Abstract
Introduction
Currently pulmonary fibrosis in post-COVID individuals represents a crucial milieu of investigation due to long-term associated complications and worse clinical outcome. Lack of studies in Indian population confers a crucial need for elucidating possible targets and mechanisms to explore better management and outcome. Hence, this study aimed to explore the role of circulating miRNA-21 in patients from South India after COVID-19 recovery, while targeting TGF-β signaling pathway involved in the development of pulmonary fibrosis.
Methods
This prospective, single centre, hospital-based study enrolled a total of 50 participants in the age group of 50 to 60 years including 25 non-infected controls and 25 patients who were recovered after 3–6 months of COVID-19 infection and presented radiological pulmonary abnormalities. Quantification of miRNA-21 and selected gene transcripts (TGF-β, Col1A2, Col3A1, and α-SMA) was performed in plasma samples of both patients and controls.
Results
Significantly increased expression levels of miRNA-21 was observed in patient samples compared to controls (4.50 ± 1.03 vs 12.60 ± 3.52, p < 0.0001) with 72.10% sensitivity and 80.10% specificity. Further, significantly increased levels of central fibrosis regulatory gene transcript TGF-β (0.56 ± 0.27 vs 1.83 ± 0.98), two crucial collagen transcripts Col1A2 (0.62 ± 0.19 vs 1.56 ± 1.00) and Col3A1 (0.61 ± 0.27 vs 1.54 ± 0.89), and α-SMA (0.46 ± 0.17 vs 1.20 ± 0.78) was observed in patients compared to controls. Western-blot analysis also showed almost similar observations at proteins levels.
Conclusion
Circulating miRNA-21 may provide crucial insights for elucidating TGF-β mediated pulmonary remodeling involved in the fibrosis development and achieve better clinical outcome for post-COVID patients after recovery, in real-time with high diagnostic accuracy.
Keywords: SARS-CoV-2, COVID-19, Pulmonary fibrosis, TGF-β
1. Introduction
Pulmonary fibrosis is a genetically predisposed, and fibroproliferative disease with a well-known impediment of acute respiratory distress syndrome (ARDS). Since the pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease (COVID-19) has been reported to induce ARDS in 17.2 to 31% of infected patients (McDonald, 2021). Recent studies have demonstrated that more than one-third of patients who recovered after COVID-19 often encompass pulmonary sequelae at 12 weeks after discharge, and may lead to irreversible fibrotic lesions in some cases (Baratella et al., 2021; Korkmaz and Kele¸s, 2021; Zou and Li, 2021). A nationwide, multicentre, and observational study in 600 COVID-19 patients in Indian population has reported 13.66% pulmonary fibrosis after six weeks of discharge post-COVID-19 (Patil et al., 2021). Men had predominantly higher rate of pulmonary fibrosis compared to women participants (85.36% vs 14.64%, p < 0.0001). Similarly, individuals above 50 years of age showed significantly higher proportion of pulmonary fibrosis compared to age below 50 years (80.49% vs 19.51%, p < 0.0003). Furthermore, duration of illness has been greatly associated with the development of pulmonary fibrosis and requires time-dependent analysis of such individuals in population-based studies (Rumende, 2021).
Based on these findings, the occurrence of post-COVID pulmonary fibrosis is estimated to be 2–6% after moderate disease, and it can be assumed that pulmonary fibrosis could be one of the major long-term consequences of COVID-19, even among asymptomatic individuals (Bazdyrev et al., 2021). Despite the utmost efforts of the global medical community, currently there is no appropriate treatment modality for COVID-induced pulmonary fibrosis. The relevance of anti-fibrotic medication therapy for patients with active SARS-CoV-2 infection or those who have been cured of residual pulmonary fibrosis is still being defined and remains uncertain; the scientific basis for introducing, maintaining, or terminating therapy is also obscure (Vitiello et al., 2020; Bazdyrev et al., 2021). These findings warrant further evaluation of recovered COVID-19 individuals in longer duration with pulmonary involvement to determine potential genetic fingerprints.
Recent studies have demonstrated involvement of epithelial and endothelial injury as a starting point for the fibrotic development (Mostafaei et al., 2021; Spagnolo and Oldham, 2021). Transforming growth factor-β (TGF-β) has been identified as one of the most crucial pro-fibrotic factors released during the endothelial injury which also helps to maintain fibrotic processes throughout the life cycle (Leach et al., 2013). Hence, TGF-β has been proposed as the most crucial target to disclose the development and progress of pulmonary fibrosis. However, the role of TGF-β in COVID-19 recovered individuals remains elusive and requires revelation of more comprehensive targets affected by TGF-β pathway. The current advancements in identifying circulating cell-free microRNAs (miRNAs) have revealed its potential utility in non-invasive and real-time assessment of several disease conditions including pulmonary fibrosis while influencing TGF-β signaling by regulating the expression of TGF-β1, and TGF-β receptor (Ong et al., 2017a, Ong et al., 2017b). Among different miRNAs, miRNA-21 has been reported to amplify TGF-β signaling in a positive feedback fashion (Xu et al., 2021a, Xu et al., 2021b), and represents a crucial target to be investigated. Hence, in this study we aim to investigate differential expression of miRNA-21 in plasma samples of patients from South India after 3–6 months of COVID-19 and evaluated involvement of well-known TGF-β signaling to explore a possible mechanism responsible for the development of pulmonary fibrosis.
2. Materials and methods
This prospective hospital-based study was conducted in individuals from South India after taking approval from Institutional Review Board (IRB) of Deccan College of Medical Sciences, Hyderabad, Telangana, India. Signed Informed Consent forms were collected from each study participant before collecting samples.
2.1. Study population
A total of 25 individuals who were recovered after 3–6 months of COVID-19 infection and considered healthy and tested negative for severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) were included as patients in this study. The patients were enrolled only after confirmation of the persisting radiological pulmonary abnormalities using a chest X-ray and high resolution computed tomography (HRCT) scan according to the revised guidelines on the clinical management of COVID–19 (Government of India, 2020). Study participants reported for positive CT chest findings of COVID-19 along with the confirmed negative diagnosis by RT-qPCR test. CT imaging features included fibrotic strips; bronchovascular bundle distortion, traction bronchiectasis, interlobar septal thickening, and architectural distortion were recorded as suggestive of pulmonary fibrosis. Pregnant women, with severe respiratory motion artifacts on CT images, patients with history of chronic interstitial lung disease, and patients with any other chronic medical conditions such as DM, hypertension, and autoimmune disease were excluded from the study. Furthermore, 25 age- and gender-matched non-infected individuals were enrolled as controls for comparative analysis. All the study participants included both men and women of age range between 50 and 60 years.
2.2. Sample collection
A total of 5 mL of peripheral blood samples were collected in EDTA-coated vacutainer tubes. From each blood sample, plasma was separated within 30 min after blood collection through centrifugation at 1200 xg for 15 min at room temperature and processed within 4 h.
2.3. RNA extraction and complementary DNA synthesis
The extraction of miRNA was performed using mirVanaTM RNA Isolation Kit (Applied Biosystems, CA, USA) according to the manufacturer's instructions in both patient and control plasma samples. While, total RNA was extracted using GITC method for quantifying selected gene transcripts. Both the types of extracted RNAs were eluted or dissolved using 50 μL of nuclease-free water. The concentration and purity of extracted RNA was determined using a NanoDrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) at 260 and 280 nm wavelength.
To quantify miRNA-21 expression, complementary DNA (cDNA) was prepared using universal stem loop and U6 primers, while OligodT was used for analyzing selected gene transcripts separately. In brief, both the types of RNA from each sample was mixed with the primers as described above and incubated at 65 °C for 10 min in a thermal cycler followed by 2 min of snap cooling of the reaction. Further, a reaction mixture of 10 mM dNTPs, 1× reaction buffer with DTT and 1 unit of MMLV reverse transcriptase enzyme was added in each reaction mixture and incubated at 42 °C for 45 min followed by 72 °C for 10 min. The integrity of constructed cDNA was checked on 1% agarose gel and 2 μL of cDNA was used for SYBR Green-based real-time quantitative polymerase chain reaction (RT-qPCR) to quantify miRNA-21 and selected gene transcripts expression using respective cDNA.
2.3.1. RT-qPCR of miRNA-21, and targeted gene transcripts
RT-qPCR was performed using a Real-time PCR machine (ABI 7500, Applied Biosystems, USA) using specific oligonucleotide sequences such as miRNA-21, forward: 5′-CGGGATCCTGGGGTTCGATCTTAACAGGC-3′; reverse: 5′- CGGAATTCCCACAATGCAGCTTAGTTTTCC-3′), and endogenous U6 snRNA, forward: 5′-CGCTTCGGCAGCAGCACATATACTA-3′, reverse: 5′- CGCTTCACGAATTTGCGTGTCA-3′; TGF-β, forward: 5′-CCCAGCATCTGCAAAGCTC-3′, reverse: 5′-GTCAATGTACAGCTGCCGCA-3′; Col1A2: forward: 5’-GGTGGTGGTTATGACTTTGG-3′, reverse: 5′-TCTGGGTGGCTGAGTCTCAA-3′); Col3A1, forward: 5’-GCTCTGCT TCATCCCACTATTA-3′, reverse: 5′-AACATTCTCCAAATGGAATT-3′; Col3A1, forward: 5′-GCTCTGCTTCATCCCACTATTA-3′, reverse: 5′-AACATTCTCCAAATGGAATT-3′; α-SMA, forward: 5′-CATCACCAACTGGGACGACATGGAA3′, reverse: 5′-GCATAGCCCTCATAGATGGGGACATTG-3′; and β-actin, forward: 5′-GTCCTCTCCCAAGTCCACAC-3′, reverse: 5′-GGGAGACCAAAAGCCTTCAT-3. Each reaction was performed in triplicate for each sample in a total of 20 μL reaction mixture separately. Following reaction conditions were set to determine cycle threshold (Ct) values of each miRNA: A single step of initial denaturation at 94 °C for 2 min followed by 40 cycles of denaturation at 94 °C for 30s, annealing at 54 °C–58 °C for 30s and extension at 72 °C for 30s. Further a single step of 10 min of melting curve was set to differentiate between primer-dimer and amplicons.
2.4. Western blot analysis
Western-blot of all 4 selected proteins was performed in plasma samples of both patients and controls using standard protocol. Briefly, protein concentration in each plasma sample was determined using BCA protein assay kit (Catalog #: 23225, ThermoFisher Scientific, USA). Fixed volume of plasma protein samples were mixed with 2× Laemmli sample buffer, boiled at 100 °C for 5 min and loaded into the wells of 12% SDS-PAGE gel. Proteins were allowed to run for 1–2 h at 100 V until proper resolution. Polyvinylidene fluoride (PVDF) membranes were activated with methanol for 60s and rinsed with transfer buffer before assembly into the cassette. Proteins were transferred from SDS-Page gel to PVDF membrane under constant voltage. Membrane was removed from the cassette and blocked for 60 min at room temperature using blocking buffer and incubated with appropriate dilutions of primary antibodies (β-actin, Catalog #: MAB8929-SP, R&D System, USA; TGF-β, Catalog #: ab92486, abcam, USA; Col1A2, Catalog #: ab96723, abcam, USA; Col3A, Catalog #: ab27099, abcam, USA; α-SMA, Catalog #: ab5694, abcam, USA) in blocking buffer overnight at 4 °C. Following washing the membrane with TBST buffer, membranes were incubated at room temperature with the recommended dilutions of conjugated secondary antibody (HRP–conjugated goat anti-rabbit secondary antibody, dilution 1:5000) in blocking buffer for 60 min. Membrane was washed thrice with TBST buffer. Recommended substrate was added to develop the color and images were captured.
2.5. Statistical analysis
All the data are presented as mean ± standard deviation (SD). Student t-test was used to compare two groups while one way ANOVA was used to compare multiple groups and calculate the statistical significance (i.e. p value at 95% confidence interval [CI]). Relative operative curve (ROC) analysis was performed to predict diagnostic value of oncomiR-21 expression in both tissue and plasma samples. A total of three experimental repeats and two biological repeats were performed for each analysis to enhance the reproducibility of the data. All the statistical analysis was performed and data were presented using GraphPad Prism software (version 8.4.2). The statistical significance for all the groups was set ≤0.05 at 95% CI.
3. Results
3.1. Difference in cell-free circulating miRNA-21 expression levels
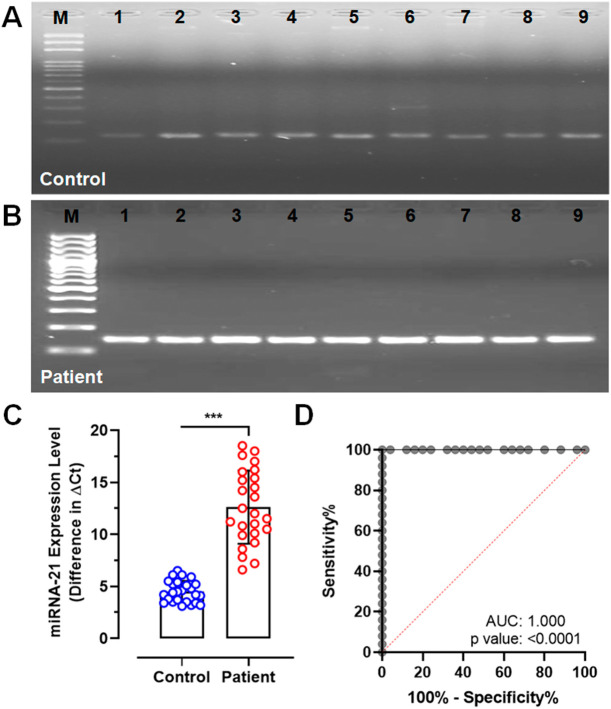
Qualitative analysis of cell-free circulating miRNA-21 showed consistently low expression, identified by the presence of low intensity amplicons on agarose gel in all the control samples following to PCR (Fig. 1A). In contrast, persistently higher expression levels of miRNA-21 were observed in all the post-covid patient samples which was identified by intensified amplicons on agarose gel (Fig. 1B). These findings were validated using SYBR-Green based quantitative PCR which clearly demonstrated significantly increased expression levels of miRNA-21 in patient samples compared to controls (mean ± SD, 4.50 ± 1.03 vs 12.60 ± 3.52; CI, 4.08–4.93 to 11.15–14.06; p < 0.0001, Fig. 1C). Further, ROC analysis showed circulating miRNA-21 expression level as an ideal clinical discriminator between control and patient samples with 72.10% sensitivity and 80.10% specificity (Fig. 1D).
Fig. 1.
Qualitative and quantitative PCR analysis of miRNA-21 expression. (A) Gel image showing miRNA-21 amplicons in control samples (B) Gel image showing miRNA-21 amplicons in patient samples (C) RT-qPCR analysis plot showing difference in Ct values of miRNA-21 expression compared to U6 between control and patient samples (D) ROC curve showing diagnostic significance of analyzing miRNA-21 between control and patient samples (***p < 0.0001).
3.2. Validation of miRNA-21 targeted fibrotic pathways
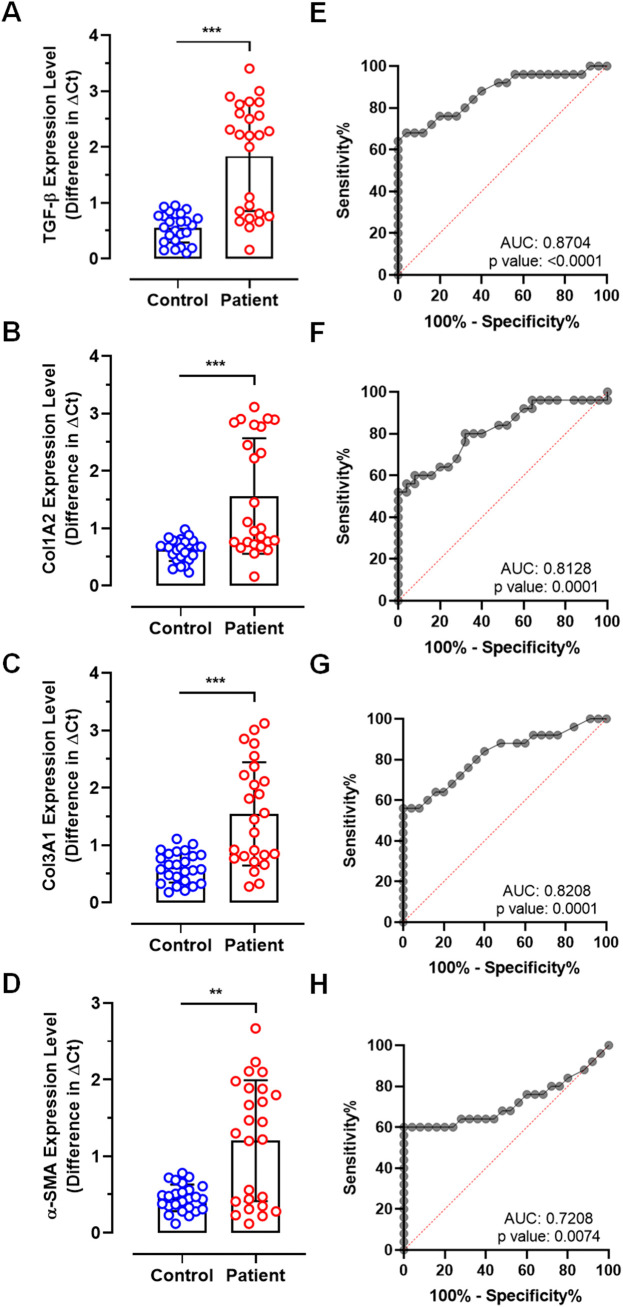
We validated quantitative expression of 4 crucial gene transcripts involved in pulmonary fibrotic pathways. RT-qPCR analysis showed significantly increased levels of central fibrosis regulatory gene transcript TGF-β (0.56 ± 0.27 vs 1.83 ± 0.98, p < 0.0001), 2 crucial collagen transcripts Col1A2 (0.62 ± 0.19 vs 1.56 ± 1.00, p < 0.0001) and Col3A1 (0.61 ± 0.27 vs 1.54 ± 0.89, p < 0.0001), and α-SMA (0.46 ± 0.17 vs 1.20 ± 0.78, p < 0.001) in patient samples compared to controls (Fig. 2A–D). However, ROC curve analysis showed relatively lower levels of sensitivity and specificity for all the 4 selected gene transcripts when compared between control and patient samples. ROC of TGF-β showed 65.33% sensitivity and 70.35% specificity with 0.8704 AUC (Fig. 2E). Similarly, other transcripts such as Col1A2 showed 63.1% sensitivity and 67.9% specificity with 0.8128 AUC (Fig. 2F), Col3A1 showed 59.64% sensitivity and 72.47% specificity with 0.8208 AUC (Fig. 2G), and α-SMA showed 55.68% sensitivity and 70.63%% specificity with 0.7208 AUC (Fig. 2H).
Fig. 2.
RT-qPCR analysis of fibrotic pathway genes between control and patient samples. Plots showing difference in expression levels of (A) TGF-β, (B) Col1A2, (C) Col3A1, and (D) α-SMA. ROC curves showing predictive diagnostic significance of (E) TGF-β, (F) Col1A2, (G) Col3A1, and (H) α-SMA. (***p < 0.0001, **p < 0.001).
Next, we validated selected 4 targeted protein expressions through western-blot analysis in serum samples between control and patients (Fig. 3 ). This analysis showed almost similar observation to RT-qPCR analysis. The protein expression levels of TGF-β, Col1A2, Col3A1, and α-SMA was found enhanced in patient samples compared to controls which are evident by protein intensity on the blotted membranes.
Fig. 3.
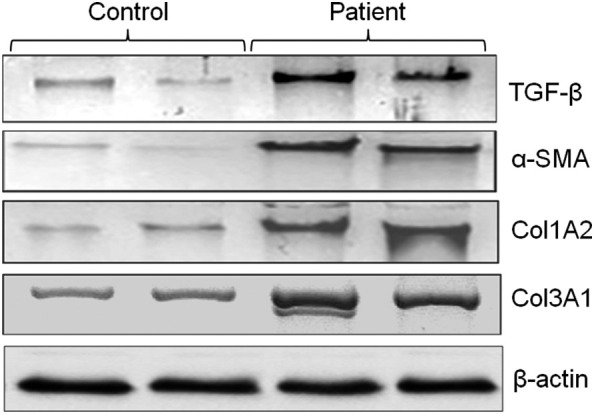
Western-blot showing difference in protein levels of TGF-β, Col1A2, Col3A1, α-SMA, and β-actin between control and patient samples.
4. Discussion
The present study describes a crucial link between circulating cell-free miRNA-21 levels and TGF-β signaling cascades, and elucidates possible diagnostic significance in the development of pulmonary fibrosis in individuals after recovery from COVID-19. This study was conducted due to long-term pulmonary sequelae identified in form of radiologic pulmonary abnormalities in discharged individuals after COVID-19 infection, and was tested negative for COVID-19. Earlier studies have reported that men at older ages are at higher risk for worse outcomes in patients with COVID-19 (Chen et al., 2020; Patil et al., 2021). However, it remains unclear about the gender-based distinctive outcomes in such individuals. Importantly, age still continues to be the most crucial risk factor due to involvement of several comorbidities including diabetes, hypertension, and other age-related conditions. Furthermore, it remains unclear why certain individuals recover from COVID-19 without development of pulmonary fibrosis.
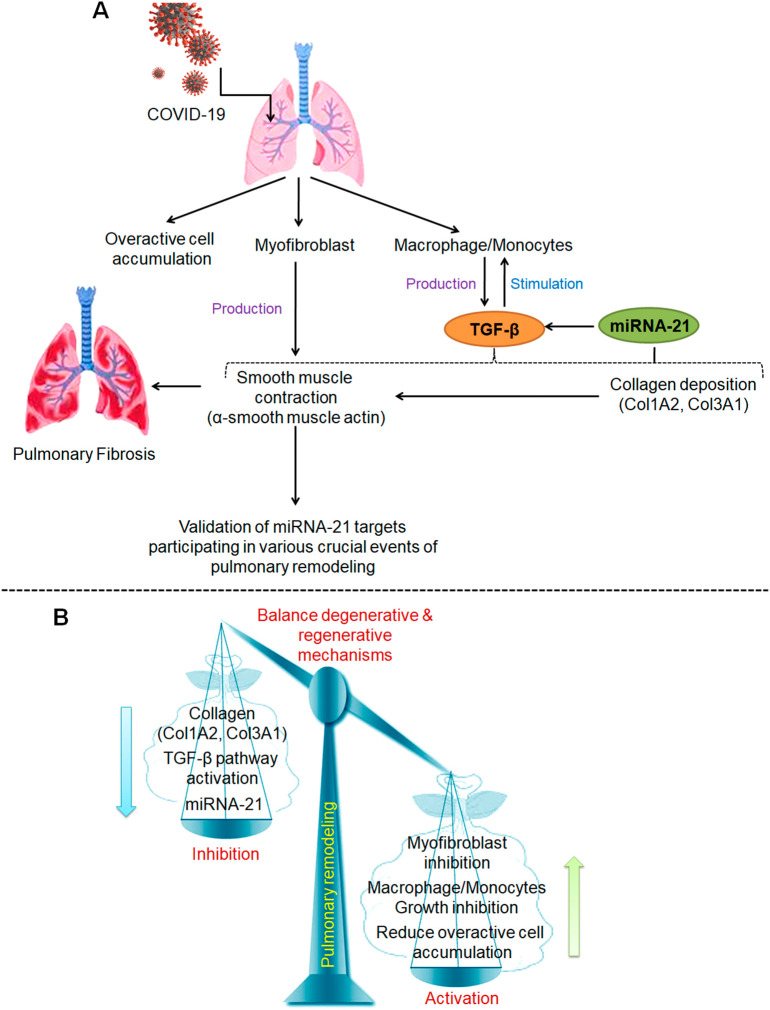
Our study is the first in South Indian population which explores crucial molecular fingerprints in recovered COVID-19 individuals in age range of 50–60 years who presented with radiologic abnormalities between 3 and 6 months of discharge from the hospital. Our interest in TGF-β pathway relies on its involvement in the accumulation of myofibroblasts and extracellular matrix (ECM) which represent major contributing factors in the development of fibrosis (Fig. 4A). Moreover, studies have reported that miRNA-21 is directly in TGF-β signaling cascades through regulation of macrophage/monocyte activity in different organs/tissues (Liu et al., 2010; Cho et al., 2011; Loboda et al., 2016). Smooth muscle contraction is another landmark feature of pulmonary fibrosis which is due to production of α-SMA from the myofibroblasts (Sun et al., 2016). Hence, post-COVID pulmonary fibrosis is now considered as a potential concerning sequela among survivors due to the development of permanent lung architectural distortion and irreversible pulmonary impairment (Ojo et al., 2020; Georg et al., 2020). Recent studies have also provided evidence for the involvement of several canonical and noncanonical signaling pathways in ECM deposition and the development of fibrosis (Finnso et al., 2020; Xu et al., 2021a, Xu et al., 2021b). Among noncanonical pathways miRNAs have been shown to play central role in the fibrotic pathogenesis and treatment; hence investigation of miRNA-based regulatory mechanisms has gained enormous attention in recent years (Yang et al., 2016; Henry et al., 2019). Similar to our findings, miRNA-21 has been shown to play crucial role in the development of lung fibrosis through interaction with diverse pathways (Cutroneo et al., 2007; Liu et al., 2016). Yamada et al. (2013) has also demonstrated that miRNA-21 is enhanced in lung epithelial cells during the development of pulmonary fibrosis and it promotes epithelial-mesenchymal transition which further worsen the disease condition. This study further provided evidence that exogenous injection of a miRNA-21 inhibitor suppresses the up-regulated production of vimentin and alpha-smooth muscle actin under conditions that induce epithelial-mesenchymal transition.
Fig. 4.
(A) Schematic representation showing different cascades of cellular and molecular events participating in pulmonary fibrosis which act as crucial targets of miRNA-21. (B) Representation showing regulation of crucial signaling pathways bases on our study findings to evolve better strategy for reducing the progress of pulmonary fibrosis in patients post-COVID to achieve better outcome.
Our study findings elucidated possible involvement of such crucial signaling pathways in pulmonary fibrosis while regulating ECM remodeling through miRNA-21 expression leading to increased production of TGF-β signaling proteins. We found >8-fold increased expression levels of miRNA-21 in overall patient samples compared to controls (p < 0.0001); while, gender-wise difference was not observed. This finding also showed high diagnostic value with >72% sensitivity and over 80% specificity. Although our results from validation studies at transcript expression and protein levels showed significantly higher in patients similar to miRNA-21, the difference was comparatively less and several values were superimposed with controls representing their poor diagnostic significance. Our findings of TGF-β signaling transcripts expression showed 1.27 fold increased expression of TGF-β, 0.94 fold of Col1A2, 0.93 fold of Col3A1, and 0.74 fold of α-SMA in patients compared to control individuals. These analyses also had relatively lower sensitivity and specificity than miRNA-21 analysis; however, represented a crucial role in activation of TGF-β signaling leading towards pulmonary remodeling and development of fibrosis. These findings were supported by earlier studies wherein altered TGF-β-induced miRNA regulation and differential expression of miRNAs has shown one of the mechanisms underlying aberrant tissue repair and ECM remodeling in pulmonary fibrosis (Ong et al., 2017a, Ong et al., 2017b, Ong et al., 2019).
Although several recent studies have explored several pleiotropic effects of miRNA-21 in ECM remodeling, none of them have provided such crucial targeted mechanistic approach in COVID patients in the development of pulmonary fibrosis (Jiang et al., 2019; Derda et al., 2021; Dingsdag et al., 2021). Our study explored crucial involvement of miRNA-21 in regulating TGF-β pathway essential for the development of pulmonary fibrosis. More importantly, early quantification of miRNA-21 in circulation may provide crucial diagnostic value in real-time disease progress along with radiological findings in clinical settings.
Despite, our study provided essential clue to understand pulmonary fibrosis in post-COVID patients with high diagnostic value; the study was limited to the enrollment of very less number of participants in only age range of 50 to 60 years in a single centre which avoids population-bias in different regions of the country. Furthermore, study couldn't discriminate gender-wise significance similar to other studies (Patil et al., 2021). Quantifying miRNA-21 in large number of patients in multi-centre studies may provide more authentic value based on the gender and different age range.
In summary, our study demonstrated that miRNA-21 may play an important role in pulmonary remodeling, making it a good candidate miRNA for further research in COVID-19 patients after getting discharged from the hospital for 3–6 months. We tried to derive role of miRNA-21 in pulmonary remodeling from a variety of clues specifically targeting TGF-β pathway which provides a direct evidence for its involvement of pulmonary fibrosis. In accordance with the findings of current study, we postulated that maintaining balance between degenerative and regenerative mechanisms by inhibition of miRNA-21 and its target molecules such as Col1A2, Col3A1, α-SMA, including TGF-β pathway such as myofibroblast activation and macrophase/monocyte growth, and accumulation of overactive cells may provide crucial strategy to eliminate pulmonary fibrosis development in early stages with better treatment outcome (Fig. 4B). Therefore, our study represents need for an experimental exploration into the aforementioned factors, with the goal that such unique insights may be valuable for future research in this field.
5. Conclusion
Our study findings demonstrated that quantifying circulating miRNA-21 may provide crucial insights for elucidating TGF-β mediated pulmonary remodeling involved in fibrosis developments and achieve better clinical outcome in real-time with high diagnostic accuracy. Hence, by modulating miRNA-21 and their targets, it may be possible to explore better diagnostic, prognostic and therapeutic approaches that may influence post-COVID complications in the future.
Credit author statement
Mohammad Ali, Fahed Abdullah, Aleemuddin Naveed, Aleem Ahmed Khan: Collected samples, Performed experiments, Data acquisition, and Manuscript writing.
Syed Mahmood Ahmed, Aleem Ahmed Khan, Ashfaq Hasan: Designed and coordinated the study, Data validation, Edited the manuscript, and Arranged resources for conducting the study.
Declaration of Competing Interest
All the authors have declared no conflict of interest for publication of this study.
Acknowledgements
Authors thank to study participants for providing their samples and useful clinical information for the study. Manuscript writing, formatting, and publication support was provided from Dr. Sandeep Kumar Vishwakarma (CLRD, DCMS, Hyd).
References
- Baratella E., Ruaro B., Marrocchio C., Starvaggi N., Salton F., Giudici F., et al. Interstitial lung disease at high resolution CT after SARS-CoV2-related acute respiratory distress syndrome according to pulmonary segmental anatomy. J. Clin. Med. 2021;10:3985. doi: 10.3390/jcm10173985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazdyrev E., Rusina P., Panova M., Novikov F., Grishagin I., Nebolsin V. Lung fibrosis after COVID-19: treatment prospects. Pharmaceuticals (Basel) 2021;14(8):807. doi: 10.3390/ph14080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.H., Gelinas R., Wang K., et al. Systems biology of interstitial lung diseases: integration of mRNA and microRNA expression changes. BMC Med. Genet. 2011;4:8. doi: 10.1186/1755-8794-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Cutroneo K.R., White S.L., Phan S.H., Ehrlich H.P. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J. Cell. Physiol. 2007;211:585–589. doi: 10.1002/jcp.20972. [DOI] [PubMed] [Google Scholar]
- Derda A.A., Garg A., Bär C., Thum T. Reply to ‘COVID-19 severity, miR-21 targets, and common human genetic variation’. Eur. J. Heart Fail. 2021;23(11):1987–1988. doi: 10.1002/ejhf.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingsdag S.A., Clay O.K., Quintero G.A. COVID-19 severity, miR-21 targets, and common human genetic variation. Letter regarding the article ‘Circulating cardiovascular microRNAs in critically ill COVID-19 patients’. Eur. J. Heart Fail. 2021;23(11):1986–1987. doi: 10.1002/ejhf.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnso K.W., Almadani Y., Philip A. Non-canonical (non-SMAD2/3) TGF-β signaling in fibrosis: mechanisms and targets. Semin. Cell Dev. Biol. 2020;101:115–122. doi: 10.1016/j.semcdb.2019.11.013. [DOI] [PubMed] [Google Scholar]
- Georg P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of India Ministry of Health & Family Welfare Directorate General of Health Services (EMR Division) 2020. Revised Guidelines on Clinical Management of COVID – 19. 31st March; pp. 1–18.https://www.mohfw.gov.in/pdf/RevisedNationalClinicalManagementGuidelineforCOVID1931032020.pdf [Google Scholar]
- Henry T.W., Mendoza F.A., Jimenez S.A. Role of microRNA in the pathogenesis of systemic sclerosis tissue fibrosis and vasculopathy. Autoimmun. Rev. 2019;18(11) doi: 10.1016/j.autrev.2019.102396. [DOI] [PubMed] [Google Scholar]
- Jiang C., Guo Y., Yu H., Lu S., Meng L. Pleiotropic microRNA-21 in pulmonary remodeling: novel insights for molecular mechanism and present advancements. Allergy, Asthma Clin. Immunol. 2019;15:33. doi: 10.1186/s13223-019-0345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz I., Kele¸s F. COVID-19-related lung involvement at different time intervals: evaluation of computed tomography images with semiquantitative scoring system and COVID-19 reporting and data system scoring. Cureus. 2021;13 doi: 10.7759/cureus.18554. doi:10.7759/cureus. 18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach H.G., Chrobak I., Han R., Trojanowska M. Endothelial cells recruit macrophages and contribute to a fibrotic milieu in bleomycin lung injury. Am. J. Respir. Cell Mol. Biol. 2013;49(6):1093–1101. doi: 10.1165/rcmb.2013-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Friggeri A., Yang Y., Milosevic J., Ding Q., Thannickal V.J., Kaminski N., Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.H., Ning B., Ma X.E., Gong W.M., Jia T.H. Regulatory roles of microRNA-21 in fibrosis through interaction with diverse pathways (review) Mol. Med. Rep. 2016;13:2359–2366. doi: 10.3892/mmr.2016.4834. [DOI] [PubMed] [Google Scholar]
- Loboda A., Sobczak M., Jozkowicz A., Dulak J. TGF-β1/Smads and miR-21 in renal fibrosis and inflammation. Mediat. Inflamm. 2016;2016:8319283. doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald L.T. Healing after COVID-19: are survivors at risk for pulmonary fibrosis? Am. J. Phys. Lung Cell. Mol. Phys. 2021;320(2):L257–L265. doi: 10.1152/ajplung.00238.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafaei S., Sayad B., Azar M.E.F., Doroudian M., Hadifar S., Behrouzi A., et al. The role of viral and bacterial infections in the pathogenesis of IPF: a systematic review and meta analysis. Respir. Res. 2021;22(1):01650-x–x. doi: 10.1186/s12931-021-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo A.S., Balogun S.A., Williams O.T., Ojo O.S. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulm. Med. 2020;2020:6175964. doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J., Timens W., Rajendran V., Algra A., Spira A., Lenburg M.E., et al. Identification of transforming growth factor-beta-regulated microRNAs and the microRNA-targetomes in primary lung fibroblasts. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0183815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J., Timens W., Rajendran V., Algra A., Spira A., Lenburg M.E., Campbell J.D., van den Berge M., Postma D.S., van den Berg A., Kluiver J., Brandsma C.A. Identification of transforming growth factor-beta-regulated microRNAs and the microRNA-targetomes in primary lung fibroblasts. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0183815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J., Faiz A., Timens W., van den Berge M., Terpstra M.M., Kok K., van den Berg A., Kluiver J., Brandsma C.A. Marked TGF-β-regulated miRNA expression changes in both COPD and control lung fibroblasts. Sci. Rep. 2019;9(1):18214. doi: 10.1038/s41598-019-54728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S.V., Gondhali G., Patil R. Post-covid-19 lung fibrosis: study of 600 cases in tertiary care setting in India. Eur. Respir. J. 2021;58 doi: 10.1183/13993003.congress-2021.PA3776. [DOI] [Google Scholar]
- Rumende C.M. Pulmonary fibrosis caused by severe COVID-19 infection: discharge may not be the end of treatment. Acta Med. Indones. 2021;53(2):141–142. (PMID: 34251340) [PubMed] [Google Scholar]
- Spagnolo P., Oldham J.M. Fibrotic lung disease: a molecular glimpse into severe Covid-19? EBioMedicine. 2021;69 doi: 10.1016/j.ebiom.2021.103470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K.H., Chang Y., Reed N.I., Sheppard D. α-Smooth muscle actin is an inconsistent marker of fibroblasts responsible for force-dependent TGFβ activation or collagen production across multiple models of organ fibrosis. Am. J. Phys. Lung Cell. Mol. Phys. 2016;310(9):L824–L836. doi: 10.1152/ajplung.00350.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A., Pelliccia C., Ferrara F. COVID-19 patients with pulmonary fibrotic tissue: clinical pharmacological rational of antifibrotic therapy. SN Compr. Clin. Med. 2020;27:1–4. doi: 10.1007/s42399-020-00487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Hong P., Wang Z., Tang Z., Li K. MicroRNAs in transforming growth factor-beta signaling pathway associated with fibrosis involving different systems of the human body. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.707461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Hong P., Wang Z., Tang Z., Li K. MicroRNAs in transforming growth factor-beta signaling pathway associated with fibrosis involving different systems of the human body. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.707461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Kubo H., Ota C., Takahashi T., Tando Y., Suzuki T., Fujino N., Makiguchi T., Takagi K., Suzuki T., Ichinose M. The increase of microRNA-21 during lung fibrosis and its contribution to epithelial-mesenchymal transition in pulmonary epithelial cells. Respir. Res. 2013;14(1):95. doi: 10.1186/1465-9921-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Zheng S.D., Wu H.J., Chen S.J. Regulatory mechanisms of the molecular pathways in fibrosis induced by MicroRNAs. Chin. Med. J. 2016;129(19):2365–2372. doi: 10.4103/0366-6999.190677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Li S.Q. Pulmonary fibrosis in critically ill patients with novel coronavirus pneumonia during the convalescent stage and a proposal for early intervention. Acta Pharmacol. Sin. 2021;42(8):1376–1378. doi: 10.1038/s41401-020-00566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]