a perfect candidate for modulating intestinal microflora. From the above context, we speculate that FPE can effectively modulate human intestinal microflora and thereby positively influence various physiological functions, especially in relation to the lipid metabolism, oxidative status, and overall health status of mildly hypercholesterolemic individuals. Therefore, the objective of the current intervention was to assess the regulatory efficacy of FPE by evaluating anthropometric parameters, lipid profile, oxidative indexes, and intestinal microflora contents in mild hypercholesterolemic volunteers.
2. Materials and methods
2.1. Commercial FPE and placebo
Yamato Enzyme, Japan provided the commercial FPE and placebo beverage. The major ingredients of FPE were summarized as follows; each serving of FPE beverages contained 58.8% plant materials (with 45.4% vegetables, 32% of fruit, 12% of seaweed and processed food, 10.5% of herbal products from different plant materials), and 41.2% was composed of oligosaccharides (inulin, fructo-oligosaccharides), yeast, plum extract, brown sugar, raw sugar, maltose, glucose, fructose, and sucrose. The placebo beverage contains plum extract, brown sugar, raw sugar, maltose, glucose, fructose, and sucrose. Both samples were similar in color, flavor, appearance, size, and shape.
2.2. In vitro studies
2.2.1. Total phenolics and flavonoids contents
The total phenolic contents were determined using the method described by Julkunen-Tiiodtto [11]. The contents of total phenolics were expressed as milligrams of gallic acid equivalents (GAE) per milliliter. The total flavonoid contents were determined using the method of Wang and Hwang [12]. Total flavonoid contents were expressed as milligrams of quercetin equivalents per milliliter.
2.3. In vivo studies (clinical trial)
2.3.1. Participants
The ethical permission for the present randomized, double-blind, placebo-controlled study was sanctioned by the Institutional Review Board f Chung Shan Medical University Hospital, Taichung, Taiwan (CSMUH: CS11103), and procedures followed the guidelines of the Declaration of Helsinki and Good Clinical Practice. Sixty-four healthy volunteers were recruited by distributing flyers and displaying posters in public places and Chung Shan Medical University Hospital, Taiwan. Initial screening was done by several questionnaires (medical history, the pattern of work, lifestyle—exercise as well as smoking and drinking habits) with some biochemical analysis to confirm the health status of potential participants. The major inclusion criterion was mild hypercholesterolemia (serum cholesterol 180–220 mg/dL), and the exclusion criteria included history of drinking, smoking, pregnant, chronic diseases, gastrointestinal diseases, hepatic or renal dysfunction, as well as intake of dietary supplements or any other medications related to metabolic syndrome. All participants were asked to fill in and sign the study consent form (Supplementary file) prior to the intervention.
2.3.2. Study design
Forty-seven healthy, mildly hypercholesterolemic individuals aged 30–60 years were chosen for this current intervention based on the preliminary assessment. These participants were randomly segregated into two groups—experimental group (n = 24; 10 male and 14 female) or placebo group (n = 23; 11 male and 12 female)—using digital arbitrary number codes (computerized) obtained from independent researchers. All participants were asked to consume either 60 mL of FPE (2 × 30 mL with 180 mL of water) or placebo for 8 weeks (after lunch or dinner) and followed by 2 weeks of follow-up period without any sample. During the intervention, volunteers were instructed to maintain their normal dietary habits and lifestyle. Anthropometric measurements were done along with collection of both fecal and blood samples at the initial, 4th, 8th, and 10th weeks. The average consumption rate of FPE was about 88% at the end of the experiment, which was calculated based on patient records. During the intervention period, two female participants in the FPE group as well as one male from the placebo group dropped out owing to antibiotic intake and occasional visits, and thus only 44 participants managed to complete the study.
2.3.3. Blood collection
The fasting blood samples were collected (initial, 4th, 8th, and 10th weeks) in two tubes, one with EDTA coated for plasma and another without anticoagulant for serum preparation. Plasma was separated and used for determining various antioxidant indexes. The serum samples were used for assaying the lipid profile. All blood samples were stored at −80°C until analysis.
2.3.4. Fecal sample collection and bacterial enumeration
Fecal samples were collected into the sterile plastic anaerobic bag at the initial, 4th, 8th, and 10th weeks to check the major intestinal microflora. One gram of fecal sample was weighed and diluted with 9 mL of peptone saline (contain peptone and sodium chloride) and homogenized in different dilution (1:10) ratio with peptone saline in anaerobic condition. To determine the different microflora, the homogenized fecal sample (20 μL) was plated on acidified different agar medium (incubated for 48 hours under aerobic and anaerobic condition at 37°C) based on different types of bacterial enumeration. Enumeration of Escherichia coli, Clostridium perfringens, Lactobacillus spp. (anaerobic bacteria), Bifidobacteria spp. (aerobic bacteria), and total anaerobic bacteria was performed on MacConkey agar (Merck, Darmstadt, Germany) using the method of Manafi and Kneifel [13], TSC agar by Harmon et al [14], Rogosa SL agar (Merck) by Rogosa et al [15], BIM 25 agar, and Brucella agar (Creative Microbiologicals, Taipei, Taiwan) by Beerens [16]. After the incubation period, colonies were characterized by Gram staining and catalase reaction, as well as biochemically characterized by API gallery system (Biomerieux, Paris, France) and were calculated using a formula with dilution factor and dry weight of feces. The colony counts were reported as colony-forming units (CFU/g dry weight of feces).
2.3.5. Lipid profiles in serum
TC, triglyceride (TG), and high-density lipoprotein cholesterol (HDL-c) were determined using commercial lipid profile assay kits from Abcam (Cambridge, UK). Low-density lipoprotein cholesterol (LDL-c) was calculated by using the Friedewald equation.
2.3.6. Determination of total phenolics contents and various oxidative indexes in plasma
The total phenolic contents in plasma were determined by the method of Serafini et al [17] using Folin–Ciocalteu's phenol reagent. The total antioxidant capacity in plasma was determined using the method described by Arnao et al [18] with a slight modification according to Miller et al [19]. The total thiobarbituric acid reactive substances (TBARS) in plasma was measured by reacting with 2-thiobarbituric acid using the Draper and Hadley method [20]. The total thiol in plasma was determined using the Halliwell and Gutteridge method [21].
2.3.7. Lag time of LDL oxidation (ex vivo)
LDL was isolated using an ultracentrifugation process, and the oxidation of LDL was measured by monitoring the formation of conjugated dienes at 234 nm using a Hitachi U 2100 spectrophotometer at 5-minute intervals at 37°C after Cu2+ catalyzed oxidation by the Esterbauer method [22].
2.4. Statistical analysis
The results were expressed as mean ± standard deviation. The paired t test was used to compare the difference in the same group, and Student t test was used to compare the difference between the FPE and placebo groups; the variables were analyzed via one-way analysis of variance using Statistical Package for the Social Sciences (SPSS) version 23.0 (IBM, Chicago, IL, USA). All results with a p of value less than 0.05 are considered statistically significant.
3. Results and discussion
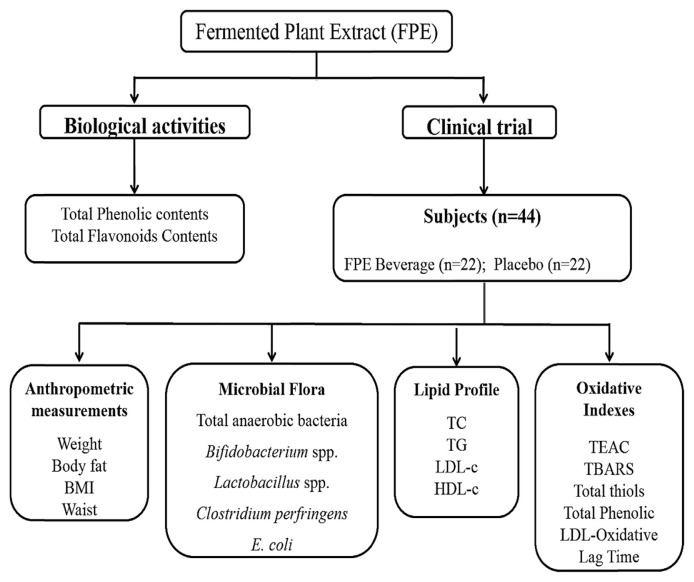
For several decades, fermentative processing has been used in the food industry to fortify or enhance the nutrient value of food products [23]. Thus, for the present study, we used the fermentation process to improve the beneficial and nutritive value of plant extracts (to release active components) in the form of health-promoting beverage. The main concept of the present study was to demonstrate the correlation between the FPE and human intestinal microflora with its subsequent physiological functions, especially in terms of lipid metabolism and oxidative status to maintain the health conditions of mildly hypercholesterolemic individuals. The flowchart of the present study is exemplified in Figure 1. Table 1 epitomizes the contents of the total phenolics and flavonoids in placebo and FPE beverage. Both phenolic and flavonoids contents in FPE (0.54 ± 0.03 mg GAE/mL; 107.71 ± 8.07 μg QE/mL) were far superior (p < 0.01) to placebo (0.09 ± 0.01 mg GAE/mL; 31.18 ± 2.07 μg QE/mL).
Figure 1.
The experimental design of the present trial. BMI = body mass index; HDL-c = high-density lipoprotein cholesterol; LDL-c = low-density lipoprotein cholesterol; TBARS = thiobarbituric acid reactive substances; TC = total cholesterol; TEAC = trolox equivalent antioxidant capacity; TG = triglyceride.
Table 1.
Contents of total phenolics and total flavonoids in the fermented plant extract (FPE) and placebo.
| Placebo | FPE | |
|---|---|---|
| Total phenolicsa (mg/mL) | 0.09 ± 0.01 | 0.54 ± 0.04** |
| Total flavonoidsb (μg/mL) | 31.18 ± 2.07 | 107.71 ± 8.07** |
Values are expressed as means ± standard deviation.
p < 0.01 (vs. placebo).
mg gallic acid equivalent (GAE)/mL.
μg quercetin equivalent (QE)/mL.
The effect of FPE on anthropometric parameters and plasma total phenolic contents in healthy mildly hypercholesterolemic participants are shown in Table 2. Marked alteration (p < 0.05) was observed in the levels of body weight, body mass index (BMI), and body fat in participants who consumed FPE, which indicates that FPE might have a direct impact on energy utilization. FPE contains dietary fibers, yeast, starch, and oligosaccharides, which would account for the decrease in body weight, fat, and BMI as they are well known for decreasing the total energy intake by lowering appetite (increased satiety value). Our results were in correlation with the results of other investigators [24]. Recently, Gullon and his coworkers [25] also proved that probiotics could activate gluconeogenesis and thereby bring down the body weight. However, no significant changes in the waist circumference were noted between FPE and placebo participants. Table 2 also shows the plasma phenolic contents. Administration of FPE for 8 weeks showed a considerable increase (p < 0.05) in the levels of plasma phenolic contents in comparison with volunteers who consumed placebo. This could be attributed to the increased total phenolic contents in FPE than in placebo beverage.
Table 2.
Effect of FPE on anthropometric parameters and plasma total phenolic contents in healthy mildly hypercholesterolemic participants.
| Placebo | FPE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Weight (kg) | Body fat (%) | BMI (kg/m2) | Waist (cm) | Phenolic content1 | Weight (kg) | Body fat (%) | BMI (kg/m2) | Waist (cm) | Phenolic content1 | |
| Initial | 59.85 ± 6.44a | 29.00 ± 2.89a | 25.81 ± 2.83a | 84.57 ± 12.15a | 2.10 ± 0.34a | 60.10 ± 4.73a | 30.13 ± 4.36a | 24.62 ± 3.84a | 83.89 ± 10.16a | 2.17 ± 0.23b |
| 4th week | 59.88 ± 7.03a | 28.83 ± 2.10a | 25.83 ± 3.99a | 84.69 ± 12.79a | 2.10 ± 0.15a | 59.29 ± 5.75b | 27.61 ± 3.44bc | 24.30 ± 3.86b* | 83.39 ± 11.94a | 2.22 ± 0.21c* |
| 8th week | 59.54 ± 6.80a | 29.17 ± 3.39a | 25.68 ± 4.96a | 84.41 ± 12.25a | 2.12 ± 0.23a | 58.61 ± 6.57c* | 27.07 ± 3.24cd** | 24.11 ± 3.79c* | 82.68 ± 11.60a | 2.35 ± 0.21a** |
| Follow-up2 | 59.56 ± 6.65a | 28.32 ± 2.66a | 25.70 ± 2.93a | 84.64 ± 11.73a | 2.09 ± 0.35a | 58.95 ± 7.83c | 26.90 ± 2.21d* | 24.12 ± 3.87c* | 82.58 ± 11.17a | 2.21 ± 0.23c* |
Values are expressed as means ± SD. Data within the same group bearing different superscripts are significantly different (p < 0.05).
p < 0.05,
p < 0.01 (vs. placebo).
BMI = body mass index; FPE = fermented plant extract; SD = standard deviation.
mg gallic acid equivalent/mL.
Follow-up: 2 weeks after the end of the experimental period.
The intestinal microflora is a collection of various micro-organisms such as bacteria, viruses, and protozoan. Usually, intestinal bacteria are taken into account as they are recognized as a health-promoting factor and are subdivided into beneficial and harmful bacteria [26]. Bifidobacterium spp. and Lactobacillus spp. are beneficial in that they render various health-promoting functions by helping digestion and absorption of nutrients, production of short-chain fatty acids (SCFAs) and vitamins; inhibiting the growth of harmful bacteria or pathogens, immune-stimulant; and lowering cholesterol and ammonia levels. By contrast, Clostridium perfringes spp. and E. coli are considered harmful bacteria, which favor deleterious effects to human host [27,28]. The prebiotic/probiotic efficiency of any products are evaluated by checking major components of microflora (anaerobic and aerobic bacteria) such as E. coli, C. perfringens, Bifidobacterium spp., Lactobacillus spp., and total anaerobic bacteria of intestinal microflora [29].
Therefore, the influence of intestinal microbiota by FPE was assessed by bacterial enumeration. After 8 weeks of drinking with FPE, an exponential improvement was observed in intestinal microflora especially Bifidobacterium and Lactobacillus bacterial colonies (Table 3). FPE acted as a good prebiotic and thus favored the proliferation and growth of microflora. Meanwhile, C. perfringens and E. coli were substantially lowered by the ingestion of FPE in comparison with the placebo group. Despite the decrease in the C. perfringens and E. coli group, the population of total anaerobic bacteria rose slightly, which might be attributable to the increase in the Bifidobacterium and Lactobacillus bacterial group (anaerobic). However, during the follow-up period (2 weeks without sample), C. perfringens and E. coli started to increase gradually, whereas Bifidobacterium and Lactobacillus spp. began to decline, thus indicating that FPE has a direct influence on intestinal microbiota.
Table 3.
Effect of FPE on the intestinal microflora count in healthy mild hypercholesterolemic individuals.
| Placebo | FPE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| E. coli (CFU/g) | C. perfringens (CFU/g) | L. spp. (CFU/g) | B. spp (CFU/g) | TAB (CFU/g) | E. coli (CFU/g) | C. perfringens (CFU/g) | L. spp. (CFU/g) | B. spp. (CFU/g) | TAB (CFU/g) | |
| Initial | 7.36 ± 0.43a | 8.32 ± 0.65a | 7.44 ± 0.61a | 8.69 ± 0.53a | 10.23 ± 0.90a | 7.33 ± 0.87a | 8.26 ± 0.62b | 7.48 ± 0.76c | 8.74 ± 0.61b | 10.21 ± 1.04a |
| 4th week | 7.42 ± 0.59a | 8.36 ± 0.62a | 7.39 ± 0.75a | 8.42 ± 0.84a | 10.25 ± 0.83a | 7.38 ± 0.64a | 8.24 ± 0.91b | 7.79 ± 0.77b | 8.89 ± 0.92ab* | 10.25 ± 1.23a |
| 8th week | 7.43 ± 0.43a | 8.39 ± 0.51a | 7.62 ± 0.45a | 8.41 ± 0.67a | 10.34 ± 0.89a | 7.21 ± 0.85b* | 7.99 ± 0.87c* | 8.17 ± 0.51a* | 9.25 ± 0.74a** | 10.44 ± 0.95ab* |
| Follow-up1 | 7.38 ± 0.36a | 8.29 ± 0.79a | 7.69 ± 0.65a | 8.56 ± 0.54a | 10.36 ± 0.91a | 7.40 ± 0.91a | 8.42 ± 0.78a | 7.99 ± 0.50ab | 8.71 ± 0.91b | 10.40 ± 0.81b |
Values are expressed as means ± SD. Data within the same group bearing different superscripts are significantly different (p < 0.05).
p < 0.05,
p < 0.01 (vs. placebo).
B. spp. = Bifidobacterium species; CFU = colony-forming unit; C. perfringens = Clostridium perfringens; E. coli = Escherichia coli; FPE = fermented plant extract; L. spp. = Lactobacillus species; SD = standard deviation; TAB = total anaerobic bacteria.
Growing evidence suggested that oral administration of polyphenol-rich prebiotics will increase the numbers of intestinal Bifidobacterium and Lactobacillus spp., which may concurrently decrease the production or number of harmful coliform bacteria such as E. coli and C. perfringens [5,10,27]. Intake of fermented Oolong tea significantly inhibited the colonization of C. perfringens and other Clostridium spp., whereas the colonization of Bifidobacterium and Lactobacillus groups are markedly elevated, thus indicating its proliferative action only on beneficial bacteria [30]. Furthermore, Parkar and colleagues [31] also pointed out that dietary polyphenols and flavonoids could increase the binding capacity of probiotics in the gut and thus suppress harmful bacteria attachment rate to intestinal mucosa that may contribute to the well-balanced intestinal microbiota.
Hypercholesterolemia has been identified as a pivotal risk factor for CVD. It is widely accepted that maintaining a normal level of blood cholesterol (lipid) would be the best strategy for preventing or lowering the incidence of CVD [1]. The TC and LDL-c levels were significantly (p < 0.05) blunted on supplementation with FPE for 8 weeks, whereas the levels of HDL were slightly altered, and no changes were noted in TG levels (Table 4). During the follow-up period, the levels of TC, TG, and LDL-c were again increased (slightly), suggesting that FPE can directly alter the intestinal microbiota and thereby influence the overall plasma lipid profile. Similar observations were reported by Connolly et al [32] and Mountzouris et al [33], who proved that fermented products and oligosaccharides could efficaciously lower the cholesterol level by improving the bacterial genera Bifidobacteria and Lactobacilli spp. Few experimental data suggest that changes in the intestinal microflora might have a direct impact on the concentration of serum cholesterol, but the mechanism is unclear [34,35].
Table 4.
Effect of FPE on the plasma lipid profile in healthy mildly hypercholesterolemic participants.
| Placebo | FPE | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| TG (mg/dL) | TC (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | TG (mg/dL) | TC (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | |
| Initial | 101.05 ± 14.93a | 226.68 ± 29.22a | 52.22 ± 10.92a | 142.52 ± 13.60a | 98.95 ± 10.39a | 230.05 ± 27.73a | 53.10 ± 10.93b | 146.60 ±1 4.18ab |
| 4th week | 102.91 ± 11.58a | 222.77 ± 21.33a | 52.63 ± 08.82a | 140.35 ± 15.75a | 102.68 ± 14.90a | 219.23 ± 34.36b | 54.95 ± 08.15ab* | 140.22 ± 16.96b |
| 8th week | 100.36 ± 10.50a | 220.18 ± 24.91a | 53.23 ± 06.65a | 138.67 ± 12.92a | 106.18 ± 11.46a* | 211.55 ± 31.97b** | 54.35 ± 10.76ab* | 130.69 ± 17.71b* |
| Follow-up1 | 101.52 ± 14.28a | 228.90 ± 24.72a | 53.51 ± 08.54a | 139.28 ± 19.59a | 100.41 ± 12.11a | 225.45 ± 30.51a | 54.64 ± 09.59a | 142.31 ± 19.34a |
Values are expressed as means ± SD. Data within the same group bearing different superscripts are significantly different (p < 0.05).
p <0.05,
p <0.01 (vs. placebo).
FPE = fermented plant extract; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SD = standard deviation; TC = total cholesterol; TG = triglyceride.
Follow-up: 2 weeks after the end of the experimental period.
Numerous animal and human studies have postulated some possible cholesterol-lowering mechanisms of prebiotics and probiotics, which are attributable to the increased viscosity of the gastrointestinal tract, thus inhibiting the bile salt conjugation, bile acid reabsorption, or micelle formation and shortening the gastric transit time by increasing the bulk to feces as well as increasing the fecal cholesterol excretion [36,37]. In addition, oligosaccharides, starch, and fibers (prebiotics) would increase the production of SCFAs such as acetate, propionate, and lactate. These SCFAs (especially propionate) are transferred from the intestine to the liver via the hepatic portal vein and subsequently inhibit 3-hydroxy-3-methyl-glutarylcoenzyme A (HMG-CoA) reductase, a rate-limiting enzyme in endogenous cholesterol synthesis, as well as act as an energy source for the host by activating gluconeogenesis [7,25,38]. Owing to several phytocomponents (mixtures) in FPE, we cannot prove the exact mechanism behind the hypocholesterolemic activity of FPE.
Hypercholesterolemia is highly implicated in the excessive generation of free radicals, which results in various detrimental effects and finally ends up in CVD. Once the free radical generation increases, it leads to oxidative stress and subsequently results in elevated lipid peroxidation [39]. Epidemiological studies have shown that intake of plant-based products probably lowers the incidence of CVD owing to its antioxidant efficiency [5]. Table 5 typifies the effect of FPE on the oxidative indexes in healthy mildly hypercholesterolemic volunteers. FPE-administered participants showed a pronounced increase (p < 0.05) in the levels of total antioxidant capacity (trolox equivalent antioxidant capacity: TEAC), with a significant decline (p < 0.05) in lipid peroxidation product (TBARS) levels, but no substantial changes in thiol levels neither with FPE nor placebo. FPE supplementation improved the plasma phenolic content (antioxidant property), which might positively elevate the antioxidant capacity (TEAC) and negatively decrease the lipid peroxidation product (TBARS) production. Some researchers also demonstrated that significant increases in the concentrations of antioxidant capacity were noted in the prebiotic-treated group [30,40]. Furthermore, inulin exhibits antioxidant properties independent of altering the gut bacterial growth and can scavenge free radicals and thus lower the lipid peroxidation in the stomach [41].
Table 5.
Effect of FPE on oxidative indexes in healthy mild hypercholesterolemic volunteers.
| Placebo | FPE | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| TEAC (μM) | TBARS (μM) | Thiols (mM) | TEAC (μM) | TBARS (μM) | Thiols (mM) | |
| Initial | 493.66 ± 70.09a | 0.91 ± 0.10a | 0.11 ± 0.02a | 491.47 ± 62.01b | 0.91 ± 0.16a | 0.11 ± 0.01a |
| 4th week | 494.80 ± 50.20a | 0.88 ± 0.15a | 0.12 ± 0.01a | 499.62 ± 52.46a | 0.74 ± 0.17b** | 0.12 ± 0.01a |
| 8th week | 491.06 ± 72.74a | 0.87 ± 0.18a | 0.12 ± 0.01a | 499.65 ± 50.54a* | 0.70 ± 0.13b** | 0.12 ± 0.02a |
| Follow-up1 | 493.34 ± 54.51a | 0.89 ± 0.10a | 0.11 ± 0.02a | 494.07 ± 53.91b | 0.82 ± 0.13ab | 0.12 ± 0.01a |
Values were expressed as means ± SD. Data within the same group bearing different superscripts were significantly different (p<0.05).
p < 0.05,
p < 0.01 (vs. placebo).
FPE = fermented plant extract; SD = standard deviation; TBARS = thiobarbituric acid reactive substances; TEAC = trolox equivalent antioxidant capacity.
Follow-up: 2 weeks after the end of experimental period.
As pointed out previously during hypercholesterolemia, excessive free radicals are generated, which may tend to easily oxidize LDL-c (Oxi-LDL), because of the rich content of fatty acids and phospholipids. Once the Oxi-LDL is increased, this probably damages the endothelium and leads to atherosclerosis by forming plaques and blocking the blood supply, and results in CVD. The LDL-oxidation lag time was determined to check the oxidation resistance of LDL, based on the formation of conjugated dienes [3]. Drinking FPE for 8 consecutive weeks exponentially increased (p < 0.05) the levels of lag time of LDL oxidation from 51.41 ± 12.07 to 69.87 ± 9.05, which indicated a 1.35-fold increase in lag time and thus improved the integrity of LDL from oxidation (Table 6). Also, FPE may increase SCFA production that was proved to display antioxidant activity in the colonic mucosa [42]. Therefore, by prolonging the LDL-oxidation process, FPE can effectively reduce the risk of CVD. In the placebo group, the beverage did not alter any of these parameters.
Table 6.
Effect of FPE on lag time of LDL oxidation in healthy mild hypercholesterolemic volunteers.
| Placebo (min) | FPE (min) | |
|---|---|---|
| Initial | 52.60 ± 4.54a | 51.81 ± 11.07b |
| 8th week | 53.76 ± 4.75a | 69.87 ± 9.05a** |
Values were expressed as means ± SD. Data within the same group bearing different superscripts were significantly different (p < 0.05).
p < 0.01 (vs. placebo).
FPE = fermented plant extract; LDL = low-density lipoprotein; SD = standard deviation.
As discussed earlier, FPE can effectively improve beneficial bacterial count and thereby increase the biotransformation of active components of various plant materials present in it. This would be the major reason for the increased bioavailability of different phenolic compounds, which were reflected in the results of increased plasma phenolic contents. Zhang and coworkers [30] also demonstrated that phenolic and flavonoids contents (active components) were increased with the elevation of the beneficial bacterial count on treatment with FPE. Therefore, we inferred that FPE could improve the intestinal microflora and thereby render an effective antioxidant and hypocholesterolemic effect.
This study has several limitations including the fact that no nonfermented plant extract was used for comparison, and microflora changes were not analyzed by flow cytometry or r-RNA gene sequencing, which would have given a clear picture about the microbiota prior to and after the intervention.
4. Conclusions
The current intervention clearly demonstrates that FPE could significantly lower body weight, fat, BMI, TC, LDL-c, TBARS, and harmful bacteria such as E. coli and C. perfringens as well as substantially improved the levels of TEAC, total phenolic content, and beneficial bacteria such as Bifidobacterium and Lactobacillus spp. Moreover, FPE prolonged the LDL-oxidation lag time owing to its antioxidant capacity and thus exhibited a cardioprotective effect. Hence, we hypothesize that FPE might positively regulate the human intestinal flora and thus maintain the balance between the beneficial and harmful bacteria and thereby favor various health benefits of acting as good probiotics. In the future, the mechanism behind the FPE regulatory effect can be examined in various metabolic syndrome and abnormal gastrointestinal diseases.
Acknowledgments
The financial support rendered by Chung Shan Medical University (CS110013), Taiwan is greatly appreciated. The authors also thank all the volunteers who participated in this trial.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2016.10.008.
Funding Statement
The financial support rendered by Chung Shan Medical University (CS110013), Taiwan is greatly appreciated.
Footnotes
Conflicts of interest
The authors declare that there is no conflict of interest to disclose.
REFERENCES
- 1. Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kamesh V, Sumathi T. Antihypercholesterolemic effect of Bacopa monniera Linn. on high cholesterol diet induced hypercholesterolemia in rats. Asian Pac J Trop Med. 2012;5:949–55. doi: 10.1016/S1995-7645(12)60180-1. [DOI] [PubMed] [Google Scholar]
- 3. Lu TM, Chiu HF, Shen YC, Chung CC, Venkatakrishnan K, Wang CK. Hypocholesterolemic efficacy of quercetin rich onion juice in healthy mild hypercholesterolemic adults: a pilot study. Plant Foods Hum Nutr. 2015;70:395–400. doi: 10.1007/s11130-015-0507-4. [DOI] [PubMed] [Google Scholar]
- 4. Sandhya V, Rajamohan T. Comparative evaluation of the hypolipidemic effects of coconut water and lovastatin in rats fed fat–cholesterol enriched diet. Food Chem Toxicol. 2008;46:3586–92. doi: 10.1016/j.fct.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 5. Etxeberria U, Fernández-Quintela A, Milagro FI, Aguirre L, Martínez JA, Portillo MP. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J Agric Food Chem. 2013;61:9517–33. doi: 10.1021/jf402506c. [DOI] [PubMed] [Google Scholar]
- 6. El-Gawad IAA, El-Sayed E, Hafez S, El-Zeini H, Saleh F. The hypocholesterolaemic effect of milk yoghurt and soy-yoghurt containing bifidobacteria in rats fed on a cholesterol-enriched diet. Int Dairy J. 2005;15:37–44. [Google Scholar]
- 7. Scheid MMA, Moreno YMF, Junior MRM, Pastore GM. Effect of prebiotics on the health of the elderly. Food Res Int. 2013;53:426–32. [Google Scholar]
- 8. Reza MA, Hossain MA, Lee SJ, Kim JC, Park SC. In vitro prebiotic effects and quantitative analysis of Bulnesia sarmienti extract. J Food Drug Anal. 2016;24:822–30. doi: 10.1016/j.jfda.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alonso VR, Guarner F. Linking the gut microbiota to human health. Br J Nutr. 2013;109:S21–6. doi: 10.1017/S0007114512005235. [DOI] [PubMed] [Google Scholar]
- 10. Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5:1241–9. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- 11. Julkunen-Tiitto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem. 1985;33:213–7. [Google Scholar]
- 12. Wang CK, Hwang LS. Analysis of the phenolic compounds in betel quid. J Chin Agric Chem Soc. 1993;31:623–32. [Google Scholar]
- 13. Manafi M, Kneifel W. A combined chromogenic–fluorogenic medium for the simultaneous detection of coliform groups and E. coli in water. Zentralb Hyg Umweltmed [Int J Hyg Environ Med] 1989;189:225–34. [PubMed] [Google Scholar]
- 14. Harmon SM, Kautter DA, Peeler JT. Comparison of media for the enumeration of Clostridium perfringens. Appl Microbiol. 1971;21:922–7. doi: 10.1128/am.21.5.922-927.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rogosa M, Mitchell JA, Wiseman RF. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J Bacteriol. 1951;62:132. doi: 10.1128/jb.62.1.132-133.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beerens H. Detection of bifidobacteria by using propionic acid as a selective agent. Appl Environ Microbiol. 1991;57:2418. doi: 10.1128/aem.57.8.2418-2419.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serafini M, Maiani G, Ferro-Luzzi A. Alcohol-free red wine enhances plasma antioxidant capacity in humans. J Nutr. 1998;128:1003–7. doi: 10.1093/jn/128.6.1003. [DOI] [PubMed] [Google Scholar]
- 18. Arnao M, Casas J, Del Rio J, Acosta M, Garcia-Canovas F. An enzymatic colorimetric method for measuring naringin using 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) in the presence of peroxidase. Anal Biochem. 1990;185:335–8. doi: 10.1016/0003-2697(90)90304-r. [DOI] [PubMed] [Google Scholar]
- 19. Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci. 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 20. Draper H, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 21. Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 22. Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res. 1989;6:67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- 23. Kantachote D, Charernjiratrakul W, Umsakul K. Antibacterial activities of fermented plant beverages collected in southern Thailand. J Biol Sci. 2008;8:1280–8. [Google Scholar]
- 24. Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr. 2006;60:567–72. doi: 10.1038/sj.ejcn.1602350. [DOI] [PubMed] [Google Scholar]
- 25. Gullon B, Pintado ME, Barber X, Fernández-López J, Pérez-Álvarez JA, Viuda-Martos M. Bioaccessibility, changes in the antioxidant potential and colonic fermentation of date pits and apple bagasse flours obtained from co-products during simulated in vitro gastrointestinal digestion. Food Res Int. 2015;78:169–76. doi: 10.1016/j.foodres.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 26. Hooper LV, Midtvedt T, Gordon JI. How host–microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 27. Vendrame S, Guglielmetti S, Riso P, Arioli S, Klimis-Zacas D, Porrini M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J Agric Food Chem. 2011;59:12815–20. doi: 10.1021/jf2028686. [DOI] [PubMed] [Google Scholar]
- 28. Mitsou EK, Turunen K, Anapliotis P, Zisi D, Spiliotis V, Kyriacou A. Impact of a jelly containing short-chain fructo-oligosaccharides and Sideritis euboea extract on human faecal microbiota. Int J Food Microbiol. 2009;135:112–7. doi: 10.1016/j.ijfoodmicro.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 29. Gibson GR, Fuller R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J Nutr. 2000;130:391S–5S. doi: 10.1093/jn/130.2.391S. [DOI] [PubMed] [Google Scholar]
- 30. Zhang X, Zhu X, Sun Y, Hu B, Sun Y, Jabbar S, Zeng X. Fermentation in vitro of EGCG, GCG and EGCG3″ Me isolated from Oolong tea by human intestinal microbiota. Food Res Int. 2013;54:1589–95. [Google Scholar]
- 31. Parkar SG, Stevenson DE, Skinner MA. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int J Food Microbiol. 2008;124:295–8. doi: 10.1016/j.ijfoodmicro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 32. Connolly ML, Lovegrove JA, Tuohy KM. Konjac glucomannan hydrolysate beneficially modulates bacterial composition and activity within the faecal microbiota. J Funct Foods. 2010;2:219–24. [Google Scholar]
- 33. Mountzouris KC, Balaskas C, Fava F, Tuohy KM, Gibson GR, Fegeros K. Profiling of composition and metabolic activities of the colonic microflora of growing pigs fed diets supplemented with prebiotic oligosaccharides. Anaerobe. 2006;12:178–85. doi: 10.1016/j.anaerobe.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 34. Song MY, Wang JH, Eom T, Kim H. Schisandra chinensis fruit modulates the gut microbiota composition in association with metabolic markers in obese women: a randomized, double-blind placebo-controlled study. Nutr Res. 2015;35:655–63. doi: 10.1016/j.nutres.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 35. Bernini LJ, Simao ANC, Alfieri DF, Lozovoy MAB, de Souza CHB, Dichi I, Costa GN. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: a randomized trial. Nutrition. 2016;6:716–9. doi: 10.1016/j.nut.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 36. Lim S, Jeong JJ, Woo KH, Han MJ, Kim DH. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota LPS production and inducing colon tight junction protein expression. Nutr Res. 2015;36:337–48. doi: 10.1016/j.nutres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 37. Yen CH, Tseng YH, Kuo Y-W, Lee MC, Chen HL. Long-term supplementation of isomalto-oligosaccharides improved colonic microflora profile, bowel function, and blood cholesterol levels in constipated elderly people—a placebo-controlled, diet-controlled trial. Nutrition. 2011;27:445–50. doi: 10.1016/j.nut.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 38. Yeo SK, Ooi LG, Lim TJ, Liong MT. Antihypertensive properties of plant-based prebiotics. Int J Mol Sci. 2009;10:3517–30. doi: 10.3390/ijms10083517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiu HF, Shen YC, Huang TY, Venkatakrishnan K, Wang CK. Cardioprotective efficacy of red wine extract of onion in healthy hypercholesterolemic subjects. Phytother Res. 2015;30:380–5. doi: 10.1002/ptr.5537. [DOI] [PubMed] [Google Scholar]
- 40. Pourghassem Gargari B, Dehghan P, Aliasgharzadeh A, Asghari Jafar-Abadi M. Effects of high performance inulin supplementation on glycemic control and antioxidant status in women with type 2 diabetes. Diabetes Metab J. 2013;37:140–8. doi: 10.4093/dmj.2013.37.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stoyanova S, Geuns J, Hideg É, Van Den Ende W. The food additives inulin and stevioside counteract oxidative stress. Int J Food Sci Nutr. 2011;62:207–14. doi: 10.3109/09637486.2010.523416. [DOI] [PubMed] [Google Scholar]
- 42. Saa DT, Turroni S, Serrazanetti DI, Rampelli S, Maccaferri S, Candela M, Severgnini M, Simonetti E, Brigidi P, Gianotti A. Impact of Kamut® Khorasan on gut microbiota and metabolome in healthy volunteers. Food Res Int. 2014;63:227–32. [Google Scholar]