Abstract
The incidence of COVID-19 gastrointestinal manifestations has been reported to range from 3% to 61%. There are limited data on the incidence rates and risk factors associated with gastrointestinal bleeding (GIB) in patients with COVID-19. A rapid review has been designed to investigate whether there is a relationship between COVID-19 and GIB in adult patients. PubMed, CINAHL, EMBASE, Cochrane Library, and Scopus databases have been analyzed. A total of 129 studies were found; 29 full texts were analyzed, and of these, 20 were found to be relevant to the topic.
The key findings of the included studies present an overall GIB rate in COVID-19 patients ranging from 1.1% to 13%. The bleeding involves mucosal damage of the duodenum, stomach, colon, and rectum. The management of gastrointestinal bleeding could be conservative. The use of fecal diversion systems for the management of diarrhea in COVID-19 patients should be minimized and closely evaluated for the risk of rectal mucosal damages and erosions. It is recommended to provide an accurate nutritional assessment; an early setting up of enteral nutrition, if not contraindicated, can help protect the gut mucosa of patients and restore normal intestinal flora. Larger cohort studies are needed to increase the information about this topic.
The COVID-19 pandemic has threatened global health and continues to cause excess mortality. Over 6 million people have died from COVID 19 so far, according to a report by the World Health Organization (2022). The typical clinical manifestations of this illness are fever, cough, dyspnea, and myalgia or fatigue (Guan et al., 2020). Digestive symptoms such as nausea, vomiting, diarrhea, and abdominal pain usually accompany the respiratory symptoms (Maculotti, Spena, & Villa, 2020; Pan, Shao, et al., 2020; Yang, Zheng, et al., 2020; Ye, Yang, Liu, Liao, & Wang, 2020). The incidence of gastrointestinal (GI) manifestations has ranged from 3% to 61% in patients with COVID-19 (Gupta et al., 2020; Ye et al., 2020). The prevalence and characteristics of GI symptoms in these patients remain largely unknown. The focus on understanding and managing the severe and fatal respiratory symptoms may have resulted to underreporting of GI manifestations. This could be due to an underreporting of GI symptoms in the first published studies. (Parasa et al., 2020).
Emerging data have shown that the GI tract could represent a target organ for SARS-CoV-2 (Mao et al., 2020). The cell entry receptor for SARS-CoV-2 is the angiotensin-converting enzyme 2 (ACE2) (Xu et al., 2020; Zhou et al., 2020), which is broadly distributed in body tissues and highly expressed in renal, cardiovascular, and GI tissues. This indicates that COVID-19 may affect multiple organs, which may explain the various extrapulmonary symptoms (Zhang et al., 2020). SARS-CoV-2 may primarily enter the cells of the lungs, but the enterocytes in the small bowel are rich in ACE2 receptors. This presents a potential primary entrance for the virus, infecting the host and causing the COVID-19 GI symptoms (Monkemüller, Fry, & Rickes, 2020).
According to Human Protein Atlas portal, the small intestine is the tissue where the protein expression level of ACE 2 is mainly expressed (Zhang et al., 2020). Histopathological evidence of diffuse endothelial inflammation in the submucosal vessels of the small intestine of patients with COVID-19 and mesenteric ischemia suggest microvascular small bowel injury (Caramaschi et al., 2021). The presence of infiltrating plasma cells and lymphocytes and interstitial edema in the lamina propria of the stomach, duodenum, and rectum explains the inflammation mediated damage. (Gupta et al., 2020). It has also been hypothesized that there is an alteration of the intestinal flora by the virus, which could result in GI symptoms (mainly diarrhea) (Pan, Mu, et al., 2020).
The intestine is the largest immune organ in the body. Changes in the composition and performance of the digestive tract flora affect the respiratory tract through the common mucosal immune system. Respiratory tract flora disorders also affect the digestive tract through immune regulation. This effect is called the “gut–lung axis” (Dhar & Mohanty, 2020; Pan, Mu, et al., 2020). Particularly concerning is the presence of living virus in patients' stool, and the fecal shedding seems to continue for days after hospitalization (Parasa et al., 2020).
COVID-19 patients are at a high risk of venous thromboembolism (VTE) because of dehydration caused by fever and/or GI symptoms, in particular diarrhea or anorexia. This condition leads to the release of a large number of inflammatory mediators in severe or critically ill patients and may lead to blood hypercoagulability. Obesity, other comorbidities (especially in the elderly), the onset of a new infection, hypotension, or simply deep sedation and other conditions related to decreased mobility call for conscientious and other conditions related to decreased mobility call for conscientious VTE risk assessment (Zhai et al., 2020). The International Society on Thrombosis and Haemostasis guidance on the detection and treatment of coagulopathy in COVID-19 patients advised to start prophylactic anticoagulation as early as possible to prevent thrombotic events and organ damage (Thachil et al., 2020). Bleeding or coagulation abnormalities may occur following the administration of anticoagulant drugs. Even if the bleeding risk seems less important than the thrombotic risk, a bleeding risk assessment should be taken into full consideration in COVID-19 patients treated with prophylactic anticoagulation.
Patients admitted into the intensive care unit (ICU) have an increased risk of stress-related mucosal disease (SRMD) from GI bleeding (GIB). This is related to well-known clinical risk factors: coagulopathy, number of comorbidities, need for renal replacement therapy, and chronic liver disease (Granholm et al., 2019). Thanks to lung-protective mechanical ventilation, pharmacology, and also enteral nutrition (EN) as prophylaxis of SRMD. The bleeding rate has been reported to decrease significantly during the last decades, from 17% without prophylaxis to 1% or lower. (Buendgens, 2016).
GIB is clinically apparent, as visible blood loss may manifest as hematemesis, melena, or hematochezia. GIB reflects one of the most common and important medical emergencies, with a mortality rate of 2%–10% (Mujtaba et al., 2020; Schmiderer et al., 2020). Hemorrhage from the GI tract is classified as upper GIB, lower GIB, or small bowel bleeding (“obscure GIB”) (Mujtaba et al., 2020). Overt upper GIB usually presents as melena or hematemesis, or also as hematochezia. Lower GIB appears as visible blood loss from the GI tract, with red or brown stool (Kamboj, Hoversten, & Leggett, 2019).
This review aims to describe the relationship between COVID-19 and GIB. The results will provide some reflections and practical considerations that can be useful in the management of COVID-19 patients.
Material and Methods
Study Design
A rapid literature review has been designed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Moher, Liberati, Tetzlaff, & Altman, 2009) and the Rapid Review Guidebook—Steps for Conducting a Rapid Review (Dobbins, 2017). Rapid reviews follow the principles of knowledge synthesis, including a clear statement of the aims, eligibility criteria, and systematic presentation and synthesis of results. The methods of a rapid review are like those of a systematic review, but the method adopted does not require the depth and breadth of a full systematic review (Langlois, Straus, Antony, King, & Tricco, 2019; Tricco et al., 2020). Rapid reviews have proved to be an efficient way to help policy makers make informed decisions based on high-quality evidence generated promptly.
The review has been guided by the following research question: “Is there a relationship between COVID-19 and gastrointestinal bleeding in adult patients?” A PO study design was applied to the review: Population: COVID-19 adult patients; Outcome: gastrointestinal bleeding.
Eligibility Criteria
Types of Participant
This review considered all studies that involved adult human subjects affected by COVID-19 and GIB. Articles involving the pediatric population were excluded.
Types of Outcome Measure
The primary outcome of interest was GIB. Secondary outcomes included GIB rates in COVID-19 patients, patient characteristics, and bleeding management. No restrictions were applied concerning the designs of the eligible studies or the time of publication.
Information Sources
The literature search was conducted in the PubMed, CINAHL, EMBASE, Cochrane Library, and Scopus databases. Both Medical Subject Headings (MeSH) and free-text terms, as well as variations of root words, were searched with no limits set. The last search was performed on February 15, 2021.
Search
Search Strategy
The search strategy was designed to access published materials and comprised three stages:
A limited search in PubMed to identify relevant key words contained in the title, abstract, and subject descriptors.
The terms and the synonyms identified were used in an extensive search of the literature.
Reference lists of the articles collected in Stage 2 were screened for relevant articles to be searched and included in this review.
The references search included the following key words: “gastrointestinal bleeding,” “gastrointestinal haemorrhage,” “COVID-19,” and “SARS-CoV-2.” Full texts of articles meeting the inclusion criteria based on their title, abstract, and subject descriptors were obtained for data synthesis. To include additional significant contributions, the references of the eligible articles were evaluated.
Study Selection
The review considered all the types of study design evaluating the GIB in patients affected by COVID-19. Identified studies that met the inclusion criteria were grouped into one of the following categories: review, case report/case series, observational, and commentaries/letter to the editor. Gray literature and non-English-language written articles were excluded. The selection process was initially performed by two reviewers by reading the titles and the abstracts. The full texts of the articles that met the inclusion criteria were then read independently by the same reviewers. The decision to include or exclude an article was reached jointly following a discussion. In case of disagreement, a third researcher was involved.
Data Extraction
The data of the included studies were checked for quality (Table 1) and extracted (see Supplemental Digital Content Table 2, available at: http://links.lww.com/GNJ/A77) by the two independent reviewers. Data were analyzed in three steps: (1) quality assessment of included articles; (2) tabulation of extracted data: author, year of publication and country, study design, aim, sample, GIB, management, and implications for practice; and (3) synthesis of the results of the selected studies, as well as the systematic organization of the literature indications/recommendations (evidence synthesis). Data were checked for consistency by the other authors.
TABLE 1. Quality Appraisal According to the Checklist by Dixon-Woods et al. (2006).
| Author(s), Year | Are the Aims and Objectives of the Research Clearly Stated? | Is the Research Design Clearly Specified and Appropriate for the Aims and Objectives of the Research? | Do the Researchers Provide a Clear Account of the Process by Which Their Findings Were Reproduced? | Do the Researchers Display Enough Data to Support Their Interpretations and Conclusions? | Is the Method of Analysis Appropriate and Adequately Explicated? |
|---|---|---|---|---|---|
| Al-Samkari et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Barrett et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Carvalho et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Cavaliere et al., 2020 | No | No | Yes | No | No |
| Chen et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Fraissé et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| González González et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Gadiparthi et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Gulen & Satar, 2020 | Yes | Yes | Yes | Yes | Yes |
| Guotao et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Li et al., 2020 | No | Yes | Yes | Yes | Yes |
| Lin et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Massironi et al., 2020 | Yes | Yes | No | Yes | Yes |
| Patel & Sengupta, 2020 | Yes | No | No | Yes | Yes |
| Su et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Trindade et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Yang, Yu, et al., 2020 | Yes | Yes | Yes | Yes | Yes |
| Xiao et al., 2020 | Yes | No | No | Yes | No |
| Wan et al., 2020 | Yes | Yes | No | Yes | No |
| Wang et al., 2021 | Yes | Yes | Yes | Yes | Yes |
Synthesis of the Results
We performed a narrative synthesis of the evidence of the included studies, according to the definition developed by Dobbins (2017). We performed a description of the key findings of the included studies. Then, we organized the findings to map the evidence and synthesize the results of the included articles.
Results
Characteristics of the Studies
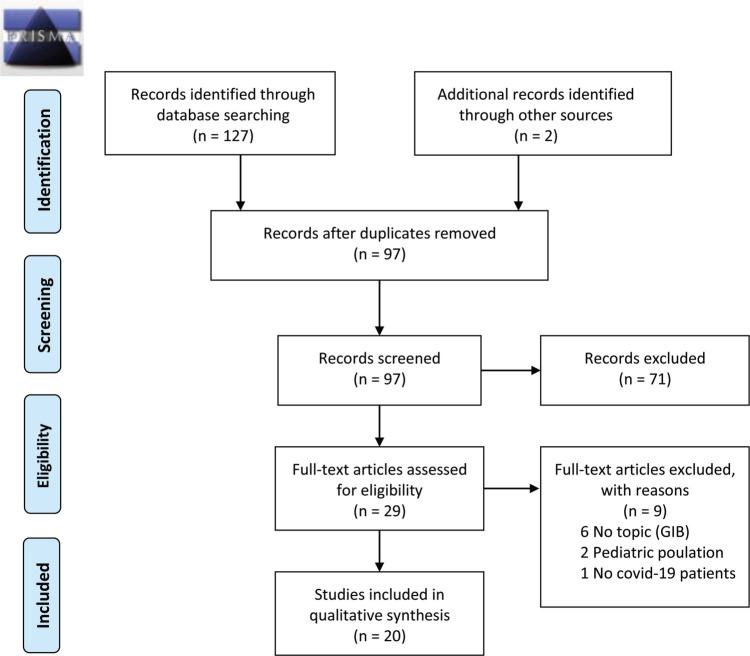
In the initial search, 127 primary studies were found Through reading of journals on the topic under study, two other articles were found. Removing the duplicates left 97 articles. After reading titles and abstracts, 29 full texts were analyzed, and among these, 20 were found to be relevant to the inclusion criteria (Figure 1). The quality evaluation of the articles was carried out independently using the Dixon-Woods checklist (Dixon-Woods et al., 2006) (Table 1). The 20 included studies were conducted in different countries, mainly China (N = 8) and the United States (N = 7). The studies had different study designs: observational studies (N = 9), case reports/case series (N = 9), and reviews (N = 2). The narrative synthesis of the evidence of the included studies based on the data extraction table is shown in Supplemental Digital Content Table 2 (available at: http://links.lww.com/GNJ/A77).
FIGURE 1.
PRISMA 2009 flow diagram. From “Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement,” By D. Moher, A. Liberati, J. Tetzlaff, D. G. Altman, and the PRISMA Group, 2009, PLoS Medicine, 6(6), p. e1000097. doi:10.1371/journal.pmed1000097. Copyright 2009 Moher et al. For more information, visit www.prisma-statement.org.
Characteristics of GIB and Rates in COVID-19 Patients
The overall GIB rate in COVID-19 patients ranged from 1.1% (González González et al., 2020) to 13% (Xiao et al., 2020). Most studies reported an average bleeding rate of 4% (Al-Samkari et al., 2020; Wan et al., 2020; Yang, Yu, et al., 2020). Most patients with GIB were critically ill men with a mean age of 67.5 years.
The bleeding involves mucosal damage with multiple, round herpetic erosions and ulcers in the esophagus with white moss and blood clots (Barrett, Lo, Stanek, & Walter, 2020; Henley & Wilson, 2014; Li et al., 2020a; Lin et al., 2020; Su et al., 2020; Xiao et al., 2020; Yang, Yu, et al., 2020). It also involves the stomach, duodenum, colon, and rectum (Carvalho et al., 2020; Gadiparthi et al., 2020; Gulen & Satar, 2020; Guotao, Xingpeng, Zhihui, & Huirui, 2020; Lin et al., 2020; Su et al., 2020; Wan et al., 2020; Wang, Mu, Qi, & Zhang, 2021; Yang, Yu, et al., 2020).
GIB could be related to the concomitant low-molecular-weight heparin therapy, severe infection, or disseminated intravascular coagulation (Barrett et al., 2020; Massironi et al., 2020). Risk factors for GIB in patients initiated on anticoagulants have included older age, a history of GIB, chronic renal impairment, Helicobacter pylori (H. pylori) infection, concomitant use of antiplatelet drugs, and pre-existing GI tract lesions (Fraissé et al., 2020; Patel & Sengupta, 2020). COVID-19 rates of thrombosis and bleeding were similar to those reported in hospitalized patients with comparable degrees of critical illness (Al-Samkari et al., 2020; Fraissé et al., 2020; Trindade et al., 2021). Elevated D-dimer levels at initial presentation predicted bleeding complications, thrombotic complications, critical illness, and death. Beyond D-dimer levels, thrombosis was primarily associated with inflammatory markers rather than coagulation parameters (Al-Samkari et al., 2020).
Bleeding Management
COVID-19-positive patients with GIB can be managed conservatively with intravenous (IV) high-dose proton pump inhibitor (PPI) therapy (Chen, Yang, & Duan, 2020; Gadiparthi et al., 2020; Gulen & Satar, 2020; Wang et al., 2021; Xiao et al., 2020), IV therapy with octreotide (Chen et al., 2020; Xiao et al., 2020), and packed red blood cells (Gadiparthi et al., 2020; Gulen & Satar, 2020; Wang et al., 2021). In critically ill patients, GI management could include a topical mucosal protective agent of aluminum phosphate gel 20 g three times a day and hemostatic therapy (thrombin 16 units/ml in ice-cooled water 50 ml every 2 hours) through a nasogastric tube for 72 hours (Li et al., 2020a). Management also required the monitoring of vital signs, GI symptoms, and hemoglobin value (Cavaliere, Levine, Wander, Sejpal, & Trindade, 2020; Chen et al., 2020), with the head of the bed raised at 30° to prevent gastric reflux (Li et al., 2020b). Lack of response over 24 hours may indicate a need for endoscopy (Cavaliere et al., 2020; Wang et al., 2021).
Discussion
Manifestations and Risk Factors
This study aimed to describe the relationship between COVID-19 and GIB to suggest some reflections and practical considerations useful in the management of COVID-19 patients. The literature showed an increased GIB rate from 1.1% to 13% in COVID-19 patients (González González et al., 2020; Xiao et al., 2020) with multiple, round herpetic erosions and ulcers in the esophagus (Barrett et al., 2020; Chen et al., 2020; Li et al., 2020a; Lin et al., 2020; Su et al., 2020; Xiao et al., 2020). This situation confirms that the mucosal damage of the whole GI tract seems to be a target organ for SARS-CoV-2 (Lin et al., 2020).
Dehydration caused by fever and/or GI symptoms, in particular diarrhea or anorexia, increases the risk of VTE in COVID-19 patients (Zhai et al., 2020). However, the COVID-19 rates of thrombosis and bleeding were similar to those reported in hospitalized patients with comparable degrees of critical illness (Al-Samkari et al., 2020).
Management
The International Society on Thrombosis and Haemostasis guidance on the detection and treatment of coagulopathy in COVID-19 patients has stated that it is advisable to offer prophylactic anticoagulation as early as possible to prevent thrombotic events and organ damage (Thachil et al., 2020).
COVID-19 patients can experience diarrhea as emerging symptoms or during hospitalization and common in ICU patients. The proportion of ICU patients experiencing diarrhea is described as being between 3.3% and 78% (Hay et al., 2019). Fecal diversion systems for patients with liquid or semiliquid stool are used in the management of fecal incontinence, infection control, and prevention of associated skin damage. Fecal management systems are usually not intended for use longer than 29 days. Clinicians should observe caution in the insertion and use of these devices due to the possible device-related mucosal pressure ulcer risk.
COVID-19 patients are at a greater risk of bleeding, and they present mucosal ischemic signs, both characteristics excluding a priori the use of a closed catheter system for the management of liquid or semi-liquid stool. The fecal diversion system is associated with local bleeding events in anticoagulated patients (Mulhall & Jindal, 2013). The plan should ensure a daily assessment of criteria for the withdrawal of these systems. If signs of rectal bleeding occur, the device should be immediately removed and the patient should be notified. There is no description of the practical management of diarrhea in COVID-19 patients in the recent literature. We have no available data on the use of fecal management systems and the potential augmentation of mucosal pressure ulcer risk related to these devices.
GI symptoms related to COVID-19 and related therapies can impair nutrient delivery, digestion, absorption, and mucosal integrity, raising important questions about nutrition. The attachment of the SARS-CoV-2 virus to the ACE2 receptors of the digestive system is believed to disrupt the normal intestinal flora, leading to different GI symptoms, especially diarrhea. The shortage of nutrient contact with intestinal mucosa may lead to an atrophy of lymphoid tissue and functional decline of the immune system, as well as intensification in bacterial translocation. It is important to emphasize that different medications used for COVID-19, such as antibiotics and antivirals, could lead to alteration of the gut microbiota.
According to the existing nutrition guidelines for COVID- 19, either oral or enteral nutrition (EN) is advised to maintain the gut barrier and immune function (Mechanick et al., 2021). However, in the actual clinical setting, it can be challenging to accomplish these recommendations for patients undergoing noninvasive ventilation (NIV) for many hours. These patients are frequently in a prone position, with altered taste, who are exhausted by the body fatigue and the loneliness caused by social isolation. Efforts should be made to evaluate and manage every patient's nutrition needs in order to provide the feeding through adequate timing, delivery method, typology of nutrients, food consistency, and texture. A healthy mucosa can sustain the GI tract to defend against the bleeding risk of COVID-19 patients.
Limitations
This rapid review has some limitations. There was a clinical heterogeneity across eligible trials that enrolled different populations and often did not identify the risk factors for bleeding in the participating individuals. The studies did not make clear distinctions between patients in the ICU and other clinical settings. No articles focused on the issue of oral cavity bleeding in COVID-19 patients. Finally, time for writing and revising may have delayed the sharing of the topic with the scientific community.
Clinical Implications
While waiting for more evidence from the scientific literature, it seems reasonable to protect the gut mucosa of critically ill patients by providing an accurate nutritional state assessment and an early setting up of EN (if not contraindicated), along with checking gastric residual volume at intervals (e.g., every 4 hours), searching for signs of bleeding. Furthermore, careful interventions to maintain EN should be established in patients undergoing NIV support (Bambi, Mati, De Felippis, & Lucchini, 2017) or prone position (Bruni et al., 2020). Even if the bleeding risk seems less important than the thrombotic risk, a bleeding risk assessment should be taken into full consideration in COVID-19 patients treated with prophylactic anticoagulation.
Research Implications
Larger cohort studies are needed to increase the information about this issue, and COVID-19 patients with GI manifestations during their stay in hospital should be adequately re-evaluated through follow-up programs after their discharge from the hospital.
Conclusion
There are limited data on the incidence rates and risk factors associated with gastrointestinal bleeding in patients with COVID-19. This rapid review showed that the overall gastrointestinal bleeding rate in COVID-19 patients ranging from 1.1% to 13%. Some interventions were described in order to manage this complication.
Footnotes
Authors' Contributions: Planning review: Alessandra Negro, Giulia Villa, and Stefano Bambi; Conducting review: Alessandra Negro, Giulia Villa, and Stefano Bambi; Drafting the manuscript: Alessandra Negro, Giulia Villa, and Stefano Rolandi; Supervising the manuscript: Alberto Lucchini and Stefano Bambi.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare compliance with Ethical Standards. All authors approved the final draft submitted.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.gastroenterologynursing.com).
Contributor Information
Alessandra Negro, Email: negro.alessandra@hsr.it.
Giulia Villa, Email: villa.giulia@hsr.it.
Stefano Rolandi, Email: rolandi.stefano@hsr.it.
Alberto Lucchini, Email: alberto.lucchini@unimib.it.
Stefano Bambi, Email: stefano.bambi@unifi.it.
REFERENCES
- Al-Samkari H., Karp Leaf R. S., Dzik W. H., Carlson J. C. T., Fogerty A. E., Waheed A., Rosovsky R. P. (2020). COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood, 136(4), 489–500. doi:10.1182/blood.2020006520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambi S., Mati E., De Felippis C., Lucchini A. (2017). Enteral nutrition during noninvasive ventilation: We should go deeper in the investigation. Respiratory Care, 62(8), 1118–1119. doi:10.4187/respcare.05509 [DOI] [PubMed] [Google Scholar]
- Barrett L. F., Lo K. B., Stanek S. R., Walter J. W. (2020). Self-limited gastrointestinal bleeding in COVID-19. Clinics and Research in Hepatology and Gastroenterology, 44(4), e77–e80. doi:10.1016/j.clinre.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni A., Garofalo E., Grande L., Auletta G., Cubello D., Greco M., Longhini F. (2020). Nursing issues in enteral nutrition during prone position in critically ill patients: A systematic review of the literature. Intensive and Critical Care Nursing, 60, 102899. doi:10.1016/j.iccn.2020.102899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendgens L. (2016). Prevention of stress-related ulcer bleeding at the intensive care unit: Risks and benefits of stress ulcer prophylaxis. World Journal of Critical Care Medicine, 5(1), 57. doi:10.5492/wjccm.v5.i1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramaschi S., Kapp M. E., Miller S. E., Eisenberg R., Johnson J., Epperly G., Giannico G. A. (2021). Histopathological findings and clinicopathologic correlation in COVID-19: A systematic review. Modern Pathology, 34(9), 1614–1633. doi:10.1038/s41379-021-00814-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A., Alqusairi R., Adams A., Paul M., Kothari N., Peters S., DeBenedet A. T. (2020). SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: Implications for detection and transmission of COVID-19 disease. American Journal of Gastroenterology, 115(6), 942–946. doi:10.14309/ajg.0000000000000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere K., Levine C., Wander P., Sejpal D. V., Trindade A. J. (2020). Management of upper GI bleeding in patients with COVID-19 pneumonia. Gastrointestinal Endoscopy, 92(2), 454–455. doi:10.1016/j.gie.2020.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Yang Q., Duan H. (2020). A severe coronavirus disease 2019 patient with high-risk predisposing factors died from massive gastrointestinal bleeding: A case report. BMC Gastroenterology, 20(1), 318. doi:10.1186/s12876-020-01458-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar D., Mohanty A. (2020). Gut microbiota and Covid-19—Possible link and implications. Virus Research, 285, 198018. doi:10.1016/j.virusres.2020.198018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon-Woods M., Cavers D., Agarwal S., Annandale E., Arthur A., Harvey J., Sutton A. J. (2006). Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Medical Research Methodology, 6(1), 35. doi:10.1186/1471-2288-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins M. (2017). Rapid review guidebook. Steps for conducting a rapid review. Hamilton, ON, Canada: National Collaborating Centre for Methods and Tools, McMaster University. [Google Scholar]
- Fraissé M., Logre E., Pajot O., Mentec H., Plantefève G., Contou D. (2020). Thrombotic and hemorrhagic events in critically ill COVID-19 patients: A French monocenter retrospective study. Critical Care, 24(1), 275. doi:10.1186/s13054-020-03025-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadiparthi C., Perisetti A., Sayana H., Tharian B., Inamdar S., Korman A. (2020). Gastrointestinal bleeding in patients with severe SARS-CoV-2. American Journal of Gastroenterology, 115(8), 1283–1285. doi:10.14309/ajg.0000000000000719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González González R., Jacob J., Miró Ò., Llorens P., Jiménez S., González del Castillo J., Piñera-Salmerón P. (2020). Incidence, clinical characteristics, risk factors, and outcomes of upper gastrointestinal bleeding in patients with COVID-19. Journal of Clinical Gastroenterology, 56(1), e38–e46. doi:10.1097/MCG.0000000000001465 [DOI] [PubMed] [Google Scholar]
- Granholm A., Zeng L., Dionne J. C., Perner A., Marker S., Krag M., Alhazzani W. (2019). Predictors of gastrointestinal bleeding in adult ICU patients: A systematic review and meta-analysis. Intensive Care Medicine, 45(10), 1347–1359. doi:10.1007/s00134-019-05751-6 [DOI] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Zhong N. (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382(18), 1708–1720. doi:10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulen M., Satar S. (2020). Uncommon presentation of COVID-19: Gastrointestinal bleeding. Clinics and Research in Hepatology and Gastroenterology, 44(4), e72–e76. doi:10.1016/j.clinre.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guotao L., Xingpeng Z., Zhihui D., Huirui W. (2020). SARS-CoV-2 infection presenting with hematochezia. Médecine et Maladies Infectieuses, 50(3), 293–296. doi:10.1016/j.medmal.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Madhavan M. V., Sehgal K., Nair N., Mahajan S., Sehrawat T. S., Landry D. W. (2020). Extrapulmonary manifestations of COVID-19. Nature Medicine, 26(7), 1017–1032. doi:10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- Hay T., Bellomo R., Rechnitzer T., See E., Ali Abdelhamid Y., Deane A. M. (2019). Constipation, diarrhea, and prophylactic laxative bowel regimens in the critically ill: A systematic review and meta-analysis. Journal of Critical Care, 52, 242–250. doi:10.1016/j.jcrc.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Henley C. E., Wilson T. E. (2014). Use of beat-to-beat cardiovascular variability data to determine the validity of sham therapy as the placebo control in osteopathic manipulative medicine research. Journal of the American Osteopathic Association, 114(11), 860–866. doi:10.7556/jaoa.2014.172 [DOI] [PubMed] [Google Scholar]
- Kamboj A. K., Hoversten P., Leggett C. L. (2019). Upper gastrointestinal bleeding: Etiologies and management. Mayo Clinic Proceedings, 94(4), 697–703. doi:10.1016/j.mayocp.2019.01.022 [DOI] [PubMed] [Google Scholar]
- Langlois E. V., Straus S. E., Antony J., King V. J., Tricco A. C. (2019). Using rapid reviews to strengthen health policy and systems and progress towards universal health coverage. BMJ Global Health, 4(1), e001178. doi:10.1136/bmjgh-2018-001178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Huang S., Lu J., Lai R., Zhang Z., Lin X., Shan H. (2020a). Upper gastrointestinal bleeding caused by SARS-CoV-2 infection. American Journal of Gastroenterology, 115(9), 1541–1542. doi:10.14309/ajg.0000000000000757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Huang S., Lu J., Lai R., Zhang Z., Lin X., Shan H. (2020b). Upper gastrointestinal bleeding caused by SARS-CoV-2 infection. American Journal of Gastroenterology, 115(9), 1541–1542. doi:10.14309/ajg.0000000000000757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., Shan H. (2020). Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut, 69(6), 997–1001. doi:10.1136/gutjnl-2020-321013 [DOI] [PubMed] [Google Scholar]
- Maculotti D., Spena P., Villa G. (2020). Position statement on care of ostomy patients during COVID-19 pandemic. Gastroenterology Nursing, 43(4), 324–326. doi:10.1097/SGA.0000000000000539 [DOI] [PubMed] [Google Scholar]
- Mao R., Qiu Y., He J. S., Tan J. Y., Li X. H., Liang J., Chen M. H. (2020). Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. The lancet. Gastroenterology & Hepatology, 5(7), 667–678. doi:10.1016/S2468-1253(20)30126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massironi S., Viganò C., Dioscoridi L., Filippi E., Pagliarulo M., Manfredi G., Invernizzi P. (2020). Endoscopic findings in patients infected with 2019 novel coronavirus in Lombardy, Italy. Clinical Gastroenterology and Hepatology, 18(10), 2375–2377. doi:10.1016/j.cgh.2020.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanick J. I., Carbone S., Dickerson R. N., Hernandez B. J. D., Hurt R. T., Irving S. Y., McKeever L. (2021). Clinical nutrition research and the COVID-19 pandemic: A scoping review of the ASPEN COVID-19 Task Force on Nutrition Research. Journal of Parenteral and Enteral Nutrition, 45(1), 13–31. doi:10.1002/jpen.2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkemüller K., Fry L., Rickes S. (2020). COVID-19, coronavirus, SARS-CoV-2 and the small bowel. Revista Española de Enfermedades Digestivas, 112(5), 383–388. doi:10.17235/reed.2020.7137/2020 [DOI] [PubMed] [Google Scholar]
- Mujtaba M., Kueht M., Merwat S., Hussain S., Gamilla-Crudo A. K., Kulkarni R., Fair J. (2020). Organ transplantation during the COVID-19 pandemic: Making the best patient care decision. American Journal of Transplantation, 20(11), 3259–3260. doi:10.1111/ajt.16116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhall A. M., Jindal S. K. (2013). Massive gastrointestinal hemorrhage as a complication of the Flexi-Seal fecal management system. American Journal of Critical Care, 22(6), 537–543. doi:10.4037/ajcc2013499 [DOI] [PubMed] [Google Scholar]
- Pan H., Shao N., Yan Y., Luo X., Wang S., Ye L., Chen W. (2020). Multi-chain Fudan-CCDC model for COVID-19—A revisit to Singapore's case. Quantitative Biology, 8(4), 325–335. doi:10.1007/s40484-020-0224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J., Tu L. (2020). Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. American Journal of Gastroenterology, 115(5), 766–773. doi:10.14309/ajg.0000000000000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasa S., Desai M., Thoguluva Chandrasekar V., Patel H. K., Kennedy K. F., Roesch T., Sharma P. (2020). Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019. JAMA Network Open, 3(6), e2011335. doi:10.1001/jamanetworkopen.2020.11335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Sengupta N. (2020). PPIs and beyond: A framework for managing anticoagulation-related gastrointestinal bleeding in the era of COVID-19. Digestive Diseases and Sciences, 65(8), 2181–2186. doi:10.1007/s10620-020-06408-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiderer A., Schwaighofer H., Niederreiter L., Profanter C., Steinle H., Ziachehabi A., Tilg H. (2020). Decline in acute upper gastrointestinal bleeding during COVID-19 pandemic after initiation of lockdown in Austria. Endoscopy, 52(11), 1036–1038. doi:10.1055/a-1178-4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Shen J., Zhu L., Qiu Y., He J.-S., Tan J.-Y., Liang J. (2020). Involvement of digestive system in COVID-19: Manifestations, pathology, management and challenges. Therapeutic Advances in Gastroenterology, 13, 175628482093462. doi:10.1177/1756284820934626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., Iba T. (2020). ISTH interim guidance on recognition and management of coagulopathy in COVID-19. Journal of Thrombosis and Haemostasis, 18(5), 1023–1026. doi:10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco A. C., Garritty C. M., Boulos L., Lockwood C., Wilson M., McGowan J., Straus S. E. (2020). Rapid review methods more challenging during COVID-19: Commentary with a focus on 8 knowledge synthesis steps. Journal of Clinical Epidemiology, 126, 177–183. doi:10.1016/j.jclinepi.2020.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade A. J., Izard S., Coppa K., Hirsch J. S., Lee C., Satapathy S. K. & Northwell COVID-19 Research Consortium. (2021). Gastrointestinal bleeding in hospitalized COVID-19 patients: A propensity score matched cohort study. Journal of Internal Medicine, 286(6), 887–894. doi:10.1111/joim.13232 [DOI] [PubMed] [Google Scholar]
- Wan Y., Li J., Shen L., Zou Y., Hou L., Zhu L., Lan P. (2020). Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. The Lancet Gastroenterology & Hepatology, 5(6), 534–535. doi:10.1016/S2468-1253(20)30118-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-L., Mu J.-S., Qi X.-B., Zhang W.-H. (2021). A case of COVID-19 complicated by massive gastrointestinal bleeding. Gastroenterology Report, 9(1), 85–87. doi:10.1093/gastro/goaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2022). WHO coronavirus disease (COVID-19). Situation by country, territory & area. Retrieved from https://covid19.who.int/table
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. (2020). Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology, 158(6), 1831–1833.e3. doi:10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Chen Q. (2020). High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science, 12(1), 8. doi:10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Zhou Y. (2020). Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. International Journal of Infectious Diseases, 94, 91–95. doi:10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Shang Y. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Medicine, 8(5), 475–481. doi:10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Yang Z., Liu J., Liao L., Wang F. (2020). Digestive system manifestations and clinical significance of coronavirus disease 2019: A systematic literature review. Journal of Gastroenterology and Hepatology, 36(6), 1414–1422. doi:10.1111/jgh.15323 [DOI] [PubMed] [Google Scholar]
- Zhai Z., Li C., Chen Y., Gerotziafas G., Zhang Z., Wan J., Wang C. (2020). Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: A consensus statement before guidelines. Thrombosis and Haemostasis, 120(6), 937–948. doi:10.1055/s-0040-1710019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li H.-B., Lyu J.-R., Lei X.-M., Li W., Wu G., Dai Z.-M. (2020). Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. International Journal of Infectious Diseases, 96, 19–24. doi:10.1016/j.ijid.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Shi Z.-L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. doi:10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]