Abstract
Background
Anatomic total shoulder arthroplasty (TSA) has been continuously developed and current designs include stemless or canal-sparing humeral components. In the literature stemless and canal sparing TSA showed good clinical and radiographic results, which were comparable to stemmed TSA.
Objective
The aim of this study was to determine the short-term clinical and radiological outcomes of a new stemless TSA design.
Methods
A prospective multicentre study including 154 total shoulder arthroplasty patients with a follow up of 12 months was performed. At the time of follow up 129 patients were available for review. The adjusted Constant Murley score,1 Oxford Shoulder Score, EQ-5D-5L score and radiographs were examined preoperatively, 3 and 12 months after the implantation of the new stemless TSA implant GLOBAL ICON™ (DePuy Synthes, Warsaw, IN, USA). Complications were documented.
Results
Implant Kaplan-Meier survivorship was 98.7% at 12 months. From baseline to 12 months follow-up, all scores showed a progressive significant mean improvement. The mean adjusted Constant score increased from 42.3 to 96.1 points (p<0.001). The Oxford Shoulder Score showed an increase of 21.6 points (p<0.001). The postoperative radiographs showed no continuous radiolucent lines, subsidence, aseptic loosening or progressive radiolucency, but one osteolytic lesion was observed. Only 2 prostheses were revised.
Conclusion
The new GLOBAL ICON stemless TSA showed good clinical and radiographic results at short-term follow up which were comparable to early results of other stemless TSA. Further studies with longer follow up are needed in the future.
Keywords: Stemless, total shoulder arthroplasty, osteoarthritis, canal-sparing
Introduction
Anatomic total shoulder arthroplasty (TSA) was first described and introduced in 1955 by Neer et al.2 The main indication for TSA at the time was fractures of the proximal humerus. Over the years indications have expanded to include primary osteoarthritis, secondary osteoarthritis after instability, rheumatoid arthritis, fracture sequelae, and humeral osteonecrosis. Nowadays primary osteoarthritis of the glenohumeral joint is the main indication for TSA. Since the first monobloc Neer prosthesis, TSA has been continuously improved in order to reconstruct the individual natural anatomy of the glenohumeral joint.3–5 The first prosthetic designs provided a humeral stem for fixation. The stemmed humeral implants were associated with complications like intraoperative humeral shaft fractures, loosening, stress shielding, and postoperative traumatic periprosthetic fractures.6–13 The prevalence of intraoperative periprosthetic fractures in stemmed TSA is estimated to be 1.5% and postoperative between 1.6% and 2.4%.6,11 In cases of head-shaft malunions in fracture sequelae the implantation of a stemmed implant can be difficult and lead to intraoperative fractures, malalignment as well as the failure of restoration of the centre of rotation.14 The stem often is also difficult in revision surgery, because implant removal can be challenging and necessitate a humeral circular access osteotomy with the risk of intraoperative fracture.7,8,10,13,14
The first stemless anatomic shoulder was the TESS implant introduced by Biomet (Warsaw, IN, USA) in 2004.4,15 As a result, other stemless designs were developed by several manufacturers. The main advantages of the stemless prostheses compared to stemmed implants were bone preservation without violating the humeral shaft, shorter operative time, decreased stress shielding and easier removal in revision surgery.3,4,16 The current literature showed that the clinical results of stemmed and stemless total shoulder arthroplasty are comparable.4,17–20
The aim of this prospective multicentre study was to determine the short-term clinical and radiologic outcome of a new stemless design.
Materials and methods
A prospective, single-arm, multicentre study including 157 consecutive patients, of which 154 received total shoulder arthroplasty. These patients had met the inclusion criteria (Tab. 1) and consented to receive a TSA with the stemless GLOBAL ICON humeral implant (DePuy Synthes, Warsaw, IN, USA) and participate in the study. The study was internationally performed at 12 specialised shoulder centres (Germany, Canada, the Netherlands and the United Kingdom). The operations were performed between 17DEC2017 and 2JUL2019. The study protocol was followed for all patients at the study centres. The protocol included the inclusion and exclusion criteria and a preoperative evaluation (Tab. 1). The metaphyseal bone quality was evaluated intraoperatively.
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
| Severe painful and disabled osteoarthritis or post traumatic arthritis | Age < 21 and > 80 years |
| No skeletal maturity, regardless of age | |
| Pre- or intraoperative inadequate bone stock in the proximal humerus or glenoid for supporting the GLOBAL ICON | |
| Too soft or porous bone intraoperative to support the implant or too hard or brittle to allow proper preparation and fixation | |
| Fractures of the proximal humerus that could compromise the fixation | |
| Previous operative treatment that may comprimise the fixation | |
| Revision of a failed hemi, total or reverse shoulder arthroplasty | |
| Active local or systemic infection | |
| Absent, irreparable or nonfunctional rotator cuff or other essential muscles | |
| Treatment, which effects the bone quality | |
| Patients with a Global ICON on the contralateral side | |
| pregnancy or breastfeeding | |
| drug or alcohol abusers or psychological disorders | |
| Medical conditions, which impact the study | |
| Known polyethylene and/or metal sensitivity or allergy |
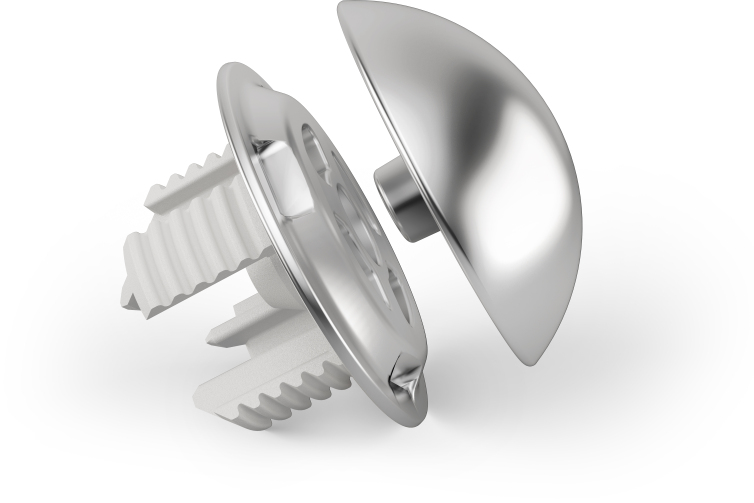
The humeral component of the Global ICON device (DePuy Synthes, Warsaw, IN, USA) consists of an anchor plate with 4 T-shaped legs, which are each arranged at 90 degrees to each other. This circular anchor plate leads to a press-fit fixation into the peripheral metaphyseal bone (Fig. 1) where the bone quality and density has been demonstrated to be superior to the central bone21,22 The legs of the anchor plate are hydroxyapatite coated to facilitate bone ingrowth for long-term stability. The anchor plate and humeral head implants are available from 40 mm to 56 mm in 2mm increments in order to resemble the natural anatomical conditions. The humeral device was combined with a pegged all-poly GLOBAL™ Anchor Peg Glenoid implant (DePuy Synthes, Warsaw, IN, USA). All operative procedures were performed by experienced shoulder surgeons.
Figure 1. The stemless Global ICON implant (DePuy Synthes, Warsaw, IN, USA) with the anchor plate and the humeral head.
Ethical consideration
Each investigational site received local institutional ethical review board to participate in this study. The Ethics Committee of the Medical School Hannover approved the study (No. 7525 Hannover). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or with comparable ethical standards.
Surgical technique
After a standard deltopectoral approach a tenodesis or tenotomy of the long head of the biceps was performed. The rotator cuff was evaluated and the subscapularis tendon was detached by lesser tuberosity osteotomy, tenotomy, or subscapularis peel-off technique according to preference of the surgeon. In the next step the osteophytes were removed from the posterior and inferior aspect of the humeral head. The proximal humerus was resected using the resection guide with an oscillating saw in the correct inclination and retroversion. This ring of the resection guide was placed on the superior and posterior rotator cuff insertion and the top of the cutting surface was aligned with the anatomic neck in native retroversion. At this point the metaphyseal bone quality was evaluated by visual inspection and attempted compression of the bone with the thumb. If the bone quality was sufficient, the stemless prosthesis was indicated. During glenoid preparation the humeral cut was protected by a metallic cover plate. To expose the glenoid the anterior capsule was separated from the subscapularis tendon and a periglenoidal capsular release and labrum resection was performed. An Anchor Peg Glenoid device (DePuy Synthes, Warsaw, IN, USA) was implanted on the glenoid. The glenoid template of correct size was placed on the glenoid and a k-wire was inserted into the centre of the glenoid in the desired retroversion angle between 0 and 10°. A central and peripheral reaming was performed. For the central peg the k-wire was over-drilled and the cancellous bone was harvested for later central bone grafting of the glenoid implant. Using another aiming device the peripheral peg holes were drilled. The peripheral peg holes were filled with bone cement and a cancellous bone paste was applied to the central interference-fitted peg of the glenoid implant. The Anchor Peg device was then implanted. After exposure of the proximal humerus the correct size of the humeral anchor plate was chosen. For the preparation of the 4 t-shaped legs the trial anchor plate was fixed on the proximal humerus and the punch was inserted for the legs. The central hole of the anchor plate was drilled in cases with hard bone quality. After the implantation of the anchor plate the humeral trial head was tested for the correct size and the humeral head was implanted. The reattachment of the subscapularis was performed according to the previous detachment procedure. The wound was closed in standard fashion according to the preference of the surgeon.
The postoperative rehabilitation protocol included an abduction pillow for immobilisation for four weeks. Passive mobilisation started one day after surgery. Flexion and abduction were limited to 90° for 6 weeks. Internal rotation was permitted in front of the body without resistance and external was limited to 0-40° according to the individual tension of the subscapularis tendon after reattachment for 6 weeks in order to protect the subscapularis repair. Physiotherapy with passive and assisted range-of-motion was carried out for at least 12 weeks and was extended if necessary.
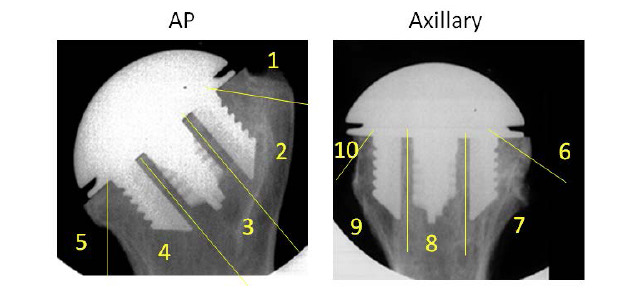
The follow up visits were at three and twelve months after surgery. The follow up examination included the measurement of the range-of-motion with a goniometer, the assessment of the Constant-Murley score (CS), Oxford Shoulder Score and EQ-5D-5L score. Radiographs in two planes (Grashey and axial view) were taken directly postoperative, 3 and 12 months postoperative. The radiographs were evaluated for radiolucency, osteolysis, loosening, migration, fractures, device condition, glenohumeral subluxation and subsidence by an independent radiologist. For evaluation of radiolucencies of the humeral component 10 zones were defined (Fig. 2). The radiolucencies were classified as “absent” or “present” with a detection limit of radiolucencies at least 1 mm in width. If radiolucency was present, it was measured perpendicular to the surface of the humeral component. Osteolysis was defined as a progressive radiolucency ≥ 4 mm that was not present on the baseline radiographs. Subsidence was defined as a caudal change in position of the humeral component ≥ 5 mm. The vertical distance between the most superior aspects of the humeral component and the greater tuberosity was measured. Aseptic loosening was defined as the presence of progressive radiolucencies, stress shielding or other imaging appearances indicative of loosening. The device condition was divided into three categories, intact, fractured and dissembled on the glenoidal or humeral side. Glenohumeral subluxation was evaluated according to the classification of Sperling et al.12
Figure 2. A: Five defined zones for the evaluation of radiolucency of the anchor plate in the anteroposterior radiograph. B: Five defined zones for the evaluation of radiolucency of the anchor plate in the axial radiograph.
Statistical analysis
Descriptive statistics were calculated for all variables and outcomes of interest, including the following: demographics, operative characteristics, adverse events, radiographic data, adjusted and unadjusted Constant-Murley score, Oxford Shoulder Score, and EQ-5D-5L score. The mean, standard deviation (SD), median, and minimum and maximum values were calculated for all numeric variables, whereas frequency and percent were reported for all categorical variables. Kaplan-Meier (KM) survivorship was used to assess time to revision. KM survivorship was not reported if <40 shoulders were at risk. For the adjusted Constant-Murley Score, Oxford Shoulder Score, and EQ-5D-5L value score outcomes, post-hoc comparisons between baseline and follow-up visits were performed using a paired t-test with a significance level of 0.05. Box plots were created to visually assess the distribution of these outcomes over time. All analyses were completed using statistical software, SAS version 9.4.
Results
Of 172 consented patients, 15 patients were withdrawn from the study without having received the GLOBAL ICON Stemless Shoulder device (8 of whom did not undergo surgery whereas 7 underwent surgery but no attempts were made to implant the Global ICON device). Three hemi arthroplasty patients were excluded from analyses; therefore, the collective consisted of 75 men and 79 women with a mean age of 66.6 years (SD 8.1). At 12 months follow up 2 patients had been revised, 2 had withdrawn consent and 21 were overdue; 129 (129/154 = 83.8%) patients returned for clinical evaluation. The mean height of the patients was 170.4 cm (SD 9.3) and the mean weight was 88.2 kg (SD 18.3). This resulted in a body mass index of 30.3 kg/m2 (SD 5.6). In 97.4% (150) of the patients the preoperative diagnosis was primary osteoarthritis and in 2.6% (4) post-traumatic arthritis. Eighty-two were right and 72 were left shoulders. The dominant hand was right in 94.8% (146) and left in 5.2%.7
In all patients the standard deltopectoral approach was used with a mean wound length of 12.1 cm (SD 2.4). Further, general anaesthesia was performed in 97.4% (150) patients. Regional anaesthesia, such as an interscalene pain catheter, was performed in 84.4% (130) of patients. The mean surgery time was 93.8 min (SD 26.2) with a mean anaesthesia time of 107.5 min (SD 49.7).
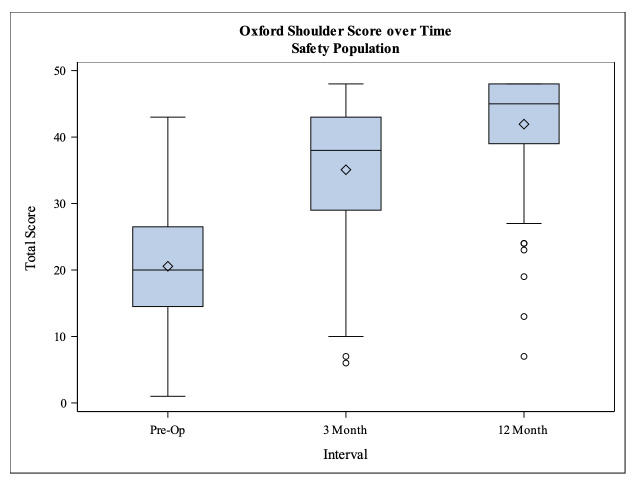
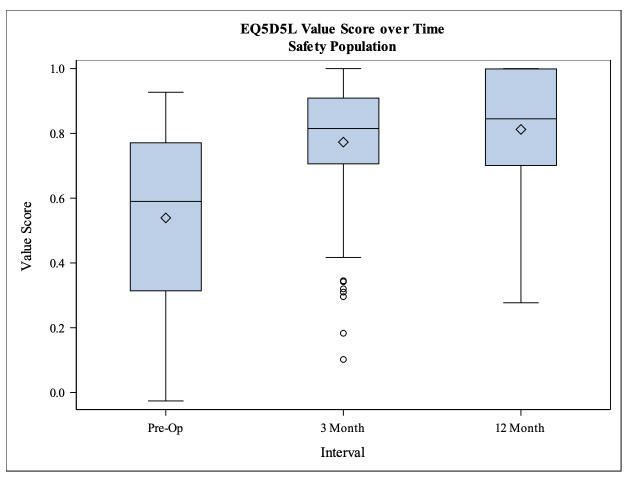
All clinical outcome scores (Constant-Murley score, Oxford shoulder score and EQ-5D-5L score) showed a significant increase from the baseline to 3 months follow up as well as from 3 months follow up to 12 months follow up. The unadjusted CS increased from preoperatively 32.1 to 72.3 points at 12 months follow up and in the same time period the adjusted CS also increased from 42.3 to 96.1 points (p<0.001) (Tab. 2). The Oxford shoulder score showed a total increase of 21.6 points from preoperatively 20.4 to 42.0 points at 12 months follow up (p<0.001) (Fig. 3). The EQ-5D-5L value score improved significantly between baseline and the two follow ups (p<0.001) as well as between the 3 and 12 months follow up (p=0.0227) (Fig. 4).
Table 2. Constant score of baseline, 3 and 12 months follow up of the comparison between baseline and each follow up.
| Characteristic | Baseline | 3 months | 12 months |
| Constant score (points) | 32.1 ±17.6 | 53.3 ±17.2 | 72.3 ±17.1 |
| Adjusted Constant score (%) | 42.3 ±22.6 | 70.8 ±23.3 | 96.1 ±22.8 |
| Pain (points) | 3.1 ±3.4 | 11.0 ±4.2 | 12.7 ±3.4 |
| Activities of daily living (points) | 7.9 ±3.4 | 14.1 ±4.3 | 17.9 ±3.4 |
| Strength (points) | 6.3 ±7.6 | 6.8 ±5.8 | 12.2 ±7.3 |
| Range of motion (points) | 14.9 ±7.6 | 21.4 ±7.8 | 29.6 ±7.6 |
Figure 3. Boxplot of the Oxford Shoulder Score.
Figure 4. Boxplot of the EQ-5D-5L score.
At 12 months, the KM survivorship was 98.7% (95%-CI 94.8%, 99.7%); there were 133 remaining with further follow-up beyond 1-year (Mean EQ-5D-5L baseline: 0.5; mean EQ-5D-5L 3 months: 0.8; mean EQ-5D-5L 12 months: 0.8).
The radiological measurements did not show continuous radiolucent lines, subsidence, aseptic loosening or progressive radiolucency. In one patient, an osteolysis was observed. In all cases the device condition was intact.
Postoperatively, two glenoid fractures and one proximal humerus fracture were observed. Intraoperatively, one proximal humerus fracture was observed. No operative revisions were indicated for the glenoid fractures. In addition to the two glenoid fractures, one patient showed radiographic loosening of the glenoid component with no revision yet performed; otherwise, no further complications were observed on the glenoid side. The intraoperative proximal humerus fracture was an anterior wall fracture that occurred during implantation of the humeral anchor plate component; however, it was possible to implant the study device. No operative site infections were observed. Revision was defined as removal of any humeral component for any reason. Of the 154 Subjects, 2 revisions were documented, resulting in a revision rate of 1.3% at 1-year (100% minus a KM device survivorship rate of 98.7% at 1-year). One periprosthetic fracture of the proximal humerus was observed 6 months postoperatively following trauma and revision to a reverse total shoulder arthroplasty was performed. One patient sustained a subscapularis tear, and an exchange of the prosthetic humeral head and subscapularis repair was performed 8 months after surgery.
A total of 32 local post-operative complications were reported in 22 study subjects (Tab. 3).
Table 3. Operative site complication.
| Operative Site Complication | Number of Events* |
| Pain and Musculoskeletal discomfort | 10 |
| Tendonitis and other tendon disorders | 3 |
| Rash and other skin disorders | 3 |
| Hematoma | 3 |
| Dorsal Subluxation** | 2 |
| Bursitis | 2 |
| Rotator cuff syndrome | 2 |
| Neuralgia/Paresthesia | 2 |
| Humeral fracture | 1 |
| Scapula fracture | 1 |
| Musculoskeletal stiffness | 1 |
| Periarthritis | 1 |
| Breast swelling | 1 |
| Total Number Operative Site Complications | 32 |
*All post-operative AEs are reported. Events unrelated to either the procedure/hospitalization or device implant were excluded. Please note: 1 additional complication (fall) was reported at the operative site but removed from Table 1 since the outcome of this complication is captured above as “Breast swelling”. Total number of subjects with reported AEs = 22 **N = 1 subject
Discussion
The aim of this prospective multicentre study was to determine clinical and radiological short-term results of a new stemless TSA. After 12 months we were able to show a significant increase of the mean adjusted CS from 42.3 to 96.1 points. Further, in the radiological measurements only one osteolytic lesion was observed, but no continuous radiolucent lines, subsidence, aseptic loosening or progressive radiolucencies were found in our collective. The clinical findings were comparable to other studies. Churchill et al., who observed 157 patients after the implantation of a stemless shoulder arthroplasty system (Wright Medical Simpliciti, Bloomington MN, USA), showed an increase of the adjusted CS from 44.3 to 79.4 points 12 months after surgery; however, patients with a preoperative CS lower than 20 points were excluded from participation.23 It is assumed that patients with a very low preoperative CS also achieve lower postoperative scores. Further, long-term results of the stemless shoulder arthroplasty designs also showed a significant increase of the postoperative scores.24,25 Hawi et al., who examined 49 stemless shoulders (Arthrex Eclipse, Naples FL, USA) with 32 hemiarthroplasties and 17 TSA, showed a significant increase of the age and gender adjusted CS from 52 % preoperatively to 79 points at 9 years follow up.25 Beck et al. found a significant improvement in the CS from 14.7 to 68.8 points in 31 shoulders after 94.7 months follow up with stemless TSA (Biomet TESS, Warsaw, IN, USA).24
The results of stemmed TSA and stemless or canal sparing TSA were often compared. A current review and meta-analysis by Liu et al. with the comparison of stemless and stemmed TSA found similar functional outcomes and complications rates for both procedures.4 Twenty-two studies with 962 patients were included in this review and stemless TSA demonstrated significantly decreased operative time and less blood loss than stemmed TSA.4 Many studies compared stemless TSA to stemmed TSA with described postoperative values of the CS of stemless TSA in these studies between 48.0 and 88 points.17–20,26 The low results of 48.0 points were caused by a short mean follow up time of only 6 months18 but the clinical results of the other studies were comparable to our findings.
In our study, there were two glenoid fractures, one intraoperative and one postoperative proximal humerus fracture, one asymptomatic osteolysis, and one subscapularis tear. Because of these complications two patients had to be revised. The complications of the humeral component were the anterior wall fracture during implantation and one osteolysis, but neither patient had to be revised. Brunner et al., who observed 233 patients after the implantation of stemless TSA, described only one radiological and asymptomatic loosening after 24 months.27 In five of 63 patients an intraoperative small lateral crack of the cortex was documented by Huguet et al.28 All fractures healed with conservative treatment.
The strengths of the study were the prospective multicentre design and a large sample size with 154 patients who received TSA with the study device and 129 available for analysis at the 1 year postoperative timepoint. Further, we were able to show a progressive improvement of the CS, Oxford Shoulder Score and EQ-5D-5L at three and twelve months follow up. The 12 month follow up rate of 87.2 % also was a strength of the study.
Limitation
Of course, the study has limitations. First, the follow up time of 12 months is short, but the stemless device is now a widely used implant design, making these short-term results of a new device valuable and novel. Second, to assess the safety and efficacy of the new stemless implant only a single-armed study design was chosen. Without a control group our results could not be directly compared to other systems. Third, there was no objective measurement or assessment of the bone quality of the proximal humerus to evaluate whether the proximal humerus was suitable for stemless TSA. Fourth, the high number of study centres could influence the outcome; however, it is possible the variety of study centres could reflect the general outcome of the procedure better than a single centre study.
Conclusion
In conclusion, the present study showed good clinical results of a new stemless shoulder implant after 12 months. The results of the Constant Murley, EQ-5D-5L and Oxford Shoulder Scores as well as the complications were comparable to other established stemmed and stemless TSA designs. Further investigations are needed to evaluate intermediate and long-term results and survivorship of this new stemless implant.
Completing interests
Tomas Smith is a consultant of Depuy Synthes. Marc-Frederic Pastor receives speaker fees from Depuy Synthes Nina Bowsher is an employee of Depuy Synthes Roman Karkosch, Alexander Ellwein, Hauke Horstmann and Spiros Tsamassiotis declare to have no conflicts of interest.
Ethical approval
The Ethics Committee of the Medical School Hannover gave approval for the study (No. 7525 Hannover).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Contribution
TS corrected and wrote the manuscript, operated the patients in our clinic, analyzed the data HH collected data by examining the patients, analyzing the data RK collected data by examining the patients AE collected data by examining the patients, correcting of manuscript, NB did the statistical analysis and the figures MFP major contributor in writing the manuscript, data collecting, analyzing data
Funding Statement
The study was funded by Depuy Synthes (Warsaw, IN, USA). Depuy Synthes was involved in the design of the study and data collection as well as statistical analysis. Funding was received to assist with the preparation of the manuscript.
References
- 1. Katolik LI, Romeo AA, Cole BJ, Verma NN, Hayden JK, Bach BR. Normalization of the Constant score. Journal of Shoulder and Elbow Surgery. 2005;14(3):279-285. doi:10.1016/j.jse.2004.10.009 [DOI] [PubMed]
- 2. Neer CS. Articular replacement for the humeral head. J Bone Joint Surg Am. 1955;37(2):215-228. doi:10.2106/00004623-195537020-00001 [PubMed]
- 3. Harmer L, Throckmorton T, Sperling JW. Total shoulder arthroplasty: re the humeral components getting shorter? Curr Rev Musculoskelet Med. 2016;9(1):17-22. doi:10.1007/s12178-016-9313-3 [DOI] [PMC free article] [PubMed]
- 4. Liu EY, Kord D, Horner NS, Leroux T, Alolabi B, Khan M. Stemless anatomic total shoulder arthroplasty: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2020;29(9):1928-1937. doi:10.1016/j.jse.2019.12.022 [DOI] [PubMed]
- 5. Petriccioli D, Bertone C, Marchi G. Stemless shoulder arthroplasty: a literature review. Joints. 2015;3(1):38-41. doi:10.11138/jts/2015.3.1.038 [PMC free article] [PubMed]
- 6. Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Periprosthetic humeral fractures during shoulder arthroplasty. J Bone Joint Surg Am. 2009;91(3):594-603. doi:10.2106/jbjs.h.00439 [DOI] [PubMed]
- 7. Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/jbjs.f.00125 [DOI] [PubMed]
- 8. Chin PYK, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22. doi:10.1016/j.jse.2005.05.005 [DOI] [PubMed]
- 9. Deshmukh AV, Wilson DR, Zilberfarb JL, Perlmutter GS. Stability of Acromioclavicular Joint Reconstruction Biomechanical Testing of Various Surgical Techniques in a Cadaveric Model. Am J Sports Med. 2004;32(6):1492-1498. doi:10.1177/0363546504263699 [DOI] [PubMed]
- 10. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005 [DOI] [PubMed]
- 11. Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(4):680-689. doi:10.2106/00004623-200404000-00003 [DOI] [PubMed]
- 12. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613. doi:10.1016/j.jse.2004.03.013 [DOI] [PubMed]
- 13. Wirth MA, Rockwood CA. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616. doi:10.2106/00004623-199604000-00018 [DOI] [PubMed]
- 14. Hawi N, Tauber M, Messina MJ, Habermeyer P, Martetschläger F. Anatomic stemless shoulder arthroplasty and related outcomes: a systematic review. BMC Musculoskelet Disord. 2016;17(1):376. doi:10.1186/s12891-016-1235-0 [DOI] [PMC free article] [PubMed]
- 15. Churchill RS, Athwal GS. Stemless shoulder arthroplasty—current results and designs. Curr Rev Musculoskelet Med. 2016;9(1):10-16. doi:10.1007/s12178-016-9320-4 [DOI] [PMC free article] [PubMed]
- 16. Athwal GS. Spare the Canal: Stemless Shoulder Arthroplasty Is Finally Here: Commentary on an article by R. Sean Churchill, MD, et al.: “Clinical and Radiographic Outcomes of the Simpliciti Canal-Sparing Shoulder Arthroplasty System. A Prospective Two-Year Multicenter Study. J Bone Joint Surg Am. 2016;98(7):e28. doi:10.2106/jbjs.15.01350 [DOI] [PubMed]
- 17. Berth A, Pap G. Stemless shoulder prosthesis versus conventional anatomic shoulder prosthesis in patients with osteoarthritis. J Orthop Traumatol. 2012;14(1):31-37. doi:10.1007/s10195-012-0216-9 [DOI] [PMC free article] [PubMed]
- 18. Maier MW, Lauer S, Klotz MC, Bülhoff M, Spranz D, Zeifang F. Are there differences between stemless and conventional stemmed shoulder prostheses in the treatment of glenohumeral osteoarthritis? BMC Musculoskelet Disord. 2015;16(1):275. doi:10.1186/s12891-015-0723-y [DOI] [PMC free article] [PubMed]
- 19. Mariotti U, Motta P, Stucchi A, Ponti di Sant’Angelo F. Stemmed versus stemless total shoulder arthroplasty: a preliminary report and short-term results. Musculoskelet Surg. 2014;98(3):195-200. doi:10.1007/s12306-014-0312-5 [DOI] [PubMed]
- 20. Spranz DM, Bruttel H, Wolf SI, Zeifang F, Maier MW. Functional midterm follow-up comparison of stemless total shoulder prostheses versus conventional stemmed anatomic shoulder prostheses using a 3D-motion-analysis. BMC Musculoskelet Disord. 2017;18(1):478. doi:10.1186/s12891-017-1835-3 [DOI] [PMC free article] [PubMed]
- 21. Barvencik F, Gebauer M, Beil FT, et al. Age- and sex-related changes of humeral head microarchitecture: histomorphometric analysis of 60 human specimens. J Orthop Res Off Publ Orthop Res Soc. 2009;28(1):18-26. doi:10.1002/jor.20957 [DOI] [PubMed]
- 22. Liew AS, Johnson JA, Patterson SD, King GJW, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426. doi:10.1067/mse.2000.107089 [DOI] [PubMed]
- 23. Churchill RS, Chuinard C, Wiater JM, et al. Clinical and Radiographic Outcomes of the Simpliciti Canal-Sparing Shoulder Arthroplasty System: A Prospective Two-Year Multicenter Study. J Bone Joint Surg Am. 2016;98(7):552-560. doi:10.2106/jbjs.15.00181 [DOI] [PubMed]
- 24. Beck S, Beck V, Wegner A, Dudda M, Patsalis T, Jäger M. Long-term survivorship of stemless anatomical shoulder replacement. Int Orthop. 2018;42(6):1327-1330. doi:10.1007/s00264-018-3779-0 [DOI] [PubMed]
- 25. Hawi N, Magosch P, Tauber M, Lichtenberg S, Habermeyer P. Nine-year outcome after anatomic stemless shoulder prosthesis: clinical and radiologic results. J Shoulder Elbow Surg. 2017;26(9):1609-1615. doi:10.1016/j.jse.2017.02.017 [DOI] [PubMed]
- 26. Uschok S, Magosch P, Moe M, Lichtenberg S, Habermeyer P. Is the stemless humeral head replacement clinically and radiographically a secure equivalent to standard stem humeral head replacement in the long-term follow-up? A prospective randomized trial. J Shoulder Elbow Surg. 2017;26(2):225-232. doi:10.1016/j.jse.2016.09.001 [DOI] [PubMed]
- 27. Brunner UH, Fruth M, Rückl K, et al. Die schaftfreie Eclipse-Prothese – Indikation und mittelfristige Ergebnisse. Obere Extrem. 2012;7(1):22-28. doi:10.1007/s11678-011-0152-y
- 28. Huguet D, DeClercq G, Rio B, Teissier J, Zipoli B. Results of a new stemless shoulder prosthesis: radiologic proof of maintained fixation and stability after a minimum of three years’ follow-up. J Shoulder Elbow Surg. 2010;19(6):847-852. doi:10.1016/j.jse.2009.12.009 [DOI] [PubMed]