Abstract
Carrageenan, a sulfated polysaccharide, was produced by certain species of marine red seaweeds, which have been used as a significant source of food, feed, and antibiotic agent throughout history due to their alleged human health benefits. The present study aimed to derive the polysaccharides from Hypnea valentiae and describe the biological applications. Carrageenan was characterized by FT-IR, C-NMR, AFM, and their antimicrobial, antioxidant, and anticoagulant capabilities; furthermore, the larvicidal effect of methanol extract was generated from the seaweed against Aedes aegypti larvae at various concentrations. The molecular docking experiments were carried out computationally for finding the molecular insight of the macromolecules and small molecules' interaction using GLIDE docking by using Schrodinger software. Antibacterial zones of inhibition in different concentrations are compared with the 40 mg/mL higher activity against bacterial pathogens. Carrageenan is strong in all antioxidant activities, with the overall antioxidant (70.1 ± 0.61%) of radical at 250 μg/mL concentration being exhibited. The DPPH scavenging is effective in the inhibition of (65.74 ± 0.58%) radical at a concentration of 160 μg/mL and the hydroxyl scavenging (65.72 ± 0.60%) of activity at a concentration of 125 μg/mL being exhibited. Anticoagulant activities (APPT and PT) of carrageenan fraction were tested. H. valentiae and heparin sulphate shows higher activity of APTT (106.50 IU at 25 μg/mL) in comparison with the PT test (57.86 IU at 25 μg/mL) and the methanol extraction of higher larvicidal activity on A. aegypti (LC50 = 99.675 μg/mL). In this study, the carrageenan was exploited through in vitro and in silico molecular docking studies against antimicrobial, antioxidant, and anticoagulant properties. The results were establishing the potentiality of the carrageenan which is an alternative source to control the mosquitocidal property in the future. Moreover, molecular docking of carrageenan against multiple targets results in −7 to −6 Kcal/mol binding score. Findings of carrageen from in vitro to in silico studies are needed for further validation of clinical pieces of evidence.
1. Introduction
Marine macroorganisms are a rich source of functionally diversified bioactive compounds that play an active role in human nutrition and health. Seaweeds are a major source of sulfated polysaccharides, particles which are widely employed in food, feed, and medicine due to their rheological qualities as gelling and stabilizing agents [1, 2]. Sulfated polysaccharides have numerous biological and physiological activities, including antithrombotic [3], anticoagulant [4], antioxidant [5], antidiabetic [6], antibacterial [7], immunomodulatory [8, 9], antiviral [10], antiinflammatory [11], antinociceptive [12, 13], antitumor [14], and proinflammatory effects [15, 16]. Sulfated polysaccharides including agarans, galactans, and carrageenans are also available abundantly. Among these, carrageenan is a generic deviation of linear, sulfated galactans derived from species of red seaweeds [17]. Various investigations on the antioxidant and anticoagulant properties of seaweeds or their extracts have been published [18, 19]. Algal polysaccharides must be shown to serve a significant role as free-radical scavengers and antioxidants in the protection of oxidative damage in living organisms in current administration, and anticoagulants have long been used to treat blood during dialysis and surgery, as well as to treat disseminated intravascular coagulation and thrombosis in a variety of disorders and for in vitro blood testing [20, 21]. Mosquitoes, such as Aedes aegypti, are the deadliest since they act as a vector for a variety of diseases, including dengue fever, malaria, yellow fever, filariasis, and other types of chikungunya as well as Zika virus [22–25]. Furthermore, seaweed sulfated polysaccharide extracts contain primary and secondary metabolites including bioactive chemicals which are biodegradable into harmless products; it may be effective in mosquito larval control [26, 27].
The standard new therapeutic approach is complicated, exhausting, expensive, time-consuming, and laborious. Computational methods such as molecular docking have played a critical role in rationalizing the road to drug discovery in order to overcome these obstacles. A molecular docking feature becomes a promising pharmaceutical research tool for screening candidates from drug libraries effectively [28]. In such studies, the ultimate goal was to find effective therapeutics for the carrageenan molecule extracted from marine algae. It is evaluated against antimicrobial, anticoagulant, and antioxidant activity using GLIDE docking in the maestro platform of Schrodinger software (Schrödinger Release 2021-2: Maestro, Schrödinger, LLC, New York, NY, 2021). Furthermore, the efficiency of the carrageenan compound was exploited with in vitro validation against the foresaid targets. Carrageenan is a sulfated polysaccharide, which is obtained by extraction from red seaweed species. It has been widely utilized in the food industry, agricultural, drug delivery, tissue engineering, and biosensor applications. Therefore, the present study investigated the isolation and biofunctional activities of carrageenan extracted from H. valentiae, characterization of structural properties using Fourier transform infrared (FT-IR) spectroscopy, 13C nuclear magnetic resonance (NMR), and atomic force microscopy (AFM) spectroscopy and evaluation of the biological properties such as antioxidant, antimicrobial, and anticoagulant activities with in silico molecular docking analysis.
2. Materials and Methods
2.1. Collection and Extraction of Sample
The red seaweed, H. valentiae (Turnur), was collected from Mandapam (Lat. 09°28′177N, Long. 79°18′536E) located on the southeast coastline of Ramanathapuram, Tamil Nadu, India. The seaweed specimen identification number was 1759. The sample was properly cleaned with seawater to remove all undesired pollutants such as sand particles and epiphytes and then thoroughly cleaned with tap water to discard all salt on the surface. The water was drained away, and the seaweed was laid out on the blotting paper to absorb any remaining moisture before being shade-dried at room temperature for 3 days and crushed into a fine powder. Following the completion of the sulfated polysaccharide extraction, the procedure was evaluated for 100 g of seaweed powder, which was soaked separately in acetone and methanol solvent in 7:3 for two days in a shaker at 200 rpm for 10 min. The process was repeated twice to ensure the dry biomass. This biomass was dried into a powder and dispersed in 1 L of 0.1 M HCL for 24 h with constant stirring at room temperature. The pellet was reextracted as above, and the supernatants were pooled. The supernatant was kept at 4°C overnight and precipitated with two volumes of absolute 1:1 and stirred continuously for 15 min, and then the precipitate was collected to separate carrageenan gel and distilled water. Carrageenan gel was then completely soaked in 96% alcohol for 1 h and stirred continuously. The carrageenan gel was separated from alcohol and distilled water by filtration. Subsequently, the mixture was centrifuged at 5000 rpm for 10 min, and the supernatant was neutralized with 1.0 M NaOH and poured in 100 mL of methanol. The carrageenan was freeze-dried by using a membrane at 70°C for a 24 h duration which was performed for further processing [29].
2.2. Structural Analysis
Using an alpha FT-IR spectrophotometer and its technique, FT-IR was used to determine the functional groups of the carrageenan polysaccharides [30]. The characterization has been complemented by the 13C-NMR spectrum. The 13C-NMR spectrum of 10 mg of the carrageenan was dissolved in 0.5 mL solution in D2O which was recorded at 27°C on an NMR spectra Bruker model MHz 400 spectrometer. The proton chemical shift was expressed in parts per million (ppm). The substance was diluted in distilled water before being mixed with a solution of 200 μg/mL carrageenan in 0.2 M NaOH. The carrageenan solutions were allowed to equilibrate for 1 h with NaOH prior to neutralization with HCl (pH∼6-7). Solutions were then passed through a 0.22 μm filter and deposited onto 1 cm of a freshly cleaned glass plate surface (sample volume ∼60 μL). Samples were dried for approximately 16 h in ambient conditions. Imaging of AFM was performed within 24 h of carrageenan deposition to avoid sample contamination.
2.3. Antibacterial Activity
Antibacterial studies were carried out on two Gram-positive bacteria (Enterococcus faecalis (MTCC 439) and Staphylococcus aureus (MTCC 96)) and Gram-negative bacteria (Escherichia coli (MTCC 443) and Pseudomonas aeruginosa (MTCC 741)). All the bacteria were obtained from the Research Laboratory, Microbiology Department, Periyar University, India. Positive control includes streptomycin. All bacterial cultures were incubated at 35°C for 24 h [31].
2.4. Antibiofilm Activity
To determine the effect of seaweed methanol extracts against antibiofilm activity, about 3.5 mL of nutrient broth and 1.5 mL of bacterial culture were added into sterile test tubes. 10 mg of sample was added to different concentrations of methanol extract (10, 20, 30, 40, and 50 μg/mL) and additionally given into each tube. All tubes were incubated in a shaking incubator at 37°C for 24 h. After incubation, all tubes were washed with distilled water. All the tubes were breeze-dried for a few minutes and added 5 mL of crystal violet and then incubated at 37°C for 1 h. After incubation, we discarded the crystal violet from all the tubes and washed them with distilled water. About 5 mL of 95.0% ethanol was added into all test tubes and taken OD at 595 nm by UV-visible spectrophotometer [32], respectively. The antibiofilm activity was calculated using the formula of percentage:
| (1) |
2.5. Antioxidant Activity Measurement
2.5.1. Total Antioxidant Property
The antioxidant activities of samples were evaluated by phosphomolybdenum complex formation according to the method [33]. About 0.5 mL sample extracts were added with 3 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The test tubes were covered with foil and incubated in a water bath at 95°C for 90 min. After the samples had cooled to room temperature, the absorbance of the mixture was measured at 695 nm against the reagent blank. The reported results were mean values expressed as mg AAE/g sample.
2.6. Assay for Radical Scavenging In Vitro
2.6.1. Scavenging Activity of DPPH (2,2-Diphenyl-1-picryl Hydrazyl)
Purified polysaccharides' DPPH-free radical-scavenging activity was assessed using Q-sepharose anion-exchange chromatography, as previously described by the technique [29, 34] with slight modifications. A solution of 3 mL of 0.1 mM methanolic solution DPPH was prepared. A respective blank sample of BHA and L-ascorbic acid was prepared by adding 100 μg/mL. The discoloration of the sample was measured with a proper blank at 517 nm after incubation for 30 min at 30°C in the dark using a UV-Vis spectrophotometer. The samples' free radical scavenging activity was calculated using the following equation:
| (2) |
where A1 is the absorption of the control and A2 is the absorption of the sample.
2.6.2. Hydroxyl Radical Scavenging Assay
The capacity of the seaweed polysaccharides against the scavenging hydroxyl radical was evaluated using Fenton's reaction (Fe2++H2O2⟶Fe3++OH−+OH) according to the modification method described [35]. The extracts of methanol were evaporated in vials. The reaction mixture contained 1 mL of (EDTA) solution, 0.5 mL of EDTA (0.018%), and 1 mL of DMSO (0.85% v/v in 0.1 M phosphate buffer, pH 7.4), and 0.5 mL of ascorbic acid (0.22%) was added to each tube. The solution was incubated at 37°C for 15 min, and the presence of yellow color was measured spectrophotometrically at 510 nm against the blank sample. The mixture without the sample was treated as a control. The scavenging activity was calculated by the following equation:
| (3) |
where A0 is the control, A1 is the absorption of the sample, and A2 is absorption without sodium salicylate.
2.7. Anticoagulant Activity
2.7.1. Activated Partial Thromboplastin Time (APTT)
For carrageenan fractions, APTT was calculated using a modified version of the methodology reported [36]. The positive and negative controls were heparin at a concentration of 5 g/mL and 0.9 percent w/v NaCl, respectively.
2.7.2. Prothrombin Time (PT)
The methodology of [37] was also used to determine PT, with some modifications. The device was also programmed to perform the same procedure in APTT determination. Each poor-platelet plasma and carrageenan solution mixture was incubated for 3 min at 37°C. Then, 0.6 mL of prewarmed PT reagent was added, and the time for clot formation was also observed and repeated three times. For positive and negative control, heparin and NaCl were utilized, respectively.
2.8. Molecular Docking
Ligand preparation: for molecular docking studies, carrageenan structure was retrieved from the PubChem database (71597331) in a three-dimensional structure file (SDF) format, and furthermore, the structure was refined using the LigPrep module in Schrodinger's Maestro (v 12.8). The OPLS4 force field was applied, and 32 different states of stereoisomerism were derived (Schrödinger Release 2021-2: LigPrep, Schrödinger, LLC, New York, NY, 2021).
Protein preparation: we need to evaluate the antioxidant (2C0D & 5B6M), antimicrobial (1JIJ & 2XCT), and anticoagulant (5E8E) activity against sulfated polysaccharide computationally. The three-dimensional structure of proteins was retrieved from the database of Protein Data Bank (PDB). The X-ray crystallographic structures were imported into Maestro using the protein preparation wizard, and this module helps to solve the missing hydrogen bonds, create the disulfide bonds, and optimize (Schrödinger Release 2021-2: Protein Preparation Wizard; Epik, Schrödinger, LLC, New York, NY, 2021; Impact, Schrödinger, LLC, New York, NY; Prime, Schrödinger, LLC, New York, NY, 2021).
The molecular docking was performed using the Glide package (ligand docking) in the Schrodinger suite. The standard precision docking method was applied and performed postdocking minimization and analyzed the results in pose viewer Schrödinger Release 2021-2: Glide, Schrödinger, LLC, New York, NY, 2021.
2.9. Mosquito Culture and Larvicidal Activity
A. aegypti mosquito larvae were collected from the Indian Council of Medical Research—Vector Control Research Centre, Madurai, Tamil Nadu, India. The mosquito larvae were fed a finely powdered mixture containing a 3 : 1 ratio of dog biscuits and Brewer's yeast for repeated generations at 25–30°C. According to the procedure used, adult mosquitoes were kept under standard environmental conditions as larvae [38]. Following this procedure, a mortality assay was carried out on fourth instar larvae [22, 39]. The test for the larvae effect of methanol extract derived from seaweed against mosquito larvae A. aegypti involved 10 mg of the sample in different concentrations for 50, 100, 200, and 500 μL. Twenty larvae were added to 250 mL distilled water in triplicates, with 1 mL DMSO serving as a negative control H2O. Dead larvae were counted, and the proportion of dead larvae was estimated for the replicates after 24 and 48 h of exposure. Profit analysis was utilized to calculate average LC50 and LC90 (lethal concentration) values from the duplicates.
| (4) |
2.10. Statistical Analysis
The results were examined using one-way analysis to calculate LC50, LC90, and 95% fiducial limits of upper confidence and lower confidence of variance and standard values presented as the mean SD (ANOVA). The asterisk (∗, (∗∗, and ∗∗∗) denotes a significant difference from the control (p < 0.01, (p < 0.05, and p < 0.001).
3. Results
The FT-IR spectra of the carrageenan were isolated from the red seaweed H. valentiae and the absorption bands typical of carrageenan between the wave numbers 1000 and 3500 cm−1 clearly highlighted the functional groups in all of the analyzed samples (Figure 1, Table 1). The peaks at 3457.74 cm−1 correlate with O-H stretching H-bonding vibrations which indicated alcohols and phenols. The existence of alkanes was revealed by the similarity of the peaks found at 2349.36 cm−1. Furthermore, the peaks observed at 1687.22 cm−1 were attributed to alkenes. The aliphatic amines had an absorption peak position at 1187.22 cm−1 confirming the -C≡C- stretch. The spectral observations at 1222.18 cm−1 indicated asymmetric stretching of S-O, revealing the presence of sulfate [40]. The band at 1222.18–1030.72 cm−1 stretching of C-O was attributed to alcohols, carboxylic acids, esters, and ethers. C-H stretching vibrations indicated the existence of alkanes in the band between 1444.61 cm−1. The C-O-C of 3, 6-anhydro-L-galactose vibrations has been assigned to the peak at 920.72 cm−1. Aromatic group C-H stretching vibrations were as described to the peak 845.44 cm−1. The last peak which appears to be 650.30 cm−1 was related to -C≡C-H: C-H bending the presence of alkanes. The NMR spectra of the carrageenan provided more structural characterization, which is shown in Figure 2, respectively, and band assignment for a sample of H. valentiae polysaccharide. The NMR can be used to demonstrate the existence of different carrageenan units. The NMR spectrum was also a complex polymer signal from the anomeric proton at (78.30 ppm), and ring carbons (70.16 ppm) were assigned to 3,6-α-L-anhydrogalactose. The signal was assigned from the 75.02 ppm which was assigned to H-1 of β-D-galactose linked to α-L-galactose-6-sulfate. The signal at 102.86 ppm was attributed to carboxyl β-D-galactose.
Figure 1.
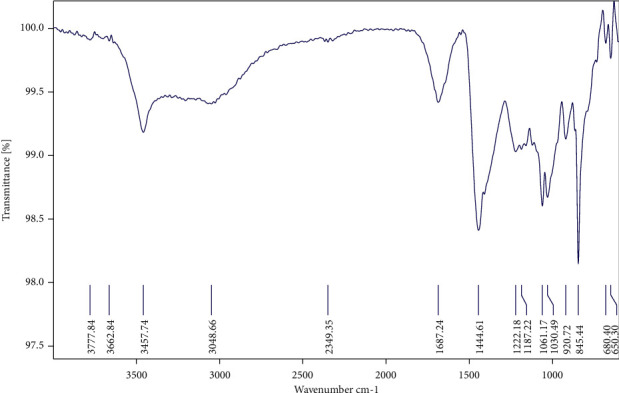
FT-IR spectrum of the carrageenan from Hypnea valentiae.
Table 1.
Interpretation of functional groups in the carrageenan using FT-IR.
| Peak position (wave number cm−1) | Spectroscopic assignments | Functional groups |
|---|---|---|
| 3457.74 cm−1 | O-H stretch, H-bonding | Alcohol, phenol |
| 2349.36 cm−1 | C-H stretch | Alkanes |
| 1687.22 cm−1 | -C≡C- stretch | Alkenes |
| 1187.22 cm−1 | C-N stretch | Aliphatic amines |
| 1222.18-1030.72 | C-O stretch | Alcohols, carboxylic acids, esters, and ethers |
| 1444.61 cm−1 | C-H bend | Alkanes |
| 920.72 cm−1 | C-O-C stretch | 3, 6-anhydro-L-galactose |
| 845.44 cm−1 | C-H stretch | Aromatics |
| 650.30 cm−1 | -C≡C-H : C-H bend | Alkynes |
Figure 2.
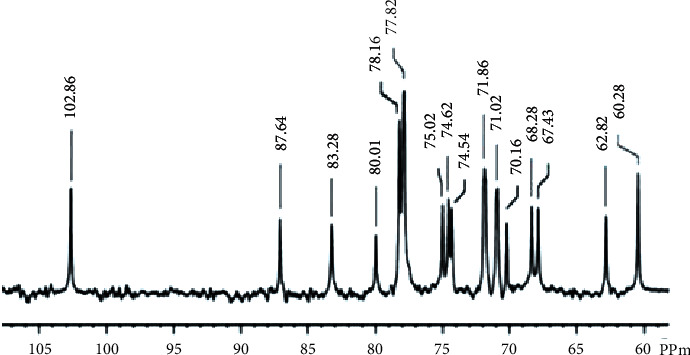
13C-NMR spectrum of the carrageenan from Hypnea valentiae.
The surface topography of the carrageenan was studied with the help of AFM measurements in a contact mode. The AFM images of the carrageenan soft template and bright spot are shown in (Figure 3(a)). Moreover, the topography of the carrageenan can be observed with peaks and valleys distributed across the surface. Apart from this, huge numbers of deformed shapes with larger sizes can also be seen in the AFM image of the carrageenan (Figure 3(b)). The AFM is a significant source for detecting the morphology and size of the carrageenan macromolecules originating from 0.0 to 0.7 μm. The carrageenan particles' average height which ranged from 0.00 to 0.72 μm was also noted.
Figure 3.
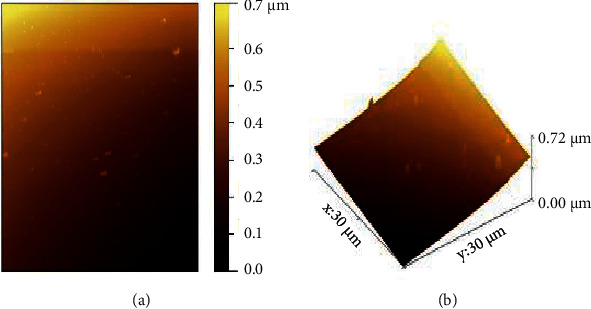
AFM of the carrageenan from Hypnea valentiae.
The antibacterial activity of the methanol extract was evaluated for the resistance to pathogenic bacteria which may cause infection in human beings. The methanol extract of H. valentiae inhibitory activity against E. faecalis, S. aureus, E. coli, and P. aeruginosa was identified. The pathogenic bacterial zone of inhibition in various concentrations of the methanol extract was compared to the 40 mg/mL higher inhibitory activity against four bacterial pathogens obtained in the results shown in (Figure 4) and Table 2, respectively. The antibacterial potential of methanol extracts depends upon the ability of permeation to the bacterial cell through the cell wall of bacteria. Moreover, the methanol extract had a minimum inhibitory bacterial effect against the Gram +ve strain rather than the Gram −ve strain. Therefore, the present study focused that both extracts demonstrated high significant antibacterial inhibition activity against Gram-negative bacteria rather than Gram-positive bacteria.
Figure 4.
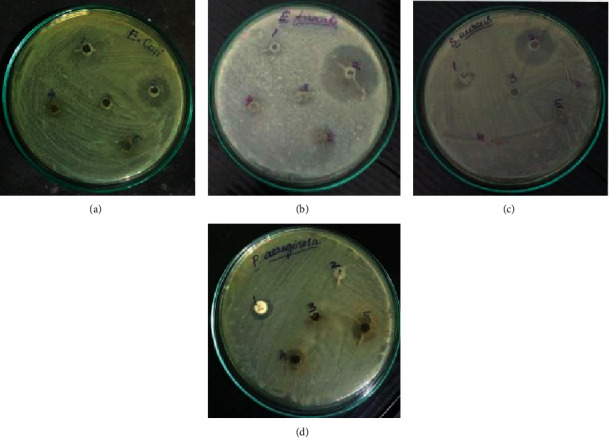
The zone of inhibition for methanol extracts of Hypnea valentiae activity against (a) E. coli, (b) E. faecalis, (c) P. aeruginosa, and (d) S. aureus. 1—methanol, 2—antibiotic streptomycin, 3—methanol extract 10 mg/mL, 4—methanol extract 20 mg/mL, and 5—methanol extract 40 mg/mL.
Table 2.
The zone of inhibition for methanol extracts of Hypnea valentiae activity against bacterial species.
| Bacteria | Zone of inhibition (mm) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| E. coli | 3.0 ± 0.577 | 5.0 ± 0.577 | 0 | 1.0 ± 0.577 | 1.0 ± 0.274 |
| E. faecalis | 1.0 ± 0.577 | 9.0 ± 1.155 | 0.00 | 1.0 ± 0.288 | 2.0 ± 0.577 |
| P. aeruginosa | 2.0 ± 0.320 | 0.0 ± 0.000 | 0.00 | 3.0 ± 0.111 | 5.0 ± 0.412 |
| S. aureus | 1.0 ± 0.000 | 8.0 ± 1.155 | 0 | 0.0 ± 0.000 | 0.0 ± 0.000 |
∗ Data are mean=SE (n-3). 1—methanol, 2—antibiotic Streptomycin, 3—methanol extract 10 mg/mL,4—methanol extract 20 mg/mL, and 5—methanol extract 40 mg/mL.
The antibiofilm activity of methanol extract of H. valentiae has been investigated with bacterial potential against stains based on E. faecalis Gram +ve and P. aeruginosa Gram −ve bacterial activity obtained in the results shown in Table 3, respectively. The different concentrations of methanol extract (10, 20, 30, 40, and 50 μg/mL) were tested. The highest activity against antibiofilm activity (11.1 ± 0.885 at 50 μg/mL) methanol extract was recorded for E. faecalis. In P. aeruginosa, the inhibition of the biofilm activity rate as 14.3 ± 0.979 at 50 μg/mL concentration of methanol extract was found. The percentage of inhibition in E. faecalis activity effect methanol extract was significantly higher than that in P. aeruginosa. The current study investigates the antibiofilm activity of H. valentiae extracts bacterial activity against two different bacterial strains.
Table 3.
The effect of Hypnea valentiae methanol extracts on antibiofilm activity E. faecalis and P. aeruginosa.
| Pathogens | Concentrations of methanol extracts of H. valentiae (μg/mL) | ||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | |
| E. faecalis | 1.0 ± 0.211 | 4.2 ± 0.392 | 7.5 ± 0.555 | 10.0 ± 0.709 | 11.1 ± 0.885 |
| P. aeruginosa | 4.0 ± 0.380 | 7.0 ± 0.885 | 9.1 ± 0.692 | 12.0 ± 0.883 | 14.3 ± 0.979 |
∗ Data are mean=SE (n-3).
Total antioxidant activity was measured to evaluate the antioxidant capacity of sulfated polysaccharides from H. valentiae extracts. The carrageenan extract was shown to reduce the total antioxidant scavenging activity of radicals (70.1 ± 0.61%) at 250 μg/mL concentration rather than at the other concentrations of 50–250 μg/mL of the displayed activities. Based on the findings visualized (Table 4) and noticed, the carrageenan was found to have significantly higher total antioxidant activities as compared with L-ascorbic acid (87.22 ± 0.80%) and BHA (81.99 ± 0.75%). The antiradical assay was determined by measuring the absorbance of the inhibition of DPPH radicals. The seaweed extract of sulfated polysaccharide showed that higher inhibition activity of these radicals (65.74 ± 0.58%) values for DPPH scavenging at 160 μg/mL concentration rather than at the other concentrations. According to the results shown in (Table 4), carrageenan has the potential for radical scavenging activity when compared to the compounds of L-ascorbic acid (82.05 ± 0.73%) and BHA (79.01 ± 0.70%) that demonstrated effective DPPH neutralizing activity.
Table 4.
Antioxidant activity of Hypnea valentiae methanol extract.
| Extract/positive control | Antioxidant activity, % | ||
|---|---|---|---|
| DPPH, μg/mL | OH, μg/mL | Total antioxidant, μg/mL | |
| Carrageenan | 65.74 ± 0.58 | 65.72 ± 0.60 | 70.1 ± 0.61 |
| BHA | 79.01 ± 0.70 | 77.93 ± 0.70 | 81.99 ± 0.75 |
| L-ascorbic acid | 82.05 ± 0.73 | 81.14 ± 0.73 | 87.22 ± 0.80 |
The hydrogen radical scavenging assay was used to determine the inhibition of hydroxyl (OH) radical formation, and the results showed that the scavenging activity of sulfated polysaccharide was significant with increasing concentrations. The extract of carrageenan inhibited the hydrogen peroxide scavenging activity of the OH radical (65.72 ± 0.60%) at 125 μg/mL concentration rather than at the other concentrations of 25–125 μg/mL of the exhibited activities. Furthermore, as shown in Table 4, carrageenan exhibited significantly higher hydroxyl radical activities when compared to L-ascorbic acid (81.140.73%) and BHA (77.930.70%).
The study of anticoagulant activity was analyzed by the APTT and PT assays which demonstrated that the anticoagulant mechanism of carrageenan inhibits plasma coagulation release during the regular phase of the coagulation process blood clotting time (Table 5). The anticoagulant activity of carrageenan was exhibited higher in APTT (106.50 IU at 25 μg/mL) when compared with the PT test (57.86 IU at 25 μg/mL) indicating the pathway to inhibition.
Table 5.
Anticoagulant activity of Hypnea valentiae.
| Extraction/control | Clotting time | |
|---|---|---|
| APTT, 25 μg/mL | PT, 25 μg/mL | |
| Carrageenan | 106.4 ± 0.65 | 57.3 ± 0.70 |
| Heparin-sulfate | 175.2 ± 0.85 | 126.5 ± 0.88 |
The carrageenan molecule was subjected to small molecular (ligand) docking, the antioxidant targets chosen as mitochondrial 2-cys peroxiredoxin (2C0D) from Plasmodium falciparum and crystal structure of human peroxiredoxin 6 (5B6M). The docking score results of −7 Kcal/mol indicate strong affinity among the complex molecules, and the interaction diagrams of 3D and 2D are represented in Figure 5. The antimicrobial target protein chosen as S. aureus tyrosyl-t RNA synthetase (1JIJ) and S. aureus gyrase topoisomerase II (2XCT) against the small molecule showed a strong affinity in their complex molecule with the least binding score of −6.894 Kcal/mol and with their interactive sites represented (Figure 6). The phytochemical exploited against antioxidant activity using auto dock tool results in −3.3 to −6 Kcal/mol binding score with in vitro validation, wherein in our case, it was shown as −7 Kcal/mol using Glide docking, Schrodinger [41]. The anticoagulant target protein was chosen as the crystal structure of thrombin (5E8E) from humans against carrageenan, showing the amino acid interaction as SER546 forms a hydrogen bond with the NH- group, and PO4 forms a hydrogen bond with the O- group with a docking score of −6.639. The structure was illustrated with 2D and 3D interaction maps with a pocket binding site (Figure 7, Table 6). Carrageenan extracted from H. valentiae was found to have the highest larvicidal activity against A. aegypti (LC50 = 99.675 μg/mL; LC90 = 491. 453 mg/L) shown (Figure 8, Table 7). The larvicidal activity of seaweeds to the larvae of A. aegypti was determined to the inhibition effect of seaweed mortality larvae. Seaweed is a natural product, and particularly, halogenated terpenes might be exploited for the improvement of new larvicidal compounds and its prototype insecticidal agents.
Figure 5.
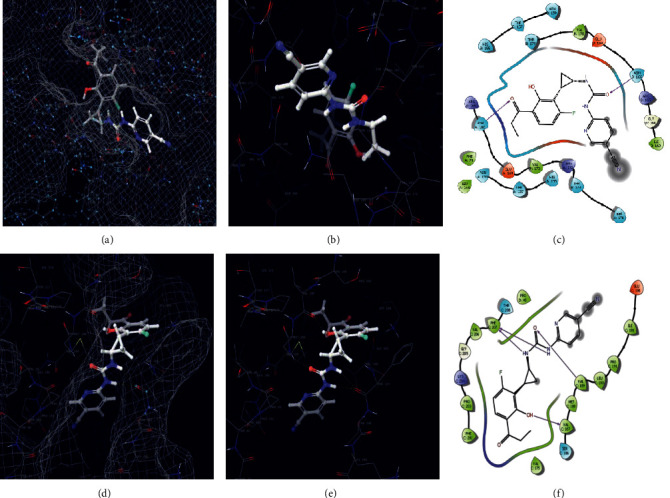
Molecular docking of the carrageenan against antioxidant targets (i) 2COD protein. (a) 3D surface mess map interaction site. (b) 3D complex interaction. (c) 2D complex interaction. (ii) 5B6M. (d) 3D surface mess map interaction site. (e) 3D complex interaction. (f) 2D complex interaction.
Figure 6.
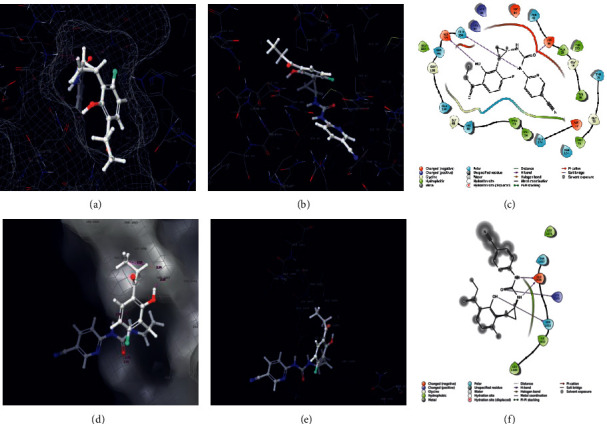
Staphylococcus aureus tyrosyl-tRNA synthetase docked with carrageenan. (a) 3D surface mess map interaction site. (b) 3D interaction site. (c) 2D interaction map. S. aureus gyrase topoisomerase II docked with carrageenan. (d) 3D surface mess map interaction site. (e) 3D interaction site. (f) 2D interaction map.
Figure 7.
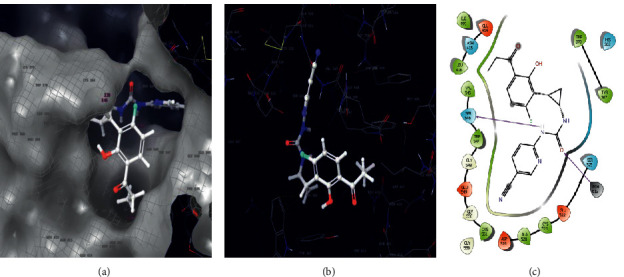
Molecular docking of the carrageenan against anticoagulant target proteins of 5EBE. (a) pocket 3D interaction site. (b) 3D complex interaction. (c) 2D complex interaction.
Table 6.
Molecular docking of the carrageenan molecule against three different activities of 5 multiple targets, the bond length in which the range below 3 Å is hydrogen bond interaction.
| S No | PDB | Target activity | Amino acid interaction site | Bond length, Å | Docking score |
|---|---|---|---|---|---|
| 1 | 2C0D | Antioxidant | GLY164, ASN166, ASN165 | 2.22, (2.07, 2.67), 2.50 | −7.465 |
| 2 | 5B6M | Antioxidant | VAL189, PHE207, LYS204, VAL187, PHE202 | (2.54, 2.25), (2.32, 2.25), 2.13, 1.97, (2.58, 2.45) | −6.227 |
| 3 | 5E8E | Anticoagulant | ASN415, SER546, TRP370 | 2.38, 2.28, (2.42, 2.39) | −6.639 |
| 4 | 1JIJ | Antimicrobial | GLN196, ASP40, LYS84, ASP195 | 2.53, 1.95, (1.66 and 2.38) and 1.82 | −6.894 |
| 5 | 2XCT | Antimicrobial | GLU1456, LYS1375, SER1453 | (1.60, 2.01), (1.78, 2.21) and (2.34, 2.10) | −4.363 |
Figure 8.
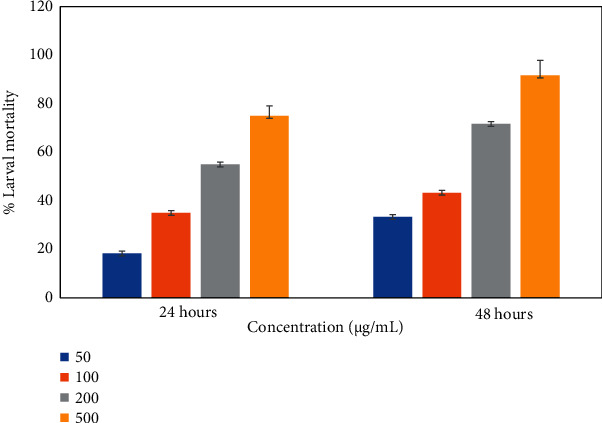
Larvicidal activity of Hypnea valentiae.
Table 7.
The effect of aqueous and methanol extract of the Hypnea valentiae against Aedes aegypti larvae.
| Mosquito species | Extract | Hours | LC50 (μg/mL) | 95% confidence limit | LC90 (μg/mL) | 95% confidence limit | x 2 | df | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||||
| Aedes aegypti | Control (H2O) | 24 | 316.083 | 236.821 | 488.167 | 2322.664 | 1171.696 | 8645.237 | 2.823 | 10 |
| 48 | 289.440 | 208.435 | 486.467 | 3219.817 | 1359.596 | 20753.085 | 2.514 | 10 | ||
| Methanol (40 μg/250 mL) | 24 | 171.464 | 134.073 | 224.234 | 1081.894 | 657.746 | 2603.426 | 3.415 | 10 | |
| 48 | 99.675 | 76.920 | 123.824 | 491.453 | 345.703 | 876.642 | 6.845 | 10 | ||
4. Discussion
The present study on methanol extract of H. valentiae was tested for yield, antioxidant, antimicrobial, anticoagulant with in-silico molecular docking, and larvicidal activity properties. The extract effect is an important parameter for the chemical compounds which have been used in the screening of bioactive substances from red seaweeds enriched with secondary metabolites and have potential antioxidant, antimicrobial, and anticoagulant activities [42]. The FT-IR spectrum is one of the most important tools for polysaccharides and their spectroscopic assignments of functional groups. The O-H stretching of alcohol and phenol vibration from the intramolecular hydrogen bond corresponded to the absorption peak position at 3457.74 cm−1 [43]. The absorption peaks at 2349. 36–1687.22 cm−1 is attributable to C-H stretch and -C≡C- stretch alkane functional groups, which probably confirmed the polymer-bound water which is a characteristic feature of red seaweed polysaccharides [44]. These absorption bands were described as enhancing the activation of molecular chain movements. Furthermore, the absorption peaks at 1222.18–1030.72 cm−1 correspond to assignments of functional groups. C-O stretches alcohols, carboxylic acids, esters, and ethers from lipid triglycerides and fatty acids [45]. The absorption peak position at 1444.61 cm−1 is attributable to C-H symmetric stretching alkanes. Another characteristic peak 920.72 cm−1 is attributable to C-O-C stretch 3,6-anhydro-L-galactose in the presence of high sulfated polysaccharides [42]. The absorption peak 650.30 cm−1 is attributed to -C≡C-H: C-H sulfate group alkynes. 1030.72 cm−1 corresponds to the high molecular weight skeleton of sulfated D-galactans and carrageenan bands found in H. valentiae [46]. These absorption bounds of oxygen, nitrogen, and biofunctional groups confirmed the presence of polyphenol compounds, polysaccharides, and protein, primary, and secondary metabolites.
The NMR spectroscopy is one of the efficient techniques for determining the structural characters of seaweed polysaccharides [47]. Recently, the report has demonstrated that the NMR spectrum used in the presence of different carrageenan components was observed to indicate the conversion of 3,6-α-L-anhydrogalactose units into its alditol derivatives anomeric protons containing μ and ν carrageenan [48]. Previous studies illustrate the occurrence of β-D-galactospectroscopic functional group of sulphated polysaccharides in the sugar residues, which are in conform that the trisaccharide branches on bacterial polysaccharide [49, 50]. Collectively, the literature has reported that Gracilaria caudata analyzes the structural features of β-D-galactos linked to α-L- galactose-6-sulfate, and methyl or pyruvate molecules are substituted by carrageenan polysaccharide [47, 51]. These reports indicated that the α-glycosidic peaks in the carrageenan backbone were partially broken [52]. NMR spectroscopy is also useful for recognizing the conformations of polysaccharides.
The AFM is single molecular spectroscopic technology to detect the conformation of morphological structures of polysaccharides in AFM images which provides a perfect observation of molecular assembling. The AFM provides visualization of the different types of sulfated polysaccharides such as curdlan [53], xanthan [54], carrageenan [55], xyloglucan [56], pectin [57], arabinoxylan [58], and starch [59]. The previous literature has functionalized the AFM based on the purpose of many fundamental food systems of carrageenan polysaccharides [48]. Similar reports have identified the height range in the structure of the food particles [49]. Based on the findings, the fibrous height of carrageenan was topographically similar to sulfated polysaccharides derived from the red seaweed [44]. The majority of the results from AFM analysis have reviewed the macromolecules of functional food polymers [60]. Therefore, AFM is nanostructure characteristics of polysaccharides from different food products. The conformation of polymers to identify numerous environmental conditions controlled the temperature [61].
The potential antibacterial activity of carrageenan polysaccharides from H. valentiae derivatives was evaluated to affect microbial pathogens of (E. faecalis and S. aureus (Gram +ve) and E. coli and P. aeruginosa (Gram −ve) organisms. The antibacterial activity was revealed to be strong against biologically multidrug-resistant pathogens S. aureus and E. faecalis [52]. In addition, numerous reports have described the antibacterial potential using algae extracts' strong effect on various bacterial pathogens such as E. coli, S. aureus, and E. faecalis. These pathogenic bacterial activities to enhancing the biostimulants of medicinal properties valuable for enhancing the mint and basil antimicrobial activity against bacteria [62]. Previous reports have analyzed the antibacterial activity against bacteria Spatoglossum asperum biological and pharmaceutical useful for valuable drugs [50]. The antibacterial activities of highly potential biomedical applications such as drug delivery, wound healing, and tissue engineering. The antibacterial capabilities could improve rapid healing by making a barrier against microbial contamination [63].
In the present study, H. valentiae were tested for antibiofilm activity potential against pathogenic strains that the reports revealed the methanol extract inhibited biofilm formation. Based on the reports, we have identified the biofilm activity inhibition of Sargassum wightii and Halimeda gracillis seaweed activity against antimicrobial pathogens and antibiofilm activity against various clinically significant pathogenic microorganisms [64]. The previous literature reported that the biofilm activities could be applied in different medical treatments to the prevention of various biofilm-related infections [65]. The biofilm pathogens are eco-friendly to presently used metal-based antifouling agents [66]. Collectively, the results have indicated the significant antibiofilm potential of the Ulva lactuca microbiocidal effect of different microorganisms that lead to the formation of cariogenic biofilm against the environment. It has been useful to the need for clinical studies to completely enhance the antibiofilm and mechanical properties of the new product [67].
The antioxidant potential of polysaccharides for various free radical scavenging ability of higher polyphenol and flavonoids content was extracted, and they could have excellent antioxidant activity [68]. The antioxidant activity having higher polyphenol content in soluble fraction is observed to precipitate. The highly effective of polyphenol and flavonoids compounds with their hydroxyl group for scavenging free radicals [64]. The antioxidant activity of polysaccharides could protect the human system from reactive oxygen species which affect such macromolecules as membrane lipids, proteins, and DNA and lead to many functional disorders of the human body. In the current study, phenolic content was higher in methanol extract from H. valentiae which were screened for total antioxidant activity at 250 μg/ml concentration. The highest total antioxidant activity is present in the methanol precipitate from H. valentiae (70.1 ± 0.61%). Previous literature reports have indicated the highest total antioxidant activity in the fractionated polysaccharides from Turbinaria conoides (246.6 mg AscAE/g) Gracilaria filiforms (353.3 AscAE mg/g), and Enteromorpha compressa (326.6 mg AscAE/g) [27]. Similar results have reported the highest total antioxidant activity occurred in extract sulfated polysaccharides Sargassum tenerrimum (22.0 ± 06) [69]. Similarly, the study reported the total antioxidant activity in aqueous extract of codium sp which showed the activity of 85.53 ± 0.25% [70]. The results revealed that the polysaccharides conformed that the total antioxidant activity which was the highest inhibition in the methanol extract from the red seaweed. The potential antioxidant activity screened for DPPH radical scavenging was due to their hydrogen donating ability. The free radical scavenging activity indicated a higher value in methanol extract from H. valentiae (65.74 ± 0.58%). Based on the results, the highest scavenging ability was present in the ethanolic precipitate of S. wightii (78.3 ± 0.25%) [64]. The antioxidant activity of polysaccharides extracted from Undaria pinnatifida showed that the highest inhibition of hydrogen peroxide screening ability [71]. In our study, the occurrence of the sulfate group to activate the hydrogen atom of the anomeric carbon through the sulfate content to adsorb the antioxidant exhibited the scavenging ability of Sargassum thunbergii [72]. The same other results demonstrated that the sulfated polysaccharide effect on inhibiting the formation of these radicals was higher in the extract from Laminaria japonica [73].
The results showed that the exhibited hydroxyl radical formations determine the scavenging activity of sulfated polysaccharides was significantly higher than the extract from H. valentiae (65.72 ± 0.60%). The hydroxyl radical result reveals the damage to the biomolecules including protein, deoxyribonucleic acid, and polyunsaturated fatty acids in the human cell membrane. These costs must lead to the expansion of various infections including cancer (Undaria pinnatifida) [71]. Previous literature reports have demonstrated that sulfate groups of algal polysaccharides are various kinds of biological activities as antioxidant activity in radicals scavenging (Sargassum thunbergii) [72]. These results indicate the antioxidant activity of sulfate content had a significant source of hydroxyl radical scavenging ability [20].
The present investigation continues to the methanol extract screening anticoagulant activity. Anticoagulant activity has evaluated the APTT and PT assay that showed higher inhibition of the coagulant in the soluble fraction. The APTT and PT assay demonstrate the anticoagulant mechanism of the carrageenan as blood coagulation. The methanol precipitate of H. valentiae inhibited higher APTT (106.50 IU at 25 μg/mL) and PT (57.86 IU at 25 μg/mL). The anticoagulant property of the carrageenan was assessed using human plasma from healthy donors. The results found in the APTT assay showed that the carrageenan had higher coagulation properties. The main source of the anticoagulant property of the carrageenan appears could be antithrombic activity [16]. The anticoagulant pathway indicated the potentiality of the sulfated polysaccharides in spreading the coagulation time that paves the technique for more possibility for their application in pharmaceutical industry for drugs [74]. The ray skin dermatan sulfated DS showed higher anticoagulant activity of the skin DS as shown by APTT and PT anticoagulant drug of interest potentially useful in therapy. The report suggested the higher content of the sulfate group which presented higher coagulant property [75].
Sulfated polysaccharides are heterogeneous natural polymers found in massive quantities in marine algae with a wide range of therapeutic applications due to their chemical structure and composition. The identification of chemicals that reduce inflammation is of importance because inflammation plays a role in illnesses. Antiinflammation activity has been determined using a variety of approaches. With results of in vitro assays, several derivatives of carrageenan oligosaccharides had better antioxidant activity than carrageenan oligosaccharides, indicating that chemical modification of carrageenan oligosaccharides could improve their antioxidant activity [76]. The investigations of these studies showed noticeable significance, and the resultant complex molecules amino acid interaction with bond length are depicted in Table 7. Carrageenan had been exploited with antimicrobial, antiviral, and anticancer activity. The current research observed a significant interaction of the carrageenan with multiple targets resulting majorly around −7 to −6 Kcal/mol binding score [28, 77].
The mosquito larvicidal activity of methanol extract from H. valentiae has been found in the highest larvicidal activity than the control A. aegypti (LC50 = 99.675 μg/mL; LC90 = 491.453 mg/L). The present investigation focused on the larvicidal efficacy of the potential effect on the development of H. valentiae against A. aegypti. Based on the results observed in Padina australis (LC50 = 82.55 mg/mL), Sargassum binderi (LC50 = 160.07) mg/mL, Bryopsis pennata (LC50 = 192.43 mg/mL), the methanol extract was much effective on A. aegypti larvicidal activities. The report provided information on numerous effects using different seaweed extracts of A. aegypti [78]. Gracilaria filiforms has been reviewed in the literature larvicidal activity against the larvae Anopheles stephensi in which the higher inhibition is LC50 = 0.255867 and LC90 = 3.434589 mg/L [27]. The mosquito activity of the current research reported damage and shrinkage of cells from the midgut epithelium of the seaweed treated larvae Anopheles stephensi and A. aegypti which is (LC50: 58.34; LC90: 114.91). The application of the seaweed extracts is derived from terrestrial aromatic and medicinal values [64]. Halimeda macroloba has been reported in the literature in the past (LC50—1119.0; LC90—1890.3) and similarly Ulva lactuca (LC50—952.9; LC90—1830.4) and Caulerpa racemosa (LC50—886.0; LC90—1790.1) [79]. The same was reported to show the activity against the larvicidal activity of Hypnea muciformis and Padina gymnospora [80]. Based on the results responsible for the larvicidal action, demonstrating the use of seaweed extracts has high potential as a mosquito control strategy.
5. Conclusion
The findings of this study suggested that the natural carrageenan derivatives with high sulfate content have greater radical scavenging antioxidant and antibacterial properties. Carrageenan from red seaweeds has also been shown to have in vitro anticoagulant functions, confirming its explicit role in the coagulation pathway. However, more research is needed to confirm that seaweeds have high mosquitocidal activity in the future. In addition, the carrageenan was evaluated computationally using glide ligand—molecular docking with multiple targets of antimicrobial, antioxidant, and anticoagulant activity. The minimal binding score explicits the strong affinity among the complex molecules. However, our potential candidate needs to further validate the in vivo model and extend it to clinical trials.
Acknowledgments
The authors are thankful to their respective institutes for their support. Also, the authors were grateful to the authority of the International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi, for computation analysis.
Contributor Information
Wen-Chao Liu, Email: liuwc@gdou.edu.cn.
Maruthupandian Arumugam, Email: maruthumdu82@gmail.com.
Data Availability
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors hereby declare that they have no conflicts of interest.
Authors' Contributions
This research article is done with the help of collaboration among the authors. Conceptualization was carried out by A.M., W.C., and B.B.; writing of the original manuscript was conducted by K.P and B.B.; methodology, data curation, and formal analysis were performed by K.P., K.P., and V.M.; molecular docking, software, and revision were conducted by M.A.; review and editing were carried out by V.A.M., N.A.A., and V.A.A.; organization of the working groups was conducted by B.B.; interpretation and review/revision were conducted by W.C., V.A.A., B.B., and A.M. All authors revised and approved the final article. Balamuralikrishnan Balasubramanian contributed equally to this work.
References
- 1.Liang W., Mao X., Peng X., Tang S. Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohydrate Polymers . 2014;101:776–785. doi: 10.1016/j.carbpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Radjasegarin A., Perumal A. Synergetic effects of seaweed extract and rhizobium on cowpea. Natura Resource for Human Health . 2021;1(1):43–50. [Google Scholar]
- 3.Dore C. M. P. G., Faustino Alves M. G. d. C., Pofírio Will L. S. E., et al. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydrate Polymers . 2013;91(1):467–475. doi: 10.1016/j.carbpol.2012.07.075. [DOI] [PubMed] [Google Scholar]
- 4.Mansour M. B., Dhahri M., Hassine M., et al. Highly sulfated dermatan sulfate from the skin of the ray Raja montagui: anticoagulant activity and mechanism of action. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology . 2010;156(3):206–215. doi: 10.1016/j.cbpb.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Sellimi S., Younes I., Ayed H. B., et al. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a tunisian brown seaweed. International Journal of Biological Macromolecules . 2015;72:1358–1367. doi: 10.1016/j.ijbiomac.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Ben Gara A., Ben Abdallah Kolsi R., Jardak N., et al. Inhibitory activities of Cystoseira crinita sulfated polysaccharide on key enzymes related to diabetes and hypertension: in vitro and animal study. Archives of Physiology and Biochemistry . 2016;123(1):31–42. doi: 10.1080/13813455.2016.1232737. [DOI] [PubMed] [Google Scholar]
- 7.Jin M., Zhao K., Huang Q., Xu C., Shang P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (oliv.) diels: a review. Carbohydrate Polymers . 2012;89(3):713–722. doi: 10.1016/j.carbpol.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 8.Senthilkumar K., Manivasagan P., Venkatesan J., Kim S. K. Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. International Journal of Biological Macromolecules . 2013;60:366–374. doi: 10.1016/j.ijbiomac.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Wu L., Sun J., Su X., Yu Q. L., Yu Q. Y., Zhang P. A review about the development of fucoidan in antitumor activity: progress and challenges. Carbohydrate Polymers . 2016;154:96–111. doi: 10.1016/j.carbpol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Bhatti H. A., Rubina R., Rashid F., Zaib S., Iqbal J., Hameed A. Synthesis and antitumor activities of novel mannich base derivatives derived from natural flavonoids. Natural Resources for Human Health . 2022;2(2):100–106. doi: 10.53365/nrfhh/141866. [DOI] [Google Scholar]
- 11.Coura C. O., de Araujo I. W. F., Vanderlei E. S. O., et al. Antinociceptive and anti-inflammatory activities of sulphated polysaccharides from the red seaweed Gracilaria cornea. Basic and Clinical Pharmacology and Toxicology . 2012;110(4):335–341. doi: 10.1111/j.1742-7843.2011.00811.x. [DOI] [PubMed] [Google Scholar]
- 12.Assreuy A. M. S., Gomes D. M., da Silva M. S. J., et al. Biological effects of a sulfated-polysaccharide isolated from the marine red algae Champia feldmannii. Biological and Pharmaceutical Bulletin . 2008;31(4):691–695. doi: 10.1248/bpb.31.691. [DOI] [PubMed] [Google Scholar]
- 13.Viana G. S., Freitas A. L. P., Lima M. M., Vieira L., Andrade M. C., Benevides N. M. B. Antinociceptive activity of sulfated carbohydrates from the red algae Bryothamnion seaforthii (turner) kutz and B. triquetrum (S.G. gmel.) M. howe. Brazilian Journal of Medical and Biological Research . 2002;35(6):713–722. doi: 10.1590/s0100-879x2002000600012. [DOI] [PubMed] [Google Scholar]
- 14.Lins K. O. A. L., Bezerra D. P., Alves A. P. N. N., et al. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (diaz-pifferer) Journal of Applied Toxicology . 2009;29(1):20–26. doi: 10.1002/jat.1374. [DOI] [PubMed] [Google Scholar]
- 15.Assreuy A. M. S., Pontes G. C., Rodrigues N. V. F. C., et al. Vascular effects of a sulfated polysaccharide from the red marine alga Solieria filiformis. Natural Product Communications . 2010;5(8) doi: 10.1177/1934578x1000500825.1934578X1000500 [DOI] [PubMed] [Google Scholar]
- 16.Silva F. R. F., Dore C. M. P. G., Marques C. T., et al. Anticoagulant activity, paw edema and pleurisy induced carrageenan: action of major types of commercial carrageenans. Carbohydrate Polymers . 2010;79(1):26–33. doi: 10.1016/j.carbpol.2009.07.010. [DOI] [Google Scholar]
- 17.de Araújo I. W. F., Vanderlei E. D. S. O., Rodrigues J. A. G., et al. Effects of a sulfated polysaccharide isolated from the red seaweed Solieria filiformis on models of nociception and inflammation. Carbohydrate Polymers . 2011;86(3):1207–1215. doi: 10.1016/j.carbpol.2011.06.016. [DOI] [Google Scholar]
- 18.Je J. Y., Park P. J., Kim E. K., et al. Antioxidant activity of enzymatic extracts from the brown seaweed Undaria pinnatifida by electron spin resonance spectroscopy. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology . 2009;42(4):874–878. doi: 10.1016/j.lwt.2008.10.012. [DOI] [Google Scholar]
- 19.Chandini S. K., Ganesan P., Bhaskar N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chemistry . 2008;107(2):707–713. doi: 10.1016/j.foodchem.2007.08.081. [DOI] [Google Scholar]
- 20.Bartolomeu W. S., Miguel A., Ana I., et al. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed. Gracilaria birdiae. Food Hydrocolloids . 2012;27:287–292. [Google Scholar]
- 21.Wang J., Zhang Q., Zhang Z., Song H., Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from laminaria japonica. International Journal of Biological Macromolecules . 2010;46(1):6–12. doi: 10.1016/j.ijbiomac.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Dengue and Severe Dengue . Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 23.Al-Shami S. A., Mahyoub J. A., Hatabbi M., Ahmad A. H., Rawi C. S. M. An update on the incidence of dengue gaining strength in Saudi Arabia and current control approaches for its vector mosquito. Journal of Parasites & Vectors . 2014;7 doi: 10.1186/1756-3305-7-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiboutot M. M., Kannan S., Kawalekar O. U., et al. Chikungunya: A Potentially Emerging Epidemic. Neglected Tropical Diseases . 2010;4:p. e623. doi: 10.1371/journal.pntd.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salvador-Neto O., Gomes S. A., Soares A. R., et al. Larvicidal potential of the halogenated sesquiterpene (+)-obtusol, isolated from the alga Laurencia dendroidea J. agardh (Ceramiales: rhodomelaceae), against the dengue vector mosquito Aedes aegypti (Linnaeus)(diptera: culicidae) Marine Drugs . 2016;14(2):p. 20. doi: 10.3390/md14020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu K. X., Jantan I., Ahmad R., Wong CL. The major bioactive components of seaweeds and their mosquitocidal potential. Parasitology Research . 2014;113(9):3121–3141. doi: 10.1007/s00436-014-4068-5. [DOI] [PubMed] [Google Scholar]
- 27.Venkatesan M., Arumugam V., Pugalendi R., et al. Antioxidant, anticoagulant and mosquitocidal properties of water soluble polysaccharides (WSPs) from Indian seaweeds. Process Biochemistry . 2019;84:196–204. doi: 10.1016/j.procbio.2019.05.029. [DOI] [Google Scholar]
- 28.Arunkumar M., Gunaseelan S., Kubendran Aravind M., et al. Marine algal antagonists targeting 3CL protease and spike glycoprotein of SARS-CoV-2: a computational approach for anti-COVID-19 drug discovery. Journal of Biomolecular Structure and Dynamics . 2021:1–28. doi: 10.1080/07391102.2021.1921032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suresh V., Senthilkumar N., Thangam R., et al. Separation, purification and preliminary characterization of sulfated polysaccharidesfrom sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process Biochemistry . 2013;48(2):364–373. doi: 10.1016/j.procbio.2012.12.014. [DOI] [Google Scholar]
- 30.Li X., Wang L., Wang Y., Xiong Z. Effect of drying method on physicochemical properties and antioxidant activities of Hohenbuehelia serotina polysaccharides. Process Biochemistry . 2016;51(8):1100–1108. doi: 10.1016/j.procbio.2016.05.006. [DOI] [Google Scholar]
- 31.Vetrivel C., Balamuralikrishnan B., Durairaj K., et al. Fabrication and characterization of noble crystalline silver nanoparticles from ceropegia bulbosa roxb root tuber extract for antibacterial, larvicidal and histopathology applications. Nanoscience and Nanotechnology Letters . 2019;11:11–21. doi: 10.1166/nnl.2019.2845. [DOI] [Google Scholar]
- 32.Balaraman P., Balasubramanian B., Kaliannan D., et al. Phyco-synthesis of silver nanoparticles mediated from marine algae sargassum myriocystum and its potential biological and environmental applications. Waste and Biomass Valorization . 2020;11(10):5255–5271. doi: 10.1007/s12649-020-01083-5. [DOI] [Google Scholar]
- 33.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry . 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 34.Spector D. L., Goldman R. D., Leinwand L. A. Cells:A Laboratory Manual . NewYork, NY, USA: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- 35.Leong E. C., He L., Rahardjo H. Factors affecting the filter paper method for total and matric suction measurements. Geotechnical Testing Journal . 2002;25(3):322–332. [Google Scholar]
- 36.Andersson L.-O., Barrowcliffe T. W., Holmer E., Johnson E. A., Sims G. E. C. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin III and by gel filtration. Thrombosis Research . 1976;9(6):575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- 37.Quick A. J. The clinical application of the hippuric acid and the prothrombin tests. American Journal of Clinical Pathology . 1940;10(3):222–233. doi: 10.1093/ajcp/10.3.222. [DOI] [Google Scholar]
- 38.Cittrarasu V., Kaliannan D., Dharman K., et al. Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Scientific Reports . 2021;11(1) doi: 10.1038/s41598-020-80327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elumalai D., Hemalatha P., Kaleena P. Larvicidal activity and GC–MS analysis of Leucas aspera against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. Journal of the Saudi Society of Agricultural Sciences . 2017;16(4):306–313. doi: 10.1016/j.jssas.2015.10.003. [DOI] [Google Scholar]
- 40.Hifney A. F., Fawzy M. A., Abdel-Gawad K. M., Gomaa M. Industrial optimization of fucoidan extraction from Sargassum sp. and its potential antioxidant and emulsifying activities. Food Hydrocolloids . 2016;54:77–88. doi: 10.1016/j.foodhyd.2015.09.022. [DOI] [Google Scholar]
- 41.Salaria D., Rolta R., Sharma N., et al. In vitro and in silico antioxidant and anti-inflammatory potential of essential oil of cymbopogon citratus (DC.) stapf. of north-western Himalaya. Journal of Biomolecular Structure and Dynamics . 2021:1–15. doi: 10.1080/07391102.2021.2001371. [DOI] [PubMed] [Google Scholar]
- 42.Arulkumar A., Rosemary T., Paramasivam S., Rajendran R. B. Phytochemical composition, in vitro antioxidant, antibacterial potential and GC-MS analysis of red seaweeds Gracilaria corticata and Gracilaria edulis from palk bay. Indian, Biocatalysis and agricultural Biotechnology . 2018;15:63–71. doi: 10.1016/j.bcab.2018.05.008. [DOI] [Google Scholar]
- 43.Baleta F. N., Bolaños J. M., Ruma O. C., Baleta A. N., Cairel J. D. Phytochemicals screening and antimicrobial properties of Sargassum oligocystum and Sargassum crassifolium extracts. Journal of Medicinal Plants Studies . 2017;5(1):382–387. [Google Scholar]
- 44.Khan B. M., Qiu H. M., Xu S. Y., Liu Y., Cheong K. L. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. International Journal of Biological Macromolecules . 2020;145:1155–1161. doi: 10.1016/j.ijbiomac.2019.10.040. [DOI] [PubMed] [Google Scholar]
- 45.Leri A. C., Dunigan M. R., Wenrich R. L., Ravel B. Particulate organohalogens in edible brown seaweeds. Food Chemistry . 2019;272:126–132. doi: 10.1016/j.foodchem.2018.08.050. [DOI] [PubMed] [Google Scholar]
- 46.Cunha L., Grenha A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Marine Drugs . 2016;14:14–42. doi: 10.3390/md14030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barros F. C., da Silva D. C., Sombra V. G., et al. Structural characterization of polysaccharide obtained from red seaweed Gracilaria caudata (J Agardh) Carbohydrate polymers . 2013;92(1):598–603. doi: 10.1016/j.carbpol.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Yang D., Yang H. The temperature dependent extraction of polysaccharides from eucheuma and the rheological synergistic effect in their mixtures with kappa carrageenan. Lebensmittel-Wissenschaft & Technologie . 2020;129 doi: 10.1016/j.lwt.2020.109515.109515 [DOI] [Google Scholar]
- 49.Yang L., Zhang L. M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydrate Polymers . 2009;76(3):349–361. doi: 10.1016/j.carbpol.2008.12.015. [DOI] [Google Scholar]
- 50.Palanisamy S., Vinosha M., Marudhupandi T., Rajasekar P., Prabhu N. M. In vitro antioxidant and antibacterial activity of sulfated polysaccharides isolated from spatoglossum asperum. Carbohydrate Polymers . 2017;170:296–304. doi: 10.1016/j.carbpol.2017.04.085. [DOI] [PubMed] [Google Scholar]
- 51.Tabarsa M., Lee S. J., You S. Structural analysis of immunostimulating sulfated polysaccharides from Ulva pertusa. Carbohydrate Research . 2012;361:141–147. doi: 10.1016/j.carres.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Junior E. H., Gonçalves A. G., Noseda M. D., Duarte M. E. R., Murakami F. S., Ducatti D. R. Semi-synthesis of N-alkyl-kappa-carrageenan derivatives and evaluation of their antibacterial activity. Carbohydrate Research . 2021;499 doi: 10.1016/j.carres.2021.108234.108234 [DOI] [PubMed] [Google Scholar]
- 53.Xiao M., Jiang M., Wu K., et al. Investigation on curdlan dissociation by heating in water. Food Hydrocolloids . 2017;70:57–64. doi: 10.1016/j.foodhyd.2017.03.018. [DOI] [Google Scholar]
- 54.Gulrez S. K., Al-Assaf S., Fang Y., Phillips G. O., Gunning A. P. Revisiting the conformation of xanthan and the effect of industrially relevant treatments. Carbohydrate Polymers . 2012;90(3):1235–1243. doi: 10.1016/j.carbpol.2012.06.055. [DOI] [Google Scholar]
- 55.Necas J., Bartosikova L. Carrageenan: a review. Veterinarni Medicina . 2013;58(4):187–205. doi: 10.17221/6758-vetmed. [DOI] [Google Scholar]
- 56.Lucyszyn N., Lubambo A. F., Ono L., Jo T. A., De Souza C. F., Sierakowski M. R. Chemical, physico-chemical and cytotoxicity characterisation of xyloglucan from Guibourtia hymenifolia (Moric.) J. leonard seeds. Food Hydrocolloids . 2011;25(5):1242–1250. doi: 10.1016/j.foodhyd.2010.11.012. [DOI] [Google Scholar]
- 57.Willats W. G., Knox J. P., Mikkelsen J. D. Pectin: new insights into an old polymer are starting to gel. Trends in Food Science & Technology . 2006;17(3):97–104. doi: 10.1016/j.tifs.2005.10.008. [DOI] [Google Scholar]
- 58.Gunning A. P., Mackie A. R., Kirby A. R., Kroon P., Williamson G., Morris V. J. Motion of a cell wall polysaccharide observed by atomic force microscopy. Macromolecules . 2000;33(15):5680–5685. doi: 10.1021/ma000331w. [DOI] [Google Scholar]
- 59.Maley J., Asare E. K., Båga M., Rossnagel B. G., Chibbar R. N., Sammynaiken R. Application of aerosol-spray deposition for determination of fine structure of barley starch using atomic force microscopy. Starch Staerke . 2010;62(12):676–685. doi: 10.1002/star.201000005. [DOI] [Google Scholar]
- 60.Khan B. M., Zheng L. X., Khan W., Shah A. A., Liu Y., Cheong K. L. Antioxidant potential of physicochemically characterized Gracilaria blodgettii sulfated polysaccharides. Polymers . 2021;13(3):p. 442. doi: 10.3390/polym13030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J., Nie S. Application of atomic force microscopy in microscopic analysis of polysaccharide. Trends in Food Science & Technology . 2019;87:35–46. doi: 10.1016/j.tifs.2018.02.005. [DOI] [Google Scholar]
- 62.Elansary H. O., Yessoufou K., Shokralla S., Mahmoud E. A., Skalicka-Woźniak K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Industrial Crops and Products . 2016;92:50–56. doi: 10.1016/j.indcrop.2016.07.048. [DOI] [Google Scholar]
- 63.Sellimi S., Maalej H., Rekik D. M., et al. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. International Journal of Biological Macromolecules . 2018;119:633–644. doi: 10.1016/j.ijbiomac.2018.07.171. [DOI] [PubMed] [Google Scholar]
- 64.Suganya S., Ishwarya R., Jayakumar R., et al. New insecticides and antimicrobial derived from Sargassum wightii and Halimeda gracillis seaweeds; toxicity against mosquito vectors and anti-biofilm activity against microbial pathogens. South African Journal of Botany . 2019;125:466–480. doi: 10.1016/j.sajb.2019.08.006. [DOI] [Google Scholar]
- 65.Viszwapriya D., Prithika U., Deebika S., Balamurugan K., Pandian S. K. In vitro and in vivo antibiofilm potential of 2, 4-Di-tert-butylphenol from seaweed surface associated bacterium Bacillus subtilis against group A. Microbiological Research . 2016;191:19–31. doi: 10.1016/j.micres.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Salem D. M., Ismail M. M., Tadros H. R. Evaluation of the anti-biofilm activity of three seaweed species and their biosynthesized iron oxide nanoparticles (Fe3O4-NPs) Egyptian Journal of Aquatic Research . 2020;46(4):333–339. doi: 10.1016/j.ejar.2020.09.001. [DOI] [Google Scholar]
- 67.Pourhajibagher M., Salehi-Vaziri A., Noroozian M., et al. An orthodontic acrylic resin containing seaweed ulva lactuca as a photoactive phytocompound in antimicrobial photodynamic therapy: assessment of anti-biofilm activities and mechanical properties. Photodiagnosis and Photodynamic Therapy . 2021;35:102–295. doi: 10.1016/j.pdpdt.2021.102295. [DOI] [PubMed] [Google Scholar]
- 68.Rajivgandhi G. N., Kanisha C. C., Ramachandran G., et al. Phytochemical screening and anti-oxidant activity of Sargassum wightii enhances the anti-bacterial activity against Pseudomonas aeruginosa. Saudi Journal of Biological Sciences . 2021;28(3):1763–1769. doi: 10.1016/j.sjbs.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohan M. S. G., Achary A., Mani V., Cicinskas E., Kalitnik A. A., Khotimchenko M. Purification and characterization of fucose-containing sulphated polysaccharides from Sargassum tenerrimum and their biological activity. Journal of Applied Phycology . 2019;31(5):3101–3113. doi: 10.1007/s10811-019-01797-7. [DOI] [Google Scholar]
- 70.Kallswari G., Mahendran S., Subalakshmi P., Shankar T., Ponmanickam P. Purification, characterization and antioxidant activity of green seaweed Codium sp. Advances in Pharmacology and Pharmacy . 2016;4(2):16–21. doi: 10.13189/app.2016.040202. [DOI] [Google Scholar]
- 71.Koh H. S. A., Lu J., Zhou W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydrate Polymers . 2019;212:178–185. doi: 10.1016/j.carbpol.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 72.Kang M. C., Lee H., Choi H. D., Jeon Y. J. Antioxidant properties of a sulfated polysaccharide isolated from an enzymatic digest of Sargassum thunbergii. International Journal of Biological Macromolecules . 2019;132:142–149. doi: 10.1016/j.ijbiomac.2019.03.178. [DOI] [PubMed] [Google Scholar]
- 73.Duan Z., Duan W., Li F., Li Y., Luo P., Liu H. Effect of carboxymethylation on properties of fucoidan from Laminaria japonica: antioxidant activity and preservative effect on strawberry during cold storage. Postharvest Biology and Technology . 2019;151:127–133. doi: 10.1016/j.postharvbio.2019.02.008. [DOI] [Google Scholar]
- 74.Shobharani P., Nanishankar V. H., Halami P. M., Sachindra N. M. Antioxidant and anticoagulant activity of polyphenol and polysaccharides from fermented Sargassum sp. International Journal of Biological Macromolecules . 2014;65:542–548. doi: 10.1016/j.ijbiomac.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Ben Mansour M., Balti R., Ollivier V., Ben Jannet H., Chaubet F., Maaroufi R. M. Characterization and anticoagulant activity of a fucosylated chondroitin sulfate with unusually procoagulant effect from sea cucumber. Carbohydrate Polymers . 2017;174:760–771. doi: 10.1016/j.carbpol.2017.06.128. [DOI] [PubMed] [Google Scholar]
- 76.Yuan H., Song J., Zhang W., Li X., Li N., Gao X. Antioxidant activity and cytoprotective effect of κ-carrageenan oligosaccharides and their different derivatives. Bioorganic & Medicinal Chemistry Letters . 2006;16(5):1329–1334. doi: 10.1016/j.bmcl.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 77.Pham T. V., Hoang H. N. T., Nguyen H. T., et al. Anti-inflammatory and antimicrobial activities of compounds isolated from Distichochlamys benenica. BioMed Research International . 2021;2021:10. doi: 10.1155/2021/6624347.6624347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu K. X., Wong C. L., Ahmad R., Jantan I. Larvicidal activity, inhibition effect on development, histopathological alteration and morphological aberration induced by seaweed extracts in Aedes aegypti (Diptera: Culicidae) Asian Pacific Journal of Tropical Medicine . 2015;8(12):1006–1012. doi: 10.1016/j.apjtm.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 79.Adaikala Raj G., Jayaraman M., Krishnamoorthy S., Chandrasekaran M., Venkatesalu V. Screening of different extracts of marine macro algae for larvicidal activity against dengue fever mosquito, Aedes aegypti (dipptera: culiadae) International Letters of Natural Sciences . 2017;62:44–51. doi: 10.18052/www.scipress.com/ilns.62.44. [DOI] [Google Scholar]
- 80.Guedes E. A. C., Carvalho C. M. D., Ribeiro Junior K. A. L., et al. Larvicidal activity against Aedes aegypti and molluscicidal activity against Biomphalaria glabrata of Brazilian marine algae. Journal of Parasitology Research . 2014;2014:1–6. doi: 10.1155/2014/501328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.


