Abstract
Using the Murine Hepatitis Virus (MHV) A59 coronavirus as a SARS-CoV-2 animal surrogate, we validated that methylene blue (MB) in combination with sunlight exposure is a robust, fast, and low-cost decontamination method for PPE that should be added to the toolbox of practical pandemic preparedness.
Key Words: Decontamination, Photochemistry, Next generation sequencing, Murine hapatitis virus, Pandemic preparedness
Introduction
COVID-19 has not only highlighted the unpreparedness of the world to handle a global pandemic, but it has also unmasked the inequity of resources available.1, 2, 3 A major issue from the beginning of the pandemic has been the availability of personal protective equipment (PPE), including surgical masks and respirators,1, 2, 3 which still remains problematic in the same areas of the world where vaccines are not readily available to the general public.2 Consequently, efforts have been made to sanitize and re-use PPE.4, 5 Published sanitization techniques are on the hours’ time scale and often require equipment that is not readily available to low-resource settings.4, 5 Therefore, there is a need for cost-effective and time-efficient methods for the safe decontamination of PPE.
A recent global collaboration, which included our group, participated in a study on the Development and Methods for N95 Respirators and Mask Decontamination (DeMaND) and validated methylene blue (MB) in combination with visible indoor light to efficiently disinfect PPE contaminated with infectious SARS-CoV-2 or surrogate animal viruses.6 Herein, we undertake a follow-up study and demonstrate the efficacy of MB in combination with sunlight to decontaminate surgical masks, solidifying the position of MB as a robust decontamination method for all settings.
Materials and methods
Virus and cell
The murine hepatitis coronavirus (MHV) strain A59 and the delayed brain tumor (DBT) cells were kindly supplied by Dr Alain Lamarre, INRS, Quebec, Canada. All cultures were grown at 37 °C in a humidified incubator with 5% CO2 according to literature protocols.7 The infectious activity of the viral cultures was determined in 6-well plates (VWR) by plaque counting assays.8 MHV stocks with titers ranging from 5.18 ±0.22 log10 pfu/mL to 6.28±0.23 log10 pfu/mL were used in subsequent steps.
Surgical masks
Razor Medical Surgical Disposable Face Masks: ASTM F2100 Level 1 were purchased from IDA pharmacy at the University of Calgary.
Simulated sunlight
Sunlight was simulated with a SolSim 2 photoreactor (Luzchem, Canada) that matches the air mass AM1.5 solar spectrum to within 1% total power difference between 300-800 nm, equivalent to the yearly average irradiance measure at mid-latitude (41.81° above the horizon) on an inclined plane at 37° tilt towards the equator, with a solar zenith angle of z=48.2°. The AM1.5 spectrum has an integrated power of 1000W/m2 (280-4000 nm).
Methylene blue treatment in solution
Solutions of MHV were made at a 10−2 dilution in MEM without phenol red (Quality Biological) in the absence (control) and in the presence of 10 μM MB (treated). The solutions, with and without MB, were irradiated under simulated sunlight for various times, except for the solution referred to as time 0 min, which was kept in the dark. Virus inactivation at different irradiation time points was quantified via a plaque counting assay.8 Integrity of the genetic material was assessed by next generation sequencing (Illumina MiSeq, 300 cycle nano flowcell, paired-end). Viral RNA was extracted from selected samples using a QIAamp Viral RNA kit (Qiagen) according to manufacturer protocol on triplicate samples. Library input was evaluated using D1000 ScreenTape (Agilent). Sequencing libraries were prepared using the Ultra II RNA kit (NEB) with no fragmentation, and adapter ligation efficiency was evaluated using the KAPA Library Quantification Kit (Roche). The raw sequences for the RNA sequencing experiment have been deposited in the NCBI SRA under accession PRJNA806985, while mapping and mutation statistics were generated using scripts and reference datasets available at http://github.com/nodrogluap/damage.
Virus inoculation and elution on a surgical mask
Surgical masks were cut into 1 cm2 square coupons. A 10 μM aqueous MB solution was sprayed onto several mask cut-outs (treated). Each coupon was placed down on a bench and sprayed 3 times (1 mL/spray) from 10 cm above to ensure full identical coverage of the coupons treated with MB. The coupons were then allowed to dry overnight in the dark, while control coupons were left untreated. To all coupons, 20 μL of the virus was inoculated. After sunlight exposure, the virus was eluted from the mask according to previously developed protocols.6 Virus inactivation was quantified via plaque counting assay.6 , 8
Data analysis
All data were computed and analyzed using GraphPad Prism 9 (Graph-Pad Software).
Results
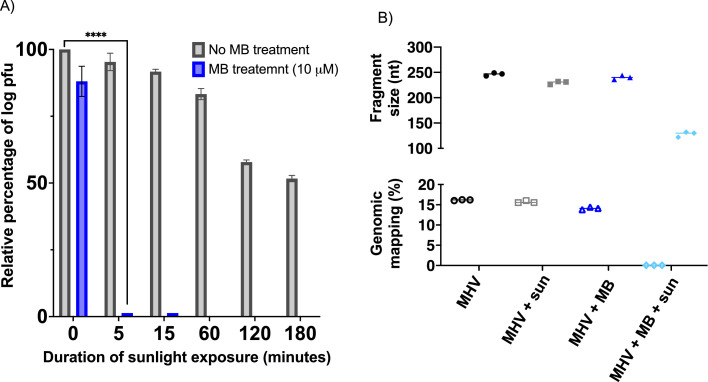
As shown in Figure 1 A, complete viral inactivation was observed within 5 minutes of sunlight exposure for solutions treated with 10 μM MB, while it required at least 3 hours of sunlight exposure to reach a 50% reduction in MHV log pfu in the absence of MB. The chemical integrity of the viral RNA was assessed via next generation sequencing on triplicate samples. Viral RNA for samples treated with 10 μM MB in combination with 15 minutes of sunlight exposure showed a decreased sequencing library prep yield and poorer adapter ligation efficiency (Mann-Whitney test P=.0091 for both). Furthermore, as shown in Figure 1B, in an equimolar pooling of all libraries, only the sample treated with MB plus sunlight showed a significantly smaller mean fragment size (∼239nt vs ∼128nt) and much lower percentage mapping to the MHV genome (0.05% for samples treated with MB plus sunlight vs 15.3% for other samples). This is consistent with MB plus sunlight treatment causing severe damage to all RNA backbones, and especially depleting intact viral RNA. Interestingly, no treatment (sunlight exposure and/or presence of MB) consistently caused a significant change in the observation of T mutations in the most common UV-lesion (YCY, n=1375) and 8-oxoG (CGG, n=163) contexts of the MHV reference genome vs corresponding background mutation rates.
Fig 1.
(A) Antiviral activity of MB and/or sunlight exposure against MHV expressed as relative log pfu (%) to that for the untreated virus-infected control cells, which was defined as 100%. The data shown are the mean ± SD from three replicated experiments. P-value<.0001 (****). (B) Next generation sequencing statistical data for the median virus mapped fragment size (nucleotide, nt – top), and reads mapping to MHV-A59 genome (percentage, % - bottom). MHV is an untreated viral sample kept in the dark (control); MHV + sun corresponds to the viral sample irradiated for 180 minutes under simulated sunlight exposure; MHV + MB represents the viral sample treated with 10 μM MB kept in the dark; MHV + MB + sun is the viral sample treated with 10 μM MB in combination with 15 minutes of simulated sunlight exposure.
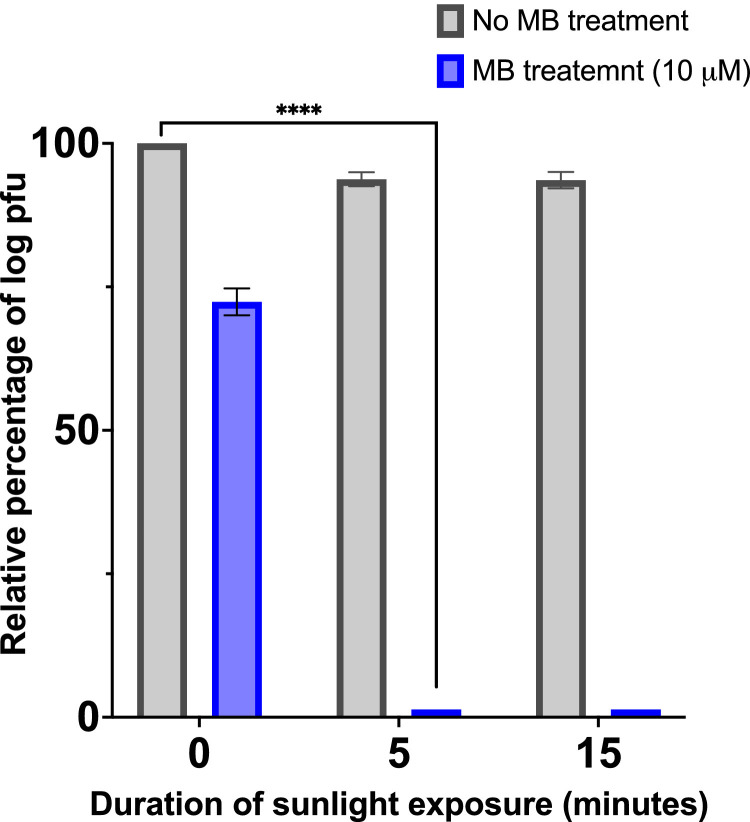
Importantly, pre-treatment with 10 μM MB resulted in the complete viral inactivation on surgical masks after only 5 minutes of sunlight exposure (Fig 2 ).
Fig 2.
Antiviral activity of MB and/or sunlight exposure against MHV contaminated masks expressed as relative log pfu (%) to that for the untreated virus-infected control cells, which was defined as 100%. The data shown are the mean ± SD from three replicated experiments. P-value<.0001 (****).
Discussion
As demonstrated in our previous study, methylene blue in combination with bright indoor light (50,000 lux) generated by a white LED lamp consistently inactivates SARS-CoV-2, and other animal surrogates, including MHV, in 30 minutes or less.6 While this decontamination method is time and cost-effective, it still requires the light source to be powered, and thus it relies on the need for electricity, which remains unavailable to nearly 16% of the world population. Therefore, we set to validate that sunlight can be used to activate MB and disinfect masks contaminated with MHV, a SARS-CoV-2 animal surrogate.6 , 9 Sunlight itself has already been reported to inactivate SARS-CoV-2, with time ranging from as little as 8 minutes to several hours.10, 11, 12 Results shown in Figure 1A show that MHV is more resistant to sunlight, making it an ideal surrogate for the combination treatment of MB and sunlight. Herein, we showed the superiority of combining MB with sunlight. Indeed, total inactivation of MHV was achieved within 5 minutes of sunlight irradiation in solution, while it required several hours for sunlight to display antiviral activity (Fig 1A). Interestingly, the observed fundamental genetic damage (low viral RNA proportion, short fragments, and poor ligation efficiency, Fig 1B) when the virus is treated with MB in combination with sunlight exposure strongly suggests that this synergistic approach will also work in depleting viable genomic RNA in other sarbecoviruses such as SARS-CoV-2. In the absence of light, a small reduction of viral titers was also found for samples treated with MB, which was already observed in our past study, and can be attributed to the antiviral activity of MB itself.6 Importantly, we demonstrated that surgical masks’ material contaminated with MHV can be fully decontaminated within 5 minutes of sunlight exposure when treated with 10 μM MB, while significant infectious titers remain on the masks’ material exposed solely to sunlight (Fig 2).
Conclusion
This brief report validates the use of methylene blue in a combination of sunlight, as a robust decontamination method for PPE, such as surgical masks, that can be deployed in any setting, especially in remote areas where electricity is not readily available.
Acknowledgment
The authors would like to thank Drs D. H. Evans and Y-C. Lin (University of Alberta) for fruitful discussions regarding experimental protocols.
Footnotes
Funding/Support: The authors acknowledge that funds for the project were provided by WHO based on a grant from the German Federal Ministry of Health (BMG). This work has been supported in part by the Natural Sciences and Engineering Research Council (NSERC) of Canada. BH acknowledges NSERC of Canada Discovery Grant (RGPIN/03824-2017) for funding. Open access of this article is sponsored by the World Health Organization.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Burki T. Global shortage of personal protective equipment. Lancet Infect Dis. 2020;20:785–786. doi: 10.1016/S1473-3099(20)30501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon D.E., Peters G.A., Ivers L.C., Freeman E.E. Global resource shortages during COVID-19: Bad news for low-income countries. PLOS Neglect Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranney M.L., Griffeth V., Jha A.K. Critical Supply Shortages — The need for ventilators and personal protective equipment during the covid-19 pandemic. N Engl J Med. 2020;382:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig-Begall L.F., Wielick C., Dams L., et al. The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus. J Hosp Infect. 2020;106:577–584. doi: 10.1016/j.jhin.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz A., Stiegel M., Greeson N., et al. Decontamination and Reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS-CoV-2 (COVID-19) pandemic. Appl Biosaf. 2020;25 doi: 10.1177/1535676020919932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lendvay T.S., Chen J., Harcourt B.H., et al. Addressing personal protective equipment (PPE) decontamination: Methylene blue and light inactivates severe acute respiratory coronavirus virus 2 (SARS-CoV-2) on N95 respirators and medical masks with maintenance of integrity and fit. Infect Control Hosp Epidemiol. 2021:1–10. doi: 10.1017/ice.2021.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel C., Lamarre A., Talbot P.J. Increased viral titers and enhanced reactivity of antibodies to the spike glycoprotein of murine coronavirus produced by infection at pH 6. J Virol Methods. 1994;50:237–244. doi: 10.1016/0166-0934(94)90180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano N., Fujiwara K., Hino S., Matumoto M. Replication and plaque formation of mouse hepatitis virus (MHV-2) in mouse cell line DBT culture. Archiv für die gesamte Virusforschung. 1974;44:298–302. doi: 10.1007/BF01240618. [DOI] [PubMed] [Google Scholar]
- 9.Körner R.W., Majjouti M., Alcazar M.A.A., Mahabir E. Of Mice and Men: The coronavirus MHV and mouse models as a translational approach to understand SARS-CoV-2. Viruses. 2020;12:880. doi: 10.3390/v12080880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raiteux J., Eschlimann M., Marangon A., et al. Inactivation of SARS-CoV-2 by simulated sunlight on contaminated surfaces. Microbiol Spectr. 2021;9:e0033321. doi: 10.1128/spectrum.00333-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuit M., Ratnesar-Shumate S., Yolitz J., et al. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J Infect Dis. 2020;222:564–571. doi: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloan A., Cutts T., Griffin B.D., et al. Simulated sunlight decreases the viability of SARS-CoV-2 in mucus. PLOS ONE. 2021;16 doi: 10.1371/journal.pone.0253068. [DOI] [PMC free article] [PubMed] [Google Scholar]