Abstract
We report a case of unusual human anaplasmosis in the Amazon rainforest of French Guiana. Molecular typing demonstrated that the pathogen is a novel Anaplasma species, distinct to all known species, and more genetically related to recently described Anaplasma spp. causing infections in rainforest wild fauna of Brazil.
Keywords: anaplasmosis, Amazon rainforest, bacteria, vector-borne infections, zoonoses, French Guiana
Anaplasmoses are emerging tickborne zoonoses caused by intracellular bacteria of the Anaplasma genus. In total, 8 Anaplasma species and several candidate species have been described, including at least 5 species infecting humans (1,2). Of particular concern, the agent of human granulocytic anaplasmosis, A. phagocytophilum, has a specific tropism to polymorphonuclear neutrophils (1,3). Another species, provisionally named A. capra, recently described from asymptomatic goats, is now recognized as an agent of human intraerythrocytic anaplasmosis in China (4). The 3 other species detected in humans are major veterinary agents sporadically identified in few patients worldwide: A. ovis and A. bovis in erythrocytes and A. platys in platelets (1,5). Human anaplasmosis are consistently associated with persons who live in rural areas in habitats favorable to ticks or who work closely with domestic animals (1,6). However, recent surveys report the presence of novel Anaplasma species of undetermined zoonotic potential in wild fauna (1,2).
The Study
We assessed the presence of Anaplasma in blood samples of clandestine gold miners working in the Amazon rainforest of French Guiana. This 83,000 km2 territory, located between Suriname and Brazil, is one of the regions of highest biodiversity in the world, with >280 species of wild mammals (7). The human population of French Guiana (≈284,000 inhabitants) is concentrated principally in a handful of towns spread along the coastline and the 2 main rivers (8). The interior is largely uninhabited and covered by dense rainforest, where illegal gold mining camps are located (9,10).
We examined 363 archived DNA extracts obtained from human blood samples. We primarily collected these samples in 2019 as part of Malakit, a malaria survey in remote mining camps in French Guiana (11). To characterize the whole bacterial diversity, we typed DNA blood samples by using a high-throughput bacterial 16S rDNA (rrs) sequencing approach (bacterial barcoding) (12). Bacteria were characterized as operational taxonomic units (OTUs) and amplicon sequence variants (ASVs) and taxonomically assigned by using the Silva database (https://www.arb-silva.de).
Examination of OTUs and ASVs revealed the presence of Anaplasma sequences in 1 DNA sample. No OTU or ASV assigned to the Anaplasma genus or to the Anaplasmataceae family was detected in the 362 other samples. We further conducted 2 independent Anaplasma-specific PCRs targeting a region of the 16S rDNA gene (544 bp) and the 23S–5S (ITS2) intergenic region (423 bp) using techniques described by Calchi et al. (13) and obtained amplicons of correct sizes for the positive sample. The Sanger sequencing of amplicons obtained with each pair of primers confirmed the presence of Anaplasma. These sequences have been deposited to GenBank (accession nos. ON513878, ON521229).
We used BLAST (https://www.ncbi.nlm.nih.gov/blast/Blast.cgi) to compare the 16S rDNA and ITS2 nt sequences with Anaplasma sequences available in GenBank. None of the nucleotide sequences observed in this study are 100% identical to known Anaplasma sequences. The 16S rRNA sequence showed highest identities with Anaplasma found in wild fauna of Brazil, including an Anaplasma sp. detected in Amblyomma coelebs ticks collected on South American coatis, Nasua nasua (99.8%; GenBank accession no. MT019560); another Anaplasma sp. of black rats, Rattus rattus (99.8%; GenBank accession no. KY391803); and Candidatus Anaplasma amazonensis (13) of brown-throated sloths (Bradypus variegatus) and two-toed sloths (Choloepus didactylus) (99.1%; GenBank accession no. MT199827). All other Anaplasma species showed identities <99%. The ITS2 sequences showed highest nucleotide identity with Candidatus A. amazonensis of sloths (96.8%; GenBank accession no. MT267354) and lower identities with other Anaplasma species or strains (<92%). On account of these distinct genetic traits, we propose the designation Candidatus Anaplasma sparouinense for this novel bacterium. The specific name refers to the Sparouine River, where the infected patient lived.
We conducted phylogenetic analyses on the basis of these 16S rDNA and ITS2 nucleotide sequences by using the maximum-likelihood method. We obtained trees of similar topologies with a robust clustering of Candidatus A. sparouinense with some Anaplasma associated with Brazilian wild fauna: Candidatus A. sparouinense is phylogenetically related to Anaplasma sp. infections detected in ticks of coatis, black rats, and, to a lesser extent, to Candidatus A. amazonensis of sloths (Figure 1). Altogether, they delineate a clade of neotropical Anaplasma divergent to all other Anaplasma species (Figure 1).
Figure 1.
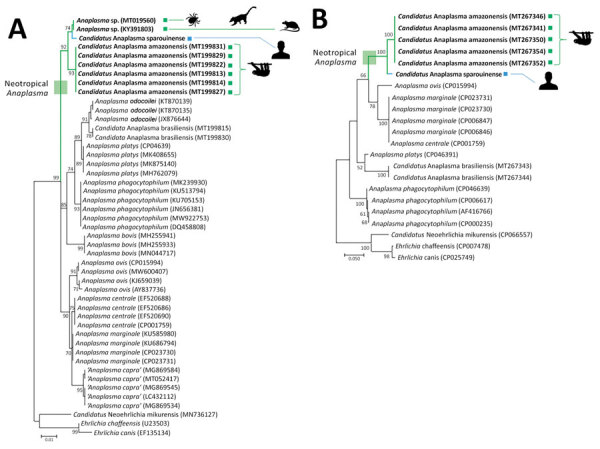
Anaplasma phylogenies for the Candidatus Anaplasma sparouinense species characterized from French Guiana and reference sequences. Trees were constructed by using maximum-likelihood estimations based on best-fit approximation for the evolutionary model Hasegawa-Kishino-Yano plus invariant sites for 16S rDNA with 485 unambiguously aligned bp (A) and ITS2 sequences with 387 unambiguously aligned bp (B). Bold indicates Anaplasma species and strains specific to the Neotropics. GenBank accession numbers of sequences used in analyses are shown on the phylogenetic trees. Numbers at nodes indicate percentage support of 1,000 bootstrap replicates. The scale bar is in units of substitution per site.
The DNA sample positive for Candidatus A. sparouinense was from a 58-year-old man who had a history of posttraumatic splenectomy and malaria attacks caused by Plasmodium vivax. This patient originated from Maranhão, Brazil, but had been working exclusively in the rainforest of French Guiana for the past 3 years. The Sparouine anaplasmosis was retrospectively diagnosed in September 2021 on the basis of PCR survey of previous blood samples (October 2019 and May 2021) and blood smears (October 2019).
We primarily detected the presence of Candidatus A. sparouinense in a blood sample collected in October 2019. At that time, the patient was asymptomatic, including no fever and blood pressure at reference levels; tests were negative for agents of diseases usually tested for in French Guiana (serologic assays for yellow fever, Q fever, hepatitis B and C, HIV, and syphilis and molecular tests for malaria and leptospirosis). He displayed anemia, a hemoglobin level of 10.5 g/dL. The reexamination of Giemsa-stained thin blood film taken for malaria diagnosis at that time revealed the presence of intraerythrocytic bodies, which could be Candidatus A. sparouinense. No infection was detected in granulocytes and platelets, but around one third of erythrocytes harbored 1 or 2 small, round, dark purple inclusions located at their periphery, which could be Anaplasma (Figure 2). We also detected the presence of Howell–Jolly bodies in erythrocytes (Figure 2, panel B), which could be a consequence of splenectomy.
Figure 2.
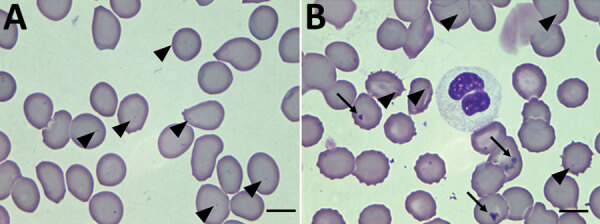
Thin films of a blood sample collected in October 2019 from a patient in French Guiana. Inclusions of Candidatus Anaplasma sparouinense are located at the periphery of the red blood cells as small round dots of 0.3–0.4 µm (arrowheads). Other red blood cells contain Howell-Jolly bodies of various shapes and sizes >1 µm (arrows). Some Howell-Jolly bodies are found in the background of the smears. Wright-Giemsa stain; original magnification ×100.
Eighteen months later (May 2021), the patient was admitted to the Cayenne Hospital Center with fever, myalgia, headache, epistaxis, and severe anemia (hemoglobin 6.6g/dL). A broad microbiologic investigation ruled out COVID-19, dengue, chikungunya virus, Zika virus, influenza, malaria, HIV, hepatitis B and C, and leptospirosis. The only positive test was a subnormal level of Coxiella burnetii IgM and IgG (phase II IgG 64, IgM 96; phase I negative), which led to the introduction of antibiotic treatment (doxycycline 100 mg 2×/d for 21 d and ceftriaxone 1 g/d for 5 d). The anemia was considered autoimmune hemolytic because of a positive Coombs test and was thus treated with prednisolone with decreasing doses from 60 mg/day to 10 mg/day for 3 months. The patient recovered within 3 weeks; symptoms resolved, and his hemoglobin level improved to 9.4 g/dL at discharge. Our a posteriori Anaplasma PCR survey of May 2021 blood samples (before and at day 7 of antibiotic treatment) again revealed the presence of Candidatus A. sparouinense. No further blood sample was preserved; thus, the disappearance of the Anaplasma infection at the end of antibiotic treatment could not be confirmed.
Conclusions
We characterized Candidatus A. sparouinense as a novel human intraerythrocytic pathogen. The infection arose over at least 18 months in a patient living in the rainforest of French Guiana who was potentially more susceptible because of a previous splenectomy. The phylogenetic proximity of Candidatus A. sparouinense to other Anaplasma associated with Amazon ticks and wild mammals highlights that a genetic cluster of Anaplasma is circulating in French Guiana and Brazil. These neotropical Anaplasma species might represent a source of novel infections to humans. Better documentation of the diversity and transmission cycles of Anaplasma in the Amazon rainforest is needed, as recently highlighted for other novel tickborne pathogens described in French Guiana (14,15).
Acknowledgments
We thank the medical staffs of health centers in French Guiana, especially the staff of Grand Santi and the field team that collected the data (Louise Hureau-Mutricy, Audrey Godin, Mylène Cebe, and Alan Ribeiro).
The research was supported by funding from Investissements d’Avenir grants managed by the Agence Nationale de la Recherche (ANR, France, Laboratoire d’Excellence CEBA, ref. ANR-10-LABX-25-01).
Biography
Dr. Duron is an evolutionary ecologist in the MIVEGEC Research Unit, French National Centre for Scientific Research, France. His research interest includes the molecular study of tickborne microbes and their epidemiologic relevance.
Footnotes
Suggested citation for this article: Duron O, Koual R, Musset L, Buysse M, Lambert Y, Jaulhac B, et al. Novel chronic anaplasmosis in splenectomized patient, Amazon rainforest. Emerg Infect Dis. 2022 Aug [date cited]. https://doi.org/10.3201/eid2808.212425
References
- 1.Rar V, Tkachev S, Tikunova N. Genetic diversity of Anaplasma bacteria: Twenty years later. Infect Genet Evol. 2021;91:104833. 10.1016/j.meegid.2021.104833 [DOI] [PubMed] [Google Scholar]
- 2.Battilani M, De Arcangeli S, Balboni A, Dondi F. Genetic diversity and molecular epidemiology of Anaplasma. Infect Genet Evol. 2017;49:195–211. 10.1016/j.meegid.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 3.Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29:341–55. 10.1016/j.idc.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyer AR, Carlyon JA. Of goats and men: rethinking anaplasmoses as zoonotic infections. Lancet Infect Dis. 2015;15:619–20. 10.1016/S1473-3099(15)70097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu M, Li F, Liao Y, Shen J-J, Xu J-M, Chen Y-Z, et al. Epidemiology and diversity of Rickettsiales bacteria in humans and animals in Jiangsu and Jiangxi provinces, China. Sci Rep. 2019;9:13176. 10.1038/s41598-019-49059-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atif FA. Alpha proteobacteria of genus Anaplasma (Rickettsiales: Anaplasmataceae): Epidemiology and characteristics of Anaplasma species related to veterinary and public health importance. Parasitology. 2016;143:659–85. 10.1017/S0031182016000238 [DOI] [PubMed] [Google Scholar]
- 7.Hollowell T, Reynolds RP. Checklist of the terrestrial vertebrates of the Guiana Shield. Natl Mus Nat Hist MRC. 2013;106. [Google Scholar]
- 8.Institut National de la Statistique et des Études Économiques (INSEE). Bilan démographique de Guyane 2018. Une croissance démographique toujours soutenue. Report no. 121. 2020. [cited 2021 Oct 7]. https://www.insee.fr/fr/statistiques/4285434
- 9.Douine M, Musset L, Corlin F, Pelleau S, Pasquier J, Mutricy L, et al. Prevalence of Plasmodium spp. in illegal gold miners in French Guiana in 2015: a hidden but critical malaria reservoir. Malar J. 2016;15:315. 10.1186/s12936-016-1367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douine M, Mosnier E, Le Hingrat Q, Charpentier C, Corlin F, Hureau L, et al. Illegal gold miners in French Guiana: a neglected population with poor health. [Erratum in: BMC Public Health. 2017;17:736]. BMC Public Health. 2017;18:23. 10.1186/s12889-017-4557-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douine M, Lambert Y, Galindo MS, Mutricy L, Sanna A, Peterka C, et al. Self-diagnosis and self-treatment of malaria in hard-to-reach and mobile populations of the Amazon: results of Malakit, an international multicentric intervention research project. Lancet Reg Health Am. 2021;4:100047. 10.1016/j.lana.2021.100047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binetruy F, Buysse M, Lejarre Q, Barosi R, Villa M, Rahola N, et al. Microbial community structure reveals instability of nutritional symbiosis during the evolutionary radiation of Amblyomma ticks. Mol Ecol. 2020;29:1016–29. 10.1111/mec.15373 [DOI] [PubMed] [Google Scholar]
- 13.Calchi AC, Vultão JG, Alves MH, Yogui DR, Desbiez ALJ, De Santi M, et al. Ehrlichia spp. and Anaplasma spp. in Xenarthra mammals from Brazil, with evidence of novel ‘Candidatus Anaplasma spp.’. Sci Rep. 2020;10:12615. 10.1038/s41598-020-69263-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binetruy F, Garnier S, Boulanger N, Talagrand-Reboul É, Loire E, Faivre B, et al. A novel Borrelia species, intermediate between Lyme disease and relapsing fever groups, in neotropical passerine-associated ticks. Sci Rep. 2020;10:10596. 10.1038/s41598-020-66828-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binetruy F, Buysse M, Barosi R, Duron O. Novel Rickettsia genotypes in ticks in French Guiana, South America. Sci Rep. 2020;10:2537. 10.1038/s41598-020-59488-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


